Supplemental Digital Content is available in the text.
Keywords: cardiac, critical care, intensive care unit, mechanical ventilation, noninvasive ventilation
Objectives:
The medical complexity and critical care needs of patients admitted to cardiac ICUs are increasing, and prospective studies examining the underlying cardiac and noncardiac diagnoses, the management strategies, and the prognosis of cardiac ICU patients with respiratory failure are needed.
Design:
Prospective cohort study.
Setting:
The Critical Care Cardiology Trials Network is a research collaborative of cardiac ICUs across the United States and Canada.
Patients:
We included all medical cardiac ICU admissions at 25 cardiac ICUs during two consecutive months annually at each center from 2017 to 2019.
Measurements:
We evaluated the use of advanced respiratory therapies including invasive mechanical ventilation, noninvasive ventilation, and high-flow nasal cannula versus no advanced respiratory support across admission diagnoses and the association with in-hospital mortality.
Main Results:
Of 8,240 cardiac ICU admissions, 1,935 (23.5%) were treated with invasive mechanical ventilation, 573 (7.0%) with noninvasive ventilation, and 281 (3.4%) with high-flow nasal cannula. Admitting diagnoses among those with advanced respiratory support were diverse including general medical problems in patients with heart disease as well as primary cardiac problems. In-hospital mortality was higher in patients who received invasive mechanical ventilation (38.1%; adjusted odds ratio, 2.53; 2.02–3.16) and noninvasive ventilation or high-flow nasal cannula (8.8%; adjusted odds ratio, 2.25; 1.73–2.93) compared with patients without advanced respiratory support (4.6%). Reintubation rate was 7.6%. The most common variables associated with respiratory insufficiency included heart failure, infection, chronic obstructive pulmonary disease, and pulmonary vascular disease.
Conclusions:
One-third of cardiac ICU admissions receive respiratory support with associated increased mortality. These data provide benchmarks for quality improvement ventures in the cardiac ICU, inform cardiac critical care training and staffing patterns, and serve as foundation for future studies aimed at improving outcomes.
The contemporary cardiac ICU (CICU) has evolved from providing care for patients mainly with complications of acute myocardial infarction to delivering complex and multidisciplinary intensive care for cardiac patients with multiple comorbidities (1, 2). Acute respiratory failure necessitating invasive or noninvasive ventilatory support in patients with acute cardiac illness is increasing and associated with an elevated risk of mortality (3–7). Major knowledge gaps remain in addressing this important and high-acuity patient population (8, 9).
The primary contributors to respiratory failure in CICU patients are not well defined, including the overlap of primary cardiac and primary pulmonary pathologies. There are few data addressing the use and outcomes of newer modes of respiratory support in CICU patients such as high-flow nasal cannula (HFNC) devices that provide gas flow from 30 to 60 L/min (10). Other knowledge gaps include the role of noninvasive respiratory support prior to intubation and after extubation in CICU patients, benchmark reintubation rates in medical cardiac patients, and the optimal use of hemodynamic monitoring strategies. Finally, available outcome data on respiratory failure in the CICU have limitations including retrospective design (5), single-center populations (6), and reliance on claims data, and focus on single cardiac disease states (3, 4, 7). An accounting of the diversity of cardiac pathologies treated with respiratory support is needed. Multicenter prospective studies of the epidemiology and outcomes of invasive ventilation and noninvasive ventilation (NIV) in the CICU are needed to assess the management, monitoring strategies, and prognosis for cardiac patients with respiratory failure. Addressing these knowledge gaps would inform cardiac critical care staffing and training, provide benchmarks for CICU quality, and inform prospective intervention trials.
To address these knowledge gaps, we performed a prospective cohort study of CICU patients across 25 centers using data from the Critical Care Cardiology Trials Network (CCCTN). We report the use of noninvasive positive pressure ventilation, invasive mechanical ventilation (IMV), and HFNC, including the interhospital practice variation, clinical characteristics, and outcomes associated with respiratory support. We hypothesized that CICU patients treated with respiratory support would be at high risk of adverse outcomes and that specific treatments rendered and contributors to respiratory failure would be heterogeneous.
MATERIALS AND METHODS
Study Population
The CCCTN is a prospective research collaborative of American Heart Association level 1 (1) CICUs across the United States and Canada with the goal of advancing knowledge in the field of cardiac critical care. Details of the network and data collection methods have been previously published (11). The 25 centers were mostly academic with “closed” ICU staffing models. All had mechanical circulatory support capacity. The network is overseen by the Executive and Steering Committees and coordinated by the Thrombolysis in Myocardial Infarction Study Group (Boston, MA). Each center contributes clinical data for all medical CICU admissions annually over 2 consecutive months. Admissions originating as “overflow” admissions from other medical ICU and postcardiac surgical admissions were excluded. The study population in this report represents a prospective cohort enrolled across 2 years from 2017 to 2019. Centers could elect to contribute data for additional consecutive admissions outside of the 2-month window. The Institutional Review Board at the Coordinating Center as well as each participating site approved the CCCTN protocol and waiver of informed consent.
