Abstract
Introduction
Due to the rarity of malignant insulinoma, a lack of the literature describing factors affecting outcomes exists. Our aim was to review malignant insulinoma incidence, characteristics and survival trends.
Methods
We identified all patients with malignant insulinoma in the SEER registries from 1973 to 2015. Incidence, neoplasm characteristics and factors affecting cancer-specific survival (CSS) were described.
Results
A total of 121 patients were identified. The crude annual overall incidence was low (range 0.0–0.27 cases per million person years). The largest proportion had localized disease (40%), while 16% had regional disease, 39% distant metastatic disease, and stage was unreported in 5%. Most neoplasms were in the body/tail of the pancreas, followed by the head of the pancreas. Grade was reported in 40% of patients; only a single patient reported as having grade IV with the remainder all grades I/II. Surgical resection was performed in 64% of patients. Within surgical patients, the median primary neoplasm size was 1.8 cm. Regional lymph nodes were examined in 57.1% of surgical patients, while 34% of examined nodes were positive. The median CSS was 183 months. On multivariable analysis, surgical resection, male sex and absence of metastatic disease were associated with superior survival.
Conclusion
While the greatest proportion of patients with malignant insulinoma present with localized disease, regional lymph node involvement was found in 34% of whose nodes were tested. Further studies are needed to assess the role of lymph node dissection in improving survival and preventing recurrence given the observed frequency of lymph node involvement.
Introduction
Insulinoma is a functioning pancreatic neuroendocrine tumor (PNET) that was first described in 1927, when a patient who developed hypoglycemic symptoms was found to have a metastatic pancreatic tumor, extracts of which produced hypoglycemia in laboratory animals [1]. While the overall incidence of insulinoma is rare, with an estimated incidence of 1–4 cases per 1 million person years, it has increased over time [2, 3]. Interestingly, autopsy studies suggest even higher rates of insulinoma, suggesting that many may be undiagnosed or of minimal clinical significance [4, 5].
Although the first described insulinoma case was malignant, the majority is benign [2, 3]. While benign insulinoma is well understood as many studies have assessed its epidemiology, treatment and prognosis, data about malignant insulinoma are still limited. Given its rarity, much remains unknown regarding trends and factors affecting survival for malignant insulinoma, but it is accepted that overall survival for malignant insulinoma is significantly worse than for benign disease [3].
Complete surgical resection is the treatment of choice to cure the debilitating hypoglycemic symptoms associated with insulinoma [6, 7]. However, surgery can be challenging in the presence of malignant disease as these patients can present with unresectable lesions. On the other hand, as the few case series assessing malignant insulinoma looked only at distant metastatic disease, data about the frequency and prognosis of other stages are still underreported [3, 8]. Very few studies have provided meaningful evidence regarding malignant insulinoma characteristics, trends and outcomes. Our aim was to use the Surveillance, Epidemiology, and End Results (SEER) set of population-based cancer registries to better understand this rare disease through assessing patient characteristics, tumor characteristics and survival.
Methods
Case selection
Malignant insulinoma cases diagnosed during 1973–2015 were obtained from the Surveillance, Epidemiology, and End Results (SEER) set of population-based cancer registries. The SEER registries cover approximately 34.6% of the US population and provide a good estimate of the cancer status across the USA [9]. The SEER program provides information on cancer statistics and is maintained by the National Cancer Institute (NCI). This dataset includes information from 18 registries (SEER-18; Alaskan Native, Metropolitan Atlanta, Connecticut, Detroit, Rural Georgia, Greater Georgia, San Francisco-Oakland, San Jose-Monterey, Greater California, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, New Mexico, New Jersey, Seattle-Puget Sound, Utah) [8].
Cases were identified using International Classification of Diseases for Oncology, Third Edition, ICD-O-3; a combination of topography code of the pancreas (C73) along with histology code (8151) was used to identify the cases of malignant insulinoma.
Demographics
Demographic characteristics reviewed for this study included sex, race and age at diagnosis. Information regarding age at death and underlying cause of death were abstracted from death certificates by the SEER program [8].
Tumor characteristics
Information about primary neoplasm location, size, grade, stage, involvement of lymph nodes and presence of metastases as well as surgical resection was extracted. Tumor grade was reported as defined by SEER database: grade I for well-differentiated, grade II for moderately differentiated, grade III for poorly differentiated and grade IV for undifferentiated/anaplastic tumors [10]. Tumor stage was reported by SEER as localized if it is confined to the pancreas, regional if the neoplasm extended beyond the pancreas directly into surrounding organs or tissues, or into regional lymph nodes by way of lymphatic system or a combination of extension and regional lymph nodes, or reported as distant if distant metastases were present. Localized insulinoma can be determined malignant either if lymphovascular invasion is present or if the histological features meet the World Health Organization (WHO) criteria for pancreatic neuroendocrine carcinomas. Please see appendix for more information about the WHO criteria.
