Abstract
Diffusion MRI is extensively used to investigate changes in white matter microstructure. However, diffusion measures within white matter tissue can be affected by partial volume effects due to cerebrospinal fluid and white matter hyperintensities, especially in the aging brain. In previous aging studies, the cingulum bundle that plays a central role in the architecture of the brain networks supporting cognitive functions has been associated with cognitive deficits. However, most of these studies did not consider the partial volume effects on diffusion measures. The aim of this study was to evaluate the effect of free water elimination on diffusion measures of the cingulum in a group of 68 healthy elderly individuals. We first determined the effect of free water elimination on conventional DTI measures and then examined the effect of free water elimination on verbal fluency performance over 12 years. The cingulum bundle was reconstructed with a tractography pipeline including a white matter hyperintensities mask to limit the negative impact of hyperintensities on fiber tracking algorithms. We observed that free water elimination increased the ability of conventional DTI measures to detect associations between tissue diffusion measures of the cingulum and changes in verbal fluency in older individuals. Moreover, free water content and mean diffusivity measured along the cingulum were independently associated with changes in verbal fluency. This suggests that both tissue modifications and an increase in interstitial isotropic water would contribute to cognitive decline. These observations reinforce the importance of using free water elimination when studying brain aging and indicate that free water itself could be a relevant marker for age-related cingulum white matter modifications and cognitive decline.
Introduction
Aging is associated with widespread brain structural modifications including neurodegeneration of white and grey matter [1–3]. In the last few years, diffusion tensor imaging (DTI) has been widely used to indirectly explore microstructural properties of white matter and constitutes a sensitive technique to describe age-related white matter microstructural changes [4,5]. Parameters quantified by DTI such as Fractional Anisotropy (FA), Mean Diffusivity (MD), Radial Diffusivity (RD) and Axial Diffusivity (AD) can be used to indirectly infer changes in axonal integrity and myelination [6]. In older adults, previous DTI studies reported a decrease in FA and an increase in MD and RD in major white matter tracts [6–8] that correlated with cognitive impairment [9–12]. Age-related modifications for AD were less consistent. Previous studies showed that both increases [4,13–16] and decreases [14,15,17] in AD occur along aging that inconsistently associated with cognition [13,18–20].
However, diffusion measures within brain tissues can be affected by partial volume effects due to cerebrospinal fluid [21], especially in aging individuals with atrophied brains and ventriculomegaly [22–25]. When voxels contain cerebrospinal fluid (e.g. partial volume), diffusion measures can be biased towards a pattern of high diffusivity (MD, AD, RD) and reduced FA [26,27]. This effect has been particularly observed in the fornix and the corpus callosum because of their close proximity to periventricular regions [26,28,29]. In addition, the neuroinflammation process occurring in aging [30] can also affect the interstitial space and contribute to an increase of free extracellular water content [31]. To overcome this problem, the Free Water Elimination (FWE) method was used to differentiate the contribution of the surrounding free water extracellular from the diffusion properties of brain tissues (including hindered water) such as white matter bundles[21,32]. Elimination of extracellular free water contamination improves the sensitivity and specificity of most measures derived from conventional DTI [24,32–34]. Although such correction has been used in pathological conditions [35–40], the majority of diffusion MRI studies do not consider free water effects of aging. Aging is associated with grey and white matter tissue loss, and the resulting enlargement of interstitial space could lead to increase in free water [41–44]. In addition, free water content in white matter fibers was recently associated with the presence of white matter hyperintensities (WMHs) in clinical studies [38,45–48]. In older adults, the prevalence and severity of WMH burden increases after age 60 [49,50]. Previous studies reported that the presence of WMH lesions was not only associated with age-related white matter changes evaluated with DTI [51–53] or tractography [54–56], but also with cognitive impairments mainly in memory and executive functions [5,56,57].
The cingulum bundle is one of the most studied white matter tracts running from the anterior to the posterior part of the brain that interconnects frontal, parietal, and temporal areas [58–60]. Due to this central location, the cingulum plays a central role in white matter architecture and functional networks that support and contribute to optimal cognitive function [60]. Some previous studies [59,61–63] but not all [64,65] described a decrease in FA and an increase in AD, RD and/or MD along the cingulum bundle. These changes within this bundle due to aging were correlated with executive and memory deficits [26,59,61,66–68]. However, these observations still remain controversial considering that most of these studies did not consider CSF partial effect due to atrophy or WMH burden. Recent tractography studies consistently observed that atrophy-related partial volume effects [24,26] and age-related ventricular enlargement [23] or WMH burden [54] affect diffusion measures of the cingulum in healthy older individuals. In this regard, Reginold and colleagues reported higher RD in cingulum tracts that crossed WMH than those outside WMH regions [23,54]. Atrophy and WMH burden might therefore impact cingulum diffusion measures suggesting a source of significant bias in studies exploring the integrity of this bundle and its association with cognitive functions in aging.
In this study, we hypothesized that free water elimination would provide more accurate white matter diffusion measures of the cingulum, which in turn would be better correlated with verbal fluency changes in 68 elderly individuals. To do so, the cingulum bundle was reconstructed with a tractography pipeline that includes a WMH mask to limit the negative impact of hyperintensities on fiber tracking algorithms. We first described the effect of FW-correction on diffusion measures of the cingulum derived from the conventional tensor DTI model. Then, we explored the association between FW-corrected diffusion measures and cognitive changes as evaluated using the Isaacs Set Test over 12 years. The Isaacs Set Test was chosen since it specifically assesses semantic memory and executive functioning.
Materials and methods
Dataset
Participants were selected from the Bordeaux sample of the Three-City study [69], an ongoing longitudinal multicenter population-based elderly cohort designed to evaluate risk factors for dementia. The study protocol was approved by the ethics committee of Kremlin-Bicêtre University Hospital (Paris, France), and all participants provided written informed consent. At baseline, subjects were non-institutionalized, randomly recruited from electoral lists, and were older than 65 years. Since the 1999–2000 baseline inclusion, an extended neuropsychological assessment was administered by trained psychologists at each follow-up occurring at 2, 4, 7, 10 and 12 years. An MRI scan was performed at the 10-year follow-up for every subject. All 239 subjects were screened, and individuals were excluded if they had dementia (n = 8), brain pathologies (n = 27) or if MRI images were either unavailable (n = 62), distorted by artefacts (n = 24), or failed pre-processing (n = 15). In addition, cingulum reconstruction failed in 16 subjects and tractography quality control revealed suboptimal data in 19 additional subjects. Out of the 239 initially screened subjects, 68 subjects were included in the study. All participants were right-handed and had a Mini Mental State Examination (MMSE) score greater than or equal to 24.
Cognitive and clinical variables
The Isaacs Set Test (IST) [70] chosen for the study consists of a test on verbal fluency, where subjects are asked to cite the highest number of words in four semantics categories. Three scores of the IST were used, corresponding to the number of words cited by the subject at 15-seconds (IST 15s), 30-seconds (IST 30s) and 60-seconds (IST 60s). Verbal fluency was evaluated over 12 years, using a subject-specific slope for each IST score computed using a linear mixed model with time as a continuous variable, random intercept and slope. Over the 12-year follow-up period, the negative mean annual slope indicated a decrease in the number of given words.
Some clinical variables were also collected: depressive symptoms evaluated using the Center for Epidemiologic Studies-Depression scale (CESD) [71], vascular risk factors including hypertension as defined in patients with a blood pressure > 140/90 mm Hg or taking anti-hypertensive medication, diabetes as defined in patients with a blood glycemic levels > 7 mmol/l or taking hypoglycemic medication, and body mass index (BMI).
MRI acquisition
MRI scanning was performed using a 3T Achieva system (Philips Medical Systems, The Netherlands) equipped with a 8-channel SENSE head coil. Head motions were minimized by using tightly padded clamps attached to the head coil. Anatomical MRI volumes were acquired using a 3D magnetization-prepared rapid gradient-echo (MPRAGE) T1-weighted sequence with the following parameters: repetition time (TR)/ echo time (TE) = 8.2 ms/3.5 ms, flip angle 7°, field of view (FOV) 256x256 mm2, 180 slices of 1 mm of thickness, voxel size 1x1x1 mm3. Fluid-attenuated inversion recovery (FLAIR) images were obtained with the following parameters: TR = 11000 ms; TE = 140 ms, inversion time = 2800 ms, 90-degree flip angle, FOV 230x172 mm2, 24 slices of 5 mm of thickness, voxel size 0.72x1.20x5 mm3. Diffusion weighted images were acquired using a spin echo single shot EPI sequence composed of one b0 map (b-value = 0 s/mm2) followed by 21 diffusion gradients maps (b-value = 1000s/mm2) homogenously spread over a half sphere and 21 opposite directions spread over the opposite half sphere. To increase signal-to-noise ratio, a second series of two b0 and 42 DWI volumes was acquired. Sixty axial slices were acquired with the following parameters: TR = 9700 ms, TE = 60 ms, flip angle 90°, FOV 224×224 mm2, 60 slices, no gap and voxel size 2x2x2 mm3. All acquisitions were aligned on the anterior commissure-posterior commissure plan.
