Abstract
Kaposi’s sarcoma-associated herpesvirus (KSHV) infection causes several human cancers, including Kaposi’s sarcoma (KS), one of the most common AIDS-associated tumors. The involvement of the oral cavity represents one common clinical manifestation of AIDS-KS Individuals with periodontal diseases and an oral carriage of a variety of pathogenic bacteria, including Porphyromonas gingivalis. In the current study, we report clinical relevance of P. gingivalis and KSHV co-infection in the oral cavity of a cohort of HIV+ patients. Furthermore, we found that P. gingivalis conditioned medium or derived lipopolysaccharide (LPS) effectively induced KSHV lytic reactivation from infected oral cells. This reactivation requires TLR4 as well as the activities of p38 and JNK MAPK signaling pathways. Our findings reveal the mechanisms through which co-infected periodontal pathogens potentially promote oncogenic virus pathogenesis in the unique niche of immunocompromised patients.
Keywords: KSHV, Kaposi’s Sarcoma, HIV, Porphyromonas gingivalis
Introduction
Kaposi’s sarcoma-associated herpesvirus (KSHV) is the etiologic agent of several human cancers such as Kaposi’s sarcoma (KS) and Primary effusion lymphoma (PEL). 1 Despite the reduced incidence of KS in the era of combined antiretroviral therapy (cART) for HIV infection, KS remains one of the most common AIDS-associated tumors and a leading cause of morbidity and mortality in this setting. 2 Oral cavity involvement represents the initial manifestation of KS in 20–60% of HIV-associated cases. 3 Oral KS lesions contain higher KSHV viral loads relative to skin KS lesions and may portend higher mortality for HIV+ patients. 4 Interestingly, one recent study about oral shedding of herpesviruses in HIV+ patients has indicated that KSHV is similarly detectable across all levels of CD4 counts in these patients. 5 The existing clinical data suggest that KSHV dissemination within and from the oral cavity are critical factors for KSHV infection and oral KS progression in HIV+ patients. 6 It has also been noted that saliva sharing is more common in areas where KSHV is highly endemic; infection is acquired in childhood through practices such as the premastication of food for infants, candy sharing among children and the sharing of toothbrushes. 7
Periodontitis is characterized by chronic inflammation associated with oral bacteria and fungi, resulting in the destruction of periodontal ligaments and the supporting bone tissue of the tooth ultimately leading to tooth loss, high cost of oral health care, and significant morbidity. 8 Several studies indicate a significantly higher prevalence of severe oral inflammation and periodontal disease for HIV+ patients. 9,10 Among those periodontal pathogens, Porphyromonas gingivalis is a well-known bacteria responsible for the development of chronic periodontitis. Published data indicate that bacteria and viruses in the oral cavity interact to facilitate periodontal disease. 11 One very recent study reported impoverishment of oral microbial diversity and enrichment of specific microbiota in oral KS individuals. 12 Interestingly, it has been reported that P. gingivalis can mediate epithelial cell entry of HIV-1, which represents a receptor-independent behavior. 13 P. gingivalis can also induce HIV-1 in monocytes/macrophages through Toll-like receptors (TLRs). 14 We recently reported that pre-treatment of primary human gingival fibroblasts (HGF) and periodontal ligament fibroblasts (PDLF) with two prototypical pathogen-associated molecular patterns (PAMPs)—lipoteichoic acid (LTA) from Staphylococcus aureus and lipopolysaccharide (LPS) from P. gingivalis— increases KSHV entry and subsequent viral latent gene expression in oral cells. 15 We also revealed that S. aureus derived-products, such as conditioned medium and LTA, are able to induce KSHV lytic reactivation and its co-infection with KSHV in cohort HIV+ patients. 16 In this brief report, we will explore whether P. gingivalis and its products affect KSHV lytic reactivation from oral cells, how this process works, and the potential clinical relevance of these findings.
