Abstract
Objective
To assess the performance of a fecal immunochemical test (FIT) among participants of a population-based colorectal cancer (CRC) screening program with one or more first-degree relatives (FDR) with CRC.
Methods
Asymptomatic 50 to 74 years olds with a FDR diagnosed with CRC, enrolled in a colon screening program completed FIT (two samples, cut-off 20 µg Hemoglobin/gram feces) and underwent colonoscopy. FIT-interval CRCs were identified from the British Columbia cancer registry. Logistic regression analysis was used to identify variables associated with the detection of CRC and high-risk polyps (nonmalignant findings that required a 3-year surveillance colonoscopy) in those patients undergoing FIT and colonoscopy.
Results
Of the 1387 participants with a FDR with CRC, 1244 completed FIT with a positivity rate of 10.8%, 52 declined FIT but underwent colonoscopy and 90 declined screening. Seven CRCs were identified: six in patients with a positive FIT, one in a patient who only had colonoscopy. No CRCs were found in patients with a negative FIT. The positive and negative predictive values of FIT in the detection of CRC were 4.8% and 100%, respectively. On multivariate logistic regression, positive FIT, and not type of family history, was the only variable associated with detection of CRC or high-risk polyps. At 2-year follow-up, there was no FIT interval cancer detected in the study cohort.
Conclusion
FIT is more strongly associated with high-risk findings on colonoscopy than type of family history. FIT may be an alternative screening strategy to colonoscopy in individuals with a single FDR with CRC.
Keywords: Advanced neoplasia, Colonoscopy, Family history of colon cancer, Fecal immunochemical test
INTRODUCTION
Individuals with a first-degree relative (FDR) with colorectal cancer (CRC) are at increased risk of developing CRC compared to the general population (1). The risk increases as the number of family members with CRC increases and the age of diagnosis of CRC decreases (2). A positive family history, excluding known hereditary syndromes is linked to approximately 20% of cases of CRC (3).
The recently published Canadian Association of Gastroenterology Consensus Guidelines on CRC screening in individuals with a family history of nonhereditary CRC highlight the importance of screening but state that the evidence is not of high enough quality to recommend a specific screening strategy in these individuals (2). Due to the high risk for individuals with multiple FDRs diagnosed with CRC, the Consensus Group recommended that individuals with two or more FDRs undergo screening with colonoscopy every 5 years commencing at 40 years of age or 10 years younger than the age of diagnosis of the FDR, whichever is earlier. The recommendations for individuals with one FDR with CRC are to perform colonoscopy every 5 to 10 years commencing at 40 to 50 years of age or 10 years younger than the age of diagnosis of the FDR, whichever is earlier. Fecal immunochemical test (FIT) every 1 or 2 years commencing at 40 to 50 years or 10 years younger than the age of diagnosis of the FDR, whichever is earlier, was recommended as a second line strategy. The Consensus Group stressed that the risk appears to be higher the younger the FDR was at the time of CRC diagnosis and then decreases on a continuum.
CRC screening programs generally do not distinguish individuals with a family history of CRC, who are either screened in an opportunistic fashion outside of programs or grouped with those of average risk. The British Columbia (BC) Colon Screening Program does offer primary screening colonoscopy to participants with a FDR with CRC diagnosed at younger age than 60 or two or more FDRs diagnosed with CRC at any age. Additionally, in the first 3 years of the program, a participant with a FDR with CRC, regardless of age of diagnosis, was offered colonoscopy. In an effort to understand the utility of FIT in this population, participants with a family history of CRC were also requested to complete FIT.
The objective of this study is to demonstrate the performance of FIT amongst participants with one or more FDRs with CRC in a population-based screening program.
METHODS
Eligible subjects were asymptomatic men and women, 50 to 74 years of age with a FDR with CRC living in one of three BC communities participating in a colon cancer-screening program. The family history was divided into three groups: (i) 1 FDR diagnosed ≥ 60 years old, (ii) 1 FDR diagnosed < 60 years old and (iii) ≥ 2 FDRs with CRC diagnosed at any age. The program nurse coordinators confirmed the family history before enrolment. Exclusion criteria were rectal bleeding, personal history of CRC, personal history of inflammatory bowel disease or colonoscopy/sigmoidoscopy within the last 5 years.
From January 1, 2009 to April 1, 2011, all participants with at least one FDR with CRC were recommended to undergo colonoscopy and were also asked to complete FIT prior to colonoscopy. From April 1, 2011, those individuals with a single FDR diagnosed with CRC at age 60 years or older were offered a colonoscopy only if the FIT was positive. Each participant received two FIT kits in the mail and was instructed to take one sample each from two consecutive bowel movements. The kits were transported to a central laboratory for analysis. A semi-automated quantitative FIT, OC-Auto Micro 80 (Polymedco Inc. New York, USA and Somagen, Canada) was used. The FIT was considered positive, if either test was ≥ 20 mcg Hb/g of stool.
