Abstract
Background and purpose
During the COVID-19 outbreak, the presence of extensive white matter microhemorrhages was detected by brain MRIs. The goal of this study was to investigate the origin of this atypical hemorrhagic complication.
Methods
Between March 17 and May 18, 2020, 80 patients with severe COVID-19 infections were admitted for acute respiratory distress syndrome to intensive care units at the University Hospitals of Strasbourg for whom a brain MRI for neurologic manifestations was performed. 19 patients (24%) with diffuse microhemorrhages were compared to 18 control patients with COVID-19 and normal brain MRI.
Results
The first hypothesis was hypoxemia. The latter seemed very likely since respiratory failure was longer and more pronounced in patients with microhemorrhages (prolonged endotracheal intubation (p = 0.0002), higher FiO2 (p = 0.03), increased use of extracorporeal membrane oxygenation (p = 0.04)). A relevant hypothesis, the role of microangiopathy, was also considered, since patients with microhemorrhages presented a higher increase of the D-Dimers (p = 0.01) and a tendency to more frequent thrombotic events (p = 0.12). Another hypothesis tested was the role of kidney failure, which was more severe in the group with diffuse microhemorrhages (higher creatinine level [median of 293 µmol/L versus 112 µmol/L, p = 0.04] and more dialysis were introduced in this group during ICU stay [12 versus 5 patients, p = 0.04]).
Conclusions
Blood–brain barrier dysfunction secondary to hypoxemia and high concentration of uremic toxins seems to be the main mechanism leading to critical illness-associated cerebral microbleeds, and this complication remains to be frequently described in severe COVID-19 patients.
Keywords: COVID-19, Microhemorrhages, MRI, Hypoxemia, Kidney failure
Introduction
Recently, some cerebral complications related to COVID-19 were depicted on brain MRIs. Diffuse microbleeds, located in the white matter (WM) with an atypical distribution affecting, in particular, the corpus callosum, has recently been described in critically ill patients with COVID-19 [1–9]. A recent neuropathological study has also described hemorrhagic lesions disseminated throughout cerebral WM in a patient with severe COVID-19 [10]. Another pathological study [11] described the autopsies of six patients with COVID-19, and among them, four presented diffuse petechial hemorrhages in the brain. These observations can be paralleled with the «critical illness-associated cerebral microbleeds (CIAM)» described in a 10 year follow-up on 12 patients [12].
However, the underlying pathophysiological mechanisms leading to diffuse microbleeds in the COVID-19 pandemic context remain unknown. As summarized in Table 1, several hypotheses were proposed, notably those concerning the potential role of hypoxemia, microangiopathy, or coagulopathy. Our aim in this study is to discuss and address the origin of this atypical hemorrhagic complication in the context of COVID-19, by studying in particular, the three main mechanisms above-mentioned.
Table 1.
Review of studies reporting disseminated white matter microhemorrhages during COVID-19 outbreak
| Author | Study area | Number of cases | Hypotheses raised |
|---|---|---|---|
| Radmanesh [1] | New York (monocentric) | 7 | Hypoxemia/small vessel vasculitis |
| Kremer [2] | France (multicentric) | 9 | Hypoxemia/small vessel vasculitis |
| Vattoth [3] | Qatar (monocentric) | 1 | Hypoxemia |
| Chougar [4] | Paris (monocentric) | 8 | ECMO/anticoagulation overdose/microangiopathy |
| Klironomos [5] | Stockholm (monocentric) | 23 | Hypoxemia/microangiopathy/endothelial injury/ECMO |
| Lin [6] | New York [2hospitals] | 3 | Mechanical ventilation |
| Paterson [7] | UK (multicentric) | 4 | Endothelial injury |
| Fitsiori [8] | Geneva (monocentric) | 9 | Endothelial injury related to SARS-CoV-2/microangiopathy/ECMO/Hypoxemia/disseminated intravascular coagulation/hypercoagulable state |
| Freeman [9] |
Philadelphia (monocentric) |
4 | Thrombotic microangiopathy/hypoxemia |
Material and methods
This retrospective monocentric study was approved by the ethical committee of Strasbourg University Hospital (CE-2020–37) and was in accordance with the 1964 Helsinki Declaration and its later amendments. Informed consents were waived.
