Abstract
One of the potential mechanisms of motor cortex stimulation by non-invasive brain stimulation (NIBS) effects on pain is through the restoration of the defective endogenous inhibitory pain pathways. However, there is still limited data on quantitative sensory testing (QST), including conditioned pain modulation (CPM), supporting this mechanism. This systematic review and meta-analysis aimed to evaluate the effects of non-invasive motor cortex stimulation on pain perception as indexed by changes in QST outcomes. Database searches were conducted until July 2019 to included randomized controlled trials that performed sham-controlled NIBS on the motor cortex in either healthy and/or pain population and assessed the QST and CPM. Quality of studies was assessed through the Cochrane tool. We calculated the Hedge’s effect sizes of QST and CPM outcomes, their 95% confidence intervals (95% CI) and performed random-effects meta-analyses. Thirty-eight studies were included (1178 participants). We found significant increases of pain threshold in healthy subjects (ES=0.16, 95% CI=0.02 to 0.31, I2=22.2%) and pain population (ES=0.48, 95% CI=0.15 to 0.80, I2=68.8%); and homogeneous higher CPM effect (pain ratings reduction) in healthy subjects (ES=−0.39, 95% CI=−0.64 to −0.14, I2=17%) and pain population (ES=−0.35, 95% CI=−0.60 to −0.11, I2=0%) in active NIBs group compared with sham. These results support the idea of top-down modulation of endogenous pain pathways by motor cortex stimulation as one of the main mechanisms of pain reduction assessed by QST, which could be a useful predictive and prognostic biomarker for chronic pain personalized treatment with NIBS.
Introduction
Pain perception is a complex process influenced by sensory, cognitive, and emotional dimensions [72]; the multidimensional nature of pain requires different measurement approaches to understand the pathophysiology underlying pain syndromes [44]. Quantitative sensory testing (QST) assessments have been used to objectively measure pain in both healthy and pain populations [44]. Static QST – measured by pain threshold (PT) – assess the basal state of the nociceptive system, while dynamic QST evaluates the pain processing system: i) pain facilitation – measured by temporal summation (TS) – and pain inhibitory systems (the endogenous pain inhibitory system) – assessed by conditioned pain modulation (CPM) protocols [5; 51]. This latest evaluates the phenomena known as “pain inhibits pain” by testing the functioning and integrity of the endogenous descending inhibitory pathways [7]. The changes on QST measurements are useful to understand pain processes in healthy subjects, and they could be applied in pain populations as diagnostic biomarkers, and as a predictor of responsiveness to analgesic treatments [68].
Non-invasive motor cortex stimulation has shown an effect on pain facilitatory and inhibitory systems due to the activation of subcortical structures related to the endogenous pain modulation system as thalamus, cingulate gyrus, periaqueductal gray, subnucleus reticularis dorsalis (SRD), among others [30; 31]. Transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) might restore the balance in the endogenous pain pathways as a top-down regulation, through subcortical nuclei such as thalamic nuclei and SRD) while preventing or reversing maladaptive plasticity leading to a decrease of pain perception [16]. tDCS delivers a subthreshold current from anode to cathode by two electrodes over the scalp, whereas rTMS uses magnetic fields in order to induce electrical changes in the brain activity [16]. Both of them are non-invasive brain stimulation (NIBS) techniques that have shown some efficacy in healthy subjects and pain-related syndromes [45; 46; 61]. Hence, these tools are appropriate options to modulate the pain perception processes reflected on QST changes. The NIBS effects on CPM can be related to endogenous pain pathways modulation [24], which can be used to understand this system disruption on different chronic pain conditions better and clarify the NIBS mechanism of action. Moreover, their effects on PT and TS can give us a better understanding of its impact on peripheral and central sensitization [70].
Even though the utility that would have to understand the NIBS effects on pain processes indexed by QST and that non-invasive motor cortex stimulation has been extensively studied in chronic pain [2; 27; 61], the knowledge on their effects on QST is still limited; especially the pooled effect of two commonly used techniques for motor cortex stimulation (TDCS and rTMS) and in healthy and in subjects with pain. Thus, with this systematic review and meta-analysis, we aim to evaluate the effects that previous studies have shown of motor cortex stimulation on pain perception processes indexed by changes in static and dynamic QST outcomes, including PT, TS, and CPM.
Methods
A systematic review of the literature and meta-analysis was conducted following the recommendation of the Cochrane handbook [33], including the PRISMA guidelines (online supplementary material 1)[54].
Literature search and study selection
We searched in MEDLINE, EMBASE, Web of Science, Lilacs and Cochrane Central until July 31st, 2019 using a search strategy with the following search terms: “noninvasive brain stimulation” OR “transcranial magnetic stimulation” OR “transcranial direct current stimulation” AND “Diffuse Noxious Inhibitory Control” OR “Pain Threshold”. The full research strategy is shown in online supplementary Material 2. Duplicates were eliminated before selection. Previous to the title and abstract selection, two experienced reviewers (KPB and AC-R) agreed on a standard approach. Two random samples of fifty search results were pre-selected for the training and standardization process. After pre-selecting the articles based on the title and abstract, four reviewers (LC, ML, PM, and SG-L) selected the same articles for calibration purposes. Subsequently, we calculated the inter-rater agreement and kappa estimator, aiming for an inter-rater agreement of at least 90% (online supplementary material 3). Afterward, the citations were independently screened by the four reviewers (LC, ML, PM, and SG-L) in terms of titles and abstracts. Discrepancies between reviewers were resolved by a fifth reviewer (AC-R). Then, the four main reviewers independently assessed the full text of selected studies, and again the fifth reviewer resolved discrepancies.
Eligibility criteria
We searched for full-text articles restricted to English. Included articles had to have: a) enrolled either healthy subject and/or with a pain condition; b) performed NIBS such as tDCS or rTMS on the motor cortex compared to their respective sham; c) assessed the quantitative sensory testing including pressure (PPT), heat (HPT), cold (CPT) or electrical (EPT) pain thresholds; CPM; and TS; and d) designed as randomized controlled trials (RCTs), included parallel-group, crossover designs, and pilot studies.
Data extraction
In total, eight reviewers participated in the extraction process. Two of them (AC-R and SG-L) developed the extraction matrix. The extraction was performed in pairs (PM and ML; JB and SG-L; MG and LC) that extracted the same articles independently. All discrepancies between reviewers were solved by a seventh reviewer (KP-B). For each study, we extracted in a standardized spreadsheet the following: i) participant characteristics (sample size, condition, age, gender, drop-outs), ii) NIBS intervention protocol characteristics (stimulated area, electrode size, current intensity, pulse frequency, number of sessions, and session duration), and iii) outcomes of interest (pain threshold, CPM, or/and Temporal summation). In case of missing or unclear information, we requested by email the values from the authors. We used WebPlotDigitizer v.3.11[66] to extract data from relevant graphs, and if a study only reported postintervention data, we determined whether to include the data in the analysis by studying baseline comparability on the graphs. If we were unable to contact the authors or extract the data graphically, we excluded the study from the quantitative analysis. Some of the included studies measured multiple variables to assess the QST outcome within-subjects (more than one body location for PT assessments – left arm, right arm, left leg, etc.). We were aware that computing different effect sizes for the same sample or overlapping sets of participants and treating them as completely unrelated effect sizes violate the basic assumptions of the traditional meta-analytic method. In those cases, we calculated a weight mean of the multiple variables to compute a unique measurement of the outcome of interest, in order not to lose relevant information.
Static QST outcomes
PT: Corresponded to the smallest stimulus that was reported by subjects as painful. This could be measured by the different stimuli such as pressure with an algometer, heat, cold, or electrical stimulus. It was reported a lower pain threshold in different chronic pain conditions [44; 68]. We extracted and analyzed changes in stimulus units (kPA, centigrade degrees, etc.) and Standard deviation (SD) as a measurement of PT changes [59; 60].
Dynamic QST outcomes
TS: This protocol measured pain facilitation and was calculated as the difference between the pain rating after series of stimuli and the rating after a single stimulus after the series, expecting more pain after the application of stimuli in series [44; 68]. We extracted and analyzed changes in pain ratings and SD as a measurement of TS effect [59; 60]
CPM: This protocol involved two conditions, the test stimulus (painful sensation) and the conditioned stimulus (cold water sensation). This protocol could be measured by the difference between pain threshold or pain rating after the test stimulus and after the conditioned stimulus. In healthy patients, we expected a decrease in pain score after the conditioned stimulus; however, in pain conditions, as the endogenous pain modulation system was impaired, higher pain scores would be perceived after the conditioned stimulus [58]. We extracted and analyzed changes in pain ratings and SD as a measurement of CPM effect [59; 60]
Risk of bias assessment
The risk of bias of the selected studies was evaluated by two reviewers (AC-R and SG-L) using Cochrane Risk of Bias Scale for RCTs [33]. In order to classify in the low, high, and unclear risk of bias, we followed the instructions stated in the Cochrane handbook for systematic reviews of interventions for RCTs [33]. In the event of any discrepancies between the two reviewers, a consensus was attempted to be reached by discussion. If a full consensus could not be reached between the two reviewers after an exhaustive discussion, the opinion of a third reviewer was obtained (KP-B), and the proceeding majority consensus was taken.
Data Synthesis
The RCTs were presented separately according to the condition (healthy versus pain population), given the differences in the pain perception processes between these two groups. The QST outcomes were categorized according to the type of stimulation (tDCS or rTMS). Then, the effect sizes of QST outcomes and their 95% confidence intervals (95% CI) were calculated, and an exploratory meta-analysis was performed. Although with-in the treatment categories were interventions with different parameters, we decided to do an exploratory synthesis to compare across the spectrum of the available non-invasive motor cortex stimulation techniques. We adjusted Cohen’s d to Hedge’s g by applying a correction factor as Cohen’s d has a slight bias to overestimate in small sample sizes. We assessed heterogeneity using an I2 statistical, and we considered low heterogeneity when I2 <40% [33]. We considered it appropriate to use random-effects models due to the overall heterogeneity evaluation (in population and intervention) [23]. Moreover, we performed subgroup analysis, sensitivity analysis, and meta-regression as further evaluations of sources of heterogeneity. The publication bias was evaluated visual assessment (funnel plot) and by the Egger test. The data were analyzed using Stata v15.1 software (StataCorp LLC).