Data Collection, Exposures, and Outcomes
Data collected included demographics, admitting diagnosis, indications for ICU level of care, ICU therapies provided during the CICU stay, laboratory data and Sequential Organ Failure Assessment (SOFA) score (12, 13), hemodynamic variables, length of stay, and inhospital outcome including death or discharge to home or skilled nursing facility. CICU use of advanced respiratory therapies was classified in a hierarchical manner (IMV > NIV > HFNC) versus no advanced respiratory support. During the second year of data acquisition, details on the use of noninvasive respiratory support prior to intubation and upon extubation were also reported, along with the duration of mechanical ventilation and requirement for reintubation. All staff entering data received standardized training on variable definitions and data capture using the dedicated case report form (Research Electronic Data Capture [14]), and data were reviewed and queried by the coordinating center to ensure internal validity.
Statistical Analysis
The exposure variable of interest was use of advanced respiratory therapy, categorized hierarchically as IMV, NIV, HFNC, or no support. Demographic and baseline clinical data are reported as n (%) for categorical data and medians (interquartile range) for continuous variables across the exposure. We performed logistic regression for the primary outcome of in-hospital mortality adjusting for factors of a priori clinical interest including age, sex, race, body mass index (BMI), cardiac arrest, SOFA score, history of pulmonary disease, and shock. Survival curves were generated using the Kaplan-Meier method. To assess findings in more homogenous study subgroups, we performed predefined subgroup analyses of patients with primary admitting diagnoses of acute heart failure (AHF) and those with acute coronary syndrome (ACS). Results were considered statistically significant at a two-sided p value of less than 0.05. Analyses were performed using SAS System V9.4 (SAS Institute, Cary, NC).
RESULTS
Use of Respiratory Support in the CICU
A total of 8,240 CICU admissions (4,352 from campaign 1 and 3,888 from campaign 2) were included from 25 individual CICUs. Any advanced respiratory support was used in 2,789 patients (32.7%), including 1,935 (23.5%) who received IMV, 573 (7.0%) NIV, and 281 (3.4%) HFNC. Demographics and baseline characteristics for all CICU patients across each category of respiratory support are shown in Table 1. CICU patients treated with NIV had greater comorbidity burden including chronic lung disease and chronic heart failure than patients receiving no support and those receiving IMV. Baseline characteristics of the ACS and AHF subgroups are displayed in Supplemental Tables S1 and S2 (http://links.lww.com/DCR/B375) and http://links.lww.com/DCR/B375. ACS patients treated with noninvasive respiratory support were older and had more chronic comorbidities and more AHF than those treated with IMV and those treated with no support. AHF patients treated with noninvasive support were similarly older with more chronic lung disease than those treated with IMV and no support.
Table 1.
Demographics and Baseline Characteristics for Patients Across Each Category of Respiratory Support
| Hierarchal Categorization: IMV-NIV-HFNC, Median (IQR) and n (%), n = 8,240 | IMV, n = 1,935 | NIV, n = 573 | HFNC, n = 281 | No Respiratory Support, n = 5,451 |
|---|---|---|---|---|
| Age (yr) | 65.0 (55.0–74.0) | 71.0 (61.0–79.0) | 68.0 (57.0–78.0) | 66.0 (55.0–75.0) |
| Weight (kg) | 83.0 (68.9–99.5) | 82.4 (67.0–101.4) | 76.3 (61.4–93.9) | 81.7 (68.7–97.0) |
| Body mass index (kg/m2) | 28.4 (24.2–33.8) | 28.7 (24.3–35.2) | 26.9 (22.8–33.2) | 28.0 (24.2–32.5) |
| Female | 722 (37.3) | 257 (44.9) | 139 (49.5) | 2,003 (36.7) |
| Caucasian | 1,258 (73.3) | 377 (71.4) | 192 (77.4) | 3,299 (71.3) |
| Smoking status | ||||
| Current | 347 (18.2) | 70 (12.3) | 39 (14.1) | 923 (17.1) |
| Former | 630 (33.0) | 267 (47.1) | 109 (39.4) | 1,981 (36.8) |
| Never | 680 (35.6) | 206 (36.3) | 105 (37.9) | 2,087 (38.8) |
| Unknown | 252 (13.2) | 24 (4.2) | 24 (8.7) | 391 (7.3) |
| Hypertension | 1,257 (65.0) | 442 (77.1) | 174 (61.9) | 3,581 (65.7) |
| Diabetes mellitus | 749 (38.7) | 254 (44.3) | 90 (32.0) | 1,794 (32.9) |
| Chronic kidney disease | 546 (28.2) | 233 (40.7) | 79 (28.1) | 1,346 (24.7) |
| Dialysis-dependent | 139 (25.5) | 36 (15.5) | 14 (17.7) | 299 (22.3) |
| Significant pulmonary disease | 412 (21.3) | 194 (33.9) | 88 (31.3) | 732 (13.4) |
| Significant liver disease | 81 (4.2) | 12 (2.1) | 7 (2.5) | 190 (3.5) |
| Significant dementia | 31 (1.6) | 16 (2.8) | 9 (3.2) | 96 (1.8) |
| Active cancer | 129 (6.7) | 49 (8.6) | 31 (11.0) | 384 (7.0) |
| Coronary artery disease | 755 (39.0) | 269 (46.9) | 118 (42.0) | 2,116 (38.8) |
| Cerebrovascular disease | 212 (11.0) | 82 (14.3) | 28 (10.0) | 542 (9.9) |
| Peripheral artery disease | 201 (10.4) | 75 (13.1) | 39 (13.9) | 519 (9.5) |
| Heart failure | 826 (42.7) | 359 (62.7) | 139 (49.5) | 1,969 (36.1) |
| Atrial fibrillation | 483 (25.0) | 223 (38.9) | 76 (27.0) | 1,403 (25.7) |
| Ventricular arrhythmia | 158 (8.2) | 26 (4.5) | 11 (3.9) | 386 (7.1) |
| Severe valvular disease | 305 (15.8) | 111 (19.4) | 54 (19.2) | 918 (16.8) |
| Pulmonary hypertension | 143 (7.4) | 70 (12.2) | 54 (19.2) | 254 (4.7) |
HFNC = high-flow nasal cannula, IMV = invasive mechanical ventilation, IQR = interquartile range, NIV = noninvasive ventilation.
