Abstract
Background
Studies have suggested that there is increased risk of thromboembolism (TE) associated with coronavirus disease 2019 (COVID-19). However, overall arterial and venous TE rates of COVID-19 and effect of TE on COVID-19 mortality is unknown.
Methods
We did a systematic review and meta-analysis of studies evaluating TE in COVID-19. We searched PubMed, Cochrane, and Embase for studies published up to June 12, 2020. Random effects models were used to produce summary TE rates and odds ratios (OR) of mortality in COVID-19 patients with TE compared to those without TE. Heterogeneity was quantified with I2.
Findings
Of 425 studies identified, 42 studies enrolling 8271 patients were included in the meta-analysis. Overall venous TE rate was 21% (95% CI:17–26%): ICU, 31% (95% CI: 23–39%). Overall deep vein thrombosis rate was 20% (95% CI: 13–28%): ICU, 28% (95% CI: 16–41%); postmortem, 35% (95% CI:15–57%). Overall pulmonary embolism rate was 13% (95% CI: 11–16%): ICU, 19% (95% CI:14–25%); postmortem, 22% (95% CI:16–28%). Overall arterial TE rate was 2% (95% CI: 1–4%): ICU, 5% (95%CI: 3–7%). Pooled mortality rate among patients with TE was 23% (95%CI:14–32%) and 13% (95% CI:6–22%) among patients without TE. The pooled odds of mortality were 74% higher among patients who developed TE compared to those who did not (OR, 1.74; 95%CI, 1.01–2.98; P = 0.04).
Interpretation
TE rates of COVID-19 are high and associated with higher risk of death. Robust evidence from ongoing clinical trials is needed to determine the impact of thromboprophylaxis on TE and mortality risk of COVID-19.
Funding
None.
Keywords: Covid-19, Venous thromboembolism, Arterial thromboembolism, Deep vein thrombosis, Pulmonary embolism
Research in context.
Evidence before this study
Early reports indicated that in COVID-19 may be associated with coagulation dysfunction. Studies have reported varying rates of thromboembolism. We searched PubMed, Cochrane and Embase for systematic reviews and meta-analyses evaluating thromboembolism rates in COVID-19 published until June 12, 2020. The search terms were “COVID-19”, “SARS-CoV-2” or “novel coronavirus” and “venous thromboembolism”, “arterial thromboembolism”, “deep vein thrombosis” or “pulmonary embolism”. There were varying rates of venous and arterial thromboembolism rates reported by several articles. Some studies noted TE rates in the range of 20–30% while others reported rates as high as 40–70%. However, there were no published systematic reviews and meta-analyses evaluating thromboembolism in COVID-19. We assessed and provided summary estimates of the overall thromboembolism rates of COVID-19 and further evaluated the impact of thromboembolism on COVID-19 mortality risk.
Added value of this study
This is the first systematic review and meta-analysis to provide pooled estimates of both the venous and arterial thromboembolism rates of COVID19 and the associated mortality risk. We evaluated the evidence of 42 studies. We found that the overall arterial and venous and thromboembolism rates of COVID-19 were significantly high. COVID-19 patients who developed thromboembolism were at a significantly higher odds of mortality compared to those who did not.
Implications of all the available evidence
The available evidence indicates that COVID-19 poses a significant risk of thromboembolism and that strategies that succeed in preventing the development of thromboembolism could reduce COVID-19 mortality. This underscores the need for clinicians to implement thromboprophylaxis protocols in order to reduce the thromboembolism risk among COVID-19 patients and to potentially reduce the mortality risk of thromboembolism. Further research is however needed to determine the optimal dosing of anticoagulation and its mortality benefit among COVID-19 patients.
Alt-text: Unlabelled box
1. Introduction
In December 2019, the first case of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) was described and by March 2020, the World Health Organization had declared the disease (Coronavirus disease 2019, COVID-19) a pandemic [1,2]. There is much that remains to be known about the virus and the disease it causes. However, in terms of disease manifestation, it is clear that while some infected people experience a life-threatening severe acute respiratory syndrome, others experience a mild respiratory illness and some others are completely asymptomatic [1]. Whereas respiratory symptoms are the fundamental feature of the disease, evidence is emerging which indicates that the disease is associated with coagulation dysfunction which predisposes patients to an increased risk of both venous and arterial thromboembolism (TE) and potentially increased mortality risk as a consequence [3].
The rate of TE reported in the literature is varied. Some studies have reported TE rates in the range of 20–30% [4], [5], [6] while others have reported rates as high as 40–70% [7], [8], [9]. The presence of hypercoagulation and thromboembolic complications been noted to correlate with a more severe course of the disease involving the need for admission into intensive care units and potentially, death. The association of the increased thrombotic risk of COVID-19 with mortality is however not well characterized. While some studies found a higher risk of mortality in COVID-19 patients with TE [10], others did not find any association [5].
With studies reporting varying rates of TE among patients with COVID-19, the overall rate of venous and arterial TE and the extent to which TE in COVID-19 may increase mortality remains unknown. Therefore, the objective of this systematic review and meta-analysis is to estimate the overall rates of TE of COVID-19 and further determine the association of TE with mortality among patients with COVID-19.
2. Methods
2.1. Search strategy and selection criteria
The systematic review was conducted in line with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [11]. A systematic search was conducted in PubMed, Cochrane and Embase database for studies evaluating vascular events in COVID-19. “COVID-19”, “SARS-CoV-2” or “novel coronavirus” and “venous thromboembolism”, “arterial thromboembolism”, “deep vein thrombosis” or “pulmonary embolism” were the key words used in all searches to identify studies. No language filters were applied in the search. Forward reference searching was used to identify additional studies. The literature search was conducted on June 12, 2020 and all studies published in English or with an English translated version available up to that date of search were eligible for screening.
