Abstract
INTRODUCTION:
Celiac disease is an autoimmune disorder where intestinal immunopathology arises after gluten consumption. Previous studies suggested that hookworm infection restores gluten tolerance; however, these studies were small (n = 12) and not placebo controlled.
METHODS:
We undertook a randomized, placebo-controlled trial of hookworm infection in 54 people with celiac disease. The 94-week study involved treatment with either 20 or 40 Necator americanus third-stage larvae (L3-20 or L3-40) or placebo, followed by escalating gluten consumption (50 mg/d for 12 weeks, 1 g intermittent twice weekly for 12 weeks, 2 g/d sustained for 6 weeks, liberal diet for 1 year).
RESULTS:
Successful study completion rates at week 42 (primary outcome) were similar in each group (placebo: 57%, L3-20: 37%, and L3-40: 44%; P = 0.61), however gluten-related adverse events were significantly reduced in hookworm-treated participants: Median (range) adverse events/participant were as follows: placebo, 4 (1–9); L3-20, 1 (0–9); and L3-40, 0 (0–3) (P = 0.019). Duodenal villous height:crypt depth deteriorated similarly compared with their enrolment values in each group (mean change [95% confidence interval]: placebo, −0.6 [−1.3 to 0.2]; L3-20, −0.5 [−0.8 to 0.2]; and L3-40, −1.1 [−1.8 to 0.4]; P = 0.12). A retrospective analysis revealed that 9 of the 40 L3-treated participants failed to establish hookworm infections. Although week 42 completion rates were similar in hookworm-positive vs hookworm-negative participants (48% vs 44%, P = 0.43), quality of life symptom scores were lower in hookworm-positive participants after intermittent gluten challenge (mean [95% confidence interval]: 38.9 [33.9–44] vs 45.9 [39.2–52.6]).
DISCUSSION:
Hookworm infection does not restore tolerance to sustained moderate consumption of gluten (2 g/d) but was associated with improved symptom scores after intermittent consumption of lower, intermittent gluten doses.
INTRODUCTION
Celiac disease (CeD) is an acquired autoimmune enteropathy with a complex pathogenesis (1). Dietary gluten is normally catalyzed to form glutamine-rich peptides that are deamidated by tissue transglutaminase (tTG). Genetically susceptible people express HLA-DQ2 or HLA-DQ8 proteins on antigen-presenting cells, which can bind deamidated gluten epitopes and result in the induction of proinflammatory T lymphocytes that populate the small intestine and induce epithelial cell apoptosis, chronic inflammation, and villous atrophy (2). Autoantibodies induced against tTG correlate with mucosal inflammation (3,4). The condition is becoming increasingly diagnosed and is estimated to affect 2% of many ethnically diverse populations (5,6). Strict compliance with a gluten-free diet is the only effective treatment, but the diet is inconvenient and expensive, and inadvertent gluten exposures are common (7), which can cause debilitating symptoms such as vomiting, diarrhea, and diminished well-being (8).
Helminth parasites are recognized as global pathogens that have coevolved with humans (9). Necator americanus, once ubiquitous, has exquisitely adapted to its human host, such that most infections in well-nourished populations are subclinical (10). A survival strategy used by helminths is to induce regulatory immune responses, immunomodulation that has a collateral effect on concomitant inflammatory diseases of the host (11,12). Previously, we undertook a N. americanus vs placebo-controlled trial in people with CeD (13), where participants were abruptly challenged with gluten (16 g of gluten daily); however hookworm infection was not associated with improved outcomes after this abrupt challenge. Subsequent observation of the immunomodulatory impact of hookworm infection on gluten tolerance (14–16) was the impetus for a follow-up study to address whether hookworm treatment, when combined with gluten desensitization immunotherapy akin to that trialed for CeD (17) and food allergies (18), promotes a beneficial regulatory immune response in the intestine and restores gluten tolerance (19). In that open-label study, 12 participants were primed with daily consumption of trace gluten (50 mg daily), after which 10 of the 12 comfortably tolerated 12 weeks of intermittent gluten bolus challenges (1 g/d twice weekly). Duodenal histologic scores at completion were similar to baseline assessments; tTG values improved, as did their quality of life (QoL) scores, suggesting that gluten microchallenge in the presence of a hookworm infection might help restore gluten tolerance.
This study expands on the open-label study, by comparing gluten microchallenge alone vs hookworm inoculation plus microchallenge, for restoring tolerance to escalating gluten consumption. The primary outcome was the relative rates of critically monitored safe completion after 30 weeks of gluten challenges (concluding with 2 g/d for 6 weeks). Detailed safety evaluations were also conducted at interim assessment visits to identify tolerance to 50 mg of gluten daily and twice weekly 1-g challenge, which mimics exaggerated real-life exposures to gluten boluses.
METHODS
Study sites and regulatory approvals
Between March 2017 and October 2019, we conducted a phase 1b randomized, double-blinded, placebo-controlled trial of experimental hookworm infection in otherwise healthy people with documented CeD. The study included 4 clinical sites: 3 within Queensland, Australia (The Prince Charles Hospital, Brisbane; Logan Hospital, Logan; and Townsville University Hospital, Townsville) and 1 in New Zealand (Christchurch Hospital, New Zealand). The study designated HREC/16/QPCH/206 (Australia) and HDEC/16/CEN/117 (New Zealand) was approved on August 31, 2016, and October 25, 2016, respectively, by the Human Research Ethics Committee at the Prince Charles Hospital, Chermside Queensland, Australia, and the Central Health and Disability Ethics Committee, Wellington, New Zealand. The trial was registered with ClinicalTrials.gov, Protocol Record NCT02754609 on April 28, 2016.
