Summary
The interiors of plants are colonized by diverse microorganisms that are referred to as endophytes. Endophytes have received much attention over the past few decades, yet many questions remain unanswered regarding patterns in their biodiversity at local to global scales. To characterize research effort to date, we synthesized results from ~600 published studies. Our survey revealed a global research interest and highlighted several gaps in knowledge. For instance, of the 17 biomes encompassed by our survey, 7 were understudied and together composed only 7% of the studies that we considered. We found that fungal endophyte diversity has been characterized in at least one host from 30% of embryophyte families, while bacterial endophytes have been surveyed in hosts from only 10.5% of families. We complimented our survey with a vote counting procedure to determine endophyte richness patterns among plant tissue types. We found that variation in endophyte assemblages in above-ground tissues varied with host growth habit. Stems were the richest tissue in woody plants, whereas roots were the richest tissue in graminoids. For forbs, we found no consistent differences in relative tissue richness among studies. We propose future directions to fill the gaps in knowledge we uncovered and inspire further research.
Introduction
In 1887, Galippe reported that soil-derived microbes could reside within the above-ground tissues of healthy plants. At the time, this work was underappreciated, perhaps because of the long-prevailing attitude that microbial assemblages solely comprised deleterious pathogens (Compant et al., 2012), this notwithstanding the work of Frank and others demonstrating the mutualistic nature of the mycorrhizal-host relationship (Frank, 1885, 2005; Trappe, 2005). Nevertheless, Galippe’s observations set the stage for an exploration of the non-pathogenic portion of the plant microbiome that took place from the late 1800s into the mid-1900s (Laurent, 1889; Janse, 1897; Rayner, 1915; Auret, 1930; Hardoim et al., 2015). During those decades, knowledge began to accumulate regarding the diversity, prevalence and ecological roles of the so-called ‘endophytes’ (Box 1; Campbell, 1908; Hyde and Soytong, 2008), with much early work focused on the fungi living within grasses (e.g. Sampson, 1937; Neill, 1940). Seminal research in the 1970s and 1980s led to widespread acknowledgement of the ubiquitous nature of non-pathogenic fungi and bacteria in plant tissues, particularly within leaves (Carroll and Carroll, 1978; Carroll, 1988; Petrini, 1991). These studies have inspired an evergrowing interest from microbial ecologists (Fig. 1), yet answers to many basic questions regarding the natural history, biogeography, ecology and evolution of endophytes remain elusive.
Box 1. What, exactly, is an endophyte?
The term ‘endophyte’ is believed to have originated with de Bary (1866), who so dubbed pathogenic, plant-inhabiting microbes because of their habitat (also see Link, 1809). Since then, the term endophyte has been expanded to invoke both a habitat and a non-pathogenic lifestyle (at least in some hosts and life history stages) and encompasses fungal (Petrini, 1991; Rodriguez et al., 2009), bacterial (Ryan et al., 2008; Griffin and Carson, 2015) and archael taxa (Müller et al., 2015; Moissl-Eichinger et al., 2018). The term endophyte can be used sensu lato to refer to those taxa that live inside of plant tissues, either inside of or between host cells. However, in our experience, contemporary microbial ecologists most often use the term sensu stricto to refer to those taxa, which over some portion of their life history do not cause obvious harm to their hosts, such as inducing a hypersensitive response (Petrini, 1991; Wilson, 1995; Stone et al., 2000). The lack of precision in this definition is somewhat unsatisfying, but does hint at the complex life histories of many endophytic taxa (Rodriguez et al., 2009). Indeed, for perhaps the majority of endophytic taxa, individuals are horizontally transmitted among hosts and, consequently, may exist outside of the plant corpus for some time, for instance as spores or endospores, free living cells or colonies, or as epiphytic fruiting bodies on decaying tissue (Malloch and Blackwell, 1992; Rodriguez et al., 2009). The term endophyte is particularly strained by the mycorrhizal fungi, which possess a mycelium that grows externally to the host and also penetrates the root epidermis (Jumpponen, 2001; Schulz and Boyle, 2006). The categorization of these fungi as endophytes seems to be on an author-by-author basis (Schulz and Boyle, 2006). These examples illustrate how the term endophyte is useful for communication, but not biologically well delineated.
Fig. 1.
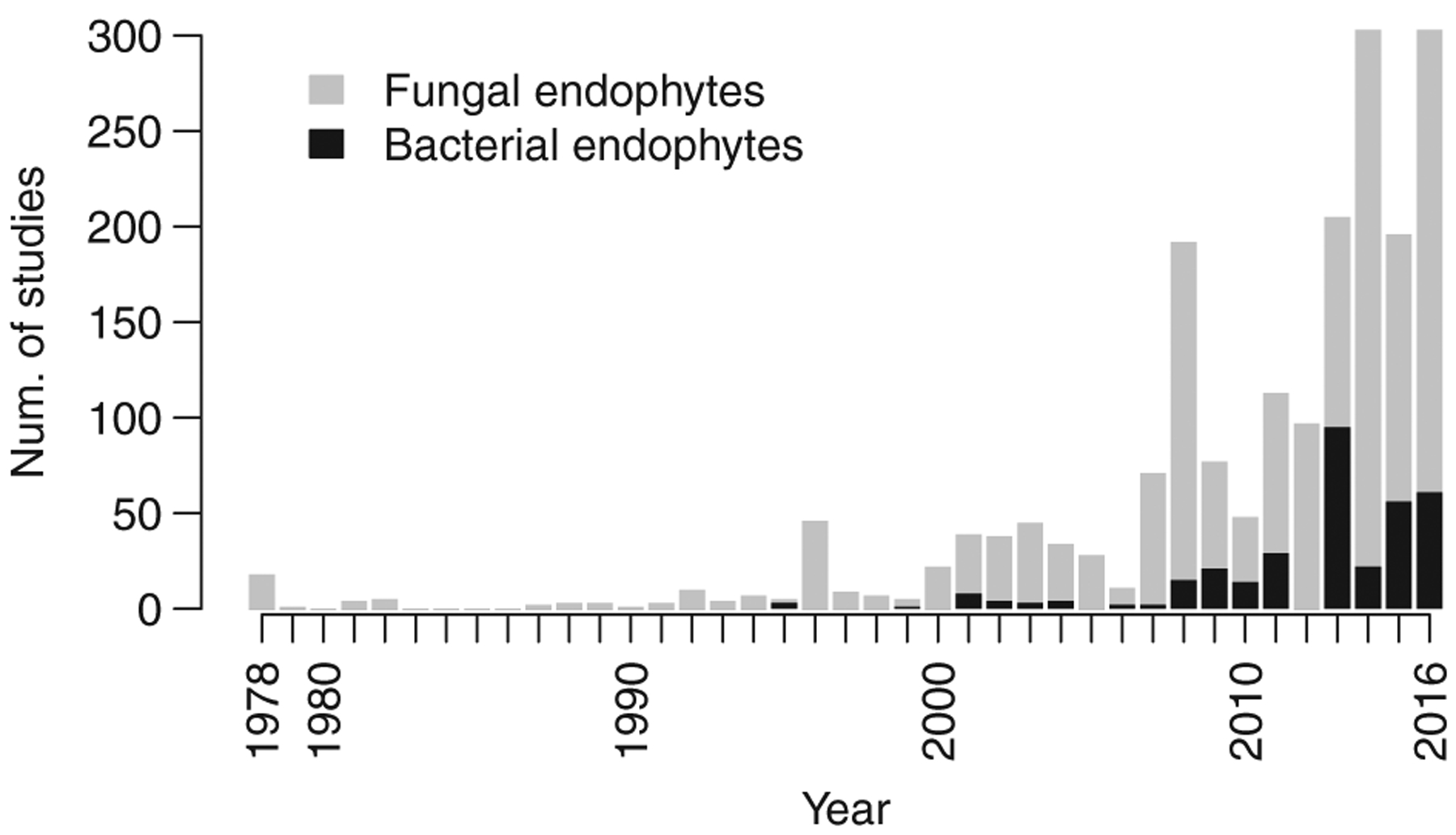
The number of studies characterizing endophyte biodiversity published each year since the late 1970s. Studies are parsed by taxonomy with fungal studies in grey and bacterial studies in black.
What is clear, however, is that fungal and bacterial endophytes are important - even critical - components of the world’s ecosystems. Endophytes can affect plant phenotype, including decreasing disease susceptibility (Arnold et al., 2003; Compant et al., 2005; Herre et al., 2007; Busby et al., 2016; Christian et al., 2017), increasing resistance to abiotic stressors (Redman et al., 2002; Márquez et al., 2007; Rodriguez et al., 2008), shaping phytochemical profiles (Kusari et al., 2012; Panaccione et al., 2014) and mediating plant functional trait expression (Friesen et al., 2011; Griffin et al., 2016). Recent work has demonstrated how these various effects of endophytes can influence whole ecosystem level processes (Clay and Holah, 1999; Kivlin et al., 2013; Griffin et al., 2017; Laforest-Lapointe et al., 2017b; Christian et al., 2019). Importantly, endophytes are often erroneously assumed to have predominantly mutualistic associations with their hosts. Reality is much more complex and the influence of endophyte taxa is highly context dependent (Carroll, 1988; Rodriguez et al., 2009), with interactions between hosts and endophytes ranging from mutualism through commensalism to latent or mild antagonism (Saikkonen et al., 1998; Schulz and Boyle, 2005; Hardoim et al., 2008).
Much of the focus on endophytes has been driven by the desire to harness these taxa to manipulate plant phenotype (e.g. increase growth, Doty, 2011) and prevent pathogen colonization of crops (Busby et al., 2017). Endophytes have also attracted attention from natural products chemists who survey the world’s organisms for useful compounds (Strobel and Daisy, 2003; Aly et al., 2010). This is motivated by the capacity of various endophytes to synthesize an impressive array of bioactive small molecules (Newman et al., 2003; Strobel et al., 2004; Verma et al., 2009). Indeed, a number of endophyte-synthesized compounds are of medicinal value (Strobel et al., 1996; Kharwar et al., 2011).
Both basic and applied research regarding endophytes have been hampered by a lack of knowledge regarding the distribution of endophyte biodiversity at any spatial scale - from global, interbiome scales to among the tissues of individual host plants. Similarly, almost nothing is known regarding how endophyte biodiversity maps onto the plant phylogeny. Characterizing such broad patterns in endophyte biodiversity is extremely logistically challenging because of the outlay of effort required for large culturing and sequencing projects (Arnold and Engelbrecht, 2007; Nilsson et al., 2019; Carini, 2019). Moreover, determining the causes of patterns in endophyte diversity is difficult because they result from the interplay of many forcings, including both contemporary and historical ecological drivers (i.e. niche determination, ecological drift, dispersal limitation) and, at longer timescales, evolutionary processes (i.e. divergence and extinction, Hanson et al., 2012; Wiens and Donoghue, 2004).
