Abstract
Elagolix is a novel oral gonadotropin releasing hormone receptor antagonist, that can suppress estradiol in a dose‐dependent manner. It is indicated for management of moderate‐to‐severe pain associated with endometriosis. A population exposure‐response model describing the relationship between elagolix exposure and changes in bone mineral density (BMD) was developed using data from four phase III studies in premenopausal women with endometriosis‐associated pain. Elagolix pharmacokinetic exposure‐dependent changes in BMD were described by an indirect‐response maximum effect (Emax) model through stimulation of bone resorption. African American race, higher body mass index (BMI), and lower type‐I collagen C‐telopeptide concentrations were significantly associated with higher baseline BMD. Higher BMI was significantly associated with higher bone formation rates. Simulations using the final model demonstrated that elagolix 150 mg q.d. dosing for 24 months is predicted to result in −1.45% (−2.04 to −0.814) decrease from baseline in BMD and were used to support corresponding dosing recommendations in the label.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Current treatment with gonadotropin releasing hormone (GnRH) agonists for endometriosis can have deleterious effects on the bone mineral density (BMD), whereas elagolix, a GnRH antagonist, has the potential to manage endometriosis‐associated pain, while minimizing the effects on the bones.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What are the main predictors of elagolix‐mediated changes in BMD, and what are the longer‐term effects of elagolix 150 mg q.d. dosing on BMD?
WHAT DOES THIS STUDY ADDS TO OUR KNOWLEDGE?
☑ African American race, body mass index, and baseline type‐1 collagen c‐telopeptide levels are significant predictors of BMD. Elagolix exposures are significantly associated with increased changes in BMD with 24‐month treatment with 150 mg q.d. predicted to result in 1.45% decrease from baseline BMD.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The developed model sets a framework for future evaluation of untested dosing and treatment scenarios as well as the effects of GnRH antagonists on BMD with the potential to extrapolate to other patient populations (e.g., uterine fibroids).
Endometriosis is a chronic, estrogen‐dependent inflammatory disease that results from implantation of endometrial‐like tissue outside the uterus and affects ~ 5–10% of women of reproductive age. 1 Symptoms associated with endometriosis include dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia, and less commonly pain with ovulation, constipation, and painful urination. 1 Currently, first‐line treatment options include nonsteroidal anti‐inflammatory medications and oral contraceptives, whereas second‐line treatment options include progestins and gonadotropin‐releasing hormone (GnRH) agonists. The use of nonsteroidal anti‐inflammatory medications has not been supported by consistent evidence of clinical efficacy in women with endometriosis. 2 Progestins are an effective treatment for endometriosis‐associated pain, but patients receiving progestin therapy may experience undesirable side effects, such as weight gain, mood changes, and irregular bleeding. 1 , 3 , 4 GnRH agonists are also effective for managing pain associated with endometriosis; however, these agents can result in significant hypoestrogenic effects, such as hot flush and significant loss of bone mineral density (BMD), which limits the duration of use.
Elagolix, an orally active non‐peptide GnRH antagonist, 5 has recently been approved for the management of moderate‐to‐severe pain associated with endometriosis based on its ability to suppress estrogen levels in a dose‐dependent fashion. 6 , 7 , 8 In four phase III clinical studies (Elaris EM‐I, Elaris EM‐II, Elaris EM‐III, and Elaris EM‐IV), elagolix doses of 150 mg q.d. and 200 mg b.i.d. reduced dysmenorrhea and nonmenstrual pelvic pain in premenopausal women with moderate‐to‐severe pain associated with endometriosis. 1 , 9 In Elaris EM‐I and Elaris EM‐II studies, elagolix 150 mg q.d. treatment groups showed mean changes from baseline lumbar spine BMD at month 6 of −0.32% (95% confidence interval: −0.70 to 0.07) and −0.72% (95% confidence interval: −1.09 to −0.35), respectively. The elagolix 200 mg b.i.d. treatment groups showed mean changes from baseline lumbar spine BMD at month 6 of −2.61% (95% confidence interval: −3.00 to −2.22) and −2.49% (95% confidence interval: −2.85 to −2.13), respectively. 2 Dose‐dependent changes in lumbar spine BMD were observed with both the elagolix dose of 150 mg q.d. and the 200 mg b.i.d. dose. 1 Elagolix exhibits linear pharmacokinetics over the efficacious dose range (150 mg q.d. to 200 mg b.i.d.) with an apparent terminal elimination half‐life of ~ 4–6 hours in healthy subjects. 10 The population mean apparent clearance of elagolix is 118 L/h with interindividual variability (IIV) of elagolix apparent clearance being 42.5%. 11
The analyses reported herein describe the relationship between elagolix exposure and changes in BMD. A population exposure‐response model was developed using data from the four phase III studies in premenopausal women with moderate‐to‐severe pain associated with endometriosis. The exposure‐response model was used to evaluate the effect of subject covariates on baseline and changes in BMD, and to run simulations to predict BMD changes upon treatment with elagolix 150 mg q.d. for 24 months.
METHODS
Participants and study design
The relationship between elagolix exposure and lumbar spine BMD was evaluated using data from premenopausal women with moderate‐to‐severe endometriosis‐associated pain who participated in two pivotal phase III studies (Elaris EM‐I and Elaris EM‐II), with subsequent optional enrollment in two open‐label extension studies (Elaris EM‐III and Elaris EM‐IV). A description of the studies included in the analyses is provided in Table 1 , and details of the study designs and BMD assessments have been reported previously. 1 , 9 Briefly, in each of the pivotal studies, eligible women were randomly assigned in a 2:2:3 ratio to receive elagolix 150 mg q.d., elagolix 200 mg b.i.d., or placebo for a 6‐month treatment period. Subjects were then monitored during a follow‐up period (no elagolix treatment) for up to 12 months, unless the subject was enrolled in the corresponding 6‐month extension study. In the extension studies, subjects who were initially randomized to elagolix treatment arms in the original pivotal study continued on the same dose; whereas subjects originally randomized to placebo were randomized in a 1:1 ratio to receive either elagolix 150 mg q.d. or 200 mg b.i.d.