RESULTS
Overview
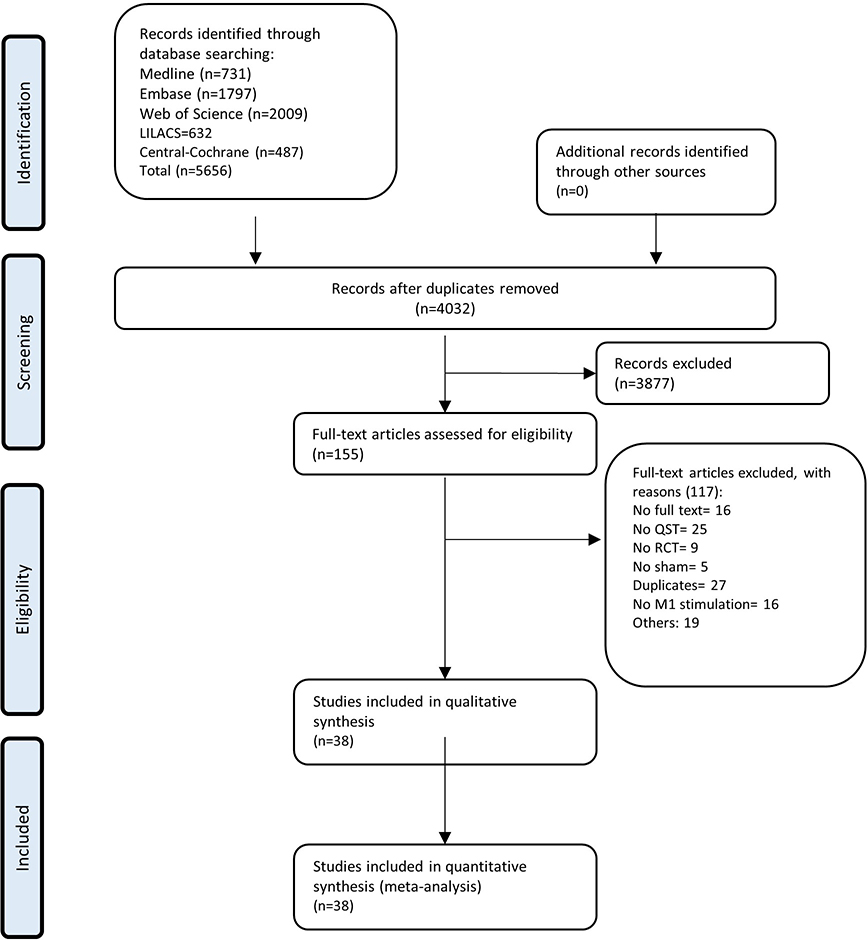
The search retrieved 5656 results; after removing duplicates, 4032 titles and abstracts were screened, and of these, 3877 were excluded. One hundred fifty-five studies were evaluated in full-text, 117 studies were excluded (online supplementary material 4). And finally, 38 studies were included [1; 6; 8-12; 14; 15; 18; 20-22; 25; 26; 32; 34; 36-38; 40; 41; 43; 49; 52; 53; 55-57; 62; 64; 65; 69; 73; 74; 76; 78], reporting 71 comparisons (1178 participants). A flow diagram of the search process is presented in Figure 1.
Figure 1.
Flow diagram of the search and selection process.
Regarding NIBS, 28 studies evaluated the effects of tDCS and 10 of rTMS. From them, nine studies (23.7%) assessed other interventions together with NIBS. Three evaluated the effects of exercise (7.6%), while the other six evaluated melatonin, intramuscular electrical stimulation, naloxone, ketamine, and remifentanil (2.6% each). In terms of QST outcomes data, 33 studies reported PT: 20 in the healthy population (35 comparisons, 629 subjects) and 13 in pain conditions (17 comparisons, 462 patients); two reported TS (three comparisons, 38 patients); and 13 reported CPM outcomes: seven in the healthy population (ten comparisons, 169 subjects) and six in pain conditions (eight comparisons, 239 patients); and 11 reported more than one outcome. Besides, 23 (60.5%) performed the QST protocols in upper limbs, seven (18.4%) in lower limbs, two (5.3%) in both upper and lower, and six (15.8%) in other body areas. The included pain populations [1; 6; 12; 15; 20; 22; 37; 40; 41; 49; 52; 53; 62; 65; 69; 76] were heterogeneous including fibromyalgia four (25%), knee osteoarthritis three (18.8%), peripheral neuropathy two (12.5%), temporomandibular disorder one (6.3%), post-stroke pain one (6.3%), myofascial pain one (6.3%), postoperative pain one (6.3%), and others three (18.8%) studies. Only seven (43.8%) reported pain duration and two (12.5%) sensory profile. A qualitative summary of included articles is provided in Tables 1 and 2.
Table 1.
General information from the tDCS studies included in the meta-analysis
| Author | Country | Design | Condition | Pain duration | Sensory Profile Per Patient N (%) | Participants | Motor cortex stimulation parameters | QST protocol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anode | Cathode | Intensity (mA), Sponge area (cm2), duration (min) | Number of sessions | Device | Concomitant therapy | Pain threshold | TS | CPM | Device | |||||||
| Mendoca et al. (2011) | Brazil | Parallel | Fibromyalgia | Not reported | Not reported | 30 participants. A. 6 participants in c-tDCS M1 (83.4% females) Mean age: 41.8 yrs B. 6 participants in c-tDCS SO (100% females) Mean age: 43.5 yrs. C. 6 participants in a-tDCS M1 (83.4% females) Mean age: 44.5 yrs. D. 6 participants in a-tDCS SO (100% female) Mean age: 42.6 yrs. E. 6 participants in s-tDCS (100% female) Mean age: 43.5 yrs | Cervical and thoracic spine (between the scapulas) | C3 | 2 mA, 16 cm2 on cranially sponges. 80 cm2 on extracephalic sponges,, 20 min | 1 | Universal Pulse generator (941 NEMESYS, Quark Medical Products, Brazil) | No | PPT was assessed by a pressure algometer in the 18 pre-established points for the diagnosis of fibromyalgia | Pressure algometer (Pain Diagnostics& Thermographics Corporation, Great Neck, NY | ||
| C3 | cervical and thoracic spine (between the scapulas) | 2 mA, 16 cm2 on cranially sponges. 80 cm2 on extracephalic sponges,, 20 min | 1 | No | ||||||||||||
| Kim et al. (2013) | Korea | Parallel | Painful diabetic polyneuropathy | 2–5 years: 29 (48.3%)
patients >5 years 31 (61.7%) |
Not reported | 60 participants. A. 20 participants in a-tDCS M1 ( 55% females) Mean age: 59.6 yrs. B. 20 participants in s-tDCS ( 60% females). Mean age: 61.6 yrs. C. 20 participants in a-tDCS DLPFC ( 60% females). Mean age: 63.5 | C3 | SO | 2 mA, 25 cm2, 20 min | 5 | Phoresor II PM850 anodal tDCS was used (IOMED,Salt Lake City, UT, USA | No | PPT was measured by an algometer over the more painful sole region | Commander algometer(JTECH Medical Industries, Salt Lake City, UT, USA | ||
| Villamar et al. (2013) | US | Crossover | Fibromyalgia | 10.7 years (with Fibromyalgia) | Not reported | 18 participants (83% females) Mean age: 50.3 yrs. A. a-HD-tDCS. B. c-HD-tDCS. C. s-HD-tDCS | C3 | (Cz, F3, T7, P3) | 2 mA, 20 min | 1 | Model 1224-B; Soterix Medical Inc | No | PPT was measured by a pressure algometer over 1) the area of the forearm 2 cm distal to the lateral epicondyle; 2) the supraspinatus muscle; 3) the occiput | Difference of PPT before and after the immersion of the left hand in cold water (10–12 C) | Commander Algometer (JTECHMedical, Salt Lake City, UT) | |
| (Cz, F3, T7, P3) | C3 | 2 mA, 20 min | 1 | No | ||||||||||||
| Bae et al. (2014) | Korea | Parallel | Central post stroke pain | Not reported | Tingling 7 (50) Burning 4 (28.6) Numbness 1 (7.1) |
14 participants: A. 7 participants in a-tDCS ( 43 % females). Mean age: 51.1. B. 7 participants in s-tDCS ( 57 % females). Mean age: 52.3. | C3/C4 contralateral to paralyzed side | SO | 2 mA, 35 cm2, 20 min | 9 | Phoresor II AutoModel PM850; USA | No | Cold and heat pain over the thenar area of hand | Thermal sensory analyzer (TSA-II, MEDOC Co. Ltd., Israel) | ||
| Souto et al. (2014) | Brazil | Parallel | Pain in HTLV-1 | Not reported | Neuropathic pain 13 (65) Nociceptive 7 (35) |
20 participants. A. 10 participants in a-tDCS. (80% females) Mean age: 47.8 yrs. B. 10 participants in s-tDCS. (70% female). Mean age: 56.1 | C3 | SO | 2 mA, 25 cm2, 20 min | 5 | Electro-stimulator (Striat, Ibramed, Brazil) | No | PPT was assessed by a pressure algometer over the tibial tuberosity | Pressure algometer (Pain Diagnostic & Thermographics Great Neck,New York) | ||
| Oliveira et al. (2015) | Brazil | Parallel | Temporomandibular disorder | 31.75 (SD 20.15) months | Not reported | 32 participants. A. 16 participants in a-tDCS and exercises ( 94% females). Mean age: 23.8 yrs. B. 16 participants in s-tDCS and exercise ( 88 % females). Mean age: 25.5 yrs. | C3/C4 contralateral to pain | SO | 2 mA, 20 min | 5 | Striat, Ibramed, Sao Paulo Brazil. | Exercise | PPT was assessed by a pressure algometer over the anterior and posterior regions of the TMJ condyle, suboccipital muscles, upper portion of the trapezium and levator scapulae | Mechanical pressure algometer (Force Dial, Wagner Instruments, Greenwich, CT, USA) | ||
| Brietzke et al. (2016) | Brazil | Parallel | Chronic Hepatitis C | Not reported | Not reported | 28 participants: A. 14 participants in a-tDCS (35% female) Mean age:53.86 yrs. B. 14 participants in s-tDCS (14% female) Mean age: 56.57 yrs. | C3 | SO | 2 mA, 25–35 cm2, 20 min | 5 | Not specified | No | PPT was meassure with an algometer over the right antecubital fossa | Algometer device (JTECH Medical Industries,Salt Lake City, UT). | ||
| Mendoca et al. (2016) | Brazil | Parallel | Fibromyalgia | 138.5 (SD 95.91) | Not reported | 45 subjects (44 females/01 male) were assigned to 1 of 3 groups: tDCS + AE, AE + sham tDCS, and tDCS only. Mean age:142,2 | C3 | SO right | 2mA, 35cm2, 20min | 5 | Monophasic current device (DC stimulator, NeuroCom, Germany) | Aerobic exercise | PPT was measured with a pressure algometer over the thenar region of the hand and the uppermost portion of the anterior tibialis. | Pressure algometer (Wagner Instruments, USA) | ||