Surgery characteristics
Primary neoplasm size and the number of examined lymph nodes were reported for patients who underwent surgical resection. Patients who did not undergo surgery, or who underwent incisional biopsy of other than primary site only, or if it is unknown if surgery was done, were excluded from this sub-analysis.
Data analysis
The SEER Incidence Database was used to identify population denominators and calculate incidence trends. Overall survival (OS) and cancer-specific survival (CSS) were assessed using Kaplan-Meier analysis, and a Cox proportional hazards regression was used for both univariate and multivariable survival analysis to adjust for confounding variables. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Demographics
A total of 121 cases of malignant insulinoma were identified between 1973 and 2015. Alaska, Rural Georgia and Greater Georgia registries are part of the SEER 18, but did not have patients with malignant insulinoma. The average age at diagnosis was 56.0 ± 16.8 years, range 19–93. There was a slight female predominance with 69 females (57.0%) and 52 males (43.0%). The majority of patients were white (82.6%) (Table 1).
Table 1.
Patient and tumor baseline characteristics
| Total (N = 121) | |
|---|---|
| Age | |
| Mean (SD) | 56.0 (16.8) |
| Median | 56.0 |
| Q1, Q3 | 44.0, 71.0 |
| Range | (19.0–93.0) |
| Sex | |
| Male | 52 (43.0%) |
| Female | 69 (57.0%) |
| Race | |
| Non-Hispanic white | 100 (82.6%) |
| Hispanic | 9 (7.4%) |
| Non-Hispanic black | 2 (1.7%) |
| Non-Hispanic Asian or Pacific Islander | 10 (8.3%) |
| Cause of death | |
| Alive | 64 (52.9%) |
| Esophagus cancer | 1 (0.8%) |
| Pancreas cancer | 33 (27.3%) |
| Ovary cancer | 1 (0.8%) |
| Miscellaneous malignant cancer | 5 (4.1%) |
| In situ, benign, or unknown behavior neoplasm | 6 (5.0%) |
| Diabetes mellitus | 1 (0.8%) |
| Diseases of heart | 6 (5.0%) |
| Cerebrovascular diseases | 2 (1.7%) |
| Symptoms, signs, and Ill-defined conditions | 1 (0.8%) |
| Other cause of death | 1 (0.8%) |
| Tumor size | |
| N | 87 |
| Mean (SD) | 3.02 (3.0) |
| Median | 2.0 |
| Ql, Q3 | 1.3, 3.0 |
| Range | (0.0–13.5) |
| Primary site | |
| Head of pancreas | 22 (18.2%) |
| Body of pancreas | 14 (11.6%) |
| Tail of pancreas | 42 (34.7%) |
| Overlapping lesion of pancreas | 5 (4.1%) |
| Pancreas nos. | 38 (31.3%) |
| Stage | |
| Localized | 48 (39.7%) |
| Regional | 19 (15.7%) |
| Distant | 47 (38.8%) |
| Unstaged | 7 (5.8%) |
| Grade | |
| Grade I: well-differentiated | 39 (32.2%) |
| Grade II: moderately differentiated | 8 (6.6%) |
| Grade IV: undifferentiated | 1 (0.8%) |
| Cell type not determined, or not stated | 73 (60.3%) |
| Regional nodes examined | |
| Missing | 12 |
| No | 65 (59.6%) |
| Yes | 44 (40.4%) |
| Nodes positive | |
| Missing | 74 |
| No | 32 (68.1%) |
| Yes | 15 (31.9%) |
| Surgery type | |
| Unknown if surgery done | 6 (5.0%) |
| No surgical procedure | 33 (27.3%) |
| Incisional biopsy of other than primary site | 5 (4.1%) |
| Local or partial surgical excision of pancreas | 5 (4.1%) |
| Subtotal gastrectomy, duodenectomy with complete/partial pancreatectomy | 15 (12.4%) |
| Radical regional pancreatectomy | 2(1.7%) |
| Surgery of regional and/or distant sites only | 1 (0.8%) |
| Local excision of tumor nos. | 21 (17.4%) |
| Partial pancreatectomy | 28 (23.1%) |
| Total Pancreatectomy | 3 (2.5%) |
| Surgery nos. | 2 (1.7%) |
Incidence
The crude annual overall incidence was extremely low, but increased over the study period, from 0.0 to 0.17 cases per million person years. The annual overall incidence reached its peak in 2011 and 2012, at 0.27 cases per million person years. Most recently, the incidence was reported to be 0.17 cases per million person years in 2015.