Table 1.
Phase III studies included in the elagolix exposure‐response model for changes in BMD
| Study | N a | Study design | Elagolix dose/treatment duration | DXA scan assessments b |
|---|---|---|---|---|
|
Elaris‐EM I (pivotal) |
871 | Multicenter, double‐blind, placebo‐controlled, randomized | 150 mg q.d., 200 mg b.i.d., placebo/6 months | Screening, treatment period month 6, follow‐up period months 6 and 12 |
|
Elaris EM‐II (pivotal) |
814 | Multicenter, double‐blind, placebo‐controlled, randomized | 150 mg q.d., 200 mg b.i.d., placebo/6 months | Screening, treatment period month 6, follow‐up period months 6 and 12 |
|
Elaris EM‐III (extension of Elaris EM‐I) |
504 | Multicenter, double‐blind, open‐label, randomized | 150 mg q.d., 200 mg b.i.d./6 months | Treatment period month 6, follow‐up period months 6 and 12 |
|
Elaris EM‐IV (extension of study Elaris EM‐II) |
495 | Multicenter, double‐blind, open‐label, randomized | 150 mg q.d., 200 mg b.i.d./6 months | Treatment period month 6, follow‐up period months 6 and 12 |
BMD, bone mineral density; DXA, dual energy X‐ray absorptiometry; EM, endometriosis.
Premenopausal women with moderate‐to‐severe endometriosis‐associated pain. Subjects with at least one elagolix concentration and one BMD measurement were included in the analysis.
DXA scans of the lumbar spine, femoral neck, and total hip were performed utilizing GE Lunar or Hologic equipment and read by a central reader.
Study protocols were approved by the institutional review boards of the study sites, and all the participants gave written informed consent before participation. The studies were conducted according to International Conference on Harmonization Guidelines for Good Clinical Practice and the ethical principles that have their origin in the Declaration of Helsinki.
BMD and bone biomarker assessments
Dual energy X‐ray absorptiometry (DXA) scans of the lumbar spine, femoral neck, and total hip utilizing GE Lunar or Hologic equipment were performed at baseline and at 6‐month intervals during the treatment and follow‐up periods of the 4 studies and sent to a central reader for review and analysis. Laboratory assessments of bone turnover biomarkers, including osteocalcin, type I collagen C‐telopeptide (CTX), and procollagen type I N‐propeptide, were performed at baseline, month 3, and month 6 of the treatment periods, and at months 3, 6, and 12 of the follow‐up periods.
Model development
Elagolix exposures (monthly average concentrations (Cavg) imputed from the model‐predicted monthly area under the curve (AUC)) were used to conduct the exposure‐BMD analysis. The exposures in this analysis were derived from a previously developed population pharmacokinetic model for elagolix that was based on the same phase III studies. 11 Due to correlation between BMD measurements at different sites (femoral neck, hip, and lumbar spine), analyses were only conducted for lumbar spine BMD as the most sensitive site for changes in BMD with GnRH antagonist therapy (largest change from baseline in phase III studies). 1
The exposure‐BMD model was built using nonlinear mixed effects modeling in NONMEM version 7.3 compiled with the GNU Fortran compiler (version 4.8.3). The infrastructure for model development and evaluation of the final model was a cluster featuring 42 Hewlett‐Packard ProLiant servers under the OpenSUSE operating system with MOSIX Cluster and Grid Management (version 4.4.0). Model parameters were estimated using the first order conditional estimation method with η‐ε interaction as implemented in NONMEM.
Exposure‐BMD modeling was conducted in a step‐wise manner, first developing the appropriate structural model with the appropriate residual error model, followed by models for IIV, and then testing of clinically relevant covariates.
The model was conceptualized as an indirect response model that described the change from baseline BMD and assumed a baseline steady‐state between bone formation and resorption as follows:
| (1) |
| (2) |
and at baseline:
| (3) |
where dR(t)/dt is the change in BMD over time, Kin is a zero‐order rate constant reflecting bone formation, Kout is a first‐order rate constant reflecting bone resorption, BMD(t) is the BMD at time (t), and R(t) is the change in BMD from baseline (BLBMD) at time (t).
Baseline BMD was modeled as a typical value for the population with its associated IIV, and a different baseline value was estimated for each type of DXA scan machine (Hologic and GE Lunar) used.
| (4) |
Where BLBMDi is the BLBMD for subject (i), TVBLBMD is the population estimate for BLBMD, and ηi is the IIV term assumed to rise from a normal distribution with mean 0 and variance ω 2 (i.e., η ~ N(0, ω 2)).
In order to characterize changes in BMD in women treated with placebo, a placebo model was first developed using data from the placebo arms in Elaris EM‐I and Elaris EM‐II.