Patient cohort
Between March 17 and May 18, 2020, 80 patients with severe COVID-19 infections were admitted for acute respiratory distress syndrome (ARDS) to intensive care units (ICUs) at the University Hospitals of Strasbourg. A cerebral MRI was performed to explain the neurologic manifestations. The diagnosis of COVID-19 was validated with the detection of SARS-CoV-2 by reverse transcriptase–polymerase chain reaction (RT-PCR) assays on upper or lower respiratory tract swabs.
Brain MRI interpretation
All MR studies were performed on a 3 T MR scanner, and all patients performed a 3-dimensional susceptibility-weighted imaging sequence with phase mapping. A standardized imaging protocol was made including in addition: 3D T1-weighted spin-echo MRI with and without contrast enhancement, diffusion-weighted imaging, dynamic susceptibility contrast MR perfusion, 3D FLAIR after administration of gadolinium-based contrast agent, and delayed post-contrast FLAIR.
All brain MRIs were retrospectively reviewed independently by two neuroradiologists (S.K, and F.L. with 20 and 9 years of experience in neuroradiology), who reached a consensus concerning the final diagnosis.
The diagnosis of CIAM was made when brain MRIs showed diffuse brain microhemorrhages, which predominantly involved the corpus callosum (especially the genu and the splenium of the corpus callosum), the subtentorial juxtacortical WM, the internal capsule, the brainstem, the middle cerebellar peduncles, and the cerebellum. The cortex, the basal ganglia, and the thalamus were spared.
In our study, the involvements of the corpus callosum, internal capsule, and middle cerebellar peduncles were mandatory for the diagnosis of CIAM.
The main differential diagnoses were: cerebral amyloid angiopathy (but the extensive corpus callous involvement was atypical, and none of the patients showed cortical superficial siderosis), chronic hypertension (yet the grey matter was spared), and diffuse axonal injury (however, none of our patients had a history of head injury).
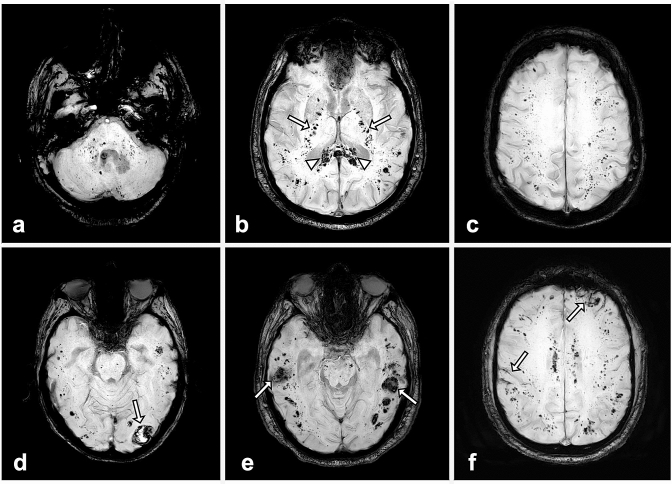
Among the 80 patients above-mentioned, 19 (24%) presented diffuse microhemorrhages located in the WM (Fig. 1), compatible with CIAM.
Fig. 1.
Susceptibility-weighted MR images in four different critically ill COVID-19 patients: a–c: diffuse microhemorrhages in a 66-year-old man appearing as multiple small hypointense foci within the brainstem and cerebellum (a), the splenium of the corpus callosum (arrowheads) and the internal capsules (arrows) (b), the juxtacortical white matter (c). d, e: Juxtacortical hematomas (arrows) associated with diffuse microhemorrhages in men of 60 (d) and 67 years (e). f subarachnoid hemorrhages (arrows) associated with diffuse microhemorrhages in a 57-year-old man.
Study design
We retrospectively compared these 19 patients to 18 other ICU patients with COVID-19, similar ARDS, and neurological manifestations, but with a normal cerebral MRI, to understand the underlying pathophysiological mechanisms. The control group's brain MRIs were normal, except for leptomeningeal enhancement, common findings during the COVID-19 outbreak, and present in seven patients in the control group and six patients in the CIAM group. Clinical and laboratory data were extracted from the patients’ electronic medical records in the Hospital Information System. Clinical and biological data were reviewed by neurologists and intensivists. The laboratory findings were analyzed at the time of admission to the ICU and during ICU stay before brain MRI. In this latter situation, the worst value has been kept in the case of redundancy of the tests.
