Abstract
Background:
Current guidelines recommend early invasive intervention (<24 hours) for high risk patients with non-ST-segment elevation myocardial infarction (NSTEMI). A delayed invasive strategy (24–72 hours) is considered reasonable for low risk patients. The real-world effectiveness of this strategy is unknown.
Methods:
The ARIC Study has conducted hospital surveillance of acute myocardial infarction (MI) since 1987. NSTEMI was classified using a validated algorithm. We limited our study to patients undergoing early (<24 hours of the event onset), or late (≥24 hours) percutaneous coronary intervention (PCI). Patients were stratified into low (TIMI score 2–4), and high risk (TIMI score 5–7, or presence of cardiogenic shock, ventricular fibrillation, or cardiac arrest). Associations between early vs. late PCI and mortality were analyzed using multivariable logistic regression adjusted for demographics, hospitalization year, TIMI score, and comorbidities.
Results:
From 1987–2012, 6,746 patients were hospitalized with NSTEMI and underwent PCI. Most were white (79%), male (68%), with mean age 61 years. The 28-day and 1-year mortality were 2% and 5%, respectively. Most revascularizations (65%) were late. After accounting for potential confounders, early PCI was associated with a 58% reduced 28-day mortality (OR = 0.42; 95% CI: 0.21 – 0.84) for the entire population, and 57% reduced mortality (OR = 0.43; 95% CI: 0.21 – 0.88) for high risk patients. By 1-year of follow up, there was no significant difference in mortality with respect to early vs. late PCI.
Conclusion:
In hospitalized NSTEMI patients with high risk of clinical events, early PCI is associated with improved 28-day survival.
Keywords: Revascularization, early, delayed, percutaneous, coronary
Introduction
The optimal timing of revascularization in patients with non ST-segment elevation myocardial infarction (NSTEMI) continues to be rigorously debated. Early intervention has the potential to prevent ischemic events during the waiting time from event to revascularization (1). Conversely, a delayed intervention may avoid procedure-related complications by allowing plaque to stabilize during the waiting period, as the patient undergoes medical therapy (2, 3). The current American Heart Association/American College of Cardiology clinical practice guidelines advocate an early invasive strategy (angiography with intent to revascularize within 24 hours) over a delayed invasive strategy for initially stabilized high-risk patients with NSTEMI (4). Evidence for this strategy is based on clinical trials in controlled settings with selected patients, using composite endpoints (3, 5–9).
The benefit of early revascularization reported by clinical trials is largely driven by lower incidence of refractory ischemia (5) or new MI (1,6), rather than survival. This is likely due to limitations recruiting a large enough sample with sufficient power to analyze mortality alone, especially in light of the low mortality following percutaneous revascularization. Additionally, definitions of MI and recurrent ischemia used by randomized trials have varied (1, 3, 5–9), reducing generalizability of these results. In the present study, we attempt to circumvent these limitations by analyzing the real-world effectiveness of early vs. late percutaneous coronary intervention (PCI) for reduction of mortality. To accomplish this aim, we analyzed data captured from 21 hospitals in four US communities by the Atherosclerosis Risk in Communities (ARIC) Surveillance study.
Methods
ARIC Study Community Surveillance
Since 1987, the ARIC study has conducted ongoing surveillance of hospitalized acute myocardial infarction (MI) in 4 geographically defined regions of the US: Forsyth County, North Carolina; Washington County, Maryland; Jackson, Mississippi; and 8 northwest suburbs of Minneapolis, Minnesota. As previously described (10, 11), eligible hospitalizations were selected on the basis of age (35–74 years of age), residence in the community, and discharge code (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 402 (hypertensive heart disease); 410 to 414, (acute MI, other acute and subacute ischemic heart disease, old MI, angina pectoris, and other forms of chronic ischemic heart disease); 427 (cardiac dysrhythmias); 428 (heart failure); and 518.4 (acute edema of the lung), through random sampling within discharge code strata. Clinical data were collected from the hospital record by trained abstractors, using the physician notes, laboratory reports, patient histories, and discharge summaries. The study sample for this analysis is limited to hospitalizations with discharge dates between January 1st, 1987 – December 31st, 2012, and at least one year of follow up for mortality outcomes.
