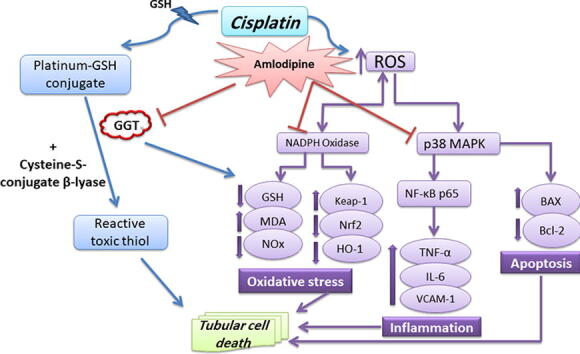
Graphical abstract
Keywords: Cisplatin nephrotoxicity, Amlodipine, GGT inhibition, Anti-oxidant defense, Anti-inflammatory response, Renal cell survival
Abbreviations: GGT, gamma-glutamyl transpeptidase; BUN, Blood urea nitrogen; ROS, Reactive oxygen species; NADPH, Nicotinamide adenine dinucleotide phosphate; Keap1, Kelch-like ECH-associated protein 1; Nrf2, Nuclear factor erythroid 2-related factor 2; HO-1, Heme oxygenase-1; GSH, Reduced glutathione; NO, Nitric oxide; NOx, Total nitrate/nitrite; MDA, Malondialdehyde; MAPK, Mitogen-activated protein kinase; NF-κB, Nuclear factor-kappa B; TNF-α, Tumor necrosis factor-alpha; IL-6, Interleukin-6; VCAM-1, vascular cell adhesion molecule-1; HGF, Hepatocyte growth factor; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; CMC, Carboxymethyl cellulose; H & E, Hematoxylin and eosin
Abstract
Background
The therapeutic utility of the effective chemotherapeutic agent cisplatin is hampered by its nephrotoxic effect. We aimed from the current study to examine the possible protective effects of amlodipine through gamma-glutamyl transpeptidase (GGT) enzyme inhibition against cisplatin nephrotoxicity.
Methods
Amlodipine (5 mg/kg, po) was administered to rats for 14 successive days. On the 10th day, nephrotoxicity was induced by a single dose of cisplatin (6.5 mg/kg, ip). On the last day, blood samples were collected for estimation of kidney function, while kidney samples were used for determination of GGT activity, oxidative stress, inflammatory, and apoptotic markers, along with histopathological evaluation.
Results
Amlodipine alleviated renal injury that was manifested by significantly diminished serum creatinine and blood urea nitrogen levels, compared to cisplatin group. Amlodipine inhibited GGT enzyme, which participates in the metabolism of extracellular glutathione (GSH) and platinum-GSH-conjugates to a reactive toxic thiol. Besides, amlodipine diminished mRNA expression of NADPH oxidase in the kidney, while enhanced the anti-oxidant defense by activating Nrf2/HO-1 signaling. Additionally, it showed marked anti-inflammatory response by reducing expressions of p38 mitogen-activated protein kinase (p38 MAPK) and nuclear factor-kappa B (NF-κB), with subsequent down-regulation of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and vascular cell adhesion molecule-1 (VCAM-1). Moreover, amlodipine reduced Bax/Bcl-2 ratio and elevated hepatocyte growth factor (HGF), thus favoring renal cell survival.
Conclusions
Effective GGT inhibition by amlodipine associated with enhancement of anti-oxidant defense and suppression of inflammatory signaling and apoptosis support our suggestion that amlodipine could replace toxic GGT inhibitors in protection against cisplatin nephrotoxicity.
1. Introduction
The essential need for cancer treatments has been aggravated the last years. Over 40 years, cisplatin has been utilized to treat various types of cancer, and it is still administered as one of the most effective antitumor drugs (Khasabova et al., 2014, Ghosh, 2019, Volarevic et al., 2019). The clinical use of cisplatin has been constrained by its nephrotoxic consequences, which show up even few days after initiation of treatment (Oh et al., 2014). Currently, cisplatin nephrotoxicity is controlled by hydration with isotonic saline and magnesium supplementation (Crona et al., 2017). In addition, forced diuresis by mannitol is applied in patients treated with high doses of cisplatin, resulting in over-diuresis and consequently increased risk of dehydration (Djokovic et al., 2019, Volarevic et al., 2019). Therefore, seeking for tolerable and effective new adjunct treatments that can alleviate the toxicity of cisplatin high dose throughout cancer’s combat is seriously insisting.
Mounting evidence underlines that the molecular mechanisms of cisplatin-triggered tubular damage and subsequent acute kidney injury are multifactorial. These mechanisms are related to the accumulation of cisplatin in renal proximal tubular cells, its metabolic biotransformation to a toxic metabolite, alongside the interference with nuclear DNA, mitochondrial dysfunction, oxidative stress, the activation of inflammatory and apoptotic responses leading eventually to cell cycle arrest or cell death (Peres et al., 2013, Manohar and Leung, 2018, Volarevic et al., 2019).
The metabolic biotransformation of cisplatin to a potent nephrotoxin during its transport into renal tubular cells has been regarded as one of the important factors that contribute to cisplatin nephrotoxicity (Townsend et al., 2003, Wilmes et al., 2015, Dugbartey et al., 2016). Platinum-glutathione (Pt-GSH)-conjugates at the surface of the proximal tubules are metabolized by gamma-glutamyl transpeptidase (GGT) enzyme, that subsequently leads to the formation of a reactive toxic thiol (Townsend et al., 2003).
Amlodipine is a dihydropyridine calcium channel blocker, which is well-established in the treatment of hypertension, as well as other cardiovascular diseases (Fares et al., 2016). There is also increasing proof that calcium channel blockers, especially amlodipine, exhibit nephroprotective effect beyond their antihypertensive effect (Duan et al., 2000, Li et al., 2009, Chinwe et al., 2019). The vasodilator effect of amlodipine on renal arterioles and the improvement of glomerular blood flow (Kimura et al., 1997, Ott et al., 2013) certainly contribute to its protective effects. Interestingly, it has been elucidated that amlodipine could counteract renal vasoconstriction after renal adrenergic receptor stimulation in cisplatin treated rats, resulting in reduced renal failure (Khan et al., 2007, Hye Khan et al., 2008, Khan et al., 2009). Besides, the protective effects of amlodipine could be attributed to inhibition of nuclear factor-kappa B (NF-κB) and subsequently inflammatory cytokines (Ikeda et al., 2019, Qasim et al., 2020), enhancement of anti-oxidant defense (Salehi et al., 2012, Nili-Ahmadabadi et al., 2018), as well as its anti-apoptotic effect (Bian et al., 2011, Xu et al., 2016). Furthermore, amlodipine has been reported as a GGT inhibitor that could disturb the key step of the enzymatic conversion of Pt-GSH-conjugates into nephrotoxic metabolites (Maj and Tomaszewski, 1997). Thereby, amlodipine is expected to be a promising protective agent to limit the deleterious effects of cisplatin on the kidney.
2. Materials and methods
2.1. Animals
Male Wistar rats (120–150 g) were obtained from the Animal House of the Holding Company for Biologics and Vaccines-VACSERA (Giza, Egypt). Rats were maintained at temperature 23 ± 2 °C, humidity 60 ± 10%, and under 12-h light/12-h dark cycles, with free access to standard diet and water. All experimental procedures related to animals were in accordance with the National Institutes of Health guide for the care and use of laboratory animals and have been approved by Institutional Animal Care and Use Committee, Beni-Suef University, Egypt (BSU-IACUC 018–45).
