Abstract
Purpose
To describe coronavirus disease 2019 (COVID-19) mortality in Chicago during the spring of 2020 and identify at the census-tract level neighborhood characteristics that were associated with higher COVID-19 mortality rates.
Methods
Using Poisson regression and regularized linear regression (elastic net), we evaluated the association between neighborhood characteristics and COVID-19 mortality rates in Chicago through July 22 (2514 deaths across 795 populated census tracts).
Results
Black residents (31% of the population) accounted for 42% of COVID-19 deaths. Deaths among Hispanic/Latino residents occurred at a younger age (63 years, compared with 71 for white residents). Regarding residential setting, 52% of deaths among white residents occurred inside nursing homes, compared with 35% of deaths among black residents and 17% among Hispanic/Latino residents. Higher COVID-19 mortality was seen in neighborhoods with heightened barriers to social distancing and low health insurance coverage. Neighborhoods with a higher percentage of white and Asian residents had lower COVID-19 mortality. The associations differed by race, suggesting that neighborhood context may be most tightly linked to COVID-19 mortality among white residents.
Conclusions
We describe communities that may benefit from supportive services and identify traits of communities that may benefit from targeted campaigns for prevention and testing to prevent future deaths from COVID-19.
Keywords: COVID-19, Prevention, Social determinants of health, Built environment, Health disparities
Introduction
Coronavirus disease 2019 (COVID-19), the illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in the fall of 2019 and was declared a pandemic by the World Health Organization in March 2020 [1]. No natural immunity exists, and while severely ill patients can benefit from supportive care [2,3], no medical cure is available. In the United States, more than 200,000 Americans have died of the disease before the middle of September 2020 [4]. The risk of COVID-19–related morbidity and mortality increases with age [5,6], and is higher in individuals with pre-existing conditions, including respiratory disease, cardiovascular disease, recent cancer history, kidney disease, liver disease, vascular disease, immune disorders, and diabetes [7].
As the pandemic has progressed in the United States, it has become clear that, the impact of COVID-19 has been felt more acutely in some communities [8,9], most clearly among black Americans, who acquire the disease and die at disproportionate rates [[10], [11], [12]]. Social determinants of health may drive some of this disparity, and neighborhood traits may be particularly relevant, given that infectious disease spread is often influenced by the built environment [13]. Determining neighborhood factors that are associated with COVID-19 could identify modifiable risk factors that may directly influence COVID-19 risk, and recent research has postulated associations with air quality [14,15], population density [16], and public transit use [17]. Further, identifying neighborhood traits that are associated with COVID-19 risk, even if they are not causally related, can identify communities with high need for support and testing and prevention services to mitigate the continued impact of COVID-19.
In the present analysis, we describe COVID-19 mortality in the spring and early summer of 2020 in Chicago, the third most populous city in the United States [18]. Further, with a focus on deaths that occurred outside of nursing homes, we identify at the census-tract level neighborhood characteristics that are associated with COVID-19 mortality in Chicago.
Methods
Deaths related to COVID-19 in Chicago
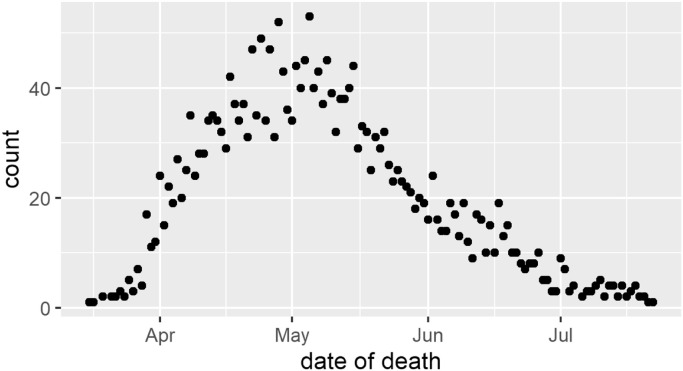
The Cook County Medical Examiner provides a public record of deaths in Cook County [19]. Beginning on March 16, 2020, there was a rapid increase in daily deaths from COVID-19; this count peaked in early May, at which point deaths declined through July (eFig. 1). Between March 16 and July 22, 2020, 4834 deaths were recorded with “novel corona virus” listed as a primary or secondary cause of death. Along with the age and race of the decedent, each record includes an incident address (populated in 97% of COVID-19 deaths); when the cause of death is an infectious disease, this almost always reflects home address. Addresses were standardized and geocoded [20], and Chicago residents were included in the subsequent analysis.
Figure s1.
Daily deaths from novel coronavirus in Chicago through July 22, 2020.
Deaths that occurred in nursing homes or assisted living facilities were identified using three methods: address match with Medicare's Nursing Home Compare database [21] (836 deaths), address match with Medicaid-managed care facilities contracted with Cook County Health and Hospital System (33 additional deaths identified), and a manual review of address in which more than four deaths occurred (21 additional deaths identified).
Neighborhood characteristics
Following the framework laid out by others [22], we curated a list of characteristics of neighborhoods that may be associated with (1) increased chance of COVID-19 infection or (2) increased risk of mortality if infected. In total, we considered 33 neighborhood characteristics, detailed in Table 1 . Unless otherwise noted, characteristics were estimated at the census-tract level from the American Community Survey 2018 five-year estimates [23]. Characteristics putatively associated with infection risk included the following: crowded living conditions, transportation habits, dense housing, and sociodemographic characteristics related to heightened barriers to social distancing. Characteristics putatively associated with risk of mortality included the following: health care access, comorbid conditions and demographic traits associated with more severe COVID-19 infection, indicators of poverty, and chronic exposure to poor air quality.
Table 1.
Neighborhood characteristics evaluated with COVID-19 infection and mortality
| Infection risk | Crowded living conditions | Residences without complete kitchens, residences with more than one occupant per room, grandparents living with children under 18 years. |
| Transportation habits | Residences without a car available, commuting primarily by public transit, commuting primarily by carpool. | |
| Dense housing | Housing units in buildings with more than 20 units, population density. | |
| Sociodemographic characteristics that might be associated with heightened barriers to social distancing | SNAP use, broadband Internet at home, educational attainment, ability to work from home. | |
| Mortality Risk | Health care access | Health insurance status (American Community Survey), access to a primary care provider (Chicago Health Atlas estimate at the community area level 2016–2018 [[24]]). |
| Presence of comorbid conditions suspected to be associated with more severe disease | Rate of heart disease deaths per 100,000, rate of diabetes-related deaths per 100,000, rate of nephrotic disease deaths per 100,000, and rate of tobacco-related deaths per 100,000 (all from Chicago Health Atlas [[24]] estimate at the community area level 2013–2017). | |
| Age and biological sex | Male sex, population aged 65–74 years, population aged 75+ years. | |
| Indicators of poverty | Poverty rate, unemployment rate, households spending more than 35% of their income on rent, historical redlining of the neighborhood (University of Richmond Mapping Inequality [[25]]). | |
| Air quality | Concentration of nitrogen dioxide (NO2), ozone (O3), and particulate matter smaller than 2.5 microns (PM2.5). | |
| Structural | Structural racism | Percent population that is non-Hispanic black, percent of population that is non-Hispanic white, percent of population that is Hispanic/Latino, percent of population that is non-Hispanic Asian. |
To assess air quality at the census tract scale, high-resolution (1.3 km2) simulations from the two-way coupled community multiscale air quality-weather research and forecasting [[26]] (CMAQ-WRF) model were performed. Our air quality exposure analysis included nitrogen dioxide (NO2) and particulate matter less than 2.5 microns (PM2.5) and ozone (O3) pollutant data. While air quality varies seasonally, and air quality was impacted by the widespread stay-at-home directives during the spring of 2020 [[25], [26], [27]], our aim was to model the influence of long-term exposure to air quality, rather than acute exposure as the pandemic was ongoing. As such, for PM2.5 and NO2, we averaged the exposure from two seasonally representative pre-COVID-19 months (August 2018 and January 2019). For ozone, given its high dependency on temperature, we averaged 8-hour daytime max values during a relevant representative pre-COVID summertime month (August 2018).