Biomarkers
Laboratory values for available cardiac biomarkers (total creatine kinase and myocardial band fractions, total lactate dehydrogenase, troponin I and troponin T) were recorded for the first 4 days of hospitalization, or the 4 days after the event if occurring in-hospital. The laboratory-reported upper limit of normal (ULN) was recorded, and biomarker values were abstracted chronologically, recording up to 3 measurements per day. In the uncommon event that >3 labs were drawn on a given day, the highest 3 consecutive measurements were retained.
Electrocardiography
Available 12-lead electrocardiograms (ECG) were reviewed for quality, rejecting any with missing leads, muscle tremor artifact, or technical errors. The first, third, and last ECG tracings meeting quality standards were coded electronically at the Minneapolis ECG Reading Center (12).
Chest Pain
Presence of chest pain was abstracted from the medical record, with origin determined by review of the physician notes. Any mention of substernal pressure, tightness, or pain precipitated by exertion or excitement was considered evidence for chest pain of cardiac origin. Chest pain specified in the physician notes as “unknown origin” or “undiagnosed” was considered “unknown”. Chest pain of cardiac origin was classified as either present or absent.
Acute Myocardial Infarction Classification
Hospitalized MI events were classified by the ARIC study as definite, probable, suspect, or no MI, based on ECG evidence (evolving diagnostic, diagnostic, evolving ST-T changes, equivocal, or absent/uncodable), presence or absence of chest pain, and cardiac biomarkers (which were considered “equivocal” if exceeding the ULN but <2 times the ULN, and “abnormal” if ≥2 times the ULN). Definite or probable MI was classified using a validated computer algorithm (10). Classification criteria remained constant over the study period and are described in detail in the ARIC Study surveillance manual (13). Cases with disagreements between the computer diagnosis and discharge diagnosis codes were reviewed by physicians on the ARIC Mortality and Morbidity Classification Committee for final classification. In order to qualify as definite or probable MI, one of the following conditions was required: (1) evolving diagnostic ECG pattern, (2) diagnostic ECG pattern and biomarkers ≥2 times ULN, (3) cardiac pain and biomarkers ≥2 times ULN, (4) cardiac pain and slightly elevated biomarkers (> ULN but <2 times ULN) with evolving ST-T pattern or diagnostic ECG pattern, or (5) biomarkers ≥2 times ULN with evolving ST-T pattern (12). Definite or probable MI was further classified as either non ST-segment elevated myocardial infarction (NSTEMI) or ST-segment elevated myocardial infarction (STEMI), based on the Minnesota code. For the purposes of this analysis, patients identified with STEMI were excluded.
Symptom Onset to Admission Time
The elapsed time from onset of chest pain until hospital admission was estimated by reviewing the clinical notes. Out-of-hospital symptom onset was defined by timing of acute pain anywhere in the chest, left arm, jaw, back, shoulder, right arm, or abdomen in the 72 hours preceding hospital arrival. For patients with chronic angina, symptom onset was defined by change in pain prompting medical attention. Onset of in-hospital chest pain was ascertained by reviewing the emergency room sheet, admission notes, and patient history.
Coronary Revascularization Procedures
Revascularizations by PCI (angioplasty, stent, or coronary atherectomy), coronary artery bypass graft (CABG) surgery, or intracoronary thrombolytics (streptokinase, urokinase, anistreplace, or tissue plasminogen activator) were abstracted from the hospital record. The elapsed time from symptom onset to PCI was derived, using the timing of acute pain precipitating hospitalization. For the purposes of this analysis, patients not undergoing PCI were excluded. We considered revascularization performed <24 hours after symptom onset to be “early”, and revascularizations ≥24 hours to be “late”. In the event that multiple revascularizations were attempted during the hospital stay, the timing to revascularization was based on the first procedure.