2.2. Drugs and chemicals
Cisplatin vials (50 mg/50 ml) were purchased from Mylan (Rockford, USA). Amlodipine was obtained as a gift from Global Napi Pharmaceuticals (6th of October, Giza, Egypt). The chemicals used for estimation of oxidative stress biomarkers including, thiobarbituric acid, Ellman's reagent, vanadium trichloride, and sulfanilamide were purchased from Sigma-Aldrich (USA), while N-(1-naphthyl) ethylenediamine dihydrochloride was purchased from Park Scientific Ltd (UK). The suspending agent carboxymethyl cellulose (CMC) was obtained from Loba Chemie (India).
2.3. Experimental design
Animals were divided in a random manner into four groups, with 8 animals in each group. The first and second groups received the vehicle (0.5% CMC) po for 14 consecutive days, and on the 10th day received a single ip injection of isotonic saline or cisplatin (6.5 mg/kg) (Szentmihályi et al., 2014), serving as control and cisplatin groups, respectively. The third and fourth groups received amlodipine (5 mg/kg/day; po) (Liu et al., 2007, Li et al., 2009) for 14 consecutive days, and on the 10th day the third group received a single ip injection of cisplatin (6.5 mg/kg), while the fourth group received a single ip injection of isotonic saline.
At the end of the 14 days, blood and kidney tissue samples were collected for estimation of kidney function, as well as determination of different biochemical and molecular markers. Kidney samples were subjected to rapid snap freezing in liquid nitrogen, then stored at −80 °C until their use. One kidney was used to get one-gram tissue that was homogenized in phosphate–buffered saline (PBS) with protease inhibitor on ice. The kidney homogenates were used for determination of GGT activity, oxidative stress and inflammatory markers, as well as hepatocyte growth factor (HGF). The remaining portion of the kidney was used for PCR and western blot analysis. The other kidney was soaked in 10% neutral buffered formalin to be used for histopathological examination.
2.4. Biochemical tests for kidney function determination
Using Diamond Diagnostics kits (Egypt), serum creatinine and blood urea nitrogen (BUN) were determined according to the manufacturer’s procedures.
2.5. Estimation of renal GGT activity
Gamma-glutamyl transpeptidase activity was determined in the kidney samples using BioVision’s kit (USA). GGT enzyme is responsible for the transfer of gamma-glutamyl functional groups. L-γ-Glutamyl-p-nitroanilide (l-γ-Glu-pNA) was used as a specific substrate to be recognized by GGT in the samples yielding a proportional color. The intensity of the color product was estimated at 418 nm as an indicator for GGT activity.
2.6. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Separation of total RNA was carried out using Thermo Fisher Scientific kit (GeneJET, Germany), while qRT-PCR was assessed by Bioline kit (SensiFAST™ SYBR® Hi-ROX One-Step Kit, UK). The thermal cycling profile of qRT-PCR was 45 °C/15 min for reverse transcription cycle, followed by 40 cycles of denaturation at 95 °C/15 s, 60 °C/30 s annealing, and 72 °C/30 s extension, as described previously (Abdel-Razek et al., 2020). Finally, cycle threshold (Ct) values of the target genes (Keap-1, NADPH oxidase, NF-κB, MAPK, VCAM-1) were normalization to those of the housekeeping gene (β-actin) (Livak and Schmittgen, 2001). The sequences of primers for the target genes and the housekeeping gene are presented as supplementary data.
2.7. Western blot
Kidney tissue protein extraction was carried out using RIPA lysis buffer (Bio Basic Inc., Canada), with added protease inhibitor cocktail. SDS-PAGE electrophoretic system via acrylamide Kit (TGX Stain-Free™ FastCast™, Bio-Rad Laboratories, USA) was used for the separation of equivalent volumes of the extracted proteins from all studied groups. The membranes were blocked with 5% non-fat dry milk, Tris-HCL, 0.1% Tween 20 for 1 h, and then incubated with the primary antibodies at 4 °C overnight. The utilized antibodies were p38 MAPK (Creative Biolabs, USA, Cat.# CBMAB-Z0329-LY), Nrf2 (Santa Cruz Biotechnology, USA, Cat.# (A-10): sc-365949), HO-1 (Santa Cruz Biotechnology, USA, Cat.# (A-3): sc-136960), Bax (Santa Cruz Biotechnology, USA, Cat.# (2D2): sc-20067), and Bcl-2 (Santa Cruz Biotechnology, USA, Cat.# (C-2): sc-7382). That was followed by incubation with the suitable secondary antibodies at room temperature for 2 h. Densitometric analysis of the immunoblots was carried out to estimate the amounts of p38 MAPK, Nrf2, HO-1, Bax, and Bcl-2 against β-actin using ChemiDocTM MP imaging system (Bio-Rad Laboratories, USA) with image analysis software (version 3).
2.8. Determination of renal oxidative stress biomarkers
Renal GSH content was determined via the intensity of the yellow color resulting from the reaction with Ellman's reagent, as previously determined (Azouz et al., 2015). Nitric oxide (NO) content was assessed as total nitrate/nitrite (NOx) following the procedure of (Miranda et al. 2001). Additionally, lipid peroxidation was measured as thiobarbituric acid reacting substances and expressed as malondialdehyde (MDA), as previously determined (Azouz et al., 2015). Moreover, renal heme oxygenase-1 (HO-1) level was assessed using BioVision’s ELISA kit (USA).
2.9. Assessment of kidney inflammatory markers
Tumor necrosis factor-alpha (TNF-α) was estimated in tissue homogenates using Sigma-Aldrich ELISA kit (USA), while interleukin-6 (IL-6) content was determined by RayBio ELISA kit (USA), according to the instructions of the manufacturer.
2.10. Determination of hepatocyte growth factor (HGF)
Renal content of HGF was determined quantitatively using RayBio ELISA kit (USA), following the manufacturer’s instructions.
2.11. Histopathological assessment
Kidney samples were fixed in 10% formol saline for 24 h. Graded ethanol concentrations (50–100%) were used to dehydrate the paraffin blocks, that were cleared after that in xylene. Sections (4–5 µm) were prepared, and then stained with Hematoxylin and Eosin (H & E) dye, following the principles of Bancroft and Gamble (2008). The degree of severity of the histopathological lesions was evaluated by numerical scoring (0: no changes, 1: mild, 2: moderate, and 3: severe changes). These degrees were determined by percentage of change following Arsad et al. (2014) principles: mild changes (>30%), moderate changes (30–50%), and severe changes (>50%).
2.12. Statistical analysis
Results are presented as the mean ± standard error of the mean (SEM). One-way Analysis of Variance (ANOVA) followed by Tukey's post hoc test, were used for detecting statistical significance between different groups. Statistical significance between values was considered at probability less than 0.05. Statistical analysis was carried out by Prism software, version 8 (GraphPad Software Inc, USA).
3. Results
3.1. Ameliorative effect of amlodipine on cisplatin-induced deterioration in kidney function
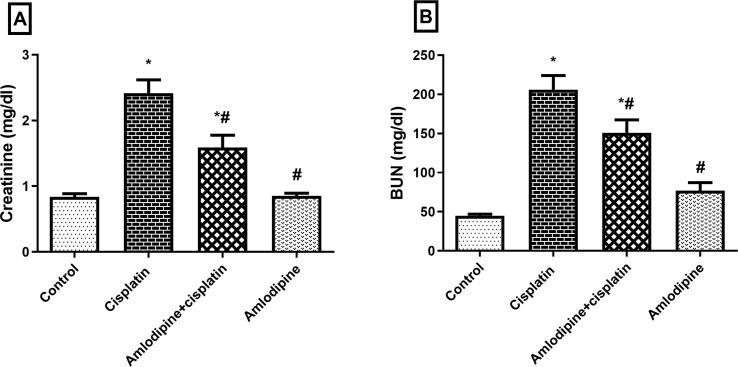
Cisplatin-induced nephrotoxicity was evidenced by a significant elevation of BUN (5-fold) and serum creatinine (3-fold) levels in comparison to control group (Fig. 1). Prophylactic treatment with amlodipine revealed an improvement in kidney function via a significant reduction in BUN and serum creatinine levels to about 73% and 66%, respectively compared to the group administered cisplatin alone.