MRI preprocessing
Diffusion MRI preprocessing
Diffusion MRI (dMRI) images were pre-processed using FMRIB’s Diffusion toolbox [72,73] in order to produce individual FA, MD, AD and RD maps in native space. For each subject, dMRI images were co-registered to the b0 reference image with an affine transformation and were corrected for motion and eddy current distortions. Brain Extraction Tool (BET) [74] was applied to eliminate non-brain voxels and resulting dMRI images were denoised using the non-local mean denoising method [75]. To increase signal-to-noise ratio, the successive runs were then combined into a dMRI image using FSL tools. Finally, the fiber Orientation Distribution Functions (fODF) map was computed using the spherical constrained deconvolution [76–78]. Visual quality check was performed and did not reveal any processing failure for the included subjects.
White matter hyperintensity segmentation
White matter hyperintensity volumes were automatically segmented by the lesion growth algorithm implemented in the Lesion Segmentation Tool (LST, v2.0) [79] of SPM12. For each participant, the T1-weighted image was used to generate partial volume estimation and three tissue probability maps (grey matter, white matter and cerebrospinal fluid). Then, each FLAIR image was co-registered on the corresponding T1 image to compute lesion belief maps for the three tissue classes. These maps were finally summed up and a lesion growth model with a pre-chosen initial threshold (κ = 0.3) was applied to create lesion maps in native space. Visual inspection of the lesion probability maps was performed and manually corrected when inaccuracies were found. Finally, WMH volumes were extracted, normalized by white matter volume and log transformed (total WMH). The volume of WMH within the cingulum was estimated by crossing the WMH mask with the cingulum bundle (cingulum WMH).
Free water elimination
Free Water Elimination (FWE) [21] was used to estimate and remove the free water components from diffusion images. To isolate the fractional volume of freely diffusing extracellular water molecules from tissue compartments (i.e. reflecting intracellular processes), FWE fits a bi-tensor model within each voxel: a first one models free water diffusion, defining as an isotropic diffusion, and a second one models the tissue compartment [34]. The isotropic compartment models extracellular water which is characterized by freely and not hindered diffusion. A fixed diffusivity of 3.10−3 mm2/s (i.e. diffusion coefficient of water at body temperature) is used for this compartment. The fraction of free water content per voxel provided a native free water (FW) map for each subject and varied between 0 to 1. In contrast, the tissue compartment models water molecules close to cellular membranes of brain tissue, which are defined by restricted or hindered diffusion using a diffusion tensor. Thereby, the volume of freely diffusing extracellular water molecules is removed from tissue compartments. Consequently, the FW-corrected measures were expected to be more sensitive and specific to tissue changes than the measures derived from the single tensor model [33,37,39]. The FW-corrected DTI maps were called FA tissue (FAt), RDt, ADt and MDt [80].
Cingulum tractography and measures
For each participant, a local tracking using probabilistic algorithm based on fODF maps was performed. The tractography was performed using twenty tracking seeds per voxels included in the white matter mask. The seeding and tracking masks were modified to include WMH masks (see S1 Appendix). Next, the left and right cingulum bundles were extracted using the RecoBundles approach (see [81], Fig 1). RecoBundles is a model-based algorithm performing a registration of a Cingulum model on each subject to extract the subject specific cingulum tract from the whole tractogram. Five different Cingulum models were used in this study (RecoBundles). Finally, after visual inspection of cingulum bundles using dmriqc tool (https://github.com/GuillaumeTh/dmriqcpy), 13 subjects were excluded due to the absence of right or left cingulum bundles and 6 subjects were excluded because of miss-segmentation of hippocampal and anterior parts of the cingulum bundle, mainly because of the age range of our population (older than 85 years of age) at the time of MRI sessions. Therefore, the cingulum bundle was considered as a whole bundle and the different subdivisions of the cingulum were not investigated in this study.
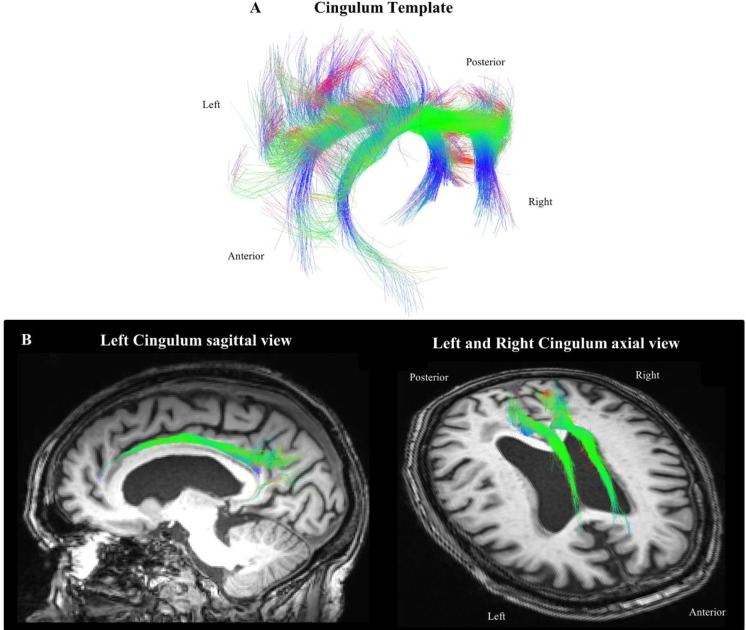
Fig 1.
Left and right cingulum templates used to extract the cingulum tract for each participant (A); Example of the cingulum bundle obtained for one subject displayed on the corresponding T1-weighted image (B). Along the fibers, color represents the RGB scale.
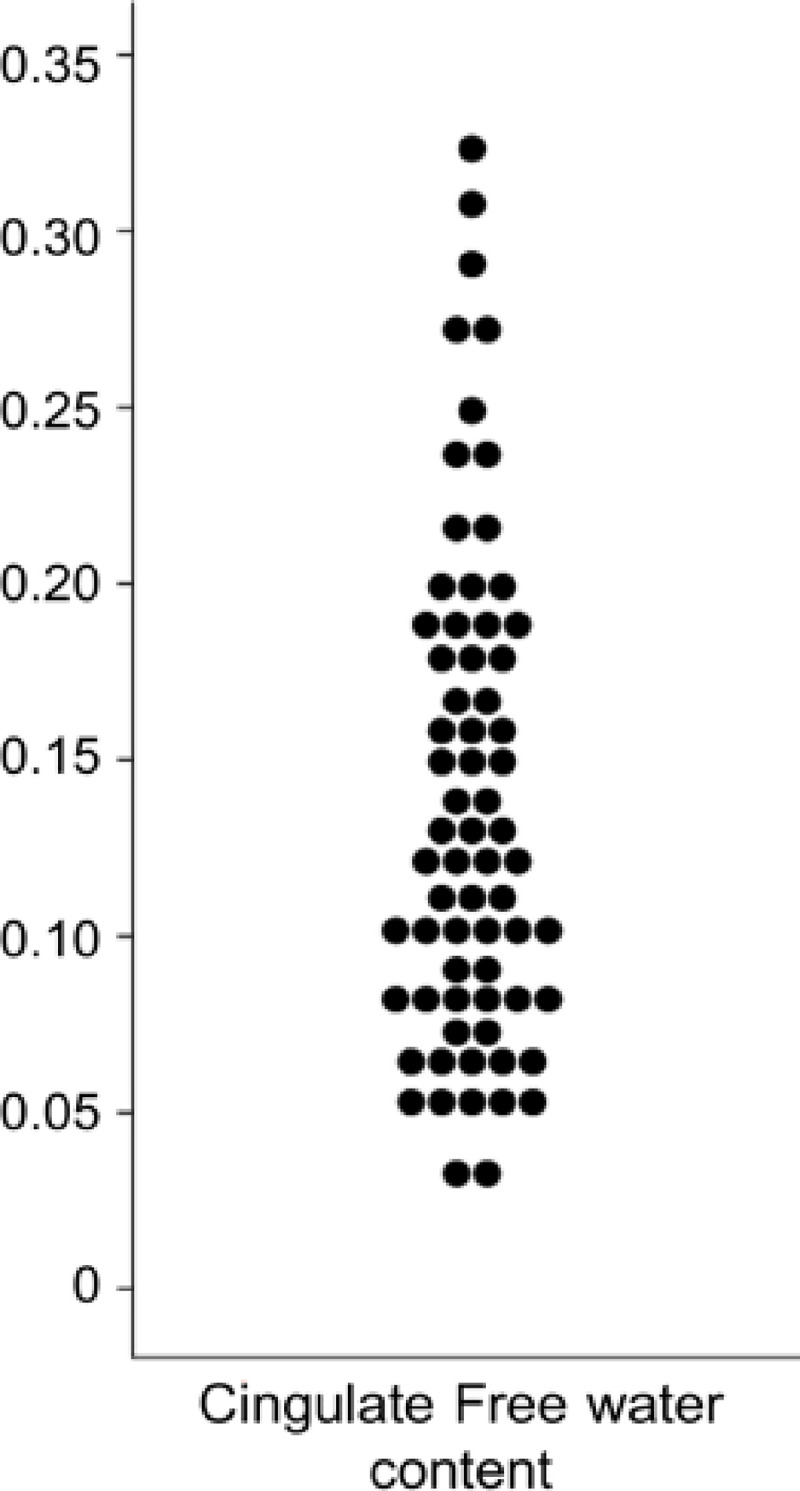
Cingulum diffusion measures were extracted before (FA, MD, RD and AD) and after FW-correction (FAt, MDt, RDt and ADt). The diffusion measures without free water correction are defined as conventional DTI measures and reflect the weighted average of all compartments including free water, whereas diffusion measures after correction of free water are defined as FW-corrected measures. The cingulum free water content was also extracted (Fig 2). Measures of the left and right cingulum were averaged for the analysis.
