Abstract
Since the coronavirus disease 2019 (COVID-19) pandemic, attention has been drawn to the possible interactions between the deadly disease and a few other infections. Although schistosomiasis and other neglected tropical diseases have been proposed to influence susceptibility to COVID-19, no study has looked into this. This study therefore investigated the impact of schistosomiasis on the transmission of COVID-19 and also evaluated the role of praziquantel treatment coverage on COVID-19 outcomes in African countries. The schistosomiasis endemicity and the preventive chemotherapy coverage index statuses were obtained from the World Health Organization databank. COVID-19 data were obtained from the Worldometer COVID-19 report. The data were adjusted and the percentage of COVID-19 cases confirmed, and active cases, recovery and deaths were computed. The COVID-19 outcomes were evaluated relative to schistosomiasis endemicity and treatment coverage. COVID-19 outcomes, especially active cases and recovery rates, were significantly improved in schistosomiasis nonendemic African countries (p < 0.05). While COVID-19 confirmed cases were significantly higher in countries with >75% schistosomiasis preventive chemotherapy coverage index (p < 0.05), improved COVID-19 outcomes were observed relative to active cases and recovery in countries with >75% preventive chemotherapy coverage index (p > 0.05). Schistosomiasis endemicity may be associated with negative COVID-19 outcomes, and higher praziquantel treatment coverage could reduce COVID-19 active cases and improve the recovery rate.
Keywords: African countries, COVID-19 outcomes, interactions, interventional programmes, schistosomiasis PCT coverage
Introduction
Coronavirus disease 2019 (COVID-19), which became a pandemic early in the year 2020, has caused a serious public health problem with significant global disease burden and mortality. The COVID-19 pandemic has reached every corner of the world and has inflicted serious economic loss, strained healthcare systems and negatively impacted education in countries worldwide [1]. The disease is currently rapidly spreading in many African countries most affected by the neglected tropical diseases (NTDs). There is currently transmission of COVID-19 in 57 countries in Africa, with over 5 million active cases and about 600 000 deaths [2].
In the wake of the COVID-19 pandemic, the World Health Organization's COVID-19 guidelines have urged countries to pause the ongoing NTDs intervention programmes in many African countries, including that of schistosomiasis. Schistosomiasis is a waterborne disease and ranks among the top 5 NTDs that require mass drug administration (MDA) [3]. About 93% of the world's total cases of schistosomiasis, totaling approximately 207 million, are from sub-Saharan African countries [4].
Although COVID-19 has been widely linked to several noncommunicable diseases, with a rise in case fatality with comorbidity [5,6], little information is available on the interaction of the disease with other infectious agents. Case series of HIV patients with COVID-19 so far in different countries have shown no clear evidence of an association between the two diseases [[7], [8], [9]]. Besides the setbacks that countries working towards the elimination of NTDs may face as a result of the COVID-19 pandemic, it has been proposed that there is a possibility of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causal agent of COVID-19, to interact with parasitic infections, thus changing the rate of severe outcomes [10]. This type of interaction with COVID-19 was recently hypothesized to exist in malaria and NTDs [11]. This may not be unusual because of the well-established interactions these tropical parasitic diseases have with several virus-associated diseases.
Unlike in the past, when the roles of NTDs in infection control were trivialized, NTDs have garnered some attention during the COVID-19 pandemic because both diseases can largely be prevented by taking the same preventative measures. Clean water, good hygiene and adequate sanitation are the foundations for fighting NTDs as well as COVID-19 [12]. However, in a scenario of already established NTD infections, the specific roles of NTDs on transmission and outcomes of COVID-19 remain to be determined.
In this study, we attempted to evaluate the impact of schistosomiasis's (one of the NTDs) endemicity and preventive treatment on COVID-19 in African countries. We hope to spur policy makers to prioritize schistosomiasis and other NTD interventional programmes in the post–COVID-19 era.
Methods
Study area
The study utilized data from African countries where transmission of COVID-19 is currently ongoing. A total of 42 African countries including 15 East, 13 West, 6 Central, 5 South and 3 North African countries were included in the study. Only African countries with data for COVID-19 testing capacity were included in the study. Of these 42 African countries, 33 are currently endemic for schistosomiasis, while nine are not. As of 15 July 2020, the total number of COVID-19 confirmed cases in Africa was 13 659 210, with 5 099 766 active cases, 7 973 858 recovered cases and 585 586 deaths.