Further complicating matters is the disconnection between the spatial scale of sampling endophyte assemblages, as dictated by logistical constraints, and the size of the focal organisms. This problem was well illustrated by Remus-Emsermann and Schlechter (2018) who pointed out that the disparity in size between a single bacterium of 2 μm3 and a leaf mirrors the ratio of sizes between a person and a mid-sized country. Indeed, using traditional culturing and sequencing methodologies, we can only sample what are in effect whole regions of endophytes that may include multiple assemblages that never directly interact, that have been shaped by differing community assembly processes and that may even have divergent evolutionary histories. This complicates the study of endophyte biogeography and community ecology because the scale of sampling is so much larger than many covariates that may affect the membership of endophytes in a particular assemblage. For instance, microhabitat variation within leaves (such as proximity to upper or lower leaf surfaces, veins) may have effects on endophyte assemblages akin to those of shifting elevation across a mountainside on forest composition, and those forcings are unavailable for study when the unit of replication is an entire leaf or even a leaf section (Lodge et al., 1996; Herre et al., 2007; Vacher et al., 2016; Remus-Emsermann and Schlechter, 2018). Adding further complexity, some bacterial endophytes can live inside endophytic fungi (Shaffer et al., 2016); thus, for these bacteria, the habitat covariates most relevant for explaining interassemblage variation may be the traits of the host fungus, not the traits of the host plant.
Although we have much to learn regarding the distribution of endophyte biodiversity, patterns observed so far generally follow the predictions of community ecology theory and are similar to those observed for metazoan and plant assemblages (Nemergut et al., 2013; Christian et al., 2015). For instance, sampling of endophyte assemblages recapitulates the positive species-area relationship observed in so many natural systems - as one samples a larger area, one encounters more taxa (e.g. Suryanarayanan et al., 2002). A necessary correlate of this observation is that the similarity among endophyte assemblages declines with distance, which also has been demonstrated numerous times (e.g. Davis and Jonathan Shaw, 2008; Nemergut et al., 2013; Higgins et al., 2014; Vacher et al., 2016). Although its causes are multifarious and poorly understood (Martiny et al., 2006; Vellend, 2010), the existence of distance decay suggests that endophytic microbes are influenced not only by deterministic forcings, but also by what are typically regarded as neutral processes, such as dispersal limitation and ecological drift (Hubbell, 2001; MacArthur and Wilson, 2001; Nemergut et al., 2013). Also following what is known for most large, multicellular organisms, foliar fungal endophyte biodiversity seems to follow a latitudinal gradient, with a higher diversity at lower latitudes, as shown by Arnold and Lutzoni (2007). The reasons for this pattern remain unknown, but likely include both contemporary and historical drivers (Pianka, 1966; Mittelbach et al., 2007). Importantly, it is unclear if this pattern holds for non-fungal taxa and if non-foliar tissues harbour higher fungal endophyte richness at lower latitudes. Indeed, ectomycorrhizal fungi appear to be at their richest in temperate zones (Tedersoo et al., 2014), which suggests the possibility that other root-associated microbes may be the richest at intermediate latitudes as well.
Much of what is known regarding the patterns of endophyte biodiversity demonstrates the influence of contemporary ecological contingencies at either regional or local spatial scales (i.e. niche determinism). For instance, Zimmerman and Vitousek (2012) reported greater fungal endophyte richness at wetter, low elevation sites on a Hawaiian mountainside, and Bowman and Arnold (2018) found that Pinus ponderosa hosted more diverse foliar fungal endophyte communities at mid-to-high elevations compared with lower elevations in southwestern Arizona (also see Giauque and Hawkes, 2013; Glynou et al., 2016; Lau et al., 2013). Furthermore, it is clear that endophyte assemblages shift among coexisting host species although the effect of host on endophyte assemblage divergence can be quite modest in some cases (Redford et al., 2010; Vincent et al., 2016; Griffin et al., 2019). Although evidence reported to date suggests that many cultivable endophytes are host generalists (e.g. Arnold and Lutzoni, 2007; Suryanarayanan et al., 2018), specialist endophytes do exist, as demonstrated by the fidelity of vertically transmitted (seed-borne) Epichloë fungi to the members of Poaceae (Clay and Schardl, 2002; Rudgers et al., 2009) and of swainsonine-producing Alternaria fungi to certain Fabaceous taxa (Cook et al., 2014; Panaccione et al., 2014). However, the host range of the myriad endophytes that occur at low relative abundance is unknown (Arnold and Lutzoni, 2007) - an important gap in knowledge given that these taxa likely comprise the bulk of endophyte biodiversity (Arnold and Lutzoni, 2007; Lynch and Neufeld, 2015).
Even within an individual plant, niche determinism can shape endophyte assemblages as many studies have confirmed that endophyte assemblages vary among tissue types (e.g. the endophyte assemblages in roots often differ from those in leaves; Coleman-Derr et al., 2016; Haruna et al., 2018; Massoni et al., 2019), though general patterns in endophyte richness among tissue types have not been described.
Niche determinism is not the only force affecting endophyte biodiversity within a particular substrate, though it is likely the best studied. For instance, it is clear from research within non-endophytic taxa that microbes can be dispersal limited, and therefore the longstanding Baas Becking hypothesis for microbial biogeography, namely that ‘everything is everywhere, but the environment selects’ (Baas Becking, 1934) is too simplistic (Martiny et al., 2006; Vellend, 2010; Hanson et al., 2012; Nemergut et al., 2013). It is still unclear, however, how dispersal limitation and ecological drift (stochastic changes in community membership due to aggregated life history events) shape endophyte community assembly. Similarly, little is understood regarding the influence of historical factors, including divergence and extinction, on endophyte biogeography, though it seems likely that these factors will manifest in differences in endophyte assemblages across biomes and geographical regions, just as they do for other taxa (Mittelbach et al., 2007). How often these forces act at what are, to our sensibilities, small spatial scales, such as within a forest or even a single-long-lived tree, remains unknown.
A final challenge to the study of endophyte distribution is the likelihood that most patterns in biodiversity will be taxon specific, because taxa respond differently to ecological contingencies and are on varying evolutionary trajectories. For example, Coleman-Derr and colleagues (2016) suggest that prokaryote taxa are more influenced by plant tissue type than fungal taxa, which are affected more by host habitat and biogeography. At the order level, Jumpponen and colleagues (2017) suggested that Helotiales fungal root endophytes are most abundant in forested ecosystems and Pleosporales fungi are more common in grasslands. Studies such as these are very rare; little is known regarding the geographical or host ranges of endophytes at any level of the biological hierarchy - from phylum to subspecies.
Although daunting, the study of endophyte biogeography and community assembly will likely provide important benefits for both basic and applied research. An exemplar is provided by Higginbotham and colleagues (2013) who isolated over 3000 endophytic fungi from numerous tropical angiosperms and ferns and tested these cultures against common diseases, including malaria, Chagas disease and cancer. They report that 30% of the fungi showed strong activity against at least one of the focal diseases and that bioactivity against a specific target was non-randomly distributed across the fungal phylogeny. Intriguingly, they also reported a generally higher degree of bioactivity in taxa sourced from cloud forests compared with lowland tropical forests - thus providing a biogeographic road map for natural product discovery in tropical forests that demonstrates an important role of both biome and host phylogeny (also see Schulz et al., 2002).
The first step towards a working knowledge of endophyte distributions across spatial scales is the description of broad patterns in their biodiversity. To understand the scope of relevant research, we scoured the literature and extracted basic metadata from 596 studies characterizing endophyte assemblages. Our goal was to synthesize the metadata from representative studies, with the hopes of highlighting particular portions of the plant phylogeny and specific biomes that need further exploration and to determine how information could be shared among studies. Additionally, we paired our survey with a vote-counting procedure where we compared patterns of endophyte richness among tissue types. The synthesis process illustrated the challenges of pooling information among studies and, consequently, we offer specific guidelines for data sharing and research reproducibility moving forward. For our purposes in this article, we did not consider obligate pathogens, epiphytes or mycorrhizae; nor did we include a review of the large body of literature examining Rhizobia and their associations with legumes, as others have already done so (e.g. Peter et al., 1996; Willems, 2006).
Methods
We searched Google Scholar and Web of Science for the term ‘endophyte’ in conjunction with ‘fungal’, ‘bacterial’, ‘diversity’ or ‘community’. All publications in which the authors characterized endophyte assemblage biodiversity were collated. As we were primarily interested in studies characterizing endophyte biodiversity, we did not consider research involving manipulative experiments where no survey of microbial diversity was conducted. We also made the choice to omit studies that did not distinguish between epiphytes and endophytes through performing some form of surface sterilization. Searches were performed periodically from 2016 to 2018, and additional studies were added to our database as we became aware of them until the beginning of 2019. We apologize to those authors whose work we missed and to those who have published their work in non-English language journals, which typically did not appear in our searches and were inaccessible to us because of our linguistic backgrounds.
From each study, we collected information on host organism(s) studied, research location(s), tissue type(s) surveyed and various metadata describing the nature of the survey conducted - for instance, if the endophyte assemblage was characterized via sequencing or culturing, if spatial or temporal replication was employed, host and culture vouchers deposited, and data made available. We considered studies spatially replicated if they involved two or more sampling locations separated by ≥1 km. We chose this threshold because of work by Higgins and colleagues (2014) who reported rapid distance decay in endophyte assemblage similarity within tropical grasses within 1 km. It is likely that the strength of distance decay depends upon biome, host plant, endophyte taxon and other ecological conditions, thus determining what constitutes sufficient spatial replication is challenging and study dependent. We use a 1 km threshold here because most studies that were replicated at a smaller spatial scale were within a single field, forest or greenhouse and thus were likely exposed to similar endophyte inoculum, at least over longer timescales. If the study location was not explicitly provided, we extrapolated an estimate based on the city or country reported by the authors. We assigned studies to biomes following the nomenclature of Olson and colleagues (2001). Host plants collected from urban, agricultural or areas that were otherwise managed were classified as coming from ‘cultivated’ landscapes and counted independently from those studies that occurred in unmanaged landscapes within the same biome. We chose this approach because managed areas experience ecological contingencies divergent from their surroundings (e.g. irrigation). We considered studies of ‘stems’ as those involving sampling of woody branches, twigs or grass shoots. Studies of ‘roots’ included any survey of below-ground plant tissue, but excluded rhizosphere soil surveys and studies, that did not attempt to surface sterilize roots. We considered studies of ‘leaves’ to be those sampling leaf sections or whole leaves/leaflets (including needles) and did not consider studies that combined petioles with leaf blade tissue.
To understand the phylogenetic breadth of host plants surveyed, we calculated the total number of hosts examined for each plant family and plotted this information on a phylogeny of the Embryophyta (algal endophyte hosts were thus omitted from this portion of our analysis) generated using phyloT (online software accessible at https://phylot.biobyte.de/). The National Center for Biotechnology Information taxonomy database was used to generate the tree (database accessed March 15, 2019; Federhen, 2012). iTOL v4.3.2 was used for tree visualization (Letunic and Bork, 2016). Manipulation of all data was performed in the R statistical computing environment (R Core Team, 2019).