Fig 2. Dot plot depicting distribution of cingulum free water content.
A percentage of measure changes (% change) for each participant was determined by computing the difference between the measure before and after FW-correction divided by the measure before FW-correction [i.e. % change = ((FW-corrected measure–DTI measure)/DTI measure) × 100].
Statistical analysis
Because of non-normality of the continuous and categorical variables, Mann-Whitney U-tests and Spearman’s correlations were used to evaluate the associations between cingulum diffusion measures as well as verbal fluency with continuous variables (age, global cognition score, depressive symptoms score, body mass index and WMH volume) and categorical variables (gender, education level, presence/absence of diabetes, of hypertension) respectively. In a supplementary analysis, the associations between WMH volumes and cingulum diffusion measures were evaluated (see S1 Appendix and S2 Table). To compare the effects of FW-correction to conventional DTI on cingulum diffusion measures, a Paired t-Test was performed.
Linear regression models were then computed to describe the extent to which the FW-correction affects the association between cingulum diffusion measures and changes in verbal fluency. Diffusion measures were included as independent variables (conventional and FW-corrected FA, MD, AD and RD in separate models) and the verbal fluency slope set as the dependent variable in model adjusted for age and WM volume of the cingulum. Similar linear regression models were performed to examine the relationship between free water content and changes in verbal fluency. Finally, FW-corrected MD and free water content were concomitantly included in the linear regression to evaluate the independency of these two variables on the IST decline.
In a second step, total WMH volume was added to previous models to evaluate the effect of WMH burden on the observed associations (see S3 Table). In a supplementary analysis, specific WMH burden of the cingulum bundle was added to the models (see S1 Appendix and S4 Table).
A false discovery rate (FDR) multiple-comparison correction method was systematically applied for each analysis. Results were considered significant for p<0.05, FDR corrected [82]. All statistical analyses were performed using IBM SPSS Statistics v.23 software (IBM Corporation, Armonk, NY, USA).
Results
Sample characteristics
Characteristics of participants are presented in Table 1. Significant age effects on verbal fluency changes were observed for all IST scores (IST 15s, r = -0.212, p = 0.022; IST 30s, r = -0.207, p = 0.038 and IST 60s, r = -0.252, p = 0.019). WMH volumes correlated with age (total WMH: r = 0.447, p<0.001; cingulum WMH: r = 0.201, p = 0.038) and changes in verbal fluency scores (r = -0.218, p = 0.038; r = -0.293, p = 0.016 and r = -0.328 p = 0.008 respectively). None of clinical variables were related to age, verbal fluency or WMH volumes (p > 0.05). Finally, no association was observed between demographic or clinical variables and cingulum diffusion measures (see S1 Table).
Table 1. Characteristics of participants, 12-year verbal fluency decline and volumetric variables.
| Sample n = 68 | |
|---|---|
| Sociodemographic variables | Mean ± SD or % |
| Age | 81.2 ± 0.48 |
| Male gender | 36.8% |
| High level of education | 37% |
| Clinical variables | |
| MMSE | 27.3 ± 0.3 |
| CES-D | 8.3 ± 0.9 |
| BMI (kg/m2) | 25.2 ± 0.5 |
| Diabetes | 10.3% |
| Hypertension | 35.3% |
| Verbal fluency decline | |
| IST at 15 seconds | -0.489 ± 0.02 |
| IST at 30 seconds | -0.697 ± 0.04 |
| IST at 60 seconds | -0.924 ± 0.06 |
| Volumetric variables | |
| Total WMH volume ml | 15.9 ± 4.1 |
| Cingulum bundle volume (% TIV) | 0.46 ± 0.06 |
| Cingulum WMH volume (%) | 3.2 ± 0.65 |
MMSE, Mini Mental State Examination; BMI, Body Mass Index; CES-D, Center for Epidemiologic Studies-Depression scale; IST, Isaacs Set Test; WMH, White Matter Hyperintensity.
Effect of FW-correction on conventional DTI parameters
Cingulum diffusion measures are presented in Table 2. All FW-corrected measures were significantly different from their conventional counterparts (paired t-test, p<0.05 FDR-corrected, Table 2). After FW-correction, a mean increase of 1.52% of FAt and a mean decrease of 1.61% of MDt, 2.5% of RDt and 1.08% of ADt were observed (Table 2, Fig 2).
Table 2. Cingulum diffusion measures before (conventional DTI) and after FW-correction and % of change between both measures for each group.
| Cingulum diffusion measures | |||||
|---|---|---|---|---|---|
| DTI | FW-corrected | t | p-FDR | % Change | |
| mean ± SD | mean ± SD | mean ± SD | |||
| FA | 0.543 ± 0.049 | 0.567 ± 0.054 | -3.367 | 0.019* | 1.52 ± 0.50 |
| MD (10−3 mm2/s) | 0.768 ± 0.0041 | 0.755 ± 0.0034 | -3.196 | 0.024* | -1.61 ± 0.22 |
| RD (10−3 mm2/s) | 0.506 ± 0.0047 | 0.493 ± 0.0049 | -5.315 | 0.010* | -2.50 ± 0.19 |
| AD (10−3 mm2/s) | 1.29 ± 0.0683 | 1.27 ± 0.0638 | -2.975 | 0.044* | -1.08 ± 0.34 |
| FW | - | 0.141 ± 0.017 | |||
* p<0.05 FDR corrected
Paired Student’s t-test
Effect of FW-correction on relationships between cingulum diffusion measures and verbal fluency
Associations with DTI conventional measures
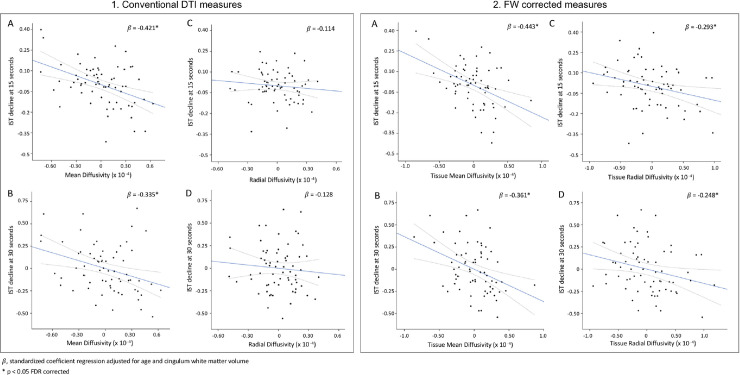
A higher MD value was related to a higher IST decline at 15 and 30 seconds in a model adjusted for age and white matter volume of the cingulum (p < 0.05 FDR-corrected, Table 3 and Fig 3). No association was observed with FA, RD and AD.