We also included indicators of the racial and ethnic makeup of the census tract as a proxy for the influence of structural racism that may not be captured by the other characteristics [22].
Statistical approach
In institutionalized settings, the community in which the facility is located may have a more distant relationship with COVID-19 mortality, as the traits of the facility itself most directly influence the risk of COVID-19 [[30]]. As such, we next focused on deaths that occurred outside of nursing homes (also excluding the five deaths that occurred at the Cook County jail).
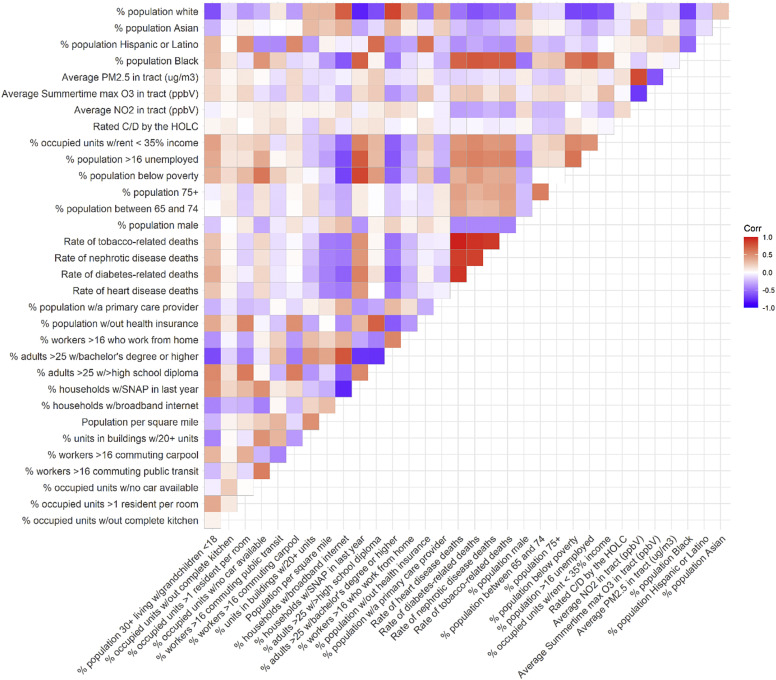
In Chicago, many neighborhood characteristics are highly correlated with one another (eFig. 2). In recognition of this, two analytical approaches were taken. The aim of the first was to describe Chicago census tracts with high COVID-19 mortality rates. The count of COVID-19 deaths was modeled as a Poisson distribution, and regressed upon each of the 33 neighborhood characteristics sequentially, with the log of the population of the census tract as an offset. The P-values from these bivariate regressions were corrected using the Benjamini-Hochberg procedure. In each regression, no other tract-level descriptors were included as covariates. As such, while this analysis well-described communities that were heavily impacted by COVID-19 deaths, any given association may be explained by the presence of other correlated characteristics.
Figure s2.
Correlation between tract-level characteristics in Chicago.
The aim of the second approach was to account for the correlation between the neighborhood traits and identify those that continued to be robustly associated with COVID-19 mortality. In the second approach, all 33 neighborhood characteristics were included as predictors in a regularized generalized linear regression model with elastic net penalty. We used the R package glmnet [[31],[32]] with the elastic net mixing parameter fixed at 0.5 and 20-fold cross-validation to select the tuning parameter. COVID-19 deaths were again modeled as a Poisson distribution with the log of the population of the census tract as an offset. We used 200 bootstrap replications to obtain 95% confidence intervals and to obtain the frequency selection of each trait. Empirically, all penalization penalties >0 produced substantively similar results (i.e., a ridge penalty was found to be inappropriate, and a least absolute shrinkage and selection operator (LASSO) penalty produced substantively similar results), and a tuning parameter of 0.5 was chosen to make use of the flexibility of the elastic net.
Results
Between March 16 and July 22 2020, 2514 COVID-19 deaths were recorded within Chicago. Although non-Hispanic black residents comprise 31% of Chicago's population, they accounted for 42% of the COVID-19 deaths. All other racial and ethnic groups were under-represented: non-Hispanic white residents comprise 32% of Chicago's population, Hispanic/Latino residents 29%, and Asian residents 6%.
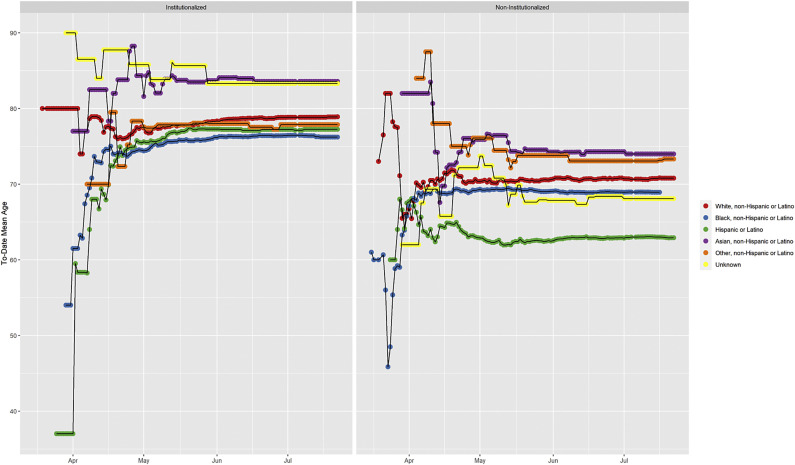
We identified 895 deaths (36%) among people living in an institutionalized setting. Selected demographic characteristics of individuals who died are shown in Table 2 . The percentage of deaths that occurred in nursing homes differed by race and ethnicity: 52% of those who died and were white lived in a nursing home, compared with 18% of those who died and were Hispanic/Latino. Outside of nursing homes, the proportion of deaths by race and ethnicity changed throughout the course of the spring of 2020 (eFig. 3). Before mid-May, more than 50% of the deaths occurred among black individuals. While initially few deaths occurred among Hispanic/Latino residents (12% of deaths before April 1), the proportion of deaths among this demographic steadily rose (32% of deaths by July 22).
Table 2.
Demographic characteristics of COVID-19 deaths in Chicago through July 22 by residential setting
| Overall n = 2514 | Institutionalized n = 885 | Noninstitutionalized n = 1619 | |
|---|---|---|---|
| Mean age (SD) | 71 (15) | 78 (12) | 67 (15) |
| Race and ethnicity | |||
| Black, non-Hispanic/Latino | 1053 (42%) | 374 (42%) | 679 (42%) |
| White, non-Hispanic/Latino | 663 (26%) | 343 (38%) | 320 (20%) |
| Hispanic/Latino | 644 (26%) | 113 (13%) | 531 (33%) |
| Asian, non-Hispanic/Latino | 85 (3%) | 38 (4%) | 47 (3%) |
| Other, non-Hispanic/Latino | 35 (1%) | 17 (2%) | 18 (1%) |
| Unknown | 34 (1%) | 10 (1%) | 24 (1%) |
| Gender | |||
| Female | 1002 (40%) | 393 (44%) | 609 (38%) |
| Male | 1507 (60%) | 501 (56%) | 1006 (62%) |
| Unknown | 5 (0%) | 1 (0%) | 4 (0%) |
Figure s3.
Trends in racial and ethnic composition of COVID-19 deaths in Chicago.
The mean age at death remained relatively stable over the same time (eFig. 4). For those who died outside of nursing homes, the age at death differed by a decade by race and ethnicity. The mean age at death among Hispanic/Latino residents was 63 years, 69 years for black residents, 71 years for white residents, and 74 years for Asian residents.
Figure s4.
Trends in age of death from COVID-19 deaths in Chicago.