Title/abstract screening was done independently by two investigators (IN and NE) to identify studies which evaluated thromboembolic events in COVID-19. Studies screened in full text were eligible for inclusion in the meta-analysis if the rate of a thromboembolic event could be calculated based on the number of vascular events and the number of patients in the overall cohort [12]. All disagreements between the two independent investigators after title/abstract and full-text screening were resolved by consensus and/or discussion with a third investigator (MM).
2.2. Data extraction
Data were extracted from eligible studies by 2 independent investigators (IN, NE). Discrepancies were resolved by discussions to reach a consensus. Data collected included study specific information (first author name, year of publication, country in which study was conducted, setting of study, number of patients), demographic information (mean/median age and gender of study participants), comorbidities [hypertension, diabetes mellitus (DM), coronary artery disease (CAD) and cardiovascular disease (CVD) when reported as a composite], and outcomes (venous and arterial TE), deep vein thrombosis (DVT) and pulmonary embolism (PE) as individual endpoints.
2.3. Methodological quality assessment
The methodological quality of the non-comparative studies was assessed with a tool for evaluating the methodological quality of case reports and case series [13], whereas that of comparative studies was assessed using the Newcastle Ottawa tool [14].
2.4. Outcomes
Primary outcomes were venous and arterial TE as well as DVT and PE as individual endpoints and mortality in patients who develop TE compared to those who do not. Secondary outcomes were myocardial infarction (MI), cerebrovascular accident (CVA) and acute limb ischemia (ALI). Venous TE was defined as a composite of DVT or PE or as defined by the individual studies. Arterial TE was defined as a composite of MI, CVA, ALI or mesenteric ischemia, or as defined by the individual studies. All individual endpoints were captured as per the definitions in the individual studies. Outcomes were reported stratified by the setting of the study/disease (ICU vs. non-ICU vs. postmortem).
2.5. Statistical methods
We reported the outcomes from the individual studies as cumulative event rates with corresponding 95% confidence intervals estimated using the binominal distribution. The pooled log transformed rates of events and Wald 95% confidence intervals were estimated using DerSimonian and Laird random effects model [15]. Freeman–Tukey double arcsine transformation or logistic-normal random-effects model was used as needed for continuity correction in order to ensure that studies with zero events were not excluded from the meta-analysis. To evaluate whether TE increases the risk of mortality among patients with COVID-19, we estimated pooled Mantel–Haenszel odds ratios (OR) for mortality using a random effects model. In this comparative analysis, patients with COVID-19 who developed TE were compared with those who did not develop TE using studies that reported mortality in the two groups of interest. Heterogeneity among studies was quantified with the I2 statistic, with I2>50% (P value<0.05) considered to indicate significant heterogeneity among studies. Given the significant heterogeneity between the included studies, random effects models were used throughout. Publication bias and small study effects was assessed with visual inspection of funnel plots and formally testing with Egger test. All analyses were performed using Stata/SE version 16.1 statistical software (StataCorp LLC, College Station, Tex). The meta, metaprop and metaprop_one commands of Stata were used as appropriate.
2.5.1. Ethics review
This study was exempt from Institutional Review Board review since no individual level data were used.
Role of funding source: No funding was received to support this study.
3. Results
3.1. Study identification, and characteristics of included studies
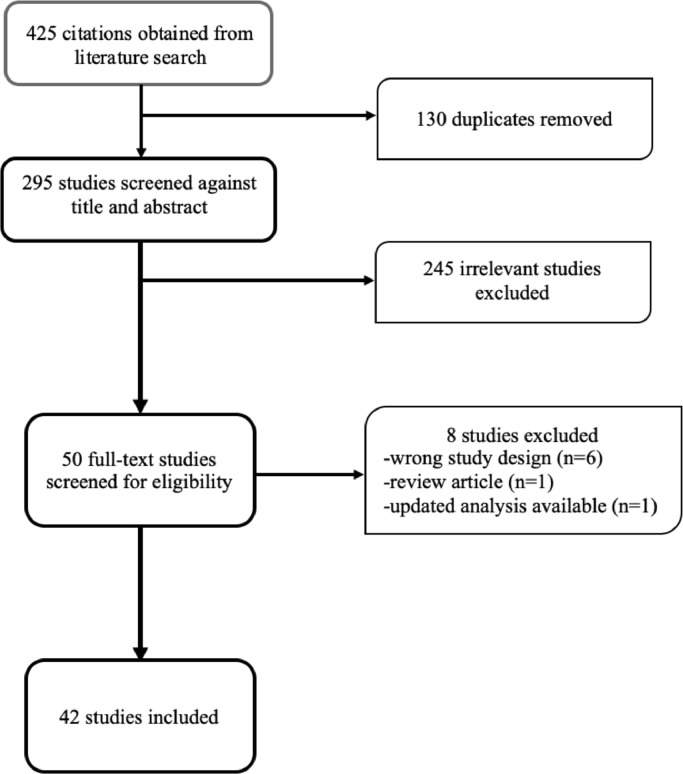
The literature search yielded 425 studies, of which 295 remained after duplicates were removed and therefore were screened. Fifty (50) studies were reviewed in full text and 42 studies [[4], [5], [6], [7],9,10,[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]] enrolling 8271 patients were eventually included in the meta-analysis. No studies were excluded from the meta-analysis on the basis of the language in which they were originally published. The study design of 6 of the 8 studies excluded after full-text screening did not allow an event rate to be estimated. These were small case series in which all patients had the event of interest but the sample size of the overall cohort was not available to enable a rate to be calculated. Also, one study was a review article and an updated analysis was available for the other. Fig. 1 depicts the process of study selection. Four (4) of the 42 included studies evaluated thromboembolic events at autopsy while the rest studied antemortem outcomes. The characteristics of the included studies are shown in Table 1. The methodological quality of included studies was accessed to be adequate. In the 4 postmortem studies, autopsies were performed on consecutive deaths and not on a selective basis. In 18 of the 38 antemortem studies, systematic screening was done to look for TE. Diagnosis of TE was based on symptom driven diagnostics in the remaining studies.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart of study selection.
