Abstract
Atorvastatin (ATO) is of the statin class and is used as an orally administered lipid-lowering drug. ATO is a reversible synthetic competitive inhibitor of 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase thus leading to a reduction in cholesterol synthesis. It has recently been demonstrated that ATO has different pharmacological actions, which are unrelated to its lipid-lowering effects and has the ability to treat chronic airway diseases. This paper reviews the potential of ATO as an anti-inflammatory, antioxidant, and anti-proliferative agent after oral or inhaled administration. This paper discusses the advantages and disadvantages of using ATO under conditions associated with those found in the airways. This treatment could potentially be used to support the formulating of ATO as an inhaler for the treatment of chronic respiratory diseases.
Keywords: Atorvastatin, Respiratory diseases, Inhale, Oral, Oxidation, Inflammation
Abbreviations: AA, Allergic asthma; AP-1, Activator protein-1; ATO, Atorvastatin; BALF, Bronchoalveolar lavage fluid; °C/min, Temperature in degrees per minutes; CCL7, Chemokine ligand 7; CI, Confidence interval; COPD, Chronic obstructive pulmonary disease; CRP, C-reactive protein; CS, Cigarettes smoke; CYP3A4/5, Cytochrome Metabolic enzymes3A4/5; FPP, Farnesylpyrophosphate; G, Gram; GEF, Guanine nucleotide exchange factors; GGPP, Geranylgeranylpyrophosphate; IL, Interleukins; log D, Coefficient values octanol/water; Log P, Partition coefficient; m2, Square meter; mg, Milligram; mg/day, Milligram per day; ml, Millilitres; MMPs, Matrix-metalloprotease; MVA, Mevalonic acid; NADPH, Nicotinamide adenine dinucleotide phosphate; NaOH, Sodium hydroxide; NCSCL, Non-small cell lung cancer; NF-κB, Nuclear factor kappa; NOS, Nitric oxide synthase; OATP, Organic anion transporting polypeptide; PEG, Polyethylene glycol; pH, Measure of the acidity or basicity of an aqueous solution; pKa, Dissociation constant; PPE, Porcine pancreatic elastase; ROS, Reactive oxygen species; s, Second; SAS, Supercritical antisolvent; SphK1, Sphingosine kinase 1; TGF, Transforming growth factor; TNF-a, Tumour necrosis factor alpha; TSC, Tuberous sclerosis; UDP, Uridine diphosphate; μg, Microgram; μg/day, Microgram per day; μg/mL, Microgram per millilitre; μM, Micromolar; UV, Ultraviolet light; v/v, Volume per volume; VEGF, Vascular endothelial cell growth factor; VLDL, Very low-density lipoproteins; WHO, World Health Organization; %, Percentage
1. Introduction
1.1 Chronic pulmonary diseases and statin
According to a World Health Organization (WHO) report, chronic respiratory diseases including bronchiectasis, cystic fibrosis, chronic obstructive pulmonary disease (COPD), and asthma are expected to be the third most common cause of mortality (Barnes, 2008). Chronic pulmonary diseases have continued to increase despite the available therapeutic advancements (Athanazio, 2012) because of the increasing number of cigarette smokers and the increasing amount of environmental pollution (Barnes, 2008). Hyper-mucus secretion, breathlessness, airflow limitation, coughing, wheezing, and bronchoconstriction are the main symptoms of these diseases (Barker, 2002, Guerra et al., 2009, James and Wenzel, 2007, Murray et al., 2007, Szilasi et al., 2006). In addition, hyper-mucus production in the lungs has been shown to be associated with airway infection and inflammation, thus impacting disease development (Hovenberg et al., 1996, Kim, 1997, Kirkham et al., 2002, Livraghi and Randell, 2007, Lundgren and Shelhamer, 1990, Reid et al., 1997, Williams et al., 2006). Convenient treatments including antibiotics, non-steroidal, anti-inflammatory drugs, β-adrenergic agonists, and steroids focus on symptom improvement (Marin et al., 2011, Yang et al., 2011), as an alternative to therapeutic remedy. Additionally, prolonged administration of convenient drugs in some patients causes a drug-resistant refractory effect (Barnes et al., 2004). Therefore, there is an immediate need for newer treatments that can replace the conveniently available and highly effective treatment.