Participants
Entry criteria included otherwise healthy men and women aged 18–80 years with verified documentation of CeD including pretreatment Marsh 3 histology score and elevated serum immunoglobulin A (IgA)-anti-tTG result. Exclusion criteria in brief included pregnancy, a history of substance abuse or regular medications likely to affect immune function, and/or poorly controlled diseases that might interfere with trial outcomes. Applicants were initially screened for eligibility through interview, a review of their documentation relating to CeD, and confirmation of current and uninterrupted adherence to a gluten-free diet for >6 months. Applicants were then assessed for compliance with inclusion/exclusion criteria, and confirmation of current CeD inactivity determined by serology (IgA-anti-tTG <7 U/mL), histology (Marsh 0 or 1 with a Vh:Cd ratio ≥2), and a CeD symptom index (CSI) <35.
Randomization and administration of treatments
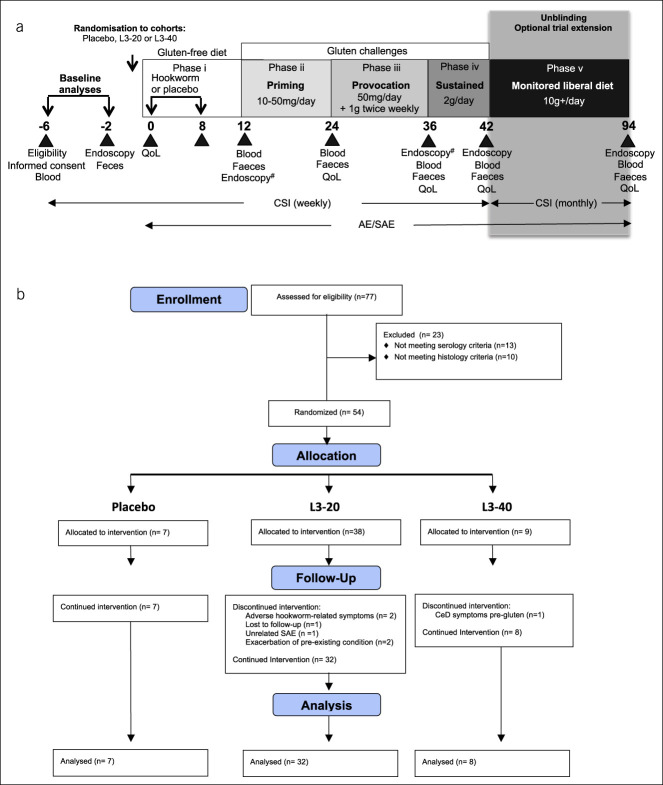
Sixty participants were to be included in the trial, randomly allocated on a 1:4:1 ratio according to a computer-generated sequence to 1 of the 3 study arms: placebo (n = 10), 20× Necator americanus third-stage larvae (L3-20; n = 40) or 40× larvae (L3-40; n = 10), stratified evenly by baseline Vh:Cd ratio (over or below Vh:Cd of 3.0). Participants and investigators were blinded to the treatment, other than the assigned producer of the inocula (coauthor L.B.) and a medical monitor (coauthor G.R.S.). The inocula were prepared freshly at James Cook University in Cairns, Australia, as previously described (19) (see Supplementary Methods, Supplementary Digital Content 2, http://links.lww.com/CTG/A451). Two doses of inocula were administered 8 weeks apart, with L3-20 and L3-40 participants receiving 10× or 20× L3 on each occasion (Figure 1a). Hookworm infection status was monitored at weeks 12 and 24 using quantitative PCR on fecal samples (20).
Figure 1.
Study time line, interventions, and CONSORT flow chart. (a) After screening and baseline analyses, participants with CeD were allocated to receive either placebo (chilli pepper solution) or hookworm larvae (L3-20 or L3-40) delivered over 2 occasions, at week 0 and week 8 (phase i). L3 denotes Necator americanus stage 3. At week 12, subjects received a 12-week gluten priming challenge (phase ii: 10–50 mg/d for 12 weeks), followed by a gluten provocation challenge (phase iii: 50 mg/d +1 g twice weekly for 12 weeks) and sustained gluten challenge (phase iv: 2 g/d for 6 weeks). At week 42, following evaluation of patient symptoms, the study became unblinded and participants in active L3-treated cohorts were given the option of undertaking a monitored “liberal diet” of at least 10 g of gluten/day, with freedom of food choice for 12 months (phase v). Participants underwent regular clinic visits as denoted by the black triangles, where various biological samples and survey assessments were taken for analysis of safety and CeD pathology. #Denotes endoscopy performed at week 12 instead of week 36 in the L3-40 cohort. (b) CONSORT chart showing flow of patients through the clinical trial. AE, adverse event; CeD, celiac disease; CSI, CeD symptom index survey; QoL, CeD quality of life survey; SAE, serious AE.
Gluten challenges
Gluten challenge commenced approximately 4 weeks after the final treatment. The study was conducted with 5 phases designed to test tolerance to different levels of gluten consumption (Figure 1a). Beginning with a 12-week treatment phase while consuming a gluten-free diet (phase i), there followed 4 sequential challenge phases where gluten was provided as wheat pasta (Barilla Spaghettini No. 3; 12% protein, estimated 6% dry weight of gluten). Gluten challenges were as follows: phase ii—12 weeks priming microchallenge with 10 mg daily for 2 weeks then 50 mg daily for 10 weeks (1.5 spaghetti straws); phase iii—12 weeks provocation with 50 mg of gluten daily and 1 g twice weekly (25–30 spaghetti straws); and phase iv—6 weeks sustained challenge with approximately 2 g of gluten daily (50–60 spaghetti straws or, in some instances, 1 slice of bread with comparable gluten content). After completion of the primary 42-week study and unblinding, participants in the L3-20 and L3-40 arms without CeD symptoms were offered an optional 12-month extension to the study that involved consumption of an unrestricted gluten-containing diet exceeding 10 g/d (phase v). All participants displayed consistent adherence to gluten-free diet before and during the trial (other than trial-prescribed gluten), which was documented weekly on a gluten score card.