A model that assumes no change in BMD (Eq. 5) from baseline was then fitted to the placebo data.
| (5) |
The model was then compared with another model that included BMD change in subjects on placebo, described by the parameter (PLAC_EFF, Eq. 6) to reflect changes that were not related to elagolix treatment.
| (6) |
Once the placebo model that best described observed BMD changes in the placebo arm was selected, the exposure‐BMD model was then built by fixing the model parameters that described the placebo effect and adding the response to elagolix treatment via an indirect response model that utilized data from the active treatment arms from the four phase III studies. Individual Cavg values were used as the exposure metric for the exposure‐BMD model based on preliminary exposure‐BMD regression analyses showing that elagolix average concentrations are better predictors of BMD changes compared with other exposure measures, such as peak or trough concentrations (data not shown). In addition, an exposure‐response relationship driven by elagolix average exposures was deemed most appropriate given the mechanism of action of elagolix, which results in changes in BMD due to sustained suppression of estrogen levels over time.
The effects of elagolix on BMD were modeled using a stimulatory maximum effect (Emax) function on the bone resorption process (Kout), as follows:
| (7) |
where Emax is the elagolix maximum stimulatory effect on Kout, EC50 is the elagolix monthly average concentration producing 50% of maximum stimulation, and HILL is the stimulatory Emax curve shape factor.
IIVs in model parameters were modeled using an exponential model (similar to Eq. 4 and as indicated in Eq. 8), and were only included if a statistically significant improvement of the model fit was achieved (P < 0.01) and model stability was maintained (successful minimization and covariance step achieved).
Covariate effects were then investigated for influence on the BMD model parameters. These were selected based on clinical relevance to the BMD safety end point, demographics (age, weight, body mass index (BMI), race (African American vs. non‐African American), tobacco use (yes or no), alcohol use (yes or no), region (non‐United States vs. United States)), baseline characteristics (bone turnover biomarkers (osteocalcin, CTX, and procollagen type I N‐propeptide), hormones (estradiol, progesterone, luteinizing hormone, and follicle stimulating hormone concentration), screening Z‐score, calcium use (yes or no), vitamin D use (yes or no), and prior GnRH therapy (yes or no). Covariate relationships were included in the model in a multiplicative fashion. Continuous covariates, except for the screening Z‐score (SCZSCOR), were normalized to a reference value (median value of the analyzed population) and included in the model via a power function. The screening Z‐score was tested using a linear model with a slope (θ SCZSCOR) instead of a power function because negative values can be observed. Dichotomous covariates were tested with a multiplicative model in order to obtain the fractional difference of the model parameters between the tested groups. The clinical importance of covariate effects was inferred based on the magnitude and precision of covariate parameter estimates.
Finally, individual model parameters were modeled as follows:
| (8) |
where is the value of the kth parameter in the ith subject, is the typical value of the kth parameter, is the number of continuous covariates, is the pth continuous covariate value in the ith subject, is the median values for the pth continuous covariate, is the pth continuous covariate parameter estimate for the kth parameter, is the number of dichotomous covariates, is the qth categorical covariate parameter estimate for the fractional change of the kth parameter, is the qth categorical covariate indicator value (0 or 1) for the ith subject, and is the individual‐specific random effects for the kth parameter in the ith subject. The values are assumed to be multivariate normally distributed: η ~ N(0, ω 2), with mean vector 0 and variance elements denoted by for the kth parameter.
Residual variability was modeled using an additive, proportional, or a combination of additive and proportional error models as follows:
| (9) |
| (10) |
| (11) |
where BMDij is the jth observed BMD measurement in individual i, ij is the jth model‐predicted BMD value in individual i, and εij is the residual random error for individual i and measurement j. The ε values were assumed to be independently and normally distributed with a means of 0 and variances of σ 2: ε ~ N(0, σ 2).
Relevant covariate‐parameter relationships were investigated using forward inclusion (P < 0.01) and backward elimination (P > 0.001) covariate model building as implemented in Perl Speaks NONMEM (version 4.6.0).
Model evaluation
The models were evaluated both during development and after the model development was completed. Methods used in model evaluation and selection included plausibility of model parameter estimates, goodness‐of‐fit plots, visual predictive checks (VPCs), and bootstrap evaluation. Details of VPCs and bootstrap evaluations are provided in the Supplementary Methods .
BMD simulations for 2 years of treatment
The final exposure‐BMD model was used to run simulations to predict BMD changes upon continuous treatment with elagolix 150 mg q.d. for 24 months. The simulations were implemented in MATLAB (R2015b 64 bit) and were run as 100 trials of 100 subjects each (total of 10,000 simulated subjects) to have a sufficiently large simulation dataset to calculate summary statistics. For the simulation datasets, patient demographics and baseline characteristics (baseline BMD, race, and machine type (lunar or hologic)) were simulated to resemble those of all subjects screened for enrollment in the phase III studies as a representative sample of the general endometriosis patient population. Baseline Z‐score was calculated from sampled baseline BMD, age, race, and machine type in order to capture the correlation between baseline BMD and Z‐score. IIV was sampled according to the final model covariance matrix and the model‐estimated residual error was added to the simulation results.
In order to calculate summary statistics, the mean change in BMD and the mean Z‐score was calculated for each simulated trial replicate. Afterward, the mean and confidence interval of the means by replicate was determined.
RESULTS
Demographics
Data from all subjects who received elagolix and had at least one elagolix monthly average concentration and one observation record for BMD (N = 1684) were included in the analysis. Summaries of demographic data for subjects included in the analysis are presented in Table 2 . The mean age and BMI at baseline were 32 years and 27 kg/m2, respectively, mean BMD at screening was 1.2 g/cm2, and the mean Z‐score was 0.4. Of the enrolled subjects, 9% were African American. A total of 5,467 DXA observations were included in the final exposure‐BMD analysis. Sixty‐four observations were excluded (from a total of 5,531) because they were scanned using a different type of machine from that used at screening for the same patient.
Table 2.