Tumor characteristics
Tumor size was reported for 87 patients; the mean (SD) size was 3.0 ± 3.0 cm. Within patients who underwent surgical resection, the size of the primary neoplasm was reported for 90% of patients, with a median size of 1.8 cm, (range 0.7–13.5 cm). Tumor characteristics are summarized in Table 1.
The greatest proportion of neoplasms (56 cases, 46.3%) was in the body/tail of the pancreas, followed by the head of the pancreas (22 cases, 18.2%), while neoplasm location was unspecified in 38 cases (31.3%) and reported as an overlapping lesion of pancreas in 5 cases (4.1%).
At the time of diagnosis, the largest proportion of patients (48 patients) had localized disease (39.7%); 19 patients (15.7%) had regional disease, and 47 patients (38.8%) had distant metastatic disease, while stage was unreported in 7 cases (5.8%). Grade was reported in 48 patients (39.7%); all but one were grade I/II, with only a single patient reported as having grade IV disease.
Surgery
Surgery was performed in 77 patients as shown in Table 1. Partial pancreatectomy was the most common performed procedure (23.1% of cases), while Whipple procedure was performed in 12.4% of cases. Regional lymph nodes were examined in 44 patients (57.1% of patients who underwent surgery), and of those patients, 15 patients (34%) had positive nodes.