Outcome measures
At each designated visit, safety assessments were conducted to identify adverse events (AEs) and serious AEs. The site principle investigator and the medical monitor were immediately notified of an adverse result. Each participant was required to complete a CSI weekly until week 42 and monthly thereafter. Blood, fecal, and duodenal biopsy samples were taken at weeks 12, 24, 36, and 42. The biopsy schedule was designed to limit interventions to a maximum of 2 per individual after baseline, with L3-40 participants scheduled for biopsy at weeks 12 and 42, and the L3-20 and placebo cohorts at weeks 36 and 42. The alternative L3-40 endoscopy schedule was to evaluate hookworm infection in isolation on intestinal biology. The primary outcome assessed at week 42 was the safety of sequential gluten challenges (phases ii–iv), assessed by adverse events, symptoms, anti-tTG serology, and qualitative and quantitative histological parameters, defined as a binary variable (pass or fail). Specific criteria were as follows:
Pass if week 42 visit successfully completed with tTG <10 and Vh:Cd ≥2.0 (Marsh 0 or 1);
Fail if dropout occurs before week 42, or tTG ≥10, or Vh:Cd <2.0 (Marsh 3).
Secondary endpoints to evaluate tolerance to lower levels of gluten consumption included serological, histological (as available), and symptom scores completed after phases ii and iii (see Supplementary Methods, Supplementary Digital Content 2, http://links.lww.com/CTG/A451). During the liberal diet, each participant was monitored monthly by tTG and CSI.
Statistical analyses
Associations between treatment groups for the primary outcome and categorical secondary measures were examined using χ2 tests of independence or the Fisher exact test, where >20% of the expected values were <5. Associations for the change in continuous secondary measures from baseline to week 42 between groups were examined using a 1-way ANOVA or Kruskal-Wallis test, where the assumptions of data normality were not met. Where differences between treatment groups were not important, evidence of an overall change for measures were assessed using a 1 sample t test or Wilcoxon signed-rank test, where the assumptions of data normality were not met. Categorical clinical measures were summarized by frequency and percentage and continuous measures were summarized by mean and 95% confidence interval (CI) or median and interquartile range for nonnormally distributed data. Where appropriate, exploratory analysis of the change from baseline to week 42 of secondary outcomes between participants in alternative 2 treatment groups was assessed using a 2-sample t test. The Kaplan-Meier analysis was used to estimate the cumulative probability of participant dropouts from baseline to the week 42, with associations between treatment groups assessed using the log-rank test statistic. Statistical analyses were performed in Stata version 15 (StataCorp, College Station, TX).
RESULTS
Study population
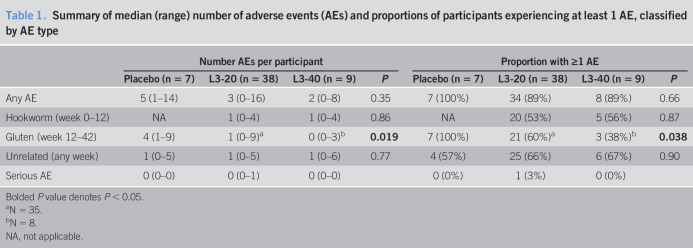
Seventy-seven applicants with CeD whose diagnosis met validation criteria were screened for eligibility based on protocol inclusion/exclusion criteria (see Supplementary Methods, Supplementary Digital Content 2, http://links.lww.com/CTG/A451). Twenty-three participants were excluded: 13 with tTG ≥7 U/mL and 10 with duodenal histopathology (Marsh 2–3, or Vh:Cd < 2) (Figure 1b). Fifty-four participants (90% of the recruitment target) were randomized and constituted the intention-to-treat population (see Supplementary Table 1, Supplementary Digital Content 2, http://links.lww.com/CTG/A451). Seven participants were allocated to receive placebo treatment, 38 to receive L3-20 treatment, and 9 to receive L3-40 (Figure 1b). HLA imputation analysis (see Supplementary Methods and Supplementary Table 2, Supplementary Digital Content 2, http://links.lww.com/CTG/A451) confirmed the presence of the DQ2/DQ8 CeD risk alleles for all study participants who consented to genotyping analysis (mean posterior probability of 0.97 for both HLA-DQA1 and HLA-DQB1; see Supplementary Table 3, Supplementary Digital Content 2, http://links.lww.com/CTG/A451).
AEs and serious AEs
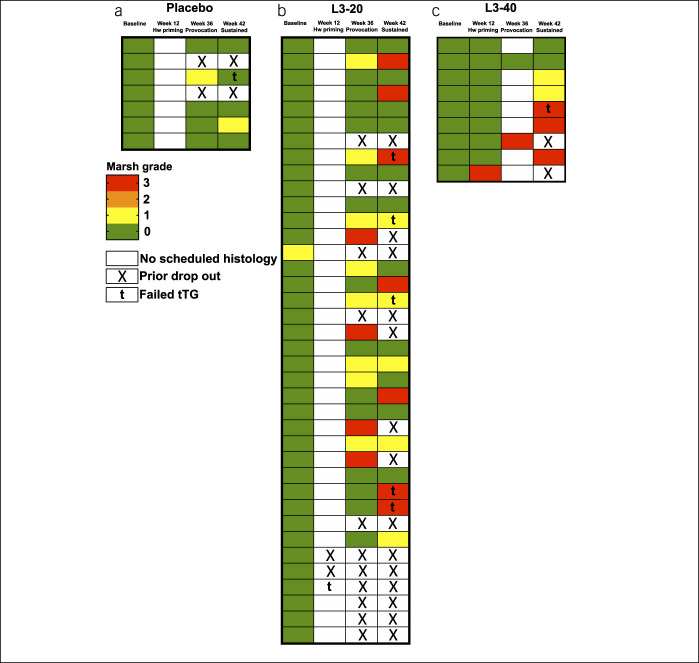
The most common AEs recorded in the first 12 weeks (hookworm priming—phase i) were abdominal pain, diarrhea, fatigue, nausea, appetite loss, flatulence, or bloating (Table 1). During the gluten challenge phases (weeks 12–42), the most common AEs were diarrhea, abdominal pain, nausea, fatigue, headache, vomiting, bloating, and muscle aches. Compared with placebo-treated participants, hookworm-treated participants exhibited significantly fewer AEs during the gluten challenge period (Table 1). Similarly, the percentage of participants in the hookworm treatment groups who experienced at least 1 AE during the gluten challenge phases was reduced, compared with that in the placebo-treated participants (Table 1). No participant developed iron deficiency or anemia (see Supplementary Figure 1A and B, Supplementary Digital Content 1, http://links.lww.com/CTG/A450), the major clinical effect of hookworm infection.