Fig. 1.
Improvement of kidney function by amlodipine in cisplatin-treated rats. Results are presented as the mean ± SEM (n = 6–8). One-way ANOVA followed by Tukey's post hoc test, were used for statistical analysis.* p < 0.05 versus control, #p < 0.05 versus cisplatin group.
3.2. Reduction of renal GGT enzyme activity by amlodipine
The activity of GGT in renal tissue was markedly elevated to about 3-fold after treatment with cisplatin, while in amlodipine pretreated rats GGT activity was significantly decreased to about 62% compared to cisplatin group (Table 1).
Table 1.
Amlodipine inhibits GGT activity, alleviates oxidative stress biomarkers and augments anti-oxidant defense in cisplatin-treated rats.
| Groups | GGT (mU/g tissue) |
GSH (µM) |
MDA (µM) |
NOx (µM) |
HO-1 (ng/g tissue) |
|---|---|---|---|---|---|
| Control | 17.17 ± 1.35 | 4.75 ± 0.31 | 1.69 ± 0.14 | 17.38 ± 0.35 | 2.70 ± 0.74 |
| Cisplatin | 58.00 ± 3.78* | 1.38 ± 0.05* | 4.70 ± 0.38* | 4.38 ± 0.29* | 0.73 ± 0.06* |
| Amlodipine + Cisplatin | 35.83 ± 1.01*# | 4.33 ± 0.28# | 1.41 ± 0.15# | 22.48 ± 1.28*# | 4.32 ± 0.09*# |
| Amlodipine | 19.17 ± 1.52# | 4.51 ± 0.3# | 1.66 ± 0.16# | 15.73 ± 0.28# | 2.72 ± 0.24# |
Results are presented as the mean ± SEM (n = 6). One-way ANOVA followed by Tukey's post hoc test, were used for statistical analysis. * p< 0.05 versus control, #p< 0.05 versus cisplatin group.
3.3. Modulatory effects of amlodipine on NADPH oxidase, Keap-1, MAPK, NF-κB, and VCAM-1 mRNA expressions
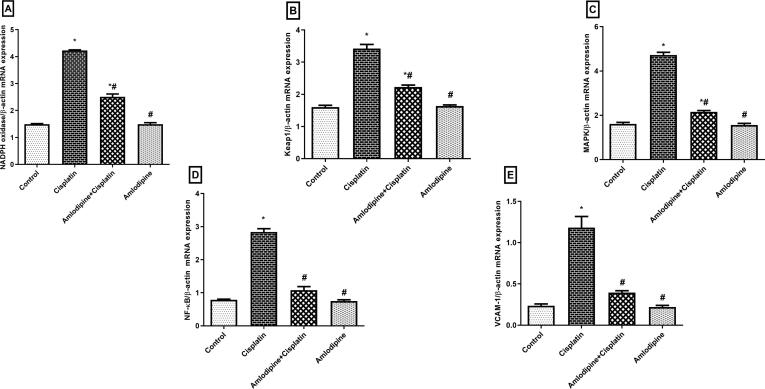
Cisplatin injection enhanced oxidative stress, which was indicated by up-regulation of NADPH oxidase mRNA expression (3-fold), in addition to the expression of the inhibitory Keap-1 (2-fold) which controls the release and nuclear translocation of Nrf2. The involvement of inflammatory events in cisplatin-induced acute renal damage was confirmed by the significantly enhanced mRNA expressions of MAPK (3-fold), NF-κB (4-fold), and VCAM-1 (5-fold) in cisplatin group. Our results elucidated that pretreatment with amlodipine significantly ameliorated the expressions of NADPH oxidase, Keap1, MAPK, NF-κB, and VCAM-1 to about 59%, 65%, 46%, 38%, and 33%, respectively, compared to cisplatin administered rats (Fig. 2).
Fig. 2.
Modulatory effects of amlodipine on mRNA expressions of NADPH oxidase, Keap-1, MAPK, NF-κB, and VCAM-1 in cisplatin-treated rats. Results are presented as the mean ± SEM (n = 3–6). One-way ANOVA followed by Tukey's post hoc test, were used for statistical analysis.* p < 0.05 versus control, #p < 0.05 versus cisplatin group.
3.4. Amlodipine reduces the protein expression of p38 MAPK and Bax/Bcl-2 ratio, while enhances Nrf2 and HO-1 expressions
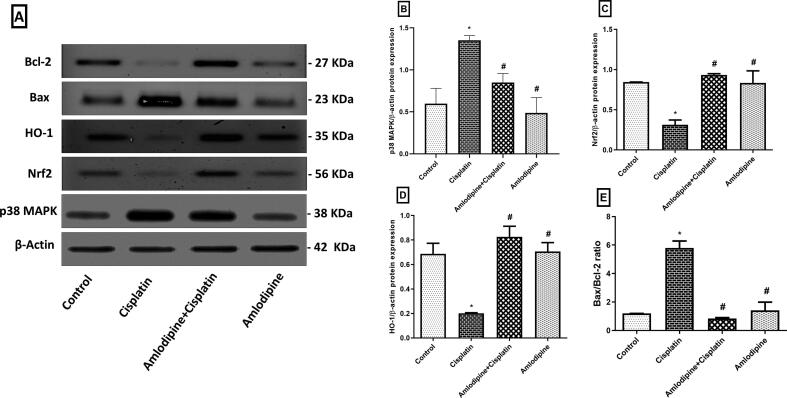
Western blot analysis (Fig. 3A) showed that cisplatin evoked an inflammatory response that was evident by the enhanced protein expression of p38 MAPK. In addition, there was a marked decline of renal anti-oxidant defense mechanisms associated with down-regulation of the transcription factor Nrf2 and the anti-oxidant protein HO-1 expressions. Pretreatment with amlodipine afforded a significant protection against inflammation and oxidative stress, that was indicated by reduced expression of p38 MAPK to about 63% (Fig. 3B), while enhanced expression of Nrf2 to about 3-fold (Fig. 3C), and HO-1 to about 4- fold (Fig. 3D), compared to the group administered cisplatin only.
Fig. 3.
Modulatory effects of amlodipine on the protein expressions of p38 MAPK, Nrf2, HO-1, Bax, and Bcl-2 in cisplatin treated rats. A. Blots demonstrating the reduced protein expressions of renal p38 MAPK and Bax, while enhanced Nrf2, HO-1, and Bcl-2 expressions by amlodipine. B-E. Graphical representations displaying changes in the relative protein expressions. B: p38 MAPK, C: Nrf2, D: HO-1, E: Bax/Bcl-2 ratio. Results are presented as the mean ± SEM (n = 3). One-way ANOVA followed by Tukey's post hoc test, were used for statistical analysis. * p < 0.05 versus control, #p < 0.05 versus cisplatin group.
Cisplatin administration elevated the expression of the pro-apoptotic effector Bax and decreased the expression of the anti-apoptotic protein Bcl-2 in kidney tissues. These events were markedly alleviated by administration of amlodipine, which significantly reduced Bax/Bcl-2 ratio to about 14.5%, compared to cisplatin group (Fig. 3E).