The colonoscopies were performed by community physicians, who completed a standard reporting form documenting colonoscopy quality indicators, polyp morphology and type of resection. Tissue specimens were assessed by BC Cancer Agency pathologists, and reported in a standardized format. All data were collected prospectively, and stored in a centralized database.
Review of the BC Cancer Registry was conducted on November 12, 2018 to determine whether any of the participants in this study were diagnosed with FIT interval CRC following a negative FIT or colonoscopy. There is mandatory reporting of all cancers diagnosed in to the BC Cancer Registry.
Pathology was classified based upon the most significant lesion. High-risk polyps (HRPs) referred to nonmalignant findings that required a 3-year surveillance colonoscopy (4): adenomas ≥10 mm in size, ≥ 25% villous features, adenomas with high-grade dysplasia, sessile serrated adenomas/polyps, traditional serrated adenomas and ≥ 3 tubular adenomas. At the time of the pilot, there were no guidelines for surveillance of serrated lesions and the program decided to recommend surveillance colonoscopy 3 years following polypectomy of a sessile serrated adenoma or traditional serrated adenoma until 2013 when surveillance was altered to fit the 2012 United States Multi-Society Task Force guidelines on postpolypectomy surveillance (5).
Statistical Analysis
Univariate and multivariate logistic regression were used to investigate the association of different variables with neoplasia detection. Results are reported using odds ratio and the corresponding 95% confidence intervals. All statistical tests used were two sided with the P-value ≤ 0.005 considered statistically significant. All analysis was performed using SAS 9.3 (SAS Institute, Cary, USA) and R 3.1.2 (6).
The Human Ethics Board at the BC Cancer Agency reviewed and approved this study.
RESULTS
From January 2009 to April 2013, 17,031 men and women, 50 to 74 years of age were enrolled in the program, of which 1387 participants had at least one FDR with CRC. The outcomes of the average risk cohort have been previously published (7).
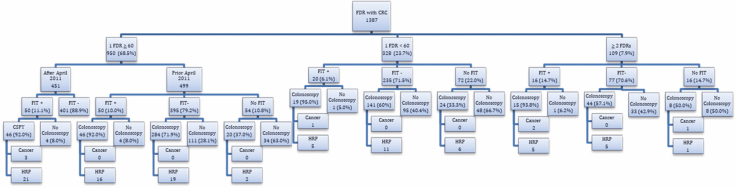
The median age of the 1387 participants was 62 years (10th, 90th percentile: 53, 72) and 61.0% of the participants were male. The family history of the cohort was as follows: 950 (68.5%) had 1 FDR ≥ 60 years of age at time of CRC diagnosis, 328 (23.7%) had 1 FDR less than 60 years of age and 109 (7.9%) had 2 FDRs with CRC.
One thousand two hundred and forty-four participants completed at least one FIT and the positivity rate was 10.9%. Fifty-two underwent colonoscopy and declined FIT, and 90 underwent neither FIT nor colonoscopy and are excluded from further analysis (Figure 1).
Figure 1.
Fecal immunochemical test and colonoscopy results by family history.
Colonoscopies were performed on 647 participants. A total of 98 high-risk findings were detected, including 7 participants with CRC and 91 with HRPs. Six of the cancers were identified in patients with a positive FIT and one in a patient who declined to undergo FIT. Considering the 126 participants with a positive FIT who underwent colonoscopy, the positive predictive values for the detection of CRC and HRPs were 4.8% and 37.3%, respectively. Considering the 469 participants with a negative FIT who underwent colonoscopy, the negative predictive values of FIT in the detection of CRC and HRP were 100% and 92.5%. There was no FIT interval CRC in the study cohort with a minimum of 2 years of follow-up through the BC Cancer Registry.
Regression analysis was performed to identify variables associated with CRC or HRPs at colonoscopy. The 549 participants included in this analysis were those who underwent both FIT and colonoscopy. The 451 participants with a single FDR ≥ 60 years who were enrolled in the program after April 1, 2011 were not included in the regression analysis as colonoscopy was performed only for a positive FIT. Logistic univariate regression analysis is shown in Table 1. Positive FIT (OR: 8.1; 95% CI: 4.3, 15.0) and male gender (OR: 1.8; 95% CI: 1.0, 3.3) were significantly associated with detection of CRC or HRPs. A statistically nonsignificant association was observed between increasing age and detection of CRC or a HRP. The type of family history did not increase the likelihood of CRC or HRPs.