Table 1.
Summary of median (range) number of adverse events (AEs) and proportions of participants experiencing at least 1 AE, classified by AE type
| Number AEs per participant | Proportion with ≥1 AE | |||||||
| Placebo (n = 7) | L3-20 (n = 38) | L3-40 (n = 9) | P | Placebo (n = 7) | L3-20 (n = 38) | L3-40 (n = 9) | P | |
| Any AE | 5 (1–14) | 3 (0–16) | 2 (0–8) | 0.35 | 7 (100%) | 34 (89%) | 8 (89%) | 0.66 |
| Hookworm (week 0–12) | NA | 1 (0–4) | 1 (0–4) | 0.86 | NA | 20 (53%) | 5 (56%) | 0.87 |
| Gluten (week 12–42) | 4 (1–9) | 1 (0–9)a | 0 (0–3)b | 0.019 | 7 (100%) | 21 (60%)a | 3 (38%)b | 0.038 |
| Unrelated (any week) | 1 (0–5) | 1 (0–5) | 1 (0–6) | 0.77 | 4 (57%) | 25 (66%) | 6 (67%) | 0.90 |
| Serious AE | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0 (0%) | 1 (3%) | 0 (0%) | ||
Bolded P value denotes P < 0.05.
N = 35.
N = 8.
NA, not applicable.
Primary outcome measure: successful study completion at week 42
Of the 54 participants included in the intention-to-treat analysis, 7 participants were withdrawn from the study because reasons not attributed to prescribed gluten. Five of the 7 were removed before the introduction of the gluten challenge: 2 participants in the L3-20 cohort with suspected hookworm-related enteric symptoms from 6 to 8 weeks (Figure 1b), both of whom received anthelmintic treatment that resulted in a prompt resolution; 2 participants in the L3-20 cohort withdrew at week 12, one without explanation and one because of a recurrence of Graves thyrotoxicosis; and one L3-40 participant with a pretrial histology graded Marsh 0 was excluded after the week 12 biopsy established Marsh 3 pathology (Figure 2). The remaining 2 participants were withdrawn due to unrelated conditions: one because of a recurrence of a depressive illness; and one with an unrelated gynecological event (serious AE) (Figure 1b).
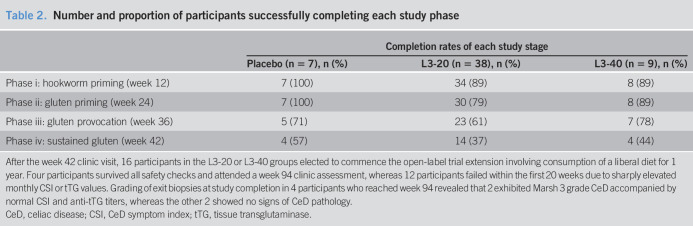
Figure 2.
Heat map displaying safety assessment outcomes and completion of study phases. Color-coded heat map displaying Marsh score, IgA-tTG safety assessment results and dropouts in the intention-to-treat (a) placebo, (b) L3-20, and (c) L3-40 cohorts at baseline and at designated visits after escalating gluten challenge. Marsh 3 represents failed histology; t denotes failed tTG assessment. Each row represents an individual participant. tTG, tissue transglutaminase.
Twelve participants displayed symptomatic gluten toxicity (placebo: n = 2, L3-20: n = 5) or developed Marsh 3 histology (L3-20: n = 4, L3-40: n = 1, Figure 2) before the week 42 assessment and, thus, failed to meet the primary endpoint. Fourteen participants failed the week 42 assessment: 4 (placebo: n = 1, L3-20: n = 3) due to raised tTG alone; 6 (L3-20: n = 4, L3-40: n = 2) due to failed histology alone; and 4 (L3-20: n = 3, L3-40: n = 1) due to failed tTG and histological results (Figure 2).
Four of 7 placebo-treated participants successfully completed the study, maintaining Marsh 0 or 1 grade pathology and normal tTG (Figure 2a). Similarly, 14 of the 38 in the L3-20 group and 4 of the 9 in the L3-40 group successfully completed the week 42 safety assessments (Figure 2b,c). Primary analysis of the intention-to-treat study population at week 42 revealed that successful completion rates were similar between the 3 study groups (Table 2; P = 0.61). Rates of completion of the interim study phases did not differ between the 3 treatment groups (Table 2).
Table 2.
Number and proportion of participants successfully completing each study phase
| Completion rates of each study stage | |||
| Placebo (n = 7), n (%) | L3-20 (n = 38), n (%) | L3-40 (n = 9), n (%) | |
| Phase i: hookworm priming (week 12) | 7 (100) | 34 (89) | 8 (89) |
| Phase ii: gluten priming (week 24) | 7 (100) | 30 (79) | 8 (89) |
| Phase iii: gluten provocation (week 36) | 5 (71) | 23 (61) | 7 (78) |
| Phase iv: sustained gluten (week 42) | 4 (57) | 14 (37) | 4 (44) |
After the week 42 clinic visit, 16 participants in the L3-20 or L3-40 groups elected to commence the open-label trial extension involving consumption of a liberal diet for 1 year. Four participants survived all safety checks and attended a week 94 clinic assessment, whereas 12 participants failed within the first 20 weeks due to sharply elevated monthly CSI or tTG values. Grading of exit biopsies at study completion in 4 participants who reached week 94 revealed that 2 exhibited Marsh 3 grade CeD accompanied by normal CSI and anti-tTG titers, whereas the other 2 showed no signs of CeD pathology.
CeD, celiac disease; CSI, CeD symptom index; tTG, tissue transglutaminase.