Table 1.
Baseline characteristics of study participants.
| Study | Country | Setting | N | Fem, % | Mean age | BMI, % | DM, % | HTN, % | CAD, % | CVD, % |
| Al-Samkari, 2020 | USA | Non-ICU | 256 | 47.3 | 60.0 | – | 25.4 | – | – | 32.0 |
| ICU | 144 | 35.4 | 65.0 | – | 40.3 | – | – | 29.9 | ||
| Artifoni, 2020 | France | Non-ICU | 71 | 39.4 | 64.0a | 27.3 | 20.0 | 41.0 | – | – |
| Benzakoun, 2020 | France | PM | 64 | 23.4 | 65.0a | – | – | – | – | – |
| Betoule, 2020 | France | Non-ICU | 76 | 50.0 | 62.0 | – | 13.1 | 32.9 | – | – |
| Bompard, 2020 | France | Non-ICU | 111 | – | – | – | – | – | – | – |
| ICU | 24 | – | – | – | – | – | – | – | ||
| Cantador, 2020 | Spain | Non-ICU | 1419 | – | – | – | – | – | – | – |
| Criel, 2020 | Belgium | Non-ICU | 52 | 44.0 | 64.0 | – | 17.0 | 38.0 | – | – |
| ICU | 30 | 33.0 | 65.0 | – | 17.0 | 33.0 | – | – | ||
| Cui, 2020 | China | ICU | 81 | 54.0 | 60.0 | – | 10.0 | 25.0 | 12.0 | – |
| D-Rodríguez, 2020 | Spain | Non-ICU | 156 | 34.6 | 68.0 | 26.9 | – | – | – | – |
| Desborough, 2020 | UK | ICU | 66 | 27.0 | 59.0a | 28 | 41.0 | 45.0 | – | – |
| Edler, 2020 | Germany | PM | 80 | 38.0 | 79.0 | 25.9 | 21.0 | – | – | – |
| Faggiano, 2020 | Italy | Non-ICU | 25 | 16.0 | 71.0 | – | 8.0 | 32.0 | 52.0 | – |
| Fraissé, 2020 | France | ICU | 92 | 21.0 | 61.0a | – | 38.0 | 64.0 | 10.0 | – |
| Galeano-Valle, 2020 | Spain | Non-ICU | 785 | – | – | – | – | – | – | – |
| Gervaise, 2020 | France | Non-ICU | 72 | 25.0 | 62.0 | 26.7 | – | – | – | – |
| Grandmaison, 2020 | Switzerland | Non-ICU | 29 | – | – | – | – | – | – | – |
| ICU | 29 | – | – | – | – | – | – | – | ||
| Grillet, 2020 | France | Non-ICU | 61 | – | – | – | – | – | – | – |
| Hekimian, 2020 | France | ICU | 39 | – | – | – | – | – | – | – |
| Helms, 2020 | France | ICU | 150 | 18.7 | 63.0a | – | 20.0 | – | – | 48.0 |
| Hippensteel, 2020 | USA | ICU | 91 | 41.8 | 56.0 | 32.3 | 30.8 | – | – | 22.0 |
| Klok, 2020 | Netherlands | ICU | 184 | 24.0 | 64.0 | – | – | – | – | – |
| Leonard-Lorant, 2020 | France | Non-ICU | 58 | – | – | – | – | – | – | – |
| ICU | 48 | – | – | – | – | – | – | – | ||
| Llitjos, 2020 | France | ICU | 26 | 23.1 | 68.0a | – | – | 85.0 | – | – |
| Lodigiani, 2020 | Italy | Non-ICU | 314 | 34.3 | 68.0a | – | 23.5 | 47.7 | 14.4 | – |
| ICU | 48 | 19.7 | 61.0a | – | 18.0 | 44.3 | 11.5 | – | ||
| Longchamp, 2020 | Switzerland | ICU | 25 | 36 | 68.0 | 27.5 | 4.0 | 40.0 | – | 12.0 |
| Louhaichi, 2020 | Tunisia | Non-ICU | 20 | 55.0 | 61.0a | – | 30.0 | 55.0 | – | – |