3.5. Alleviation of cisplatin-induced oxidative stress by amlodipine
Rats subjected to cisplatin showed significant increase in renal MDA content (3-fold), while GSH (29%), NOX (25%), and HO-1 (27%) contents were declined, compared to the control group. Prophylactic treatment with amlodipine afforded a renoprotective effect against oxidative stress induced by cisplatin, which was indicated by lowering MDA content to about 30%, along with enhanced GSH content to about 3-fold, NOx and HO-1 contents to about 5- and 6-fold, respectively compared to cisplatin group (Table 1).
3.6. Suppression of cisplatin-induced inflammatory cytokines by amlodipine
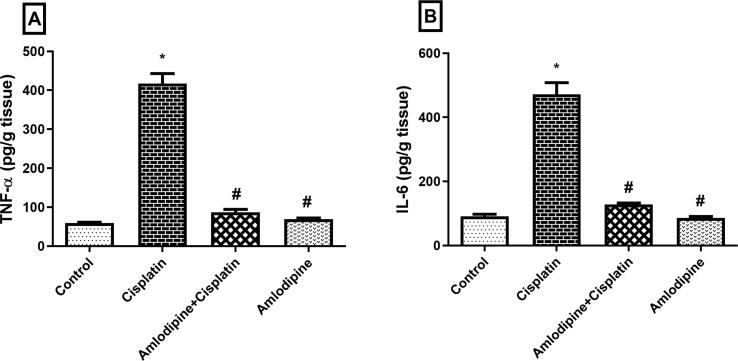
Cisplatin stimulated an acute inflammatory response as indicated by markedly increased renal content of the pro-inflammatory cytokines TNF-α and IL-6 (Fig. 4). Prophylactic treatment with amlodipine significantly reduced renal TNF-α and IL-6 to about 21% and 27%, respectively, compared to cisplatin group, thus conferring an anti-inflammatory effect.
Fig. 4.
Amlodipine suppresses cisplatin-induced inflammatory cytokines in renal tissues. Results are presented as the mean ± SEM (n = 6). One-way ANOVA followed by Tukey's post hoc test, were used for statistical analysis.* p< 0.05 versus control, #p< 0.05 versus cisplatin group.
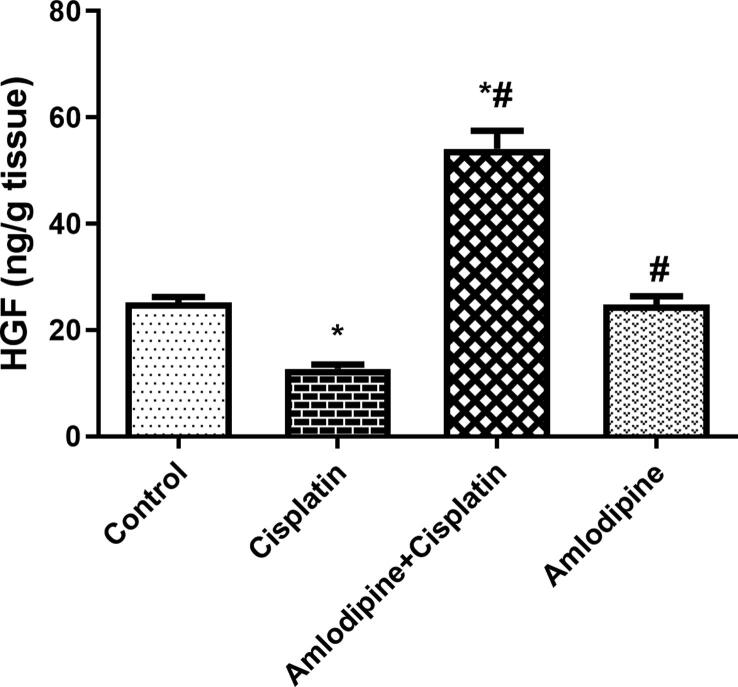
3.7. Enhancement of renal HGF content by amlodipine in cisplatin-treated rats
One of the mechanisms by which cisplatin encourages apoptotic cascade is decreasing renal HGF content, which is regarded as one of the survival factors. Renal HGF content was decreased to about 51% in cisplatin treated group compared to the control group. Pretreatment with amlodipine markedly augmented renal HGF content to about 4-fold compared with cisplatin group (Fig. 5).
Fig. 5.
Amlodipine augments renal content of HGF in cisplatin-treated rats. Results are presented as the mean ± SEM (n = 6). One-way ANOVA followed by Tukey's post hoc test, were used for statistical analysis.* p < 0.05 versus control, #p < 0.05 versus cisplatin group.
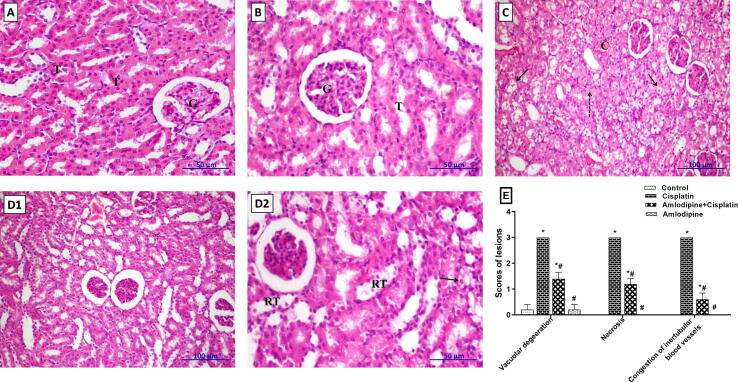
3.8. Amelioration of cisplatin-induced histopathological aberrations in renal tissues by amlodipine
The examination of kidney sections from control group revealed that the histological structures of glomeruli and tubules were normal (Fig. 6A). Similarly, kidney tissue of rats administered amlodipine alone showed high integrity with normal structure of glomeruli and tubules (Fig. 6B). On the other hand, cisplatin-treated rats showed congestion of inter-tubular blood vessels, vacuolar degeneration and necrosis of renal tubular epithelial lining (Fig. 6C). Concerning amlodipine-treated group, a good degree of regeneration of renal parenchyma against the action of cisplatin was apparent (Fig. 6D1), with mild vacuolar degeneration and few scattered necrotic cells (Fig. 6D2). The scores for histopathological alterations were presented in Fig. 6E.
Fig. 6.
Amlodipine alleviates cisplatin-induced renal histopathological injury in rats. A. Kidney section of control rat showing normal histological structure of glomeruli (G) and tubules (T) (H&E, X400). B. Section of amlodipine group showing high integrity of tissue with normal appearance of tubules (T) and glomeruli (G) (H&E, X400). C. Kidney section of amlodipine + cisplatin group showing congestion of the intertubular blood vessels (C), widespread vacuolar degeneration (arrow) and necrosis (dashed arrow) of the renal tubular epithelial lining (H&E, X200). D1, D2. Kidney sections of cisplatin administered rats that were treated with amlodipine showing, D1: good degree of restoration of the renal parenchyma against the action of cisplatin (H&E, X200), D2: mild degree of vacuolar degeneration (arrow) with few scattered necrotic cells and regenerated tubules (RT) (H&E, X400). E. Scoring of the main histopathological findings in various groups. One-way ANOVA followed by Tukey's post hoc test, were used for the statistical analysis of each lesion. * p < 0.05 versus control, #p < 0.05 versus cisplatin group.
4. Discussion
Nephrotoxicity is well-documented as the dose-limiting factor in cisplatin treatment, that prevents the increase of cisplatin dose for higher efficacy and hinders its clinical application in cancer chemotherapy (Hoek et al., 2016). Therefore, searching for protective agents against cisplatin nephrotoxicity is essential.