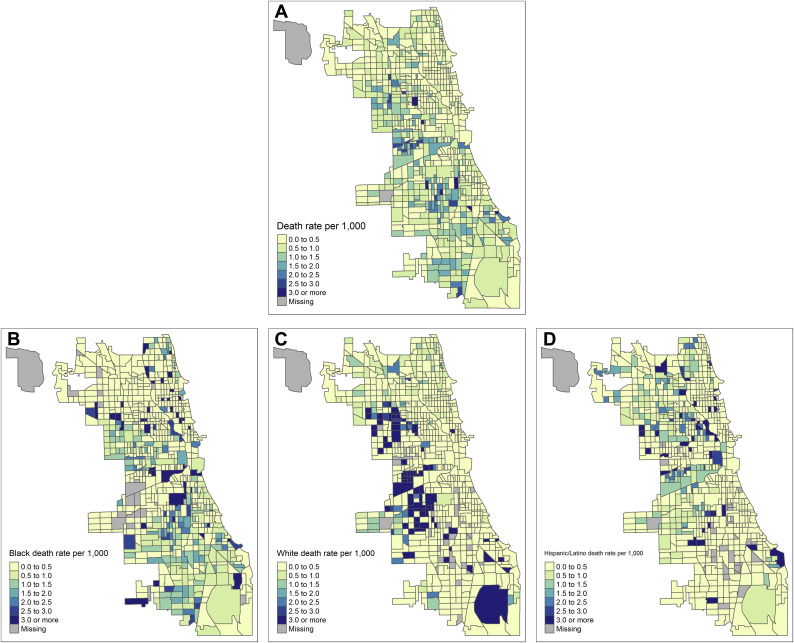
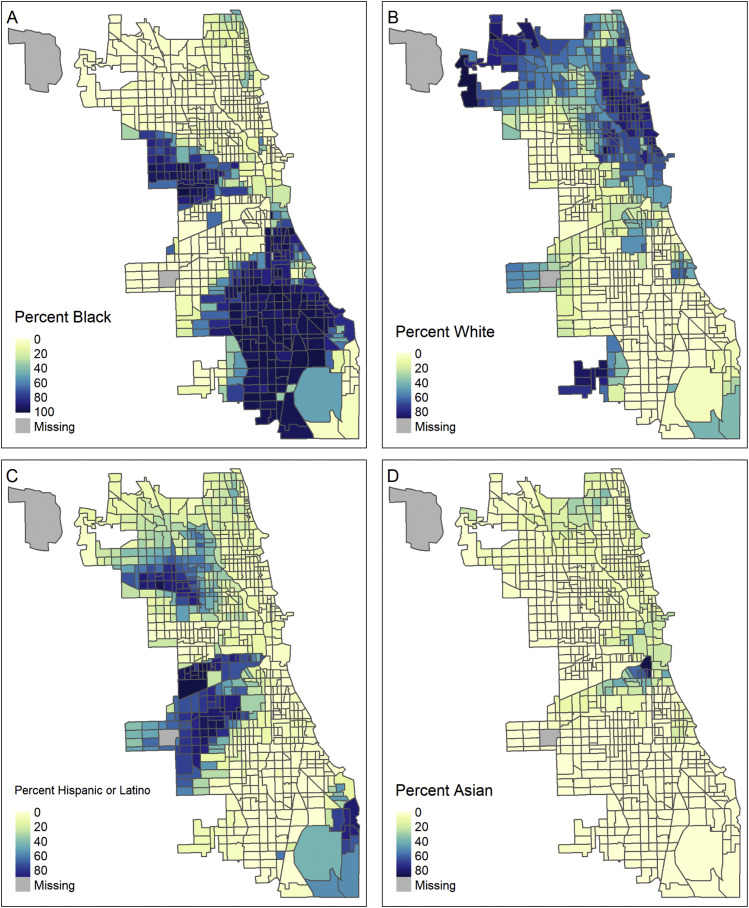
Figure 1 maps the rate of COVID-19 deaths that occurred among individuals in noninstitutionalized residential settings in each of Chicago's 795 populated census tracts per thousand residents [[33]]. Panel A displays this death rate overall, and panels B–D are restricted to deaths that occurred among residents who were black (B), white (C), or Hispanic/Latino (D) (as recorded by the medical examiner), each as a fraction of the number of residents in that tract of the given race/ethnicity. Chicago is a city with high levels of residential segregation (eFig. 5) [[34]]. In the overall Chicago population, areas with higher death rates were located on the west and south sides of Chicago, with the highest death rates occurring in majority-minority census-tracts. This pattern was particularly pronounced when focusing on deaths that occurred to white residents; in neighborhoods that were majority-minority, the proportion of white residents who died from COVID-19 (as a proportion of the white population in that neighborhood) was generally higher than it was in neighborhoods who were majority white. In contrast, high rates of COVID-19 mortality among black and Hispanic/Latino residents were found throughout the city.
Fig. 1.
COVID-19 death rates among noninstitutionalized population per thousand residents. For noninstitutionalized population, the rate of COVID-19 deaths per 1000 population (A), and subset of deaths in the black population (B), white population (C), and Hispanic/Latino population (D). Census-tracts with missing rates are census-tracts with no population (for A) or census-tracts with no population of the given race (for B–D).
Figure s5.
Racial and ethnic composition of Chicago.
Bivariate Poisson regression associations
Table 3 (column A) summarizes the bivariate associations of neighborhood characteristics with COVID-19 death rate. Among neighborhood characteristics putatively associated with increased risk of infection Table 3A, top), higher death rates were seen in neighborhoods with heightened barriers to social distancing (high proportion of supplemental nutrition assistance program (SNAP) recipients, fewer households with broadband Internet, lower educational attainment, and fewer workers able to work from home). Crowded living conditions were similarly associated with higher COVID-19 mortality, whereas neighborhoods with dense housing had lower rates. Higher COVID-19 death rates were also seen in neighborhoods where fewer residents had cars or where more residents carpooled; no association was seen with public transportation.
Table 3.
Bivariate and regularized linear regression associations between neighborhood characteristics and COVID-19 death rates in the noninstitutionalized population of Chicago (n = 1619 deaths)
| Domain | Neighborhood characteristic | (A) Bivariate association | (B) Regularized linear regression | |
|---|---|---|---|---|
| Infection | Crowded living conditions | % population 30+ living w/grandchildren <18 | 1.27 (1.22–1.33); P ≤ .0001∗ | 1 (12%; 0.989 to 1.000) |
| % occupied units w/out complete kitchen | 1.10 (1.05–1.14); P ≤ .0001∗ | 1 (13%; 1.000 to 1.022) | ||
| % occupied units >1 resident per room | 1.21 (1.16–1.27); P ≤ .0001∗ | 1 (32%; 1.000 to 1.017) | ||
| Transportation | % occupied units w/no car available | 1.09 (1.04–1.15); P = .0004∗ | 1 (1%; 1.000 to 1.000) | |
| % workers >16 commuting public transit | 0.97 (0.93–1.02); P = .3157 | 1 (29%; 1.000 to 1.007) | ||
| % workers >16 commuting carpool | 1.23 (1.18–1.28); P ≤ .0001∗ | 1 (18%; 1.000 to 1.005) | ||
| Dense housing | % units in buildings w/20+ units | 0.76 (0.71–0.80); P ≤ .0001∗ | 1 (36%; 0.997 to 1.000) | |
| Population/m2 | 0.82 (0.78–0.87); P ≤ .0001∗ | 1 (30%; 0.000 to 1.000) | ||
| Barriers to social distancing | % households w/broadband internet | 0.63 (0.60–0.67); P ≤ .0001∗ | 0.992 (99%; 0.986 to 0.999)† | |
| % households w/SNAP in last year | 1.50 (1.43–1.57); P ≤ .0001∗ | 1 (44%; 1.000 to 1.006) | ||
| % adults >25 w/> high school diploma | 1.39 (1.34–1.45); P ≤ .0001∗ | 1.004 (82%; 1.000 to 1.011)ˆ | ||
| % adults >25 w/bachelor's degree or higher | 0.57 (0.54–0.61); P ≤ .0001∗ | 0.999 (68%; 0.995 to 1.000)ˆ | ||
| % workers >16 who work from home | 0.79 (0.74–0.83); P ≤ .0001∗ | 1 (8%; 1.000 to 1.006) | ||
| Mortality | Health care access | % population without health insurance | 1.35 (1.30–1.41); P ≤ .0001∗ | 1.013 (98%; 1.000 to 1.025)† |
| % population w/a primary care provider | 0.81 (0.77–0.85); P ≤ .0001∗ | 1 (14%; 0.996 to 1.000) | ||
| Comorbid conditions | Rate of heart disease deaths | 1.19 (1.13–1.25); P ≤ .0001∗ | 1 (0%; 1.000 to 1.000) | |
| Rate of diabetes-related deaths | 1.31 (1.24–1.37); P ≤ .0001∗ | 1 (30%; 1.000 to 1.002) | ||
| Rate of nephrotic disease deaths | 1.26 (1.20–1.31); P ≤ .0001∗ | 1.001 (62%; 1.000 to 1.009)ˆ | ||
| Rate of tobacco-related deaths | 1.20 (1.14–1.26); P ≤ .0001∗ | 1 (4%; 1.000 to 1.000) | ||