Table 3. Correlations between cingulum diffusion measures and verbal fluency score (IST) before (conventional DTI) and after FW-correction.
| DTI | |||||||
| IST 15s | IST 30s | IST 60s | |||||
| β | R2 | β | R2 | β | R2 | ||
| Model unadjusted for total WMH | FA | 0.015 | 0.001 | 0.047 | 0.002 | 0.067 | 0.004 |
| MD | -0.421* | 0.17 | -0.335* | 0.12 | -0.174 | 0.07 | |
| AD | -0.186 | 0.036 | -0.135 | 0.018 | -0.060 | 0.004 | |
| RD | -0.114 | 0.013 | -0.128 | 0.016 | -0.129 | 0.017 | |
| FW-corrected | |||||||
| IST 15s | IST 30s | IST 60s | |||||
| β | R2 | β | R2 | β | R2 | ||
| Model unadjusted for total WMH | FAt | 0.047 | 0.003 | 0.098 | 0.01 | 0.095 | 0.009 |
| MDt | -0.443* | 0.19 | -0.361* | 0.13 | -0.226 | 0.08 | |
| ADt | -0.198 | 0.04 | -0.134 | 0.018 | -0.061 | 0.004 | |
| RDt | -0.293* | 0.11 | -0.248* | 0.13 | -0.199 | 0.039 | |
| FW | -0.353* | 0.12 | -0.241* | 0.10 | -0.196 | 0.061 | |
β, standardized coefficient regression adjusted for age and cingulum white matter volume
R2, R square value
* p < 0.05 FDR corrected
Fig 3.
Relationships between cingulum diffusion measures before (1) and after (2) FW-correction for (A, B) mean diffusivity and (C, D) radial diffusivity and verbal fluency decline. For the scatterplot the regression line is represented in blue and the 95% confidence interval in grey dotted line.
Associations with FW-corrected measures and cingulum free water content
After FW-correction, high values of MDt and RDt were strongly associated with IST decline at 15 and 30 seconds in a model adjusted for age and white matter volume of the cingulum (p < 0.05, Table 3, Fig 3). No association was observed with FAt and ADt.
High free water content was associated with changes in IST score at 15 and 30 seconds in a model adjusted for age and white matter volume of the cingulum (p < 0.05 FDR-corrected, Table 3, Fig 4).
Fig 4.
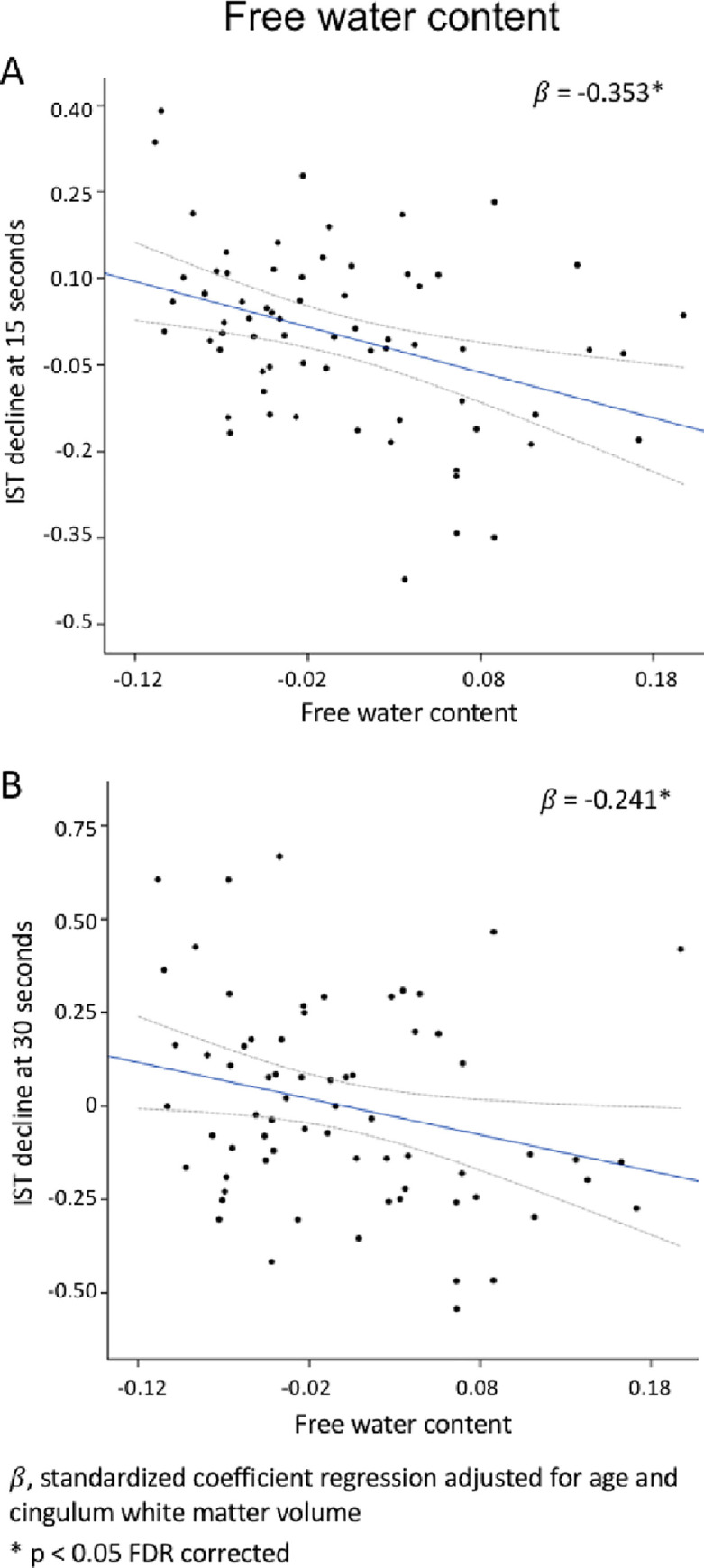
Relationships between cingulum free water content and verbal fluency at 15 (A) and 30 seconds (B). For the scatterplot the regression line is represented in blue and the 95% confidence interval in grey dotted line.
In a model including FW and MDt, both diffusion measures remained significantly associated with IST at 15 (FW: β = -0.236, p = 0.031 and MDt: β = -0.258, p = 0.027) and 30 seconds (FW: β = -0.216, p = 0.039 and MDt: β = -0.237, p = 0.033) indicating that both free water content and MDt independently contributed to the cognitive variances.
Impact of WMH burden on the association between cingulum diffusion measures and verbal fluency
In regression models adjusted for total WMH burden, correlations between conventional DTI measures (MD) and IST decline were not significant anymore. In contrast, MDt and free water content correlations were preserved when total WMH volume (see S3 Table) as well as WMH volume within the cingulum (see S4 Table) were added as covariate in regression models (p < 0.05 FDR-corrected). In a global model, both diffusion measures remained significantly associated with IST at 15 (FW: β = -0.232, p = 0.036 and MDt: β = -0.240, p = 0.034) and 30 seconds (FW: β = -0.211, p = 0.041 and MDt: β = -0.229, p = 0.038) when adjusted for total WMH volume.
Discussion
In this study, we examined the effect of free water elimination on conventional DTI measures of white matter within the cingulum tract and the effect of such correction on the decline in verbal fluency measured over a 12-year period in elderly subjects. In a group of 68 older participants, FW-correction significantly impacted all conventional DTI measures and following such correction measures correlated with decline in verbal fluency performances at 15 and 30 seconds. Free water content was also associated with changes in verbal fluency. In addition, free water content and mean diffusivity measures were independently related to changes in verbal fluency. Finally, in models adjusted for WMH volumes, correlations between MDt and free water content were preserved.
Free water elimination effect
In accordance with recent findings in young adults [32], older adults [80] and clinical studies [38,83], FW-correction was associated with an increase in FA, and a decrease in diffusivity measures (MD, RD and AD). These changes in DTI measures after elimination of the free water compartment suggested not only a non-null volume of the extracellular water [6,21,38,84], but also indicate that white matter microstructural changes were less pronounced than previously suggested by conventional DTI measures [53,85–87].