| Maatman, 2020 | USA | ICU | 109 | 43 | 61.0 | 34.8 | 39.0 | 68.0 | – | 15.0 |
| Menter, 2020 | Switzerland | PM | 21 | 19.0 | 76.0 | – | 35.0 | 100.0 | – | 71.0 |
| Middeldorp, 2020 | Netherlands | Non-ICU | 123 | 41.0 | 60.0 | 28.0 | – | – | – | – |
| ICU | 75 | 23.0 | 62.0 | 27.0 | – | – | – | – | ||
| Nahum, 2020 | France | ICU | 34 | 22.0 | 62.2 | 31.4 | 44.0 | 38.0 | – | – |
| Pavoni, 2020 | France | Non-ICU | 40 | 40.0 | 61.0 | 28.4 | 40 | 40 | – | – |
| Poissy, 2020 | France | ICU | 107 | – | – | – | – | – | – | – |
| Poyiadji, 2020 | USA | Non-ICU | 246 | – | – | – | – | – | – | – |
| ICU | 82 | – | – | – | – | – | – | – | ||
| Ren, 2020 | China | ICU | 48 | 45.8 | 70.0a | – | 27.1 | 39.6 | – | 22.9 |
| Rey, 2020 | Spain | Non-ICU | 2021 | – | – | – | – | – | – | – |
| Stoneham, 2020 | UK | Non-ICU | 274 | – | – | – | – | – | – | – |
| Thomas, 2020 | UK | ICU | 63 | 31.0 | – | – | – | – | – | – |
| Voicu, 2020 | France | ICU | 56 | 25.0 | – | – | 45 | 46 | 20 | – |
| Wichmann, 2020 | Germany | PM | 12 | 25.0 | 73.0a | 28.7 | 33.3 | 25.0 | 50.0 | – |
| Xing, 2020 | China | Non-ICU | 9 | – | – | – | – | – | – | – |
| ICU | 11 | – | – | – | – | – | – | – | ||
| Zerwes, 2020 | Germany | ICU | 20 | 30.0 | 62.0 | 28.1 | 10.0 | 65.0 | – | – |
| Zhang, 2020 | China | Non-ICU | 128 | – | – | – | – | – | – | – |
| ICU | 15 | – | – | – | – | – | – | – | ||
– Data not reported/applicable.
Median age rather than mean age reported.
Abbreviations: BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; CAD, coronary artery disease; CVD, cardiovascular disease; ICU, intensive care unit; PM, postmortem.
3.2. Outcomes
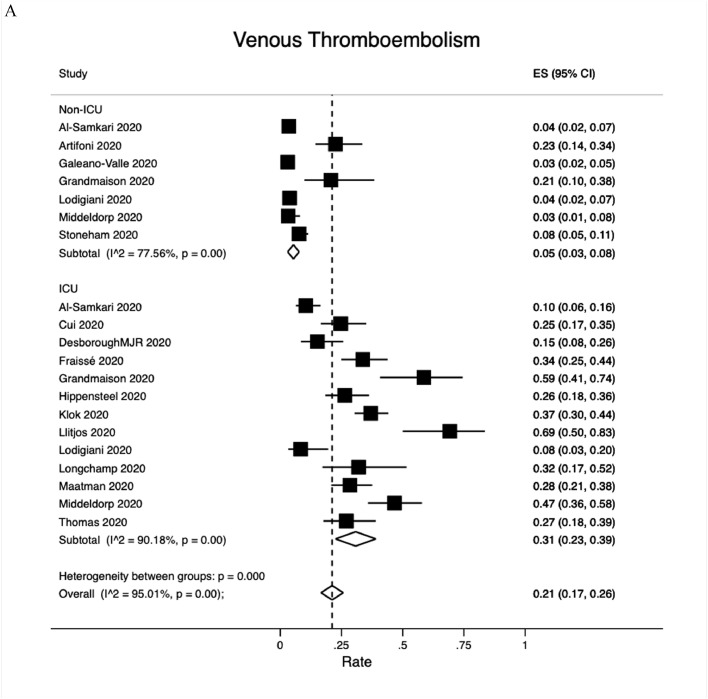
3.2.1. Venous thromboembolism (VTE)
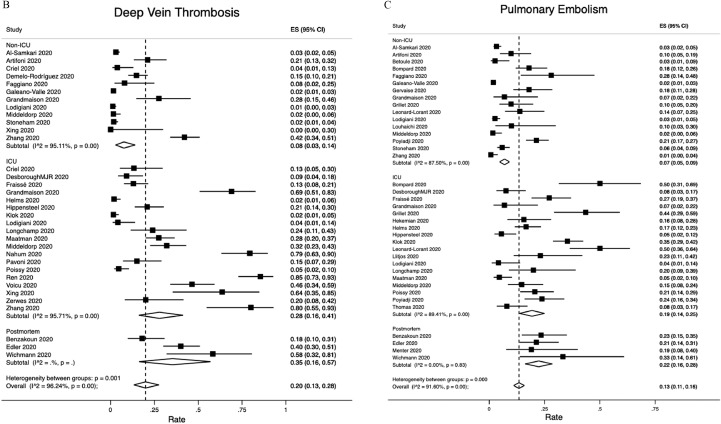
The composite outcome of VTE was reported by 16 studies (Fig. 2A). The overall VTE rate was 21% (95% CI:17−26%); 5% (95% CI: 3−8%) among non-ICU patients but as high as 31% (95% CI: 23−39%) among ICU patients. DVT events were reported by 28 studies, 3 of which studied postmortem outcomes (Fig. 2B). The overall DVT rate was 20% (95% CI: 16−23%). The pooled DVT rate was 8% (95% CI:3−14%) among 12 non-ICU studies, 28% (95% CI: 16−41%) among 19 ICU studies and 35% (95% CI:16−57%) among the 3 postmortem studies. PE events were reported by 31 studies, 4 of which were post mortem studies (Fig. 2C). The overall PE rate was 13% (95% CI: 11−16%): 7% (95% CI:5−9%) among 16 non-ICU studies, 19% (95% CI: 14−25%) among 18 ICU studies and 22% (95% CI:16−28%) among postmortem studies.