Materials and Methods
Cell culture, reagents and infection protocols
KSHV+ PEL cell line BCBL-1 was kindly provided by Dr. Dean Kedes (University of Virginia) and cultured in RPMI 1640 media with supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 0.05 mM β-mercaptoethanol, and 0.02% (wt/vol) sodium bicarbonate. Primary human gingival fibroblasts (HGF) and periodontal ligament fibroblasts (PDLF) were purchased from ScienCell and maintained in DMEM supplemented with 10% FBS, 10 mM HEPES, 100 U/mL of penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B. P. gingivalis ATCC33277 and Escherichia coli ATCC25922 strains were purchased from American Type Culture Collection (ATCC), and grown as recommended by ATCC. P. gingivalis and E. coli LPS were purchased from InvivoGen, with purities more than 99.5%. SB203580 and JNK-IN-8 were purchased from Selleck Chemicals. To obtain KSHV for the infection experiments, BCBL-1 cells were incubated with 0.6 mM valproic acid for 4–6 days, and KSHV was purified from the culture supernatant by ultracentrifugation at 20,000 × g for 3 h, 4°C. The viral pellets were resuspended in 1/100 the original volume with the appropriate culture media. The infectious titers were determined as described previously. 15
Patients and ethics statement
This study was approved by the Institutional Review Boards for Human Research (IRB, No. 8079) at Louisiana State University Health Science Center. All subjects were provided written informed consent. A total of 53 HIV+ patients with antiretroviral treatment (ART) in the HIV Outpatient (HOP) Clinic were involved. There were 21 females and 32 males in this study with an average age of 49.8 y (range 21–67 y). The average CD4 T cell count was 607/mL (range 31–1903/mL), and the average HIV viral loads was 6044 copies/mL (range 24–67082 copies/mL).
Plasma and saliva preparation
Whole blood was collected in heparin-coated tubes, and plasma was isolated by centrifugation. The KSHV infection status was determined by using quantitative ELISA for identifying circulating IgG antibodies to KSHV proteins (LANA and K8.1). 17,18 To collect whole saliva, patients rinsed with mouthwash, and saliva was collected in a wide-mouth 50 mL Nalgene tube. Typical volumes ranged between 3 to 5 mL of saliva/mouthwash. The patients were requested to not eat or smoke prior to providing the samples.
ELISA
The concentrations of total bacterial LPS in saliva were quantified using LPS ELISA Kit (Cloud-Clone) as recommended by the manufacturer.
RNAi and qRT-PCR/PCR
For RNA interference assays, TLR4 ON-TARGET plus SMART pool siRNA (Dharmacon), or negative control siRNA, were delivered using the DharmaFECT transfection reagent. Total RNA was isolated using the RNeasy Mini kit (Qiagen), and cDNA was synthesized using a SuperScript III First-Strand Synthesis SuperMix Kit (Invitrogen). Primers used for amplification of target genes were listed in Table S1. Amplification was carried out using an iCycler IQ Real-Time PCR Detection System, and cycle threshold (Ct) values were tabulated in duplicate for each gene of interest in each experiment. “No template” (water) controls were used to ensure minimal background contamination. Using mean Ct values tabulated for each gene, and paired Ct values for β-actin as a loading control, fold changes for experimental groups relative to assigned controls were calculated using automated iQ5 2.0 software (Bio-rad). When PCR was used to detect bacteria and virus in saliva samples, the positive (DNAs from P. gingivalis ATCC33277 or KSHV+ BCBL-1 cells) and negative (water) template controls were used, too.
Immunoblotting
Total cell lysates (30 μg) were resolved by 10% SDS–PAGE, transferred to nitrocellulose membranes, and incubated with 100–200 μg/mL of antibodies for TLR4, phosphor- or total-p38, phosphor- or total-JNK, β-Actin (Cell Signaling) and KSHV-RTA (ABBIOTEC).
Statistical analysis
Significance for differences between experimental and control groups was determined using the two-tailed Student’s t-test (Excel 2016).