The percentage of patients treated with each modality of respiratory support varied by CICU admission diagnosis (Fig. 1), and the primary cardiac diagnoses of respiratory support patients were diverse. Of the 332 patients with cardiac arrest, 86.2% received IMV, 0.9% NIV, and 1.2% HFNC. Otherwise, the disease requiring the greatest fraction of IMV use was cardiogenic shock (IMV use in 41% of cases). The diseases most commonly requiring NIV included AHF (19% of cases) and hypertensive urgency/emergency (17%). HFNC was most commonly used among patients with pulmonary embolism (15%) (Fig. 1).
Figure 1.
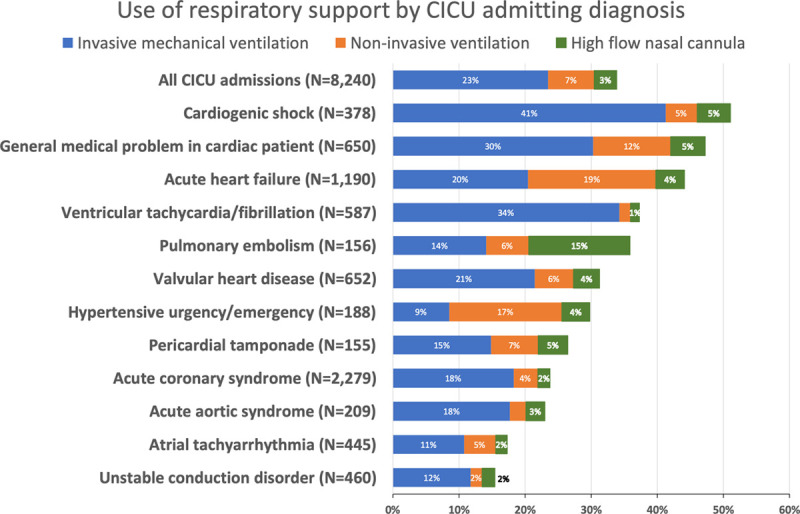
Use of respiratory support in the cardiac ICU (CICU) and across admitting diagnosis: The percentage of patients treated with each modality of respiratory support varied by CICU admission diagnosis and the primary cardiac diagnoses of respiratory support patients were diverse.
Center-Specific Variation in Use of Respiratory Support
There was significant interhospital variation (15–56%) in the proportion of patients managed with respiratory support, as shown in Supplemental Figure 1 (http://links.lww.com/DCR/B375). This heterogeneity was associated, in part, with higher disease severity: for centers using respiratory support in less than 30% of admissions, the median SOFA score was 3.0 (1.0–5.0) versus 4.0 (2.0–7.0) for those using respiratory support in 30–40% of admissions versus 5.0 (3.0–9.0) for those using respiratory support in greater than 40% of admissions (p < 0.0001 for difference across center category).
ICU Management of CICU Patients Managed With Advanced Respiratory Support
Over half of CICU patients treated with IMV received some form of invasive hemodynamic monitoring with either a central venous line, pulmonary artery catheter, and/or arterial line, compared with less than 25% for each among those treated with NIV and HFNC (Supplemental Fig. 2, http://links.lww.com/DCR/B375). Table 2 displays the ICU therapies delivered, worst laboratory values, and overall outcomes of CICU patients requiring respiratory support, compared with no support. SOFA score, concomitant ICU therapies, length of stay, and mortality were all higher in CICU patients requiring respiratory support. Findings were similar in the subgroups of patients with ACS and with AHF (Supplemental Tables 3 and 4, http://links.lww.com/DCR/B375). In the ACS subgroup, 50.2% of patients treated with IMV also received some form of mechanical circulatory support. In the AHF subgroup, 19.8% of those treated with IMV also received renal replacement therapy and 35.8% received mechanical circulatory support.
Table 2.
