Sir,
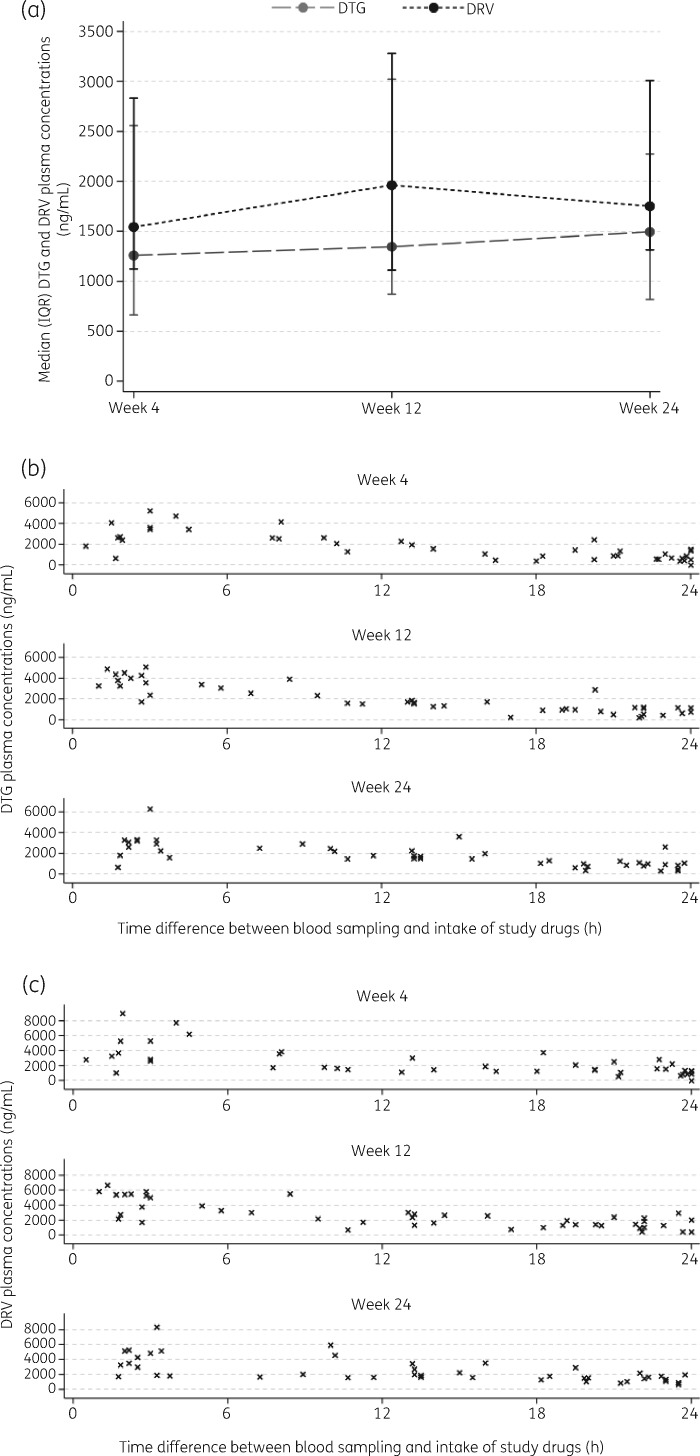
Successful combination ART (cART) has substantially increased the life expectancy of patients infected with HIV.1 Although triple therapy has been the standard of care since 1996, recent studies have demonstrated the non-inferiority of a number of dual ARTs.2,3 Although dolutegravir in combination with the NRTI lamivudine has demonstrated safety and efficacy for therapy-naive patients and after switch of therapy in patients with HIV,3,4 only limited evidence exists for the combination of dolutegravir and the ritonavir-boosted PI, darunavir.5,6 This gap was addressed by the DUALIS study, a large prospective, interventional, multicentre, randomized controlled trial (DUALIS, Eudra-CT: 2015-000360-34), which demonstrated the safety and efficacy of a switch to once-daily dolutegravir (50 mg) and darunavir boosted with ritonavir (800/100 mg) in virally suppressed participants. Data of intensive pharmacokinetic (PK) sampling from a substudy of 10 subjects were published in this journal; some subjects were included in both the PK I and PK II substudies.7 The additional substudy presented here, PK II, aims to describe untimed dolutegravir and darunavir plasma concentrations and changes in safety parameters in a larger subgroup of subjects from the DUALIS study. Approval was obtained from the ethics committee of the University Hospital rechts der Isar, Munich, Germany (approval number 162/15AF) and the study was carried out in accordance with the Declaration of Helsinki. After obtaining written informed consent from all subjects, their medications were switched to dolutegravir (50 mg) and darunavir boosted with ritonavir (800/100 mg) once daily. Study medication was recommended to be taken with a fatty meal. For the PK II substudy, serum blood was collected on scheduled visits but not at a set time after last pill intake at Week 4, 12 and 24. Blood samples were centrifuged at 4500 rpm for 10 min and plasma samples were stored at −80°C; drug concentrations were measured using modified liquid chromatography.7 Carbamazepine and quinoxaline were employed as internal standards for dolutegravir and darunavir, respectively. Lower limits of quantification were 150 ng/mL for dolutegravir and 125 ng/mL for darunavir (limits of detection were 2.0 and 1.8 ng/mL for dolutegravir and darunavir, respectively). All data are