| Chang et al. (2017) | Australia | Parallel | Knee Osteoarthritis | 8.10 (SD 6.38) years | Not reported | 30 participants: A. 15 participants in a-tDCS + exercise (73% female). Mean age: 59.8 yrs. B. 15 participants in s-tDCS+exercise. Mean age: 64.1 yrs. | C3/C4 contralateral | SO | 1 mA, 35 cm2, 20 min | 16 | DC-STIMULATOR,neuroConn,Ilmenau,Germany | Exercise | PPT was meassure with an algometer the ipsilateral tibilialis anterior and ipsilateral extensor carpi radialis longus and eight sites at the worst knee (inferomedial, inferolateral, lateral, superolateral, superior, superiomedial, medial, centre of the patella). HPT was measured at the worst knee (medial knee joint line, patella and lateral knee joint line) and both forearm | Difference of PPT over the worst knee and the contralateral forearm, before and after heat conditioned stimulation | Hand-held pressure algometer (FORCE TEN FDX
compact digital force gauge, Wagner Instruments,
USA). Conditioned pain modulation system (Thermal Sensory Analyser, TSA-2001, Q-Sense-CPM Medoc Ltd, Ramat Yishai, Israel). |
|
| Khedr et al. (2017) | Egypt | Parallel | Fibromyalgia | 6.08 (SD 2.59) years | Not reported | 36 participants. A. 18 participants in a-tDCS (94% female) Mean age: 31.3 yrs. B. 18 participants in s-tDCS (94% female). Mean age: 33.9 yrs. | C3 | Contralateral arm (extra-cephalic) | 2 mA, 24 cm2, 20 min | 10 | Not specified | No | MPT was measured by an electronic model of Von Frey unit. | Von Frey electronic device (BIO-CIS software) | ||
| Ribeiro et al. (2017) | Brazil | Parallel | Chronic foot pain going on hallux valgus surgery | Not reported | Not reported | 40 female particpants. A. 20 participants in a-tDCS. Mean age: 46 yrs. B. 20 participants in s-tDCS. Mean age: 48.36 yrs | C3 | SO | 2 mA, 35 cm2, 20 min | 2 | Not specified | No | HPT measure over the ventral mid forearm with a temperature going from 32C to 52 C | Difference of HPT before and after the immersion of the non-dominant hand in cold water (0–1 C) | Computer Peltier-based device thermode (30×30mm) | |
| Ahn et al. (2018) | US | Parallel | Knee Osteoarthritis | Not reported | Not reported | 40 participants: A. 20 participants with aTDCS ( 50 % females). Mean age: 60.6yrs. B. 20 participants in the sham group ( 55 % females). Mean age: 59.3 yrs. | C3/C4 contralateral | SO | 2 mA, 35 cm2, 20 min | 5 | Soterix Clinical Trial direct current stimulator (Soterix Medical Inc., New York, NY, USA | No | HPT over the ipsilateral forearm and index knee and PPT over the index knee, quadriceps and trapezius | Determine the changes of the PPT on the trapeciuz and during the hand in the cold water immersion | Handheld digita pressure algometer (Wagner,
Greenwich, CT, USA). Computer-con-trolled TSA-II. Analyzer (Medoc Ltd., Ramat Yishai, Israel) |
|
| Lewis et al. (2018) | New Zealand | Parallel | Upper limb neuropathic pain | 7.05 (SD 7.94) years | Not reported | 28 participants. A. 14 participants in a-tDCS ( 21% females) Mean age: 59 yrs. B. 14 participants in a s-tDCS ( 78% females) Mean age: 60 yrs. | C3/C4 contralateral to the affected upper limb | SO | 1 mA, 35 cm2, 20 min | 5 | HDCell (MagStim Co, UK) | No | PPT measured by a handheld transducer over the abductor pollicis brevis and to the contralateral tibialis anterior. | Ten standardized punctuate stimuli were applied using 225.1 g of Von Frey filament (1 Hz of frequency) to the abductor pollicis brevis muscle. | Handheld transduce (Somedic, Sweden) | |
| da Graça-Tarragó et al. (2019) | Brazil | Parallel | Knee Osteoarthritis | Not reported | Not reported | 60 female participants. A. 15 participants in a-tDCS + a-EIMS. B. a-tDCS + sEIMS. C. s-tDCS + aEIMS. D. s-tDCS + EIMS | C3/C4 contralateral | SO | 2 mA, 35 cm2, 30 min | 5 | Not specified | No | PPT was assessed by an electronic algometer in the patellar tendon of the leg with the sclerotomal hyperalgesia. | Difference of PPT in the patellar tendon before and after the immersion of the nondominant hand in cold water (0–1 C) for 1 min. | Electronic algometer (J-Tech MedicalIndustries, Midvale, UT, USA) | |
| C3/C4 contralateral | SO | 2 mA, 35 cm2, 30 min | 5 | Intramuscular electrical stimulus | ||||||||||||
| Boggio et al. (2008) | Brazil | Crossover | Healthy | - | - | 20 participants ( 65% females). Mean age: 21 yrs. | C3 | SO | 2 mA, 35 cm2, 5 min | 5 | Schneider Electronic, Gleichen, Germany | No | Electrical stimulus was applied to the right index finger | Digitimer DS-7A electrical stimulator (Hert-fordshire, England) | ||
| Borckardt et al. (2012) | US | Parallel | Healthy | - | - | 24 participants (75% female). Mean age: 26.58 A. 13 participants with active HD-tDCS B. 11 participants in the sham group | C4 | 4 (7 cm radius from anode) | 2 mA, 20 min | 1 | Not specified | No | HPT and CPT where assessed over the left arm (5 cm from the wrist) and PPT was assessed with an electric von frey anesthesiometer over the dorsal surface of the distal phalange of left digiti minimi | 20 brief suprathreshold thermal stimuli to the left forearm. the mean of the first and the last 3 seconds of the 30-second wind-up trial were assessed | HPT and CPT via ATS thermode of
the Medoc Ad-vanced Medical Systems Ltd, Durham, NC PPT: IITC Life Sciences (Woodland Hills, CA) Electric vonFrey Anesthesiometer with rigid tips. Thermal-wind up: CHEPS thermode from the Medoc Pathway System |
|
| Jürgens et al. (2012) | Germany | Crossover | Healthy | - | - | 17 participants (47% females) Mean age: 24.9 yrs. A. a-tDCS. B. c-tDCS. C. s-tDCS | C3 | C4 | 2 mA, 35 cm2, 15 min | 1 | Battery-powered constant current stimulator (DC-Stimulator, neuro-Conn, Ilmenau, Germany) | No | PPT was assessed by a pressure gauge device. MPT was assessed by a weighted pinprick stimuli over the thenar part of both hands | 10 pinprick stimuli (256 mN, repeated at a 1/s rate within a small area of about 1 cm2) | Pressure gauge device (FDN100, Wagner Instruments,Greenwich, CT, USA | |
| Reidler et al. (2012) | US | Crossover | Heatlhy | - | - | 15 participants (60% females). Mean age: 36.7. Groups: A. a-tDCS. B. s-tDCS | C3 | SO | 2 mA, 35 cm2, 20 min | 1 | DC generator (Activa Dose, Salt lakeCity, UT | No | PPT was applied over the right thenar region | Difference of PPT measured before and after the immersion of the hand into cold water (10–12 C) | Commander Algometer, JTECH Medical, Salt
Lake City, UT |
|
| Zandieh et al. (2012) | Iran | Crossover | Healthy | - | -- | 22 participants (45% females) Mean age: 27.9 yrs. Groups: A. a-tDCS. B. c-tDCS. C.s-tDCS | C3 | SO | 2 mA, 35 cm2, 15 min | 1 | Not specified | No | CPT was assessed on the right hand (up to elbow) with a tank with cold water 3(+− 0.5) C. | Not specified | ||
| SO | C3 | 2 mA, 35 cm2, 15 min | 1 | No | ||||||||||||
| Moloney et al. (2013) | Ireland | Crossover | Healthy | - | - | 20 male participants. Mean age: 21.5 yrs. Groups: A. a-tDCS and real rTMS. B. c-tDCS and real rTMS. C. s-tDCS and real rTMS. D. s-tDCS and sham rTMS. | C3 | SO | 1 mA, 25 cm2, 10 min | 1 | tDCS (NewRonika, Italy) | stimulation of C3 with 1 Hz rTMS | CPT and HPT were assessed over the palmar thenar in both sides. | TSA-2001 NeuroSensory Analyzer apparatus (Medoc, Ramat Yishai, Israel) | ||
| SO | C3 | 1 mA, 25 cm2, 10 min | 1 | stimulation of C3 with 1 Hz rTMS | ||||||||||||
| Ihle et al (2014) | Germany | Crossover | Healthy | - | - | 16 participants (62% female) Mean age:27 yrs. Groups: A. c-tDCS. B. a-tDCS. C. s-tDCS | C3 | SO | 2 mA, 16 cm2, 15 min | 1 | DC-Stimulator (NeuroConn, Ilmenau, Germany) | No | HPT over the right volar forearme | Peltier device (TSAII, Medoc,Israel) | ||
| da Silva et al. (2015) | Brazil | Crossover | Healthy | - | - | 20 male participants divided in 3 random sequences of: A. a-tDCS +melatonin. B. s-tDCS + melatonin C. s-tDCS+placebo. First group: Mean age: 25.37 yrs. Second group: Mean age:25.67 yrs. Third group: Mean age: 25.60 yrs. | C3 | SO | 2 mA, 35 cm2, 20 min | 1 | Not specified | No | HPT over the mid-forearm (from 32 C to a maximum of 52 C). | Difference of HPT over the forearm, before and after cold pressor task as a conditioning stimulus (0 –1 C) | Computer Peltier-based devicethermode (30×30 mm) | |
| C3 | SO | 2 mA, 35 cm2, 20 min | 1 | Melatonin | ||||||||||||