Cardiac ICU Therapies Delivered, Worst Laboratory Values, and Overall Outcomes of Cardiac ICU Patients Requiring Respiratory Support, Compared With No Support
| Hierarchal Categorization: IMV-NIV-HFNC, Median (IQR) and n (%), n = 8,240 | IMV, n = 1,935 | NIV, n = 573 | HFNC, n = 281 | No Respiratory Support, n = 5,451 |
|---|---|---|---|---|
| Sequential Organ Failure Assessment score | 9.0 (6.0–12.0) | 5.0 (3.0–7.0) | 5.0 (4.0–8.0) | 3.0 (1.0–5.0) |
| Days of CICU care | 4.6 (2.1–9.4) | 2.6 (1.5–4.8) | 2.3 (1.2–5.0) | 1.9 (1.0–3.5) |
| Days of hospital stay | 9.2 (3.9–18.3) | 7.2 (4.2–12.0) | 8.9 (4.8–15.8) | 4.9 (2.6–9.8) |
| CICU LOS among hospital survivors | 5.3 (2.8–10.4) | 2.5 (1.5–4.6) | 2.3 (1.3–5.0) | 1.9 (1.0–3.3) |
| Hospital LOS among hospital survivors | 13.0 (7.0–22.2) | 7.6 (4.6–12.0) | 9.2 (5.6–16.2) | 4.8 (2.6–9.6) |
| Renal replacement therapy | 351 (18.1) | 29 (5.1) | 24 (8.5) | 179 (3.3) |
| Invasive hemodynamic monitoring | 1,443 (74.6) | 160 (27.9) | 94 (33.5) | 1,280 (23.5) |
| Central venous line | 1,023 (52.9) | 81 (14.1) | 55 (19.6) | 630 (11.6) |
| Pulmonary artery catheter | 602 (31.1) | 58 (10.1) | 31 (11.0) | 502 (9.2) |
| Arterial line | 1,259 (65.1) | 122 (21.3) | 71 (25.3) | 857 (15.7) |
| Number of inotropes/vasopressor | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Mechanical circulatory support | 523 (27.0) | 27 (4.7) | 17 (6.0) | 364 (6.7) |
| Mortality in CICU | 639 (33.0) | 48 (8.4) | 27 (9.6) | 117 (2.1) |
| Mortality in hospital | 738 (38.1) | 79 (13.8) | 50 (17.8) | 248 (4.5) |
| Lactate (mmol/L) | 3.5 (1.8–7.6) | 1.8 (1.2–2.9) | 2.1 (1.3–3.3) | 1.7 (1.2–2.7) |
| Lactate > 2 mmol/L | 1,203 (70.6) | 179 (42.6) | 100 (50.5) | 1,047 (38.3) |
| Arterial pH | 7.30 (7.19–7.36) | 7.37 (7.28–7.42) | 7.39 (7.34–7.45) | 7.39 (7.33–7.43) |
| Arterial pH < 7.25 | 611 (37.0) | 45 (13.8) | 9 (6.5) | 74 (6.5) |
| Creatinine (mg/dL) | 1.8 (1.2–3.1) | 1.6 (1.2–2.7) | 1.4 (1.1–2.3) | 1.2 (0.9–1.8) |
| eGFR (mL/min/1.73 m2) | 37.7 (19.7–60.9) | 40.9 (23.7–60.6) | 46.7 (26.2–71.1) | 61.4 (36.5–82.5) |
| eGFR < 60 | 1,432 (74.1) | 425 (74.3) | 193 (68.9) | 2,632 (48.4) |
CICU = cardiac ICU, eGFR = estimated glomerular filtration rate, HFNC = high-flow nasal cannula, IMV = invasive mechanical ventilation, IQR = interquartile range, LOS = length of stay, NIV = noninvasive ventilation
For the subgroup of data collected in the second campaign, more details as to the indications for respiratory therapy were obtained for 1,355 patients, including 903 treated with IMV, 286 with NIV, 136 with HFNC, and 30 patients with respiratory insufficiency but who received no advanced support. Of 903 patients treated with IMV, 54% had respiratory insufficiency, 25% had cardiac arrest, 7% were intubated solely for airway protection, and 14% for procedural sedation or general anesthesia. Among 286 patients treated with NIV, the majority were managed with bilevel ventilation (n = 239, 83.6%). Heart failure, infection, chronic obstructive pulmonary disease (COPD), and pulmonary vascular disease were the most common contributors to respiratory insufficiency as adjudicated by the individual site at the point of data entry (Fig. 2). Of these, AHF was the most common. Acute respiratory distress syndrome was rarely present as adjudicated by the individual site.
Figure 2.