Thrombolysis in Myocardial Ischemia (TIMI) Score
TIMI risk scores were derived, based on the abstracted medical histories and laboratory values. Clinical inputs for the TIMI risk score for unstable angina / NSTEMI include age ≥ 65, presence of 3 or more coronary artery disease (CAD) risk factors (family history of CAD, hypertension, hypercholesterolemia, diabetes, or tobacco use), known history of CAD (stenosis ≥ 50%), aspirin use within the past 7 days, severe angina, ST-segment deviation on ECG, and elevated cardiac biomarkers (14). For the purposes of this analysis, hypertension was defined by known history of hypertension, antihypertensive medication use, systolic blood pressure ≥140 or diastolic blood pressure ≥90 mmHg. Diabetes was defined by known history of diabetes or diabetic medication use. Diagnosis of hypercholesterolemia was not abstracted by the ARIC surveillance, but was inferred by statin medication use. However, statin use prior to 2000 was uncommon. Likewise, family history of CAD was not abstracted from the medical record. Known history of CAD was inferred by history of prior angioplasty or CABG. Severe angina was inferred by acute chest pain precipitating hospitalization. Aspirin usage on a regular basis (not PRN) was abstracted from the medical record, and from this aspirin use within the past 7 days was inferred. Patients with a TIMI score of 2–4 were considered “low risk”, and patients with either a TIMI score of 5–7, or presence of cardiogenic shock, cardiac arrest, or ventricular fibrillation were considered “high risk”.
Mortality Outcomes
Mortality was ascertained within 28 days and 1 year of hospital admission by the ARIC Community Surveillance, by linking hospitalizations with the national death index.
Statistical Analysis
All statistical analyses were carried out using SAS 9.4 (SAS Institute; Cary, NC) and were weighted by the inverse of the sampling probability. Continuous variables were assessed for normality, and compared using 2 sample t-tests. Categorical variables were compared using Rao-Scott χ2 tests. Associations between early vs. late revascularization and 28-day or 1-year mortality were analyzed using multivariable logistic regression. Models were adjusted for demographics (age, race, sex, and year of hospitalization), TIMI score, clinical comorbidities (pulmonary edema/congestive heart failure, diabetes, smoking, heart rate, ventricular fibrillation, cardiac arrest, and cardiogenic shock), aspirin use, weekend admissions, and transfer status. Separate models were constructed to assess mortality outcomes of early vs. late PCI among high and low risk patients. Because potential benefit of early PCI may be confounded by time to presentation following symptom onset (e.g. patients arriving early would start appropriate medical therapy sooner than those presenting late) we conducted 2 separate sensitivity analyses, which were limited to patients presenting to the hospital either <6 hours of symptom onset, or <24 hours after symptom onset. These last 2 models were additionally adjusted for duration of symptoms until admission (<1 hour, 1 to <2 hours, 2 to <4 hours, 4 to <6 hours, 6 to < 12 hours, and 12 to <24 hours). We also conducted a subgroup analysis limited to patients admitted from 2000 – 2012, to examine outcomes of early vs. late PCI for the more recent years. Potential modifications of mortality outcomes associated with early PCI were examined within sex, age (<65 or ≥65 years), and race (white or non-white) categories, using multiplicative interaction terms.
Results
From January 1, 1987 – December 31, 2012, there were 16,383 NSTEMI hospitalizations with available follow up for mortality outcomes. Of these 1,793 were excluded due to missing abstractions of the medical history. Out of the 14,590 NSTEMI patients with sufficient clinical data, 4,419 underwent PCI, corresponding to 6,746 weighted hospitalizations. The majority were white (79%), and male (68%), with a mean age of 61 years. Over half (55%) were classified as low risk.