Summary of demographics and baseline characteristics of subjects included in the analysis
| Variable | Total (N = 1,684) |
|---|---|
| Age, years | |
| Mean (SD) | 32.3 (6.5) |
| Range | 18.0–49.0 |
| Weight, kg | |
| Mean (SD) | 74.4 (18.0) |
| Range | 40.0–148.0 |
| Body mass index, kg/m2 | |
| Mean (SD) | 27.6 (6.5) |
| Range | 16.2–55.6 |
| Race, N (%) | |
| Non‐African Americans | 1,538 (91.3%) |
| African Americans | 146 (8.7%) |
| BMD (lumber spine) at screening, g/cm2 | |
| Mean (SD) | 1.2 (0.2) |
| Range | 0.8–1.7 |
| Z‐score at screening | |
| Mean (SD) | 0.4 (1.0) |
| Range | –2.0 to 4.4 |
| Estradiol at baseline, (pg/mL | |
| Mean (SD) | 79.6 (73.2) |
| Range | 3.2–624.0 |
| Progesterone at baseline, ng/mL | |
| Mean (SD) | 0.6 (2.2) |
| Range | 0.1–26.4 |
| Luteinizing hormone at baseline, IU/L | |
| Mean (SD) | 8.0 (9.6) |
| Range | 0.2–118.8 |
| Follicle stimulating hormone at baseline, IU/L | |
| Mean (SD) | 8.4 (7.7) |
| Range | 0.9–126.6 |
| CTX at baseline, pg/mL | |
| Mean (SD) | 320.5 (153.4) |
| Range | 35.0–1,057.0 |
| P1NP at baseline, ng/mL | |
| Mean (SD) | 51.7 (20.9) |
| Range | 14.0–234.0 |
| Osteocalcin at baseline, ng/mL | |
| Mean (SD) | 19.4 (7.1) |
| Range | 1.3–52.5 |
| Concomitant use of calcium, n | |
| No | 431 (25.6%) |
| Yes | 1,253 (74.4%) |
| Concomitant use of vitamin D, n | |
| No | 469 (27.9%) |
| Yes | 1,215 (72.1%) |
| Tobacco use, n | |
| Never, ex‐user, unknown | 1,291 (76.7%) |
| User | 393 (23.3%) |
| Alcohol use, n | |
| Never, ex‐user | 522 (31.0%) |
| User | 1,162 (69.0%) |
| Prior GnRH therapy, n | |
| No | 1,244 (73.9%) |
| User | 440 (26.1%) |
BMD, bone mineral density; CTX, C‐terminal telopeptide; GnRH, gonadotropin‐releasing hormone; P1NP, procollagen type I N‐propeptide.
Exploratory BMD plots
The observed BMD data indicated that the distribution of % BMD change from baseline at month 6 for placebo and elagolix 150 mg q.d. dosing were very similar (Figure S1 ). For the elagolix 150 mg q.d. dose at month 12, the distribution of % BMD change from baseline continued to substantially overlap with that observed with placebo and the elagolix 150 mg q.d. at month 6 (Figure S2 ). For the elagolix 200 mg b.i.d. dose, there was a change in BMD of the lumbar spine observed at month 6 compared with placebo and to the elagolix 150 mg q.d. dose (Figure S1 ).
Exposure‐BMD final model
The initial placebo model describing observed BMD changes over time in subjects in the placebo arm was a constant baseline model. The constant value was estimated as a population mean (for each type of DXA machine used), with an exponential IIV term, and a proportional residual error term. An alternative model that included a linear slope for increase in BMD over time in subjects on placebo was found to improve the model fit. The model predicted an increase in BMD of 0.405% (coefficient of variation = 124%) after 6 months on placebo. These estimates were fixed for later parts of the analysis when the treatment effects were introduced. An attempt to simultaneously estimate the placebo effect together with the treatment effect rendered a placebo estimate that predicted a bone change of –0.20%, which resulted in worse model predictive performance.
The placebo response model was extended by incorporating the effect of elagolix monthly average concentrations on bone resorption, as reflected by Kout, to describe the observed BMD changes in the active treatment arms from the four phase III studies. An indirect‐response model was used to describe the effects of elagolix on BMD. In addition to IIV random effects already included on the baseline BMD and placebo effect, log‐normally distributed IIVs were also added on the EC50 estimate. Inclusion of a Hill factor other than one did not result in a significant decrease in the objective function value; thus, a Hill factor was not included in the model.
Baseline body weight and BMI were both significant covariates on baseline BMD and represented the most statistically significant covariates in the univariate covariate selection process. BMI was selected for further model development steps due to its clinical relevance for endogenous estradiol production and BMD changes. 12 , 13 , 14
The full model included BMI, race, and baseline CTX as covariates on baseline BMD, BMI as a covariate on Kin, and screening Z‐score on Emax. Subsequent backward elimination procedures resulted in removal of screening Z‐score on Emax from the final model. The parameter estimates from the final exposure‐BMD model are listed in Table 3 .
Table 3.