Among patients whose lymph nodes were examined, there was no difference in average patients’ age, sex, tumor location or grade between patients who had positive and negative lymph nodes, all p > 0.05. The average tumor size was statistically similar in those with positive and negative lymph nodes; mean (SD) tumor size for patients with positive lymph node was 4.03 (4.12) cm compared to 2.75 (2.96) cm for those with negative lymph nodes, p = 0.22.
Survival
The median overall survival (OS) was 143 months, with 5-and 10-year OS observed in 58% and 55%, respectively (Fig. 1). On the other hand, the median cancer-specific survival was 183 months. Overall survival and cancer-specific survival were better in patients who underwent surgery as shown in Fig. 2; 5-year OS was 84% and 14% for the surgery and no-surgery groups, respectively, p < 0.001. On multivariable analysis, surgical resection, male sex, and absence of metastatic disease were associated with greater overall and cancer-specific survival as shown in Table 2.
Fig. 1.
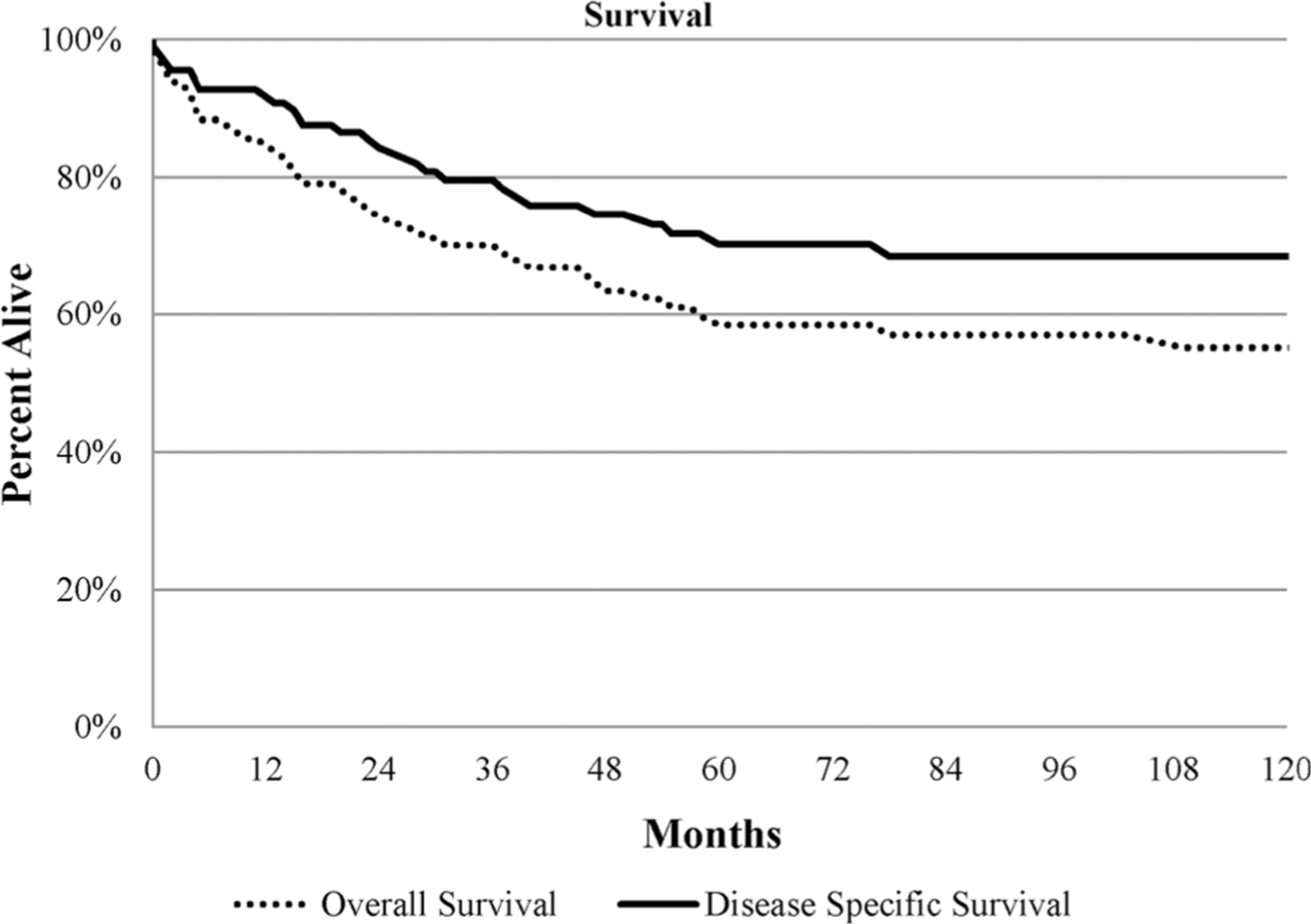
Kaplan-Meier curve for overall and cancer-specific survival
Fig. 2.
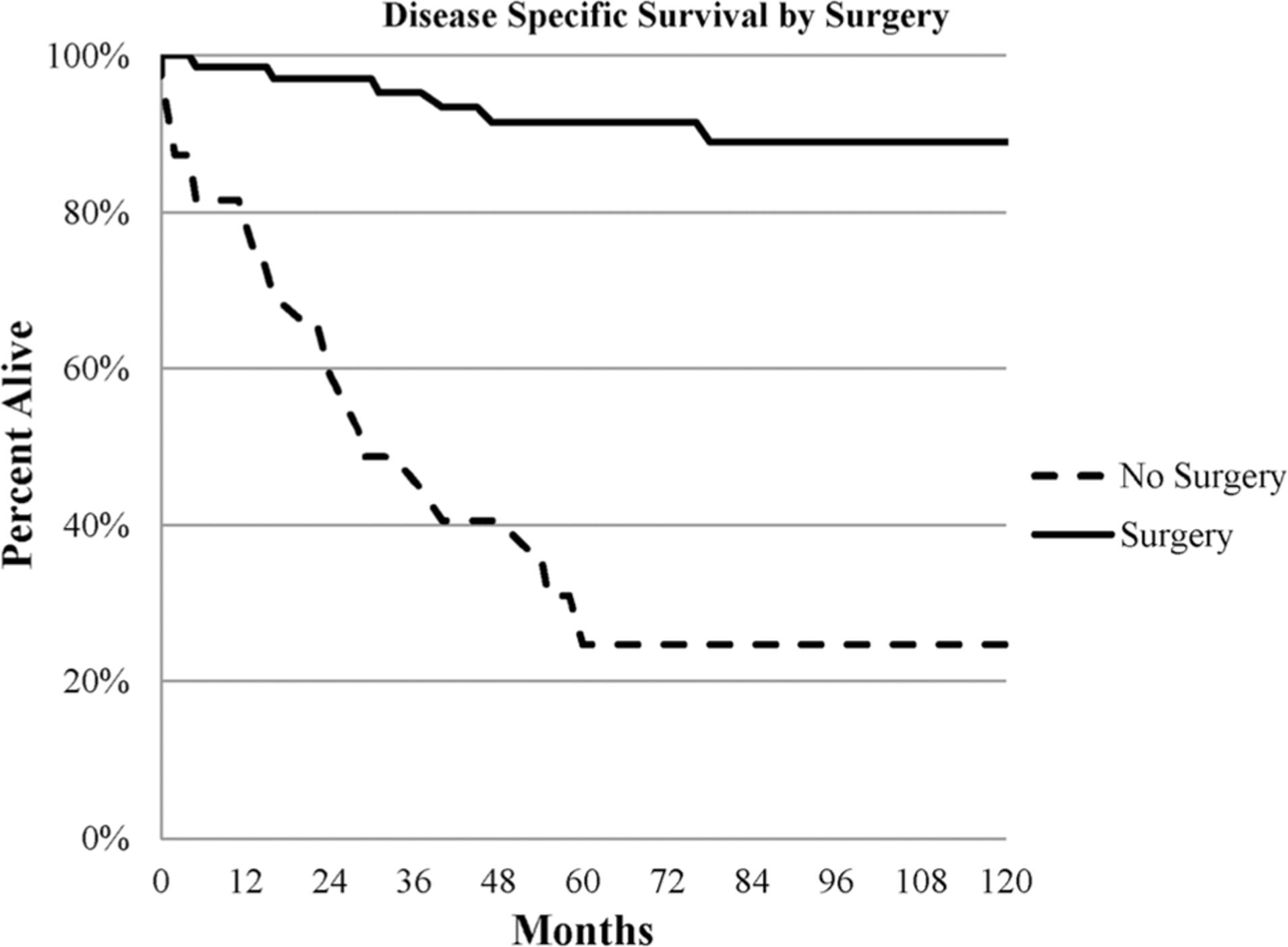
Kaplan-Meier curve for cancer-specific survival by surgical resection
Table 2.
Cox proportional hazards regression for cancer-specific survival among all patients
| Variable | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Female | 1.12 | 0.56–2.25 | 0.75 | 2.26 | 1.02–1.96 | 0.04 |
| Age ≥ 50 | 1.73 | 0.86–3.52 | 0.12 | 1.12 | 0.52–2.41 | 0.77 |
| No surgery | 7.45 | 3.58–15.53 | <0.01 | 3.53 | 1.14–10.06 | 0.03 |
| Tumor size | ||||||
| 0–2 cm | Ref | |||||
| Missing | 8.86 | 3.00–26.15 | <0.01 | 1.09 | 0.28–1.20 | 0.90 |
| ≥ 2 cm | 6.11 | 1.94–19.25 | <0.01 | 1.79 | 0.50–6.42 | 0.37 |