Data sources
Schistosomiasis endemicity status and preventive chemotherapy (PCT) coverage index data were obtained from the World Health Organization's NTD databank [13]. The COVID-19 data used for the analyses were obtained from the COVID-19 Worldometer 13 July 2020 report [2]. The Worldometer provides a daily update on each country's COVID-19 situation. It provides information on the number of confirmed cases, active cases, recovery, deaths and testing capacity for each country. These four COVID-19 pandemic outcomes were used in this study.
Spatial data set
We used QGIS 3.10 software (https://qgis.org/) to generate the spatial maps for the sampling data set for schistosomiasis treatment coverage and COVID-19 outcomes in 42 African countries. The data structure, which is an essential step in data cleaning, was done in Google Sheets to normalize the data set for seamless integration into the database and visualization in QGIS. In QGIS the attribute data were merged with spatial data using the Join Attribute by Location Tool; this created a database containing the COVID-19 outcomes and schistosomiasis treatment coverage, with the Africa shape file obtained from the Natural Earth database. Each point of the variables' attribute data was visualized in QGIS using Equal Count (Quantile) mode with five classes. The classes represent the percentage of each attribute data within the database; this was visualized using different colour ramps.
Statistical analysis
Data obtained from Worldometer were exported to an Excel spreadsheet. Because the COVID-19 testing capacity for the countries is different, an adjustment was made by dividing the confirmed cases by the total number of people tested in each country. Then the percentages of confirmed cases, active cases, recoveries and deaths were calculated, as shown in equations (1), (2), (3), (4). The countries were classified into schistosomiasis-endemic and -nonendemic areas. The 33 schistosomiasis-endemic African countries were further classified according to the NTD PCT coverage index [3]. The classifications were as follows: 0 to 25% indicated not on track, >25 to 75% indicated progressing and >75% indicated on track.
| (1) |
| (2) |
| (3) |
| (4) |
Data were analysed by SPSS 23.0 software (IBM, Armonk, NY). The statistical hypothesis of difference in schistosomiasis endemicity in each of COVID-19 outcomes in the African countries was determined by the independent t test, with the t statistic defined as
| (5) |
where is the mean of observations in the endemic group for each COVID-19 outcome, is the mean of observations in the nonendemic group for each COVID-19 outcome and are the number of observations in the endemic and nonendemic groups respectively.