Vote counting to determine patterns in relative tissue richness
In addition, we asked how endophyte richness shifted among tissue types for both fungi and bacteria. We attempted two approaches to address this question - a formal meta-analysis and a simple vote-counting approach. Because few studies used the same methods, comparing the effect of tissue type on richness among studies was inappropriate (this limitation also precluded comparison of richness among taxa or across biomes, unfortunately). Thus, we only examined those studies that compared richness among multiple tissue types, and all comparisons were made within studies.
Unfortunately, very few studies provided data sufficient for a quantitatively rigorous meta-analysis (see Methods and Results in Data S1; Viechtbauer, 2010), so we conducted a simple vote-counting procedure where we considered each study independently and ranked tissue types by the relative richness reported in that study. We only considered those studies that examined multiple tissues and that standardized sampling effort among tissues. In total, we examined 243 studies: 182 studies of fungal endophytes and 61 studies of bacterial endophytes. After ranking tissues by relative richness separately for each study, we calculated, across studies, the proportion of times one tissue type had higher richness than another tissue (e.g. for what proportion of studies did leaves have higher richness than roots) and calculated the probabilities of these proportions using a binomial sign test (Cooper and Hedges, 1993). This test is simply the probability of observing a particular number, or more, of positive outcomes (in our case, one tissue type having higher richness than another), given a certain number of trials and assuming equal probability of positive and negative outcomes. For this vote-counting approach, we focused on richness because fewer studies reported diversity metrics and, when not explicitly reported by authors, relative richness was simpler to calculate and extract from published summary tables and figures than were diversity entropies. To test how growth habit influenced relative microbial richness among tissues, we conducted vote counting separately for studies of hosts with the following growth habits: woody-stemmed trees and shrubs, forbs and graminoids.
Results
Our survey highlighted the breadth of the endophyte biodiversity literature, as we extracted data from 596 unique publications. We report that interest in endophyte diversity is on the rise, with a sharp increase in studies per year since 2010 (Fig. 1). Fungi have received comparatively more attention than bacteria, though this disparity is diminishing (Figs. 1 and 2E). The majority of studies were of foliar endophytes (1694 unique combinations of study and host species), followed by root (577 combinations) and stem (540 combinations) endophytes. By comparison, floral tissues (39 combinations) and plant propagules were understudied (172 combinations; Fig. 2). Multiple host studies were not the norm - approximately 66% of studies focused on a single host taxon.
Fig. 2.
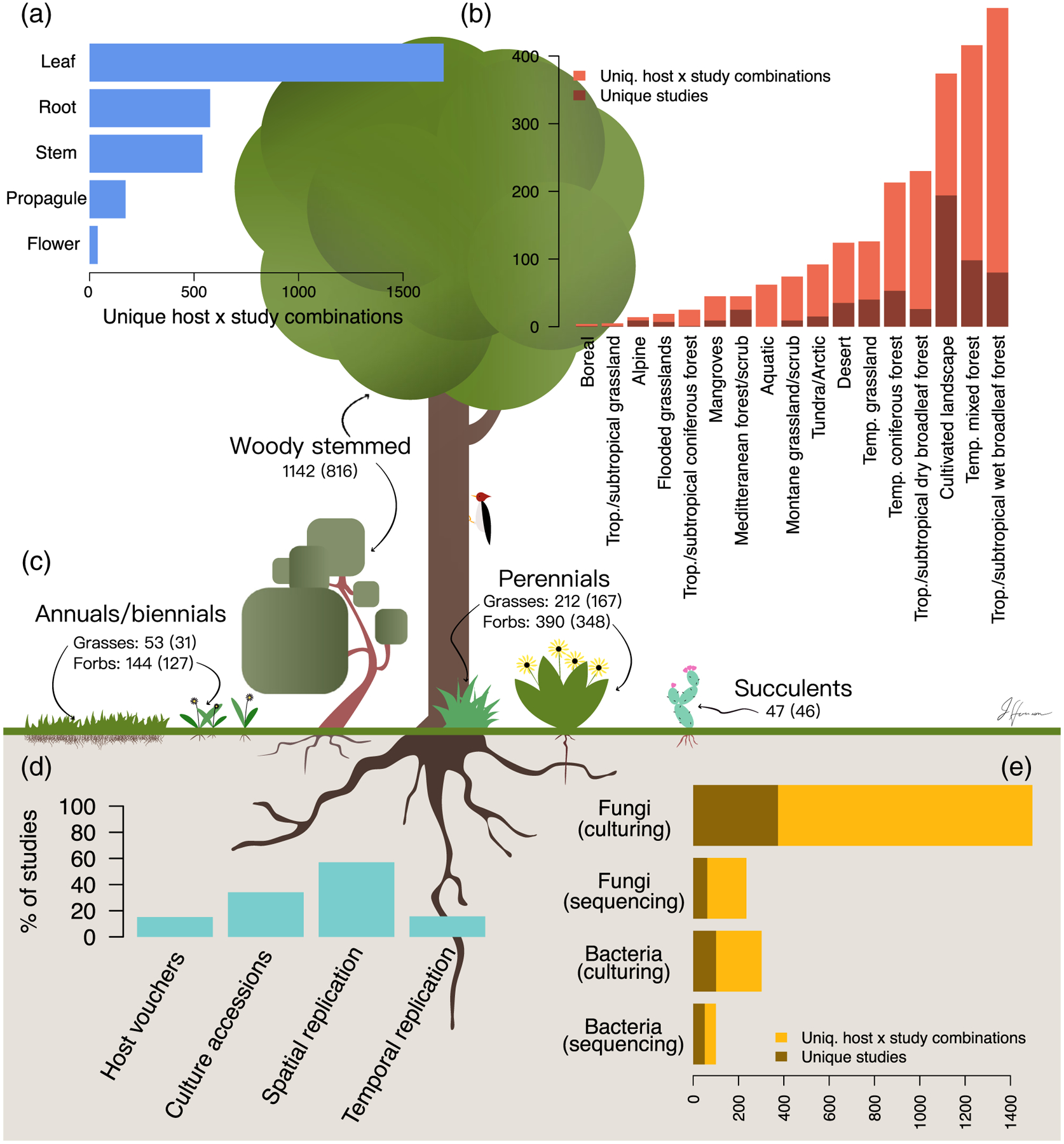
Summary of 596 publications characterizing endophyte biodiversity. Because many studies surveyed multiple hosts, we report both the number of studies and number of unique host by study combinations.
A–C. The number of studies surveying each plant compartment (A), biome (B) and host life history category (C; values in parentheses are unique hosts).
D. Information pertaining to study design and reproducibility.
E. The endophytic taxon characterized and the methodology employed.
The global extent of endophyte biodiversity research
The geographical range encompassed by the studies we considered was global; endophytes, both fungal and bacterial, have been recovered from hosts across all major biomes (Fig. 3). Temperate mixed coniferous and deciduous forests were the best studied with 96 studies (16% of total). However, the most unique combinations of host and study were reported from tropical and subtropical wet forests (471, 21% of total). This was due to several studies that surveyed many hosts within these forests (e.g. Rojas-Jimenez et al., 2016 with 92 hosts and Suryanarayanan et al., 2011 with 70 hosts). In terms of unique studies, research in tropical and subtropical forests composed a more modest 13% of studies in our survey. Many biomes were quite understudied. For instance, 50 or fewer studies (in terms of unique host by study combinations) were conducted in 7 of the 17 biomes that we considered (Fig. 2B). Overall, studies from these biomes composed only 7% of those surveyed.
Fig. 3.

Locations of studies considered. An interactive, zoomable version of this map can be found at https://jharrisonecoevo.github.io/EndophyteMap/. Black points represent studies of cultivated crops and managed landscapes, purple points represent studies of uncultivated plants in unmanaged settings. Biomes are colour coded and delineated in accordance with Olson and colleagues (2001). In some cases, multiple, proximal locations were surveyed and a single point was used to graphically represent these locations. If a study did not provide exact location information, then study location was approximated.
Across biomes, we found comparatively few studies of hosts growing in obvious wilderness, far from human development. Indeed, 33% of studies relied on hosts grown in cultivated environments, including urban locations, agricultural landscapes and greenhouses (with university campuses being particularly well sampled). This estimate may be conservative as for some studies the exact collection location was difficult to determine and so we did not include them in the ‘cultivated’ category, but sampling was likely not far from human development.
Much of the host phylogeny remains unsampled
The studies we surveyed encompassed 1702 unique taxa from 254 plant families. Poaceae was by far the most well-studied family (189 hosts studied), followed by Fabaceae (98 hosts), Pinaceae (82 hosts) and Asteraceae (79 hosts; Fig. 4). In the studies we examined, fungal endophytes have been surveyed in hosts from 30% of plant families listed in the NCBI taxonomy database for Embryophyta. By comparison, bacterial endophytes have been characterized in only 10.5% of plant families. Of particular note, very few observations of foliar microbiota have been made among bryophyte and pteridophyte families (Fig. 4; Davis and Jonathan Shaw, 2008; Alessandro et al., 2013). Additionally, we observed a mismatch between host family species richness and sampling effort. For instance, only 39 Orchidaceae species have been surveyed out of the approximately 28,000 accepted orchid taxa occurring worldwide (Chase et al., 2015; Kew and Missouri Botanical Gardens, 2019).
Fig. 4.

Survey effort across Embryophyta. Number of studies surveying fungal (blue) and bacterial (red) endophytes are shown extending outwards from the tips of the phylogeny. Tips are families. Notable taxa within Embryophyta are labelled and colour coded. Numbers in parentheses denote unique hosts surveyed. Very few surveys of bacterial endophytes have been conducted in bryophyte hosts; therefore, this portion of the figure has been abbreviated to aid visualization. An interactive, zoomable version of this phylogeny can be found at https://itol.embl.de/shared/harrisonjg.
Replication and reproducibility could be improved
We also characterized details for each study regarding sampling scheme and reproducibility (Fig. 2D and E). We found that just over half of studies were spatially replicated (sampling areas were separated by at least a kilometre) and fewer than a quarter of studies were temporally replicated. The majority of studies (~74%) relied on culturing; however, only about a third of these studies reported accessioning cultures (Fig. 2E). By comparison, 72% of studies that relied on sequence data provided clear instructions for downloading raw data, though only 23% of these studies provided processed data [such as an operational taxonomic unit (OTU) table]. Surprisingly, fewer than 20% of studies mentioned accessioning host vouchers. For cultivated plants, we considered a description of the cultivar as equivalent to an accessioned voucher.
The effects of tissue type on endophyte richness and diversity
We performed vote counting to compare the relative richness and diversity of fungal endophyte assemblages in varying tissue types across plant taxa. We resorted to vote-counting procedure because data were insufficient for a robust meta-analysis (see Methods and Results in Data S1). We found that relative tissue richness was dependent upon host growth habit. For instance, stems had richer fungal endophyte assemblages than leaves for woody-stemmed hosts, but this pattern was not observed for either forbs or graminoids (Table S1). By comparison, for graminoids, roots had richer fungal and bacterial endophyte assemblages than stems (Table S3). For forbs, no tissue type was clearly richer, on average, than other tissues (Table S2). Additionally, for fungal endophytes, we found that reproductive structures, including flowers and propagules, were relatively species poor, while bark was species rich (Tables S1 and S2), though these results are quite tentative given the few studies that compared endophyte assemblages in these tissues to those in other portions of the plant corpus.