presented as median (IQR) unless stated otherwise. To evaluate the significance of changes from baseline in safety laboratory parameters, two-sided Wilcoxon signed-rank test for paired samples was used. For PK analysis, 57 subjects (50 male and 7 female) with a median (IQR) age of 45 (37–51) years and a median BMI of 24.3 (22.6–26.2) kg/m2 were included in the substudy at the first PK measurement at Week 4. HIV RNA was <50 copies/mL in 98.1%, 96.3% and 96.3% of subjects at Week 4, 12 and 24, respectively. Median CD4 cell count remained at 638.0, 602.5 and 637.0 cells/mm3 at Week 4, 12 and 24, respectively. Adherence of the subjects to study medication measured by pill count was 78.9%, 87.5% and 89.3% for dolutegravir and 75.4%, 87.5% and 89.3% for darunavir at Week 4, 12 and 24, respectively. The median (IQR) time difference between the last reported intake of dolutegravir and darunavir boosted with ritonavir and blood sampling for PK analysis at Week 4, 12 and 24 was 20.6 (8.1–24.0), 18.3 (5.8–23.5) and 18.3 (8.9–23.0) h, respectively. Median (IQR) plasma levels at Week 4, 12 and 24 were 1258 (662–2556), 1345 (870–3021) and 1494 (816–2274) ng/mL for dolutegravir and 1543 (1123–2832), 1961 (1111–3279) and 1751 (1314–3008) ng/mL for darunavir, respectively, (Figure 1a–c). Median plasma concentrations were 0–82-fold (Week 4), 3–79-fold (Week 12) and 4–98-fold (Week 24) above the protein-adjusted IC90 (64 ng/mL, calculated for WT virus) for dolutegravir8 and 0–45-fold (Week 4), 2–33-fold (Week 12) and 1–42-fold (Week 24) above the protein-adjusted EC90 (200 ng/mL, calculated for WT virus) for darunavir.9 Liver and kidney functions remained normal during the investigation period [ALT level was 20.0 (18.0–25.0), 21.0 (16.0–28.0) and 22.0 (17.0–24.0) U/L at Week 4, 12 and 24, respectively; AST level was 22.0 (19.0–26.0), 23.0 (20.0–26.0) and 23.5 (20.0–28.0) U/L at Week 4, 12 and 24, respectively; and serum creatinine was 1.0 (0.9–1.1), 1.1 (1.0–1.1) and 1.1 (0.9–1.1) mg/dL at Week 4, 12 and 24, respectively].
Figure 1.
(a) Median (IQR) plasma concentrations for dolutegravir (DTG) and darunavir (DRV) after Week 4, 12 and 24 in a subset of 57 subjects within substudy PK II of the DUALIS study. Distribution of plasma levels of (b) dolutegravir and (c) darunavir in hours after last reported intake at Week 4, 12 and 24. Each cross represents one subject.