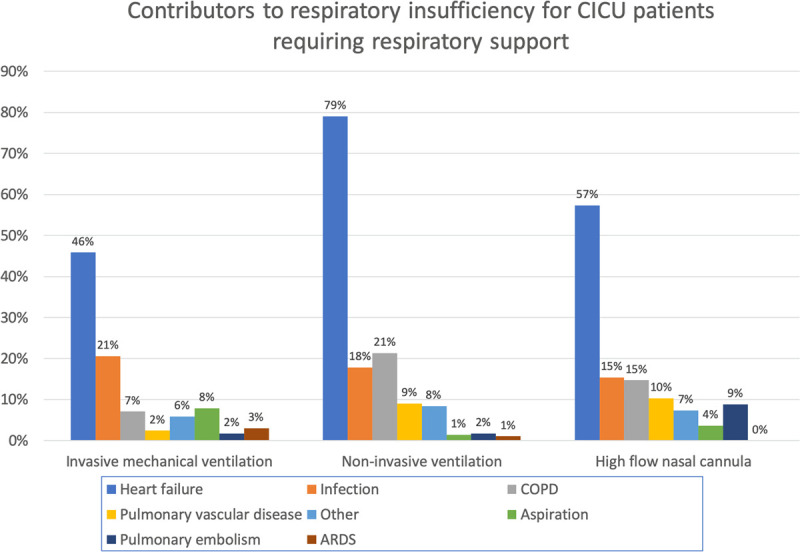
Contributors to respiratory insufficiency for cardiac ICU (CICU) patients requiring respiratory support. ARDS = acute respiratory distress syndrome, COPD = chronic obstructive pulmonary disease.
Outcomes of CICU Patients Managed With Advanced Respiratory Support
Among the 903 admissions in the second campaign treated with IMV, 117 (13.0%) had a trial of antecedent NIV and 50 (5.5%) had a trial of antecedent HFNC before intubation. Of these, 685 patients (77.5%) were extubated including 26.4% extubated to some form of noninvasive support (13.0% extubated to NIV and 13.4% extubated to HFNC). Overall, 69 patients (7.6%) were reintubated at a median of 2.0 days (0.7–3.6 d).
Unadjusted in-hospital mortality for patients treated with IMV, NIV, HFNC, and no support was 38.1%, 13.8%, 17.8%, and 4.6%, respectively (Fig. 3). Unadjusted inhospital mortality for ACS patients treated with IMV, NIV, HFNC, and no support was 35.1%, 13.3%, 11.6%, and 2.6%. Unadjusted inhospital mortality for AHF patients treated with IMV, NIV, HFNC, and no support was 31.7%, 10.9%, 17.3%, and 8.3% (Supplemental Tables 3 and 4, http://links.lww.com/DCR/B375). Excluding patients with cardiac arrest and shock, inhospital mortality was 10.0% for IMV, 7.6% for NIV, and 8.2% for HFNC compared with 2.0% for no respiratory support (Supplemental Fig. 3, http://links.lww.com/DCR/B375).. Unadjusted mortality after adjustment for age, sex, race, BMI, cardiac arrest, SOFA score, history of pulmonary disease, and shock, and use of IMV (odds ratio [OR], 2.53; 95% CI, 2.02–3.16; p < 0.0001) and NIV/HFNC (OR, 2.25; 95% CI, 1.73–2.93) were associated with higher mortality than patients with no advanced respiratory support. Findings were similar in the subgroups of patients with ACS and AHF (Supplemental Figs. 4 and 5, http://links.lww.com/DCR/B375).
Figure 3.
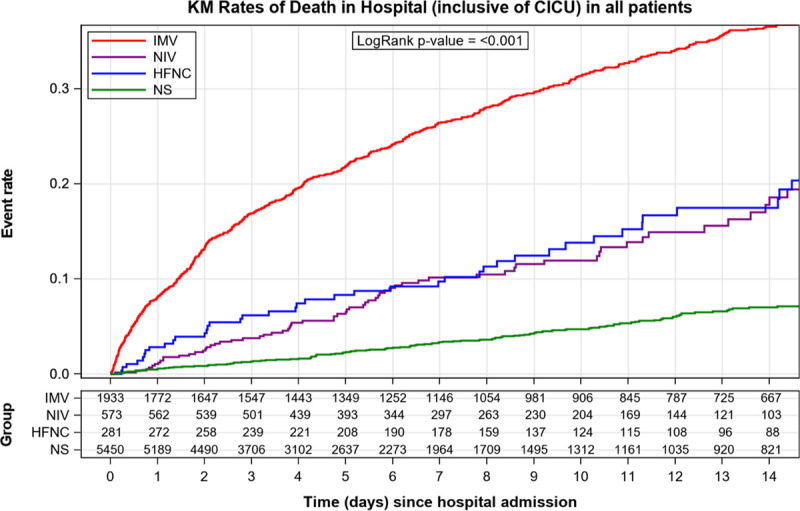
Kaplan-Meier (KM) plot of mortality in cardiac (CICU) patients stratified by respiratory support. HFNC = high-flow nasal cannula, IMV = invasive mechanical ventilation, NIV = noninvasive ventilation, NS = no support.
In patients with shock, the association remained significant for IMV (OR, 2.10; 95% CI, 1.61–2.73) and NIV/HFNC (OR, 1.93; 95% CI, 1.34–2.77). Among patients without shock, IMV (OR, 3.37; 95% CI, 2.26–5.04) and NIV/HFNC (OR, 2.57; 95% CI, 1.73–3.83) also remained significant (Supplemental Fig. 3, http://links.lww.com/DCR/B375).