Parameter estimates and bootstrap analysis results for the final exposure‐BMD model
| Parameter | Final model | Bootstrap evaluation (N = 996) | ||||
|---|---|---|---|---|---|---|
| Estimate | %RSE c | 95% CI | Mean | Median | 2.5th and 97.5th percentiles | |
| BLBMD (Hologic, g/cm3 | 1.06 | 0.36 | 1.05, 1.07 | 1.061 | 1.060 | 1.050, 1.070 |
| BLBMD (GE Lunar), g/cm3 | 1.24 | 0.30 | 1.23, 1.25 | 1.238 | 1.240 | 1.230, 1.240 |
| PLAC_EFF, 1/day | 0.00002 (fix) | – | – | – | – | – |
| Kin (and Kout), 1/day | 0.0015 | 5.84 | 0.0013, 0.0017 | 0.001 | 0.001 | 0.001, 0.002 |
| Emax | 0.30 | 14.5 | 0.22, 0.39 | 0.306 | 0.300 | 0.232, 0.401 |
| EC50, ng/mL | 240 | 20.9 | 142, 338 | 241.8 | 239.0 | 176.5, 323.5 |
| BMI effect on BLBMD | 0.10 | 10.3 | 0.08, 0.12 | 0.101 | 0.101 | 0.081, 0.119 |
| Race effect on BLBMD | 0.05 | 16.6 | 0.03, 0.07 | 0.049 | 0.049 | 0.033, 0.066 |
| BLCTX effect on BLBMD | –0.020 | 23.6 | –0.029, –0.011 | –0.020 | –0.020 | –0.029, –0.011 |
| BMI effect on Kin | –0.67 | 23.2 | 0.97, –0.36 | –0.669 | –0.664 | –1.010, –0.343 |
| IIV on BLBMD, %CV a | 0.00816 (9.1) | 3.75 | – | 0.008 | 0.008 | 0.008, 0.009 |
| IIV on PLAC_EFF, %CV a | 0.93 (fix) | – | – | – | – | – |
| IIV on EC50, %CV a | 0.77 (107) | 20.7 | – | 0.780 | 0.771 | 0.476, 1.140 |
| Proportional residual error, %CV b | 0.00032 (1.789) | 1.84 | – | – | – | – |
BLBMD, baseline bone mineral density; BLCTX, baseline C‐terminal telopeptide; BMD, bone mineral density; BMI, body mass index; CI, confidence interval; CV, coefficient of variation; EC50, elagolix average concentration at which 50% of Emax is achieved; Emax, maximum effect by elagolix; IIV, interindividual variability; Kin, bone formation rate constant; Kout, bone resorption rate constant; PLAC_EFF, parameter for effects not related to elagolix; RSE, relative standard error; SEE, standard error of the estimate.
.
.
%RSE was estimated as the SEE divided by the population estimate and multiplied by 100.
Model evaluation
The goodness‐of‐fit for the final model was evaluated graphically as displayed in Figure 1 . The plots of predicted and observed changes in lumbar spine BMD indicated that the model adequately described the observations over the entire range. It is noteworthy that because two separate baseline BMD values are estimated depending on the machine used for DXA scanning, two clusters can be seen in the predicted vs. observed BMD plot. Conditional weighted residuals did not show any major trends when plotted against time or population predictions, indicating that the model was appropriately unbiased.
Figure 1.
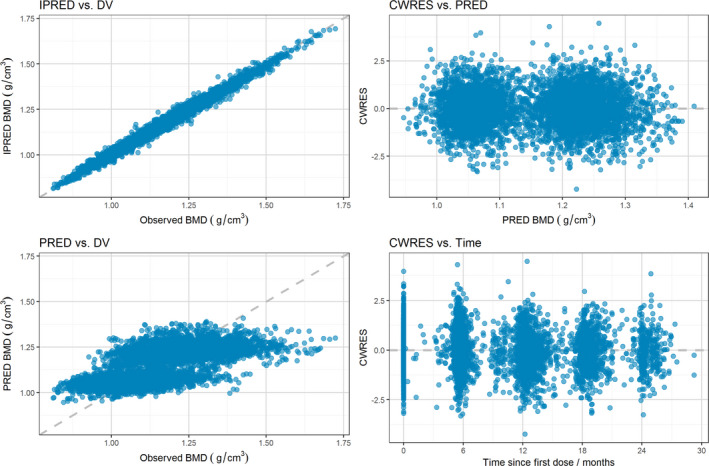
Goodness‐of‐fit plots for the final exposure‐BMD model. Note: Individual predicted (IPRED; upper left) and population predicted (PRED; lower left) vs. observed (DV) lumbar spine bone mineral density (BMD) and conditional weighted residuals (CWRES) vs. population predicted lumbar spine BMD (upper right) and vs. time (lower right).
Based on 500 simulations, the VPC for changes in the lumbar spine BMD vs. time showed that the model adequately described the central tendency as well as the variability in the observed data for the different dosing groups and treatment periods (Figure 2 ).
Figure 2.
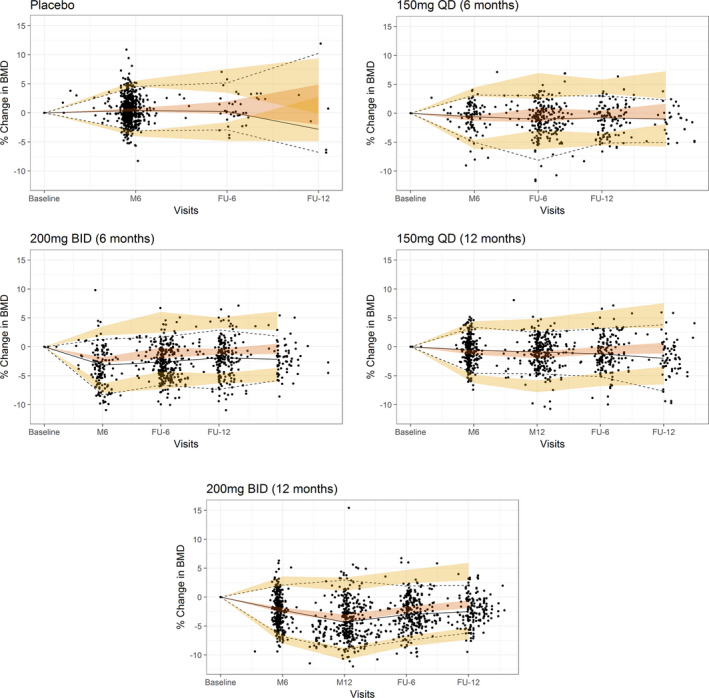
Visual predictive checks for the final exposure‐bone mineral density (BMD) model. M, month; FU, follow‐up. Note: Median (solid line), 5th, and 95th percentiles of the observed data (dashed lines) are compared to the 95% confidence intervals of the median, 5th, and 95th percentiles of the simulated data (shaded regions).