| Vaseghi et al. (2015) | Australia | Crossover | Healthy | - | - | 12 participants (67% females). Mean age: 23.6 yrs. Groups: A. c-tDCS M1. B. c-tDCS S1. C. c-tDCS DLPFC. D. s-tDCS | SO | C3 | 0.3 mA, 3 cm2 over the target area and 12 cm2 over the reference, 20 min | 1 | tDCS stimulator (IntelectAdvancedTherapy System; Chattanooga, Vista, CA, USA) | No | PPT was measured by a pressure algometer over the first dorsal interosseous | Pressure algometer (model: FDX 50, Wagner, USA | ||
| Vasegui et al. (2015) | Australia | Crossover | Healthy | - | - | 12 participants (67% females) Mean age: 23.6 yrs. Groups: A. a-tDCS M1, B. a-tDCS S1, C. a-tDCS DLPFC. D. s-tDCS. E. no tDCS | C3 | SO | 0.3 mA, 3 cm2 over the target area and 12 cm2 over the reference, 20 min | 1 | tDCS stimulator (IntelectAdvancedTherapy System; Chattanooga, Vista, CA, USA) | No | PPT was measured by a pressure algometer over the first dorsal interosseous | Pressure algometer (model: FDX 50, Wagner, USA | ||
| Flood et al. (2016) | Australia | Crossover | Healthy | - | - | 30 males. Mean age: 23.9 yrs. A. a-HD-tDCS. B. s-HD-tDCS | C3 | Cz, F3, T7, P3 | 2 mA, 20 min | 1 | A HD-tDCS multi-channel stimulation interface (Model 4X1-C2, Soterix Medical, New York, NY) attached to a 1×1 low-intensity direct current stimulator (Model 1300, Soterix Medical) | No | PPT with a handheld pressure algometer over the index finger. | Difference of PPT over the index finger, before and after the hand immersion in cold water for 4 minutes (2 +-1C) | Algometer (Wagner Force Dial FDK 20, Wagner Instruments, Greenwich, CT) | |
| Flood et al. (2017) | Australia | Crossover | Healthy | - | - | 12 males. Mean age: 24.42 yrs. | C3/C4 | Cz, F3/F4, T7, P3/P4 | 2 mA, 20 min | 1 | A HD-tDCS multi-channel stimulation interface (Model 4X1-C2, Soterix Medical, New York, NY) attached to a 1×1 low-intensity direct current stimulator (Model 1300, Soterix Medical) | No | PPT was assessed by a pressure algometer over the dorsal surface of the index finger of the non-dominant hand | Difference of PPT over the index finger before and after the cuff occlusion of the non-dominant arm (Conditioned stimulus) | Algometer (Wagner Force Dial FDK 20, Wagner Instruments, Greenwich, CT) | |
| Braulio et al. (2018) | Brazil | Parallel | Healthy | - | - | 48 participants: A. 12 male participants in a-tDCS + placebo. Mean age: 26.09. B. 12 male participants in a-tDCS + remifentanil. Mean age:27.33. C. 12 male participants in s-tDCS + placebo. Mean age: 26.09. D. 12 participants in s-tDCS plus remifentanil. Mean age: 26.08 | C3 | SO | 2 mA, 35 cm2, 20 min | 1 | Not Specified | No | HPT over the mid-forearm (from 32C to a maximum of 53 C). | Three identical nociceptive stimuli of 6 in the NPS. hand immerse in cold water for 60 s, after 30 s the pain was rated. | Difference of HPT over the forearm before and after the immersion of the non-dominant hand in cold water (0–1 C) for 60 s | Not specified |
| C3 | SO | 2 mA, 35 cm2, 20 min | 1 | Remifentanil | ||||||||||||
| Hughes et al. (2018) | UK | Crossover | Healthy | - | - | Eight participants (25% female). Mean age: 27.4 yrs. | C3/C4 contralateral to the pain testing | SO | 2 mA, 16 cm2, 20 min | 1 | Battery driven stimulator neuroConn GMBH, Ilmenau, Germany | No | Electrical stimulus (TS) | Trains of transcutaneous electrical stimulation consisted of five constant current 1-ms-duration pulses at 200 Hz at the Retromaleolar pathways of sural nerve (TS). | Current stimulator (DS7A, Digitimer, UK) | |
a-tDCS: active-tDCS; AE: Aerobic Exercise; C3:left central lobe position based on 10–20 EEG system; C4:Right central lobe position based on 10–20 EEG system; Cz Vertex position based on 10–20 EEG system; CPT: Cold Pain Threshold; CPM: Conditioned Pain Modulation; c-tDCS: cathodal-tDCS; DC: Direct Current; DLPFC: Dorsolateral Prefrontal Cortex; EIMS: Electrical Intramuscular Stimulation;F3: left DLPFC location based on 10–20 EEG system; F4:right DLPFC location based on 10–20 EEG system; HPT: Heat Pain Threshold; HD-tDCS: High Definition-tDCS;M1:Primary Motor Cortex; mA: milliampere; mN: millinewton; MPT: Mechanical Pain Threshold; P3: left Parietal lobe location based on 10–20 EEG system; P4: Right Parietal lobe location based on 10–20 EEG system; PPT: Pressure Pain Threshold; QST: Quantitative Sensory Testing; rTMS: repetitive Transcranial Magnetic Stimulation; SD: Standard Deviation; s-tDCS: sham-tDCS; SO: Supraorbital; T7: Temporal lobe location based on 10–20 EEG system; TMJ: Temporal-Mandibula Junction; r TS: Temporal Summation; tDCS: Transcranial Direct Current Stimulation.
Table 2.
General information from the rTMS studies included in the meta-analysis
| Articles | Country | Design | Condition | Pain Duration | Sensory Profile | Participants | Motor Cortex Stimulation Parameters | QST Protocol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target, coil type | frequency | Intensity % motor threshold | Total number of pulses | Device | Number of sessions | Concomitant Therapy or motor tasks | Pain threshold | TS | CPM | Device | |||||||
| Johnson et al. (2006) | Australia | Crossover | Chronic back pain | Not reported | Not reported | 17 participats (59 % females ). Mean age: 43.5 yrs. A) a-rTMS. B)s-rTMS | left M1/S1, figure 8 coil | 20 | 95 | 500 | MagStim Super Rapid stimulator | 1 | No | HPT and CPT were meassured over the thenar eminence of the right hand | Thermal Sensory Analyzer (TSA-2001) device (Medoc Ltd) | ||
| Dall Agnol et al. (2014) | Brazil | Parallel | Myofascial pain syndrome | Not reported | Not reported | 24 female participants. A. 12 participants in a-rTMS. Mean age: 45.83 yrs. B. 12 participants in s-tDCS. Mean age: 44.83 yrs. | C3, figure 8 coil | 10 | 80% RMT | 1600 | MagPro X100 (MagVentureCompany, Lucernemarken, Denmark) | 1 | No | HPT was measured with a Peltier-based thermode over the mid-forearm from 32 C up to a maximun 52 C | Difference of pain score of the heat stimuli over the forear before and after the immersion of the opposite hand in cold water (0–1 C) | Computer Peltier-based device thermode (30 × 30 mm(Heat Pain Stimulator-1.1.10, Brazil) | |
| Graff-Guerrero et al. (2005) | Mexico | Parallel | Healthy | - | - | 180 participants (45% female). Mean age: 19 yrs. Experiment 1: A)15 participants in a-rTMS L-DLPFC. B) 15 participants in a-rTMS R-DLPFC. C) 15 participants in a-rTMS R-MC. D) 15 participants in a-rTMS L-MC. E) 15 participants in s-rTMS. Experiment 2: A)15 participants in a-rTMS L-DLPFC. B) 15 participants in a-rTMS R-DLPFC. C) 15 participants in a-rTMS R-MC. D) 15 participants in a-rTMS L-MC. E) 15 participants in s-rTMS | C3, figure 8 coil | 1 Hz | 100% MT | 900 | Mc-b70 transducer, Dantec MagPro, Medical A/S,Skovlunde, Denmark | 1 | No | PPT was measured by an electronic algometer over the distal phalanx of the fifth finger. CPT was measured with a cold pressor test, by immersing the hand in cold water (8+-1) and resporting the pain threshold. HPT was assessed with an infrared generator over the forearm. | Electronic pressure algometer with a
modified Randall–Selitto test (Basile Analgesy-Meter, Milan,
Italy Infrared generator (Basile Plantar-Test, Milan, Italy) |
||
| C4, figure 8 coil | 1 Hz | 100% MT | 900 | 1 | No | ||||||||||||
| Mylius et al. (2007) | Germany | Crossover | Healthy | - | - | 12 participants (50% females) Mean age: 22.3 yrs. Group: A) a-rTMS. B) s-rTMS | C3/C4 contralateral to induced pain, figure 8 coil | 10 Hz | 80% RMT | Medtronic MagPro stimulator (Medtronic Functional Diagnostics, Skovlunde, Denmark) | 1 | No | Pain threshold by electrical stimulation on the left calf over the subcutaneous course of sural nerve. | Not specified | |||