Fig. 2.
Venous thromboembolism rates. A. Overall; B. Deep vein thrombosis; C. Pulmonary embolism. Abbreviation: ICU, intensive care unit.
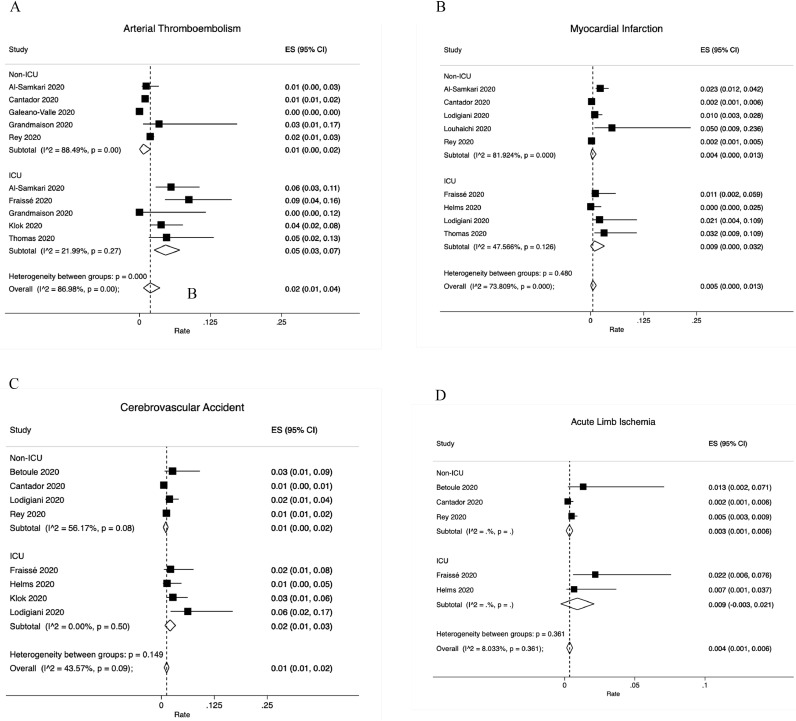
3.2.2. Arterial thromboembolism (ATE)
Overall ATE was reported by 8 studies (Fig. 3A). The overall ATE rate was 2% (95% CI: 1−4%): 1% (95% CI: 0−2% among 5 non-ICU studies and 5% (95% CI: 3−7%) among 5 ICU studies. The pooled rates of specific arterial thromboembolic events were as follows: MI, 0.5% (95% CI:0−1.3%), Fig. 3B; CVA, 1% (95% CI: 1−2%), Fig. 3C and ALI, 0.4% (95% CI:0.1−0.6%), Fig. 3D.
Fig. 3.
Arterial thromboembolism rates. A. Overall; B. Myocardial infarction; C. Cerebrovascular accident; D. Acute limb ischemia. Abbreviation: ICU, intensive care unit.
3.2.3. Comparative analysis
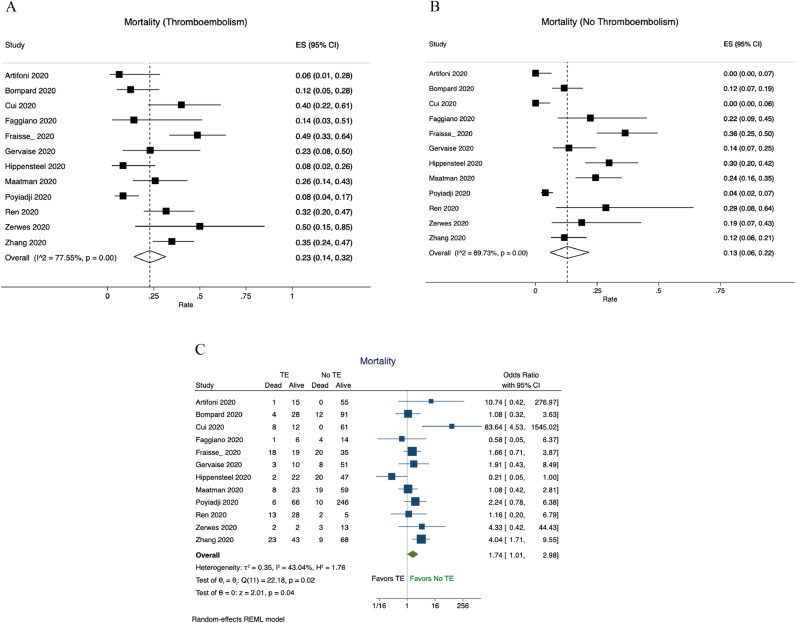
The pooled mortality rate among patients with TE was 23% (95%CI:14−32%, Fig. 4A) and among patients without TE it was 13% (95% CI:6−22%, Fig. 4B). The pooled odds of mortality were 74% higher among patients who developed TE compared to those who did not (OR, 1.74; 95%CI, 1.01–2.98; P = 0.04), (Fig. 4C).
Fig. 4.
Mortality among patients with and without thromboembolism. A. Mortality rate of patients with thromboembolism; B. Mortality rate of patients without thromboembolism; C. Pooled odds of mortality among patients with TE compared to patients without TE. Abbreviation: TE, thromboembolism.