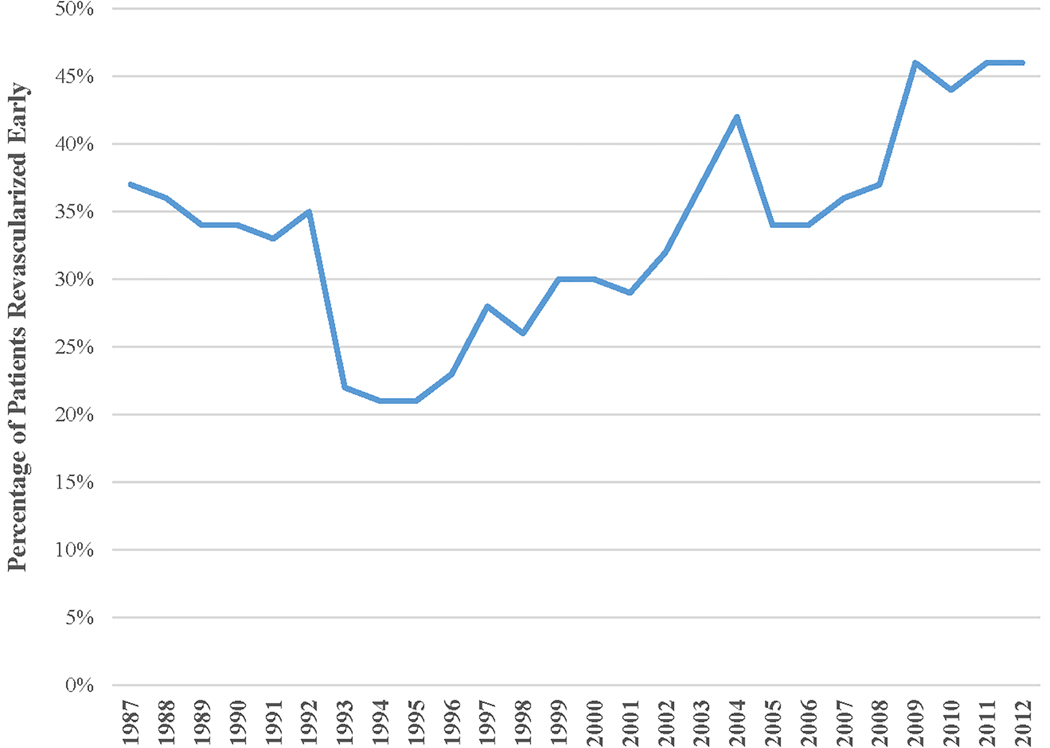
Overall, the majority of patients (65%) were revascularized late. In addition to angioplasty, most (87%) had a stent placed. A small fraction (3%) of patients underwent CABG subsequent to the PCI. The proportion of revascularizations occurring within 24 hours of symptom onset decreased from 1987 to 1996 but then increased with calendar year, reaching a high of 46% by 2012 (Figure 1). Patients undergoing early revascularization were more often male (72% vs. 66%), but similar in age and race to patients with late PCI (Table 1). Current smoking was more common with early compared to late intervention (36% vs. 33%); however, diabetes (24% vs. 28%) and chronic kidney disease (11% vs. 18% in a subsample of ~40%) were less prevalent. Compared to late PCI, an early procedure was more often associated with presence of ST-segment deviation (74% vs. 63%) and cardiogenic shock (4% vs. 3%). However, acute pulmonary edema was less common with early intervention (12% vs. 15%). Mean TIMI scores (4.4 vs. 4.3) were similar between the 2 groups.
Figure 1:
Calendar-year trends for the proportion of hospitalized NSTEMI patients undergoing percutaneous coronary intervention who were revascularized early (<24 hours after symptom onset). The Atherosclerosis Risk in Communities (ARIC) Surveillance Study, 1987–2012.