A total of 996 of the 1,000 bootstrap replicates plus the original dataset converged successfully. The estimated parameter values based on the original dataset were in good agreement with the medians of the parameter estimates from the bootstrap replicates, indicating the robustness of the final model parameter estimates (Table 3 ).
BMD simulations for 2 years of treatment
Final model simulations were conducted so that each simulated subject was treated with elagolix 150 mg q.d. for 24 months and the % BMD change from baseline was predicted over the treatment period. Figure 3 shows the mean % change in BMD over time together with 95% confidence intervals. Summary statistics for BMD % change at months 6, 12, and 24 for the same simulation are shown in Table 4 . Model simulations show that elagolix 150 mg q.d. dosing for 24 months is associated with −1.45% change in lumbar spine BMD from baseline.
Figure 3.
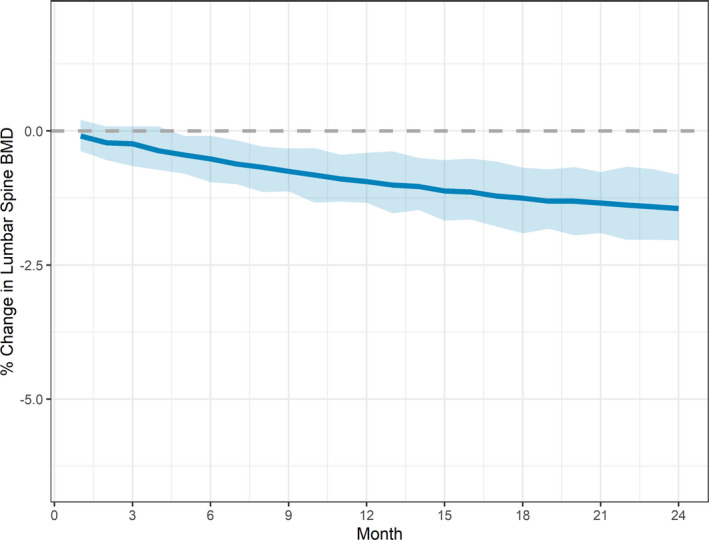
Simulated mean (95% confidence interval) for lumbar spine bone mineral density (BMD) % change over time for treatment with elagolix 150 mg q.d. for 24 months. Note: Solid line represents mean and shaded area represents 95% confidence interval of the mean.
Table 4.
Summary statistics of predicted mean (95% confidence interval) for lumbar spine BMD % change for treatment with elagolix 150 mg q.d. for 24 months
| Mean % change in BMD | 95% Confidence interval | Mean Z‐score | Month |
|---|---|---|---|
| −0.519 | (−0.955, −0.0885) | 0.269 | 6 |
| −0.942 | (−1.34, −0.408) | 0.228 | 12 |
| −1.45 | (−2.04, −0.814) | 0.179 | 24 |
BMD, bone mineral density.
DISCUSSION
A population exposure‐response model was developed to describe the effects of elagolix exposure on BMD changes in premenopausal women with moderate‐to‐severe endometriosis‐associated pain. Exposure‐BMD modeling using data from four phase III studies revealed an exposure‐response relationship between elagolix average concentrations and changes in BMD. The exposure‐BMD indirect response model with zero‐order bone formation and first‐order bone resorption rates adequately predicted the observed BMD changes during treatment and follow‐up periods of the phase III studies.
The model‐estimated slope for increase in lumbar spine BMD in subjects receiving placebo may reflect a slight gain in bone mass over time in those premenopausal women. This is consistent with previous reports of BMD changes in the perimenopausal age showing slight annual increases in lumbar spine BMD in women during the few years before menopause. 15 , 16 , 17 Conversely, other studies have reported an overall reduction in BMD in the last few years before menopause. 16 Although this was not directly evaluated in the phase III studies, the slight increase in BMD over time in subjects administering placebo could be related to administration of vitamin D and calcium supplements during the studies. The current elagolix USPI provides recommendations for adequate intake of vitamin D and calcium supplements in women with endometriosis treated with elagolix. 18
The model‐estimated EC50 of 240 ng/mL was > 5‐fold higher than the predicted exposure with 150 mg q.d. dosing (median Cavg concentrations of ~ 47 ng/mL) and was in the range of predicted elagolix exposures with the 200 mg b.i.d. dosing regimen (median (5–95th) Cavg concentrations of 120 (38–262) ng/mL). 11 This large EC50 estimate is reflected in the small BMD change observed with 150 mg q.d. dosing (~ –1% BMD change from baseline after 12 months) compared with 200 mg b.i.d. and suggests that clinically relevant BMD changes may not be expected in most women treated with the 150 mg q.d. dose of elagolix. It is important to note that the only significant covariate in the elagolix population pharmacokinetic analysis was the OATP genotype. Subjects with reduced transporter function were predicted to have 14% lower elagolix clearance compared with subjects with normal transporter function. Such difference in elagolix clearance or exposures is not expected to result in clinically relevant changes in the exposure‐safety relationship for changes in BMD or the general recommendations for treatment duration for either of the two dosing regimens. 11
Furthermore, the minimal BMD changes with elagolix 150 mg q.d. dosing reflect the milder hypoestrogenic effects with GnRH antagonist therapy compared with GnRH agonists or progesterone‐based treatments. Leuprolide acetate 3.75 mg depot injections in women with endometriosis have been shown to result in change in lumbar spine BMD from pretreatment values by −3.2% and −6.3% at 24 and 52 weeks, respectively, 19 which resulted in recommendations for co‐administration with norethindrone acetate to reduce effects on BMD. 19 Similarly, medroxy‐progesterone use in women 18–35 years of age decreased mean spine (L1–L3) BMD by 3.5% after 1 year and 5.7% after 2 years. On the other hand, mean spine BMD in untreated women changed by < 0.9% over the 2‐year period. 20