| Borckardt et al. (2011) | US | Crossover | Healthy | - | - | 75 participants (60% female). Mean age: 29.95 Groups: A. 13 participants in rTMS at 1 Hz 80% of rMT. B. 12 participants in rTMS at 1 Hz 100% of rMT. C. 15 participants in 10Hz 80%rMT. D. 13 participants in 10Hz 100% rMT. E. 12 participants in 50 Hz triplets at 90% of active motor threshold (theta burst) | C3, figure 8 coil | 1 Hz | 80% | 1200 | Neuronetics Model 2100, Neuronetics Inc.; Malvern, PA | 1 | No | PPT was measured with a electrovonfrey anesthesiometer over the dorsum of the ventral pad of the digit minimi of the right hand | TS was assessed measuring a serie of heat pulses (1 per 1.5 s) for 30 seconds over the left forearm | PPT: Digital Electrovonfrey
Anesthesiometer (IITC model Alemo 2290–4; Woodland Hills, CA,
USA) TS: The Medoc PATHWAY Pain & Sensory Evaluation System (Medoc Ltd, Israel) |
|
| C3, figure 8 coil | 1Hz | 100% | 1200 | 1 | No | ||||||||||||
| C3, figure 8 coil | 10 Hz | 80% | 1200 | 1 | No | ||||||||||||
| C3, figure 8 coil | 10 Hz | 100% | 1200 | 1 | No | ||||||||||||
| C3, figure 8 coil | 50 Hz triplets delivered at the rate of 5Hz | 90% | 1200 | 1 | No | ||||||||||||
| Ciampi de Andrade et al. (2011) | France | Crossover | Healthy | - | - | 36 participants: A. 12 participants in a-rTMS M1 (33% females). Mean age: 30.2 yrs. B. 12 participants in a-rTMS DLPFC/PMC(25% females). Mean age: 28.2 yrs. C. 12 participants in s-rTMS (42% females). Mean age: 29 yrs. | C4, figure 8 coil | 10 Hz | 80% RMT | 1500 | MagPROX100 machine (MagVenture Tonika Elektronic, Farum, Denmark) | 2 | Naloxone or placebo | CPT was measured with a Somedic thermotest over the lest thenar eminence. | Somedic thermotest(Somedic AB, Stockholm, Sweden) | ||
| Ciampi de Andrade et al. (2014) | France | Crossover | Healthy | - | - | 36 participants: A. 12 participants in a-rTMS M1 (42% females). Mean age: 30.6 yrs. B. 12 participants in a-rTMS DLPFC/PMC(33% females). Mean age: 29.2 yrs. C. 12 participants in s-rTMS (42% females). Mean age: 29 yrs. | C4, figure 8 coil | 10 Hz | 80% RMT | 1500 | MagPROX100 machine (Magventure Tonika Elektronic, Farum, Denmark) | 2 | Ketamine or placebo | CPT was measured with a Somedic thermotest over the lest thenar eminence. | Somedic Thermotest (Somedic AB, Stockholm, Sweden) |
||
| Moisset et al. (2015) | France | Crossover | Healthy | - | - | “14 participants (50% females). Mean age: 26.9 yrs. Groups: A) prolonged continuous TBS (pcTBS). B) intermittent TBS (iTBS). C) classical 10 Hz rTMS. D) sham stimulation” | C3, figure 8 coil | Prolonged cTBS: Three pulses at 50 Hz (i.e. 60 ms) repeated 400 times at intervals of 200 ms | 80% RMT | 1200 | MagPro 100 machine (Magventure Tonika Elektronic, Denmark), | 1 | No | CPT was measured with a thermotest over both thenar eminences and over the left foot. | Difference of pain score of suprathreshold stimulis over the right tenar eminence was measured before and after the immersion of the left foot in cold water (4–8 C) | Somedic thermotest (Somedic AB, Stockholm, Sweden) | |
| C3, figure 8 coil | Intermittent TBS: 20 trains (3 pulses at 50 Hz repeated 10 times at 200 ms intervals) with an intertrain interval of 8 s (total of 600 pulses in 3 min and 20 s) | 80% RMT | 600 | 1 | No | ||||||||||||
| C3, figure 8 coil | 10 Hz | 80% RMT | 1500 | 1 | No | ||||||||||||
| Lamusuo et al. (2017) | Finland | Crossover | Healthy | - | - | 10 participants (70% females). Mean age: | Right M1/S1, double eight-shaped coil | 10 | 90% RMT | 1000 | Nexstim Ltd.,Helsinki, Finland) with a double eight-shaped coilgiving biphasic pulses (Nexstim Ltd., Focal Bipulse8-coil) | 1 | No | "Warm, coheat pain and cold pain detection thresholds in the infraorbital nerve distribution" | TS device (Medoc Ltd., Rehovot,Israel) | ||
| Cavaleri et al. 2019 | Australia | Parallel | Healthy | - | - | 30 participants. Group: A. 15 participants in a-rTMS (47 % of females). Mean age: 23.9 yrs. B. 15 participants in a-rTMS (47 % of females). Mean age: 22.7 yrs. | C3, figure 8 coil | 1 Hz | 90% RMT | 1200 | MagstimSuper Rapid2(Magstim Co Ltd, Dyfed, United Kingdom | 5 | No | "PPT was measured with an algometer over the right (injected with nerve growth factor) ECRB muscle" | Difference of PPT over the right ECRB muscle, injected site, before and after the immersion of the left had in cold water (4–6 C) as the conditioning stimulus | Algometer (Somedic, 1 cm 2probe, Norra Mellby, Sweden) | |
a-tDCS: active-tDCS; a-rTMS: active repetitive Transcranial Magnetic Stimulation; C3:left central lobe position based on 10–20 EEG system; C4:Right central lobe position based on 10–20 EEG system CPM: Conditioned Pain Modulation; CPT: Cold Pain Threshold; c-tDCS: cathodal tDCS; DLPFC: Dorsolateral Prefrontal Cortex; ECRB: Extensor Carpi Radialis Brevis; EIMS: Electrical Intramuscular Stimulation; HPT: Heat Pain Threshold; iTBS: intermittent Theta Burst Stimulation; L-DLPFC: Left Dorsolateral Prefrontal Cortex; L-MC: Left-Motor Cortex; M1: Primary Motor Cortex; pcTBS: prolonged continuous Theta Burst Stimulation; PMC: Primary Motor Cortex; PPT: Pressure Pain Threshold; QST: Quantitative Sensory Testing; R-DLPFC: Right Dorsolateral Prefrontal Cortex; R-MC: Right Motor Cortex; RMT: Resting Motor Threshold; rTMS: repetitive Transcranial Magnetic Stimulation; S1: Primary Somatosensorial Cortex; SO: Supraorbital; s-rTMS: sham-repetitive Transcranial Magnetic Stimulation; s-tDCS: sham-tDCS; TBS:Theta Burst Stimulation; tDCS: Transcranial Direct Current Stimulation; TS: Temporal Summation.
Effect on outcomes
Pain threshold
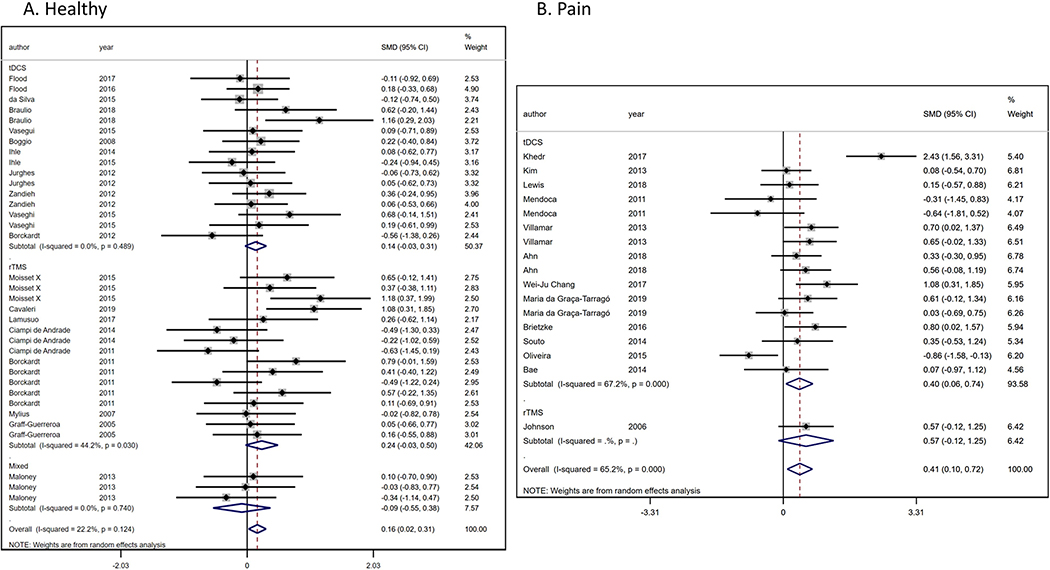
We analyzed 20 RCTs [8-11; 14; 17; 18; 21; 22; 25; 26; 32; 34; 36; 38; 43; 55-57; 64; 73; 74; 78] (35 comparisons) with healthy population (n=629) (Figure 2a) to evaluate the NIBs effects on PT. We found a significant and homogenous PT increase (ES: 0.16, 95% CI:0.02 to 0.31; I2=22.2%) in favor to NIBs intervention compared to sham. When analyzing techniques separately, results were not significant, the 12 tDCS studies results in an effect size of 0.14 (95% CI:−0.03 to 0.31), while for the eight rTMS studies, the pooled effect size was 0.24 (95% CI:−0.03 to 0.50), and the combination of both (tDCS + rTMS) effect was −0.09 (95% CI:−0.55 to 0.38). No significant difference was found in the heterogeneity test between sub-groups (p=0.426).
Figure 2.
Forest-plot of Pain threshold in A) healthy population and B) pain conditions.
Besides, we evaluated the PT changes due to NIBs interventions in pain population (n=492), from 14 RCTs [1; 6; 12; 15; 20; 37; 40; 41; 49; 52; 53; 62; 69; 76] (18 comparisons) (Figure 2b). We found a significant PT increase in favor to NIBs (ES: 0.48, 95% CI:0.15 to 0.89; I2=68.8%). However, we did not found differences (p=0.790) among tDCS (ES: 0.47, 95% CI:0.13 to 0.82) and rTMS effects (ES: 0.57, 95% CI:−0.12 to 1.25).
Conditioned pain modulation
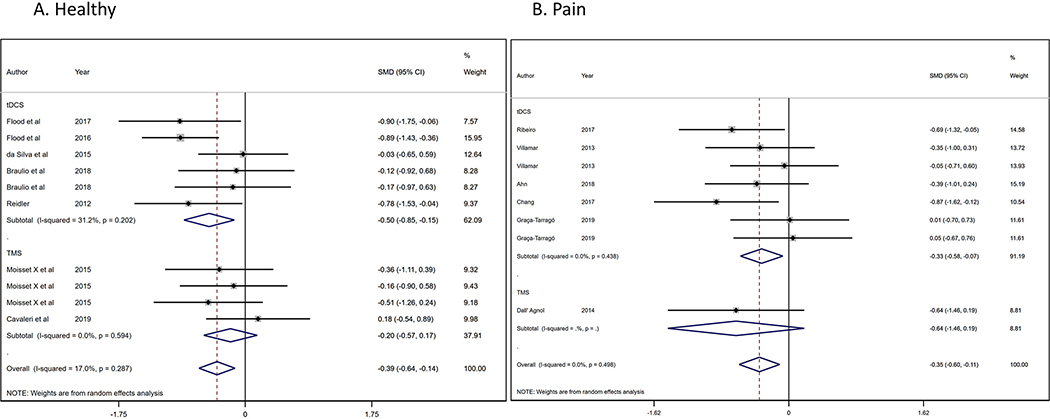
We analyzed seven RCTs [11; 14; 21; 25; 26; 55; 64] (10 comparisons) with healthy population (n=303) (Figure 3a) that evaluate the NIBs effects on CPM effect (pain ratings reduction). We found a significant and homogenous higher CPM effect (ES: −0.39, 95% CI:−0.64 to −0.14; I2=17%) in favor to NIBs intervention compared to sham. The tDCS effect size was significant (ES: −0.50, 95% CI:−0.85 to −0.15); while the rTMS was not (ES: −0.20, 95% CI:−0.57 to 0.17). No significant difference was found in the heterogeneity test between sub-groups (p=0.195). Besides, we evaluated the CPM effects due to NIBs interventions in pain population (n=184), from six RCTs [1; 15; 20; 22; 65; 76] (eight comparisons) (Figure 3b) compared with healthy subjects, we found a significant and homogeneous CPM effect in favor to NIBs (ES: −0.35, 95% CI:−0.60 to −0.11; I2=0%). We did not found differences (p=0.266) among tDCS (ES: −0.33, 95% CI:−0.58 to −0.07) and rTMS effects (ES: −0.35, 95% CI:−0.60 to −0.11).
Figure 3.
Forest-plot of conditioned pain modulation in A) pain conditions and B) healthy population.