| Stage | ||||||
| Localized | Ref | |||||
| Regional | 7.42 | 0.77–71.41 | 0.08 | 8.61 | 0.84–88.08 | 0.07 |
| Distant | 65.88 | 8.89–87.92 | <0.01 | 42.81 | 5.11–358.52 | <0.01 |
| Unstaged | 11.37 | 1.02–126.27 | 0.047 | 16.82 | 1.42–199.91 | 0.03 |
HR hazard ratio, Ref reference
On multivariable analysis including only patients who underwent resection after excluding patients who underwent surgery for incisional biopsy only or whose resection status was unknown, we found that the presence of distant metastatic disease was associated with worse survival as shown in Table 3. HR for the presence of distance metastatic disease among patients who underwent surgery was 139.21 (95% CI 8.06–2405.25, p = < 0.001).
Table 3.
Cox proportional hazards regression for cancer-specific survival among patients who underwent resection
| Multivariable | |||
|---|---|---|---|
| Variable | HR | 95% CI | P-value |
| Female | 0.68 | 0.16–2.96 | 0.60 |
| Age ≥ 50 | 2.04 | 0.48–8.61 | 0.33 |
| Tumor size | |||
| 0–2 cm | Ref | ||
| Missing | 3.12 | 0.40–24.33 | 0.28 |
| ≥2 cm | 1.57 | 0.30–8.18 | 0.59 |
| Stage | |||
| Localized | Ref | ||
| Regional | 3.10 | 0.25–38.24 | 0.38 |
| Distant | 139.21 | 8.06–2405.25 | <0.01 |
| Unstaged | 3.01 | 0.18–51.74 | 0.45 |
HR hazard ratio, Ref reference
When comparing cancer-specific survival (CSS) for patients whose regional lymph nodes were examined, the 5-year CSS was 81.5% in patients with positive lymph nodes compared to 96.7% in patients with negative lymph nodes, p = 0.21.