Based on conventional DTI, our study revealed that only MD showed significant correlations with changes in verbal fluency, especially at 15 and 30 seconds. The elimination of the free water content confirmed such strong association for MDt and revealed additional correlations for RDt that could not be fully observed without considering the free water content. The observed association is in line with previous studies reporting the role of the cingulum bundle in executive functioning in older adults [59,67,88–91]. Even if the underlying neurobiological properties of these parameters remain controversial, a high RDt, without any changes in ADt, has been described as predominantly reflecting myelin changes in animal studies [92,93] and demyelination severity in human post mortem studies on multiple sclerosis [94,95]. This suggests that changes in verbal fluency in our population might be related to myelin changes within the cingulum, rather than axonal damage [6,17,96]. Therefore, our results support previous studies reporting that the elimination of free water improves the estimation of tissue indices which were more strongly predictive of cognitive changes than conventional DTI-derived parameters [6,33,36,48,84,97].
Free water content
In our group of elderly participants, a non-null value of free water content was observed, suggesting that despite its distance from the ventricles, some free water is likely present in the extracellular space of the cingulum. This is consistent with recent studies on whole brain white matter reporting a fraction of free water in the elderly [33,80,98]. In addition, no association between the free water content within the cingulum and CSF volumes was observed in our participants (r = 0.138, p = 0.142). However, we observed that a high content of free water was related to a low cingulum volume (r = -0.251, p-FDR = 0.039), suggesting that in our population the high free water content may be due to atrophy-related processes rather than CSF contamination [99,100].
In line with previous older adults and clinical studies, we reported that a high content of free water within the cingulum was strongly associated with changes in verbal fluency performances at 15 and 30 seconds [35,38,47,48,101]. In addition, cingulum free water content and mean diffusivity measures were both associated with verbal fluency decline. This suggests that both tissue (MDt) and non-tissue parameters (free water) would contribute independently to cognitive decline. In a recent longitudinal study on 224 elderly subjects, Maillard and colleagues showed that an increase in free water was not only associated with reduced performances in executive function assessments but was the strongest predictor of cognitive decline. Taken together, these results support the idea that beyond the elimination of CSF contamination, free water could provide additional structural information that could constitute a physiological index reflecting brain changes [37,80,84]. However, the underlying microcellular events accounting for the observed free water content are far from being fully understood; its increase could be related to different pathophysiological processes such as a microvascular degeneration [38,47,102], a reduction in myelin content [98,99] or a modulation in the permeability of the blood-brain barrier [47].
White matter hyperintensities burden effect
The present study showed that when adjusting for WMH volumes, the associations between MDt and free water content and verbal fluency performances at 15 and 30 seconds remained significant, suggesting that both could contribute independently to cognitive impairment in aging. In accordance with these results, recent tractography studies reported that cingulum tracts crossing WMH exhibited significant changes in diffusion measures [54], and suggested that these modifications could extend beyond the WMH lesions [51,55,103]. Previous DTI studies also reported that WMH were associated with higher diffusion measures in the normal-appearing white matter [51,53,56,103–105]. Taken together, these results suggest that small focal WMH may lead to both local and distant effects that are large enough to impact white matter diffusion properties of the cingulum tract.
Some methodological limitations should be taken into account when interpreting our findings. First, this study was based on a moderate sample size of healthy elderly participants. However, our population exhibited sufficient inter-individual variability in structural measures and cognitive performances to detect associations between these parameters. Second, the cingulum was analyzed as a single entity despite the fact that it consists of a complex structure containing not only long association fibers, but also short tracts connecting adjacent cortices [60,106,107]. Finally, the current study was based on diffusion MRI data that was acquired with a single b-value. The algorithm used to fit the free water imaging model involved spatial regularization of data which decreases intra-group variability [21], and may hide subtle spatial features. In addition, this method is based on a bimodal model, considering isotropic versus non-isotropic diffusion to split tissue on respectively extra-cellular and intra-cellular compartments. However, part of extra-cellular compartment could be non-isotropic [108]. Even though comparable results using single- and multi-shell acquisitions have been previously reported [109], advanced acquisitions that include multiple b-values could further increase the accuracy of the free water model [84,109].
To conclude, we reported that FW-correction increases the ability to detect associations between tissue diffusion measures of the cingulum and changes in verbal fluency in elderly individuals. Moreover, free water content per se appears to be a relevant parameter to describe age-related modifications of the white matter and its association with executive functioning. In addition, we reported that free water content an index of interstitial water, and corrected mean diffusivity, an index of tissue changes both contribute to cognitive decline. These observations suggest the importance of considering the free water compartment for DTI measures in aging.
Supporting information
Yellow represents the white matter hyperintensity lesion mask displayed on the corresponding T1-weighted image. In A, the peak directions extracted from the fODF are preserved and coherent under the WMH. In B, the lesions do not impact the reconstruction of the Corpus Callosum.
(TIF)
(A) In red, tracks that stop in WMH lesion instead of grey matter and should not. (B) In blue, tracks that cross the WMH mask and reach grey matter regions. WMH mask is represented in yellow, both displayed on the corresponding T1-weighted image.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
In the interest of participant confidentiality and in keeping with data sharing guidelines imposed by the National Commission on Informatics and Liberty (CNIL), the data from 3C cohort used in this study are available upon request. Interested researchers may contact e3c.coordinatingcenter@gmail.com for access to 3C data.
Funding Statement
This research was funded by the FONDATION VAINCRE ALZHEIMER (#14733, www.vaincrealzheimer.org). This study was considered an emerging project and therefore partly funded as part of the laboratory of Excellence TRAIL ANR-10-LABX-57 (trail.labex.u-bordeaux.fr). The Three-City study is conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale (INSERM, www.inserm.fr), the University Bordeaux 2 Victor Segalen (www.u-bordeaux.fr/) and Sanofi-Aventis (www.sanofi.fr/), who funded the collection of initial data for the 3C cohort (from 1999 to 2005). The Fondation pour la Recherche Médicale funded the planning and initiation of the study (www.frm.org/). The Three-City study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés (www.ameli.fr/), Direction Générale de la Santé MGEN (solidarites-sante.gouv.fr/), Institut de la Longévité (www.ilvv.fr), Conseils Régionaux d’Aquitaine et Bourgogne, Fondation de France (www.fondationdefrance.org), Ministry of Research-INSERM Programme “Cohortes et collections de données biologiques”, Agence Nationale de la Recherche ANR PNRA 2006 and LongVie 2007 (www.anr.fr), the "Fondation Plan Alzheimer" (FCS 2009-2012) and the Caisse Nationale de Solidarité pour l'Autonomie (CNSA, www.cnsa.fr). Part of this research was also supported by the Fonds de recherche du Québec – Nature et technologies (FRQNT, www.frqnt.gouv.qc.ca), the NSERC Discovery grant (www.nserc-crsng.gc.ca), the Université de Sherbrooke institutional chair in neuroinformatics from Pr Descoteaux (www.usherbrooke.ca) and the Mitacs Accelerate program (www.mitacs.ca). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Damoiseaux JS. Effects of aging on functional and structural brain connectivity. Neuroimage. 15 October 2017;160:32–40. 10.1016/j.neuroimage.2017.01.077 [DOI] [PubMed] [Google Scholar]