The current study sheds light on the protective effects of amlodipine against cisplatin nephrotoxicity. A single dose administration of cisplatin (6.5 mg/kg) resulted in serious tubular necrosis with significant deterioration of kidney function, which was demonstrated by elevation of serum creatinine and BUN levels. We investigated the ability of amlodipine to inhibit GGT enzyme in the kidney that could augment its anti-oxidant, anti-inflammatory, and anti-apoptotic effects in retarding cisplatin nephrotoxicity.
Gamma-glutamyl transpeptidase enzyme has been reported to participate a substantial role in cisplatin toxicity through enhancing its metabolic conversion to a nephrotoxin (Townsend et al., 2003, Manohar and Leung, 2018). GGT enzyme is localized in the brush border of renal proximal tubular cells. Cisplatin conjugates with reduced GSH mainly in the liver, then reaches to the kidneys as a Pt-GSH-conjugate which is cleaved by GGT forming a cysteinyl-glycine-conjugate followed by the formation of a cysteine-conjugate by aminodipeptidases at the surface of proximal tubular cells (Townsend et al., 2003). After transport of the formed cysteine-conjugate into the tubular cells, it undergoes further metabolic conversion by cysteine-S-conjugate β-lyase into a toxic thiol which interacts with essential proteins, leading finally to nephrotoxicity and renal cell death (Zhang and Hanigan, 2003, Dugbartey et al., 2016).
Previous studies have confirmed that high expression level of GGT in renal proximal tubular cells renders them more sensitive to cisplatin toxicity, while that in tumor cells results in increased resistance to cisplatin, so inhibition of GGT activity reduces cisplatin nephrotoxicity and enhances the antitumor effect of cisplatin (Townsend and Hanigan, 2002, Hanigan and Devarajan, 2003, Corti et al., 2010, Hanigan, 2014). Moreover, it has been reported that GGT knock-out mice were more fortified against the nephrotoxic effect of cisplatin than wild-type mice (Hanigan et al., 2001), that has been confirmed by Fliedl et al. (2014) using different cell lines with either siRNA knock-down of GGT or addition of GGT inhibitor. Similarly, our results revealed an enhanced GGT activity in cisplatin treated rats with concomitant deterioration in kidney function. Whereas, pretreatment with amlodipine ameliorated the elevation in GGT activity and improved kidney function.
Gamma-glutamyl transpeptidase enzyme inhibition has been investigated in different studies to elucidate the protective mechanism of some agents against cisplatin nephrotoxicity (Townsend and Hanigan, 2002, Hausheer et al., 2010, Salama et al., 2011). One of the most powerful of these agents is the glutamine analogue acivicin, which afforded a great efficacy against cisplatin nephrotoxicity (Hanigan et al., 1994), but unfortunately the clinical trials have revealed that acivicin is too toxic to be used in humans (Kreuzer et al., 2015). Moreover, it has been reported that all the known glutamine analogue GGT inhibitors encroached the safety limits for using as treatments (King et al., 2009). On the other hand, our study demonstrated that cisplatin nephrotoxicity was attenuated via pretreatment with amlodipine, referring that to the capability of amlodipine to decrease GGT activity. Fortunately, treatment with amlodipine has not been reported to produce serious adverse effects, where it is well known to be safe for humans (Derosa and Maffioli, 2011). The reported adverse effects of amlodipine include edema, dizziness, flushing, palpitations, headache, fatigue, nausea, and abdominal pain that occur to a small percentage of the patients (1–10%) (Bulsara and Cassagnol, 2020).
Previous studies have demonstrated that accumulation of cisplatin in the mitochondria of renal cells results in reactive oxygen species (ROS) production via activation of NADPH oxidase, which elevates significantly after treatment with cisplatin (Wang et al., 2015, Meng et al., 2018). In accordance, our results revealed an elevated NADPH oxidase expression, along with increased lipid peroxidation and a decline of GSH level. Cisplatin also resulted in a significant decrease of renal NOx in our study. That has been elucidated in a previous study through amelioration of renal injury by the NO precursor L-arginine, while aggravation by the nitric oxide synthase (NOS) inhibitor L-NAME (Saleh and El-Demerdash, 2005). Therefore, the reduction of NOx by cisplatin could be attributed to the damage of endothelial cells and hence the reduction of endothelial NOS activity.
The pro-oxidant effects of cisplatin are usually accompanied by down-regulation of Nrf2 expression (Ansari, 2017), as a key transcription factor that activates the anti-oxidant defense mechanisms via enhancing expression of the anti-oxidant proteins, such as HO-1 (Tayem et al., 2014, Behiry et al., 2018). As previously reported, under basal conditions Nrf2 appears to be bound to the inhibitory protein Keap-1, which is a nuclear-cytoplasmic shuttling protein (Karapetian et al., 2005). Meanwhile, cisplatin significantly diminishes the expression level of Nrf2, and so elevates the expression of the inhibitory protein Keap-1 (Liao et al., 2017), that was also evident from our results. Hence, enhancement of Nrf2 protein expression is considered as a vital molecular target for cytoprotection.
Interestingly, treatment with amlodipine has been reported to exhibit anti-oxidant effects (Aslam et al., 2006, Li et al., 2009, Suliburska et al., 2014). That was evident in our study by reduced NADPH oxidase expression, a decline of MDA level, and a restoration of GSH level. Besides, amlodipine increased NOx, that could probably result from enhancing the release of endothelial NO and extending its duration of action by scavenging superoxide anions radicals (Berkels et al., 2004, Toma et al., 2011). Moreover, amlodipine reduced mRNA expression of the inhibitory Keap-1, leading to enhanced Nrf2 protein expression, and in turn augmented expression of the anti-oxidant protein HO-1, that was in accordance with previous studies (Toba et al., 2006, Lisk et al., 2013).
It has been documented that the acute inflammatory response triggered by cisplatin enhances its nephrotoxic effect (Manohar and Leung, 2018, Djokovic et al., 2019). Interestingly, the reactive oxygen and nitrogen species collaborate in p38 MAPK activation, which plays a crucial role in production of the pro-inflammatory cytokines, as well as regulation of the apoptotic cascade (Malik et al., 2015). In the present study, the inflammatory response evoked by cisplatin was indicated by the enhanced mRNA and protein expressions of p38 MAPK, as well as NF-κB mRNA expression. p38 MAPK acts as an upstream signal for activation and migration of NF-κB to the nucleus, that promotes transcription of specific genes which encode pro-inflammatory mediators and cytokines, such as TNF-α (Ma et al., 2015). Furthermore, TNF-α is considered as an important cytokine to coordinate the activation of a large network of pro-inflammatory cytokines such as IL-6, alongside the adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) that promotes an inflow of inflammatory cells (Yousef and Hussien, 2015). In parallel, our results demonstrated that cisplatin-induced elevation of TNF-α, IL-6, and VCAM-1 in renal tissues.
Consistent with the mitigation of oxidative stress in renal tubular cells, amlodipine afforded a remarkable anti-inflammatory response. That was manifested via suppression of the pro-inflammatory signaling triggered by p38 MAPK. Consequently, amlodipine reduced the downstream targets NF-κB, TNF-α, IL-6, and VCAM-1, giving an evidence to its anti-inflammatory actions that have been also demonstrated in other studies (Toma et al., 2011, Lu et al., 2016). These anti-inflammatory effects certainly reinforce amlodipine in the management of cisplatin nephrotoxicity.
It has been reported that cisplatin induces nephrotoxicity via its accumulation in the mitochondria of renal tubular cells, hindering mitochondrial bioenergetics, promoting excessive ROS generation, leading eventually to the activation of various downstream proteins that initiate apoptosis of renal tubular cells (Dasari and Tchounwou, 2014). Additionally, ROS produced by cisplatin are potent activators for signal transduction by protein kinases, such as p38 MAPK which has been recognized by its critical role in promoting apoptosis of renal tubular cells (Bragado et al., 2007).