DISCUSSION
In this prospective, multicenter cohort study profiling respiratory failure and use of respiratory support in the contemporary CICU, we report several important findings. First, the use of IMV, NIV, and HFNC in acute cardiac illness in contemporary CICUs is common across a diverse array of cardiovascular pathologies, highlighting the need for CICU practitioners to understand advanced respiratory therapies and heart-lung interactions in depth. Second, contributors to respiratory insufficiency included both cardiovascular and noncardiovascular causes, implying that active and evidence-based management of noncardiac organ failure is needed in the modern CICU. We provide a preliminary benchmark for rates of contemporary use of preintubation and postextubation noninvasive supports that are avenues in need of further study. Finally, our results demonstrate that CICU patients requiring respiratory support are at high risk of adverse outcomes such as reintubation and death. The very high mortality risk in admissions with IMV provides clinicians with meaningful prognostic perspective and should motivate studies to identify drivers of outcome and potential avenues to mitigate this risk in this critically ill patient population.
Use of Respiratory Support in the CICU
Our results reinforce that respiratory failure and use of respiratory support are common in the contemporary CICU. Our data are consistent with prior studies demonstrating increasing use of respiratory support in the CICU (5), in cardiac disease such as ST segment elevation myocardial infarction (4), AHF (3, 15), cardiogenic shock (7), and in patients with left ventricular assist devices (16). Our results build on the evidence base by characterizing the full spectrum of cardiac diseases that receive respiratory support. These patients include those requiring intubation solely for airway protection due to depressed mental status, those with tamponade or right ventricular failure, acute ischemia, or infection, and those with significant gas exchange deficits and poor left ventricular function. Each of these phenotypes would respond differently to the hemodynamic challenge of positive pressure ventilation (17). We also describe the equally important contributions of cardiac, pulmonary, and other noncardiac causes to respiratory insufficiency. AHF is the most common contributor, yet infection, COPD, and pulmonary vascular disease also contribute in many cases. These findings emphasize that optimal management of both primary cardiac and pulmonary conditions will be required to improve these patients’ outcomes, consistent with the evolving understanding of the fact that heart disease and pulmonary disease are synergistic (18).
Thus, our results have implications for all critical care providers delivering services to CICU patients in tertiary centers, noting that management of cardiac disease is a self-reported deficiency among some critical care trainees (19). As the provision of critical care services in the CICU often includes critical care specialists without focused cardiology training (1, 2), our data reinforce that competence in all forms of respiratory support is required in contemporary CICUs. Our data also could inform educational efforts within the specialty of critical care cardiology, a subspecialty that is receiving increased attention (20–23). Facility with invasive and noninvasive respiratory supports is now a core competency in cardiovascular training (20). Therefore, our finding that one in three CICU patients receives some form of respiratory support adds support to maintain and expand this focus on respiratory therapy in cardiovascular education and practice. Additionally, noncardiology intensivist groups providing critical care consultation in the CICU can use these data to reinforce need for multidisciplinary management. Finally, the common and heterogeneous nature of respiratory failure in the CICU should prompt studies on optimal interventions for respiratory support in each specific phenotype such as recent examples of trials to prevent ventilator-associated pneumonia after cardiac arrest (24), the optimal oxygenation target (25), and the optimal positive end-expiratory pressure for patients with RV failure (26).
Outcomes of Respiratory Support
We report that CICU patients with respiratory failure have a high rate of adverse outcomes. Although the high mortality rate is intuitive, the magnitude of association with mortality has meaningful implications for prognostication—mortality rates we report are commensurate with and exceed those of general medical critical care population (27). The overall mortality rates in patients receiving respiratory support are largely consistent with national data (3, 4, 28). High mortality persists in the nonshock, noncardiac arrest stratum and after adjusting for SOFA score and cardiac arrest. Our results can inform sample size calculations of prospective trials in this high-risk patient population that is an unmet need (8, 9). The high mortality can serve as a clarion call to optimize any reversible mediators of adverse outcome such as ventilator-induced lung injury, evidence-based sedation strategy, and other factors.
The reintubation rate we report is intermediate compared with other studies assessing reintubation rates. A national study of 185 ICUs suggested a benchmark reintubation rate of 10%, although among cardiothoracic ICUs, the rate was lower at 4.9% (29) than our reported rate of 7.6%. Therefore, our results support benchmarking the reintubation rate of medical CICUs as less than that of general medical ICUs but greater than that of a unit incorporating cardiothoracic surgical patients.
The adverse outcome associated with respiratory failure in cardiac patients is multifactorial, influenced by primary disease severity, noncardiac patient comorbidities (6, 30), iatrogenic factors related to respiratory support such as sedation strategy and delirium (31), ventilator-induced lung injury (32), and other contributors. Our results should provide impetus to better identify causes and potential innovative treatments to reduce these adverse outcomes including minimizing preventable harm (33) through protocols specific to CICU patients.