4. Discussion
In this meta-analysis of 42 studies involving 8271 patients, we showed that thromboembolic events are high in SARS-CoV-2 infected individuals. Overall VTE rate was 21%, with DVT rate of 20% and PE rate of 13% while ATE rate was 2%. Among ICU patients, the VTE rate was 31%, DVT rate was 28%, PE rate was 19% and ATE rate was 5%. Thromboembolism significantly increased the odds of mortality by as high as 74% (OR, 1.74; 95%CI, 1.01–2.98; P = 0.04).
COVID-19 morbidity and mortality continue to remain significant in parallel with rising infection rates. The pathophysiology of COVID-19 is still being understood. The disease is known to primarily affect the respiratory system but the involvement of other systems is not uncommon. Involvement of the vascular system in particular is thought to contribute significantly to morbidity and more importantly mortality. Of note is the increased risk of thromboembolism that is now known to be associated with COVID-19. There are a number of mechanisms thought to contribute to this elevated thromboembolism risk of COVID-19. Abnormally elevated levels of proinflammatory cytokines have been found in patients infected with the novel coronavirus [52]. The resultant increased systemic inflammation coupled with endothelial injury triggered by attachment of the virus to the angiotensin-2 receptor of the endothelial cells and viral replication leads to a prothrombotic endothelial dysfunction [53,54]. Platelet activation, immobilization, mechanical ventilation and the use of central venous catheters are other factors that contribute to a prothrombotic state in COVID-19. Earlier reports have linked coagulopathy and development of TE with an increased risk of death [3,52]. Autopsy studies have provided some essential insights into this prothrombotic state in COVID-19 [34,44,55]. A recent autopsy study found that almost no organ in the body is spared of thrombosis [56]. Regardless of anticoagulation status and sometimes early in the disease course, significant macrovascular and microvascular thrombosis was found in multiple organs.
In order to mitigate the attendant prothrombotic state associated with COVID-19, the International Society of Thrombosis and Hemostasis (ISTH) interim guidance on recognition and management of coagulopathy in COVID-19 recommends that in the absence of contraindications, “prophylactic dose low molecular weight heparin (LMWH) should be considered in all patients (including non-critically ill) who require hospital admission for COVID-19 infection” [57]. In a similar manner, the American Society of Hematology also recommends that “all hospitalized patients with COVID-19 should receive pharmacologic thromboprophylaxis with LMWH or fondaparinux, unless they are judged to be at increased bleeding risk” [58]. Several other consensus statements, guidelines and reviews have also made similar recommendations of thromboprophylaxis for COVID-19 patients especially for hospitalized patients [59], [60], [61].
The VTE rates found in our study, particularly among ICU patients, are significantly higher than what is expected for hospitalized patients with acute infections. Previous studies have shown that hospitalization for acute infections can be associated with an estimated VTE risk as high as 15.5% [62], especially in hospitalization for pneumococcal and influenza infections [63,64]. Moreover, these rates are higher than what is reported in the literature for other viral pandemics experienced in the past. In the pandemic H1N1 influenza of 2009, studies reported VTE rates of about 6% [65]. Of note, the pooled VTE, DVT and PE rates were consistently higher among ICU versus non-ICU patients. This is in tandem with prior research indicating there is a correlation between disease severity and the risk of thromboembolism among SARS-CoV-2 infected individuals.
That said we believe that, the VTE rates reported in this study are conservative estimates of the overall VTE risk associated with SARS-CoV-2 infection. This position is informed by the fact some studies did not systematically search for VTE in all patients and this may have led to an underestimation of some of the VTE rates reported in the individual studies included in this meta-analysis. This is further supported by the fact that DVT rates in the postmortem studies were nearly two times higher than the DVT rates of antemortem studies. It is worth noting these autopsies were mostly performed on patients who were not suspected of VTE before death [44].
Understandably, it is sometimes not feasible to conduct the imaging studies needed to diagnose VTE especially in patients who are critically ill, intubated, unstable and might be in prone position. The potential for other patients and healthcare workers acquiring the infection through contact with SARS-CoV-2 infected individuals and contaminated imaging equipment may discourage clinicians from systematically searching for TE in all patients. That said, the overall VTE rate of 21%, DVT rate of 20% and particularly PE rate of 13%, though likely conservative, are still high, and should prompt all to the heightened risk of VTE associated with COVID-19.
More importantly, whereas a prior study evaluating non-COVID-19 ICU patients found that patients with and without VTE had comparable mortality risk (16% vs 20%, P = 0.72) [66], our meta-analysis shows that concomitant TE and COVID-19 is associated with 74% increased odds of death compared to COVID-19 patients without TE (13% vs 23%, OR: 1.74, P = 0.04). The risk of death in COVID-19 posed by TE is therefore not negligible. It stands to reason that thromboprophylaxis may favorably change the clinical course and improve the prognosis of COVID-19 infected patients. In line with prior recommendations therefore, it is our position that standardized thromboprophylaxis protocols should be implemented in all COVID-19 patients in the absence of contraindications; to mitigate the risk of developing TE and to reduce the mortality risk associated with concomitant TE and COVID-19 [67]. The optimal dosing of antithrombosis drugs however remains unknown given the limited evidence in the literature [61]. In the face of life-threatening thromboembolic complications developing in the presence of standard dose thromboprophylaxis, some authors have pushed for higher anticoagulation targets in severely ill COVID-19 patients [8]. In this regard some physicians routinely increase the dose of anticoagulation beyond prophylactic dosing to intermediate or therapeutic dosing in the hope that this will reduce the widespread microvascular thrombosis and the thrombosis related mortality associated with SARS-CoV-2 infection [7,61,67]. The role and optimal dosing of anticoagulation in COVID-19 is the subject of ongoing trials. It is hoped that these clinical trials will provide the much-needed robust evidence on the impact of anticoagulation on the risk of TE and mortality among SARS-CoV-2 infected individuals.