In the PK II study, adequate and well-distributed plasma dolutegravir and darunavir levels were observed in randomly collected blood samples from subjects. PK safety of coadministration of dolutegravir and darunavir boosted with ritonavir has been discussed in the PK I substudy.7 Notably, one subject had inadequate dolutegravir and darunavir plasma concentrations at Week 4 (25 h after the last dose) and reported an issue with adherence. However, during follow-up, drug levels at Week 12 and 24 were adequate in this subject. In two other subjects, each darunavir level (155 and 172 ng/mL) was slightly below the protein-adjusted EC90 at 25 h after the last dose, although drug levels at all other times were adequate. According to the protocol, neither of these subjects had complete adherence to study medication by the pill count. For all other subjects, dolutegravir and darunavir concentrations were above the respective protein-adjusted IC90 and EC90. Liver, renal and haematological function remained unchanged during the 24 week period. Since blood samples were not taken at a fixed timepoint, a limitation of the study could be that short-time concentrations of dolutegravir and darunavir below the therapeutic target might be missed in some subjects. However, the data obtained from the PK II substudy support the safe PK combination of once-daily dolutegravir and darunavir boosted with ritonavir.
Contributor Information
the DUALIS study team:
Christoph D Spinner, Björn Jensen, Thomas Lutz, Petra Spornraft-Ragaller, Stellbrink Hans Jürgen, Martin Hower, Ulrich Bohr, Wilfried Obst, Ivanka Krznaric, Martin Sprinzl, Franz Audebert, Tim Kuemmerle, Stefan Scholten, Heribert Hillenbrand, Christiane Cordes, Heribert Knechten, Birger Kuhlmann, Heiko Jessen, Patrick Beck, Gerd Fätkenheuer, Hartwig H F Klinker, Juergen K Rockstroh, Stefan Esser, Christoph Stephan, Olaf Degen, Andreas Bellmunt-Zschäpe, Pavel Khaykin, Norbert Brockmeyer, and Albrecht Stoehr
Acknowledgements
We would like to thank the team of Munich Study Center, CRO, Technical University of Munich as well as the staff of MUC Research for extending their support to carry out this study. We thank Ms Anne Elter and Ms Diana Schirmer for their technical assistance. We also thank the study centres and participants of the DUALIS study.
Members of the DUALIS study team
Christoph D. Spinner (Technical University Munich, School of Medicine, University Hospital rechts der Isar, Department of Medicine II, Munich, Germany), Björn Jensen (University Hospital Duesseldorf, Department of Gastroenterology, Hepatology and Infectiology, Duesseldorf, Germany), Thomas Lutz (Infektiologikum Frankfurt, Frankfurt, Germany), Petra Spornraft-Ragaller (Carl Gustav Carus University Hospital of the Technical University Dresden, Dresden, Germany), Hans Jürgen Stellbrink (ICH Study Center GmbH & Co, Hamburg, Germany), Martin Hower (Hospital Dortmund gGmbH, outpatient clinic for infectious diseases, Dortmund, Germany), Ulrich Bohr (Praxis Kaiserdamm, Berlin, Germany), Wilfried Obst (University Hospital Magdeburg, Department for Gastroenterology, Hepatology and und Infectiology, Magdeburg, Germany), Ivanka Krznaric (ZIBP Center for Infectiology Berlin Prenzlauer Berg GmbH, Berlin, Germany), Martin Sprinzl (University Hospital Mainz, Germany), Franz Audebert (Praxiszentrum Alte Mälzerei, Köln, Germany), Tim Kuemmerle (Praxis am Ebertplatz, Köln, Germany), Stefan Scholten (Praxis Hohenstaufenring, Köln, Germany), Heribert Hillenbrand (Medizinisches Versorgungszentrum Berlin-Friedrichshain, Germany), Christiane Cordes (Praxis Cordes, Berlin, Germany), Heribert Knechten (Praxis Knechten, Aachen, Germany), Birger Kuhlmann (Praxis Kuhlmann, Hannover, Germany), Heiko Jessen (Praxis Jessen2 + colleagues, Berlin, Germany), Patrick Beck (Gemeinschaftspraxis - Infectomed, Stuttgart, Germany), Gerd Fätkenheuer (University Hospital Köln, Department of Medicine II, Köln, Germany), Hartwig H. F. Klinker (University of Wuerzburg Medical Center, Department of Medicine II, Division of Infectious Diseases, Wuerzburg, Germany), Juergen K. Rockstroh (University Hospital Bonn, Department of Medicine I, Bonn, Germany), Stefan Esser (University Hospital Essen, Department of Dermatology, Essen, Germany), Christoph Stephan (Hospital of Johann Wolfgang Goethe University, Department of Internal Medicine, HIV-Center, Frankfurt, Germany), Olaf Degen (University Hospital Hamburg-Eppendorf Center for Infectiology, Hamburg, Germany), Andreas Bellmunt-Zschäpe (Allgemeinmedizinische Praxis Bellmunt Zschäpe, Dortmund, Germany), Pavel Khaykin (MainFachArzt, Frankfurt/Main, Germany), Norbert Brockmeyer (Hospital Bochum WIR ‘Walk In Ruhr’ at St. Elisabeth-Hospital, Bochum, Germany), Albrecht Stoehr (ifi – Institute for interdisciplinary medicine, center for infectiology at Asklepios Hospital St. Georg, Hamburg, Germany).