Discussion
We report that endophyte biodiversity has been studied within all major biomes and continents (even Antarctica, if one counts King George Island; Fig. 3; Rosa et al., 2009). Given that widespread interest in endophytes did not occur until the 1970s, progress has been rapid. However, great swathes of the globe still remain unsurveyed. Certain biomes have been particularly understudied - either due to their high biodiversity, which makes thorough sampling exceptionally difficult (i.e. tropical rainforests); large geographical area (e.g. the boreal forest); or because they are geographically restricted and simply have not received much attention. For instance, we found few studies from coastal dunes, flooded grasslands and mangrove forests. These habitats are challenging for plants due to salinity, short intervals between disturbances and the presence of anoxic soil. Surveys of understudied biomes will help define the scope of endophyte biodiversity and functional traits. In particular, we suggest that surveys in flooded grasslands and mangroves may improve our understanding of archael endophyte biodiversity (Moissl-Eichinger et al., 2018), as this branch of life includes numerous halophiles and other extremophiles that may be able to cope with the abiotic conditions characteristic of those locations. Similarly, studies in desert and alpine biomes may uncover endophytes with unique mechanisms for coping with the severe ultraviolet exposure, temperature swings and desiccation that occurs in such harsh habitats (see e.g. Lopez et al., 2011; Massimo et al., 2015; Sangamesh et al. 2018).
We also reported a lack of studies from Africa, west and north Asia, and the interiors of Australia and South America (Fig. 3). These areas hold some of the most biodiverse and charismatic landscapes on the planet; for instance, the Congo basin is the second largest tropical rainforest in the world, with thousands of endemic plant taxa (Brenan, 1978; Linder, 2001), and it has historically experienced less deforestation than other rainforests (Koenig, 2008). Similarly, the Cape Floristic province in Africa has some of the highest levels of plant endemism in the world. Because these regions have evolutionary histories that have facilitated endemism, it seems likely that they harbour unique endophyte taxa and would be prime locations to study coevolution and codivergence between plants and endophytes. More generally, the lack of sampling outside of North American, Europe and portions of Asia precludes a robust knowledge of endophyte biogeography.
The influence of human development on endophyte biodiversity
We acknowledge the logistical challenges of sampling the remote locations that remain understudied. Indeed, we report an imprint of this challenge in even relatively well-studied regions, where we found that most studies were conducted near roadways, townships and other human development. The lack of sampling in wilderness areas likely biases our nascent understanding of endophyte biodiversity. Human development is associated with pollution, habitat fragmentation, ecosystem disturbance frequency and the abundance of introduced hosts (Dietz et al., 2007; Crowl et al., 2008) - all of which likely affect plant microbiomes. Evidence for this hypothesis is sparse; however, Laforest-Lapointe and colleagues (2017a) reported many phyllosphere bacterial taxa shift in relative abundance along an urbanization gradient, with an overall decline in dominant Alphaproteobacteria with more urbanization. Similarly, Lappalainen and colleagues (1999) reported a decline in endophyte colonization of Betula trees with proximity to a copper-nickel smelter. Variation in heavy metal concentrations (Jurc et al., 1996; Tóth et al., 2009), acid rain (Helander et al., 1994) and air pollution (Wolfe et al., 2018) have all been associated with shifts in endophyte assemblages - thus, it seems likely that the effects of pollution and urbanization are multifarious and have effects that depend upon the endophytic taxon examined and the ecological context.
In addition to pollution, habitat fragmentation also increases in proximity to human development. Very little is known regarding how habitat fragmentation affects microbial assemblages or, more generally, how metacommunity processes manifest within microbiomes (Christian et al., 2015). However, classic island biogeography theory (MacArthur and Wilson, 2001) suggests that human-caused habitat fragmentation likely shapes endophyte assemblages through determining proximity to inoculum sources. In a survey spanning islands of various sizes, Helander and colleagues (2007) reported that endophyte colonization of Betula spp. trees was greater on larger islands and islands closer to the mainland (also see Oono et al., 2017). This result, coupled with work documenting dispersal limitation in non-endophytic microbial systems (Andrews et al., 1987; Peay et al., 2007; 2010; Golan and Pringle, 2017), suggests that it is reasonable to expect variation in endophyte assemblages routinely follows the predictions of island biogeography, regardless of whether habitat fragmentation and patch size are caused by geological processes or human influence.
Another way in which endophyte assemblages may be affected by proximity to human development is through the influence of invasive plant taxa, which are often much more abundant near development than in wilderness areas. Invasive host taxa could influence endophytes in a variety of ways - from changing the inoculum pool within an area (i.e. ‘neighbourhood’ effects; Moeller et al., 2015), bringing along endophyte taxa or genotypes from the ancestral range of the host (Dickie et al., 2017), or affecting many other aspects of the local ecology [e.g. shifting fire regimes (Brooks et al., 2004), determining litter deposition rate and elemental composition (Allison and Vitousek, 2004), influencing herbivore assemblages (Forister, 2009), etc.].
Interestingly, the few studies we encountered that surveyed endophytic microbiomes of invasive plants in both their native and invaded ranges found that endophyte assemblages differed between ranges. For instance, Lu-Irving and colleagues (2019) report reduced richness in phyllosphere and endophytic root bacteria in the invaded portion of the range of Centaurea solstitialis compared with the native range. Similarly, Shipunov and colleagues (2008) report a wholesale shift in the fungal endophyte assemblage within the leaves of Centaurea stoebe in invaded versus native portions of its range. Thus, the biodiversity of endophytes within widespread, invasive plants is also influenced by host invasion history (also see Gundale et al., 2016; Sikes et al., 2016; Taylor et al., 1999).
All of these anecdotes support the idea that endophyte assemblages in relatively undisturbed areas, such as portions of the Amazon or the Siberian forest, are likely to be different from those in conspecific hosts growing near human habitation or that are being actively cultivated and thus remote locations should be the focus of further study. Even if different microbial taxa are not observed in less disturbed environments, the study of the shifts in relative abundances among endophyte assemblages along urbanization and pollution gradients could provide insight into how endophytes interact and communities assemble (e.g. Gazis and Chaverri, 2015).
Much of the host phylogeny remains unexplored: what might we be missing?
We found that members of about a third of plant families have been surveyed for fungal endophytes and only about a tenth of plant families are represented among studies characterizing bacterial endophyte assemblages. These results suggest we may be missing a large portion of endophyte biodiversity. It is true that many cultivable endophytic taxa are known to have broad host ranges (e.g. Arnold and Lutzoni, 2007; Suryanarayanan et al., 2018), thus one could argue that an understanding of endophyte biodiversity does not hinge on thorough sampling of potential host taxa. However, we note that, in the majority of multivariate studies of endophyte biogeography, host taxon is a supported predictor of assemblage variation (Griffin et al., 2019; Kivlin et al., 2019) - albeit a sometimes modest one (Vincent et al., 2016). Moreover, little is known regarding the host range of those rare endophyte taxa that compose the bulk of most assemblages (Arnold and Lutzoni, 2007).
Studies delineating host range are desperately needed to understand endophyte distributions and biodiversity; however, given the daunting nature of the sampling required, where then should we begin? We suggest targeting those plant lineages with unique traits, such as production of unusual secondary metabolites or preferences for restricted or harsh habitats (e.g. halophiles and extremophiles). As an example, certain Astragalus taxa can hyperaccumulate selenium, and recent research has suggested that these plants may harbour unusual endophytic taxa that could influence selenium uptake (Sura-de Jong et al., 2015; Lindblom et al., 2018, 2013). Following a similar rationale, we also suggest surveying those plant families that are phylogenetically distinctive. If coevolution or codivergence has occurred between hosts and their endophytes, then unusual endophytic taxa could occur in hosts from remote portions of the plant phylogeny (Hassani et al., 2019). Non-vascular plants, in particular, deserve more attention, as these plants have different evolutionary histories, physiology, growth habits and preferred habitats than vascular plants (Huang et al., 2018).
An additional justification for surveying broadly across the plant phylogeny is the discovery of specialist endophyte taxa. Surveys of seeds, in particular, could lead to the discovery of more specialist vertically transmitted endophytes (class I and II endophytes sensu Rodriguez et al., 2009), which are particularly interesting because of their capacity to influence their hosts during early ontogeny (e.g. Hodgson et al., 2014; Truyens et al., 2015; Gundel et al., 2017). An individual seed generally contains a very species poor endophyte assemblage (e.g. in many cases only a single fungus can be isolated from seeds, see, Hodgson et al., 2014; Newcombe et al., 2018; Shipunov et al., 2008), and relatively few instances of vertical transmission of endophyte taxa have been documented. However, recent work by Hodgson and colleagues (2014) provides evidence that vertical transmission of fungi may occur much more often than previously suspected (also see a review on bacterial seed endophytes by Truyens et al., 2015). Indeed, while the well-known clavicipitaceous endophytes seem to be limited to members of the Poaceae (Rudgers et al., 2009), the occurrence of vertically transmitted endophytes capable of systemic growth has been documented from throughout the plant phylogeny, including within members of the Asteraceae (Hodgson et al., 2014), Araliaceae (Soares et al., 2016), Convulvulacea (Cook et al., 2013), Ericaceae (Rayner, 1915), Fabaceae (including members of Astragalus, Oxytropis and Swainsona, Cook et al., 2009, 2014; Grum et al., 2013), Papaveraceae (Hodgson et al., 2014) and Plantaginaceae (Hodgson et al., 2014). This suggests that facultative vertical transmission may occur in numerous plant hosts and across many biomes. Cross-biome comparative studies of the seed microbiome could determine whether vertical transmission is more common in certain habitats, as might be predicted if these endophytes interact mutualistically with their hosts to ameliorate the negative effects of particular abiotic conditions (Afkhami et al., 2014; Gundel et al., 2017; Semmartin et al., 2015).
The effects of tissue type on endophyte assemblages
Our vote-counting approach suggested that in woody plants stems had higher richness than other tissues for both fungi and bacteria. However, for graminoids, roots were the richest tissue, and for forbs, intertissue patterns in richness were less clear (Tables S1–S3). These results suggest that tissues with greater lifetime inocula exposure have the highest richness across plant life histories. This hypothesis is supported by several studies that have demonstrated that older leaves typically harbour richer microbial assemblages than younger leaves, presumably because of greater exposure to inoculum and increased time for microbial growth (Ercolani, 1991; Arnold et al., 2003). Stems and bark of woody plants are exposed to inocula in air, water and dust year round and have long lifespans (indeed much bark is dead and can remain on the trunk for a lengthy period of time), whereas leaves, even for evergreen trees, do not persist for nearly as long. Similarly, roots are the longest-lived tissues of many perennial forbs and graminoids, as above-ground tissues of these hosts often senesce annually. It is true that roots of woody-stemmed plants can be quite long lived; however, roots are primarily encountering inoculum from the surrounding soil matrix, thus it is possible that there is greater variation in the inoculum encountered by stems than by roots over the lives of those tissues. Alternatively, perhaps the resources available to microbes within stems of woody plants favour higher richness compared to leaves, particularly of latent saprotrophs that catabolize lignin or other structural carbohydrates (Oses et al., 2006, 2008). These hypotheses are not mutually exclusive and await experimental testing.