This study has some limitations within which the results of the analysis should be interpreted. As noted earlier, not all studies included in the analysis systematically looked for the presence of TE. For most studies, the screening for TE was rather symptom driven, with the implication of missing asymptomatic events. This may have resulted in conservative estimates of the TE risk associated with SARS-CoV-2 infection. Also, the nature of the data available in the individual studies did not allow the meta-analysis to be stratified by some clinically relevant variables such as thromboprophylaxis status, race, and healthcare access/quality to assess their effect on the incidence of TE and mortality. The ability to stratify the analysis by thromboprophylaxis status, for instance, would have helped to determine the extent to which thromboprophylaxis reduces COVID-19-related TE and mortality.
Despite these limitations, our meta-analysis is novel in estimating the overall high TE rates of COVID-19 and the associated significant increase in mortality odds.
In this novel systematic review and meta-analysis involving 8271 SARS-CoV-2 patients, we determined the overall incidence of VTE to be 21%. Among ICU patients the VTE rate was as high as 31%. Patients who developed TE were at 74% increased odds of death compared to those who did not. Clinical trials are ongoing to elucidate the mortality benefit of thromboprophylaxis and optimal dosing in this population of patients.
Funding
No financial support received for this study.
Data sharing statement
The data used in this study were gathered from publicly available studies and available from the corresponding author upon reasonable request.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest relevant to the content of this manuscript.
Acknowledgments
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100639.
Appendix. Supplementary materials
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease (COVID-19) situation reports. Published 2020. Accessed 13 July 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hippensteel J.A., Burnham E.L., Jolley S.E. Prevalence of venous thromboembolism in critically ill patients with COVID-19. Br J Haematol. 2020 doi: 10.1111/bjh.16908. Published onlinebjh.16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14888. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intens Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llitjos J.F., Leclerc M., Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020 doi: 10.1111/jth.14869. Published onlinejth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Feng X., Zhang D. Deep vein thrombosis in hospitalized patients with Coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020 doi: 10.1161/circulationaha.120.046702. Published online. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Mathes T., Pieper D. Clarifying the distinction between case series and cohort studies in systematic reviews of comparative studies: potential impact on body of evidence and workload. BMC Med Res Methodol. 2017;17(1):107. doi: 10.1186/s12874-017-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murad M.H., Sultan S., Haffar S., Bazerbachi F., Mohammad D., Murad H. Methodological quality and synthesis of case series and case reports. BMJ. 2018;23(2) doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WELLS, G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Published online 2001.
- 15.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Cantador E., Núñez A., Sobrino P. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02176-7. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Criel M., Falter M., Jaeken J. Venous thromboembolism in SARS-CoV-2 patients: only a problem in ventilated ICU patients, or is there more to it? Eur Respir J. 2020 doi: 10.1183/13993003.01201-2020. Published online2001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demelo-Rodríguez P., Cervilla-Muñoz E., Ordieres-Ortega L. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated d-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desborough M.J.R., Doyle A.J., Griffiths A., Retter A., Breen K.A., Hunt B.J. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res. 2020;193:1–4. doi: 10.1016/j.thromres.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edler C., Schröder A.S., Aepfelbacher M. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134(4):1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faggiano P., Bonelli A., Paris S. Acute pulmonary embolism in COVID-19 disease: preliminary report on seven patients. Int J Cardiol. 2020;313:129–131. doi: 10.1016/j.ijcard.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraissé M., Logre E., Pajot O., Mentec H., Plantefève G., Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24(1):275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galeano-Valle F., Oblitas C.M., Ferreiro-Mazón M.M., Alonso-Muñoz J., del Toro-Cervera J., Demelo-Rodríguez P. Antiphospholipid antibodies are not elevated in patients with severe COVID-19 pneumonia and venous thromboembolism. Thromb Res. 2020;192:113–115. doi: 10.1016/j.thromres.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gervaise A., Bouzad C., Peroux E., Helissey C. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol. 2020 doi: 10.1007/s00330-020-06977-5. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandmaison G., Andrey A., Périard D. Systematic screening for venous thromboembolic events in COVID-19 pneumonia. TH Open. 2020;04(02):e113–e115. doi: 10.1055/s-0040-1713167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020 doi: 10.1148/radiol.2020201544. Published online201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hékimian G., Lebreton G., Bréchot N., Luyt C.E., Schmidt M., Combes A. Severe pulmonary embolism in COVID-19 patients: a call for increased awareness. Crit Care. 2020;24(1):274. doi: 10.1186/s13054-020-02931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intens Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard-Lorant I., Delabranche X., Severac F. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. Published online201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longchamp A., Longchamp J., Manzocchi-Besson S. Venous thromboembolism in critically ill patients with Covid-19: results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020 doi: 10.1002/rth2.12376. Published onlinerth2.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louhaichi S., Allouche A., Baili H. Features of patients with 2019 novel coronavirus admitted in a pneumology department: the first retrospective Tunisian case series. Tunisie Medicale. 2020;98(4):261–265. https://www.latunisiemedicale.com/article-medicale-tunisie.php?article=3695 [PubMed] [Google Scholar]