Temporal summation
We could not perform a meta-analysis due to lack of combinable data, from the 2 included studies [34; 49], one included a healthy population [34] and the other chronic pain patients [49].
Risk of bias assessment
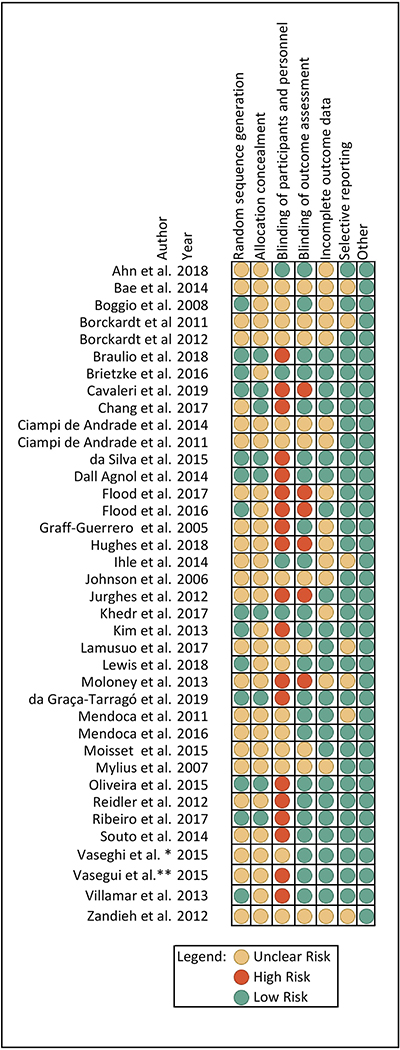
Most of the selected articles (44.74%) had low risks of bias in several categories. However, most of them (60.53%) had problems when reporting randomization sequence generation and allocation concealment, even though they specified the allocation of the subjects was done by a random method, they did not specify how the randomization sequence was generated. Besides, most of them (73.68%) did not specify how they achieved the allocation concealment; therefore, they were classified as unclear regarding this item. Furthermore, more than half of the selected articles (55.26%) presented a high risk of bias in the blinding component, as most of them did not blind the researcher performing the intervention. See Figure 4.
Figure 4.
Risk of bias
Subgroup, sensitivity, and meta-regression analysis
The sensitivity analysis showed that results did not change even if the study with the largest effect was removed from the analysis and also when we excluded one study at a time. Moreover, after subgroup analysis and meta-regression, the risk of bias level, the combination with other interventions, the number of sessions (less or more than one session), stimulation polarity (excitatory versus inhibitory) and type of stimulus (pressure, heat, cold or electrical stimulus) were not important sources of heterogeneity in the CPM analysis (online supplementary material 5). However, we identified substantial sources of heterogeneity (I2>60%) in the PT analysis (the use of NIBS combined with other interventions, the stimulation polarity [excitatory vs. inhibitory stimulation], and type of stimulus [pressure, cold, heat stimulus]). We evaluated the PT location (upper- and lower-limb) as a source of heterogeneity, we found, in the healthy population, a higher pooled effect size from studies with a lower limb as a location for the PT assessment compared with upper-limb location; however, we did not find this difference among the studies with pain population (online supplementary 5).
Also, we performed a subgroup analysis by disease with 2 or more included studies (Knee OA and Fibromyalgia), we did not find any significant difference between conditions (online supplementary 5). Finally, we performed a subgroup analysis by conditions categorized by the underlying mechanisms: neuropathic pain states (peripheral neuropathy and poststroke pain), nociceptive states (osteoarthritis and low back pain), and nociplastic states (fibromyalgia); we found no difference in the CPM response between nociceptive and nociplastic states, but the PT increase was stronger in the nociplastic states (ES: 0.81, 95% CI:−0.01 to 1.63) compared with neuropathic and nociceptive (ES: 0.15, 95% CI:−0.24 to 0.54; ES: 0.38, 95% CI:−0.01 to 0.78; respectively)(online supplementary 5).
Publication bias
We did not find publication bias in the PT and CPM meta-analysis as indexed by symmetrical funnel plots and non-significant egger test analysis (online supplementary material 6)
DISCUSSION
Summary of results
We included 38 RCTs that have evaluated the effects of Non-invasive motor cortex stimulation on QST outcomes in healthy and pain subjects. The included studies were heterogeneous, had small sample sizes, and presented a low to moderate risk of bias with no publication bias. We found a significant increase in pain threshold and homogenous higher CPM effect (small to moderate effect size) in healthy subjects and pain disorders, in favor of NIBs group compared with sham. These effects seem robust and consistent since all sensitivity analyses, subgroup analyses, and meta-regression could not identify any critical between-studies source of heterogeneity.
Motor cortex stimulation effects on pain threshold
Our meta-analysis found a small to moderate pooled effect of motor cortex stimulation by tDCS and rTMS on pain thresholds, these results are consistent with one of the postulated mechanisms of action of NIBs: modulation of pain by activation of subcortical structures related to the endogenous pain modulation system as the thalamus, cingulate gyrus, periaqueductal gray, subnucleus reticularis dorsalis, among others [30; 31]. This endogenous pain modulation system also could affect the pain threshold perception.
Regarding the type of stimuli to evaluate the PT changes, we included all the reported categories such as heat, cold, electrical, and mechanical pain stimulus. We did not find significant differences among the type of stimuli, which are consistent with previous literature [48]; this would be useful for future design experiments and to increase the comparability of different PT protocols.
Previous systematic reviews and meta-analysis [75] evaluated the effect of anodal tDCS on PT in healthy subjects compared to sham intervention. Similar to our results, they found a significant increase of PT (MD 12.57, 95% CI: 6.29 to 18.85), however, they did not report the results using a standardized effect size, which hinders the comparison with our findings.
Our findings by population show that both healthy and pain populations increased their PT values; however, we found higher increases in the pain population, these results could be explained due to a ceiling effect. In other words, pain neurocircuitry dysfunction provides a more extensive range of modulation of this system as there is a limit for the enhancement of the endogenous inhibitory pain system. This supports that non-invasive motor cortex stimulation is a brain-state dependent technique [35] with a high potential to modulate pain, especially in dysfunctional and maladaptive pain networks in chronic pain patients. However, due to the heterogeneous included pain populations, further studies are needed to elucidate the effects in specific pain conditions.
Motor cortex stimulation effects on conditioned pain modulation
In this metanalysis, both techniques (tDCS and rTMS) showed a similar direction effect in CPM effects both in health and in pain populations, although they have different mechanisms of action. In contrast to the PT results by population, the CPM effect was similar in both suggesting a higher neuroplasticity potential of the descending inhibitory pathways related to CPM effects and potentially less ceiling effect. It may also indicate that CPM is a better marker to address, understand and measure the mechanistic effects of motor cortex stimulation.
We hypothesize that non-invasive motor cortex stimulation could have modulated motor cortex excitability restoring the inhibitory effects on pain circuits, as seen in neuropathic pain and other types of pain [3; 47]. In fact, lack of inhibition by the motor cortex leads to decreased endogenous pain inhibitory pathways [13; 60]. This fact is supported by some studies that showed CPM as a possible predictor of chronic pain development [39; 60]. Also, studies advocate that CPM could be used as a possible prognosis factor for pain sensitized patients and, therefore, could be used as a predictor of higher pain levels experience. One idea is also to explore CPM as a possible prognostic variable for tDCS and rTMS, as recently proposed in another trial [71]. Also, one possibility is also to use motor cortex stimulation to enhance the endogenous pain inhibitory system as to “prevent” pain in a healthy population exposed to a nociceptive stimulus [4; 28].
More well-powered studies are needed to validate the CPM biomarker as a predictor of motor cortex stimulation effects in pain populations and to elucidate the relationship of CPM effect and pain levels in specific chronic pain populations.
Heterogeneity of methods of QST and NIBS protocols
The behavioral protocols change across the studies, measuring different variables of pain processing with different QST protocols; almost half of the studies used pressure pain threshold, while others used heat, cold, or electric stimuli. On the other hand, cold water stimuli were the most frequently used as the conditioning stimulus in CPM. We found that the type stimulus in the PT protocols is an important source of heterogeneity in the meta-analysis of pain populations, however, the pooled effect sizes are similar among those subgroups (online supplementary 5). In this context, the different QST protocols should not be a source of heterogeneity. Additionally, different anatomical parts as the forearm, hand, feet, knee, among others, were used for the QST assessment. Although the healthy population should not be affected, in different pain conditions, these different locations would be related to peripheral and central sensitization. Therefore, the information of these results might contribute to the heterogeneity and accuracy of our findings.
Another factor of heterogeneity of significant value is the difference in the stimulation parameters. Even though we selected studies that had investigated the effects of the stimulation on the same cortical area, there are known differences between the underlying mechanisms of tDCS and rTMS and the number of sessions. However, we decided to do an exploratory pooled analysis [42; 50][43; 51][43; 51][43; 51][44; 52][44; 52][43; 50][43; 50][43; 50][42; 49][42; 49] because most of the included studies used an excitatory protocol over the motor cortex.
Additionally, the statistical approach to analyze the QST outcomes (e.g. proportion vs absolute numbers changes), the presence or not of follow-up, and quantity and duration variation of QST assessments across all the included studies (see table 1) highlight the need of acquiring more standardized data for a more precise QST outcomes changes evaluation.
tDCS and rTMS mechanisms to modulate QST
The quantitative sensory testing is a more objective measurement of pain perception processes [67]. It includes different tasks as sensory pain testing, pain threshold, conditioned pain modulation and temporal summation. These different measurements assess peripheral and central sensitization. The pain signal arrives in the dorsal horn and then crosses the midline just in front of the anterior commissure and sent the signal up by the spinothalamic tract to the thalamus and then to the sensory cortex [77]. Once pain signals arrive in the sensory area, it is processed and interpreted as a pain threshold [77]. Then, the signal sends feedback via supraspinal structures as the primary motor cortex, sensory cortex, thalamus, and other structures as the cingulate gyrus, periaqueductal gyrus, rostral ventromedial medulla, subnucleus reticularis dorsalis and spinal cord, in order to enhance the endogenous pain modulation system decreasing the pain perception. In a healthy population, this mechanism is believed to contrast the pain stimulus [77]. However, in chronic pain, there is a disruption in this communication, decreasing the pain threshold and increasing pain perception [63]. This effect disrupts the endogenous pain pathway that thus can be measured with the CPM [58].