Avenues for Further Exploration
We found that outcomes of NIV and HFNC were similar in our survival analysis. HFNC has been reported to reduce work of breathing, inspiratory effort, lung volume, and compliance (34) and reduce reintubations (35). Among cardiac surgical patients, HFNC compared with conventional oxygen therapy reduced ICU readmissions (36), and HFNC was noninferior to NIV for reducing reintubation after cardiac surgery (10). However, HFNC has not been well studied in prospective trials in medical CICU patients. Our findings that approximately 19% of intubated patients receive NIV or HFNC prior to intubation and that 26% are extubated to HFNC or NIV should prompt studies of the role of HFNC as an extubation adjunct and as a primary therapy in the CICU. The variation in use of respiratory therapies across centers could prompt exploration of opportunities to standardize criteria or increase availability of noninvasive respiratory support.
Limitations
The study has several limitations. First, we did not collect reasons why a particular respiratory support strategy was chosen for each patient, the specific ventilator settings, other time-varying exposures such as sedation strategy, hemodynamic or respiratory variables at the time of ventilation initiation, or serial laboratory values. Treatment was not standardized across centers and was at the treating team’s discretion. As a prospective observational study, we describe associations not causation, and therefore could not attribute reasons why patients had worse outcomes. Future randomized trials in this patient population are needed to identify specific respiratory support for different CICU patient phenotypes. Last, the data collection was limited to in-hospital outcomes. Future studies should consider capturing postdischarge outcomes including quality-of-life metrics.
CONCLUSIONS
In conclusion, nearly one-third of contemporary level I CICU admissions received some form of respiratory support with IMV, NIV, or HFNC, across a diverse set of primary cardiac diseases. Respiratory support in cardiac patients was associated with a substantial risk of reintubation and death. HFNC has an emerging role in the CICU to manage respiratory failure. Our findings should prompt consideration of the optimal training and staffing patterns in CICUs and ongoing studies of the optimal management strategies in these high-risk patients.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Solomon received research support from the National Institutes of Health Clinical Center intramural research funds. Dr. Metkus received salary support from the National Institutes of Health-funded Institutional Career Development Core at Johns Hopkins (project number 5KL2TR003099-02). The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Morrow DA, Fang JC, Fintel DJ, et al. ; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Council on Quality of Care and Outcomes Research. Evolution of critical care cardiology: Transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: A scientific statement from the American Heart Association. Circulation. 2012; 126:1408–1428 [DOI] [PubMed] [Google Scholar]
- 2.Katz JN, Minder M, Olenchock B, et al. The genesis, maturation, and future of critical care cardiology. J Am Coll Cardiol. 2016; 68:67–79 [DOI] [PubMed] [Google Scholar]
- 3.Metkus TS, Stephens RS, Schulman S, et al. Utilization and outcomes of early respiratory support in 6.5 million acute heart failure hospitalizations. Eur Heart J Qual Care Clin Outcomes. 2020; 6:72–80 [DOI] [PubMed] [Google Scholar]
- 4.Metkus TS, Albaeni A, Chandra-Strobos N, et al. Incidence and prognostic impact of respiratory support in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2017; 119:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz JN, Shah BR, Volz EM, et al. Evolution of the coronary care unit: Clinical characteristics and temporal trends in healthcare delivery and outcomes. Crit Care Med. 2010; 38:375–381 [DOI] [PubMed] [Google Scholar]
- 6.Jentzer JC, van Diepen S, Barsness GW, et al. Changes in comorbidities, diagnoses, therapies and outcomes in a contemporary cardiac intensive care unit population. Am Heart J. 2019; 215:12–19 [DOI] [PubMed] [Google Scholar]
- 7.Vallabhajosyula S, Kashani K, Dunlay SM, et al. Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000-2014. Ann Intensive Care. 2019; 9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller PE, van Diepen S, Ahmad T. Acute decompensated heart failure complicated by respiratory failure. Circ Heart Fail. 2019; 12:e006013. [DOI] [PubMed] [Google Scholar]
- 9.van Diepen S, Granger CB, Jacka M, et al. The unmet need for addressing cardiac issues in intensive care research. Crit Care Med. 2015; 43:128–134 [DOI] [PubMed] [Google Scholar]
- 10.Stéphan F, Barrucand B, Petit P, et al. ; BiPOP Study Group. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: A randomized clinical trial. JAMA. 2015; 313:2331–2339 [DOI] [PubMed] [Google Scholar]
- 11.Bohula EA, Katz JN, van Diepen S, et al. ; Critical Care Cardiology Trials Network. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: The critical care cardiology trials network prospective North American multicenter registry of cardiac critical illness. JAMA Cardiol. 2019; 4:928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jentzer JC, Bennett C, Wiley BM, et al. Predictive value of individual sequential organ failure assessment sub-scores for mortality in the cardiac intensive care unit. PLoS One. 2019; 14:e0216177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raith EP, Udy AA, Bailey M, et al. ; Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017; 317:290–300 [DOI] [PubMed] [Google Scholar]