Table 1.
Univariate analysis of factors associated with CRC or HRPs
| Variable name | P-value | Odds ratio (95% CI) |
|---|---|---|
| FIT (Positive vs. Negative) | <0.0001 | 8.07 (4.34, 15.02) |
| Gender (Male vs. Female) | 0.04 | 1.82 (1.01, 3.26) |
| FHx type | 0.45 | |
| 1FDR<60 vs. 1FDR ≥ 60 | 0.92 (0.46, 1.82) | |
| 2FDR vs. 1FDR ≥ 60 | 1.63 (0.71, 3.76) | |
| Age category | 0.29 | |
| 55–59 vs. 50–54 | 1.37 (0.47, 3.98) | |
| 60–64 vs. 50–54 | 1.92 (0.70, 5.32) | |
| 65–69 vs 50–54 | 2.11 (0.75, 5.94) | |
| 70–74 vs. 50–54 | 2.89 (1.02–7.87) |
CRC, colorectal cancer; HRP, high-risk polyps.
Multivariate regression analysis showed positive FIT (OR: 7.3; 95% CI: 3.8, 14.0), but not gender, age or type of family history, was significantly associated with detection of CRC or HRPs (Table 2).
Table 2.
Multivariate analysis of factors associated with CRC or HRP
| Variable name | P-value | Odds ratio (95% CI) |
|---|---|---|
| FIT (Positive vs. Negative) | <0.0001 | 7.30 (3.82, 13.95) |
| Gender (Male vs. Female) | 0.31 | 1.38 (0.74, 2.60) |
| FHx type | 0.92 | |
| 1FDR<60 vs. 1FDR ≥ 60 | 0.92 (0.44, 1.91) | |
| 2FDR vs. 1FDR ≥ 60 | 1.63 (0.71, 3.76) | |
| Age category | 0.60 | |
| 55–59 vs. 50–54 | 1.20 (0.40, 3.65) | |
| 60–64 vs. 50–54 | 1.81 (0.63, 5.22) | |
| 65–69 vs. 50–54 | 2.04 (0.69, 6.01) | |
| 70–74 vs. 50–54 | 1.96 (0.66–5.79) |
CRC, colorectal cancer; HRP, high-risk polyps.
DISCUSSION
In this retrospective cohort study assessing the performance of FIT in individuals with one or more FDRs with CRC, FIT detected all of the CRCs and a positive FIT was a stronger predictor of CRC or high-risk polyps at colonoscopy than the number or age of diagnosis of affected relatives. This study reflects a Canadian population participating in a colon cancer screening program.
Several other studies have assessed the performance of FIT in screening patients with a nonhereditary family history of CRC and are well summarized in a meta-analysis by Kasoula et al. (8). The pooled sensitivity and specificity of quantitative FITs with a cut-off less than 25 mcg Hb/g feces was 91% and 92% in the detection of CRC for patients with a FDR with CRC. The validity of these results is limited by the different study designs, different FIT brands and cut-offs and variable time to follow-up colonoscopy.
In a prospective trial comparing annual FIT (OC-Sensor, cut-off 10 mcg Hb/g feces) to colonoscopy in FDR of patients with CRC, Quintero et al. demonstrated that repeated FIT over 3 years detected all CRCs and 61% of advanced adenomas (9). This is similar to the results in the current study in which all CRCs and 62% of high-risk polyps were detected. Quintero et al. concluded that repeated FIT was equivalent to colonoscopy in detecting CRC and advanced adenomas in individuals with a FDR with CRC.
Furthermore, a retrospective study comparing the performance of FIT (OC-Sensor, cut-off 20 mcg Hb/g feces) in average risk patients enrolled in the colonoscopy arm of the COLONPREV trial (10) and individuals with a FDR with CRC showed similar sensitivities and specificities for the detection of advanced neoplasia between the two patient populations (11). In addition, there was no difference in FIT performance when comparing the number of FDRs with CRC or their age of diagnosis. This is in keeping with the regression analysis in the current study, which did not show an association between findings at colonoscopy and number or age of diagnosis of FDRs with CRC. However, this may be due to an inadequate sample size in the highest risk group, those with two FDRs with CRC, which comprised only 8% of the family history cohort. In an attempt to achieve adequate power to stratify familial risk, Quintero et al. published a subsequent retrospective study with an expanded patient population with familial risk and compared colonoscopy results to average risk patients in the colonoscopy arm of the COLONPREV study (12). The distribution of family history type was similar to the present study but the sample size was larger with 3015 subjects in the family history group of which 10% had two FDRs with CRC. The study showed that the prevalence of advanced adenomas and CRC in individuals with one FDR, regardless of age, was not different from the average risk group. However, individuals with two FDRs with CRC had an increased risk of advanced neoplasia compared to those of average risk.