Statins are one such drug class that has potential as an alternative treatment for chronic lung diseases. They are anti-cholesterol drugs and act as 3-hydroxymethyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (Tobert, 2003) by blocking the conversion of HMG-CoA to mevalonate biosynthesis, a vital intermediary in the metabolism of cholesterol (Endo, 2004, Endo et al., 1976). Mevastatin was the first detected statin, which was extracted from Penicillium Citrinium (Srinivasa Rao et al., 2011). After that, atorvastatin, cerivastatin, pitavastatin, fluvastatin, and rosuvastatin were synthesised chemically, whereas others, for instance, pravastatin, simvastatin, and lovastatin were derived from the fermentation of fungi (Hoffman et al., 1986, Lee et al., 1991). Numerous studies have also underlined that statins have many pleiotropic effects besides their well-known cholesterol-lowering activity, such as muco-inhibitory (Marin et al., 2013, Tulbah et al., 2015, Tulbah et al., 2015), anti-proliferative (Shang et al., 2015, Yu et al., 2013), antithrombotic, and anti-antioxidant activity (ALKharfy et al., 2000, Endo et al., 1977, Grommes et al., 2012, Gullestad et al., 1999, Jialal et al., 2001, McAuley et al., 2013, Rezaie-Majd et al., 2002, Vaughan et al., 1996). Statins also have an anti-inflammatory effect that could potentially be used as an alternative therapy for respiratory diseases in which inflammation exists (Tulbah et al., 2016, Walsh, 2008).
1.2 Atorvastatin
Atorvastatin (ATO) is statin drugs that is common and affordable (Lea and McTavish, 1997). It was the preferred and most prescribed anti-cholesterol drug worldwide from 2002 to 2009 (Dinarello, 2010). It is also one of the most effective synthetic reversible competitive inhibitors of the HMG-CoA reductase enzyme (Dinarello, 2010, Endo, 2004, Endo et al., 1976, Lea and McTavish, 1997). Lipitor is one of ATO’s trade names and is used to reduce total cholesterol, and triglyceride levels in hypercholesterolemia patients (Lea and McTavish, 1997) and those with cardiovascular risk (Collaboration, 2015, Poli, 2007). In addition, ATO has been shown to be effective in decreasing transient ischaemic attack (Amarenco, 2007, Armitage, 2007), and reducing the death rate from coronary artery disease (Merx and Weber, 2006, Wilt et al., 2004). Some pharmacological actions of ATO, including immune-modulatory effects (Emruzi et al., 2018, Zeiser, 2018), improved endothelial cell function, and enhanced fibrinolysis were also highlighted by different studies (Fogari et al., 2004, Sakabe et al., 2008, Souza-Costa et al., 2007, Tehrani et al., 2010, Van Linthout et al., 2007), and anti-hyperalgesia effects (Pathak et al., 2013). ATO has been well-documented as an anti-oxidation and anti-inflammatory agent in the literature (Ferreira et al., 2014, Souza-Costa et al., 2007, Van Linthout et al., 2007). This suggests that ATO could serve as an alternative treatment for chronic respiratory diseases, including COPD and asthma (Bradbury et al., 2018, Mroz et al., 2015, Walsh, 2008). Therefore, the aim of this review is primarily to highlight the capability of ATO to treat chronic respiratory diseases, with a focus on its effects associated with decreasing mevalonate synthesis.
Contradictory results from clinical trials involving the oral use of ATO in asthma, COPD, bronchiectasis, and pneumonia patients over the past decade are shown in Table 1. ATO treatments have been shown to decrease sputum macrophage counts (leukotriene B4) in asthmatic patients (Hothersall et al., 2008). In addition, ATO treatment resulted in a downregulation of immune response, inflammation, and leukocyte activation in COPD patients (Mroz et al., 2015). However, a randomized and double-blind trial study found that oral ATO (10 mg/day) did not improve pulmonary function in asthmatic patients (Fahimi et al., 2009). Consequently, the lack of clinical therapeutics improvement could be due to ATO’s chemical structure, lipophilic nature, and rapid systemic clearance, as well as to the low bioavailability of oral ATO administration (Gazzerro et al., 2012, Mroz et al., 2015).
Table 1.