According to the Berlin definition [13], all patients had an ARDS, and, therefore, a PaO2/FiO2 ratio of less than 300 mmHg. The severity of an ARDS is further sub-classified into mild (PaO2/FiO2 200 to 300 mmHg), moderate (PaO2/FiO2 100 to 200 mmHg), and severe (PaO2/FIO2 ≤ 100 mm Hg) [13]. The severity of ARDS was also evaluated in our study by the higher inspiratory oxygen fraction (FiO2) delivered during ICU stay, by the number of days intubated, and by the requirement or not of extracorporeal membrane oxygenation (ECMO). Moreover, we evaluated the percentage of lung involvement in chest computed tomography with a numeric scale: 0, 1 (< 10%), 2 (10–25%), 3 (25–50%), 4 (50–75%), 5 (> 75%) [14].
Statistical analysis
Data were described using frequency and proportion (n, %) for categorical variables, using median, first and third quartile, and range for quantitative data.
Categorical data were compared using Fisher exact test. Quantitative data were compared using Mann–Whitney–Wilcoxon test. A p value lower than 0.05 was considered significant.
Results
The clinical features are summarized in Table 2.
Table 2.
Epidemiologic profile and neurologic manifestations
| Population of COVID-19 patients with extensive white matter microhemorrhages (n = 19) | Population of COVID-19 patients without extensive white matter microhemorrhages (n = 18) | p value | |
|---|---|---|---|
| Sex | |||
| Male/female | 16 (84%)/3 (16%) | 13 (72%)/5 (28%) | 0.44 |
| Age (years) | |||
| Range/median (Q1–Q3) | 44–79/66 (60–70) | 21–72/62 (55–68) | 0.17 |
| History of stroke | 3 (16%) | 1 (6%) | 0.6 |
| Cardiovascular risk factors | |||
| High blood pressure | 7 (37%) | 10 (56%) | 0.33 |
| Type 2 diabetes mellitus | 2 (11%) | 6 (33%) | 0.12 |
| Hyperlipidemia | 7 (37%) | 7 (39%) | 1 |
| Smoking | 4 (21%) | 2 (11%) | 0.65 |
| Weight (kg) | 90 (78–95) | 89 (80–97) | 0.87 |
| Body mass index | 29 (26–32) | 28 (26–33) | 1 |
| Intensive care unit stay | |||
| Duration (days) | 34 (29–45) | 17 (11–21) | 0.0004 |
| Time between intubation and brain MRI (days) | 26 (20–31) | 12 (6–18) | 0.0003 |
| Hospital length of stay (days) | 44 (40–54) | 32 (22–37) | 0.007 |
| Hospital discharge | 7 (37%) | 13 (72%) | 0.04 |
| Death of the patient | 4 (21%) | 1 (6%) | 0.33 |
| Neurologic manifestations leading to the realization of the brain MRI | |||
| Seizures | 4 (21%) | 0 | 0.1 |
| Clinical signs of corticospinal tract involvement | 3 (16%) | 4 (22%) | 0.69 |
| Disturbance of consciousness | 13 (68%) | 2 (11%) | 0.0006 |
| Confusion | 5 (26%) | 12 (67%) | 0.02 |
| Agitation | 4 (21%) | 10 (56%) | 0.04 |
| Pathological wakefulness when sedative therapies were stopped | 17 (89%) | 14 (78%) | 0.4 |
Data are number and percentage or median associated with first and third quartile
All statistical test results are in italics. Statistically significant results are in bold italics
Among the 19 patients with a diagnosis of CIAM, some of them were associated with other hemorrhagic complications, such as subarachnoid hemorrhages (5/19, 26%), and intracerebral hematomas (4/19, 21%) (Fig. 1). Five patients presented with convexal subarachnoid hemorrhage, as described in Fig. 1. The bleedings were localized to the brain's convexities with preferential frontal lobe involvement, usually multiple and bilateral, without extension into basal cisterns. Venous structures were excluded by the hyperintensities seen with FLAIR MR imaging.
Four patients presented with intracerebral hematomas, unique in three cases and multiple for one patient. Their maximum sizes for each patient were 2.3 cm, 2.5 cm, 2.7 cm, and 7.7 cm.
Neurologic manifestations (Table 2)
Even if both groups demonstrated a high prevalence of pathological wakefulness when the sedative therapies were stopped, the neurological status seemed more severe in case of extensive WM microhemorrhages: indeed, this group showed more impaired consciousness (p = 0.0006), whereas the second group was associated with less severe clinical signs such as confusion (p = 0.02) and agitation (p = 0.04). Four patients in the CIAM group had seizures, and among them, two also had subarachnoid hemorrhages, and one had a more extensive intracerebral hematoma.