| Age and gender | % population male | 0.95 (0.90–1.00); P = .0355∗ | 1 (22%; 1.000 to 1.014) | |
| % population between 65 and 74 | 1.11 (1.05–1.17); P ≤ .0001∗ | 1.005 (62%; 1.000 to 1.033)ˆ | ||
| % population 75+ | 1.11 (1.06–1.16); P ≤ .0001∗ | 1.005 (68%; 1.000 to 1.024)ˆ | ||
| Indicators of poverty | % population below poverty | 1.38 (1.32–1.45); P ≤ .0001∗ | 1 (14%; 1.000 to 1.004) | |
| % population >16 unemployed | 1.36 (1.30–1.42); P ≤ .0001∗ | 1 (18%; 1.000 to 1.004) | ||
| % occupied units w/rent > 35% income | 1.35 (1.28–1.42); P ≤ .0001∗ | 1 (13%; 1.000 to 1.002) | ||
| Rated C/D by the HOLC | 1.13 (1.08–1.19); P ≤ .0001∗ | 1 (42%; 1.000 to 1.194) | ||
| Air quality | Average NO2 in tract (ppbV) | 0.92 (0.88–0.97); P = .0012∗ | 1 (19%; 0.983 to 1.000) | |
| Average summertime O3 in tract (ppbV) | 1.13 (1.08–1.18); P ≤ .0001∗ | 1 (8%; 1.000 to 1.007) | ||
| Average PM2.5 in tract (ug/m3) | 1.01 (0.97–1.06); P = .6107 | 1 (28%; 0.901 to 1.000) | ||
| Structural | Structural racism | % population Black | 1.32 (1.25–1.38); P ≤ .0001∗ | 1 (4%; 1.000 to 1.000) |
| % population Hispanic/Latino | 1.19 (1.14–1.24); P ≤ .0001∗ | 1 (18%; 1.000 to 1.003) | ||
| % population Asian | 0.67 (0.62–0.73); P ≤ .0001∗ | 0.995 (95%; 0.987 to 1.000)† | ||
| % population white | 0.57 (0.54–0.61); P ≤ .0001∗ | 0.993 (100%; 0.990 to 0.997)† | ||
| Model metrics | R2-like statistic: 0.2518 | |||
| deviance ratio: 0.2638 |
The reported bivariate associations (Column A) are the rate ratios from a Poisson regression with counts of the noninstitutionalized deaths predicted by the given neighborhood characteristic, with the offset equal to the population of the census tract. 95% confidence intervals are reported in parentheses. Benjamini-Hochberg p-values are reported, and those BH adjusted p-values that are below 0.05 are marked with an asterisk (∗).
The reported regularized linear regression associations (Column B) are the rate ratios from a regularized (elastic net) Poisson regression with counts of the noninstitutionalized deaths of Chicago residents assigned the given race predicted by the given neighborhood characteristic, with the offset equal to the log of the population of the census-tract. In parentheses is the percentage of bootstrap replications in which the variable was selected, along with bootstrapped 95% confidence intervals for the estimate. Variables that were selected by more than 50% of the bootstrap replications are highlighted with a caret (ˆ), and variables that were selected by more than 90% of the bootstrap replications are highlighted with a dagger (†). The reported R2-like statistic is: , where
Among neighborhood characteristics associated with populations at higher risk of severe disease (Table 3A, middle), most were associated with higher rates of COVID-19 mortality. Mortality was higher in neighborhoods with worse access to health care, more comorbid conditions, older age, higher rates of poverty, and neighborhoods that had historically been redlined. Summertime concentrations of maximum O3 were associated with higher death rates, and average concentrations of NO2 were inversely associated with death rates.
Our analysis confirms that neighborhoods with a greater percentage of black and Hispanic/Latino residents saw higher COVID-19 death rates during the spring of 2020 (Table 3A, bottom). For each additional percentage point of the population that was black, there was a 32% increase in the COVID-19 death rate, and for each additional percentage point of the population that was Hispanic/Latino, there was an 19% increase in the COVID-19 death rate. Conversely, neighborhoods with a higher percentage of Asian or white residents saw lower death rates.
Regularized regression associations
The results from the regularized regression model are shown on the right of Table 3 (Table 3B). After implementing the regularized regression to further account for the correlation between the neighborhood traits, COVID-19 death rate remained robustly associated with four neighborhood traits (here, “robustly” includes traits selected by more than more than 90% of the bootstrap replications). The death rate was lower in neighborhoods with higher penetration of broadband internet service and higher percentages of white or Asian residents, and the death rate was higher in neighborhoods with more residents without health insurance. Additional characteristics were suggestively associated with COVID-19 death rate: lower education, higher rates of severe kidney disease, and a higher percentage of the population above age 65 years (here, “suggestively” includes traits selected by between 50% and 90% of the bootstrap replications).
Table 4 displays the results of the regularized regression for the race and ethnicity-specific death rates. As shown in Table 4 (left), the COVID-19 death rate among black residents was only weakly associated with neighborhood characteristics, as none were selected by more than 50% of the bootstrap replications, and the deviance ratio was essentially zero.
Table 4.