Clinical trials data on asthma, chronic obstructive pulmonary disease (COPD), pneumonia and bronchiectasis patients used oral Atorvastatin (ATO) treatment.
| Statins | Disease | Administration Route | Primary Outcomes | Ref |
|---|---|---|---|---|
| Atorvastatin | Asthma | Oral dose of 40 mg of oral atorvastatin per day and 400 μg inhaled beclomethasone for 4 weeks | Improved asthma quality of life. Airway responsiveness, lung function and inflammation did not improve | (Braganza et al., 2011) |
| Atorvastatin | Atopic Asthma | Inhaled corticosteroids combined with 40 mg of oral atorvastatin for 24 weeks | Reduces sputum macrophage counts (leukotriene B4) in mild to moderate asthma. | (Hothersall et al., 2008) |
| Atorvastatin | COPD | atorvastatin 40 mg/day for 12 weeks | Lowered sputum neutrophil count by 34%, and caused a 57% decrease in CD45+ cells biopsies from lung tissues involved in inflammatory processes, immune response, and leukocyte activation | (Mroz et al., 2015) |
| Atorvastatin | Asthma | Oral atorvastatin 40 mg per day for 8 weeks | No significant difference between the atorvastatin and control groups in controlling asthma, FEV1%, FVC%, and blood eosinophil count after treatment | (Moini et al., 2012) |
| Atorvastatin | Asthma | Oral administration of atorvastatin and inhaled corticosteroid (week) | FVC% did not change between ATO and control group. | (Fahimi et al., 2009) |
| Atorvastatin | Asthma | Oral atorvastatin combined with inhaled corticosteroids/long acting agonist therapy | Lower % predicted FVC and had a high prevalence of comorbid conditions | (Zeki et al., 2013) |
| Atorvastatin | Asthma | Oral | Reduced emergency department visit. | (Tse et al., 2014) |
| Atorvastatin | COPD | 40 mg per day for nine weeks of oral dose | Improved quality of life in sulfur mustard-injured patients with COPD. No effect on serum hs-CRP and lung functions |
(Ghobadi et al., 2014) |
| Atorvastatin, | bronchiectasis | Oral 80 mg/day for 6 months | Cough improved on patients with bronchiectasis. | (Mandal et al., 2014) |
| Atorvastatin | COPD | administration of atorvastatin | Decreased inflammatory mediators. | (Blamoun et al., 2008) |
| Atorvastatin | COPD | administration of atorvastatin | % COPD patients mortality decreased if C reactive protein level > mg/L. |
(Lahousse et al., 2013) |
| Atorvastatin | Smoker or former smoker | administration of atorvastatin | Slower decline in pulmonary function. | (Keddissi et al., 2007) |
| Atorvastatin | Patients with advanced non-small cell lung cancer | 400 mg/m2/day of chemotherapy starting on day 4 and atorvastatin starting at least 5 days before chemotherapy | Drug–drug interactions between the lipid-lowering and chemotherapeutic agent (bexarotene). | (Wakelee et al., 2012) |
| Atorvastatin | Patients with aspiration pneumonia complicated with cerebral infarction | Oral medication, 20 mg/day for 2 months | levels of TNF-α, IL-6 and IL-8 were decreased in ATO group more than the control group (P < 0.01) | (Wei and Liu, 2018) |
| Atorvastatin | Smokers, patients with asthma | Oral atorvastatin (40 mg/day) used for four weeks versus placebo, followed by inhaled beclometasone (400 μg/day) for a more four weeks. | Short-term treatment with atorvastatin alone or in combination with inhaled beclometasone reduces several sputum cytokine, chemokine, and growth factor levels unresponsive to inhaled corticosteroids alone in smokers with asthma | (Thomson et al., 2015) |
The chemical structure of ATO and its metabolites is presented in Fig. 1, which represents its lipophilicity, water solubility, and pharmacokinetic profile (absorption, distribution, metabolism, and excretion) (Castaño et al., 2003, Gee et al., 2002, Sparks et al., 2005). ATO is metabolized into two active metabolites, lactone form, 4- and 2-hydroxy-atorvastatin acids, and three corresponding inactive lactone metabolites (Jacobsen et al., 2000) by major action of P450 metabolic enzymes, CYP3A4/5 (Jacobsen et al., 2000). The lactone form is transported via passive diffusion as it is more lipophilic in nature, whereas the atorvastatin acids are substrates for P-glycoprotein (P-gp) and organic anion transporting polypeptide (OATP) cellular membrane transporters (Lennernäs, 2003). The hydroxy acid form of the drug is presented as a free acid species; it is about 15 times more soluble than the lactone form because the carboxyl group of the hydroxyl acid is ionized. This ionization has a significant effect on its solubility (Khan and Dehghan, 2011). Log D values (octanol/water coefficient) for the acid and lactone forms of the drug are 1.53 and 4.2 at pH 7.4, respectively (Ishigami et al., 2001).