Final model results showed that subject race, baseline BMI, and baseline CTX levels were significant predictors of baseline BMD. Consistent with literature showing that African American race is associated with higher BMD compared with other race groups in the United States, 21 , 22 African American subjects were estimated to have 5% higher baseline BMD. Furthermore, higher BMI was also associated with a higher BMD at baseline, with a typical subject having a BMI of 30 kg/m2 (class I obese) estimated to have 5% higher baseline BMD compared with a typical subject with a BMI of 18.5 kg/m2. This is in line with literature showing the correlation of different body size metrics, such as BMI, body fat, and body weight with higher BMD. 12 , 13 , 14 Subjects with lower CTX levels showed a higher BMD at baseline, with a drop of 7% in baseline BMD across the observed range of baseline values for CTX. A possible explanation is that lower CTX levels may be reflective of less active bone resorption processes and, hence, associated with higher BMD. 23 Overall, the effects of the significant covariates in the final model are generally consistent with previous literature reports. The predicted magnitude of effect based on the estimated covariate relationships is not expected to account for major differences in BMD levels (~ 5–7%) among individual patients with endometriosis eligible for treatment with elagolix. The current elagolix USPI provides recommendation to consider assessment of BMD in patients with a history of a low‐trauma fracture or other risk factors for osteoporosis or bone loss.
In addition to its effects on baseline BMD, BMI was also significantly associated with higher bone formation rates (Kin). These results indicated the overall positive correlation between BMI and BMD. After the above covariates were incorporated, none of the tested covariates (including baseline BMD, expressed as Z‐score) were significantly associated with BMD changes due to elagolix treatment (i.e., Emax and EC50). Based on the current dataset, BMD changes in women with endometriosis treated with elagolix may not be affected by evaluated subject demographics or baseline characteristics. However, appropriate treatment and monitoring decisions for individual subjects is still warranted based on individual subject disease burden, and other potential risk factors for bone loss.
Based on the final indirect response model, the first‐order bone resorption process predicted that women who experience larger changes in BMD by the end of the treatment period would show a faster recovery when elagolix treatment was stopped. This has also been observed in a subset of patients who had been followed up after stopping elagolix treatment during the follow‐up periods. Results from the extension studies Elaris EM‐III and Elaris EM‐IV showed that the BMD recovery rate was steepest for women who received elagolix 200 mg b.i.d. 9 A limitation of the study design was that only women who experienced relatively large changes in their BMD were scheduled to have follow‐up visits.
Simulations of elagolix 150 mg q.d. dosing for 24 months showed that the predicted mean % change from baseline BMD (before starting elagolix treatment) were −0.94% and −1.45% after 12 and 24 months, respectively, with a predicted mean Z‐score of 0.179 after 24 months of treatment. These results indicate minimal additional change in BMD during the second year of treatment and that the majority of women treated with elagolix 150 mg q.d. for long term may not experience clinically significant changes in BMD. Given that individual patients treated with elagolix may experience different BMD changes or may be at different risk for bone loss, the current elagolix USPI provides recommendations to treating clinicians to use the lowest effective dose based on severity of symptoms and treatment objectives and to consider assessment of BMD in women with other risk factors for osteoporosis or bone loss. 18
It is important to note that the elagolix phase III program was not designed to assess the risk of fracture in the endometriosis population given the low risk at this young age and the short duration of the studies. In premenopausal women with endometriosis, the 10‐year risk of fracture is quite low (2.9%) and comparable to that in women without endometriosis (2.7%). 24 In addition to the low baseline fracture risk in this population, the relationship between the observed changes in BMD and risk of fracture is unknown and commonly used fracture risk calculation tools, such as the FRAX tool (https://www.sheffield.ac.uk/FRAX/tool.aspx) by the University of Sheffield, are designed based on data from older populations (e.g., 40–90 years). Hence, the current elagolix USPI recommends assessment of BMD in patients with additional risk factors for osteoporosis or bone loss. 18
In conclusion, results from these analyses provided key insights into predictors of BMD changes in women with endometriosis treated with elagolix. Results from final model simulations were used to support elagolix labeling recommendations for continued dosing with 150 mg q.d. for 24 months. 18
Funding
This study was funded by AbbVie. AbbVie contributed to the study design, research, and interpretation of the data and the writing, review, and approval of the manuscript.
Conflicts of Interest
AbbVie provided financial support for the study and participated in the design, study conduct, analysis, and interpretation of data, as well as the writing, review, and approval of the abstract. A.A.S., A.N., I.W., D.B., A.R.P., J.N., P.N., and N.M.M. are AbbVie employees and may own stock.
Author Contributions
A.A.S., A.N., I.W., D.B., A.R.P., J.N., P.N., and N.M.M. wrote the manuscript. A.A.S., A.N., I.W., D.B., A.R.P., J.N., P.N., and N.M.M. designed the research. A.A.S., A.N., I.W., and D.B., performed the research. A.A.S., A.N., I.W., and D.B. analyzed the data.