When comparing cancer-specific survival for all patients with distant metastatic disease, we found that surgery group (n = 11) tended to have better survival compared to no-surgery group (n = 31), but the difference did not reach statistical significance (p = 0.07), as shown in Fig. 3.
Fig. 3.
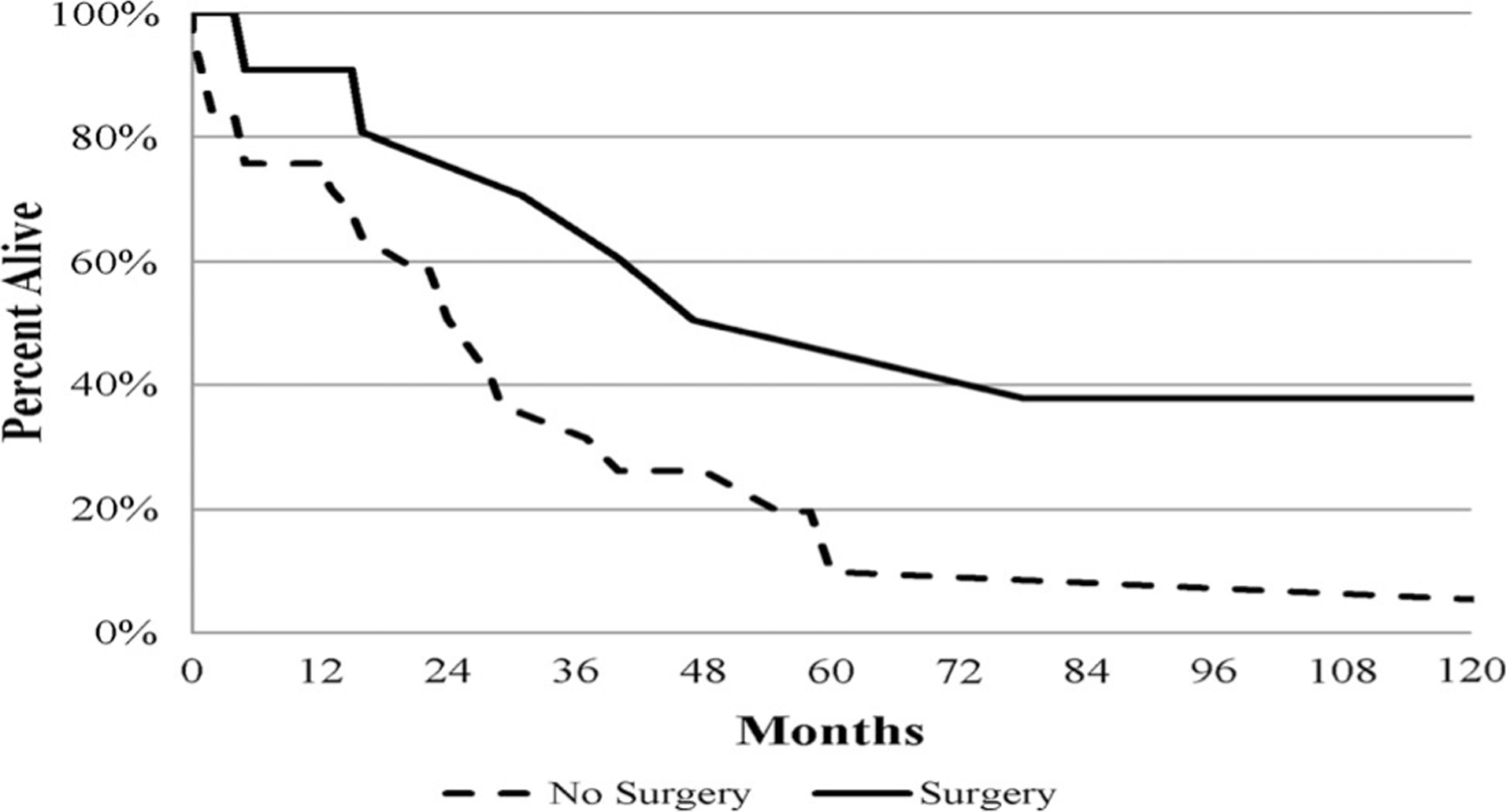
Kaplan-Meier curve for cancer-specific survival for metastatic disease by surgery
Discussion
Malignant insulinoma is a rare pancreatic neuroendocrine tumor that represents <6% of all insulinoma [3]. To our knowledge, this series of 121 patients is the largest published series of malignant insulinoma and has offered insight into incidence as well as factors associated with survival. While this neoplasm is slow growing with good overall prognosis, studying trends and factors impacting survival is important to provide more insight into this disease as symptoms of hypoglycemia can be debilitating and hard to control [3, 11]. Surgical resection improves survival, but the role of lymph node dissection is still controversial. Our findings have demonstrated that the greatest proportion of malignant insulinoma presents as localized disease and regional lymph node involvement was common. Further studies are needed to assess the role of routine lymph node dissection on survival.