Race and ethnicity-specific regularized regression (elastic net) associations between neighborhood characteristics and COVID-19 death rates in the noninstitutionalized population of Chicago
| Domain | Neighborhood characteristic | Non-Hispanic black |
Non-Hispanic white |
Hispanic/Latino |
|
|---|---|---|---|---|---|
| n = 679 | n = 320 | n = 531 | |||
| Infection | Crowded living conditions | % population 30+ years living w/grandchildren <18 | 1 (8%; 0.988 to 1.000) | 1.006 (63%; 1.000 to 1.051)ˆ | 1 (14%; 0.990 to 1.001) |
| % occupied units w/out complete kitchen | 1 (2%; 1.000 to 1.000) | 1 (22%; 1.000 to 1.050) | 1 (12%; 0.990 to 1.013) | ||
| % occupied units >1 resident per room | 1 (1%; 1.000 to 1.000) | 1.020 (86%; 1.000 to 1.063)ˆ | 1 (43%; 0.998 to 1.025) | ||
| Transportation | % occupied units w/no car available | 1 (3%; 1.000 to 1.000) | 1 (10%; 1.000 to 1.004) | 1 (5%; 1.000 to 1.001) | |
| % workers >16 commuting public transit | 1 (0%; 1.000 to 1.000) | 1 (30%; 1.000 to 1.013) | 1.001 (60%; 1.000 to 1.015)ˆ | ||
| % workers >16 commuting carpool | 1 (6%; 0.995 to 1.000) | 1.005 (62%; 1.000 to 1.030)ˆ | 1 (38%; 1.000 to 1.015) | ||
| Dense housing | % units in buildings w/20+ units | 1 (2%; 1.000 to 1.000) | 1 (36%; 0.995 to 1.000) | 1 (8%; 0.998 to 1.000) | |
| Population per square mile | 1 (6%; 0.000 to 1.000) | 0.001 (70%; 0.000 to 1.000)ˆ | 1 (5%; 1.000 to 10.543) | ||
| Barriers to social distancing | % households w/broadband Internet | 1 (20%; 0.994 to 1.000) | 0.993 (82%; 0.981 to 1.000)ˆ | 1 (47%; 0.985 to 1.000) | |
| % households w/SNAP in the last year | 1 (2%; 1.000 to 1.000) | 1.003 (73%; 1.000 to 1.018)ˆ | 1 (22%; 1.000 to 1.006) | ||
| % adults >25 w/>high school diploma | 1 (1%; 1.000 to 1.000) | 1.021 (100%; 1.005 to 1.035)† | 1.000 (53%; 1.000 to 1.011)ˆ | ||
| % adults >25 w/bachelor's degree or higher | 1 (0%; 1.000 to 1.000) | 0.995 (86%; 0.989 to 1.000)ˆ | 1 (0%; 1.000 to 1.000) | ||
| % workers >16 who work from home | 1 (1%; 1.000 to 1.000) | 1 (16%; 1.000 to 1.025) | 1 (5%; 0.994 to 1.000) | ||
| Mortality | Health care access | % population without health insurance | 1 (2%; 1.000 to 1.000) | 1.011 (79%; 1.000 to 1.029)ˆ | 1.003 (64%; 1.000 to 1.016)ˆ |
| % population w/a primary care provider | 1 (0%; 1.000 to 1.000) | 1 (12%; 0.998 to 1.006) | 0.998 (57%; 0.984 to 1.000)ˆ | ||
| Comorbid conditions | Rate of heart disease deaths | 1 (0%; 1.000 to 1.000) | 1 (2%; 1.000 to 1.000) | 1 (42%; 0.999 to 1.000) | |
| Rate of diabetes-related deaths | 1 (6%; 1.000 to 1.002) | 1 (5%; 0.999 to 1.000) | 1 (1%; 1.000 to 1.000) | ||
| Rate of nephrotic disease deaths | 1 (2%; 1.000 to 1.000) | 1 (36%; 1.000 to 1.020) | 1 (0%; 1.000 to 1.000) | ||
| Rate of tobacco-related deaths | 1 (0%; 1.000 to 1.000) | 1 (8%; 0.999 to 1.000) | 1 (30%; 0.999 to 1.000) | ||
| Age and gender | % population male | 1 (8%; 0.996 to 1.000) | 1 (15%; 0.990 to 1.013) | 1.002 (55%; 1.000 to 1.040)ˆ | |
| % population between 65 and 74 | 1 (38%; 1.000 to 1.031) | 1 (20%; 1.000 to 1.037) | 1 (2%; 1.000 to 1.000) | ||
| % population 75+ | 1 (9%; 1.000 to 1.007) | 1.002 (54%; 1.000 to 1.048)ˆ | 1 (4%; 1.000 to 1.009) | ||
| Indicators of poverty | % population below poverty | 1 (1%; 1.000 to 1.000) | 1 (10%; 1.000 to 1.004) | 1 (37%; 1.000 to 1.009) | |
| % population >16 unemployed | 1 (6%; 1.000 to 1.002) | 1 (14%; 1.000 to 1.013) | 1 (6%; 0.997 to 1.000) | ||
| % occupied units w/rent > 35% income | 1 (0%; 1.000 to 1.000) | 1.003 (70%; 1.000 to 1.012)ˆ | 1 (11%; 1.000 to 1.005) | ||
| Rated C/D by the HOLC | 1 (4%; 1.000 to 1.016) | 1 (23%; 0.975 to 1.197) | 1 (47%; 1.000 to 1.326) | ||
| Air quality | Average NO2 in tract (ppbV) | 1 (16%; 0.964 to 1.000) | 1 (41%; 0.926 to 1.000) | 1.025 (80%; 1.000 to 1.080)ˆ | |
| Average O3 in tract (ppbV) | 1 (2%; 1.000 to 1.000) | 1.016 (72%; 1.000 to 1.079)ˆ | 1 (4%; 0.996 to 1.000) | ||
| Average PM2.5 in tract (ug/m3) | 1 (4%; 0.980 to 1.000) | 1 (16%; 1.000 to 1.141) | 1 (8%; 0.890 to 1.000) | ||
| Structural | Structural racism | % population black | 1 (0%; 1.000 to 1.000) | 1 (0%; 1.000 to 1.000) | 1 (12%; 1.000 to 1.003) |
| % population Hispanic/Latino | 1 (0%; 1.000 to 1.000) | 1.005 (94%; 1.000 to 1.011)† | 1 (2%; 1.000 to 1.000) | ||
| % population Asian | 1 (0%; 1.000 to 1.000) | 0.988 (95%; 0.975 to 1.000)† | 1 (18%; 0.990 to 1.000) | ||
| % population white | 1 (0%; 1.000 to 1.000) | 0.992 (98%; 0.986 to 0.999)† | 1 (6%; 0.999 to 1.000) | ||
| Model metrics | R2-like statistic | 0.0000 | 0.2865 | 0.0547 | |
| Deviance ratio | 0.0000 | 0.3336 | 0.0198 |
The reported regularized linear regression associations (Column B) are the rate ratios from a regularized (elastic net) Poisson regression. The dependent variable was the number of noninstitutionalized deaths of Chicago residents for a given race/ethnicity (as recorded by the medical examiner) within a census tract, with the offset equal to the log of the number of residents of that race/ethnicity living in the census-tract. In parentheses is the percentage of bootstrap replications in which the variable was selected, along with bootstrapped 95% confidence intervals for the estimate. Variables that were selected by more than 50% of the bootstrap replications are highlighted with a caret (ˆ), and variables that were selected by more than 90% of the bootstrap replications are highlighted with a dagger (†). The reported R2-like statistic is: , where
Among Hispanic/Latino residents (Table 4, right), no robust associations were found, and the deviance ratio was low (0.0198). Suggestive associations were found with public transit, high school diplomas, health insurance coverage, primary care providers, gender, and exposure to NO2.
In contrast, among white residents (Table 4, middle), there were robust associations between COVID-19 death rate and four neighborhood characteristics. The death rate among white residents was higher in neighborhoods with more residents without a high school diploma, and a greater percentage of residents who were Hispanic or Latino, and the death rate was lower in neighborhoods with higher percentages of white or Asian residents. There were further suggestive associations between higher death rates and intergenerational households, crowded housing, carpool use, population density, broadband Internet access, SNAP use, bachelor degree attainment, health insurance, age, rent-to-income ratios, and summertime ozone concentration.
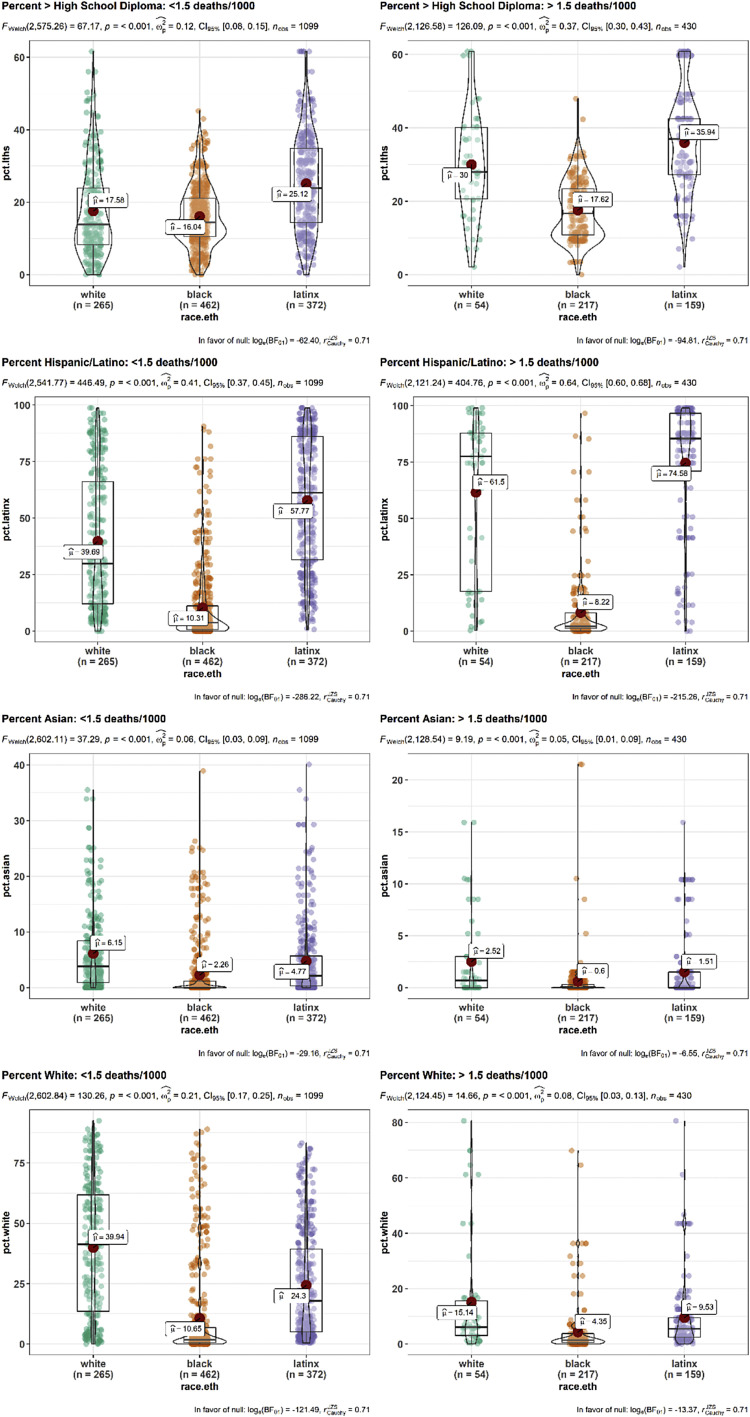
To assess whether these race-specific findings were due to more uniformity in neighborhood traits among nonwhite residents, for the traits that were robustly associated with white death rate but not with black and Hispanic/Latino death rate, we compared the variability in neighborhood traits by the race of the decedent (eFig. 6). While neighborhoods of black residents who died had less variability terms of high school completion, there was no such difference in variability in the neighborhoods of Hispanic/Latino residents who died, and further, there was broad variability in the neighborhood-level race/ethnicity makeup. As such, homogeneity in neighborhood traits could not fully explain the lack of associations among nonwhite deaths.