Results and Discussion
Clinical prevalence of P. gingivalis and KSHV shedding within oral cavity of cohort HIV+ patients
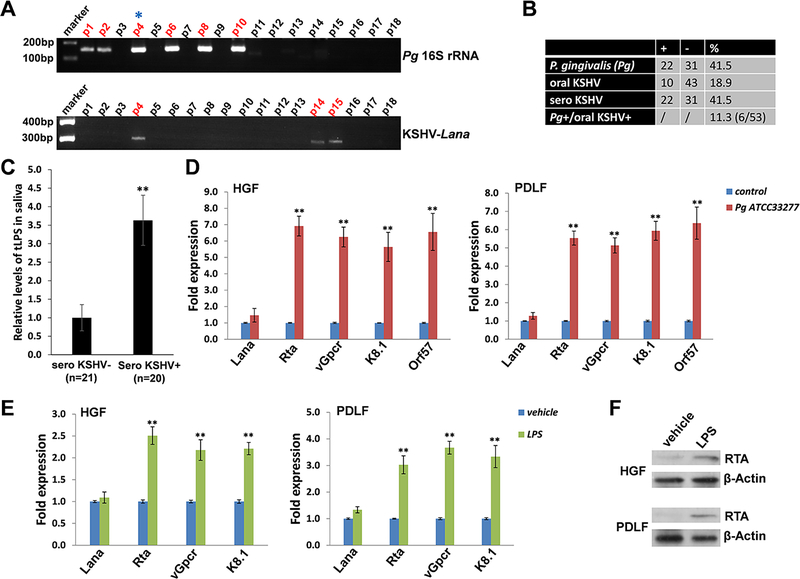
In saliva samples obtained from 53 cohort HIV+ patients, we found that 41.5% (22/53) were P. gingivalis positive, and 18.9% (10/53) were KSHV-Lana positive (a viral protein marker representing latent infection) (Figure 1A–B). Notably, 6 patients (11.3%) were P. gingivalis /KSHV double positive, providing clinical evidence for the co-infection of this specific periodontal bacterial species and oncogenic virus in the oral cavity of HIV+ patients. We noticed that 41.5% of the HIV+ patients (22/53) were KSHV seropositive, which is much higher than determined from their saliva samples (18.9%); this discrepancy is likely due to the difficultly of acquiring KSHV-infected oral cells in just one saliva collection. Therefore, we speculate that the actual rates of oral KSHV positive as well as P. gingivalis /KSHV double positive cases are potentially higher than what we found. Currently, there are no commercial kits available to measure the LPS concentration derived from a single bacteria species such as P. gingivalis, thus we can only measure total bacterial LPS levels. Our data indicates that HIV+/KSHV+ patients have higher levels of total salivary LPS than HIV+/KSHV- patients, indicating the potential clinical implication of periodontal bacterial PAMPs in oral KSHV pathogenesis and related disease progression (Figure 1C).
Figure 1. Clinical prevalence of P. gingivalis and KSHV shedding within patients’ saliva and induction of KSHV lytic reactivation from oral cells by P. gingivalis.
(A) Total DNA was extracted from saliva samples of cohort HIV+ patients by using QIAamp DNA Mini-kit (Qiagen), then PCR was performed using specific primers designed for 16S rRNA of P. gingivalis or KSHV-encoded major latent gene Lana, respectively. Amplicons were subsequently identified by ethidium bromide-loaded agarose gel electrophoresis and representative bands shown only. Asterisks represent double-positive patients. The KSHV seroprevalence was determined by using quantitative ELISAs as described in the Methods. (B) The results of total 53 HIV+ patients’ samples were calculated. (C) The salivary total lipopolysaccharides (LPS) levels in cohort HIV+ patients were measured by using ELISA. ** = p<0.01. (D) Oral fibroblasts were infected by purified KSHV (MOI~10) for 2 h. 24 h later, cells were treated with filtered conditioned medium from overnight P. gingivalis (Pg) ATCC33277 strain culture or fresh medium control (diluted as 1:50) for additional 48 h. qRT-PCR was used to quantify the expression of representative viral latent (Lana) and lytic genes (Rta, vGpcr, K8.1 and Orf57). (E-F) Cells were infected as (D), then incubated with Pg-derived LPS (5.0 μg/mL) for 48 h followed by qRT-PCR. Protein expression was detected using immunoblots. Error bars represent the S.D. for 3 independent experiments. ** = p<0.01. HGF: human gingival fibroblasts; PDLF: periodontal ligament fibroblasts.