It is still unclear whether the differential mechanism of rTMS compared to tDCS on the motor cortex would represent a different mechanism of endogenous pain inhibitory system modulation though the final effect is similar. tDCS delivers a continuous transcranial subthreshold current inducing long-lasting modulation of the neuronal activity by mechanisms of long-term potentiation (LTP) and long-term depression (LTD) and therefore changing synaptic plasticity mechanisms [19; 29]. On the other hand, rTMS induces an action potential, and thus a response of the neuronal membrane and thereby, different frequencies of pulses can enhance or inhibit excitability in the targeted region [29] although some differences in the mechanisms, both of them have shown capability of inducing long term effects related to neuroplastic mechanisms of pain [29]. The rationale behind using motor cortex stimulation relies on the ability to potentiate the endogenous pain modulation system. Motor cortex stimulation ultimately modulates other circuits such as the thalamus and other structures as the sensory cortex, cingulate gyrus, periaqueductal gyrus, and subnuclear reticularis dorsalis. These structures control the inhibition/facilitation of pain perception and, ultimately, the PT and CPM effects. Hence, these techniques have the capacity to modulate these structures by a top-down modulation in healthy and pain conditions indexed by QST outcomes changes.
The potential therapeutic implications of NIBS are plausible, specially using motor cortex stimulation, however these results seem brain-state dependent [35] and possible disease-related, even though we reported here a possible larger modulation of PT in nociplastic pain syndromes, such as fibromyalgia, the small sample size and higher heterogeneity among the current evidence difficult to draw a definitive conclusion.
Limitations
Some factors may limit these results and thus should be interpreted with caution. One crucial factor, as mentioned, is the heterogeneity of the QST measurement and the NIBS protocols. Chronic pain patients presented different syndromes across the studies that result in different mechanisms of pain and differential responsiveness to the stimulation. To address this problem, we decided to divide the results in healthy, and pain conditions as combining the pain population with healthy subjects could bias the results. Another limitation is the inclusion of pilot studies and not adequately justified sample size calculation by a statistical power analysis. Finally, as shown by Cochrane risk bias indexes, some of the studies included did not describe the randomization accurately and/or blinding procedures, thus leading to potentially lower quality of the data included in the analysis. However, the comprehensive and systematic methodology used in this study assures the high-quality summary of all the studies to date in the field and motivates the conduct of future research with improved design.
Conclusion
This meta-analysis suggests a significant small to moderate effect of non-invasive motor cortex stimulation on PT and CPM in healthy and pain populations. This supports the idea of top-down modulation of endogenous pain pathways by motor cortex stimulation as one of their primary mechanism of action on pain. These biomarkers could be useful in the treatment follow-up of chronic pain patients. However, validation requires further investigation under strict methodological settings, and with an evaluation of specific chronic pain populations.
Supplementary Material
Acknowledgments
This study was funded by NIH grant R01 AT009491-01A1.
Footnotes
Conflicts of interests: The authors declare to have no compelling interests with this article.
References
- [1].Ahn H, Suchting R, Woods AJ, Miao H, Green C, Cho RY, Choi E, Fillingim RB. Bayesian analysis of the effect of transcranial direct current stimulation on experimental pain sensitivity in older adults with knee osteoarthritis: randomized sham-controlled pilot clinical study. J Pain Res 2018;11:2071–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain stimulation 2008;1(2):97–105. [DOI] [PubMed] [Google Scholar]
- [3].Antal A, Terney D, Kühnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. Journal of pain and symptom management 2010;39(5):890–903. [DOI] [PubMed] [Google Scholar]
- [4].Arendt-Nielsen L, Jiang GL, DeGryse R, Turkel CC. Intra-articular onabotulinumtoxinA in osteoarthritis knee pain: effect on human mechanistic pain biomarkers and clinical pain. Scandinavian journal of rheumatology 2017;46(4):303–316. [DOI] [PubMed] [Google Scholar]
- [5].Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. The Journal of Pain 2009;10(6):556–572. [DOI] [PubMed] [Google Scholar]
- [6].Bae SH, Kim Gd Fau - Kim K-Y, Kim KY. Analgesic effect of transcranial direct current stimulation on central post-stroke pain. Tohoku J Exp Med 2014;234(3):189–95. [DOI] [PubMed] [Google Scholar]
- [7].Bannister K, Dickenson AH. The plasticity of descending controls in pain: translational probing. The Journal of physiology 2017;595(13):4159–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol 2008;15(10):1124–1130. [DOI] [PubMed] [Google Scholar]
- [9].Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, Madan A, Barth K, George MS. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. The journal of pain : official journal of the American Pain Society 2012;13(2):112–120. [DOI] [PubMed] [Google Scholar]
- [10].Borckardt JJ, Reeves ST, Beam W, Jensen MP, Gracely RH, Katz S, Smith AR, Madan A, Patterson D, George MS. A randomized, controlled investigation of motor cortex transcranial magnetic stimulation (TMS) effects on quantitative sensory measures in healthy adults: evaluation of TMS device parameters. Clin J Pain 2011;27(6):486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Braulio G, Passos SC, Leite F, Schwertner A, Stefani LC, Palmer ACS, Torres ILS, Fregni F, Caumo W. Effects of Transcranial Direct Current Stimulation Block Remifentanil-Induced Hyperalgesia: A Randomized, Double-Blind Clinical Trial. Front Pharmacol 2018;9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brietzke AP, Rozisky JR, Dussan-Sarria JA, Deitos A, Laste G, Hoppe PF, Muller S, Torres IL, Alvares-da-Silva MR, de Amorim RF, Fregni F, Caumo W. Neuroplastic Effects of Transcranial Direct Current Stimulation on Painful Symptoms Reduction in Chronic Hepatitis C: A Phase II Randomized, Double Blind, Sham Controlled Trial. Frontiers in neuroscience 2015;9:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Castillo Saavedra L, Mendonca M, Fregni F. Role of the primary motor cortex in the maintenance and treatment of pain in fibromyalgia. Medical hypotheses 2014;83(3):332–336. [DOI] [PubMed] [Google Scholar]
- [14].Cavaleri R, Chipchase LS, Summers SJ, Schabrun SM. Repetitive transcranial magnetic stimulation of the primary motor cortex expedites recovery in the transition from acute to sustained experimental pain: a randomised, controlled study. Pain 2019;160(11):2624–2633. [DOI] [PubMed] [Google Scholar]
- [15].Chang WJ, Bennell KL, Hodges PW, Hinman RS, Young CL, Buscemi V, Liston MB, Schabrun SM. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: A pilot randomised controlled trial. PLoS One 2017;12(6):e0180328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Front Hum Neurosci 2015;9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ciampi de Andrade DMA, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: The role of endogenous opioids. Pain 2011;152(2):320–326. [DOI] [PubMed] [Google Scholar]
- [18].Ciampi de Andrade D, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Repetitive transcranial magnetic stimulation induced analgesia depends on N-methyl-D-aspartate glutamate receptors. Pain 2014;155(3):598–605. [DOI] [PubMed] [Google Scholar]
- [19].Cirillo GDPG, Capone F, Ranieri F, Florio L, Todisco V, Tedeschi G, Funke K, Di Lazzaro V. Neurobiological after-effects of non-invasive brain stimulation. Brain stimulation 2017;10(1):1–18. [DOI] [PubMed] [Google Scholar]
- [20].da Graca-Tarrago M, Lech M, Angoleri LDM, Santos DS, Deitos A, Brietzke AP, Torres IL, Fregni F, Caumo W. Intramuscular electrical stimulus potentiates motor cortex modulation effects on pain and descending inhibitory systems in knee osteoarthritis: a randomized, factorial, sham-controlled study. J Pain Res 2019;12:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].da Silva NRJ, Laste G, Deitos A, Stefani LC, Cambraia-Canto G, Torres ILS, Brunoni AR, Fregni F, Caumo W. Combined neuromodulatory interventions in acute experimental pain: assessment of melatonin and non-invasive brain stimulation. Frontiers in Behavioral Neuroscience 2015;9(77). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dall’Agnol L, Medeiros LF, Torres ILS, Deitos A, Brietzke A, Laste G, de Souza A, Vieira JL, Fregni F, Caumo W. Repetitive Transcranial Magnetic Stimulation Increases the Corticospinal Inhibition and the Brain-Derived Neurotrophic Factor in Chronic Myofascial Pain Syndrome: An Explanatory Double-Blinded, Randomized, Sham-Controlled Trial. The Journal of Pain 2014;15(8):845–855. [DOI] [PubMed] [Google Scholar]
- [23].DerSimonian R Fau - Laird N, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- [24].Duarte D, Castelo-Branco LEC, Uygur Kucukseymen E, Fregni F. Developing an optimized strategy with transcranial direct current stimulation to enhance the endogenous pain control system in fibromyalgia. Expert review of medical devices 2018;15(12):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Flood A, Waddington G, Cathcart S. High-Definition Transcranial Direct Current Stimulation Enhances Conditioned Pain Modulation in Healthy Volunteers: A Randomized Trial. The journal of pain : official journal of the American Pain Society 2016;17(5):600–605. [DOI] [PubMed] [Google Scholar]
- [26].Flood A, Waddington G, Keegan RJ, Thompson KG, Cathcart S. The effects of elevated pain inhibition on endurance exercise performance. PeerJ 2017;5:e3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. The Lancet Neurology 2007;6(2):188–191. [DOI] [PubMed] [Google Scholar]
- [28].Fregni F, Macedo IC, Spezia-Adachi LN, Scarabelot VL, Laste G, Souza A, Sanches PRS, Caumo W, Torres ILS. Transcranial direct current stimulation (tDCS) prevents chronic stress-induced hyperalgesia in rats. Brain stimulation 2018;11(2):299–301. [DOI] [PubMed] [Google Scholar]
- [29].Fregni FP-LA. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol 2007;3(7):383–393. [DOI] [PubMed] [Google Scholar]
- [30].Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Bonnefoi F, Mauguiere F, Laurent B, Sindou M. Positron emission tomography during motor cortex stimulation for pain control. Stereotactic and functional neurosurgery 1997;68(1–4 Pt 1):141–148. [DOI] [PubMed] [Google Scholar]
- [31].Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, Convers P, Mauguiere F, Sindou M, Laurent B. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain 1999;83(2):259–273. [DOI] [PubMed] [Google Scholar]