Table 1:
Demographics and clinical characteristics of patients hospitalized with non ST-segment elevation myocardial infarction (NSTEMI) who underwent percutaneous coronary revascularization, either early (<24 hours after symptom onset) or late (≥24 hours after symptom onset). The Atherosclerosis Risk in Communities (ARIC) Surveillance Study, 1987–2012.
| Characteristic | Early Revascularization N=2,376 | Late Revascularization N=4,370 | |
|---|---|---|---|
| Mean ± SEM or No. (%) | Mean ± SEM or No. (%) | P-value | |
| Demographics | |||
| Age (years) | 59 ± 0.3 | 61 ± 0.2 | 0.01 |
| Male | 1711 (72%) | 2903 (66%) | 0.0009 |
| White | 1930 (80%) | 3416 (78%) | 0.04 |
| Medical History | |||
| Current Smoker | 856 (36%) | 1442 (33%) | 0.09 |
| Hypertension* | 1438 (99.6%) | 3017 (99.8%) | 0.3 |
| Diabetes | 571 (24%) | 1230 (28%) | 0.02 |
| Chronic Kidney Disease† | 150 (11%) | 359 (18%) | 0.003 |
| Prior Revascularization‡ | 735 (31%) | 1312 (30%) | 0.6 |
| Hospital Visit | |||
| TIMI Risk Score | 4.4 ± 0.02 | 4.2 ± 0.02 | <0.0001 |
| Chest Pain | 2354 (99%) | 4122 (94%) | <0.0001 |
| Elevated Cardiac Enzymes | 2368 (99.7%) | 4214 (96%) | <0.0001 |
| ST-Segment Deviation | 1748 (74%) | 2756 (63%) | <0.0001 |
| S3 Gallop | 57 (2%) | 84 (2%) | 0.3 |
| Pulmonary Rales | 132 (6%) | 380 (9%) | 0.0008 |
| Pulmonary Edema / CHF | 280 (12%) | 662 (15%) | 0.009 |
| Cardiogenic Shock | 100 (4%) | 110 (3%) | 0.002 |
| Weekend Admission | 532 (22%) | 1145 (26%) | 0.02 |
| Transferred from Other Hospital | 105 (4%) | 560 (13%) | <0.0001 |
| Symptom to Admission Time | <0.0001 | ||
| <1 hour | 477 (20%) | 328 (8%) | |
| 1 to <2 hours | 631 (27%) | 560 (13%) | |
| 2 to <4 hours | 551 (23%) | 554 (13%) | |
| 4 to <6 hours | 206 (9%) | 250 (6%) | |
| 6 to <12 hours | 283 (12%) | 384 (9%) | |
| 12 to <24 hours | 228 (10%) | 472 (11%) | |
| 24 to <36 hours | 0 | 848 (19%) | |
| ≥36 hours | 0 | 974 (22%) | |
Based on patients with available abstractions for hypertension classification (n=4466).
Chronic kidney disease defined by stage 3 or higher glomerular filtration rate by the CKD-Epi formula. Based on patients with available creatinine assessments (n=3,376).
Revascularization includes coronary angioplasty or bypass graft surgery
Early PCI was closely correlated with an early presentation to the hospital following symptom onset. This is congruent with our definition of early revascularization, which is based on the duration of time from symptom onset until intervention. The majority of patients undergoing early revascularization (79%) presented to the hospital within 6 hours of their symptom onset, compared to 40% of patient who were revascularized late. Of note, 41% of patients with late revascularization presented to the hospital 24 hours or later after their symptom onset. Patients revascularized late were also more likely to have been transferred from another hospital (13% vs. 4%).
The overall mortality in this population of NSTEMI patients undergoing PCI was low. There were 124 (2%) deaths occurring in-hospital and 150 (2%) deaths within 28 days. Within 1 year of hospital admission, 313 patients (5%) died. As might be expected, mortality was lower in the low-risk patients compared to high-risk patients (0.04% vs. 6% for in-hospital deaths, 0.2% vs. 7% for deaths within 28-days, and 2% vs. 11% for 1-year mortality).