- 14.Oremus M, Sharafoddini A, Morgano GP, et al. Multimedia appendix correction: A computer-assisted personal interview app in research electronic data capture for administering time trade-off surveys (REDCap): Development and pretest. JMIR Form Res. 2018; 2:e11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller PE, Patel S, Saha A, et al. National trends in incidence and outcomes of patients with heart failure requiring respiratory support. Am J Cardiol. 2019; 124:1712–1719 [DOI] [PubMed] [Google Scholar]
- 16.Miller PE, Caraballo C, Ravindra NG, et al. Clinical implications of respiratory failure in patients receiving durable left ventricular assist devices for end-stage heart failure. Circ Heart Fail. 2019; 12:e006369. [DOI] [PubMed] [Google Scholar]
- 17.Alviar CL, Miller PE, McAreavey D, et al. ; ACC Critical Care Cardiology Working Group. Positive pressure ventilation in the cardiac intensive care unit. J Am Coll Cardiol. 2018; 72:1532–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter P, Lagan J, Fortune C, et al. Association of cardiovascular disease with respiratory disease. J Am Coll Cardiol. 2019; 73:2166–2177 [DOI] [PubMed] [Google Scholar]
- 19.Hill T, Means G, van Diepen S, et al. Cardiovascular critical care: A perceived deficiency among U.S. trainees. Crit Care Med. 2015; 43:1853–1858 [DOI] [PubMed] [Google Scholar]
- 20.O’Gara PT, Adams JE, 3rd, Drazner MH, et al. COCATS 4 task force 13: Training in critical care cardiology. J Am Coll Cardiol. 2015; 65:1877–1886 [DOI] [PubMed] [Google Scholar]
- 21.Brusca SB, Barnett C, Barnhart BJ, et al. Role of critical care medicine training in the cardiovascular intensive care unit: Survey responses from dual certified critical care cardiologists. J Am Heart Assoc. 2019; 8:e011721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Malley RG, Olenchock B, Bohula-May E, et al. Organization and staffing practices in US cardiac intensive care units: A survey on behalf of the American Heart Association Writing Group on the Evolution of Critical Care Cardiology. Eur Heart J Acute Cardiovasc Care. 2013; 2:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Diepen S, Fordyce CB, Wegermann ZK, et al. Organizational structure, staffing, resources, and educational initiatives in cardiac intensive care units in the United States: An American Heart Association Acute Cardiac Care Committee and American College of Cardiology Critical Care Cardiology Working Group cross-sectional survey. Circ Cardiovasc Qual Outcomes. 2017; 10:e003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.François B, Cariou A, Clere-Jehl R, et al. ; CRICS-TRIGGERSEP Network and the ANTHARTIC Study Group. Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med. 2019; 381:1831–1842 [DOI] [PubMed] [Google Scholar]
- 25.Bray JE, Smith K, Hein C, et al. ; EXACT investigators. The EXACT protocol: A multi-centre, single-blind, randomised, parallel-group, controlled trial to determine whether early oxygen titration improves survival to hospital discharge in adult OHCA patients. Resuscitation. 2019; 139:208–213 [DOI] [PubMed] [Google Scholar]
- 26.Pinsky MR. My paper 20 years later: Effect of positive end-expiratory pressure on right ventricular function in humans. Intensive Care Med. 2014; 40:935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Checkley W, Martin GS, Brown SM, et al. ; United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study Investigators. Structure, process, and annual ICU mortality across 69 centers: United States critical illness and injury trials group critical illness outcomes study. Crit Care Med. 2014; 42:344–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metkus T, Miller PE, Alviar CL, et al. Incidence, predictors and prognosis of respiratory support in non-ST segment elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2020. Apr 23. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Miltiades AN, Gershengorn HB, Hua M, et al. Cumulative probability and time to reintubation in U.S. ICUs. Crit Care Med. 2017; 45:835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha SS, Sjoding MW, Sukul D, et al. Changes in primary noncardiac diagnoses over time among elderly cardiac intensive care unit patients in the United States. Circ Cardiovasc Qual Outcomes. 2017; 10:e003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aday AW, Dell’orfano H, Hirning BA, et al. Evaluation of a clinical pathway for sedation and analgesia of mechanically ventilated patients in a cardiac intensive care unit (CICU): The Brigham and Women’s Hospital Levine CICU sedation pathways. Eur Heart J Acute Cardiovasc Care. 2013; 2:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017; 195:438–442 [DOI] [PubMed] [Google Scholar]
- 33.van Diepen S, Sligl WI, Washam JB, et al. Prevention of critical care complications in the coronary intensive care unit: Protocols, bundles, and insights from intensive care studies. Can J Cardiol. 2017; 33:101–109 [DOI] [PubMed] [Google Scholar]
- 34.Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017; 195:1207–1215 [DOI] [PubMed] [Google Scholar]
- 35.Thille AW, Muller G, Gacouin A, et al. ; HIGH-WEAN Study Group and REVA Research Network. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: A randomized clinical trial. JAMA. 2019; 322:1465–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zochios V, Collier T, Blaudszun G, et al. The effect of high-flow nasal oxygen on hospital length of stay in cardiac surgical patients at high risk for respiratory complications: A randomised controlled trial. Anaesthesia. 2018; 73:1478–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