- [32].Graff-Guerrero A, Gonzalez-Olvera J, Fresan A, Gomez-Martin D, Mendez-Nunez JC, Pellicer F. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Brain Res Cogn Brain Res 2005;25(1):153–160. [DOI] [PubMed] [Google Scholar]
- [33].Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hughes SW, Ali M, Sharma P, Insan N, Strutton PH. Frequency-dependent top-down modulation of temporal summation by anodal transcranial direct-current stimulation of the primary motor cortex in healthy adults. Eur J Pain 2018. [DOI] [PubMed] [Google Scholar]
- [35].Hurley RML. Using tDCS priming to improve brain function: Can metaplasticity provide the key to boosting outcomes? Neurosci Biobehav Rev 2017;83:155–159. [DOI] [PubMed] [Google Scholar]
- [36].Ihle K, Rodriguez-Raecke R, Luedtke K, May A. tDCS modulates cortical nociceptive processing but has little to no impact on pain perception. PAIN® 2014;155(10):2080–2087. [DOI] [PubMed] [Google Scholar]
- [37].Johnson S, Summers J, Pridmore S. Changes to somatosensory detection and pain thresholds following high frequency repetitive TMS of the motor cortex in individuals suffering from chronic pain. Pain 2006;123(1–2):187–192. [DOI] [PubMed] [Google Scholar]
- [38].Jurgens TP, Schulte A, Klein T, May A. Transcranial direct current stimulation does neither modulate results of a quantitative sensory testing protocol nor ratings of suprathreshold heat stimuli in healthy volunteers. Eur J Pain 2012;16(9):1251–1263. [DOI] [PubMed] [Google Scholar]
- [39].Katz NP, Paillard FC, Edwards RR. Review of the performance of quantitative sensory testing methods to detect hyperalgesia in chronic pain patients on long-term opioids. Anesthesiology 2015;122(3):677–685. [DOI] [PubMed] [Google Scholar]
- [40].Khedr EM, Omran EAH, Ismail NM, El-Hammady DH, Goma SH, Kotb H, Galal H, Osman AM, Farghaly HSM, Karim AA, Ahmed GA. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain stimulation 2017;10(5):893–901. [DOI] [PubMed] [Google Scholar]
- [41].Kim YJ, Ku J, Kim HJ, Im DJ, Lee HS, Han KA, Kang YJ. Randomized, sham controlled trial of transcranial direct current stimulation for painful diabetic polyneuropathy. Ann Rehabil Med 2013;37(6):766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kniknik LMD-SJ, Rozisky JR, Torres IL, Brunoni AR, Fregni F, Caumo W. Repetitive Transcranial Magnetic Stimulation for Fibromyalgia: Systematic Review and Meta-Analysis. Pain Pract 2016;16(3):294–304. [DOI] [PubMed] [Google Scholar]
- [43].Lamusuo S, Hirvonen J, Lindholm P, Martikainen IK, Hagelberg N, Parkkola R, Taiminen T, Hietala J, Helin S, Virtanen A, Pertovaara A, Jaaskelainen SK. Neurotransmitters behind pain relief with transcranial magnetic stimulation - positron emission tomography evidence for release of endogenous opioids. Eur J Pain 2017;21(9):1505–1515. [DOI] [PubMed] [Google Scholar]
- [44].Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis research & therapy 2011;13(2):211–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lefaucheur J-P, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipović SR, Hummel FC, Jääskeläinen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schönfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology 2014;125(11):2150–2206. [DOI] [PubMed] [Google Scholar]
- [46].Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, Vanneste S, Ziemann U, Garcia-Larrea L, Paulus W. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017;128(1):56–92. [DOI] [PubMed] [Google Scholar]
- [47].Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006;67(9):1568–1574. [DOI] [PubMed] [Google Scholar]
- [48].Lewis GNHL, Rice DA, Rome K, McNair PJ. Reliability of the conditioned pain modulation paradigm to assess endogeneous inhibitory pain pathways. Pain Res Manag 2012;17(2):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lewis GN, Rice DA, Kluger M, McNair PJ. Transcranial direct current stimulation for upper limb neuropathic pain: A double-blind randomized controlled trial. Eur J Pain 2018;22(7):1312–1320. [DOI] [PubMed] [Google Scholar]
- [50].Lima MCFF. Motor cortex stimulation for chronic pain: systematic review and meta-analysis. Neurology 2008;70(24):2329–2337. [DOI] [PubMed] [Google Scholar]
- [51].Marcuzzi A, Wrigley PJ, Dean CM, Adams R, Hush JM. The long-term reliability of static and dynamic quantitative sensory testing in healthy individuals. Pain 2017;158(7):1217–1223. [DOI] [PubMed] [Google Scholar]
- [52].Mendoca MESM, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial Direct Current Stimulation Combined with Aerobic Exercise to Optimize Analgesic Responses in Fibromylagia: A Randomized Placebo-Controlled Clinical Trial. Frontiers in human neuroscience 2016;10(68). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mendonca ME, Santana MB, Baptista AF, Datta A, Bikson M, Fregni F, Araujo CP. Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high-resolution computational models. The journal of pain : official journal of the American Pain Society 2011;12(5):610–617. [DOI] [PubMed] [Google Scholar]
- [54].Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Moisset X, Goudeau S, Poindessous-Jazat F, Baudic S, Clavelou P, Bouhassira D. Prolonged continuous theta-burst stimulation is more analgesic than ‘classical’ high frequency repetitive transcranial magnetic stimulation. Brain stimulation 2015;8(1):135–141. [DOI] [PubMed] [Google Scholar]
- [56].Moloney TM, Witney AG. Transcranial direct current stimulation (tDCS) priming of 1Hz repetitive transcranial magnetic stimulation (rTMS) modulates experimental pain thresholds. Neurosci Lett 2013;534:289–294. [DOI] [PubMed] [Google Scholar]
- [57].Mylius V, Reis J, Knaack A, Haag A, Oertel WH, Rosenow F, Schepelmann K. High-frequency rTMS of the motor cortex does not influence the nociceptive flexion reflex but increases the unpleasantness of electrically induced pain. Neurosci Lett 2007;415(1):49–54. [DOI] [PubMed] [Google Scholar]
- [58].Nir RRYD. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9(2):131–137. [DOI] [PubMed] [Google Scholar]
- [59].O’Brien AT, Deitos A, Trinanes Pego Y, Fregni F, Carrillo-de-la-Pena MT. Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. J Pain 2018;19(8):819–836. [DOI] [PubMed] [Google Scholar]
- [60].O’Brien AT, El-Hagrassy MM, Rafferty H, Sanchez P, Huerta R, Chaudhari S, Conde S, Rosa G, Fregni F. Impact of Therapeutic Interventions on Pain Intensity and Endogenous Pain Modulation in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Pain medicine (Malden, Mass) 2019;20(5):1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. The Cochrane database of systematic reviews 2018;4:Cd008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Oliveira LB, Lopes TS, Soares C, Maluf R, Goes BT, Sa KN, Baptista AF. Transcranial direct current stimulation and exercises for treatment of chronic temporomandibular disorders: a blind randomised-controlled trial. J Oral Rehabil 2015;42(10):723–732. [DOI] [PubMed] [Google Scholar]
- [63].Ossipov MH, Morimura K Fau - Porreca F, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014;8(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Reidler JS, Mendonca ME, Santana MB, Wang X, Lenkinski R, Motta AF, Marchand S, Latif L, Fregni F. Effects of motor cortex modulation and descending inhibitory systems on pain thresholds in healthy subjects. J Pain 2012;13(5):450–458. [DOI] [PubMed] [Google Scholar]
- [65].Ribeiro H, Sesterhenn RB, Souza A, Souza AC, Alves M, Machado JC, Burger NB, Torres I, Stefani LC, Fregni F, Caumo W. Preoperative transcranial direct current stimulation: Exploration of a novel strategy to enhance neuroplasticity before surgery to control postoperative pain. A randomized sham-controlled study. PLoS One 2017;12(11):e0187013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rogatgi A WebPlotDigitizer, Vol. 2019, 2011. [Google Scholar]
- [67].Roldan CJ, Abdi S. Quantitative sensory testing in pain management. Pain Manag 2015;5(6):483–491. [DOI] [PubMed] [Google Scholar]
- [68].Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006;123(3):231–243. [DOI] [PubMed] [Google Scholar]
- [69].Souto G, Borges Ic Fau - Goes BT, Goes Bt Fau - de Mendonca ME, de Mendonca Me Fau - Goncalves RG, Goncalves Rg Fau - Garcia LB, Garcia Lb Fau - Sa KN, Sa Kn Fau - Coutinho MR, Coutinho Mr Fau - Galvao-Castro B, Galvao-Castro B Fau - Fregni F, Fregni F Fau - Baptista AF, Baptista AF. Effects of tDCS-induced motor cortex modulation on pain in HTLV-1: a blind randomized clinical trial. Clin J Pain 2014;30(9):809–815. [DOI] [PubMed] [Google Scholar]
- [70].Starkweather AR, Heineman A, Storey S, Rubia G, Lyon DE, Greenspan J, Dorsey SG. Methods to measure peripheral and central sensitization using quantitative sensory testing: A focus on individuals with low back pain. Applied Nursing Research 2016;29:237–241. [DOI] [PubMed] [Google Scholar]
- [71].Tavares DRB, Okazaki JEF, Rocha AP, Santana MVA, Pinto A, Civile VT, Santos FC, Fregni F, Trevisani VFM. Effects of Transcranial Direct Current Stimulation on Knee Osteoarthritis Pain in Elderly Subjects With Defective Endogenous Pain-Inhibitory Systems: Protocol for a Randomized Controlled Trial. JMIR research protocols 2018;7(10):e11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tracey I, Mantyh PW. The Cerebral Signature for Pain Perception and Its Modulation. Neuron 2007;55(3):377–391. [DOI] [PubMed] [Google Scholar]
- [73].Vaseghi B, Zoghi M, Jaberzadeh S. Does anodal transcranial direct current stimulation modulate sensory perception and pain? A meta-analysis study. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2014;125(9):1847–1858. [DOI] [PubMed] [Google Scholar]
- [74].Vaseghi B, Zoghi M, Jaberzadeh S. Differential effects of cathodal transcranial direct current stimulation of prefrontal, motor and somatosensory cortices on cortical excitability and pain perception - a double-blind randomised sham-controlled study. Eur J Neurosci 2015;42(7):2426–2437. [DOI] [PubMed] [Google Scholar]
- [75].Vaseghi B, Zoghi M, Jaberzadeh S. How Does Anodal Transcranial Direct Current Stimulation of the Pain Neuromatrix Affect Brain Excitability and Pain Perception? A Randomised, Double-Blind, Sham-Control Study. PLOS ONE 2015;10(3):e0118340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A, Fregni F. Focal modulation of the primary motor cortex in fibromyalgia using 4×1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. The journal of pain : official journal of the American Pain Society 2013;14(4):371–383. [DOI] [PubMed] [Google Scholar]
- [77].Yam MF, Loh YA-O, Tan CA-O, Khadijah Adam S, Abdul Manan N, Basir R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int J Mol Sci 2018;19(8). pii: E2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zandieh A, Parhizgar SE, Fakhri M, Taghvaei M, Miri S, Shahbabaie A, Esteghamati S, Ekhtiari H. Modulation of cold pain perception by transcranial direct current stimulation in healthy individuals. Neuromodulation 2013;16(4):345–348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.