Fig. 1.
Atorvastatin (ATO) and its metabolites, chemical structure.
2. Atorvastatin challenges
Drug bioavailability, dissolution, stability, and solubility are among the rate-limiting steps in the development of any new drug formulation and also arise during the manufacturing, storage, and shipping stages. In addition to chemical hydrolysis or oxidation, these parameters can be influenced by different factors, including temperature, light, moisture, solvents, excipients, and pH (Guideline, 2003, Kommanaboyina and Rhodes, 1999).
2.1. Chemical and physical challenges of atorvastatin
ATO must be studied under various chemical conditions if therapeutic activities for its stability are to be maintained. ATO, in the open ring hydroxy acid form, is an unstable drug. Its form of hydroxy acid is converted into a form of lactone under specific conditions of changing pH, oxidative stress, moisture, heat, and light.
One of the major factors that could affect on the chemical stability of ATO is pH (basic and acidic conditions). Oliveira et al., studied ATO degradation and its effects on basic and acidic conditions. Their findings revealed that ATO was not as stable under acidic conditions as basic conditions with the development of two degradation products under basic conditions. They also discovered that, under acidic conditions, ATO degradation employed first-order kinetic degradation compared to the zero-order kinetic degradation obtainable in the basic environments (Oliveira et al., 2013). A study by Shah et al., investigated the impact of oxidative stress on the aliquots of stock solutions of ATO in 3% hydrogen peroxide (Shah et al., 2008). Similar results have been found in which ATO was degraded, mostly with 1% hydrogen peroxide solution at 25 ± 2 °C of room temperature for 24 h (Vukkum et al., 2012) and in 3% v/v hydrogen peroxide solution at room temperature for seven days (Sherikar and Mehta, 2012). In addition, ATO sensitivity to ultraviolet light (UV) and temperature has been studied. Zaheer et al., discovered that 0.03% of ATO degraded when the drug was heated in a boiling water bath for 30 mins (Zaheer et al., 2008). With regards to light stress, a study discovered that ATO degraded when exposed to 1.2 million lux hours of visible light and 200 W hour/per square meter of UV light for 11 days (Vukkum et al., 2012). ATO can be very permeable and soluble except where there is an aqueous solution and its pH values are less than 4 (Nováková et al., 2008). Such findings underline the interrelation of the various factors that could influence ATO's stability, solubility, and bioavailability and, thus, subsequent therapeutic effectiveness.
The crystalline or amorphous solid state of most drugs has a huge effect on their stability. The amorphous state might have a reputation for not being as thermodynamically stable as its crystal counterpart (Tong and Zografi, 2004), but its solubility characteristics are generally higher than those of the crystalline form. Findings like this are important for crystalline ATO forms and low water solubility (0.1 mg/mL) for better bioavailability (Lau et al., 2006).
The physico-chemical properties of amorphous ATO formulated by lyophilization utilizing skimmed milk were characterised in a study by Choudhary et al. They discovered that the amorphous state had better solubility and in vitro drug release compared to the pure drug in its crystalline form (Choudhary et al., 2012). Another study by Aggarwal et al., formulated amorphous ATO with magnesium stearate, talc, lactose, and avicel pH 10.2 to develop its solubility, stability, and dissolution rate. The physical stability of the formulation was assessed at 40 °C (75 ± 5% relative humidity) for up to one month. It was observed that the formulation of amorphous ATO was significantly more stable with a better dissolution rate, dissolution efficiency, and solubility under this condition (Aggarwal et al., 2012). In a study done in 2016, amorphous ATO was formulated using polyethylene glycol 4000 (PEG) to improve its bioavailability and dissolution profile. The study revealed that a drug/PEG 4000 with a ratio of 1:3 significantly increased the drug dissolution rate and bioavailability because of the drug’s change from a crystalline state to an amorphous state (Shamsuddin et al., 2016).