Interestingly, during a 60-year study of insulinoma between 1927 and 1986, diagnoses of insulinoma increased significantly especially during the last two decades of the study [3]. However, it was not clear if the incidence of malignant lesions followed the same trajectory. Due to the rare incidence of malignant insulinoma, it is impossible to assess incidence and trends without using a large database. We found that the incidence of malignant insulinoma has increased over time from 0 cases during the period (1973–1976) until it reached the highest incidence of 0.27 cases per million person years during 2011 and 2012. As our study looked at all malignant insulinoma trends over four decades, it is uncertain whether this increased incidence is due to increased diagnosis as there have been significant improvements in the imaging modalities that are used to diagnose insulinoma including CT scans and endoscopic ultrasounds. A clinic-pathological analysis of 800 autopsy cases suggests that endocrine tumors of the pancreas are common and can be asymptomatic even in the presence of hormone over-secretion, which may suggest we are detecting and diagnosing more subclinical insulinoma cases than we used to [5].
Malignant insulinoma is defined by the presence of local invasion or metastatic disease [12]. Because most studies looked at distant metastatic disease only, data about the frequency and prognosis of other stages are underreported [3, 8]. Our study showed that most patients present with localized or regional disease which make surgical resection applicable. As expected, survival was better in patients who underwent surgery. As patients who present with distant metastatic disease are less likely to be offered surgical resection, we elected to compare survival between surgery vs no-surgery groups who presented with distant metastatic disease. Our results suggest that survival was better for distant metastatic disease patients who had surgery, but the difference was not statistically significant which may be due to the small number of events.
Our finding that regional disease has similar survival compared to localized disease differs from what is reported in the literature. Other studies suggest worse prognosis and increased risk of recurrence if regional lymph nodes are involved with metastatic insulinoma. Krampitz et al. [13] studied 326 patients undergoing resection for PNETs and found that patients with metastases to lymph nodes only are at higher risk of developing liver metastases than patients without lymph nodes involvement, and that risk depends of the number of involved lymph nodes. Whether these results are true for malignant insulinoma is not very clear as only 8% of patients in that study had insulinoma. In another study, Danforth et al. [14] reviewed 17 cases of metastatic insulinoma and found that 3 patients had previous disease confined to the pancreas at initial exploration before they developed liver metastases. Nikfarjam et al. [6] reported that during 25-year experience of surgical management of insulinoma at the Massachusetts General Hospital, 5 patients had malignant insulinoma, all of them had metastatic disease to lymph nodes and all of them developed recurrence or disease progression after resection. A possible explanation that our study did not show a difference in survival between localized and regional stages is that the regional disease was underreported in the SEER database. The regional lymph nodes were not examined in around half of all cases and without regional lymph nodes examination; some cases may have been downstaged to localized disease. The effect of regional lymph nodes involvement on survival or the development of distant metastatic disease is still controversial, and further studies are needed.
Previous case series studies showed that liver and lymph nodes are the most common location for metastasis, but the frequency of lymph nodes metastases is still unknown [14, 15]. In our study, among patients whose lymph nodes were sampled, over one-third of them had positive lymph nodes and these results are similar to what is reported in cases series studies. Hirshberg et al. [8] reported the National Institutes of Health (NIH) experience with malignant insulinoma over two decades by reviewing 10 patients with metastatic insulinoma; 6 patients had liver metastases, while 4 patients had lymph node metastases. Danforth et al. [14] reviewed 17 metastatic insulinoma cases and reported similar results as 12 patients had liver metastases vs 8 with lymph nodes metastases. Our findings along with other studies suggest that lymph node involvement with malignant insulinoma is very common which illustrates the importance of further assessment of prognostic value of lymph nodes involvement and the role of routine lymph node resection on improving survival or predicting outcomes.