Funding
This study was funded by Technical University of Munich with grants from Janssen-Cilag and ViiV Healthcare.
Transparency declarations
S.W. has received a travel grant from Gilead. C.B. received honoraria for lectures and/or consultancies from AbbVie, Gilead, Janssen, MSD and ViiV as well as funding from Deutsche Leberstiftung, DZIF, Hector Stiftung and NEAT ID. H.H.F.K. is part of the advisory committee or review panel for AbbVie, BMS, Gilead, Hexal, Janssen, MSD and Shionogi, and has received grant/research support from AbbVie, Arrowhead, BMS, Janssen, Gilead, MSD and Novartis. He has also received honoraria for speaking and teaching from AbbVie, BMS, Gilead, Janssen and MSD. A.Z. has been an advisor and/or received speaker’s honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, BMS, GSK and Janssen-Cilag. E.W. has received honoraria for consulting or speaking at educational events from AbbVie, Bristol Myers Squibb, Gilead, GlaxoSmithKline, Hexal, Janssen-Cilag, MSD, Roche and ViiV Healthcare. MUC Research has received grants for clinical research from AbbVie, MSD Sharp & Dohme, Pfizer und ViiV Healthcare. C.D.S. has received grants for travel and participation in advisory boards or speaker’s honoraria from AbbVie, Astellas, Bristol Meyers Squibb, Gilead, Janssen-Cilag, MSD and ViiV Healthcare. He has also received grants for investigator-sponsored studies from Gilead Sciences, Janssen-Cilag and ViiV Healthcare. J.S., A.B. and H.B. declare no conflicts of interest.
Author contributions
S.W. has drafted the manuscript. C.B., J.S., A.Z. and H.H.F.K. have supervised patient care and assisted data interpretation and editing of the manuscript. A.B. and E.W. have performed statistical analysis and data interpretation and assisted editing of the manuscript. H.B. has been involved in supervision and management of the study as well as data handling. C.D.S. has coordinated the entire study as well as supervising patient care and editing of the manuscript.
References
- 1. Teeraananchai S, Kerr SJ, Amin J et al. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med 2017; 18: 256–66. [DOI] [PubMed] [Google Scholar]
- 2. Rossetti B, Montagnani F, De Luca A. Current and emerging two-drug approaches for HIV-1 therapy in ART-naïve and ART-experienced, virologically suppressed patients. Expert Opin Pharmacother 2018; 19: 713–38. [DOI] [PubMed] [Google Scholar]
- 3. Cahn P, Madero JS, Arribas JR et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393: 143–55. [DOI] [PubMed] [Google Scholar]
- 4. van Wyk J, Ajana F, Bisshop F et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose two-drug regimen versus continuing a tenofovir alafenamide-based three- or four-drug regimen for maintenance of virologic suppression in adults with HIV-1: phase 3, randomized, non-inferiority TANGO study. Clin Infect Dis 2020; 6: doi:10.1093/cid/ciz1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vassilios P, Varvara V, Sofia K. Dolutegravir with boosted darunavir as treatment simplification for treatment-experienced HIV patients with multiple mutations. Int J STD AIDS 2019; 30: 1214–5. [DOI] [PubMed] [Google Scholar]
- 6. Vizcarra P, Fontecha M, Monsalvo M et al. Efficacy and safety of dolutegravir plus boosted-darunavir dual therapy among highly treatment-experienced patients. Antivir Ther 2019; 24: 467–71. [DOI] [PubMed] [Google Scholar]
- 7. Spinner CD, Kummerle T, Krznaric I et al. Pharmacokinetics of once-daily dolutegravir and ritonavir-boosted darunavir in HIV patients: the DUALIS study. J Antimicrob Chemother 2017; 72: 2679–81. [DOI] [PubMed] [Google Scholar]
- 8. Cottrell ML, Hadzic T, Kashuba AD. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet 2013; 52: 981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prezista Product Information. http://www.janssen.com.au/files/Products/Prezista_CMI.pdf.