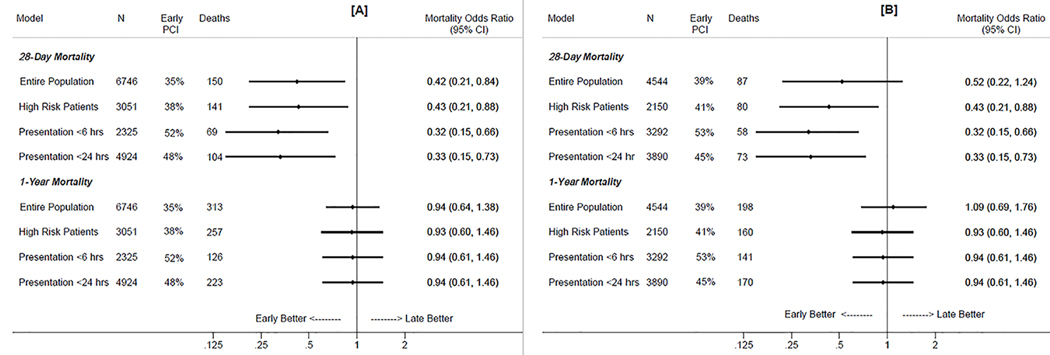
As shown in Figure 2a, early revascularization was associated with lower 28-day mortality. After adjustment for demographics (age, race, sex, and year of hospitalization), TIMI score, comorbidities (pulmonary edema / congestive heart failure, diabetes, smoking, heart rate, ventricular fibrillation, cardiac arrest, and cardiogenic shock), aspirin use, weekend admission, and transfer status, early intervention was significantly associated with a 58% lower 28-day mortality for the entire population, and a 57% lower 28-day mortality for high risk patients. Among the subset of patients admitted to the hospital within 6 hours of their symptom onset, early PCI was associated with a 68% lower 28-day mortality, after adjustments for demographics, comorbidities, aspirin, weekend admission, transfer status, and duration of time from symptom onset to admission. Similarly, a 67% lower 28-day mortality was observed among the subset of patients admitted to the hospital within 24 hours of their symptom onset. No 28-day survival benefit with early intervention was observed in low risk patients (OR=1.12, 95% CI: 0.09 – 19.1); however, statistical models were severely underpowered, due to the low number of deaths (n=9) in this group. By one year of follow up, the benefit of early revascularization had attenuated for the entire cohort, as well in the subgroups of patients with high risk or an early presentation to the hospital. No significant interaction between early PCI with age, sex, or race groups was observed, either for the 28-day morality or 1-year mortality models. (p-value for interaction >0.10 for each model). In the subset of patients admitted during between 2000 and 2012, similar 28-day and 1-year mortality outcomes were observed with early vs. late PCI (Figure 2b).
Figure 2:
Adjusted mortality odds ratios* of early (<24 hours after symptom onset) relative to late (≥24 hours after symptom onset) percutaneous revascularization in patients hospitalized with non ST-segment myocardial infarction (NSTEMI). The Atherosclerosis Risk in Communities (ARIC) Surveillance Study, 1987–2012 (2a), and subset of the ARIC Communities Surveillance Study admitted from 2000 –2012 (2b).
*Models adjusted for demographics (age, race, sex, calendar year of hospitalization), TIMI risk score, comorbidities (pulmonary edema / congestive heart failure, diabetes, smoking, heart rate, ventricular fibrillation, cardiac arrest, and cardiogenic shock), aspirin use, weekend admission, and transfer status. Models of patients presenting to the hospital <6 and <24 hours of symptom onset are additionally adjusted for time from symptom onset to admission (<1 hour, 1 to <2 hours, 2 to <4 hours, 4 to <6 hours, 6 to <12 hours, and 12 to <24 hours).
Discussion
In this real-world analysis of NSTEMI patients undergoing coronary revascularization, early percutaneous intervention was associated with better 28-day survival, both for the entire population and the subgroup of patients classified with high risk. However, by 1-year of follow up, the better survival with early PCI was no longer statistically significant. The results were largely consistent in demographic subgroups by age, sex, and race.