Two main pathways of apoptosis are predominant in the nephrotoxicity induced by cisplatin including the extrinsic or death receptor pathway, that leads to activation of death receptors via membrane ligands of cell death, such as TNF-α (Kalra et al., 2019). In the other intrinsic or mitochondrial pathway cisplatin affords up-regulation of the pro-apoptotic effector (Bax) and diminishes the anti-apoptotic protein (Bcl-2), along with translocation of Bax from the cytosol to mitochondria promoting the liberation of cytochrome c to the cytosol, that leads finally to the activation of caspases (Yang et al., 2014).
In our study, cisplatin significantly induced apoptosis in renal tubular cells, while prophylactic treatment with amlodipine markedly enhanced the anti-apoptotic cascade through down-regulation of the pro-apoptotic Bax, and up-regulation of the anti-apoptotic Bcl-2 expression. These results are in harmony with those of other studies performed on amlodipine (Liu et al., 2007, Bian et al., 2011, Xu et al., 2016). Moreover, amlodipine displayed a remarkable regenerative effect by increasing HGF level, which accelerates renal tubular repair after an acute injury with rapid recovery of tubular morphology and function, along with acting as a survival factor for renal tubular cells (Salem et al., 2018). That signifies the protective role of amlodipine in the abrogation of apoptosis induced by cisplatin.
5. Conclusion
The current study afforded a comprehensive elucidation regarding the protective effects of amlodipine and its proposed mechanisms against cisplatin nephrotoxicity. Importantly, inhibition of GGT enzyme, which is the key step responsible for most of the deleterious effects of cisplatin, is the main protective mechanism conferred by amlodipine. Besides, amlodipine exhibits a great anti-oxidant effect through augmenting Nrf2 and HO-1 expressions. Additionally, amlodipine suppresses the inflammatory response evoked by p38 MAPK, resulting in reduction of the pro-inflammatory cytokines TNF-α and IL-6. Finally, amlodipine protects the kidney from apoptotic cell death by reducing Bax/Bcl-2 ratio, as well as elevating the survival factor HGF. Therefore, effective GGT inhibition by amlodipine with subsequent enhancement of anti-oxidant defense and suppression of inflammatory signaling and apoptosis support our suggestion that amlodipine could replace toxic GGT inhibitors in protection against cisplatin nephrotoxicity.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
The Authors express their deep thanks to Dr. Sahar S. Abd El-Rahman, Professor of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt for the effective guidance in histopathological investigation.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2020.08.022.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abdel-Razek E.A.-N., Abo-Youssef A.M., Azouz A.A. Benzbromarone mitigates cisplatin nephrotoxicity involving enhanced peroxisome proliferator-activated receptor-alpha (PPAR-α) expression. Life Sci. 2020;243 doi: 10.1016/j.lfs.2020.117272. [DOI] [PubMed] [Google Scholar]
- Ansari M.A. Sinapic acid modulates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2017;93:646–653. doi: 10.1016/j.biopha.2017.06.085. [DOI] [PubMed] [Google Scholar]
- Arsad S., Esa N., Hamzah H. Histopathologic changes in liver and kidney tissues from male Sprague Dawley rats treated with Rhaphidophora decursiva (Roxb.) schott extract. Cytol. Histol. 2014;4(1):1–6. [Google Scholar]
- Aslam S., Santha T., Leone A., Wilcox C. Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int. 2006;70(12):2109–2115. doi: 10.1038/sj.ki.5001983. [DOI] [PubMed] [Google Scholar]
- Azouz A.A., Omar H.A., Abo-yousef A.M., El-Sherbiny G.A., Abdel-Latif H.A. Different protective effects of trimetazidine against renal ischemia/reperfusion injury in rats. Br. J. Pharmacol. Toxicol. 2015;6(3):64–69. [Google Scholar]
- Bancroft J.D., Gamble M. Elsevier Health Sciences; Philadelphia, USA: 2008. Theory and practice of histological techniques. [Google Scholar]
- Behiry S., Rabie A., Kora M., Ismail W., Sabry D., Zahran A. Effect of combination sildenafil and gemfibrozil on cisplatin-induced nephrotoxicity; role of heme oxygenase-1. Ren. Fail. 2018;40(1):371–378. doi: 10.1080/0886022X.2018.1455596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkels R., Taubert D., Bartels H., Breitenbach T., Klaus W., Roesen R. Amlodipine increases endothelial nitric oxide by dual mechanisms. Pharmacology. 2004;70(1):39–45. doi: 10.1159/000074241. [DOI] [PubMed] [Google Scholar]
- Bian Y.-F., Yang H.-Y., Yang Z.-M., Gao F., Zhang N.-N., Xiao C.-S. Amlodipine treatment prevents angiotensin II-induced human umbilical vein endothelial cell apoptosis. Arch. Med. Res. 2011;42(1):22–27. doi: 10.1016/j.arcmed.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Bragado P., Armesilla A., Silva A., Porras A. Apoptosis by cisplatin requires p53 mediated p38α MAPK activation through ROS generation. Apoptosis. 2007;12(9):1733–1742. doi: 10.1007/s10495-007-0082-8. [DOI] [PubMed] [Google Scholar]
- Bulsara K.G., Cassagnol M. Amlodipine. In: StatPearls. StatPearls Publishing; Treasure Island (FL): 2020. [PubMed] [Google Scholar]
- Chinwe I.I., Kingsley U.I., Blessing E.I. Effects of some calcium channel blockers againsts CCl4-induced nephrotoxicity in albino rats. PhOL. 2019;1:87–95. [Google Scholar]
- Corti A., Franzini M., Paolicchi A., Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30(4):1169–1181. [PubMed] [Google Scholar]
- Crona D.J., Faso A., Nishijima T.F., McGraw K.A., Galsky M.D., Milowsky M.I. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist. 2017;22(5):609–619. doi: 10.1634/theoncologist.2016-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa G., Maffioli P. Drug safety evaluation of amlodipine. Expert Opin. Drug Saf. 2011;10(5):795–804. doi: 10.1517/14740338.2011.585966. [DOI] [PubMed] [Google Scholar]
- Djokovic B., Jankovic M.G., Harrell C.R., Fellabaum C., Arsenijevic N., Volarevic V. New insights in the pathogenesis of cisplatin-induced nephrotoxicity. Serbian J. Exp. Clin. Res. 2019;1 doi: 10.2478/sjecr-2019-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S., Liu F., Luo J., Wu H., Liu R., Peng Y., Yang X. Nephrotoxicity of high-and low-osmolar contrast media: the protective role of amlodipine in a rat model. Acta Radiol. 2000;41(5):503–507. doi: 10.1080/028418500127345794. [DOI] [PubMed] [Google Scholar]
- Dugbartey G.J., Peppone L.J., de Graaf I.A. An integrative view of cisplatin-induced renal and cardiac toxicities: molecular mechanisms, current treatment challenges and potential protective measures. Toxicology. 2016;371:58–66. doi: 10.1016/j.tox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H., DiNicolantonio J.J., O'Keefe J.H., Lavie C.J. Amlodipine in hypertension: a first-line agent with efficacy for improving blood pressure and patient outcomes. Open Heart. 2016;3(2) doi: 10.1136/openhrt-2016-000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliedl L., Wieser M., Manhart G., Gerstl M.P., Khan A., Grillari J., Grillari-Voglauer R. Controversial role of gamma-glutamyl transferase activity in cisplatin nephrotoxicity. ALTEX-Altern. Anim. Ex. 2014;31(3):269–278. doi: 10.14573/altex.1311152. [DOI] [PubMed] [Google Scholar]
- Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019;88 doi: 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- Hanigan M.H. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Adv. Cancer Res. 2014;122:103–141. doi: 10.1016/B978-0-12-420117-0.00003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan M.H., Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- Hanigan M.H., Gallagher B.C., Taylor P.T., Large M.K. Inhibition of γ-glutamyl transpeptidase activity by acivicin in vivo protects the kidney from cisplatin-induced toxicity. Cancer Res. 1994;54(22):5925–5929. [PubMed] [Google Scholar]
- Hanigan M.H., Lykissa E.D., Townsend D.M., Ou C.-N., Barrios R., Lieberman M.W. γ-Glutamyl transpeptidase-deficient mice are resistant to the nephrotoxic effects of cisplatin. Am. J. Pathol. 2001;159(5):1889–1894. doi: 10.1016/s0002-9440(10)63035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausheer F.H., Shanmugarajah D., Leverett B.D., Chen X., Huang Q., Kochat H., Petluru P.N., Parker A.R. Mechanistic study of BNP7787-mediated cisplatin nephroprotection: modulation of gamma-glutamyl transpeptidase. Cancer Chemother. Pharmacol. 2010;65(5):941–951. doi: 10.1007/s00280-009-1101-y. [DOI] [PubMed] [Google Scholar]
- Hoek J., Bloemendal K.M., Van der Velden L.-A.A., Van Diessen J.N., Van Werkhoven E., Klop W., Tesselaar M.E. Nephrotoxicity as a dose-limiting factor in a high-dose cisplatin-based chemoradiotherapy regimen for head and neck carcinomas. Cancers. 2016;8(2):21. doi: 10.3390/cancers8020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hye Khan M., Sattar M., Abdullah N., Johns E. Influence of combined hypertension and renal failure on functional α1-adrenoceptor subtypes in the rat kidney. Br. J. Pharmacol. 2008;153(6):1232–1241. doi: 10.1038/bjp.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S., Matsushima S., Okabe K., Ikeda M., Ishikita A., Tadokoro T., Enzan N., Yamamoto T., Sada M., Deguchi H. Blockade of L-type Ca2+ channel attenuates doxorubicin-induced cardiomyopathy via suppression of CaMKII-NF-κB pathway. Sci. Rep. 2019;9(1):1–14. doi: 10.1038/s41598-019-46367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra P., Karwasra R., Gupta Y.K., Ray S.B., Singh S. Terminalia chebula supplementation attenuates cisplatin-induced nephrotoxicity in Wistar rats through modulation of apoptotic pathway. Nat. Prod. Res. 2019;33(11):1641–1645. doi: 10.1080/14786419.2018.1425843. [DOI] [PubMed] [Google Scholar]
- Karapetian R.N., Evstafieva A.G., Abaeva I.S., Chichkova N.V., Filonov G.S., Rubtsov Y.P., Sukhacheva E.A., Melnikov S.V., Schneider U., Wanker E.E. Nuclear oncoprotein prothymosin α is a partner of Keap1: implications for expression of oxidative stress-protecting genes. Mol. Cell. Biol. 2005;25(3):1089–1099. doi: 10.1128/MCB.25.3.1089-1099.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.H., Sattar M.A., Abdullah N.A., Johns E.J. Influence of cisplatin-induced renal failure on the α1-adrenoceptor subtype causing vasoconstriction in the kidney of the rat. Eur. J. Pharmacol. 2007;569(1–2):110–118. doi: 10.1016/j.ejphar.2007.04.063. [DOI] [PubMed] [Google Scholar]
- Khan A.H., Sattar M.A., Abdullah N.A., Johns E.J. Effect of calcium channel blockade on adrenergically induced renal vasoconstriction in rat models of renal impairment. Clin. Exp. Pharmacol. Physiol. 2009;36(5–6):501–508. doi: 10.1111/j.1440-1681.2008.05098.x. [DOI] [PubMed] [Google Scholar]
- Khasabova I.A., Yao X., Paz J., Lewandowski C.T., Lindberg A.E., Coicou L., Burlakova N., Simone D.A., Seybold V.S. JZL184 is anti-hyperalgesic in a murine model of cisplatin-induced peripheral neuropathy. Pharmacol. Res. 2014;90:67–75. doi: 10.1016/j.phrs.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Suzuki N., Ohba S., Mise N., Miyashita K., Tojo A., Goto A., Omata M. Effects of amlodipine, a calcium channel blocker, on rat renal arterioles. Curr. Ther. Res. 1997;58(6):375–380. [Google Scholar]
- King J.B., West M.B., Cook P.F., Hanigan M.H. A novel, species-specific class of uncompetitive inhibitors of γ-glutamyl transpeptidase. J. Biol. Chem. 2009;284(14):9059–9065. doi: 10.1074/jbc.M809608200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer J., Bach N.C., Forler D., Sieber S.A. Target discovery of acivicin in cancer cells elucidates its mechanism of growth inhibition. Chem. Sci. 2015;6(1):237–245. doi: 10.1039/c4sc02339k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li Q.-X., Xie X.-F., Ao Y., Tie C.-R., Song R.-J. Differential roles of dihydropyridine calcium antagonist nifedipine, nitrendipine and amlodipine on gentamicin-induced renal tubular toxicity in rats. Eur. J. Pharmacol. 2009;620(1–3):97–104. doi: 10.1016/j.ejphar.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Liao W., Fu Z., Zou Y., Wen D., Ma H., Zhou F., Chen Y., Zhang M., Zhang W. MicroRNA-140-5p attenuated oxidative stress in Cisplatin induced acute kidney injury by activating Nrf2/ARE pathway through a Keap1-independent mechanism. Exp. Cell Res. 2017;360(2):292–302. doi: 10.1016/j.yexcr.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Lisk C., McCord J., Bose S., Sullivan T., Loomis Z., Nozik-Grayck E., Schroeder T., Hamilton K., Irwin D.C. Nrf2 activation: a potential strategy for the prevention of acute mountain sickness. Free Radic. Biol. Med. 2013;63:264–273. doi: 10.1016/j.freeradbiomed.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.-L., Li Q.-X., Xia L., Li J., Shao L. Differential effects of dihydropyridine calcium antagonists on doxorubicin-induced nephrotoxicity in rats. Toxicology. 2007;231(1):81–90. doi: 10.1016/j.tox.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J., Liu F., Chen F., Jin Y., Chen H., Liu D., Cui W. Amlodipine and atorvastatin improve ventricular hypertrophy and diastolic function via inhibiting TNF-α, IL-1β and NF-κB inflammatory cytokine networks in elderly spontaneously hypertensive rats. Biomed. Pharmacother. 2016;83:330–339. doi: 10.1016/j.biopha.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Ma X., Dang C., Kang H., Dai Z., Lin S., Guan H., Liu X., Wang X., Hui W. Saikosaponin-D reduces cisplatin-induced nephrotoxicity by repressing ROS-mediated activation of MAPK and NF-κB signalling pathways. Int. Immunopharmacol. 2015;28(1):399–408. doi: 10.1016/j.intimp.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Maj J.G., Tomaszewski J.J. The effect of cyclosporine A and amlodipine on the activity of γ-glutamyl transpeptidase in mouse cerebral cortex. Pharmacol. Res. 1997;36(1):73–76. doi: 10.1006/phrs.1997.0209. [DOI] [PubMed] [Google Scholar]