- 33.Maatman T.K., Jalali F., Feizpour C. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe Coronavirus disease 2019. Crit Care Med. 2020 doi: 10.1097/ccm.0000000000004466. Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menter T., Haslbauer J.D., Nienhold R. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nahum J., Morichau-Beauchant T., Daviaud F. Venous thrombosis among critically ill patients with Coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poissy J., Goutay J., Caplan M. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020 doi: 10.1161/circulationaha.120.047430. Published online. [DOI] [PubMed] [Google Scholar]
- 37.Pavoni V., Gianesello L., Pazzi M., Stera C., Meconi T., Frigieri F.C. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02130-7. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poyiadi N., Cormier P., Patel P.Y. Acute pulmonary embolism and COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020201955. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren B., Yan F., Deng Z. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020 doi: 10.1161/circulationaha.120.047407. Published online. [DOI] [PubMed] [Google Scholar]
- 40.Rey J.R., Caro-Codón J., Poveda Pineda D., Merino J.L., ÁM Iniesta, López-Sendón J.L. Arterial thrombotic complications in hospitalized patients with COVID-19. Rev Española Cardiol. 2020 doi: 10.1016/j.rec.2020.05.008. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoneham S.M., Milne K.M., Nuttal E. Thrombotic risk in COVID-19: a case series and case–control study. Clin Med. 2020 doi: 10.7861/clinmed.2020-0228. Published onlineclinmed.2020-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas W., Varley J., Johnston A. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voicu S., Bonnin P., Stépanian A. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.053. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wichmann D., Sperhake J.-.P., Lütgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020 doi: 10.7326/m20-2003. Published onlineM20-2003. [DOI] [PubMed] [Google Scholar]
- 45.Xing C., Li Q., Du H., Kang W., Lian J., Yuan L. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit Care. 2020;24(1) doi: 10.1186/s13054-020-02876-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zerwes S., Hernandez Cancino F., Liebetrau D. Increased risk of deep vein thrombosis in intensive care unit patients with CoViD-19 infections?—Preliminary data. Chirurg. 2020:1–7. doi: 10.1007/s00104-020-01222-7. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Artifoni M., Danic G., Gautier G. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50(1):211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Samkari H., Karp Leaf R.S., Dzik W.H. COVID and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection. Blood. 2020 doi: 10.1182/blood.2020006520. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benzakoun J., Hmeydia G., Delabarde T. Excess out-of-hospital deaths during COVID-19 outbreak: evidence of pulmonary embolism as a main determinant. Eur J Heart Fail. 2020 doi: 10.1002/ejhf.1916. Published onlineejhf.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Betoule A., Martinet C., Gasperini G. Diagnosis of venous and arterial thromboembolic events in COVID-19 virus-infected patients. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02163-y. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bompard F., Monnier H., Saab I. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J. 2020 doi: 10.1183/13993003.01365-2020. Published online2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henry B.M., Vikse J., Benoit S., Favaloro E.J., Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann M., Kleine-Weber H., Krueger N., Mueller M.A., Drosten C., Poehlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020 doi: 10.1101/2020.01.31.929042. Published online2020.01.31.929042. [DOI] [Google Scholar]
- 55.Lax S.F., Skok K., Zechner P. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020 doi: 10.7326/M20-2566. Published onlineM20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rapkiewicz A V., Mai X., Carsons S.E. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100434. 0(0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thachil J., Tang N., Gando S. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.COVID-19 and VTE-Anticoagulation - Hematology.org. Haematology.Org. Published 2020. Accessed 6 July 2020. https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation
- 59.Moores L.K., Tritschler T., Brosnahan S. Prevention, diagnosis, and treatment of VTE in patients With COVID-19: CHEST guideline and expert panel report. Chest. 2020 doi: 10.1016/j.chest.2020.05.559. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casini A., Alberio L., Angelillo-Scherrer A. Thromboprophylaxis and laboratory monitoring for in-hospital patients with COVID-19 – a Swiss consensus statement by the Working Party Hemostasis. Swiss Med Wkly. 2020;150:w20247. doi: 10.4414/smw.2020.20247. [DOI] [PubMed] [Google Scholar]
- 61.Bikdeli B., Madhavan M V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alikhan R., Cohen A.T., Combe S. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX study. Arch Intern Med. 2004;164(9):963–968. doi: 10.1001/archinte.164.9.963. [DOI] [PubMed] [Google Scholar]
- 63.Obi A.T., Tignanelli C.J., Jacobs B.N. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord. 2019;7(3):317–324. doi: 10.1016/j.jvsv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y.-.G., Lin T.-.Y., Huang W.-.Y., Lin C.-.L., Dai M.-.S., Kao C.-.H. Association between pneumococcal pneumonia and venous thromboembolism in hospitalized patients: a nationwide population-based study. Respirology. 2015;20(5):799–804. doi: 10.1111/resp.12501. [DOI] [PubMed] [Google Scholar]
- 65.Bunce P., High S., Nadjafi M., … KS-CI, 2011. Pandemic H1N1 influenza infection and vascular thrombosis. academic.oup.com. Accessed 10 July 2020. https://academic.oup.com/cid/article-abstract/52/2/e14/376301 [DOI] [PubMed]
- 66.Beitland S., Wimmer H., Lorentsen T. Venous thromboembolism in the critically ill: a prospective observational study of occurrence, risk factors and outcome. Acta Anaesthesiol Scand. 2019;63(5):630–638. doi: 10.1111/aas.13316. [DOI] [PubMed] [Google Scholar]
- 67.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.