- 2.Ikram MA, Vrooman HA, Vernooij MW, van der Lijn F, Hofman A, van der Lugt A, et al. Brain tissue volumes in the general elderly population: The Rotterdam Scan Study. Neurobiology of Aging. 1 juin 2008;29(6):882–90. 10.1016/j.neurobiolaging.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Wen W, Jiang J, Crawford JD, Reppermund S, Levitan C, et al. Age-associated differences on structural brain MRI in nondemented individuals from 71 to 103 years. Neurobiology of Aging. 1 avr 2016;40:86–97. 10.1016/j.neurobiolaging.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, et al. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci. 12 November 2014;34(46):15425–36. 10.1523/JNEUROSCI.0203-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. mai 2009;66(5):545–53. [DOI] [PubMed] [Google Scholar]
- 6.Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease. 1 mars 2012;1822(3):386–400. 10.1016/j.bbadis.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Groot M, Ikram MA, Akoudad S, Krestin GP, Hofman A, van der Lugt A, et al. Tract-specific white matter degeneration in aging: the Rotterdam Study. Alzheimers Dement. mars 2015;11(3):321–30. 10.1016/j.jalz.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 8.Vinke EJ, de Groot M, Venkatraghavan V, Klein S, Niessen WJ, Ikram MA, et al. Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiology of Aging. 1 November 2018;71:32–40. 10.1016/j.neurobiolaging.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 9.Arvanitakis Z, Fleischman DA, Arfanakis K, Leurgans SE, Barnes LL, Bennett DA. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct Funct. 2016;221(4):2135–46. 10.1007/s00429-015-1034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW, et al. White matter in aging and cognition: a cross-sectional study of microstructure in adults aged eighteen to eighty-three. Dev Neuropsychol. 2010;35(3):257–77. 10.1080/87565641003696775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borghesani PR, Madhyastha TM, Aylward EH, Reiter MA, Swarny BR, Schaie KW, et al. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia. juill 2013;51(8):1435–44. 10.1016/j.neuropsychologia.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiseman SJ, Booth T, Ritchie SJ, Cox SR, Muñoz Maniega S, Valdés Hernández MDC, et al. Cognitive abilities, brain white matter hyperintensity volume, and structural network connectivity in older age. Hum Brain Mapp. 2018;39(2):622–32. 10.1002/hbm.23857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Tang Z, Sun Y, Tian J, Liu Z, Han Y. White matter degeneration in subjective cognitive decline: a diffusion tensor imaging study. Oncotarget. 15 juin 2016;7(34):54405–14. 10.18632/oncotarget.10091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH. Age-Related Differences in Multiple Measures of White Matter Integrity: A Diffusion Tensor Imaging Study of Healthy Aging. Hum Brain Mapp. mars 2010;31(3):378–90. 10.1002/hbm.20872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bender AR, Prindle JJ, Brandmaier AM, Raz N. White matter and memory in healthy adults: Coupled changes over two years. NeuroImage. 1 mai 2016;131:193–204. 10.1016/j.neuroimage.2015.10.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bender AR, Völkle MC, Raz N. Differential aging of cerebral white matter in middle-aged and older adults: A seven-year follow-up. NeuroImage. 15 janv 2016;125:74–83. 10.1016/j.neuroimage.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li S-C, Lindenberger U, et al. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. NeuroImage. 1 févr 2010;49(3):2104–12. 10.1016/j.neuroimage.2009.09.041 [DOI] [PubMed] [Google Scholar]
- 18.Hirsiger S, Koppelmans V, Mérillat S, Liem F, Erdeniz B, Seidler RD, et al. Structural and functional connectivity in healthy aging: Associations for cognition and motor behavior. Hum Brain Mapp. 1 mars 2016;37(3):855–67. 10.1002/hbm.23067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arfanakis K, Wilson RS, Barth CM, Capuano AW, Vasireddi A, Zhang S, et al. Cognitive activity, cognitive function, and brain diffusion characteristics in old age. Brain Imaging and Behavior. 1 juin 2016;10(2):455–63. 10.1007/s11682-015-9405-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marques PCG, Soares JMM, Magalhães RJ da S, Santos NC, Sousa NJC. Macro- and micro-structural white matter differences correlate with cognitive performance in healthy aging. Brain Imaging Behav. mars 2016;10(1):168–81. 10.1007/s11682-015-9378-4 [DOI] [PubMed] [Google Scholar]
- 21.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. September 2009;62(3):717–30. 10.1002/mrm.22055 [DOI] [PubMed] [Google Scholar]
- 22.Jbabdi S, Johansen-Berg H. Tractography: where do we go from here? Brain Connect. 2011;1(3):169–83. 10.1089/brain.2011.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurki TJI, Heiskanen LAA. Diffusion parameters of the core of cingulum are associated with age-related ventricular enlargement: a diffusion tensor tractography study. Neuroradiology. 1 October 2018;60(10):1013–8. 10.1007/s00234-018-2068-3 [DOI] [PubMed] [Google Scholar]
- 24.Salminen LE, Conturo TE, Bolzenius JD, Cabeen RP, Akbudak E, Paul RH. REDUCING CSF PARTIAL VOLUME EFFECTS TO ENHANCE DIFFUSION TENSOR IMAGING METRICS OF BRAIN MICROSTRUCTURE. Technol Innov. avr 2016;18(1):5–20. 10.21300/18.1.2016.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos SB, Jones DK, Viergever MA, Leemans A. Partial volume effect as a hidden covariate in DTI analyses. NeuroImage. 15 avr 2011;55(4):1566–76. 10.1016/j.neuroimage.2011.01.048 [DOI] [PubMed] [Google Scholar]
- 26.Metzler-Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O’Sullivan MJ. Cingulum Microstructure Predicts Cognitive Control in Older Age and Mild Cognitive Impairment. J Neurosci. 5 déc 2012;32(49):17612–9. 10.1523/JNEUROSCI.3299-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine. 2003;49(5):953–61. 10.1002/mrm.10452 [DOI] [PubMed] [Google Scholar]
- 28.Concha L, Gross DW, Beaulieu C. Diffusion Tensor Tractography of the Limbic System. American Journal of Neuroradiology. 1 October 2005;26(9):2267–74. [PMC free article] [PubMed] [Google Scholar]
- 29.Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. août 2010;23(7):803–20. 10.1002/nbm.1543 [DOI] [PubMed] [Google Scholar]
- 30.Raj D, Yin Z, Breur M, Doorduin J, Holtman IR, Olah M, et al. Increased White Matter Inflammation in Aging- and Alzheimer’s Disease Brain. Front Mol Neurosci [Internet]. 2017. [cité 8 janv 2019];10. Disponible sur: https://www.frontiersin.org/articles/10.3389/fnmol.2017.00206/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syková E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. oct 2008;88(4):1277–340. 10.1152/physrev.00027.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sepehrband F, Cabeen RP, Choupan J, Barisano G, Law M, Toga AW, et al. Perivascular space fluid contributes to diffusion tensor imaging changes in white matter. Neuroimage. 15 2019;197:243–54. 10.1016/j.neuroimage.2019.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albi A, Pasternak O, Minati L, Marizzoni M, Bartrés-Faz D, Bargalló N, et al. Free water elimination improves test-retest reproducibility of diffusion tensor imaging indices in the brain: A longitudinal multisite study of healthy elderly subjects. Hum Brain Mapp. 2017;38(1):12–26. 10.1002/hbm.23350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasternak O, Westin C-F, Bouix S, Seidman LJ, Goldstein JM, Woo T-UW, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 28 November 2012;32(48):17365–72. 10.1523/JNEUROSCI.2904-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergamino M, Kuplicki R, Victor TA, Cha Y-H, Paulus MP. Comparison of two different analysis approaches for DTI free-water corrected and uncorrected maps in the study of white matter microstructural integrity in individuals with depression. Hum Brain Mapp. 2017;38(9):4690–702. 10.1002/hbm.23694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumont M, Roy M, Jodoin P-M, Morency FC, Houde J-C, Xie Z, et al. Free water in white matter differentiates MCI and AD from control subjects. bioRxiv. 31 janv 2019;537092 10.3389/fnagi.2019.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoy AR, Ly M, Carlsson CM, Okonkwo OC, Zetterberg H, Blennow K, et al. Microstructural white matter alterations in preclinical Alzheimer’s disease detected using free water elimination diffusion tensor imaging. PLoS ONE. 2017;12(3):e0173982 10.1371/journal.pone.0173982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji F, Pasternak O, Liu S, Loke YM, Choo BL, Hilal S, et al. Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in Alzheimer’s disease with and without cerebrovascular disease. Alzheimers Res Ther. 17 août 2017;9(1):63 10.1186/s13195-017-0292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ofori E, Krismer F, Burciu RG, Pasternak O, McCracken JL, Lewis MM, et al. Free water improves detection of changes in the substantia nigra in parkinsonism: A multisite study. Mov Disord. oct 2017;32(10):1457–64. 10.1002/mds.27100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanner JJ, Amin M, Hardcastle C, Parvataneni H, Vaillancourt DE, Mareci TH, et al. Better Brain and Cognition Prior to Surgery Is Associated With Elevated Postoperative Brain Extracellular Free-Water in Older Adults. Front Aging Neurosci [Internet]. 2019. [cité 29 mai 2019];11. Disponible sur: https://www.frontiersin.org/articles/10.3389/fnagi.2019.00117/full?utm_source=F-AAE&utm_medium=EMLF&utm_campaign=MRK_999212_55_Neuros_20190528_arts_A 10.3389/fnagi.2019.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aboitiz F, Rodriguez E, Olivares R, Zaidel E. Age-related changes in fibre composition of the human corpus callosum. Neuroreport. 1 juill 1996;7(11):1761–4. 10.1097/00001756-199607290-00013 [DOI] [PubMed] [Google Scholar]