Figure s6.
Variability in neighborhood characteristics by race. Variability by race of the of two neighborhood characteristics robustly associated with COVID-19 mortality among white residents: (A, B) percent of adults without a high school diploma, (C, D) Percent of population that is Hispanic/Latino, (E, F) Percent of population that is Asian, and (G, H) percent of the population that is white. To further investigate whether neighborhoods with high death rates had an outsized influence on the results, the plots were separated based on whether they occurred in neighborhoods with low to moderate COVID-19 death rates (i.e., less than 1.5 deaths per thousand) (right side) or in neighborhoods with high COVID-19 death rates (i.e., more than 1.5 deaths per thousand, left side).
During the observed study period, the city of Chicago implemented a phased reopening policy, in which residents and businesses were able to participate in increasingly less restrictive activities. On June 3, and June 26, Chicago entered “phase 3” and “phase 4,” respectively [[35],[36]]. To assess whether the results were sensitive to this timing, we repeated the overall regularized regression analyses twice: first limiting to deaths that occurred before June 3 (2118 deaths; 1324 among the noninstitutionalized population), and those that occurred before June 26 (2417 and 1544 deaths, respectively). The results were substantively similar (eTable 1).
Discussion
During the spring of 2020, COVID-19 impacted the residents of the city of Chicago unevenly. Black residents were at the highest risk of death from COVID-19, and Hispanic/Latino residents died from COVID-19 at an appreciably younger age than all other ethnic groups. This racial disparity in COVID-19 mortality largely mirrors the already existing racial disparity that is seen in Chicago for deaths from non-COVID-related causes [[37]]. The deaths were not evenly distributed throughout Chicago's neighborhoods. We identified characteristics that were more often seen in neighborhoods with higher COVID-19 mortality, including heightened barriers to social distancing and low health care access. Conversely, neighborhoods with a higher percentage of white and Asian residents had lower COVID-19 mortality. We further found suggestive evidence that COVID-19 mortality may be associated with the educational attainment of the neighborhood, the rates of severe kidney disease, and percentage of residents who were above 65 years.
The associations between neighborhood characteristics and COVID-19 mortality differed by race and ethnicity. While the overall death rate was higher among black residents, no neighborhood characteristics were associated with COVID-19 death rates among black residents specifically, whereas among white residents, several were associated with COVID-19 death rate. This suggests that for white residents, COVID-19 mortality may be more tightly linked to neighborhood context than for black residents.
Our analysis suggests that controlling for the correlated nature of the neighborhood characteristics is necessary, as bivariate associations suggested inverse associations between COVID-19 mortality rates and air quality, density, and public transit use. These associations that generally did not persist in the regularized regression models. These paradoxical bivariate associations may be due to Chicago-specific desire of affluent residents to live close to the lake (with dense housing) or highways (which are near public transit hubs and often have poor air quality). Although additional study designs are necessary to confirm that these associations are not due to residual confounding, the regularized regression approach statistically controls for more aspects of this confounding than a standard Poisson regression.
This work is part of a growing body of literature that explores social determinants of COVID-19 severity. A study from New York City characterized neighborhood traits associated with COVID-19 infection [[38]]. Consistent with our results that study found that housing value, housing density, and income were protective against infection, whereas crowded households were associated with increased risk. Other work has compiled aggregate indices of social vulnerability [9,[39],[40]] and found that cumulative levels of social vulnerability were associated with increased mortality. Our census tract-level analysis provides additional specificity of which neighborhood characteristics may be driving that vulnerability. Our focus on deaths that occurred outside of institutionalized settings and our use of a regularized regression approach also enabled us to offer a more precise characterization of the communities with high death rates.
Although there has been speculation of the role of public transit in enabling SARS-CoV-2 infection, mainly from studies that examined county-level associations [[39], [40], [41]], in our analysis, public transit use was not robustly associated with COVID-19 mortality. Our analysis focused on the city of Chicago, which, like many urban areas in the spring of 2020, saw more widespread infection than nonurban counties [4]. Our tract-level single-city analysis allowed us to avoid comparing areas in which both the transportation habits and viral exposure were systematically different, and therefore avoid a potential source of confounding.
Air quality has also been proposed as a risk factor for COVID-19 mortality, with evidence coming from analyses that covered large geographic areas (U.S. counties and administrative units across Europe) [14,[44]]. Because our analysis examined the relationship between air quality and COVID-19 within a uniformly urban area, we avoided comparing air quality (which tends to be worse in urban areas [[45]]) between populations with different potential to be exposed to the virus. Our CMAQ-WRF simulated air quality exposure also assigned individual residential exposures at a fine level of spatial resolution. We observed no robust associations between NO2, O3, or PM2.5 and COVID-19 mortality. While there was moderate evidence for a higher risk of mortality associated with NO2 among Hispanic/Latino residents and with O3 among white residents, given that the findings were not robust and also differed by race, we are cautious of overinterpretation of the observed associations. As high-resolution air quality models are developed that estimate the air quality during the pandemic, future work will be able to estimate the effect of acute exposure to poor air quality.
Our work focused on COVID-19 mortality. While currently available data on confirmed SARS-CoV-2 infections were limited, and during the spring of 2020, the ability to confirm an infection was limited due to uneven access to testing [[46],[47]], future work that identifies separately the influence social determinants of health on risk of infection, severe disease, and mortality will bring additional context to these findings. Similarly, future work that can identify those who died while experiencing homelessness can help to identify risk factors that may be particularly relevant for that vulnerable population. We were also limited in the neighborhood characteristics that were available at the census tract level. Other characteristics would have provided additional context, such as obesity rates and percentage of residents working in “essential” businesses. While the inclusion of these additional variables (along with possible interaction terms) might produce a model with better prediction capacity, our model does clearly describe the associations between well-measured and widely available neighborhood characteristics and COVID-19 mortality. Finally, we emphasize that the identified neighborhood characteristics may not directly influence COVID-19 mortality, and the variables selected by the regularized regression may not ultimately be causally associated with COVID-19 mortality. Instead, we expect that the identified traits broadly reflect a legacy of policy choices that have negatively impacted the health of those living in the neighborhood [22], and further work is needed to examine how the identified characteristics may cooccur and possibly modify one another.