The better prognosis with early vs. late PCI among NSTEMI patients in the ARIC Surveillance is consistent with a few prior studies, such as the Randomized Study of Immediate versus Delayed Invasive Intervention in Patients with non ST-segment Elevation Myocardial Infarction (RIDDLE-NSTEMI) (6) and the Intracoronary Stenting with Antithrombotic Regimen Cooling-Off (ISAR-COOL) (1). In both trials, the 30-day benefit of early intervention was largely driven by the reduction in new MI during the pre-catheterization period. However, neither was able to demonstrate survival benefit alone with early revascularization. In this context, it is important that our analysis confirms better 28-day survival in patients with early vs. late PCI, which is independent of demographics, year or hospitalization, and comorbidities. The higher 28-day mortality observed with delayed PCI is likely due to the prolonged ischemic time prior to revascularization, leading to myocardial necrosis (15) and poor subsequent outcomes.
The attenuation of long-term survival benefit with early vs. late revascularization in the ARIC Surveillance parallels the 1-year outcomes reported by the RIDDLE-NSTEMI trial. A possible explanation may be adherence to guideline-based recommendations for acute coronary syndrome. In particular, antithrombotic and cardioprotective medications have led to a reduction in post-hospitalization mortality for NSTEMI patients, irrespective of timing of revascularization (4). Additionally, the deleterious effect of prolonged pre-catheterization ischemia may wane over time due to selective survival, with most deaths occurring within 30 days of revascularization. Patients who survive the 30-day window following revascularization may be at lower risk of death in long-term follow up.
Although there was declining trend from late 1980s to the beginning of 1990s, from 1996 onward, an upward trend was observed in the proportion of revascularized NSTEMI patients who underwent early PCI. This trend may reflect changes in medical practices in the late 1990s, following the publication of studies showing benefit of early invasive strategy over conservative medical therapy (16–18). The upward trend in the proportion of early PCIs may also relate to national trends for earlier transfers of NSTEMI patients from community to tertiary hospitals with cardiac catheterization capabilities (19). On the other hand, it is possible that the adoption of cardiac troponins by most hospitals in this time frame led to an earlier diagnosis of NSTEMI and subsequent early PCI.
Our study has a few important limitations. Although we tried to account for potential confounders, as true in any observational studies, we cannot deny the possibility of residual confounding (particularly confounding by indication), because early or late PCI was not randomly assigned. However, our purpose was to evaluate early vs. late PCI in NSTEMI in an uncontrolled real world setting. Clinical data were also limited by their availability in the medical record, and creatinine values were unavailable for half the patients. We did not have information on timing to angiography; only timing to revascularization was recorded. For this reason, we did not include patients undergoing angiography without intervention, as there was no way to classify cardiac catheterizations as “early” or “late”. Additionally, the timing for revascularization was based on the elapsed time from symptom onset to intervention. This limits comparability to early or delayed invasive strategies defined by the elapsed time from hospital admission to angiography. However, in a subanalysis of patients presenting to the hospital <24 hours after their symptom onset, early PCI remained associated with 28-day survival. Our study also has several strengths. The ARIC Surveillance provides a large, population-based sample of unselected patients, with sufficient statistical power to examine mortality alone, outside of a composite outcome. Clinical and laboratory values were meticulously collected by certified abstractors following standardized protocols, and death outcomes were ascertained by the national death index.
Conclusion:
In hospitalized NSTEMI patients at high risk of clinical events and amenable to PCI, early intervention is associated with improved 28-day survival.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Disclosures
None.