Drug surface area is another factor that will possibly affect ATO solubility and dissolution. A study prepared amorphous ATO Hemi-calcium using supercritical antisolvent (SAS) and spray-drying after which the physicochemical properties and bioavailability were evaluated. The study revealed that amorphous ATO with particle sizes of 95.7 ± 12.2 nm, 79.78 ± 0.93 m2/g and 68.7 ± 15.8 nm, 120.35 ± 1.40 m2/g appeared to have better solubilities and dissolution performances compared to unprocessed crystalline ATO. According to the findings of this study, physical modifications like the reduction of particle size and the use of spray-dying and the SAS process to generate an amorphous state can be used to improve crystalline ATO bioavailability and physiochemical properties (Kim et al., 2008). Therefore, a different approach is needed to improve the development of ATO stability, solubility, and bioavailability.
2.2. In vivo challenges
Inside the body, when ATO is administered orally for active hydroxyl acid rather than the lactone prodrug (Lennernäs, 2003), the two active ATO form are in a state of equilibrium with the inactive lactone forms. Both active forms were metabolized mostly by CYP3A4 and CYP3A5. ATO acid had a significantly lower affinity to CYP3A4 than the lactone (Km: para-hydroxy atorvastatin lactone, 1.4 ± 0.2 μM; para-hydroxy atorvastatin, 25.6 ± 5.0 μM; ortho-hydroxy atorvastatin, 29.7 ± 9.4 μM; and ortho-hydroxy atorvastatin lactone, 3.9 ± 0.2 μM). Compared with atorvastatin acid, CYP-dependent metabolism of atorvastatin lactone to its ortho-hydroxy metabolite was 20-fold higher and to its para-hydroxy metabolite was 83-fold higher (Jacobsen et al., 2000). Acylglucuronide intermediates mostly require that the active ATO form be converted to inactive, which is majorly catalysed by the UDP glucuronosyltransferase (Hoffart et al., 2012). Over 98 percent of ATO is bound to plasma proteins. A small amount is excreted in the urine, while most is excreted through bile in faecal matter. Very small concentrations of the active ATO metabolites are related to about 70% of the total plasma HMG-CoA activity. In biological fluid, only five percent of the statin dose reaches systemic circulation due to its low solubility in the plasma. The atorvastatin active metabolites are found as pg/ml in plasma levels from 0.1 to 20 ng/ml concentration. It absorbs completely when administered orally but undergoes broad first-pass metabolism while in the liver and intestine through cytochrome P450, which can lead to a low 12% (Lennernäs, 2003, Rodde et al., 2014).
However, the ATO a hepatic first-pass effect, poor solubility, low bioavailability, and drug instability that are not totally understood (Lennernäs, 2003). This effect might be due to extensive gut wall extraction and/or incomplete intestinal absorption. The upper gastrointestinal tract is not able to absorb the un-solubilised form of ATO, which is the possible absorption site of the drug (Khan and Dehghan, 2011). Consequently, the lung, which is the target site, cannot experience any therapeutic effect if ATO is taken in large oral doses, which could eventually lead to more systemic side effects. The systemic effects of ATO have been reported in a few cases when it was taken orally, which includes progression to rhabdomyolysis and myopathy (Kommanaboyina and Rhodes, 1999). The conflicting results from the clinical trial studies analysing oral ATO’s level of effectiveness as an anti-inflammatory chronic lung disease agent could be a consequence of the issues mentioned above. At the target site (pulmonary), large oral doses of ATO are essential for obtaining therapeutic effects. Consequently, systemic side effects will be increased. A few case studies have reported on the systemic side of ATO when administered orally, such as rhabdomyolysis and myopathy (Bernini et al., 2001, Hermann et al., 2006, Manoj et al., 2017). Therefore, many clinical trial studies have demonstrated conflicting results in assessing the effectiveness of oral ATO as an anti-inflammatory and antioxidant agent for chronic pulmonary diseases.