Hypoxemia hypothesis (Table 3)
Table 3.
Respiratory status during ICU stay
| Population of COVID-19 patients with extensive white matter microhemorrhages (n = 19) | Population of COVID-19 patients without extensive white matter microhemorrhages (n = 18) | p value | |
|---|---|---|---|
| Number of days intubated | 24 (19–25) | 8 (4–14) | 0.0002 |
| Higher FiO2 (%) | 100 (92–100) | 75 (63–100) | 0.03 |
| Lower PaO2/FiO2 | 81 (59–104) | 104 (80–124) | 0.07 |
| Extracorporeal membrane oxygenation | 5 (26%) | 0 | 0.04 |
| Extension of pulmonary involvement with computed tomography (score from 0 (no injury) to 5 (≥ 75%) | 4 (4–5) | 4 (2.5–4) | 0.04 |
Data are number and percentage or median associated with first and third quartile
All statistical test results are in italics. Statistically significant results are in bold italics
During ICU stay, before brain MRI, patients with CIAM showed a more severe respiratory status in comparison with those with a normal brain MRI. In fact, these patients required prolonged endotracheal intubation (median of 24 days versus 8 days, p = 0.002) for a more severe ARDS, as evidenced by a tendency in a lower PaO2/FiO2 (median of 81 mmHg versus 104 mmHg, p = 0.07), a higher FiO2 (median of 100% versus 75%, p = 0.03), and increased use of ECMO (5 patients versus 0, p = 0.04) during their stay. Similarly, the pulmonary involvement on chest computed tomography was more severe in the group with diffuse WM microhemorrhages (p = 0.04).
Microangiopathy–microthrombi hypothesis (Tables 2 and 4)
Table 4.
Laboratory findings and significant events during ICU stay
| Population of COVID-19 patients with extensive white matter microhemorrhages (n = 19) | Population of COVID-19 patients without extensive white matter microhemorrhages (n = 18) | p value | |
|---|---|---|---|
| Laboratory findings at the time of ICU admission | |||
| White blood cell count, × 109/L | 8.7 (4.9–12) | 6.9 (5.8–9.7) | 0.36 |
| Lymphocyte count, × 109/L | 0.71 (0.47–1.24) | 0.84 (0.68–1.12) | 0.6 |
| Haemoglobin, g/L | 119 (96–134) | 128 (118–137) | 0.18 |
| Platelet count, × 109/L | 200 (158–242) | 168 (160–272) | 0.68 |
| C-reactive protein, mg/L | 183 (112–281) | 119 (91–189) | 0.15 |
| Alanine aminotransferase U/L | 48 (30–63) | 43 (26–81) | 0.98 |
| Aspartate aminotransferase, U/L | 71 (59–91) | 64 (34–89) | 0.45 |
| Urea, mmol/L | 13 (8–17) | 7 (5–10) | 0.003 |
| Creatinine, µmol/L | 81 (69–248) | 79 (67–102) | 0.35 |
| Prothrombin time, s | 15.4 (14.3–16.1) | 14.8 (13.4–17.4) | 0.5 |
| Activated partial thromboplastin time, s | 39 (37–43) | 38 (36–40) | 0.43 |
| International normalized ratio (INR) | 1.17 (1.1–1.23) | 1.1 (1–1.35) | 0.35 |
| Antithrombin III (%) | 85 (72–96) | 94 (78–101) | 0.23 |
| Fibrinogen, g/L | 6.8 (6.3–7.8) | 6.2 (5.5–7.4) | 0.27 |
| d-dimers, mg/L | 3.1 (1.8–6.8) | 1.7 (1.1–2) | 0.01 |
| Laboratory findings during ICU stay and before brain MRI | |||
| Lower platelet count, × 109/L | 140 (108–204) | 163 (152–195) | 0.36 |
| Lower fibrinogen, g/L | 5.1 (4.5–5.8) | 5.8 (4.9–7.2) | 0.44 |
| Higher prothrombin time, s | 16.5 (15.9–17.5) | 17.4 (15.8–19) | 0.46 |
| Higher d-dimers, mg/L | 11.5 (7.5–20) | 4.6 (3–12) | 0.08 |
| Disseminated intravascular coagulation According to the criteria endorsed by the ISTH | 1 (5%) | 2 (11%) | 0.6 |
| Higher fibrinogen, g/L | 9 (7.6–10.4) | 8.8 (6.8–9.4) | 0.26 |
| Higher C-reactive protein, mg/L | 276 (185–382) | 196 (117–294) | 0.09 |
| Higher Creatinine, µmol/L | 293 (154–387) | 112 (91–220) | 0.04 |
| Lupus anticoagulant, data are n/N (%) | 15/19 (79%) | 10/12 (83%) | 1 |
| Thrombotic events during ICU stay | 7 (37%) | 2 (11%) | 0.12 |
| Treatment initiated during hospitalization before brain MRI | |||