Induction of KSHV lytic reactivation from infected oral cells by P. gingivalis culture and components
We found that treating latently infected primary human oral fibroblasts (HGF and PDLF) with filtered conditioned medium from P. gingivalis ATCC33277 strain induced significantly higher expression of KSHV lytic genes, including Rta, vGpcr, K8.1 and Orf57, when compared to cells treated with a fresh medium control (Figure 1D). Moreover, these inducible effects were also observed in P. gingivalis derived LPS-treated oral cells with virus infection (Figure 1E), although to a lesser extent. Furthermore, immunoblots results confirmed the induction of RTA expression from P. gingivalis derived LPS-treated oral cells (Figure 1F). However, we did not observe similar effects using conditioned medium or purified LPS from other non-periodontal Gram-negative bacteria such as Escherichia coli (Figure S1), indicating a specificity for bacterial species. In fact, P. gingivalis-derived LPS exhibits unique features compared with the LPS of other species, including differences in the structure of the O-antigen, as well as in the acylation patterns and receptor-activating capacities of the lipid A component. 19,20 Interestingly, one recent study reported that P. gingivalis LPS and E. coli LPS differently regulated cytokine production in human gingival fibroblasts. 21 Another study showed that whole blood cell cultures populations obtained from healthy and chronic periodontitis patients may differ in the cytokine response to P. gingivalis LPS but not E. coli LPS. 22
TLR4 and intracellular signaling pathways are required for induction of KSHV lytic reactivation by P. gingivalis conditioned medium and LPS
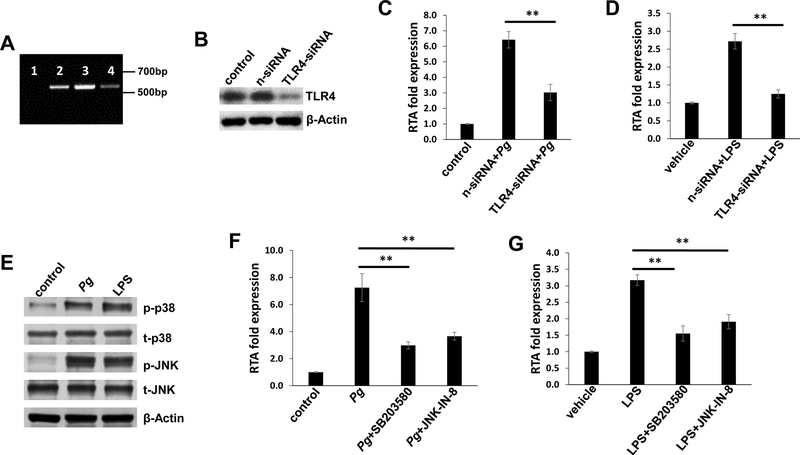
The receptor for P. gingivalis LPS has initially been reported as TLR2, although this observation remains controversial because synthetic P. gingivalis lipid A activates TLR4 but not TLR2, 23 thus the TLR2 activity of P. gingivalis LPS might be attributed to a contaminant lipoprotein. 24 Moreover, a recent study states that P. gingivalis LPS activity is mediated exclusively through TLR4. 25 Interestingly, one recent study demonstrated the role of TLR4-mediated inflammation in KSHV induced tumorigenesis. 26 In this study, we first assessed TLR4 expression in oral cells, using human monocyte THP-1 as a positive control. Our RT-PCR data indicated that TLR4 is expressed in oral fibroblasts, HGF and PDLF (Figure 2A). Silencing of TLR4 by RNAi significantly impaired KSHV lytic gene expression (e.g., Rta, a viral lytic reactivation activator), induced by P. gingivalis conditioned medium and/or LPS (Figure 2B–D). However, in contrast to near complete impairment of viral lytic gene expression induced by LPS, silencing of TLR4 had only partial blocking effects on viral lytic gene expression induced by P. gingivalis conditioned medium (Figure 2C–D). These results indicate that additional bacterial products besides LPS may induce viral lytic gene expression, such as the short-chain fatty acids reported previously. 27
Figure 2. TLR4 and related signaling pathways are required for induction of KSHV lytic reactivation by P. gingivalis conditioned medium or LPS.