Secondary outcome measures: histology, IgA anti-tTG, and symptom scores
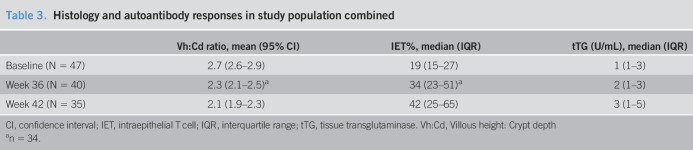
For analysis of secondary outcomes, we excluded results from the 7 participants who were removed from the study because of reasons not attributed to prescribed gluten or hookworm. Analysis of changes in histological parameters Vh:Cd and intra-epithelial T (IET) cell counts in the combined study cohort identified significant deterioration in both parameters at week 42 compared with those at baseline (P < 0.001, Table 3). Relative changes in Vh:Cd (P = 0.12) and IET% (P = 0.21) were similar between the 3 study groups (Figure 3a,b and see Supplementary Table 4, Supplementary Digital Content 2, http://links.lww.com/CTG/A451). IgA anti-tTG titers remained stable across the combined study group until week 36 but significantly rose after the sustained gluten challenge at week 42 (P < 0.001, see Supplementary Table 4, Supplementary Digital Content 2, http://links.lww.com/CTG/A451). Relative changes in anti-tTG titers among the 3 groups were similar (P = 0.49, Figure 3c and see Supplementary Table 4, Supplementary Digital Content 2, http://links.lww.com/CTG/A451). CSI scores in the combined study cohort were similar at week 42 compared with those at baseline (mean change CSI [95% CI]: −1.0 [−2.3 to 0.3], P = 0.14), with no differences between the 3 study groups (P = 0.14, see Supplementary Table 4, Supplementary Digital Content 2, http://links.lww.com/CTG/A451). QoL scores were significantly improved in the combined study cohort at week 42 compared with those at baseline (mean change QoL [95% CI]: −7.2 [−11.0 to −3.4], P < 0.001); however, there were no differences in change in QoL score among the 3 study groups (P = 0.12; see Supplementary Table 4, Supplementary Digital Content 2, http://links.lww.com/CTG/A451).
Table 3.
Histology and autoantibody responses in study population combined
| Vh:Cd ratio, mean (95% CI) | IET%, median (IQR) | tTG (U/mL), median (IQR) | |
| Baseline (N = 47) | 2.7 (2.6–2.9) | 19 (15–27) | 1 (1–3) |
| Week 36 (N = 40) | 2.3 (2.1–2.5)a | 34 (23–51)a | 2 (1–3) |
| Week 42 (N = 35) | 2.1 (1.9–2.3) | 42 (25–65) | 3 (1–5) |
CI, confidence interval; IET, intraepithelial T cell; IQR, interquartile range; tTG, tissue transglutaminase. Vh:Cd, Villous height: Crypt depth
n = 34.
Figure 3.
Histology and serum autoantibody (anti-tTG) responses. (a) Duodenal villous height:crypt depth ratio (Vh:Cd, mean ± 95% CI) and (b) intraepithelial CD3+ T cell (IET cell) frequency (median ± IQR) in each individual's duodenal biopsy specimen at baseline and each study visit after intervention and escalating gluten challenge. (c) Serum IgA-tTG titers (median ± IQR) at baseline and each study visit. Dot points represent each individual's value and grayed area indicates the normal ranges for each parameter. CI, confidence interval; Hw, hookworm; IgA, immunoglobulin A; IQR, interquartile range; n/a, not applicable; tTG, tissue transglutaminase.
Evaluation of cellular immune response and hookworm establishment
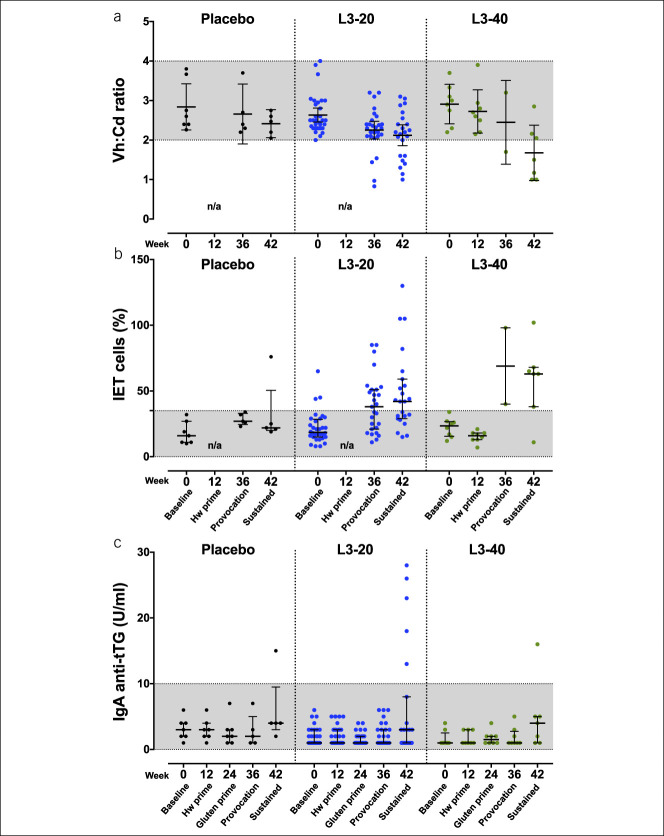
Hookworm infection is associated with induction of blood eosinophilia, peaking between weeks 6 and 12 (10,21,22). Eosinophil counts remained stable in the placebo cohort throughout but were increased in the L3-20 and L3-40 cohorts at week 12, before declining to near baseline levels by week 42 (Figure 4a). However, eosinophilia was not universal, with only 28 of the 40 individuals in the L3-20 and L3-40 groups reaching the upper limit of normal for clinical eosinophilia (0.6 × 109 cells/mL; Figure 4b), suggesting suboptimal establishment of hookworm infection. Analysis of fecal samples taken at weeks 12 and 24 revealed that 9 of 40 L3-20 or L3-40 participants did not have evidence of N. americanus infection (negative for hookworm by PCR; negative for eosinophilia; Figure 4c). Using a composite definition of hookworm infection (hookworm PCR+ and/or elevated eosinophil count), 31 of 40 participants were deemed to have established successful hookworm infections (Figure 4c). Of note, 6 of the 9 participants without evidence of infection were from the New Zealand-based clinical site.