| Dialysis | 12 (63%) | 5 (28%) | 0.04 |
| Anticoagulant therapy | 19 (100%) | 18 (100%) | 1 |
| Hydroxychloroquine | 7 (37%) | 8 (44%) | 0.74 |
| Lopinavir/ritonavir | 7 (37%) | 9 (50%) | 0.51 |
Data are number and percentage or median associated with first and third quartile
All statistical test results are in italics. Statistically significant results are in bold italics
The two groups did not differ concerning the distribution of cardiovascular risk factors. A significant proportion of our patients were at least classified as overweight (first quartile of 26 kg/m2 in the two groups).
At the ICU admission time, the D-dimers levels were higher in the group with diffuse microhemorrhages (median of 3.1 mg/L versus 1.7 mg/L, p = 0.01). A similar trend was observed during ICU stay (higher D-dimers levels of 11.5 mg/L versus 4.6 mg/L, p = 0.08).
There seemed to be more thrombotic events (7 versus 2 patients, p = 0.12), and a higher CRP level (median of 276 mg/L versus 196 mg/L, p = 0.09) during ICU stay in the group with CIAM.
The percentage of positive specimens for lupus anticoagulant was close in both groups (79% versus 83%, p = 1).
Disseminated intravascular coagulation hypothesis
According to the criteria endorsed by the International Society on Thrombosis and Haemostasis [15], only one case of disseminated intravascular coagulation was present in the group with CIAM, and two cases in the second group (p = 0.6). The other coagulation biomarkers were strictly comparable between both groups at the time of admission and during ICU stay, and all patients received anticoagulant therapy during hospitalization.
Other results
The kidney failure was more severe in the group with diffuse microhemorrhages, as evidenced by a higher creatinine level (median of 293 µmol/L versus 112 µmol/L, p = 0.04) and more dialysis were introduced in this group during ICU stay (12 versus 5 patients, p = 0.04).
The two groups were comparable concerning the use of hydroxychloroquine or lopinavir/ritonavir.
Cerebrospinal fluid (CSF) analysis (Table 5)
Table 5.
Cerebrospinal fluid analysis
| CSF analysis: data are n/N (%) | Normal range | Population of COVID-19 patients with extensive white matter microhemorrhages (n = 19) | Population of COVID-19 patients without extensive white matter microhemorrhages (n = 18) | p value |
|---|---|---|---|---|
| High white blood cell count | < 5/mm3 | 2/10 (20%) | 0/14 (0%) | 0.16 |
| Low glycorrhachia | > 50% of the concentration of blood glucose | 0/10 (0%) | 0/14 (0%) | 1 |
| High proteinorachia | 0.15–0.45 g/L | 3/10 (30%) | 1/14 (7%) | 0.27 |
| Elevated Immunoglobulin G | 10-34 mg/L | 3/10 (30%) without concomitant increase of the Tibbling-link IgG index | 1/14 (7%) without concomitant increase of the Tibbling-link IgG index | 0.27 |
| Elevated albumin | 130–350 mg/L | 1/10 (10%) | 0/14 (0%) | 0.41 |
| Increased albumin quotient | Age related | 3/10 (30%) | 2/14 (14%) | 0.61 |
| Presence of oligoclonal IgG bands | _ | 6/10 (60%) with the same pattern in the serum (type IV) | 6/14 (43%): 2 CSF-specific IgG oligoclonal bands (type II) and 4 with the same pattern in the serum (type IV) | 0.68 |
| Positive RT-PCR SARS-CoV-2 | _ | 0/10 (0%) | 1/14 (7%) | 1 |
| High Interleukin-6 | (0-13 pg/mL) | 4/6 (67%) | 4/6 (67%) | 1 |
N is the total number of patients with available data, and n the number of positive patients
10 patients underwent a lumbar puncture in the first group and 14 in the second group. The two populations were strictly comparable, and only one patient demonstrated the presence of SARS-CoV-2 on RT-PCR in the control group. In 60% of cases of CIAM, oligoclonal IgG bands identical in serum and CSF (type IV) were present.