Our survey comes with several caveats. First, it is possible that the efficacy of surface sterilization may vary with tissue type; thus, for instance, the high fungal richness in bark that we report could be because it was more difficult to surface sterilize than other tissues. Also, while we chose those studies that had the same sample size (in terms of replicates) between each tissue type, it was not always apparent that the same mass was used for each sample. Additionally, both culture and sequence-based surveys suffer from taxonomic biases (Nilsson et al., 2019; Carini, 2019) and if those biases coincide with taxonomic variation among tissue types, then richness estimates will be incorrect. Nevertheless, our analysis demonstrates the existence of clear patterns in richness among tissue types and suggests several hypotheses for those patterns that deserve further study.
How can we best share information among studies?
We report several challenges that impede meta-analysis and synthesis of the endophyte literature (e.g. Meiser et al., 2014). Most importantly, raw and processed sequence data were not always available. Moreover, it was quite rare for sufficient detail to be provided regarding sequence processing - including options and versions for software used and date accessed for taxonomy training databases, which are in constant flux. Given the challenge in reprocessing data and the influence different bioinformatic pipelines can have on results (e.g. Pauvert et al., 2019), we suggest that publication of polished data and scripts should be considered to facilitate information sharing among studies. Those data that would be most amenable to meta-analysis include replicate by taxon tables, sequences of OTUs or exact sequence variants, and the taxonomic hypotheses for those sequences. In many cases, meta-analysis will require substantial reprocessing of the data, so raw data should also be made available.
Additionally, we suggest that authors consider depositing vouchers of host taxa studied, nucleic acids extracted or cultures obtained in an herbarium whenever possible (Fig. 2D). This suggestion is motivated, in part, by fascinating new work by Daru and colleagues (2019) who have shown that endophytes within herbarium specimens can be sequenced, and, in some cases, even cultured. Thus, vouchers could act as ‘time capsules’ that preserve endophyte genotypes and could afford insight into endophyte evolution and shifts in host and geographic range over time. To best share information among vouchers, standardized protocols (such as drying time and temperature) could be helpful to adopt, though we acknowledge the challenge of implementing such standards during field collection. Deposited cultures could provide many of the same benefits as host vouchers, but would also allow researchers to grow endophytes of interest to meet various experimental goals (Huang et al., 2018; Suryanarayanan, 2019). Finally, the plant taxonomy is ever-changing, thus as future researchers interpret published work, they may wish to examine accessions to determine the most current taxonomic placement of the focal host or endophyte. In sum, we see herbaria as tremendous resources for the study of the plant microbiome, and consequently, we urge participation in their continued development.
Conclusions
To understand the evolutionary forces and ecological pressures that shape endophyte assemblages, the delineation of patterns in endophyte biodiversity across spatial scales and the host phylogeny is required. The enthusiasm among microbial ecologists for endophyte biology paired with the tools we now have at our collective disposal suggests that description of such patterns is within grasp. We hope that our survey inspires others to fill the gaps in knowledge that we report. To that end, we have made the metadata from each study that we consider available in hopes that other researchers mine them for additional insights.
Supplementary Material
Data S1 Meta-analysis methods and results
Table S1 Differences among host tissues in fungal (top panel) and bacterial (bottom panel) endophyte richness in woody plants. Each cell in the table provides the number of times the tissue type on that row (the focal tissue) had higher richness than the tissue type in that column (the comparison tissue) followed by the number of studies reviewed for each comparison in parentheses. Significance was determined using a binomial sign test. For results from herbaceous plants see Table S2, for results from graminoids see 2018Table S3
Table S2 Differences among host tissues in fungal (top panel) and bacterial (bottom panel) endophyte richness in herbaceous plants. Each cell in the table provides the number of times the tissue type on that row (the focal tissue) had higher richness than the tissue type in that column (the comparison tissue) followed by the number of studies reviewed for each comparison in parentheses. Significance was determined using a binomial sign test. For results from woody plants see Table 2017S1, for results from graminoids see Table S3
Table S3 Differences among host tissues in fungal (top panel) and bacterial (bottom panel) endophyte richness in graminoids. Each cell in the table provides the number of times the tissue type on that row (the focal tissue) had higher richness than the tissue type in that column (the comparison tissue) followed by the number of studies reviewed for each comparison in parentheses. Significance was determined using a binomial sign test. For results from woody plants see Table S1, for results from forbs see Table S2
Fig. S2 Differences in fungal endophyte diversity (exponentiated Shannon's entropy) among host tissues as determined through meta-analysis. Each panel depicts pairwise comparisons between two tissue types. Panel (a) depicts leaves versus roots, panel (b) leaves versus stems and panel (c) roots versus stems. Mean differences between tissues for each study are shown in the right margins of each plot, with confidence intervals. Results were very similar for richness, which can be seen in Fig. S1. Diversity for Unterseher and colleagues (2018) was higher than the other studies because those authors relied on sequencing data whereas the other studies considered relied on culturing data. Two hosts were studied by Granzow and colleagues (2017) and results from each host are denoted by letters a and b.
Fig. S1 Differences in fungal endophyte richness among host tissues as determined through meta-analysis. Each panel depicts pairwise comparisons between two tissue types. Panel (a) depicts leaves versus roots, panel (b) leaves versus stems and panel (c) roots versus stems. Mean differences between tissues for each study are shown in the right margins of each plot, with confidence intervals. No model was significantly supported at p ≤ 0.05. Results were very similar for Shannon's diversity, which can be seen in Fig. S2. Richness for Unterseher et al. (2018) was higher than the other studies because those authors relied on sequencing data whereas the other studies considered relied on culturing data. Two hosts were studied by Granzow and colleagues (2017) and results from each host are denoted by letters a and b.
Acknowledgements
Thanks go to Lyra Beltran for assistance extracting data from publications. This review was inspired by conversations with Betsy Arnold, to whom we offer our thanks. We appreciate comments from Leho Tedersoo and two anonymous reviewers that led to a much improved manuscript. JGH was supported by the National Science Foundation EPSCoR grant 1655726. EAG was supported by a Smithsonian Institution Secretary’s Distinguished Research Fellowship, as well as a Smithsonian Environmental Research Center Postdoc Research Fellowship, the Maryland Native Plant Society, the Washington Biologists Field Club and The New Mexico Idea Network of Biomedical Research Excellence (NM-INBRE), and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health.
Footnotes
Data availability
All scripts and processed data are available at: https://bitbucket.org/harrisonjg/endophytereview/src/master/. For collated metadata from examined studies see: https://docs.google.com/spreadsheets/d/1hNzPz7Uteto7WiLoEdFryonUMfa0nGU_7zNsejCWZPQ/edit?usp=sharing.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- Afkhami ME, McIntyre PJ, and Strauss SY (2014) Mutualistmediated effects on species’ range limits across large geographic scales. Ecol Lett 17: 1265–1273. [DOI] [PubMed] [Google Scholar]
- Alessandro D, Duckett JG, Pressel S, Villarreal JC, and Bidartondo MI (2013) Fungal symbioses in hornworts: a chequered history. Proc Roy Soc B Biol Sci 280: 20130207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison SD, and Vitousek PM (2004) Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141: 612–619. [DOI] [PubMed] [Google Scholar]
- Aly AH, Debbab A, Kjer J, and Proksch P (2010) Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Divers 41: 1–16. [Google Scholar]
- Andrews JH, Kinkel LL, Berbee FM, and Nordheim EV (1987) Fungi, leaves, and the theory of island biogeography. Microb Ecol 14: 277–290. [DOI] [PubMed] [Google Scholar]
- Arnold AE, and Engelbrecht BMJ (2007) Fungal endophytes nearly double minimum leaf conductance in seedlings of a neotropical tree species. J Trop Ecol 23: 369–372. [Google Scholar]
- Arnold AE, and Lutzoni F (2007) Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88: 541–549. [DOI] [PubMed] [Google Scholar]
- Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, and Herre EA (2003) Fungal endophytes limit pathogen damage in a tropical tree. Proc Natl Acad Sci USA 100: 15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auret TB (1930) Observations on the reproduction and fungal endophytism of Lunularia cruciata (L.) Dumortier. Trans Br Mycol Soc 15: 1–2. [Google Scholar]
- Baas Becking LGM (1934) Geobiologie of Inleiding tot de Milieukunde. W.P. Van Stockum & Zoon: The Hague, The Netherlands. [Google Scholar]
- Bowman EA, and Elizabeth Arnold A (2018) Distributions of ectomycorrhizal and foliar endophytic fungal communities associated with Pinus ponderosa along a spatially constrained elevation gradient. Am J Bot 105: 687–699. [DOI] [PubMed] [Google Scholar]
- Brenan JPM (1978) Some aspects of the phytogeography of tropical Africa. Ann Mo Bot Gard 65: 437–478. [Google Scholar]
- Brooks ML, D’Antonio CM, Richardson DM, Grace JB, Keeley JE, DiTomaso JM, et al. (2004) Effects of invasive alien plants on fire regimes. Bioscience 54: 677–688. [Google Scholar]
- Busby PE, Ridout M, and Newcombe G (2016) Fungal endophytes: modifiers of plant disease. Plant Mol Biol 90: 645–655. [DOI] [PubMed] [Google Scholar]
- Busby PE, Soman C, Wagner MR, Friesen ML, Kremer J, Bennett A, et al. (2017) Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol 15: e2001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DH (1908) Symbiosis in fern prothalia. Am Nat 42: 154–165. [Google Scholar]
- Carini P (2019) A ‘cultural’ renaissance: genomics breathes new life into an old craft. mSystems 4: e00092–e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll G (1988) Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology 69: 2–9. [Google Scholar]