- 42.Barkhof F, Fox NC, Bastos-Leite AJ, Scheltens P. Normal Ageing. In: Barkhof F, Fox NC, Bastos-Leite AJ, Scheltens P, éditeurs. Neuroimaging in Dementia [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. [cité 5 oct 2019]. p. 43–57. Disponible sur: 10.1007/978-3-642-00818-4_4 [DOI] [Google Scholar]
- 43.Meier-Ruge W, Ulrich J, Brühlmann M, Meier E. Age-related white matter atrophy in the human brain. Ann N Y Acad Sci. 26 déc 1992;673:260–9. 10.1111/j.1749-6632.1992.tb27462.x [DOI] [PubMed] [Google Scholar]
- 44.Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJG. Age-Induced White Matter Changes in the Human Brain: A Stereological Investigation. Neurobiology of Aging. 1 November 1997;18(6):609–15. 10.1016/s0197-4580(97)00155-3 [DOI] [PubMed] [Google Scholar]
- 45.Alber J, Alladi S, Bae H-J, Barton DA, Beckett LA, Bell JM, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement (N Y). 2019;5:107–17. 10.1016/j.trci.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duering M, Finsterwalder S, Baykara E, Tuladhar AM, Gesierich B, Konieczny MJ, et al. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers Dement. juin 2018;14(6):764–74. 10.1016/j.jalz.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maillard P, Mitchell GF, Himali JJ, Beiser A, Fletcher E, Tsao CW, et al. Aortic Stiffness, Increased White Matter Free Water, and Altered Microstructural Integrity: A Continuum of Injury. Stroke. 2017;48(6):1567–73. 10.1161/STROKEAHA.116.016321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maillard P, Fletcher E, Singh B, Martinez O, Johnson DK, Olichney JM, et al. Cerebral white matter free water: A sensitive biomarker of cognition and function. Neurology. 7 mai 2019;92(19):e2221–31. 10.1212/WNL.0000000000007449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habes M, Erus G, Toledo JB, Zhang T, Bryan N, Launer LJ, et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain. avr 2016;139(4):1164–79. 10.1093/brain/aww008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leeuw F-E de, Groot JC de, Achten E, Oudkerk M, Ramos LMP, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. Journal of Neurology, Neurosurgery & Psychiatry. 1 janv 2001;70(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maillard P, Fletcher E, Lockhart SN, Roach AE, Reed B, Mungas D, et al. White Matter Hyperintensities and their Penumbra Lie Along a Continuum of Injury In The Aging Brain. Stroke. juin 2014;45(6):1721–6. 10.1161/STROKEAHA.113.004084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maniega SM, Valdés Hernández MC, Clayden JD, Royle NA, Murray C, Morris Z, et al. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol Aging. févr 2015;36(2):909–18. 10.1016/j.neurobiolaging.2014.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelletier A, Periot O, Dilharreguy B, Hiba B, Bordessoules M, Chanraud S, et al. Age-Related Modifications of Diffusion Tensor Imaging Parameters and White Matter Hyperintensities as Inter-Dependent Processes. Front Aging Neurosci. 2015;7:255 10.3389/fnagi.2015.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reginold W, Itorralba J, Luedke AC, Fernandez-Ruiz J, Reginold J, Islam O, et al. Tractography at 3T MRI of Corpus Callosum Tracts Crossing White Matter Hyperintensities. American Journal of Neuroradiology. 1 September 2016;37(9):1617–22. 10.3174/ajnr.A4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reginold W, Sam K, Poublanc J, Fisher J, Crawley A, Mikulis DJ. Impact of white matter hyperintensities on surrounding white matter tracts. Neuroradiology. September 2018;60(9):933–44. 10.1007/s00234-018-2053-x [DOI] [PubMed] [Google Scholar]
- 56.Svärd D, Nilsson M, Lampinen B, Lätt J, Sundgren PC, Stomrud E, et al. The effect of white matter hyperintensities on statistical analysis of diffusion tensor imaging in cognitively healthy elderly and prodromal Alzheimer’s disease. PLOS ONE. 21 September 2017;12(9):e0185239 10.1371/journal.pone.0185239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizvi B, Lao PJ, Colón J, Hale C, Igwe KC, Narkhede A, et al. Tract-defined regional white matter hyperintensities and memory. NeuroImage: Clinical. 1 janv 2020;25:102143 10.1016/j.nicl.2019.102143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones DK, Christiansen KF, Chapman RJ, Aggleton JP. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: Implications for neuropsychological investigations. Neuropsychologia. janv 2013;51(1):67 10.1016/j.neuropsychologia.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Catheline G, Periot O, Amirault M, Braun M, Dartigues J-F, Auriacombe S, et al. Distinctive alterations of the cingulum bundle during aging and Alzheimer’s disease. Neurobiology of Aging. 1 September 2010;31(9):1582–92. 10.1016/j.neurobiolaging.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 60.Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev. 10 mai 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ezzati A, Katz MJ, Lipton ML, Zimmerman ME, Lipton RB. Hippocampal volume and cingulum bundle fractional anisotropy are independently associated with verbal memory in older adults. Brain Imaging Behav. sept 2016;10(3):652–9. 10.1007/s11682-015-9452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jang SH, Kwon YH, Lee MY, Kim J-R, Seo JP. Aging of the cingulum in the human brain: Preliminary study of a diffusion tensor imaging study. Neurosci Lett. 1 janv 2016;610:213–7. 10.1016/j.neulet.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 63.Mårtensson J, Lätt J, Åhs F, Fredrikson M, Söderlund H, Schiöth HB, et al. Diffusion tensor imaging and tractography of the white matter in normal aging: The rate-of-change differs between segments within tracts. Magn Reson Imaging. 2018;45:113–9. 10.1016/j.mri.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 64.Gunbey HP, Ercan K, Fındıkoglu AS, Bulut HT, Karaoglanoglu M, Arslan H. The Limbic Degradation of Aging Brain: A Quantitative Analysis with Diffusion Tensor Imaging [Internet]. The Scientific World Journal. 2014. [cité 7 nov 2018]. Disponible sur: https://www.hindawi.com/journals/tswj/2014/196513/ 10.1155/2014/196513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Age-related degradation in the central nervous system: assessment with diffusion-tensor imaging and quantitative fiber tracking. Radiology. avr 2008;247(1):179–88. 10.1148/radiol.2471070707 [DOI] [PubMed] [Google Scholar]
- 66.Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 5 juill 2011;77(1):26–34. 10.1212/WNL.0b013e31822313dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seiler S, Fletcher E, Hassan-Ali K, Weinstein M, Beiser A, Himali JJ, et al. Cerebral tract integrity relates to white matter hyperintensities, cortex volume, and cognition. Neurobiology of Aging. 1 déc 2018;72:14–22. 10.1016/j.neurobiolaging.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sexton CE, Mackay CE, Lonie JA, Bastin ME, Terrière E, O’Carroll RE, et al. MRI correlates of episodic memory in Alzheimer’s disease, mild cognitive impairment, and healthy aging. Psychiatry Res. 30 October 2010;184(1):57–62. 10.1016/j.pscychresns.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 69.3C Study Group. Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. déc 2003;22(6):316–25. 10.1159/000072920 [DOI] [PubMed] [Google Scholar]
- 70.Isaacs B, Kennie AT. The Set test as an aid to the detection of dementia in old people. Br J Psychiatry. October 1973;123(575):467–70. 10.1192/bjp.123.4.467 [DOI] [PubMed] [Google Scholar]
- 71.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1 juin 1977;1(3):385–401. [Google Scholar]
- 72.Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 1 janv 2007;34(1):144–55. 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. November 2003;50(5):1077–88. 10.1002/mrm.10609 [DOI] [PubMed] [Google Scholar]
- 74.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. nov 2002;17(3):143–55. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Descoteaux M, Wiest-Daesslé N, Prima S, Barillot C, Deriche R. Impact of Rician adapted Non-Local Means filtering on HARDI. Med Image Comput Comput Assist Interv. 2008;11(Pt 2):122–30. 10.1007/978-3-540-85990-1_15 [DOI] [PubMed] [Google Scholar]
- 76.Descoteaux M, Angelino E, Fitzgibbons S, Deriche R. Regularized, fast, and robust analytical Q-ball imaging. Magn Reson Med. September 2007;58(3):497–510. 10.1002/mrm.21277 [DOI] [PubMed] [Google Scholar]