Discussion
Since the discovery of cerebral microhemorrhages in patients with COVID-19, several assumptions have been made about the pathogenesis of CIAM listed in Table 1; the presence of cerebral microhemorrhages is a bad-known indicator and reports in patients with severe COVID-19 in imaging [1–9], and neuropathological studies [10, 11] increased steadily.
The goal of this study was to evaluate the more likely hypotheses that applied in patients with COVID-19.
Due to the resemblance between CIAM and what is seen in high-altitude cerebral edema [16, 17], the first hypothesis raised concerns hypoxemia. This assumption seems very likely since patients with diffuse microhemorrhages had longer or more pronounced respiratory failure.
Even if the exact mechanisms remain unclear, hypoxemia seems associated with disruptions in the blood–brain barrier, leading to extravasation of erythrocytes. In 30% of the patients for whom a lumbar puncture was realized, their results were consistent with blood–brain barrier disruption (elevated ImmunoglobulinG without a concomitant increase of the Tibbling-link IgG index, and increased albumin quotient).
It is known that ECMO may be associated with brain hemorrhagic complications [18], and the use of ECMO itself may induce, in some cases, diffuse WM microhemorrhages [19]. In our study, five patients diagnosed with CIAM were treated with ECMO and none in the second group. However, it is impossible to say whether these hemorrhagic complications are a specific complication of ECMO or only related to the respiratory state with severe hypoxemia.
The second hypothesis evaluated was the role of microangiopathy. A recent histopathologic study [20], which focused on the pulmonary vessels in patients with COVID-19, described microangiopathy with disseminated microthrombi. This assumption concerning microangiopathy with diffuse microthrombi may also explain the extensive brain microhemorrhages: patients expressed higher D-dimers levels upon ICU admission, tend to have a higher increase in D-dimers, C-reactive protein, and more thromboembolic events during their stay in the ICU.
Nevertheless, the differences between both groups were not pronounced; microthrombi typically cause punctate foci of restricted diffusion on brain MRI scans, which have not been observed in our patients.
The third new hypothesis was formulated based on our results: the role of kidney failure, which was more severe in the group with CIAM. It is known that chronic kidney disease and hemodialysis are associated with a high incidence of microbleeds [21, 22]. The underlying physiological mechanisms including increased permeability of the blood–brain barrier secondary to a high concentration of uremic toxins [21]. Indeed, the group of patients with COVID-19 diagnosed with CIAM have shown a more severe kidney failure in agreement with this new assumption.
A combination of the above assumptions should also be considered, but the number of patients in our cohort does not allow us to test the cross hypotheses. Further studies with more patients and more diversity are needed to test all cases.
Although possible, the hypothesis of coagulation disorders has not been supported by our data to be involved in the development of microbleeds on patients with COVID-19. Only one patient had a diagnosis of disseminated intravascular coagulation in the group CIAM. The other coagulation biomarkers were strictly comparable between both groups at the time of admission and during ICU stay, and all patients received anticoagulant therapy during hospitalization.
In the group CIAM, CSF analysis did not reveal any evidence of direct viral infection of the central nervous system (SARS-CoV-2 RNA was not detected, and no intrathecal synthesis was highlighted).
It is very likely that a significant number of patients with COVID-19-associated ARDS have had and will present this complication. Thereby, the diagnosis of CIAM by MRI is essential, and this entity should be known to avoid misdiagnoses such as hemorrhagic encephalitis related to SARS-CoV-2. Thus, it could have important implications for patients care. However, many uncertainties remain, particularly in regard to long-term prognosis and prevention. In other situations, outside the COVID-19 outbreak, it is recognized that cerebral microbleeds are independently associated with cognitive dysfunction [23]. The prognosis of our patients will also require further long-term follow-up studies.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CIAM
Critical illness-associated cerebral microbleeds
- CSF
Cerebrospinal fluid
- ECMO
Extracorporeal Membrane Oxygenation
- ICU
Intensive care unit
- RT-PCR
Reverse transcriptase–polymerase chain reaction
- WM
White matter
Funding
The authors declare no funding source.