- Carroll GC, and Carroll FE (1978) Studies on the incidence of coniferous needle endophytes in the Pacific Northwest. Can J Bot 56: 3034–3043. [Google Scholar]
- Chase MW, Cameron K, Freudenstein J, Pridgeon A, Salazar G, van den Berg C, and Schuiteman A (2015) An updated classification of Orchidaceae. Bot J Linn Soc 177: 151–174. [Google Scholar]
- Christian N, Herre EA, and Clay K (2019) Foliar endophytic fungi alter patterns of nitrogen uptake and distribution in Theobroma cacao. New Phytol 222: 1573–1583. [DOI] [PubMed] [Google Scholar]
- Christian N, Herre EA, Mejia LC, and Clay K (2017) Exposure to the leaf litter microbiome of healthy adults protects seedlings from pathogen damage. Proc R Soc B 284: 20170641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian N, Whitaker BK, and Clay K (2015) Microbiomes: unifying animal and plant systems through the lens of community ecology theory. Front Microbiol 6: 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay K, and Holah J (1999) Fungal endophyte symbiosis and plant diversity in successional fields. Science 285: 1742–1744. [DOI] [PubMed] [Google Scholar]
- Clay K, and Schardl C (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160.S4: S99–S127. [DOI] [PubMed] [Google Scholar]
- Coleman-Derr D, Desgarennes D, Fonseca-Garcia C, Gross S, Clingenpeel S, Woyke T, et al. (2016) Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol 209: 798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S, Duffy B, Nowak J, Clément C, and Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71: 4951–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S, Sessitsch A, and Mathieu F (2012) The 125th anniversary of the first postulation of the soil origin of endophytic bacteria - a tribute to M.L.V. Galippe. Plant Soil 356: 299–301. [Google Scholar]
- Cook D, Beaulieu T, Mott IW, Riet-Correa F, Gardner DR, Grum D, et al. (2013) Production of the alkaloid swainsonine by a fungal endosymbiont of the Ascomycete Order Chaetothyriales in the host Ipomoea carnea. J Agric Food Chem 61: 3797–3803. [DOI] [PubMed] [Google Scholar]
- Cook D, Gardner DR, and Pfister JA (2014) Swainsonine-containing plants and their relationship to endophytic fungi. J Agric Food Chem 62: 7326–7334. [DOI] [PubMed] [Google Scholar]
- Cook D, Gardner DR, Ralphs MH, Pfister JA, Welch KD, and Green BT (2009) Swainsoninine concentrations and endophyte amounts of Undifilum oxytropis in different plant parts of Oxytropis sericea. J Chem Ecol 35: 1272–1278. [DOI] [PubMed] [Google Scholar]
- Cooper H, and Hedges LV (1993) The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation. [Google Scholar]
- Crowl TA, Crist TO, Parmenter RR, Belovsky G, and Lugo AE (2008) The spread of invasive species and infectious disease as drivers of ecosystem change. Front Ecol Environ 6: 238–246. [Google Scholar]
- Daru BH, Bowman EA, Pfister DH, and Arnold AE (2019) A novel proof of concept for capturing the diversity of endophytic fungi preserved in herbarium specimens. Philos Trans Roy Soc B Biol Sci 374: 20170395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, and Jonathan Shaw A (2008) Biogeographic and phylogenetic patterns in diversity of liverwort-associated endophytes. Am J Bot 95: 914–924. [DOI] [PubMed] [Google Scholar]
- de Bary A (1866) Morphologie und Physiologie der Pilze, Flechten und Myxomyceten. Leipzig, Germany: W. Engelmann. [Google Scholar]
- Dickie IA, et al. (2017) The emerging science of linked plant-fungal invasions. New Phytol 215: 1314–1332. [DOI] [PubMed] [Google Scholar]
- Dietz T, Rosa EA, and York R (2007) Driving the human ecological footprint. Front Ecol Environ 5: 13–18. [Google Scholar]
- Doty SL (2011) Growth-promoting endophytic fungi of forest trees In Endophytes of Forest Trees: Biology and Applications. Forestry Sciences, Pirttilä AM, and Frank AC (eds). Springer Netherlands: Dordrecht, pp. 151–156. [Google Scholar]
- Ercolani GL (1991) Distribution of epiphytic bacteria on olive leaves and the influence of leaf age and sampling time. Microb Ecol 21: 35–48. [DOI] [PubMed] [Google Scholar]
- Federhen S (2012) The NCBI taxonomy database. Nucleic Acids Res 40: D136–D143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forister ML (2009) Anthropogenic islands in the arid west: comparing the richness and diversity of insect communities in cultivated fields and neighboring wildlands. Environ Entomol 38: 1028–1037. [DOI] [PubMed] [Google Scholar]
- Frank B (1885) Ueber die auf Wurzelsymbiose beruhende Ernährung gewisser Bäume durch unterirdische Pilze. Ber Deut Bot Ges 3: 128–145. [Google Scholar]
- Frank B (2005) On the nutritional dependence of certain trees on root symbiosis with belowground fungi (an English translation of A.B. Frank’s classic paper of 1885). Mycorrhiza 15: 267–275. [DOI] [PubMed] [Google Scholar]
- Friesen ML, Porter SS, Stark SC, von Wettberg EJ, Sachs JL, and Martinez-Romero E (2011) Microbially mediated plant functional traits. Annu Rev Ecol Evol Syst 42: 23–46. [Google Scholar]
- Galippe V (1887) Note sur la présence de microorganismes dans les tissus végétaux. C R Seances Soc Biol Fil 39: 410–416. [Google Scholar]
- Gazis R, and Chaverri P (2015) Wild trees in the Amazon basin harbor a great diversity of beneficial endosymbiotic fungi: is this evidence of protective mutualism? Fungal Ecol 17: 18–29. [Google Scholar]
- Giauque H, and Hawkes CV (2013) Climate affects symbiotic fungal endophyte diversity and performance. Am J Bot 100: 1435–1444. [DOI] [PubMed] [Google Scholar]
- Glynou K, Ali T, Buch A-K, Kia SH, Ploch S, and Xia X (2016) The local environment determines the assembly of root endophytic fungi at a continental scale. Environ Microbiol 18: 2418–2434. [DOI] [PubMed] [Google Scholar]
- Golan JJ, and Pringle A (2017) Long-distance dispersal of fungi. Microbiol Spect 5: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow S, Kaiser K, Wemheuer B, Pfeiffer B, Daniel R, Vidal S, and Wemheuer F (2017) The effects of cropping regimes on fungal and bacterial communities of wheat and faba bean in a greenhouse pot experiment differ between plant species and compartment. Front Microbiol 9: 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EA, and Carson WP (2015) The ecology and natural history of foliar bacteria with a focus on tropical forests and agroecosystems. Bot Rev 81: 105–149. [Google Scholar]
- Griffin EA, Harrison JG, Kembel SW, Carrell AA, Wright SJ, and Carson WP (2019) Plant host identity and soil macronutrients explain little variation in sapling endophyte community composition: is disturbance an alternative explanation? J Ecol 107: 1876–1889. [Google Scholar]
- Griffin EA, Traw M, Morin P, Pruitt J, Wright SJ, and Carson W (2016) Foliar bacteria and soil fertility mediate seedling performance: a new and cryptic dimension of niche differentiation. Ecology 97: 2998–3008. [DOI] [PubMed] [Google Scholar]
- Griffin EA, Wright SJ, Morin PJ, and Carson WP (2017) Pervasive interactions between foliar microbes and soil nutrients mediate leaf production and herbivore damage in a tropical forest. New Phytol 216: 99–112. [DOI] [PubMed] [Google Scholar]
- Grum DS, Cook D, Baucom D, Mott IW, Gardner DR, Creamer R, and Allen JG (2013) Production of the alkaloid swainsonine by a fungal endophyte in the host Swainsona canescens. J Nat Prod 76: 1984–1988. [DOI] [PubMed] [Google Scholar]
- Gundale MJ, Almeida JP, Wallander H, Wardle DA, Kardol P, Nilsson MC, … Mason B (2016) Differences in endophyte communities of introduced trees depend on the phylogenetic relatedness of the receiving forest. J Ecol 104: 1219–1232. [Google Scholar]
- Gundel PE, Rudgers JA, and Whitney KD (2017) Vertically transmitted symbionts as mechanisms of transgenerational effects. Am J Bot 104: 787–792. [DOI] [PubMed] [Google Scholar]
- Hanson CA, Fuhrman JA, Horner-Devine MC, and Martiny JB (2012) Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol 10: 497–506. [DOI] [PubMed] [Google Scholar]
- Hardoim PR, van Overbeek LS, and van Elsas JD (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16: 463–471. [DOI] [PubMed] [Google Scholar]
- Hardoim PR, Van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, … Sessitsch A (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79: 293–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruna E, Zin NM, Kerfahi D, and Adams JM (2018) Extensive overlap of tropical rainforest bacterial endophytes between soil, plant parts, and plant species. Microbial Ecol 75: 88–103. [DOI] [PubMed] [Google Scholar]
- Hassani MA, Özkurt E, Seybold H, Dagan T, and Stukenbrock EH (2019) Interactions and coadaptation in plant metaorganisms. Annu Rev Phytopathol 57: 483–503. [DOI] [PubMed] [Google Scholar]
- Helander M, Ahlholm J, Sieber TN, Hinneri S, and Saikkonen K (2007) Fragmented environment affects birch leaf endophytes. New Phytol 175: 547–553. [DOI] [PubMed] [Google Scholar]
- Helander ML, Sieber TN, Petrini O, and Neuvonen S (1994) Endophytic fungi in scots pine needles: spatial variation and consequences of simulated acid rain. Can J Bot 72: 1108–1113. [Google Scholar]
- Herre EA, Mejía LC, Kyllo DA, Rojas E, Maynard Z, Butler A, and Van Bael SA (2007) Ecological implications of anti-pathogen effects of tropical fungal endophytes and mycorrhizae. Ecology 88: 550–558. [DOI] [PubMed] [Google Scholar]
- Higginbotham SJ, Arnold AE, Ibanez A, Spadafora C, Coley PD, and Kursar TA (2013) Bioactivity of fungal endophytes as a function of endophyte taxonomy and the taxonomy and distribution of their host plants. PLoS One 8: e73192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins KL, Arnold AE, Coley PD, & Kursar TA (2014) Communities of fungal endophytes in tropical forest grasses: highly diverse host-and habitat generalists characterized by strong spatial structure. Fungal Ecol 8: 1–11. [Google Scholar]
- Hodgson S, de Cates C, Hodgson J, Morley NJ, Sutton BC, and Gange AC (2014) Vertical transmission of fungal endophytes is widespread in forbs. Ecol Evol 4: 1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Bowman EA, Massimo NC, Garber NP, U’Ren JM, Sandberg DC, and Arnold AE (2018) Using collections data to infer biogeographic, environmental, and host structure in communities of endophytic fungi. Mycologia 110: 47–62. [DOI] [PubMed] [Google Scholar]
- Hubbell S (2001) The Unified Neutral Theory of Biodiversity and Biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- Hyde KD, and Soytong K (2008) The fungal endophyte dilemma. Fungal Divers 33: e173. [Google Scholar]
- Janse JM (1897) Les endophytes radicaux de quelques plantes javanaises. Ann Jardin Bot Buitenzorg 14: 53–201. [Google Scholar]
- Jumpponen A (2001) Dark septate endophytes - are they mycorrhizal? Mycorrhiza 11: 207–211. [Google Scholar]
- Jumpponen A, H J, P-A A, and R J (2017) Biogeography of root-associated fungal endophytes In Biogeography of Mycorrhizal Symbiosis. Ecological Studies, Tedersoo L (ed). Cham: Springer International Publishing, pp. 195–222. [Google Scholar]
- Jurc M, Jurc DUSAN, Gogala NADA, and Simoncic PRIMOZ (1996) Air pollution and fungal endophytes in needles of Austrian pine. Phyton 36: 111–114. [Google Scholar]
- Kew and Missouri Botanical Gardens (2019). The Plant List, http://www.theplantlist.org/.