Given the disruptions that the COVID-19–related deaths brought, this analysis identifies communities that may have unmet needs of supportive services [[48],[49]], and may benefit from the kind of “psychiatry-palliative care liaison teams” [[50]] that were deployed in New York City in response to the pandemic. Because SARS-CoV-2 continues to spread, this research can be used to help identify communities that may also benefit from additional resources and education that would improve their capacity to detect and prevent infection [[51],[52]]. The results of this research can be incorporated into communication and training to make them more relevant to the most affected populations and begin to mitigate the COVID-19–specific health disparities experienced by these communities.
Acknowledgments
The authors would like to thank Natalia Derevyanny at the Cook County Medical Examiner for answering questions, as well as Bea Malsky for her helpful discussions about the data. The authors also thank William Trick and Keiki Hinami at Cook County Health and Hospital System for their assistance in identifying congregate living facilities. We also thank the Texas A&M geocoding services for providing geocoding services for COVID-19–related research.
Appendix
Table s1.
Regularized regression associations between neighborhood characteristics and COVID-19 death rates in the noninstitutionalized population of Chicago June 3 (1544 deaths) and June 26 (2118 deaths)
| Domain | Neighborhood characteristic | June 3 | June 26 | |
|---|---|---|---|---|
| Infection | Crowded living conditions | % population 30+ living w/grandchildren <18 | 1 (8%; 0.992 to 1.000) | 1 (10%; 0.991 to 1.000) |
| % occupied units w/out complete kitchen | 1 (20%; 0.999 to 1.022) | 1 (17%; 0.993 to 1.014) | ||
| % occupied units >1 resident per room | 1 (21%; 1.000 to 1.014) | 1 (34%; 1.000 to 1.020) | ||
| Transportation | % occupied units w/no car available | 1 (2%; 1.000 to 1.000) | 1 (2%; 1.000 to 1.000) | |
| % workers >16 commuting public transit | 1 (26%; 1.000 to 1.006) | 1 (29%; 1.000 to 1.006) | ||
| % workers >16 commuting carpool | 1 (7%; 1.000 to 1.003) | 1 (10%; 1.000 to 1.005) | ||
| Dense housing | % units in buildings w/20+ units | 1 (12%; 0.999 to 1.000) | 1 (34%; 0.997 to 1.000) | |
| Population/m2 | 1 (20%; 0.000 to 1.000) | 1 (26%; 0.000 to 1.000) | ||
| Barriers to social distancing | % households w/broadband internet | 0.988 (100%; 0.982 to 0.994)† | 0.991 (100%; 0.985 to 0.998)† | |
| % households w/SNAP in last year | 1.001 (64%; 1.000 to 1.006)ˆ | 1.001 (56%; 1.000 to 1.006)ˆ | ||
| % adults >25 w/> high school diploma | 1.002 (68%; 1.000 to 1.010)ˆ | 1.003 (79%; 1.000 to 1.010)ˆ | ||
| % adults >25 w/bachelor's degree or higher | 1 (48%; 0.997 to 1.000) | 0.999 (64%; 0.996 to 1.000)ˆ | ||
| % workers >16 who work from home | 1 (8%; 1.000 to 1.009) | 1 (7%; 1.000 to 1.005) | ||
| Mortality | Health care access | % population without health insurance | 1.015 (98%; 1.003 to 1.029)† | 1.014 (99%; 1.004 to 1.027)† |
| % population w/a primary care provider | 1 (16%; 0.996 to 1.000) | 1 (16%; 0.995 to 1.000) | ||
| Comorbid conditions | Rate of heart disease deaths | 1 (1%; 1.000 to 1.000) | 1 (0%; 1.000 to 1.000) | |
| Rate of diabetes-related deaths | 1 (26%; 1.000 to 1.003) | 1 (17%; 1.000 to 1.001) | ||
| Rate of nephrotic disease deaths | 1.003 (81%; 1.000 to 1.010)ˆ | 1.001 (62%; 1.000 to 1.008)ˆ | ||
| Rate of tobacco-related deaths | 1 (2%; 1.000 to 1.000) | 1 (4%; 1.000 to 1.000) | ||
| Age and gender | % population male | 1 (12%; 1.000 to 1.005) | 1 (24%; 1.000 to 1.017) | |
| % population between 65 and 74 | 1.006 (67%; 1.000 to 1.030)ˆ | 1.008 (72%; 1.000 to 1.040)ˆ | ||
| % population 75+ | 1.003 (62%; 1.000 to 1.025)ˆ | 1.004 (64%; 1.000 to 1.032)ˆ | ||
| Indicators of poverty | % population below poverty | 1 (12%; 1.000 to 1.002) | 1 (18%; 1.000 to 1.003) | |
| % population >16 unemployed | 1 (18%; 1.000 to 1.004) | 1 (6%; 1.000 to 1.001) | ||
| % occupied units w/rent > 35% income | 1 (12%; 1.000 to 1.002) | 1 (19%; 1.000 to 1.003) | ||
| Rated C/D by the HOLC | 1 (38%; 1.000 to 1.135) | 1 (34%; 1.000 to 1.134) | ||
| Air quality | Average NO2 in tract (ppbV) | 1 (36%; 0.967 to 1.000) | 1 (28%; 0.975 to 1.000) | |
| Average summertime O3 in tract (ppbV) | 1 (4%; 1.000 to 1.000) | 1 (8%; 1.000 to 1.009) | ||
| Average PM2.5 in tract (ug/m3) | 1 (28%; 0.913 to 1.000) | 1 (35%; 0.908 to 1.000) | ||
| Structural | Structural racism | % population black | 1 (32%; 1.000 to 1.002) | 1 (12%; 1.000 to 1.001) |
| % population Hispanic/Latino | 1 (2%; 1.000 to 1.000) | 1 (13%; 1.000 to 1.002) | ||
| % population Asian | 0.997 (88%; 0.990 to 1.000)ˆ | 0.995 (96%; 0.988 to 1.000)† | ||
| % population white | 0.994 (100%; 0.990 to 0.997)† | 0.993 (100%; 0.990 to 0.996)† | ||
| Model metrics | R2-like statistic | |||
| Deviance ratio | 0.2600 | 0.2525 |
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [accessed 07.02.20]
- 2.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 3.STAT’s Covid-19 Drugs, Vaccines Tracker. STAT 2020. https://www.statnews.com/2020/04/27/drugs-vaccines-tracker/ [accessed 09.28.20]
- 4.Home. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/ [accessed 04.21.20]
- 5.Garg S., Kim L., Whitaker M., O'Halloran A., Cummings C., Holstein R., et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein S.L., Dhakal S., Ursin R.L., Deshpande S., Sandberg K., Mauvais-Jarvis F. Biological sex impacts COVID-19 outcomes. PLOS Pathog. 2020;16:e1008570. doi: 10.1371/journal.ppat.1008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020 doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oronce C.I.A., Scannell C.A., Kawachi I., Tsugawa Y. Association Between State-Level Income Inequality and COVID-19 Cases and Mortality in the USA. J Gen Intern Med. 2020 doi: 10.1007/s11606-020-05971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S.J., Bostwick W. Social Vulnerability and Racial Inequality in COVID-19 Deaths in Chicago. Health Educ Behav. 2020 doi: 10.1177/1090198120929677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yancy C.W. COVID-19 and African Americans. JAMA. 2020 doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 11.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L.F., Chernyak Y., et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. MedRxiv. 2020 [Google Scholar]
- 12.Kullar R., Marcelin J.R., Swartz T.H., Piggott D.A., Macias Gil R., Mathew T.A., et al. Racial Disparity of Coronavirus Disease 2019 (COVID-19) in African American Communities. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinter-Wollman N., Jelić A., Wells N.M. The impact of the built environment on health behaviours and disease transmission in social systems. Philosophical Trans R Soc B: Biol Sci. 2018;373 doi: 10.1098/rstb.2017.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. MedRxiv. 2020:2020. doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocklöv J., Sjödin H. High population densities catalyse the spread of COVID-19. J Trav Med. 2020;27 doi: 10.1093/jtm/taaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dorn A., Cooney R.E., Sabin M.L. COVID-19 exacerbating inequalities in the US. Lancet. 2020;395:1243–1244. doi: 10.1016/S0140-6736(20)30893-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bureau U.C. Population Estimates and Projections (2010 - 2019). The United States Census Bur. https://www.census.gov/data/developers/data-sets/popest-popproj.html [accessed 04.21.20]