Equally, one-way analysis of variance (ANOVA) was used to test for significant differences in COVID-19 outcomes among the three schistosomiasis PCT coverage indexes. The null hypothesis that the means of the three schistosomiasis PCT groups are equal ( against the alternative that the means of the three groups are not equal using ANOVA with F statistics was defined as
| (6) |
where represent the size of observation and sample mean of each of the three PCT coverage groups respectively, while k, N and denote the three independent schistosomiasis PCT coverage groups, total number of data points and the overall mean of the total data points respectively.
Each of the hypotheses was tested at a 5% level of significance and where necessary, post hoc analysis was conducted by Duncan multiple range test.
Results
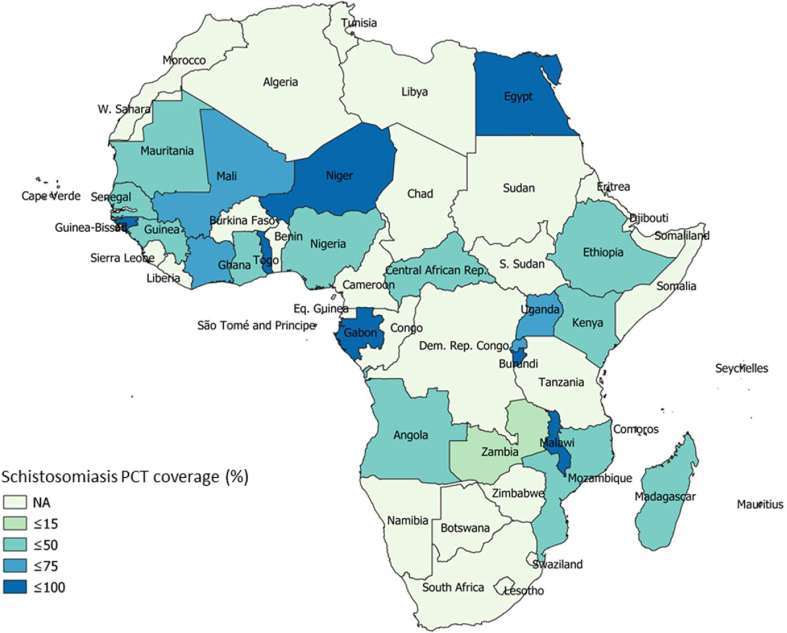
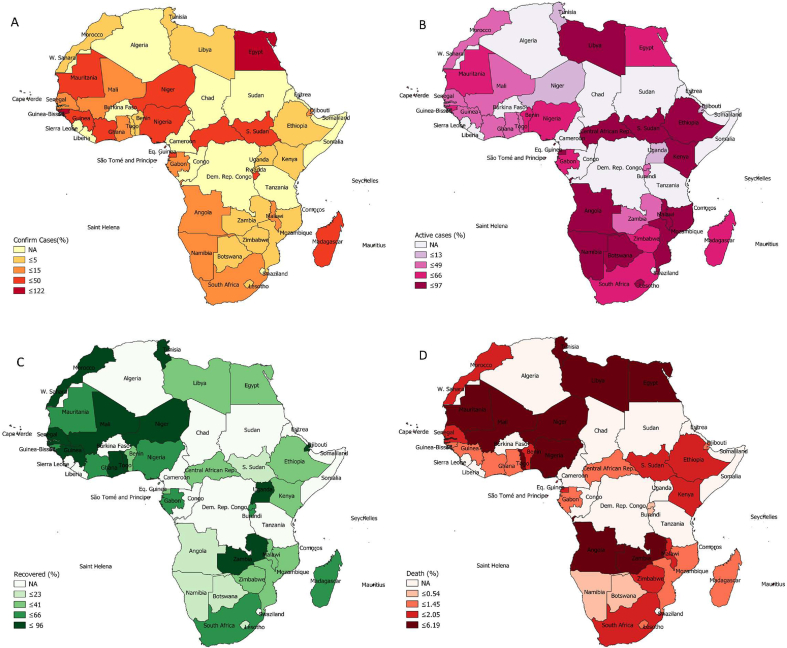
The spatial maps of schistosomiasis treatment coverage and COVID-19 pandemic outcomes in African countries are presented in Fig. 1, Fig. 2. Generally, unfavourable COVID-19 pandemic outcomes were observed in schistosomiasis-endemic African countries compared to schistosomiasis-nonendemic countries (Table 1). There was a deviation from this general observation in confirmed and active COVID-19 cases relative to schistosomiasis treatment coverage in some countries (Fig. 1, Fig. 2). The percentage of COVID-19 confirmed cases relative to the total tested was higher in schistosomiasis-endemic African countries (17.03 ± 23.55) when compared to that of nonendemic African regions (6.47 ± 9.54). However, this variation was not statistically significant (p > 0.05). More importantly, the percentage of active cases of COVID-19 relative to the confirmed cases was significantly higher in schistosomiasis-endemic countries (51.51 ± 23.04) compared to the nonendemic (30.51 ± 31.81) regions of the African continent (p < 0.05). The percentage of individuals who recovered from infections due to SARS-CoV-2 was significantly higher in schistosomiasis-nonendemic areas (67.86 ± 31.60) than in schistosomiasis-endemic areas (46.61 ± 22.81) (p < 0.05). The percentage of deaths recorded due to COVID-19 was higher in schistosomiasis-endemic areas (1.94 ± 1.56) compared to schistosomiasis-nonendemic areas (1.80 ± 1.14), although the difference was not significant (p > 0.05) (Table 1).
Fig. 1.
Schistosomiasis preventive treatment coverage (PCT) in African countries.
Fig. 2.
Coronavirus disease 2019 (COVID-19) pandemic outcomes in African countries after adjusting for testing capacity. (A) Confirmed cases. (B) Active cases. (C) Recovery. (D) Death.
Table 1.