References
- 1.Neumann FJ, Kastrati A, Pogatsa-Murray G, Mehilli J, Bollwein H, Bestehorn HP, Schmitt C, Seyfarth M, Dirschinger J, Schomig A. Evaluation of prolonged antithrombotic pretreatment (“cooling-off” strategy) before intervention in patients with unstable coronary syndromes: a randomized controlled trial. JAMA 2003;290:1593–1599. [DOI] [PubMed] [Google Scholar]
- 2.de Winter RJ, Windhausen F, Cornel JH, Dunselman PH, Janus CL, Bendermacher PE, Michels HR, Sanders GT, Tijssen JG, Verheugt FW, Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) Investigators. Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med 2005;353:1095–1104. [DOI] [PubMed] [Google Scholar]
- 3.Riezebos RK, Ronner E, Ter Bals E, Slagboom T, Smits PC, ten Berg JM, Kiemeneij F, Amoroso G, Patterson MS, Suttorp MJ, Tijssen JG, Laarman GJ, OPTIMA trial. Immediate versus deferred coronary angioplasty in non-ST-segment elevation acute coronary syndromes. Heart 2009;95:807–812. [DOI] [PubMed] [Google Scholar]
- 4.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ, ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:e344–426. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, Afzal R, Chrolavicius S, Jolly SS, Widimsky P, Avezum A, Rupprecht HJ, Zhu J, Col J, Natarajan MK, Horsman C, Fox KA, Yusuf S, TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009;360:2165–2175. [DOI] [PubMed] [Google Scholar]
- 6.Milosevic A, Vasiljevic-Pokrajcic Z, Milasinovic D, Marinkovic J, Vukcevic V, Stefanovic B, Asanin M, Dikic M, Stankovic S, Stankovic G. Immediate Versus Delayed Invasive Intervention for Non-STEMI Patients: The RIDDLE-NSTEMI Study. JACC Cardiovasc Interv 2016;9:541–549. [DOI] [PubMed] [Google Scholar]
- 7.Thiele H, Rach J, Klein N, Pfeiffer D, Hartmann A, Hambrecht R, Sick P, Eitel I, Desch S, Schuler G, LIPSIA-NSTEMI Trial Group. Optimal timing of invasive angiography in stable non-ST-elevation myocardial infarction: the Leipzig Immediate versus early and late PercutaneouS coronary Intervention triAl in NSTEMI (LIPSIA-NSTEMI Trial). Eur Heart J 2012;33:2035–2043. [DOI] [PubMed] [Google Scholar]
- 8.Montalescot G, Cayla G, Collet JP, Elhadad S, Beygui F, Le Breton H, Choussat R, Leclercq F, Silvain J, Duclos F, Aout M, Dubois-Rande JL, Barthelemy O, Ducrocq G, Bellemain-Appaix A, Payot L, Steg PG, Henry P, Spaulding C, Vicaut E, ABOARD Investigators. Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA 2009;302:947–954. [DOI] [PubMed] [Google Scholar]
- 9.Badings EA, The SH, Dambrink JH, van Wijngaarden J, Tjeerdsma G, Rasoul S, Timmer JR, van der Wielen ML, Lok DJ, van ‘t Hof AW. Early or late intervention in high-risk non-ST-elevation acute coronary syndromes: results of the ELISA-3 trial. EuroIntervention 2013;9:54–61. [DOI] [PubMed] [Google Scholar]
- 10.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 11.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation 2012;125:1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myerson M, Coady S, Taylor H, Rosamond WD, Goff DC Jr, ARIC Investigators. Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2009;119:503–514. [DOI] [PubMed] [Google Scholar]
- 13.ARIC Investigators Manual 3: surveillance component procedures manual of operations. Available at: https://www2.cscc.unc.edu/aric/sites/default/files/public/manuals/Updates%20Manual3_20151112.pdf Accessed 03/30/2017.
- 14.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 15.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 1977;56:786–794. [DOI] [PubMed] [Google Scholar]
- 16.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E, TACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)--Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001;344:1879–1887. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Lancet 1999;354:708–715. [PubMed]
- 18.Spacek R, Widimsky P, Straka Z, Jiresova E, Dvorak J, Polasek R, Karel I, Jirmar R, Lisa L, Budesinsky T, Malek F, Stanka P. Value of first day angiography/angioplasty in evolving Non-ST segment elevation myocardial infarction: an open multicenter randomized trial. The VINO Study. Eur Heart J 2002;23:230–238. [DOI] [PubMed] [Google Scholar]
- 19.Roe MT, Chen AY, Delong ER, Boden WE, Calvin JE Jr, Cairns CB, Smith SC Jr, Pollack CV Jr, Brindis RG, Califf RM, Gibler WB, Ohman EM, Peterson ED. Patterns of transfer for patients with non-ST-segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J 2008;156:185–192. [DOI] [PubMed] [Google Scholar]