(A) qRT-PCR was performed using specific primers designed for TLR4. 1: Water as the negative control; 2: THP-1 as the positive control; 3: HGF; 4: PDLF. (B) HGF were transfected with either negative control siRNA (n-siRNA) or TLR4-siRNA for 48 h, then protein expression was measured by immunoblots. (C-D) HGF were infected by purified KSHV (MOI~10) for 2 h. After 24 h, cells were transfected with siRNAs as described above, then treated with Pg filtered conditioned medium or LPS for additional 48 h followed by qRT-PCR. (E) HGF were infected as above, then treated with Pg filtered conditioned medium or LPS for additional 48 h. Protein expression was measured by using immunoblots. (F-G) After KSHV infection, HGF were treated with SB203580 (p38 inhibitor, 10 μM for 1 h) or JNK-IN-8 (JNK inhibitor, 1 μM for 1 h). Cells were then treated with Pg filtered conditioned medium or LPS for additional 48 h followed by qRT-PCR. Error bars represent the S.D. for 3 independent experiments. ** = p<0.01.
P. gingivalis infection has been found to activate several intracellular signaling pathways such as p38 and Jun N-terminal protein kinase (JNK) MAPK pathways. 28 Interestingly, the activities of p38 and JNK pathways have been found to be closely related to KSHV lytic reactivation. 29 Here we demonstrated that both P. gingivalis conditioned medium and LPS effectively increased the phosphorylation of p38 and JNK proteins from KSHV-infected oral cells (Figure 2E). Pre-treatment of specific p38 inhibitor (SB203580) or JNK inhibitor (JNK-IN-8) significantly reduced viral lytic gene expression induced by P. gingivalis conditioned medium and/or LPS (Figure 2F–G). Again, we noticed that blocking these two signaling pathways did not completely hinder viral lytic reactivation induced by P. gingivalis conditioned medium (Figure 2F), implying that there are additional mechanisms involved in this induction.
In summary, our findings demonstrate the clinical relevance of P. gingivalis and KSHV co-infection in the oral cavity of cohort HIV+ patients, which (including the whole bacteria or bacterial products) may facilitate oncogenic virus lytic reactivation and dissemination.
Supplementary Material
Acknowledgement
This work was supported by NIH/NCI R01CA228166. Support has been also provided in part by the Arkansas Bioscience Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. Funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest
All the authors have declared no conflict of interest.
References
- 1.Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupin N Update on oncogenesis and therapy for Kaposi sarcoma. Curr Opin Oncol. 2020;32(2):122–128. [DOI] [PubMed] [Google Scholar]
- 3.Bunn BK, van Heerden WF. HIV/AIDS associated malignancies of the head and neck. SADJ. 2012;67(10):590–592. [PubMed] [Google Scholar]
- 4.Rohrmus B, Thoma-Greber EM, Bogner JR, Rocken M. Outlook in oral and cutaneous Kaposi’s sarcoma. Lancet. 2000;356(9248):2160. [DOI] [PubMed] [Google Scholar]
- 5.Dittmer DP, Tamburro K, Chen H, et al. Oral shedding of herpesviruses in HIV-infected patients with varying degrees of immune status. AIDS. 2017;31(15):2077–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller CS, Berger JR, Mootoor Y, Avdiushko SA, Zhu H, Kryscio RJ. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. J Clin Microbiol. 2006;44(7):2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler LM, Neilands TB, Mosam A, Mzolo S, Martin JN. A population-based study of how children are exposed to saliva in KwaZulu-Natal Province, South Africa: implications for the spread of saliva-borne pathogens to children. Trop Med Int Health. 2010;15(4):442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6(6):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phiri R, Feller L, Blignaut E. The severity, extent and recurrence of necrotizing periodontal disease in relation to HIV status and CD4+ T cell count. J Int Acad Periodontol. 2010;12(4):98–103. [PubMed] [Google Scholar]
- 10.Mataftsi M, Skoura L, Sakellari D. HIV infection and periodontal diseases: an overview of the post-HAART era. Oral Dis. 2011;17(1):13–25. [DOI] [PubMed] [Google Scholar]
- 11.Patini R, Staderini E, Lajolo C, et al. Relationship between oral microbiota and periodontal disease: a systematic review. Eur Rev Med Pharmacol Sci. 2018;22(18):5775–5788. [DOI] [PubMed] [Google Scholar]