Outcomes of African region coronavirus disease 2019 (COVID-19) pandemic relative to endemicity of schistosomiasis
| COVID-19 outcome | Schistosomiasis status | N | Mean | Standard error | t statistic (p) |
|---|---|---|---|---|---|
| Confirmed cases to total tested (%) | Endemic | 33 | 17.03 | 4.099 | 1.306 (0.199) |
| Nonendemic | 9 | 6.47 | 3.180 | ||
| Active cases to confirmed cases (%) | Endemic | 33 | 51.51 | 4.011 | 2.230 (0.031) |
| Nonendemic | 9 | 30.51 | 10.60 | ||
| Recovery to confirmed cases (%) | Endemic | 33 | 46.61 | 3.971 | −2.258 (0.029) |
| Nonendemic | 9 | 67.68 | 10.53 | ||
| Deaths to confirmed cases (%) | Endemic | 32 | 1.94 | 0.2740 | 0.245 (0.807) |
| Nonendemic | 9 | 1.80 | 0.3804 |
Our results showed that the percentage of confirmed COVID-19 cases was significantly higher in African countries that attained >75% schistosomiasis PCT coverage index (36.14 ± 16.1) compared to countries that have only attained 0–25% (7.13 ± 1.6) and >25–75% (16.92 ± 4.34) schistosomiasis treatment coverage (p < 0.05) (Table 2). Although not significant, the percentages of active COVID-19 cases in African countries which have attained >25–75% (44.47 ± 5.76) and >75% (44.8 ± 9.22) schistosomiasis PCT coverage index were lower than those with schistosomiasis PCT coverage index 0–25% (49.02 ± 6.29) (p > 0.05). The recovery rates from COVID-19 were also higher in African countries with higher schistosomiasis PCT coverage index >25–75% (53.62 ± 5.94) and >75% (52.73 ± 8.91) than in those with lower schistosomiasis treatment index 0–25% (49.33 ± 6.23) (p > 0.05) (Table 2).
Table 2.
Coronavirus disease 2019 (COVID-19) pandemic outcomes in relation to schistosomiasis preventive chemotherapy (PCT) coverage index
| COVID-19 outcome | Schistosomiasis PCT coverage index (mean ± standard error) |
||
|---|---|---|---|
| 0–25%, not on track | >25–75%, progressing | >75%, on track | |
| Confirmed cases to total tested (%) | 7.13 ± 1.6b | 16.92 ± 4.34b | 36.14 ± 16.1a |
| Active cases to confirmed cases (%) | 49.02 ± 6.29a | 44.47 ± 5.76a | 44.8 ± 9.22a |
| Recovery to confirmed cases (%) | 49.33 ± 6.23a | 53.62 ± 5.94a | 52.73 ± 8.91a |
| Deaths to confirmed cases (%) | 1.66 ± 0.23a | 2.08 ± 0.50a | 2.47 ± 0.81a |
Values with different superscripts are significant at 5% level with a > b. Mean separation performed by Duncan multiple range test.
Discussion
Schistosomiasis continues to be a serious public health problem in Africa despite being an old disease. The inability of many governments of Africa to fully implement integrated preventive strategies, marked by shortfalls in water hygiene and sanitation, has made PCT a priority for a schistosomiasis control option [14]. Schistosomiasis has been reported to be closely related to some viral diseases such as HIV/AIDS and hepatitis [14,15]. While it is relatively difficult to promote a multidisease approach for the prevention of the aforementioned viral diseases relative to schistosomiasis, it is possible to have related platforms for research and operational control for COVID-19 and schistosomiasis. This is because clean water and good hygiene are important for the prevention of both diseases.
Clearly, our study showed unfavourable COVID-19 outcomes in African countries endemic for schistosomiasis, especially for the percentage of active cases and the recovery rate. There is currently no evidence to back up this claim, but previous studies have shown possible interactions of schistosomiasis with other viral diseases like HIV/AIDS and hepatitis. An experimental study on Schistosoma mansoni–infected rhesus macaques showed an increase in the replication of simian–human immunodeficiency virus and faster disease progression than in animals without S. mansoni infection [16]. A retrospective study among chronic hepatitis C patients revealed that although individuals with schistosomiasis had no changes in fibrosis staging, the parasitic disease was significantly associated with hepatitis treatment failure. The study further showed that schistosomiasis chemotherapy was not sufficient to improve hepatitis C management [17]. Our previous study also showed higher susceptibility to enteroviruses in S. mansoni–infected individuals [18]. These aforementioned studies in other viruses thus suggest that schistosomiasis may affect COVID-19 in two ways: by increasing increase susceptibility to SARS-CoV-2 and/or by resulting in possible treatment failure in COVID-19 active cases.
Helminths are typically associated with Th2-mediated immune responses through a variety of regulatory mechanisms [19]. The downregulation of the inflammation associated with Th2 immune response in schistosomiasis infection in endemic countries may impair immunity to COVID-19, thus resulting in increased susceptibility and higher occurrence of COVID-19 in schistosomiasis-endemic areas of Africa. It is important to state that polyparasitism is common in African countries, and the overall impact of this parasite-induced inflammation will depend on the sequence of infections and the burden of each [20]. Therefore, concomitant COVID-19 infection with schistosomiasis and parasitic NTDs could have a significant impact on the risks and severity of clinical manifestations of COVID-19 [11].