Figure 4.
Evaluation of establishment of hookworm infections. (a) Peripheral blood eosinophil counts at baseline, after treatment with placebo or hookworm L3, and after escalating gluten challenges. Each individual data point is shown along with mean ± 95% confidence interval. Grayed area indicates the normal range. (b) Peak eosinophil counts in each participant from the L3-30 or L3-40 cohorts were ranked from lowest to highest and correlated with the (c) detection of hookworm eggs in feces by quantitative polymerase chain reaction (patency). F denotes participants who demonstrated both a lack of clinical eosinophilia (<0.6 × 109/L) and an absence of a patent hookworm infection (total of 9 participants were deemed to be “hookworm treatment failures”).
Analysis of outcomes in hookworm-positive vs hookworm negative participants.
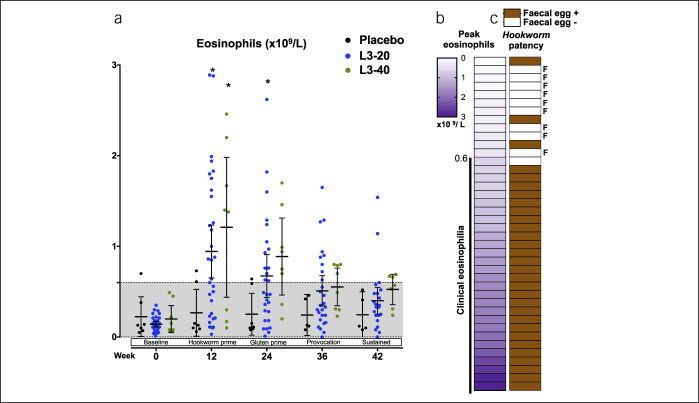
As an exploratory analysis, we investigated the effect of reclassifying the 9 participants in the active arms who lacked evidence of hookworm infection into a “hookworm-negative” group, along with the 7 placebo participants. Although successful completion rates to week 42 were comparable in the 31 hookworm-positive and 16 hookworm-negative participants (48% vs 44%, respectively, P = 0.43), rates of progression through interim phases tended to be greater in the hookworm-positive group (Figure 5a). The Vh:Cd values were comparable between hookworm-positive and -negative participants (Figure 5b). Changes in baseline to week 42 CSI scores were also similar (mean change CSI [95% CI]: hookworm-negative: 1.2 [−4.8 to 2.4], hookworm-positive: −0.9 [−2.3 to 0.5], P = 0.84, Figure 5c). Both groups also exhibited comparable changes in baseline with week 42 QoL values (mean change QoL [95% CI]: hookworm negative: −6.9 [−16.7 to 2.9], hookworm positive: −7.4 [−11.5 to 3.2], P = 0.91, Figure 5d). However, assessment of QoL scores at an interim time point (week 36) suggested that hookworm-positive participants displayed lowered QoL scores compared with their baseline value and with hookworm-negative participants (mean QoL [95% CI] baseline–week 36: hookworm positive: 46.5 [41.3–51.8] to 38.9 [33.9–44]; hookworm negative: 47.1 [39.6–54.5] to 45.9 [39.2–52.6], Figure 5d).
Figure 5.
Analysis of outcomes in hookworm-positive vs hookworm-negative participants. (a) Kaplan-Meier analysis of rates of trial continuation (%) during the various phases of the trial in the 31 hookworm-positive participants vs the 16 hookworm-negative participants, displayed as mean survival ±SEM. Survival required stable (Marsh 0 or I) intestinal pathology, subclinical immunoglobulin A-tissue transglutaminase titer (<10 units) and asymptomatic tolerance to the gluten challenge. (b) Duodenal villous height:crypt depth ratio (Vh:Cd) in hookworm-negative vs hookworm-positive participants. Each individual data point is shown along with mean ± 95% confidence interval. Grayed area indicates the normal ranges. (c) Mean CeD symptom index (CSI) in each group, averaged for each 3-week period. (d) Mean CeD quality of life (QoL) score ±95% CI determined at each clinic visit. For both CSI and QoL, a lower value indicates lessened CeD-related symptoms. CeD, celiac disease.
DISCUSSION
We postulated that treating CeD participants with hookworm N. americanus larvae would be well tolerated and that the immunomodulation induced by the worms might have a collateral effect of improving gluten intolerance in CeD, compared with placebo treatment. Although the results from this study mirror those previously reported by us (19), the ambitious primary outcome to establish tolerance to sustained gluten consumption (2 g daily for 6 weeks) was not achieved; thus, it is evident that hookworm infection does not obviate the need for a gluten-free diet.
Our hypothesis was that gradual reintroduction of gluten alone (in placebo-treated participants) would provide limited protection against sustained gluten challenge, which might be improved by hookworm treatment. Indeed, escalating gluten challenge did result in gradual deterioration of the duodenal mucosa, but this was irrespective of the treatment group. Although it was somewhat surprising that over half of the placebo-treated participants successfully completed week 42 with tTG and duodenal histology results within normal ranges, this was based on a very small sample size. Gradual gluten desensitization strategies through oral exposure or parenteral vaccination have already been trialed for CeD with limited efficacy (Nexvax2, NCT03644069) (23); hence, it remains unclear whether maintaining low-level exposure alone is beneficial for promoting gluten tolerance. It is possible that targeting mucosal antigen-presenting cells might lead to more positive outcomes, particularly for vaccination strategies. In this study, we used hookworms as an alternative strategy to target the mucosal immune system and reported that hookworm-infected participants did seem more resilient than hookworm-negative participants about gluten-related adverse events and QoL indices, particularly during the gluten provocation phase (up to week 36). This positive result is consistent with observations in both our previous trials and might relate to a sense of improved general well-being after hookworms have established in the gut (13,19). This hypothesis is supported by the decision of virtually every participant to retain the infection when exiting this and previous trials.