3. Potential for inhalation of atorvastatin
To avoid challenges related to ATO oral administration, an alternative is the administration of ATO inhaled directly into the lungs, which will provide high local pulmonary concentration, and systemic effects, decrease systemic side effects, avoid first-pass metabolism compared to oral statins and simultaneously improve the bioavailability of ATO (Irngartinger et al., 2004, Pilcer and Amighi, 2010). Hence, the soluble/stable inhaled ATO formulation would be most suitable to achieve maximum pulmonary therapeutic effects for chronic lung disease treatment (Pinho-Ribeiro et al., 2017).
An animal study after elastase-induced emphysema found that 10 min of inhaled administration of ATO (1, 5, and 20 mg) significantly decreased the leukocytes levels in BALF in all atorvastatin-treated groups, macrophages in only the A20 mg group, and tissue neutrophils in the A5 mg and A20 mg groups (Melo et al., 2018). A similar study showed that ATO administered via inhalation (15 min with 1 mg/mL once a day) was able to improve lung repair after cigarette-smoke-induced emphysema in mice (Pinho-Ribeiro et al., 2017).
At present, there are only two studies with an inhalable ATO formulation. According to Melo et al., 2018, Pinho-Ribeiro et al., 2017, pulmonary emphysema was induced by CS through oxidative stress, MMP activation, and inflammation. The objective of this study was to analyse the effect of simvastatin and ATO after emphysema was induced in a mouse lung by CS. The researchers divided male mice (C57BL/6, n = 45) into groups including 1) control (exposure to sham); 2) CSr (the mice were exposed to 12 cigarettes per day for a total of 60 days, after which another 60 day cycle was commenced using the vehicle); 3) CSr + A (the mice were made to undergo ATO for a total of 60 days); and 4) CSr + S (simvastatin was used to treat the mice for a total of 60 days). ATO and Simvastatin treatment was administered by inhalation once a day. After the mice were sacrificed physiological, morphological, and biochemical analyses were performed. The result was a decrease in cytokine and leukocyte levels. There was also a decline in the level of stress markers in the statin-treated mice and improvements in lung morphology. Lastly, lung function was ameliorated by statins. This study proved that lung repair following CS-induced emphysema in mice was improved by inhaled simvastatin and ATO (Pinho-Ribeiro et al., 2017). It also found that aerosol formulation with 1 mg/ml of ATO could improve pulmonary function and morphology. This could be attributed to the extracellular matrix restoration that reduced inflammatory cell influx and the subsequent reduction in inflammatory mediator release as well as oxidative stress (Pinho-Ribeiro et al., 2017). A similar study found that a reduction in MMP-12 and Nrf2 emphysematous mice due to the intranasal ATO formulation (5 and 20 mg) (Melo et al., 2018).
3.1. Atorvastatin activation in the lungs
Because ATO needs to be delivered via inhalation, ATO is administered as the active -open ring hydroxy acid within the lungs. This will facilitate its intracellular uptake mechanisms into epithelial cells and macrophages of the lungs (Bradbury et al., 2018). ATO is converted and metabolised into its active form via the CYP3A4 enzyme.
To understand this activation pathway, PARK et al., discovered that cytochrome P450 (CYP), the 3A5 enzyme is responsible for the metabolism of ATO into two metabolites: (1) ortho and (2) para hydroxy ATO (Park et al., 2008). Cytochrome (CY) P3A5 is present in ciliated bronchi, capillary endothelium, alveolar macrophages, and goblet cells of the bronchial glands, bronchial wall, terminal cuboidal epithelium, bronchiolar columnar epithelium and alveolar epithelium (types I and II) (Anttila et al., 1997). Subsequently, it has been assumed that ATO can be metabolized by CYP enzymes and actively move transporters into the lung cells. The lactone form of ATO is transported via passive diffusion as it is more lipophilic in nature, while the atorvastatin hydroxyl acids are substrates for cellular membrane transporters of P-gp and the OAT family and the OATP family (Bosquillon, 2010, Bradbury et al., 2018, Lennernäs, 2003).