- 19.Medical Examiner Case Archive | Cook County Open Data. Cook County. https://datacatalog.cookcountyil.gov/Public-Safety/Medical-Examiner-Case-Archive/cjeq-bs86/data [accessed 04.19.20]
- 20.Goldberg D. Texas A&M University Geoservices. http://geoservices.tamu.edu
- 21.Find and compare Nursing Homes | Nursing Home Compare. https://www.medicare.gov/nursinghomecompare/search.html [accessed 06.29.20]
- 22.Bechteler S., Kane-Willis K., Butler K., Espinosa-Ravi I. Chicago Urban League; Chicago: 2020. An Epidemic of Inequities: Structural Racism and COVID-19 in the Black Community.https://chiul.org/wp-content/uploads/2020/05/ChicagoUrbanLeague_An-Epidemic-of-Inequities_5-12-20.pdf [accessed 30.12.20] [Google Scholar]
- 23.Bureau U.C. American Community Survey (ACS). The United States Census Bureau. https://www.census.gov/programs-surveys/acs [accessed 06.29.20]
- 24.Wong D.C., Pleim J., Mathur R., Binkowski F., Otte T., Gilliam R., et al. WRF-CMAQ two-way coupled system with aerosol feedback: software development and preliminary results. Geoscientific Model Dev. 2012;5:299–312. [Google Scholar]
- 25.Horton D.E., Skinner C.B., Singh D., Diffenbaugh N.S. Occurrence and persistence of future atmospheric stagnation events. Nat Clim Change. 2014;4:698–703. doi: 10.1038/nclimate2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diffenbaugh N.S., Field C.B., Appel E.A., Azevedo I.L., Baldocchi D.D., Burke M., et al. The COVID-19 lockdowns: a window into the Earth System. Nat Rev Earth Environ. 2020;1:470–481. [Google Scholar]
- 27.Kroll J.H., Heald C.L., Cappa C.D., Farmer D.K., Fry J.L., Murphy J.G., et al. The complex chemical effects of COVID-19 shutdowns on air quality. Nat Chem. 2020;12:777–779. doi: 10.1038/s41557-020-0535-z. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Temkin-Greener H., Gao S., Cai X. COVID-19 infections and deaths among Connecticut nursing home residents: facility correlates. J Am Geriatr Soc. 2020 doi: 10.1111/jgs.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman J.H., Hastie T., Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2018. https://www.R-project.org
- 31.Datasets - Data.gov. https://catalog.data.gov/dataset?tags=shapefiles&organization=city-of-chicago [accessed 07.13.2020]
- 32.Iceland J., Weinberg D.H., Steinmetz E. Bureau of Census; Washington, DC: 2002. Racial and ethnic residential segregation in the United States 1980-2000.https://www.census.gov/content/dam/Census/library/publications/2002/dec/censr-3.pdf [Google Scholar]
- 33.Mayor Lightfoot, CDPH Reaffirm Chicago to Begin Cautious Reopening City of Chicago Office of the Mayor 2020. 2020. https://www.chicago.gov/city/en/depts/mayor/press_room/press_releases/2020/june/CautiousReopeningContinues.html [accessed 09.21.20]
- 34.Mayor Lightfoot and CDPH Announce Chicago Ready to Move to Phase Four on Friday, June 26. City of Chicago Office of the Mayor. 2020. https://www.chicago.gov/city/en/depts/mayor/press_room/press_releases/2020/june/PhaseFour.html [accessed 09.21.20]
- 35.Chicago Department of Public Health . Chicago Department of Public Health; Chicago: 2020. 2020. Healthy Chicago 2025, Closing our Life Expectancy Gap 2020-2025.https://www.chicago.gov/content/dam/city/depts/cdph/statistics_and_reports/HC2025_917_FINAL.pdf Chicago, Illinois. [Google Scholar]
- 36.Emeruwa U.N., Ona S., Shaman J.L., Turitz A., Wright J.D., Gyamfi-Bannerman C., et al. Associations Between Built Environment, Neighborhood Socioeconomic Status, and SARS-CoV-2 Infection Among Pregnant Women in New York City. JAMA. 2020 doi: 10.1001/jama.2020.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J., Waterman P., Krieger N. COVID-19 and the unequal surge in mortality rates in Massachusetts, by city/town and ZIP Code measures of poverty, household crowding, race/ethnicity, and racialized economic segregation. Harv Cent Popul Dev Stud Working Paper Ser. 2020;19 [Google Scholar]
- 38.Khazanchi R., Beiter E.R., Gondi S., Beckman A.L., Bilinski A., Ganguli I. County-Level Association of Social Vulnerability with COVID-19 Cases and Deaths in the USA. J Gen Intern Med. 2020 doi: 10.1007/s11606-020-05882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng R., Xu Y., Wang W., Ning G., Bi Y. Spatial transmission of COVID-19 via public and private transportation in China. Trav Med Infect Dis. 2020;34:101626. doi: 10.1016/j.tmaid.2020.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebrahim S.H., Ahmed Q.A., Gozzer E., Schlagenhauf P., Memish Z.A. Covid-19 and community mitigation strategies in a pandemic. BMJ. 2020;368 doi: 10.1136/bmj.m1066. [DOI] [PubMed] [Google Scholar]
- 41.McLaren J. National Bureau of Economic Research; Cambridge, MA: 2020. Racial Disparity in COVID-19 Deaths: Seeking Economic Roots with Census data. [Google Scholar]
- 42.Wu X., Nethery R.C., Benjamin Sabath M., Braun D., Dominici F. COVID-19 PM2.5: A national study on long-term exposure to air pollition and COVID-19 mortality in the United States. https://projects.iq.harvard.edu/covid-pm/home [accessed 04.24.20] [DOI] [PMC free article] [PubMed]
- 43.Karner A.A., Eisinger D.S., Niemeier D.A. Near-Roadway Air Quality: Synthesizing the Findings from Real-World Data. Environ Sci Technol. 2010;44:5334–5344. doi: 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- 44.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., et al. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 45.Rader B., Astley C.M., Sy K.T.L., Sewalk K., Hswen Y., Brownstein J.S., et al. Geographic access to United States SARS-CoV-2 testing sites highlights healthcare disparities and may bias transmission estimates. J Travel Med. 2020 doi: 10.1093/jtm/taaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompkins F., Goldblum P., Lai T., Hansell T., Barclay A., Brown L.M. A culturally specific mental health and spirituality approach for African Americans facing the COVID-19 pandemic. Psychol Trauma. 2020 doi: 10.1037/tra0000841. [DOI] [PubMed] [Google Scholar]
- 47.Novacek D.M., Hampton-Anderson J.N., Ebor M.T., Loeb T.B., Wyatt G.E. Mental health ramifications of the COVID-19 pandemic for Black Americans: Clinical and research recommendations. Psychol Trauma. 2020 doi: 10.1037/tra0000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shalev D., Nakagawa S., Stroeh O.M., Arbuckle M.R., Rendleman R., Blinderman C.D., et al. The Creation of a Psychiatry-Palliative Care Liaison Team: Using Psychiatrists to Extend Palliative Care Delivery and Access During the COVID-19 Crisis. J Pain Symptom Manage. 2020 doi: 10.1016/j.jpainsymman.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruine de Bruin W., Bennett D. Relationships Between Initial COVID-19 Risk Perceptions and Protective Health Behaviors: A National Survey. Am J Prev Med. 2020 doi: 10.1016/j.amepre.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren E., Levin A. n/a; 2020. Building a Coronavirus Containment Corps: A Plan to Expand America’s Public Health Workforce and Stop the Spread of COVID-19.https://www.warren.senate.gov/download/042120-coronavirus-containment-corps-plan_final&download=1 [Google Scholar]
- 51.Chicago Health. https://www.chicagohealthatlas.org/indicators [accessed 01.18.19]
- 52.Mapping Inequality. https://dsl.richmond.edu/panorama/redlining/ [accessed 06. 02.20]