This study incorporated multiple distinct challenge phases designed to replicate clinical scenarios. First, a dose-ranging run in was undertaken to test the safety and tolerability of inoculation with 40 L3 compared with 20 L3. Consistent with previous findings (19), hookworm infection was mostly well-tolerated, other than two L3-20 participants who withdrew due to transient symptomatic enteritis, a characteristic of primary but not chronic hookworm disease (21,22). Both promptly responded to anthelmintic therapy. Unexpectedly, establishment of hookworm infection failed in approximately one-quarter of participants. In our previous studies (13,19) and those of others (10,24), a single study site was mostly involved, and uniform batching of L3 inocula was undertaken. This study involved 4 clinical sites in 2 countries and 14 different batches of L3. Although the production is scrupulously supervised to maintain consistency, the process lacks Good Manufacturing Practice certification. Most failed inoculations occurred at the overseas site most distant from the production center, suggesting that extended transit time and handling might have compromised larval viability. Understanding of the optimal production methodology, storage, and transport conditions to maintain the integrity of infective hookworm larvae will be necessary in future studies.
Prompted by the hygiene hypothesis, studies in murine models of inflammatory bowel disease supported a role for live helminths and helminth-secreted products to mitigate disease activity (25). The initial clinical trials in patients with active inflammatory bowel disease infected with porcine whipworm ova were reported positively (26,27), as were a small study in patients with Crohn's disease infected with N. americanus (28) and a case study with Trichuris trichiura (29). Subsequent trials covering a spectrum of allergic and inflammatory diseases, including a recent trial in multiple sclerosis (30), have universally failed to show a statistically significant benefit (31). Although these negative results have resulted in the loss of professional support for “live helminth” therapy, it still garners enthusiasm as an alternative medical therapy (32). The lack of efficacy seen in some studies might relate to the application of helminths during active inflammatory disease, where helminths might be more suited as a preventative therapy given before disease induction.
This study included design and conduct limitations, including the small sample size (particularly in the placebo cohort) and the inconsistencies of outcome scores to properly reflect disease activity. Through necessity, the cohorts were small, consistent with the trial's phase 1b status. The small placebo cohort offered limited chance for delivering a statistically significant difference between the intention-to-treat cohorts. Relating to outcome scoring, no single outcome predictably quantified CeD activity. We monitored CeD-specific symptoms in response to gluten consumption (CSI), tTG, and quantitative and qualitative histologic parameters. Of concern, there existed poor uniformity between these parameters. Most concerning was that several participants who remained asymptomatic and had normal tTG had acquired Marsh 3 active CeD status. Longitudinal anti-tTG values were remarkably consistent and frequently failed to correlate with histologic evidence of disease reactivation. Quantitative histological scoring also proved an inconsistent predictor of disease activity. Several participants incongruously returned an improved Vh:Cd after gluten challenge, and in 1 case after inoculation with L3-40, the week 12 Vh:Cd score and Marsh grade had deteriorated dramatically in the absence of known gluten exposure.
In conclusion, neither trace gluten nor hookworm treatment universally protects against relaxation of a gluten-free diet. Daily consumption of 50 mg of gluten induced limited meaningful adverse events in most participants (>90% study progression beyond week 24); however, sustained gluten challenges of 2 g/d resulted in deterioration of the duodenal mucosa by week 42, irrespective of hookworm treatment. The improvement in symptom scores and wellbeing after hookworm infection during lower doses of gluten challenge seems real and might have clinical relevance. To understand these observations, further clinical trials and detailed mechanistic evaluations of the biological responses that are associated with hookworm infection and gluten ingestion are warranted.
CONFLICTS OF INTEREST
Guarantor of the article: Paul R. Giacomin, PhD.
Specific author contributions: J.C., A.L., and P.R.G.: conceptualization. J.C., G.C.M., L.M., S.L., P.O., and P.R.G.: data analysis. J.C., L.M., A.L., G.R.-S., R.B.G., T.R., and P.R.G.: funding. J.C., G.C.M., R.G., M.N., C.W., J.W.M., R.B.G., T.R., and P.R.G.: investigation. J.C., G.C.M., R.G., S.L., L.B., J.S., P.M.V., J.S.M., A.D.C., J.S.M., A.L., and P.R.G.: methodology and administration. M.L.R., A.L., J.S.M., A.D.C., and G.R.-S.: supervision. J.C., L.M., S.L., and P.R.G.: writing—original draft. All authors: writing—review and editing. All authors approved the final manuscript.
Financial support: The work was funded by an Australian National Health and Medical Research Council (NHMRC) Project Grant (J.C., P.R.G., G.R.-S, L.M., and T.R.), Advance Queensland Fellowship (P.R.G.), The Prince Charles Hospital Foundation (J.C., T.R., and P.R.G.), Celiac Australia (J.C., P.R.G., T.R., A.L., and G.R.-S.), Bowel and Liver Trust, NZ Lottery Health Research Fund (R.G.), Australian Institute of Tropical Health and Medicine (P.R.G.), NHMRC Senior Principal Research Fellowship (A.L.), NHMRC Practitioner Fellowship (J.S.M.), NHMRC Program Grant (P.M.V.), and Australian Research Council (P.M.V.). Funders had no role in the design or conduct of the study.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Hookworms suppress inflammation within the gut, the same tissue that develops immunopathology in celiac disease.
✓ A small, open-label trial showed hookworm infection enabled gradual reintroduction of gluten to patients with celiac disease.
WHAT IS NEW HERE
✓ This larger, placebo-controlled trial shows that daily low-dose gluten (<50 mg/d) induced limited adverse events, irrespective of hookworm treatment.
✓ Hookworm infection did not improve tolerance to moderate gluten challenge (2 g/d).
✓ Hookworm-treated participants had fewer adverse symptoms and improved quality of life scores during lower gluten challenges.