- 77.Tournier J-D, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage. 1 mai 2007;35(4):1459–72. 10.1016/j.neuroimage.2007.02.016 [DOI] [PubMed] [Google Scholar]
- 78.Theaud G, Houde J-C, Boré A, Rheault F, Morency F, Descoteaux M. TractoFlow: A robust, efficient and reproducible diffusion MRI pipeline leveraging Nextflow & Singularity. bioRxiv. 9 mai 2019;631952 10.1016/j.neuroimage.2020.116889 [DOI] [PubMed] [Google Scholar]
- 79.Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage. 15 févr 2012;59(4):3774–83. 10.1016/j.neuroimage.2011.11.032 [DOI] [PubMed] [Google Scholar]
- 80.Chad JA, Pasternak O, Salat DH, Chen JJ. Re-examining age-related differences in white matter microstructure with free-water corrected diffusion tensor imaging. Neurobiol Aging. nov 2018;71:161–70. 10.1016/j.neurobiolaging.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garyfallidis E, Côté M-A, Rheault F, Sidhu J, Hau J, Petit L, et al. Recognition of white matter bundles using local and global streamline-based registration and clustering. NeuroImage. 15 avr 2018;170:283–95. 10.1016/j.neuroimage.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 82.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 83.Bergamino M, Pasternak O, Farmer M, Shenton ME, Paul Hamilton J. Applying a free-water correction to diffusion imaging data uncovers stress-related neural pathology in depression. NeuroImage: Clinical. 1 janv 2016;10:336–42. 10.1016/j.nicl.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoy AR, Kecskemeti SR, Alexander AL. Free water elimination diffusion tractography: A comparison with conventional and fluid-attenuated inversion recovery, diffusion tensor imaging acquisitions. Journal of Magnetic Resonance Imaging. 2015;42(6):1572–81. 10.1002/jmri.24925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bennett IJ, Madden DJ. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience. 12 September 2014;276:187–205. 10.1016/j.neuroscience.2013.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bennett IJ, Greenia DE, Maillard P, Sajjadi SA, DeCarli C, Corrada MM, et al. Age-related white matter integrity differences in oldest-old without dementia. Neurobiology of Aging. 1 août 2017;56:108–14. 10.1016/j.neurobiolaging.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lockhart SN, DeCarli C. Structural imaging measures of brain aging. Neuropsychol Rev. sept 2014;24(3):271–89. 10.1007/s11065-014-9268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bettcher BM, Mungas D, Patel N, Elofson J, Dutt S, Wynn M, et al. Neuroanatomical substrates of executive functions: Beyond prefrontal structures. Neuropsychologia. 1 mai 2016;85:100–9. 10.1016/j.neuropsychologia.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Charlton RA, Barrick TR, Lawes INC, Markus HS, Morris RG. White matter pathways associated with working memory in normal aging. Cortex. 1 avr 2010;46(4):474–89. 10.1016/j.cortex.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 90.Chiang H-L, Chen Y-J, Shang C-Y, Tseng W-YI, Gau SS-F. Different neural substrates for executive functions in youths with ADHD: a diffusion spectrum imaging tractography study. Psychological Medicine. avr 2016;46(6):1225–38. 10.1017/S0033291715002767 [DOI] [PubMed] [Google Scholar]
- 91.Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front Neurosci [Internet]. 2013. [cité 7 nov 2018];7. Disponible sur: https://www.frontiersin.org/articles/10.3389/fnins.2013.00032/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 1 November 2003;20(3):1714–22. 10.1016/j.neuroimage.2003.07.005 [DOI] [PubMed] [Google Scholar]
- 93.Sun S-W, Neil JJ, Liang H-F, He YY, Schmidt RE, Hsu CY, et al. Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infarcted brain. Magn Reson Med. juin 2005;53(6):1447–51. 10.1002/mrm.20488 [DOI] [PubMed] [Google Scholar]
- 94.Klawiter EC, Schmidt RE, Trinkaus K, Liang H-F, Budde MD, Naismith RT, et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 15 avr 2011;55(4):1454–60. 10.1016/j.neuroimage.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmierer K, Wheeler-Kingshott CAM, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, et al. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med. févr 2008;59(2):268–77. 10.1002/mrm.21487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, et al. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. NeuroImage. 15 November 2008;43(3):470–7. 10.1016/j.neuroimage.2008.07.052 [DOI] [PubMed] [Google Scholar]
- 97.Lyall AE, Pasternak O, Robinson DG, Newell D, Trampush JW, Gallego JA, et al. Greater Extracellular Free Water in First-Episode Psychosis Predicts Better Neurocognitive Functioning. Mol Psychiatry. mars 2018;23(3):701–7. 10.1038/mp.2017.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Papadaki E, Kavroulakis E, Kalaitzakis G, Karageorgou D, Makrakis D, Maris TG, et al. Age-related deep white matter changes in myelin and water content: A T2 relaxometry study. Journal of Magnetic Resonance Imaging [Internet]. [cité 5 juin 2019];0(0). Disponible sur: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmri.26707 [DOI] [PubMed] [Google Scholar]
- 99.Oestreich LKL, Pasternak O, Shenton ME, Kubicki M, Gong X, McCarthy-Jones S, et al. Abnormal white matter microstructure and increased extracellular free-water in the cingulum bundle associated with delusions in chronic schizophrenia. NeuroImage: Clinical. 1 févr 2016;12:405–14. 10.1016/j.nicl.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang L, Goldstein FC, Levey AI, Lah JJ, Meltzer CC, Holder CA, et al. White Matter Hyperintensities and Changes in White Matter Integrity in Patients with Alzheimer’s Disease. Neuroradiology. mai 2011;53(5):373–81. 10.1007/s00234-010-0806-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ji F, Pasternak O, Ng KK, Chong JSX, Liu S, Zhang L, et al. White matter microstructural abnormalities and default network degeneration are associated with early memory deficit in Alzheimer’s disease continuum. Sci Rep. 18 mars 2019;9(1):4749 10.1038/s41598-019-41363-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rydhög AS, Szczepankiewicz F, Wirestam R, Ahlgren A, Westin C-F, Knutsson L, et al. Separating blood and water: Perfusion and free water elimination from diffusion MRI in the human brain. Neuroimage. 01 2017;156:423–34. 10.1016/j.neuroimage.2017.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Promjunyakul N-O, Lahna DL, Kaye JA, Dodge HH, Erten-Lyons D, Rooney WD, et al. Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: A multi-modal magnetic resonance imaging study. J Cereb Blood Flow Metab. 2016;36(9):1528–36. 10.1177/0271678X16651268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lange RT, Shewchuk JR, Heran MKS, Rauscher A, Jarrett M, Brubacher JR, et al. To exclude or not to exclude: further examination of the influence of white matter hyperintensities in diffusion tensor imaging research. J Neurotrauma. 15 janv 2014;31(2):198–205. 10.1089/neu.2013.2866 [DOI] [PubMed] [Google Scholar]
- 105.Leritz EC, Shepel J, Williams VJ, Lipsitz LA, McGlinchey RE, Milberg WP, et al. Associations between T1 white matter lesion volume and regional white matter microstructure in aging. Hum Brain Mapp. mars 2014;35(3):1085–100. 10.1002/hbm.22236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. sept 2008;44(8):1105–32. 10.1016/j.cortex.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 107.Lawes INC, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, et al. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage. 1 janv 2008;39(1):62–79. 10.1016/j.neuroimage.2007.06.041 [DOI] [PubMed] [Google Scholar]
- 108.Lei Y, Han H, Yuan F, Javeed A, Zhao Y. The brain interstitial system: Anatomy, modeling, in vivo measurement, and applications. Progress in Neurobiology. 1 October 2017;157:230–46. 10.1016/j.pneurobio.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 109.Pasternak O, Shenton ME, Westin C-F. Estimation of extracellular volume from regularized multi-shell diffusion MRI. Med Image Comput Comput Assist Interv. 2012;15(Pt 2):305–12. 10.1007/978-3-642-33418-4_38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Yellow represents the white matter hyperintensity lesion mask displayed on the corresponding T1-weighted image. In A, the peak directions extracted from the fODF are preserved and coherent under the WMH. In B, the lesions do not impact the reconstruction of the Corpus Callosum.
(TIF)
(A) In red, tracks that stop in WMH lesion instead of grey matter and should not. (B) In blue, tracks that cross the WMH mask and reach grey matter regions. WMH mask is represented in yellow, both displayed on the corresponding T1-weighted image.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
In the interest of participant confidentiality and in keeping with data sharing guidelines imposed by the National Commission on Informatics and Liberty (CNIL), the data from 3C cohort used in this study are available upon request. Interested researchers may contact e3c.coordinatingcenter@gmail.com for access to 3C data.