- 12.Gruffaz M, Zhang T, Marshall V, et al. Signatures of oral microbiome in HIV-infected individuals with oral Kaposi’s sarcoma and cell-associated KSHV DNA. PLoS Pathog. 2020;16(1):e1008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantri CK, Chen C, Dong X, Goodwin JS, Xie H. Porphyromonas gingivalis-mediated Epithelial Cell Entry of HIV-1. J Dent Res. 2014;93(8):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez OA, Li M, Ebersole JL, Huang CB. HIV-1 reactivation induced by the periodontal pathogens Fusobacterium nucleatum and Porphyromonas gingivalis involves Toll-like receptor 2 [corrected] and 9 activation in monocytes/macrophages. Clin Vaccine Immunol. 2010;17(9):1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai L, DeFee MR, Cao Y, et al. Lipoteichoic acid (LTA) and lipopolysaccharides (LPS) from periodontal pathogenic bacteria facilitate oncogenic herpesvirus infection within primary oral cells. PLoS One. 2014;9(6):e101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai L, Qiao J, Yin J, et al. KSHV and Staphylococcus aureus co-infection in oral cavities of HIV+ patients: a unique niche for oncogenic virus lytic reactivation. J Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mbisa GL, Miley W, Gamache CJ, et al. Detection of antibodies to Kaposi’s sarcoma-associated herpesvirus: a new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. J Immunol Methods. 2010;356(1–2):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benavente Y, Mbisa G, Labo N, et al. Antibodies against lytic and latent Kaposi’s sarcoma-associated herpes virus antigens and lymphoma in the European EpiLymph case-control study. Br J Cancer. 2011;105(11):1768–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paramonov N, Bailey D, Rangarajan M, et al. Structural analysis of the polysaccharide from the lipopolysaccharide of Porphyromonas gingivalis strain W50. Eur J Biochem. 2001;268(17):4698–4707. [DOI] [PubMed] [Google Scholar]
- 20.Paramonov NA, Aduse-Opoku J, Hashim A, Rangarajan M, Curtis MA. Structural analysis of the core region of O-lipopolysaccharide of Porphyromonas gingivalis from mutants defective in O-antigen ligase and O-antigen polymerase. J Bacteriol. 2009;191(16):5272–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrukhov O, Ertlschweiger S, Moritz A, Bantleon HP, Rausch-Fan X. Different effects of P. gingivalis LPS and E. coli LPS on the expression of interleukin-6 in human gingival fibroblasts. Acta Odontol Scand. 2014;72(5):337–345. [DOI] [PubMed] [Google Scholar]
- 22.Nogueira-Filho G, Rosa BT, Santos PF, et al. Whole-blood cultures from patients with chronic periodontitis respond differently to Porphyromonas gingivalis but not Escherichia coli lipopolysaccharide. J Periodontol. 2014;85(2):e18–23. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa T, Asai Y, Makimura Y, Tamai R. Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A. Front Biosci. 2007;12:3795–3812. [DOI] [PubMed] [Google Scholar]
- 24.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165(2):618–622. [DOI] [PubMed] [Google Scholar]
- 25.Nativel B, Couret D, Giraud P, et al. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci Rep. 2017;7(1):15789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruffaz M, Vasan K, Tan B, Ramos da Silva S, Gao SJ. TLR4-Mediated Inflammation Promotes KSHV-Induced Cellular Transformation and Tumorigenesis by Activating the STAT3 Pathway. Cancer Res. 2017;77(24):7094–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X, Shahir AM, Sha J, et al. Short-Chain Fatty Acids from Periodontal Pathogens Suppress Histone Deacetylases, EZH2, and SUV39H1 To Promote Kaposi’s Sarcoma-Associated Herpesvirus Replication. J Virol. 2014;88(8):4466–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inaba H, Amano A, Lamont RJ, Murakami Y, Matsumoto-Nakano M. Cell Cycle Arrest and Apoptosis Induced by Porphyromonas gingivalis Require Jun N-Terminal Protein Kinase- and p53-Mediated p38 Activation in Human Trophoblasts. Infect Immun. 2018;86(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie J, Ajibade AO, Ye F, Kuhne K, Gao SJ. Reactivation of Kaposi’s sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology. 2008;371(1):139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.