TRANSLATIONAL IMPACT
✓ Hookworm treatment does not cure celiac disease, but the observed improvements in symptoms may have clinical relevance for the wellbeing of people with celiac disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christian Engwerda, Marcela Montes De Oca, and Sandip Kamath for technical assistance; Ann Vandeleur, Di Jones, Leisa McCann, Leanne Rigby, Susan Netterfield, Katrina Chakradeo, Julie Randall, Rhondda Brown, Tracy Noonan, Katherine Denton, Debbi Rumble, Stephanie Pollard, Helen Legg, Ruth Lucas, Adrian Simmonds, Mark Appleyard and Tina Langford for clinical and trial operations assistance; and Marie-Claire Keogh and Michelle Woodhouse at Sullivan Nicolaides Pathology for assistance with pathology services. We thank all study participants involved in the trial. We thank the Program in Complex Trait Genomics at the Institute for Molecular Bioscience (University of Queensland) for SNP genotyping and analysis.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A450; http://links.lww.com/CTG/A451
REFERENCES
- 1.Qiao SW, Iversen R, Raki M, et al. The adaptive immune response in celiac disease. Semin Immunopathol 2012;34:523–40. [DOI] [PubMed] [Google Scholar]
- 2.Abadie V, Discepolo V, Jabri B. Intraepithelial lymphocytes in celiac disease immunopathology. Semin Immunopathol 2012;34:551–66. [DOI] [PubMed] [Google Scholar]
- 3.Di Sabatino A, Vanoli A, Giuffrida P, et al. The function of tissue transglutaminase in celiac disease. Autoimmun Rev 2012;11:746–53. [DOI] [PubMed] [Google Scholar]
- 4.Spatola BN, Kaukinen K, Collin P, et al. Persistence of elevated deamidated gliadin peptide antibodies on a gluten-free diet indicates nonresponsive coeliac disease. Aliment Pharmacol Ther 2014;39:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludvigsson JF, Rubio-Tapia A, van Dyke CT, et al. Increasing incidence of celiac disease in a North American population. Am J Gastroenterol 2013;108:818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 2009;137:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvester JA, Graff LA, Rigaux L, et al. Symptomatic suspected gluten exposure is common among patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther 2016;44:612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daveson AJM, Tye-Din JA, Goel G, et al. Masked bolus gluten challenge low in FODMAPs implicates nausea and vomiting as key symptoms associated with immune activation in treated coeliac disease. Aliment Pharmacol Ther 2020;51:244–52. [DOI] [PubMed] [Google Scholar]
- 9.Zarowiecki M, Berriman M. What helminth genomes have taught us about parasite evolution. Parasitology 2015;142(Suppl):S85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortimer K, Brown A, Feary J, et al. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. Am J Trop Med Hyg 2006;75:914–20. [PubMed] [Google Scholar]
- 11.Finlay CM, Walsh KP, Mills KHG. Induction of regulatory cells by helminth parasites: Exploitation for the treatment of inflammatory diseases. Immunological Rev 2014;259:206–30. [DOI] [PubMed] [Google Scholar]
- 12.Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 2016;138:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daveson AJ, Jones DM, Gaze S, et al. Effect of hookworm infection on wheat challenge in celiac disease: A randomised double-blinded placebo controlled trial. PLoS One 2011;6:e17366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croese J, Gaze ST, Loukas A. Changed gluten immunity in celiac disease by Necator americanus provides new insights into autoimmunity. Int J Parasitol 2013;43:275–82. [DOI] [PubMed] [Google Scholar]
- 15.Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog 2012;8:e1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McSorley HJ, Gaze S, Daveson J, et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS One 2011;6:e24092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson RP, Jabri B. Vaccine against autoimmune disease: Antigen-specific immunotherapy. Curr Opin Immunol 2013;25:410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang MLK, Lozinsky AC, Loke P. Peanut oral immunotherapy: State of the art. Immunol Allergy Clin North Am 2020;40:97–110. [DOI] [PubMed] [Google Scholar]
- 19.Croese J, Giacomin P, Navarro S, et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol 2015;135:508–16 e5. [DOI] [PubMed] [Google Scholar]
- 20.Llewellyn S, Inpankaew T, Nery SV, et al. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Negl Trop Dis 2016;10:e0004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diemert D, Campbell D, Brelsford J, et al. Controlled human hookworm infection: Accelerating human hookworm vaccine development. Open Forum Infect Dis 2018;5:ofy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell C, Hussain R, Nutman TB, et al. The clinical and immunologic responses of normal human volunteers to low dose hookworm (Necator americanus) infection. Am J Trop Med Hyg 1987;37:126–34. [DOI] [PubMed] [Google Scholar]
- 23.Crespo-Escobar P, Mearin ML, Hervás D, et al. The role of gluten consumption at an early age in celiac disease development: A further analysis of the prospective PreventCD cohort study. Am J Clin Nutr 2017;105:890–6. [DOI] [PubMed] [Google Scholar]
- 24.Feary JR, Venn AJ, Mortimer K, et al. Experimental hookworm infection: A randomized placebo-controlled trial in asthma. Clin Exp Allergy 2010;40:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinstock JV, Elliott DE. Translatability of helminth therapy in inflammatory bowel diseases. Int J Parasitol 2013;43:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Summers RW, Elliott DE, Qadir K, et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol 2003;98:2034–41. [DOI] [PubMed] [Google Scholar]
- 27.Summers RW, Elliott DE, Urban JF, Jr, et al. Trichuris suis therapy for active ulcerative colitis: A randomized controlled trial. Gastroenterology 2005;128:825–32. [DOI] [PubMed] [Google Scholar]
- 28.Croese J, O'Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors. Gut 2006;55:136–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broadhurst MJ, Leung JM, Kashyap V, et al. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med 2010;2:60ra88. [DOI] [PubMed] [Google Scholar]
- 30.Tanasescu R, Tench CR, Constantinescu CS, et al. Hookworm treatment for relapsing multiple sclerosis: A randomized double-blinded placebo-controlled trial. JAMA Neurol 2020;77:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott DE, Weinstock JV. Nematodes and human therapeutic trials for inflammatory disease. Parasite Immunol 2017;39:10.1111/pim.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Morey RA, Wilson JK, et al. Practices and outcomes of self-treatment with helminths based on physicians' observations. J Helminthol 2017;91:267–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.