Data availability
We state that the data published are available and anonymized and will be shared upon request by email to the corresponding author from any qualified investigator for purposes of replicating procedures and results.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
François Lersy and Thibault Willaume authors contributed equally to the work and are considered as co-first authors.
References
- 1.Radmanesh A, Derman A, Lui YW, et al. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020 doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kremer S, Lersy F, de Sèze J, et al. Brain MRI severe COVID-19: a retrospective observational study. Radiology. 2020 doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vattoth S, Abdelhady M, Alsoub H, Own A, Elsotouhy A. Critical illness-associated cerebral microbleeds in COVID-19. Neuroradiol J. 2020 doi: 10.1177/1971400920939229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chougar L, Shor N, Weiss N, et al. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS-CoV-2 infection and neurological manifestations. Radiology. 2020 doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klironomos S, Tzortzakakis A, Kits A, et al. Nervous system involvement in covid-19: results from a retrospective consecutive neuroimaging cohort. Radiology. 2020 doi: 10.1148/radiol.2020202791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin E, Lantos JE, Strauss SB, et al. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York City. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020 doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitsiori A, Pugin D, Thieffry C, Lalive P, Vargas MI. Unusual microbleeds in brain MRI of Covid-19 patients. J Neuroimaging. 2020 doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman CW, Masur J, Hassankhani A, Wolf RL, Levine JM, Mohan S. COVID-19-related disseminated leukoencephalopathy (CRDL): a retrospective study of findings on brain MRI. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020 doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanou EM, Coutinho JM, Shannon P, et al. Critical illness-associated cerebral microbleeds. Stroke. 2017;48(4):1085–1087. doi: 10.1161/STROKEAHA.116.016289. [DOI] [PubMed] [Google Scholar]
- 13.The ARDS Definition Task Force* (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307(23):2526–2533. 10.1001/jama.2012.5669 [DOI] [PubMed]
- 14.Revel MP, Parkar AP, Prosch H, et al. COVID-19 patients and the radiology department—advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur Radiol. 2020;30(9):4903–4909. doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor FB, Toh CH, Hoots WK, Wada H, Levi M. Scientific subcommittee on disseminated intravascular coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. doi: 10.1055/s-0037-1616068. [DOI] [PubMed] [Google Scholar]
- 16.Kallenberg K, Dehnert C, Dörfler A, et al. Microhemorrhages in nonfatal high-altitude cerebral edema. J Cereb Blood Flow Metab. 2008;28(9):1635–1642. doi: 10.1038/jcbfm.2008.55. [DOI] [PubMed] [Google Scholar]
- 17.Schommer K, Kallenberg K, Lutz K, Bärtsch P, Knauth M. Hemosiderin deposition in the brain as footprint of high-altitude cerebral edema. Neurology. 2013;81(20):1776–1779. doi: 10.1212/01.wnl.0000435563.84986.78. [DOI] [PubMed] [Google Scholar]
- 18.Xie A, Lo P, Yan TD, Forrest P. Neurologic complications of extracorporeal membrane oxygenation: a review. J Cardiothorac Vasc Anesth. 2017;31(5):1836–1846. doi: 10.1053/j.jvca.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Shah J, Armstrong MJ. Extracorporeal membrane oxygenation: uncommon cause of corpus callosal microhemorrhage. Neurology. 2015;84(6):630. doi: 10.1212/WNL.0000000000001227. [DOI] [PubMed] [Google Scholar]
- 20.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau WL, Nunes ACF, Vasilevko V, et al. Chronic kidney disease increases cerebral microbleeds in mouse and man. Transl Stroke Res. 2020;11(1):122–134. doi: 10.1007/s12975-019-00698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoyama S, Hirano H, Uomizu K, Kajiya Y, Tajitsu K, Kusumoto K. High incidence of microbleeds in hemodialysis patients detected by T2*-weighted gradient-echo magnetic resonance imaging. Neurol Med Chir (Tokyo) 2005;45(11):556–560. doi: 10.2176/nmc.45.556. [DOI] [PubMed] [Google Scholar]
- 23.Werring DJ, Frazer DW, Coward LJ, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127(Pt 10):2265–2275. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We state that the data published are available and anonymized and will be shared upon request by email to the corresponding author from any qualified investigator for purposes of replicating procedures and results.