- Malik S., Suchal K., Gamad N., Dinda A.K., Arya D.S., Bhatia J. Telmisartan ameliorates cisplatin-induced nephrotoxicity by inhibiting MAPK mediated inflammation and apoptosis. Eur. J. Pharmacol. 2015;748:54–60. doi: 10.1016/j.ejphar.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Manohar S., Leung N. Cisplatin nephrotoxicity: a review of the literature. J. Nephrol. 2018;31(1):15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- Meng X.-M., Ren G.-L., Gao L., Yang Q., Li H.-D., Wu W.-F., Huang C., Zhang L., Lv X.-W., Li J. NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab. Invest. 2018;98(1):63–78. doi: 10.1038/labinvest.2017.120. [DOI] [PubMed] [Google Scholar]
- Miranda K.M., Espey M.G., Wink D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- Nili-Ahmadabadi A., Ali-Heidar F., Ranjbar A., Mousavi L., Ahmadimoghaddam D., Larki-Harchegani A., Ghafouri-Khosrowshahi A. Protective effect of amlodipine on diazinon-induced changes on oxidative/antioxidant balance in rat hippocampus. Res. Pharm. Sci. 2018;13(4):368–376. doi: 10.4103/1735-5362.235164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh G.-S., Kim H.-J., Shen A., Lee S.B., Khadka D., Pandit A., So H.-S. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press. 2014;12(2):55–65. doi: 10.5049/EBP.2014.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott C., Schneider M.P., Raff U., Ritt M., Striepe K., Alberici M., Schmieder R.E. Effects of manidipine vs. amlodipine on intrarenal haemodynamics in patients with arterial hypertension. Br. J. Clin. Pharmacol. 2013;75(1):129–135. doi: 10.1111/j.1365-2125.2012.04336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres L.A.B., Cunha Júnior A.D.d. Acute nephrotoxicity of cisplatin: molecular mechanisms. J. Bras. Nefrol. 2013;35(4):332–340. doi: 10.5935/0101-2800.20130052. [DOI] [PubMed] [Google Scholar]
- Qasim S., Kalsoom S., Shehzad M., Irfan H.M., Zafar M.S., Bukhari I.A., Vohra F., Afzal S. Appraisal of disease-modifying potential of amlodipine as an anti-arthritic agent: new indication for an old drug. Inflammopharmacology. 2020;28(4):1121–1136. doi: 10.1007/s10787-020-00692-9. [DOI] [PubMed] [Google Scholar]
- Salama R.H., Abd-El-Hameed N.A., Abd-El-Ghaffar S.K., Mohammed Z.T., Ghandour N.M. Nephroprotective effect of Nigella sativa and Matricaria chamomilla in cisplatin induced renal injury—supportive treatments in cisplatin nephrotoxicity. Int. J. Clin. Med. 2011;2(03):185–195. [Google Scholar]
- Saleh S., El-Demerdash E. Protective effects of l-arginine against cisplatin-induced renal oxidative stress and toxicity: role of nitric oxide. Basic Clin. Pharmacol. Toxicol. 2005;97(2):91–97. doi: 10.1111/j.1742-7843.2005.pto_114.x. [DOI] [PubMed] [Google Scholar]
- Salehi I., Mohammadi M., Mirzaei F., Soufi F. Amlodipine attenuates oxidative stress in the heart and blood of high-cholesterol diet rabbits. Cardiovasc. J. Afr. 2012;23(1):18–22. doi: 10.5830/CVJA-2010-091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem N., Helmi N., Assaf N. Renoprotective effect of platelet-rich plasma on cisplatin-induced nephrotoxicity in rats. Oxid. Med. Cell. Longev. 2018;2018:9658230. doi: 10.1155/2018/9658230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliburska J., Krejpcio Z., Staniek H., Król E., Bogdanski P., Kupsz J., Hertig I. The effects of antihypertensive drugs on chromium status, glucose metabolism, and antioxidant and inflammatory indices in spontaneously hypertensive rats. Biol. Trace Elem. Res. 2014;157(1):60–66. doi: 10.1007/s12011-013-9864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentmihályi K., May Z., Szénási G., Máthé C., Sebestény A., Albert M., Blázovics A. Cisplatin administration influences on toxic and non-essential element metabolism in rats. J. Trace Elem. Med. Biol. 2014;28(3):317–321. doi: 10.1016/j.jtemb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Tayem Y., Green C.J., Motterlini R., Foresti R. Isothiocyanate–cysteine conjugates protect renal tissue against cisplatin-induced apoptosis via induction of heme oxygenase-1. Pharmacol. Res. 2014;81:1–9. doi: 10.1016/j.phrs.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Toba H., Shimizu T., Miki S., Inoue R., Yoshimura A., Tsukamoto R., Sawai N., Kobara M., Nakata T. Calcium channel blockers reduce angiotensin II-induced superoxide generation and inhibit lectin-like oxidized low-density lipoprotein receptor-1 expression in endothelial cells. Hypertens. Res. 2006;29(2):105–116. doi: 10.1291/hypres.29.105. [DOI] [PubMed] [Google Scholar]
- Toma L., Stancu C.S., Sanda G.M., Sima A.V. Anti-oxidant and anti-inflammatory mechanisms of amlodipine action to improve endothelial cell dysfunction induced by irreversibly glycated LDL. Biochem. Biophys. Res. Commun. 2011;411(1):202–207. doi: 10.1016/j.bbrc.2011.06.137. [DOI] [PubMed] [Google Scholar]
- Townsend D.M., Deng M., Zhang L., Lapus M.G., Hanigan M.H. Metabolism of cisplatin to a nephrotoxin in proximal tubule cells. J. Am. Soc. Nephrol. 2003;14(1):1–10. doi: 10.1097/01.asn.0000042803.28024.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D.M., Hanigan M.H. Inhibition of γ-glutamyl transpeptidase or cysteineS-conjugate β-lyase activity blocks the nephrotoxicity of cisplatin in mice. J. Pharmacol. Exp. Ther. 2002;300(1):142–148. doi: 10.1124/jpet.300.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volarevic V., Djokovic B., Jankovic M.G., Harrell C.R., Fellabaum C., Djonov V., Arsenijevic N. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019;26(1):1–14. doi: 10.1186/s12929-019-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Luo X., Pan H., Huang W., Wang X., Wen H., Shen K., Jin B. Pharmacological inhibition of NADPH oxidase protects against cisplatin induced nephrotoxicity in mice by two step mechanism. Food Chem. Toxicol. 2015;83:251–260. doi: 10.1016/j.fct.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Wilmes A., Bielow C., Ranninger C., Bellwon P., Aschauer L., Limonciel A., Chassaigne H., Kristl T., Aiche S., Huber C.G. Mechanism of cisplatin proximal tubule toxicity revealed by integrating transcriptomics, proteomics, metabolomics and biokinetics. Toxicol. In Vitro. 2015;30(1):117–127. doi: 10.1016/j.tiv.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Xu R., Cai A., Zheng D., Qiu R., Li L., Zhou Y., Feng Y., Mai W. Amlodipine suppresses Ang-II-induced endothelium dysfunction by diminishing ROCK1 expression. Clin. Exp. Hypertens. 2016;38(2):166–172. doi: 10.3109/10641963.2015.1081212. [DOI] [PubMed] [Google Scholar]
- Yang Y., Liu H., Liu F., Dong Z. Mitochondrial dysregulation and protection in cisplatin nephrotoxicity. Arch. Toxicol. 2014;88(6):1249–1256. doi: 10.1007/s00204-014-1239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef M.I., Hussien H.M. Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food Chem. Toxicol. 2015;78:17–25. doi: 10.1016/j.fct.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Zhang L., Hanigan M.H. Role of cysteine S-conjugate β-lyase in the metabolism of cisplatin. J. Pharmacol. Exp. Ther. 2003;306(3):988–994. doi: 10.1124/jpet.103.052225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.