- Kharwar RN, Mishra A, Gond SK, Stierle A, and Stierle D (2011) Anticancer compounds derived from fungal endophytes: their importance and future challenges. Nat Product Rep 28: 1208–1228. [DOI] [PubMed] [Google Scholar]
- Kivlin SN, Emery SM, and Rudgers JA (2013) Fungal symbionts alter plant responses to global change. Am J Bot 100: 1445–1457. [DOI] [PubMed] [Google Scholar]
- Kivlin SN, Kazenel MR, Lynn JS, Taylor DL, and Rudgers JA (2019) Plant identity influences foliar fungal symbionts more than elevation in the Colorado Rocky Mountains. Microbial Ecol 78: 688–698. [DOI] [PubMed] [Google Scholar]
- Koenig R (2008) Critical time for African rainforests. Science 320: 1439–1441. [DOI] [PubMed] [Google Scholar]
- Kusari S, Hertweck C, and Spiteller M (2012) Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem Biol 19: 792–798. [DOI] [PubMed] [Google Scholar]
- Laforest-Lapointe I, Messier C, and Kembel SW (2017a) Tree leaf bacterial community structure and diversity differ along a gradient of urban intensity. mSystems 2: e00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforest-Lapointe I, Paquette A, Messier C, and Kembel SW (2017b) Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature 546: 145–147. [DOI] [PubMed] [Google Scholar]
- Lappalainen JH, Koricheva J, Helander ML, and Haukioja E (1999) Densities of endophytic fungi and performance of leafminers (Lepidoptera: Eriocraniidae) on birch along a pollution gradient. Environ Pollut 104: 99–105. [Google Scholar]
- Lau MK, Elizabeth Arnold A, and Johnson NC (2013) Factors influencing communities of foliar fungal endophytes in riparian woody plants. Fungal Ecol 6: 365–378. [Google Scholar]
- Laurent É (1889) Sur l’existence de microbes dans les tissus des plantes supérieures. Bull Soc R Bot Belg 28: 233–244. [Google Scholar]
- Letunic I, and Bork P (2016) Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44: W242–W245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom SD, Valdez-Barillas JR, Fakra SC, Marcus MA, Wangeline AL, and Pilon-Smits EA (2013) Influence of microbial associations on selenium localization and speciation in roots of Astragalus and Stanleya hyperaccumulators. Environ Exp Bot 88: 33–42. [Google Scholar]
- Lindblom SD, Wangeline AL, Valdez Barillas JR, Devilbiss B, Fakra SC, and Pilon-Smits EA (2018) Fungal endophyte Alternaria tenuissima can affect growth and selenium accumulation in its hyperaccumulator host Astragalus bisulcatus. Front Plant Sci 9: 1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder HP (2001) Plant diversity and endemism in sub-Saharan tropical Africa. J Biogeogr 28: 169–182. [Google Scholar]
- Link HF (1809) Observationes in ordines plantarum naturales, dissertatio prima, complectens anandrarum ordines Epiphytas, Mucedines, Gastromycos et Fungos. Ges Naturf Freunde Berlin 3: 3–42. [Google Scholar]
- Lodge DJ, Fisher PJ, and Sutton BC (1996) Endophytic fungi of Manilkara bidentata leaves in Puerto Rico. Mycologia 88: 733–738. [Google Scholar]
- Lopez BR, Bashan Y, and Bacilio M (2011) Endophytic bacteria of Mammillaria fraileana, an endemic rock-colonizing cactus of the southern Sonoran Desert. Arch Microbiol 193: 527–541. [DOI] [PubMed] [Google Scholar]
- Lu-Irving P, Harenčár JG, Sounart H, Welles SR, Swope SM, Baltrus DA, and Dlugosch KM (2019) Native and invading yellow starthistle (Centaurea solstitialis) microbiomes differ in composition and diversity of bacteria. mSphere 4: e00088–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MDJ, and Neufeld JD (2015) Ecology and exploration of the rare biosphere. Nat Rev Microbiol 13: 217–229. [DOI] [PubMed] [Google Scholar]
- MacArthur RH, and Wilson EO (2001) The Theory of Island Biogeography Google-Books-ID: a10cdkywhVgC. Princeton, NJ: Princeton University Press. [Google Scholar]
- Malloch D, and Blackwell M (1992) Dispersal of fungal diaspores In The Fungal Community: Its Organization and Role in the Ecosystem, 2nd ed. New York, NY: Marcel Dekker, Inc, pp. 147–171. [Google Scholar]
- Márquez LM, Redman RS, Rodriguez RJ, and Roossinck MJ (2007) A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315: 513–515. [DOI] [PubMed] [Google Scholar]
- Martiny JBH, Bohannan BJ, Brown JH, Colwell RK, Fuhrman JA, Green JL, … Morin PJ (2006) Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4: 102–112. [DOI] [PubMed] [Google Scholar]
- Massimo NC, Devan MN, Arendt KR, Wilch MH, Riddle JM, Furr SH, … Arnold AE (2015) Fungal endophytes in aboveground tissues of desert plants: infrequent in culture, but highly diverse and distinctive symbionts. Microb Ecol 70: 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoni J, Bortfeld-Miller M, Jardillier L, Salazar G, Sunagawa S, and Vorholt JA (2019) Consistent host and organ occupancy of phyllosphere bacteria in a community of wild herbaceous plant species. ISME J 14: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser A, Bálint M, and Schmitt I (2014) Meta-analysis of deep-sequenced fungal communities indicates limited taxon sharing between studies and the presence of biogeographic patterns. New Phytol 201: 623–635. [DOI] [PubMed] [Google Scholar]
- Mittelbach GG, Schemske DW, Cornell HV, Allen AP, Brown JM, Bush MB, … McCain CM (2007) Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol Lett 10: 315–331. [DOI] [PubMed] [Google Scholar]
- Moeller HV, Dickie IA, Peltzer DA, and Fukami T (2015) Mycorrhizal co-invasion and novel interactions depend on neighborhood context. Ecology 96: 2336–2347. [DOI] [PubMed] [Google Scholar]
- Moissl-Eichinger C, Pausan M, Taffner J, Berg G, Bang C, and Schmitz RA (2018) Archaea are interactive components of complex microbiomes. Trends Microbiol 26: 70–85. [DOI] [PubMed] [Google Scholar]
- Müller H, Berg C, Landa BB, Auerbach A, Moissl-Eichinger C, and Berg G (2015) Plant genotype-specific archaeal and bacterial endophytes but similar Bacillus antagonists colonize Mediterranean olive trees. Front Microbiol 6: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JC (1940) The endophyte of rye-grass (Lolium perenne). NZ J Sci Technol Sect A 21. [Google Scholar]
- Nemergut DR, Schmidt SK, Fukami T, O’Neill SP, Bilinski TM, Stanish LF, … Ferrenberg S (2013) Patterns and processes of microbial community assembly. Microbiol Mol Biol Rev 77: 342–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe G, Harding A, Ridout M, and Busby PE (2018) A hypothetical bottleneck in the plant microbiome. Front Microbiol 9: 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM, and Snader KM (2003) Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 66: 1022–1037. [DOI] [PubMed] [Google Scholar]
- Nilsson RH, Anslan S, Bahram M, Wurzbacher C, Baldrian P, and Tedersoo L (2019) Mycobiome diversity: high-throughput sequencing and identification of fungi. Nat Rev Microbiol 17: 95–109. [DOI] [PubMed] [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GV, Underwood EC, … Loucks CJ (2001) Terrestrial ecoregions of the world: a new map of life on earth. Bioscience 51: 933–938. [Google Scholar]
- Oono R, Rasmussen A, and Lefèvre E (2017) Distance decay relationships in foliar fungal endophytes are driven by rare taxa. Environ Microbiol 19: 2794–2805. [DOI] [PubMed] [Google Scholar]
- Oses R, Valenzuela S, Freer J, Baeza J, and Rodríguez J (2006) Evaluation of fungal endophytes for lignocellulolytic enzyme production and wood biodegradation. Int Biodeter Biodegr 57: 129–135. [Google Scholar]
- Oses R, Valenzuela S, Freer J, Sanfuentes E, and Rodriguez J (2008) Fungal endophytes in xylem of healthy Chilean trees and their possible role in early wood decay. Fungal Divers 33: 77–86. [Google Scholar]
- Panaccione DG, Beaulieu WT, and Cook D (2014) Bioactive alkaloids in vertically transmitted fungal endophytes. Funct Ecol 28: 299–314. [Google Scholar]
- Pauvert C, Buée M, Laval V, Edel-Hermann V, Fauchery L, Gautier A, … Vacher C (2019) Bioinformatics matters: the accuracy of plant and soil fungal community data is highly dependent on the metabarcoding pipeline. Fungal Ecol 41: 23–33. [Google Scholar]
- Peay KG, Bidartondo MI, and Elizabeth Arnold A (2010) Not every fungus is everywhere: scaling to the biogeography of fungal-plant interactions across roots, shoots and ecosystems. New Phytologist 185: 878–882. [DOI] [PubMed] [Google Scholar]
- Peay KG, Bruns TD, Kennedy PG, Bergemann SE, and Garbelotto M (2007) A strong species-area relationship for eukaryotic soil microbes: island size matters for ectomycorrhizal fungi. Ecol Lett 10: 470–480. [DOI] [PubMed] [Google Scholar]
- Peter J, Young W, and Haukka KE (1996) Diversity and phylogeny of rhizobia. New Phytol 133: 87–94. [Google Scholar]
- Petrini O (1991) Fungal endophytes of tree leaves In Microbial Ecology of Leaves, Andrews JH, and Hirano SS (eds): Springer New York, pp. 179–197. New York, NY: Brock/Springer Series in Contemporary Bioscience. [Google Scholar]
- Pianka ER (1966) Latitudinal gradients in species diversity: a review of concepts. Am Nat 100: 33–46. [Google Scholar]
- R Core Team. (2019) R: A language and environment for statistical computing. Vienna, Austria: tex.organization: R Foundation for Statistical Computing. [Google Scholar]
- Rayner MC (1915) Obligate symbiosis in Calluna vulgaris. Ann Bot 29: 97–133. [Google Scholar]
- Redford AJ, Bowers RM, Knight R, Linhart Y, and Fierer N (2010) The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol 12: 2885–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, & Henson JM (2002) Thermotolerance generated by plant/fungal symbiosis. Science 298: 1581–1581. [DOI] [PubMed] [Google Scholar]
- Remus-Emsermann MN, and Schlechter RO (2018) Phyllosphere microbiology: at the interface between microbial individuals and the plant host. New Phytol 218: 1327–1333. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, … Redman RS (2008) Stress tolerance in plants via habitat-adapted symbiosis. ISME J 2: 404–416. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, White JF Jr, Arnold AE, and Redman ARA (2009) Fungal endophytes: diversity and functional roles. New Phytol 182: 314–330. [DOI] [PubMed] [Google Scholar]
- Rojas-Jimenez K, Hernandez M, Blanco J, Vargas LD, Acosta-Vargas LG, and Tamayo G (2016) Richness of cultivable endophytic fungi along an altitudinal gradient in wet forests of Costa Rica. Fungal Ecol 20: 124–131. [Google Scholar]
- Rosa LH, Vaz AB, Caligiorne RB, Campolina S, and Rosa CA (2009) Endophytic fungi associated with the Antarctic grass Deschampsia Antarctica Desv. (Poaceae). Polar Biol 32: 161–167. [Google Scholar]