Like other SEER-based studies, ours has multiple limitations due to the absence of information regarding presentation, biochemical diagnosis and presence of genetic testing including multiple endocrine neoplasia type 1 (MEN-1) syndrome. SEER database adopted the AJCC TNM staging system in 2004, and the eighth edition has not been adopted by SEER yet. Therefore, we are not able to report the TNM staging by the AJCC eighth edition. Tumor grade is reported in SEER database based on 4-category grading system and was missing for a significant proportion of cases. Therefore, we were not able to include grade in the Cox proportional hazards regression models due to the limited numbers. Additionally, other treatments for malignant insulinoma beside surgery were unable to be assessed as radiation and chemotherapy data are not available in SEER’s public research database. While the long time frame of this study was advantageous due to the rarity of insulinoma, it could also be a limitation as the care of this disease may have evolved over the years.
We were unable to compare our observations to benign insulinoma as those cases are not captured by SEER. However, despite these limitations, we have produced data describing the incidence trends and outcome of a very rare type of malignancy on a national level.
Conclusion
Malignant insulinoma is rare, with an incidence of 0.17 per million person years. While the majority of patients with malignant insulinoma present with localized disease, regional lymph node involvement was found in the one-third of whose nodes were tested. Further studies are needed to assess the role of lymph node dissection in improving survival and preventing recurrence given the observed frequency of lymph node involvement.
Acknowledgements
This publication was made possible by CTSA Grant No. UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Appendix
See Table 4.
Table 4.
2017 World Health Organization (WHO) classification and grading of pancreatic neuroendocrine neoplasms
| Grade | Ki-67 proliferation index (%) | Mitotic index |
|---|---|---|
| Well-differentiated | ||
| G1 | <3 | <2 |
| G2 | 3–20 | 2–20 |
| G3 | >20 | >20 |
| Poorly differentiated (G3) | >20 | >20 |
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
References
- 1.Wilder RM, Allan FN, Power M et al. (1927) Carcinoma of the islands of the pancreas: hyperinsulinism and hypoglycemia. J Am Med Assoc 89:348–355 [Google Scholar]
- 2.Crippa S, Zerbi A, Boninsegna L et al. (2012) Surgical management of insulinomas: short and long-term outcomes after enucleations and pancreatic resections. Arch Surg 147:261–266 [DOI] [PubMed] [Google Scholar]
- 3.Service FJ, Mcmahon MM, O’Brien PC et al. (1991) Functioning insulinoma: incidence, recurrence, and long-term survival of patients: a 60-year study. In: Mayo Clinic Proceedings, Elsevier, Amsterdam, 711–719. [DOI] [PubMed] [Google Scholar]
- 4.Vaidakis D, Karoubalis J, Pappa T et al. (2010) Pancreatic insulinoma: current issues and trends. Hepatobiliary Pancreat Dis Int 9:234–241 [PubMed] [Google Scholar]
- 5.Kimura W, Kuroda A, Morioka Y (1991) Clinical pathology of endocrine tumors of the pancreas. Dig Dis Sci 36:933–942 [DOI] [PubMed] [Google Scholar]
- 6.Nikfarjam M, Warshaw AL, Axelrod L et al. (2008) Improved contemporary surgical management of insulinomas: a 25-year experience at the Massachusetts General Hospital. Ann Surg 247:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boukhman MP, Karam JH, Shaver J et al. (1998) Insulinoma-experience from 1950 to 1995. West J Med 169:98. [PMC free article] [PubMed] [Google Scholar]
- 8.Hirshberg B, Cochran C, Skarulis MC et al. (2005) Malignant insulinoma. Cancer 104:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Surveillance E, and End Results (SEER) Program Overview.
- 10.Instructions for Coding Grade for (2014) https://seer.cancer.gov/tools/grade/. Accessed 2 Nov 2020
- 11.Chambers AJ, Pasieka JL (2018) Observation versus surgery for nonlocalized insulinoma Difficult decisions in endocrine surgery. Springer, Berlin, pp 459–470 [Google Scholar]
- 12.Baudin E, Caron P, Lombard-Bohas C et al. (2013) Malignant insulinoma: recommendations for characterisation and treatment. Ann Endocrinol (Paris) 74:523–533 [DOI] [PubMed] [Google Scholar]
- 13.Krampitz GW, Norton JA, Poultsides GA et al. (2012) Lymph nodes and survival in pancreatic neuroendocrine tumors. Arch Sur 147:820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danforth JD, Gorden P, Brennan M (1984) Metastatic insulin-secreting carcinoma of the pancreas: clinical course and the role of surgery. Surgery 96:1027–1037 [PubMed] [Google Scholar]
- 15.Grant CS (2005) Insulinoma. Best Pract Res Clin Gastroenterol 19:783–798 [DOI] [PubMed] [Google Scholar]