Data Sharing Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan and execution of a Data Sharing Agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our‐science/clinical‐trials/clinical‐trials‐data‐and‐information‐sharing/data‐and‐information‐sharing‐with‐qualified‐researchers.html.
Supporting information
Fig S1
Fig S2
Supplementary Methods
Model Code
Acknowledgment
Medical writing support was provided by Wesley Wayman, an AbbVie employee.
ClinicaTrials.gov identifiers: NCT01620528, NCT01931670, NCT01760954, and NCT02143713.
References
- 1. Taylor, H.S. et al Treatment of endometriosis‐associated pain with elagolix, an oral GnRH antagonist. N. Engl. J. Med. 377, 28–40 (2017). [DOI] [PubMed] [Google Scholar]
- 2. Brown, J. , Crawford, T.J. , Allen, C. , Hopewell, S. & Prentice, A. Nonsteroidal anti‐inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst. Rev. 1, CD004753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dragoman, M.V. & Gaffield, M.E. The safety of subcutaneously administered depot medroxyprogesterone acetate (104 mg/0.65 mL): a systematic review. Contraception 94, 202–215 (2016). [DOI] [PubMed] [Google Scholar]
- 4. Burney, R.O. et al Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148, 3814–3826 (2007). [DOI] [PubMed] [Google Scholar]
- 5. Chen, C. et al Discovery of sodium R‐(+)‐4‐{2‐[5‐(2‐fluoro‐3‐methoxyphenyl)‐3‐(2‐fluoro‐6‐[trifluoromethyl]benzyl)‐4 ‐methyl‐2,6‐dioxo‐3,6‐dihydro‐2H‐pyrimidin‐1‐yl]‐1‐phenylethylamino}butyrate (elagolix), a potent and orally available nonpeptide antagonist of the human gonadotropin‐releasing hormone receptor. J. Med. Chem. 51, 7478–7485 (2008). [DOI] [PubMed] [Google Scholar]
- 6. Struthers, R.S. et al Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin‐releasing hormone antagonist elagolix. J. Clin. Endocrinol. Metab. 94, 545–551 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Struthers, R.S. et al Pharmacological characterization of a novel nonpeptide antagonist of the human gonadotropin‐releasing hormone receptor, NBI‐42902. Endocrinology 148, 857–867 (2007). [DOI] [PubMed] [Google Scholar]
- 8. Ng, J. , Chwalisz, K. , Carter, D.C. & Klein, C.E. Dose‐dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J. Clin. Endocrinol. Metab. 102, 1683–1691 (2017). [DOI] [PubMed] [Google Scholar]
- 9. Surrey, E. et al Long‐term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet. Gynecol. 132, 147–160 (2018). [DOI] [PubMed] [Google Scholar]
- 10. Shebley, M. et al Clinical pharmacology of elagolix: an oral gonadotropin‐releasing hormone receptor antagonist for endometriosis. Clin. Pharmacokinet. 59, 297–309 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Winzenborg, I. et al Population pharmacokinetics of elagolix in healthy women and women with endometriosis. Clin. Pharmacokinet. 57, 1295–1306 (2018). [DOI] [PubMed] [Google Scholar]
- 12. Reid, I.R. Fat and bone. Arch. Biochem. Biophys. 503, 20–27 (2010). [DOI] [PubMed] [Google Scholar]
- 13. Norgan, N.G. The beneficial effects of body fat and adipose tissue in humans. Int. J. Obes. Relat. Metab. Disord. 21, 738–746 (1997). [DOI] [PubMed] [Google Scholar]
- 14. Lidell, M.E. & Enerback, S. Brown adipose tissue and bone. Int. J. Obes. Suppl. 5, S23–S27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaidi, M. et al FSH, bone mass, body fat, and biological aging. Endocrinology 159, 3503–3514 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greendale, G.A. et al Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women's Health Across the Nation (SWAN). J. Bone Miner. Res. 27, 111–118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finkelstein, J.S. et al Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 93, 861–868 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orilissa™ (elagolix) [US package insert]. (AbbVie Inc., North Chicago, IL, 2018). [Google Scholar]
- 19. AbbVie . Lupron Depot® 11.25 mg [US prescribing information]. (AbbVie, North Chicago, IL, 2013). [Google Scholar]
- 20. Clark, M.K. , Sowers, M.R. , Nichols, S. & Levy, B. Bone mineral density changes over two years in first‐time users of depot medroxyprogesterone acetate. Fertil. Steril. 82, 1580–1586 (2004). [DOI] [PubMed] [Google Scholar]
- 21. Nam, H.S. et al Racial/ethnic differences in bone mineral density among older women. J. Bone Miner. Metab. 31, 190–198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilkin, L.D. , Jackson, M.C. , Sims, T.D. & Haddock, B.L. Racial/ethnic differences in bone mineral density of young adults. Int. J. Exerc. Sci. 3, 197–205 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng, J. , van Schaick, E. , Wu, L.S. , Jacqmin, P. & Perez Ruixo, J.J. Using early biomarker data to predict long‐term bone mineral density: application of semi‐mechanistic bone cycle model on denosumab data. J. Pharmacokinet. Pharmacodyn. 42, 333–347 (2015). [DOI] [PubMed] [Google Scholar]
- 24. Chiuve, S.E. , Peloso, P. , Chand, D. , Patwardhan, M. , Snabes, M. & Kilpatrick, R. Risk factors for low bone mineral density in premenopausal women with endometriosis in the national health and nutrition examination survey (NHANES). Fertil. Steril. 110, e384–e385 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Supplementary Methods
Model Code