Surprisingly, our study showed a significant increase in confirmed cases of COVID-19 in African countries which are on track relative to schistosomiasis PCT (>75% PCT coverage index). While the reasons for this have not yet been ascertained, we suggest that the impact of emergence, reinfection and unsustained schistosomiasis PCT may stall the impact of even high-coverage praziquantel treatment over time. For example, Gabon, with a 90.7% schistosomiasis PCT coverage index, only had MDA once in 10 years before the 2018 MDA used for the analyses in this study (the first being in 2016), and it only covered 36.7% of the population [3]. In the same country, a 30% prevalence of schistosomiasis was recorded in 2018 [21], a situation that may be due to reinfection. In Malawi, with a 79.2% schistosomiasis PCT coverage index, there has been a sustained schistosomiasis MDA for the past 8 years (6–64.5% PCT coverage index); the country was only on track in 2015 (80% PCT coverage index) [3]. In addition, a recent study in Malawi has shown the emergence of schistosomiasis with a prevalence of 31.5% in a new district [22]. The implication of this is that favourable COVID-19 outcomes relative to confirmed cases might have been observed, had schistosomiasis MDA coverage of at least 75% been sustained for at least the past 10 years. Implementation of integrated preventive strategies in addition to chemotherapy could have prevented the emergence and reinfection of schistosomiasis common in many endemic countries.
Despite recording high COVID-19 confirmed cases in African countries with high praziquantel treatment coverage, our results showed improved COVID-19 outcomes relative to active cases and recovery rates compared to countries with very low treatment coverage (0–25%). This is encouraging. It may suggest a role of schistosomiasis PCT in modulating the immune system against SARS-CoV-2. Higher death rates in countries with higher praziquantel coverage may not be related to praziquantel administration. Because more children were likely to be enrolled onto schistosomiasis MDA (as is the usual practice in many endemic countries) than adults, the deaths observed could be as a result of COVID-19–associated complications common in the elderly population.
It is important to state that the data adopted for these analyses did not take into consideration confounders. Some of these confounding factors include the endemicity of other infectious agents, vaccination, sex ratio, and density and age of the population. These have been shown to significantly determine the outcomes of COVID-19 [23,24].
Conclusion
Many advocacies for the resumption of treatment or vaccination (post–COVID-19 era) are currently ongoing, and it is possible that diseases like HIV/AIDS, malaria, tuberculosis, cholera and measles are prioritized, as this has been a longtime practice in Africa. No doubt preventive programmes in schistosomiasis, which is second only to malaria in term of morbidity, should be prioritized in the post–COVID-19 era. Advocacy for the resumption of the PCT in the post–COVID-19 era should be extended to schistosomiasis and other NTDs. Our study has not only shown that schistosomiasis may increase the rate of unfavourable COVID-19 pandemic outcomes but also that the treatment of schistosomiasis could decrease active COVID-19 cases and increase the recovery rate. The eradication of schistosomiasis and other NTDs through PCT and other integrated approaches could enhance the management of future pandemics. Governments in African countries, the World Health Organization and other local and international partners in schistosomiasis control should ensure immediate resumption of preventive programmes in endemic countries post–COVID-19 era. These programmes should be integrated into other NTD implementation programmes in Africa.
Conflict of interest
None declared.
References
- 1.Rundle A.G., Park Y., Herbstman J.B., Kinsey E.W., Wang Y.C. COVID-19–related school closings and risk of weight gain among children. Obesity. 2020 doi: 10.1002/oby.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldometer. Available at: https://www.worldometers.info/coronavirus/#countries.