In comparing the patients in this study to the average risk cohort from the same screening program (7), the positivity rate in the family history group was higher at 10.9% versus 8.6% in the average risk group. However, the positive predictive value for CRC was similar: 4.8% in those with a FDR with CRC and 4.9% in those at average risk. Likewise, the positive predictive value for HRPs was 37.3% in the present study and 35.0% in the average risk group. Due to the higher positivity rate of FIT in the family history cohort, the overall CRC detection rate was higher at 4.8 per 1000 screened with FIT compared to 3.5 per 1000 screened in the average risk cohort.
It also bears mentioning that 33.8% of patients with a negative FIT did not undergo the recommended colonoscopy. While this is a limitation of the present study when interpreting the results, it emphasizes imperfect compliance with colonoscopy screening in individuals with a family history of CRC (13). The use of FIT for CRC screening could have important clinical implications for patients who do not comply with screening due to a reluctance to undergo colonoscopy.
Our study has other limitations that need to be considered. As mentioned above, the sample size may have precluded demonstrating an association between high-risk findings at colonoscopy and the family history arm with two FDRs with CRC. Also, the study cohort was not followed during repeat rounds of FIT screening which likely underestimated its effect on the detection of high-risk polyps, which may have been associated with a positive FIT on later rounds of screening.
In summary, an FIT-based screening strategy for individuals with a family history of CRC may be an effective alternative to primary screening colonoscopy, particularly in those with a single FDR with CRC. Possible benefits of FIT screening include increased participation and decreased colonoscopy resource utilization. Randomized trials comparing the two screening strategies over multiple rounds of FIT screening to assess uptake, CRC incidence and CRC mortality would inform future guidelines and colon screening programs.
References
- 1. Johnson CM, Wei C, Ensor JE, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013;24(6):1207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leddin D, Lieberman D, Tse F, et al. Clinical practice guidelines on colorectal cancer screening in individuals with a family history of nonhereditary colorectal cancer. Gastroenterology 2018;155:1325–47. [DOI] [PubMed] [Google Scholar]
- 3. Lin JS PM, Perdue LA, Rutter C, et al. Screening for colorectal cancer: an updated systematic seview for the U.S. Preventive Services Task Force. Evidence Synthesis No. 135. AHRQ Publication No. 14-05203-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2015. [Google Scholar]
- 4. Levin B, Lieberman DA, McFarland B, et al. ; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134(5):1570–95. [DOI] [PubMed] [Google Scholar]
- 5. Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US multi-society task force on colorectal cancer. Gastroenterology 2012;143(3):844–57. [DOI] [PubMed] [Google Scholar]
- 6. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2015. <https://www.R-project.org>. [Google Scholar]
- 7. Telford J, Gentile L, Gondara L, et al. Performance of a quantitative fecal immunochemical test in a colorectal cancer screening pilot program: A prospective cohort study. CMAJ Open 2016;4(4):E668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katsoula A, Paschos P, Haidich AB, et al. Diagnostic accuracy of fecal immunochemical test in patients at increased risk for colorectal cancer: A meta-analysis. JAMA Intern Med 2017;177(8):1110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quintero E, Carrillo M, Gimeno-García AZ, et al. Equivalency of fecal immunochemical tests and colonoscopy in familial colorectal cancer screening. Gastroenterology 2014;147(5):1021–30.e1; quiz e16–7. [DOI] [PubMed] [Google Scholar]
- 10. Quintero E, Castells A, Bujanda L, et al. ; COLONPREV Study Investigators Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012;366(8):697–706. [DOI] [PubMed] [Google Scholar]
- 11. Cubiella J, Castro I, Hernandez V, et al. ; COLONPREV study investigators Diagnostic accuracy of fecal immunochemical test in average- and familial-risk colorectal cancer screening. United European Gastroenterol J 2014;2(6):522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quintero E, Carrillo M, Leoz ML, et al. ; Oncology Group of the Asociación Española de Gastroenterología (AEG) Risk of advanced neoplasia in first-degree relatives with colorectal cancer: A large multicenter cross-sectional study. PLoS Med 2016;13(5):e1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bujanda L, Sarasqueta C, Zubiaurre L, et al. ; EPICOLON Group Low adherence to colonoscopy in the screening of first-degree relatives of patients with colorectal cancer. Gut 2007;56(12):1714–8. [DOI] [PMC free article] [PubMed] [Google Scholar]