- Rudgers JA, Afkhami ME, Rua MA, Davitt AJ, Hammer S, and Huguet VM (2009) A fungus among us: broad patterns of endophyte distribution in the grasses. Ecology 90: 1531–1539. [DOI] [PubMed] [Google Scholar]
- Ryan RP, Germaine K, Franks A, Ryan DJ, and Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278: 1–9. [DOI] [PubMed] [Google Scholar]
- Saikkonen K, Faeth SH, Helander M, and Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Annu Rev Ecol Syst 29: 319–343. [Google Scholar]
- Sampson K (1937) Further observations on the systemic infection of Lolium. Trans Br Mycol Soc 21: 84–IN9. [Google Scholar]
- Sangamesh MB, Jambagi S, Vasanthakumari MM, Shetty NJ, Kolte H, Ravikanth G, … Shaanker RU (2018) Thermotolerance of fungal endophytes isolated from plants adapted to the Thar Desert, India. Symbiosis 75: 135–147. [Google Scholar]
- Schulz B, and Boyle C (2005) The endophytic continuum. Mycol Res 109: 661–686. [DOI] [PubMed] [Google Scholar]
- Schulz B and Boyle C (2006). What are endophytes? Microbial Root Endophytes. Soil Biology Ed. by Schulz BJE, Boyle CJC, and Sieber TN. Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 1–13. [Google Scholar]
- Schulz B, Boyle C, Draeger S, Römmert AK, and Krohn K (2002) Endophytic fungi: a source of novel biologically active secondary metabolites Paper presented at the British Mycological Society symposium on Fungal Bioactive Compounds, held at the University of Wales Swansea on 22–27 April 2001. Mycol Res 106: 996–1004. [Google Scholar]
- Semmartin M, Omacini M, Gundel PE, and Hernández-Agramonte IM (2015) Broad-scale variation of fungal-endophyte incidence in temperate grasses. J Ecol 103: 184–190. [Google Scholar]
- Shaffer JP, Sarmiento C, Zalamea PC, Gallery RE, Davis AS, Baltrus DA, and Arnold AE (2016) Diversity, specificity, and phylogenetic relationships of endohyphal bacteria in fungi that Inhabit tropical seeds and leaves. Front Ecol Evol 4: 116. [Google Scholar]
- Shipunov A, Newcombe G, Raghavendra AK, and Anderson CL (2008) Hidden diversity of endophytic fungi in an invasive plant. Am J Bot 95: 1096–1108. [DOI] [PubMed] [Google Scholar]
- Sikes BA, Hawkes CV, and Fukami T (2016) Plant and root endophyte assembly history: interactive effects on native and exotic plants. Ecology 97: 484–493. [DOI] [PubMed] [Google Scholar]
- Soares MA, Li HY, Bergen M, Da Silva JM, Kowalski KP, and White JF (2016) Functional role of an endophytic Bacillus amyloliquefaciens in enhancing growth and disease protection of invasive English ivy (Hedera helix L.). Plant Soil 405: 107–123. [Google Scholar]
- Stone JK, Bacon CW, and White JF (2000) An overview of endophytic microbes: endophytism defined. Microbial Endophyt 3: 29–33. [Google Scholar]
- Strobel G, and Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67: 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel G, Daisy B, Castillo U, and Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67: 257–268. [DOI] [PubMed] [Google Scholar]
- Strobel GA, Hess WM, Ford E, Sidhu RS, and Yang X (1996) Taxol from fungal endophytes and the issue of biodiversity. J Ind Microbiol 17: 417–423. [Google Scholar]
- Sura-de Jong M, Reynolds RJ, Richterova K, Musilova L, Staicu LC, Chocholata I, … Dolinova I (2015) Selenium hyperaccumulators harbor a diverse endophytic bacterial community characterized by high selenium resistance and plant growth promoting properties. Front Plant Sci 6: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayanan TS, Devarajan PT, Girivasan KP, Govindarajulu MB, Kumaresan V, Murali TS, … Venkatesan G (2018) The host range of multi-host endophytic fungi. Curr Sci 115: 1963–1969. [Google Scholar]
- Suryanarayanan TS (2019) Repository of fungal endophytes at VINSTROM, Chennai: waiting to be harnessed. Current Science 117: 6. [Google Scholar]
- Suryanarayanan TS, Murali TS, and Venkatesan G (2002) Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Can J Bot 80: 818–826. [Google Scholar]
- Suryanarayanan TS, Murali TS, Thirunavukkarasu N, Rajulu MG, Venkatesan G, and Sukumar R (2011) Endophytic fungal communities in woody perennials of three tropical forest types of the Western Ghats, southern India. Biodivers Conserv 20: 913–928. [Google Scholar]
- Taylor JE, Hyde KD, and Jones EBG (1999) Endophytic fungi associated with the temperate palm, Trachycarpus fortunei, within and outside its natural geographic range. New Phytol 142: 335–346. [Google Scholar]
- Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, … Smith ME (2014) Global diversity and geography of soil fungi. Science 346: 1256688. [DOI] [PubMed] [Google Scholar]
- Tóth MD, Halász JL, and Balázsy S (2009) Phyllospheric microbial populations of ragweed (Ambrosia elatior L.) plant grown in toxic metal-contaminated areas. Arch Agron Soil Sci 55: 217–231. [Google Scholar]
- Trappe JM (2005) A.B. Frank and mycorrhizae: the challenge to evolutionary and ecologic theory. Mycorrhiza 15: 277–281. [DOI] [PubMed] [Google Scholar]
- Truyens S, Weyens N, Cuypers A, and Vangronsveld J (2015) Bacterial seed endophytes: genera, vertical transmission and interaction with plants. Environ Microbiol Rep 7: 40–50. [Google Scholar]
- Unterseher M, Karunarathna SC, Cruz GR, Dagamac NH, Dahl MB, Dool SE, … Schöner C (2018) Mycobiomes of sympatric Amorphophallus albispathus (Araceae) and Camellia sinensis (Theaceae) - a case study reveals clear tissue preferences and differences in diversity and composition. Mycol Prog 17: 489–500. [Google Scholar]
- Vacher C, Cordier T, and Vallance J (2016) Phyllosphere fungal communities differentiate more thoroughly than bacterial communities along an elevation gradient. Microb Ecol 72: 1–3. [DOI] [PubMed] [Google Scholar]
- Vellend M (2010) Conceptual synthesis in community ecology. Q Rev Biol 85: 183–206. [DOI] [PubMed] [Google Scholar]
- Verma VC, Kharwar RN, and Strobel GA (2009) Chemical and functional diversity of natural products from plant associated endophytic fungi. Nat Product Commun 4: 1934578X0900401114. [PubMed] [Google Scholar]
- Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36: 1–48. [Google Scholar]
- Vincent JB, Weiblen GD, and May G (2016) Host associations and beta diversity of fungal endophyte communities in New Guinea rainforest trees. Mol Ecol 25: 825–841. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, and Donoghue MJ (2004) Historical biogeography, ecology and species richness. Trends Ecol Evol 19: 639–644. [DOI] [PubMed] [Google Scholar]
- Willems A (2006) The taxonomy of rhizobia: an overview. Plant Soil 287: 3–14. [Google Scholar]
- Wilson D (1995) Endophyte: the evolution of a term, and clarification of its use and definition. Oikos 73: 274–276. [Google Scholar]
- Wolfe ER, Kautz S, Singleton SL, and Ballhorn DJ (2018) Differences in foliar endophyte communities of red alder (Alnus rubra) exposed to varying air pollutant levels. Botany 96: 825–835. [Google Scholar]
- Zimmerman NB, and Vitousek PM (2012) Fungal endophyte communities reflect environmental structuring across a Hawaiian landscape. Proc Nat Acad Sci 109: 13022–13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Meta-analysis methods and results
Table S1 Differences among host tissues in fungal (top panel) and bacterial (bottom panel) endophyte richness in woody plants. Each cell in the table provides the number of times the tissue type on that row (the focal tissue) had higher richness than the tissue type in that column (the comparison tissue) followed by the number of studies reviewed for each comparison in parentheses. Significance was determined using a binomial sign test. For results from herbaceous plants see Table S2, for results from graminoids see 2018Table S3
Table S2 Differences among host tissues in fungal (top panel) and bacterial (bottom panel) endophyte richness in herbaceous plants. Each cell in the table provides the number of times the tissue type on that row (the focal tissue) had higher richness than the tissue type in that column (the comparison tissue) followed by the number of studies reviewed for each comparison in parentheses. Significance was determined using a binomial sign test. For results from woody plants see Table 2017S1, for results from graminoids see Table S3
Table S3 Differences among host tissues in fungal (top panel) and bacterial (bottom panel) endophyte richness in graminoids. Each cell in the table provides the number of times the tissue type on that row (the focal tissue) had higher richness than the tissue type in that column (the comparison tissue) followed by the number of studies reviewed for each comparison in parentheses. Significance was determined using a binomial sign test. For results from woody plants see Table S1, for results from forbs see Table S2
Fig. S2 Differences in fungal endophyte diversity (exponentiated Shannon's entropy) among host tissues as determined through meta-analysis. Each panel depicts pairwise comparisons between two tissue types. Panel (a) depicts leaves versus roots, panel (b) leaves versus stems and panel (c) roots versus stems. Mean differences between tissues for each study are shown in the right margins of each plot, with confidence intervals. Results were very similar for richness, which can be seen in Fig. S1. Diversity for Unterseher and colleagues (2018) was higher than the other studies because those authors relied on sequencing data whereas the other studies considered relied on culturing data. Two hosts were studied by Granzow and colleagues (2017) and results from each host are denoted by letters a and b.
Fig. S1 Differences in fungal endophyte richness among host tissues as determined through meta-analysis. Each panel depicts pairwise comparisons between two tissue types. Panel (a) depicts leaves versus roots, panel (b) leaves versus stems and panel (c) roots versus stems. Mean differences between tissues for each study are shown in the right margins of each plot, with confidence intervals. No model was significantly supported at p ≤ 0.05. Results were very similar for Shannon's diversity, which can be seen in Fig. S2. Richness for Unterseher et al. (2018) was higher than the other studies because those authors relied on sequencing data whereas the other studies considered relied on culturing data. Two hosts were studied by Granzow and colleagues (2017) and results from each host are denoted by letters a and b.