- 3.Uniting to Combat Neglected Tropical Diseases . 2017. Neglected tropical diseases—profile for mass treatment of NTDs.https://unitingtocombatntds.org/wp-content/uploads/2019/02/UTC_CP_NIGERIA Available at: [Google Scholar]
- 4.Hotez P.J., Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinosa O.A., Zanetti A.S., Antunes E.F., Longhi F.G., Matos T.A., Battaglini P.F. Prevalence of comorbidities in patients and mortality cases affected by SARS-CoV2: a systematic review and meta-analysis. Rev Inst Med Trop São Paulo. 2020;62:e43. doi: 10.1590/S1678-9946202062043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;14(55):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco J.L., Ambrosioni J., Garcia F., Martínez E., Soriano A., Mallolas J. COVID-19 in HIV Investigators. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020 doi: 10.1016/S2352-3018(20)30111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W., Ming F., Dong Y., Zhang Q., Zhang X., Mo P. Social Science Research Network (SSRN); 2020. A survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China.https://ssrn.com/abstract=3550029 [DOI] [Google Scholar]
- 9.Härter G., Spinner C.D., Roider J., Bickel M., Krznaric I., Grunwald S. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020 doi: 10.1007/s15010-020-01438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutman J.R., Lucchi N.W., Cantey P.T., Steinhardt L.C., Samuels A.M., Kamb M.L. Malaria and parasitic neglected tropical diseases: potential syndemics with COVID-19? Am J Trop Med Hyg. 2020 doi: 10.4269/ajtmh.20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polo M.R. World Health Organization Regional Office for Africa; 2020. Ending neglect to drive progress: how controlling neglected tropical diseases can curb COVID-19. ESPEN Team Leader.https://www.speakupafrica.org/ending-neglect-to-drive-progress-how-controlling-neglected-tropical-diseases-can-curb-covid-19/ Available at: [Google Scholar]
- 13.World Health Organization (WHO) 2020. Neglected tropical diseases: PCT databank schistosomiasis.https://www.who.int/neglected_diseases/preventive_chemotherapy/sch/en/ Available at: [Google Scholar]
- 14.Bustinduy A., King C., Scott J., Appleton S., Sousa-Figueiredo J.C., Betson M. HIV and schistosomiasis co-infection in African children. Lancet Infect Dis. 2014;14:640–649. doi: 10.1016/S1473-3099(14)70001-5. [DOI] [PubMed] [Google Scholar]
- 15.Gasim G.I., Bella A., Adam I. Schistosomiasis, hepatitis B and hepatitis C co-infection. Virol J. 2015;12:19. doi: 10.1186/s12985-015-0251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayash-Rashkovsky M., Chenine A.L., Steele L.N., Lee S.J., Song R., Ong H. Coinfection with Schistosoma mansoni reactivates viremia in rhesus macaques with chronic simian–human immunodeficiency virus clade C infection. Infect Immun. 2007;75:1751–1756. doi: 10.1128/IAI.01703-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Rahman M., El-Sayed M., El Raziky M., Elsharkawy A., El-Akel W., Ghoneim H. Coinfection with hepatitis C virus and schistosomiasis: fibrosis and treatment response. World J Gastroenterol. 2013;19:2691–2696. doi: 10.3748/wjg.v19.i17.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adekolujo D.R., Olayinka S.O., Adeniji J.A., Oyeyemi O.T., Odaibo A.B. Poliovirus and other enteroviruses in children infected with intestinal parasites in Nigeria. J Infect Dev Ctries. 2015;9:1166–1171. doi: 10.3855/jidc.5863. [DOI] [PubMed] [Google Scholar]
- 19.Maizels R.M., McSorley H.J. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138:666–675. doi: 10.1016/j.jaci.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Supali T., Verweij J.J., Wiria A.E., Djuardi Y., Hamid F., Kaisar M.M.M. Polyparasitism and its impact on the immune system. Int J Parasitol. 2010;40:1171–1176. doi: 10.1016/j.ijpara.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Dejon-Agobé J.C., Zinsou J.F., Honkpehedji Y.J., Ateba-Ngoa U., Edoa J.R., Adegbite B.R. Schistosoma haematobium effects on Plasmodium falciparum infection modified by soil-transmitted helminths in school-age children living in rural areas of Gabon. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayuni S., O’Ferrall A.M., Baxter H., Hesketh J., Mainga B., Lally D. Primary school children on the shoreline of Lake Malawi, Mangochi District, Malawi. Research paper preprint; 2020. An outbreak of intestinal schistosomiasis, alongside increasing urogenital schistosomiasis prevalence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayse Y. Social Science Research Network (SSRN); 2020. Factors affecting the number of COVID-19 cases and the death rate: empirical evidence from the German states.https://ssrn.com/abstract=3617986 [DOI] [Google Scholar]
- 24.de Freitas E., Silva R., Pitzurra R. What are the factors influencing the COVID-19 outbreak in Latin America? Travel Med Infect Dis. 2020;35:101667. doi: 10.1016/j.tmaid.2020.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]