4. Atorvastatin pharmacological actions in the lungs
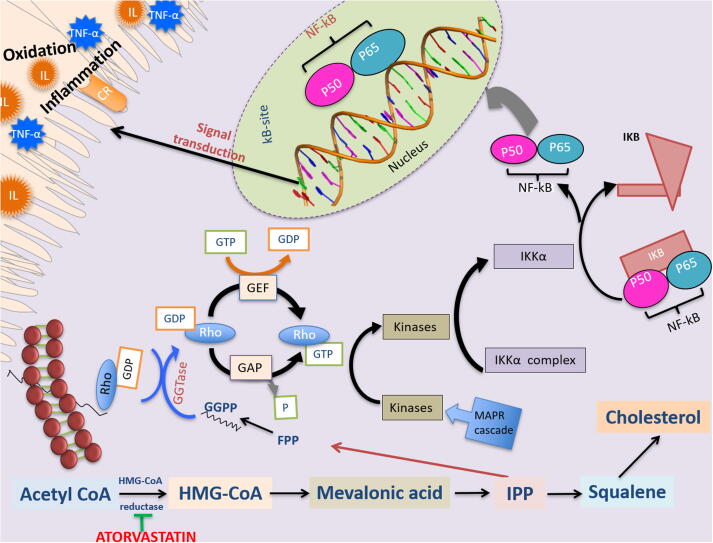
Generally, ATO is able to reduce the mevalonic acid (MVA) synthesis by inhibiting the reductase enzyme of HMG-CoA. Furthermore, MVA has been known to cause isoprenoid intermediates, like geranylgeranylpyrophosphate (GGPP) and farnesylpyrophosphate (FPP), both precursors of cholesterol (Corsini et al., 1999, Hothersall et al., 2006, Hothersall, 2008, Pryor et al., 1983, Yeganeh et al., 2014). GGPP and FPP are essential lipid anchors for carrying out post-translational modification of GTP-binding proteins (Hothersall et al., 2006, Hothersall, 2008, Marin et al., 2011), including Rho, Ras, and Rac, (Fig. 2). The GTPase can be likened to the molecular switches used for controlling the multiple signalling pathways for both the lungs and the cell functions responsible for mediating immune functions, the proliferation of smooth muscle cells, inflammation, cell apoptosis, extracellular matrix deposition, and lung inflammatory cell cytokine production (T-cells, mast cells, dendritic cells, neutrophils, eosinophils, and macrophages) (Hothersall, 2008, Yeganeh et al., 2014).
Fig. 2.
Mechanism of action “Atorvastatin (ATO)” in lung epithelial cells. ATO inhibit the HMG-CoA reductase thus affecting a reduction of isoprenoid intermediate molecules like geranylgeranylpyrophosphate (GGPP). Consequently, ATO facilitate NF-κB translocation into the nucleus and regulate Rho-signal pathways that is related to inflammation and oxidation production. HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; GGPP, geranylgeranylpyrophosphate; FPP, farnesylpyrophosphate; CR, cellular receptor; GTP, guanosine triphosphate; GDP, guanosine disphosphate; GEF, GAP, GTPase activating proteins; IL, interleukin; guanine nucleotide exchange; TNF-α, tumour necrosis factor-α; IPP, Rho, Ras-homologous; isopentenyl-5-pyrophosphate; NF-κB, nuclear factor (NF)-κB.
FPP or GGPP is then employed to prenylate the proteins using geranylgeranyltransferase and farnesyltransferase (FTase) (GGTase, and FTase) to facilitate the attachment of the protein to cell membranes. Rho proteins can now assume two different conformational statuses: 1) active GTP-bound and 2) inactive GDP-bound. Guanine nucleotide exchange factors (GEF) can facilitate GTPase activation while GTPase-activating proteins (GAP) are responsible for GTPase activity inactivation. When activated, Rho activates several kinases, which can trigger the activation of IKK complex. IKK helps facilitate nuclear factor (NF)-κB translocation into the nucleus, which automatically leads to the inflammatory production of protein (Hothersall, 2008). There is proof that Rac and Rho inactivation can reduce the migration of NF-κB into the nucleus as well as the subsequent gene transcription and DNA binding that causes inflammatory mediators. This explains the ATO’s ability to facilitate a decrease in isoprenoid intermediates while inhibiting the MVA pathway. Therefore, ATO is able to down-regulator by inhibiting the mevalonate pathway, leading to reduced oxidation and inflammation (Hothersall, 2008, Li et al., 2010, Marin et al., 2011).
4.1. Anti-inflammatory activity of atorvastatin in lung diseases
External environmental pollutants like dust combustibles, diesel particles, and cigarette smoke, are a constant source of irritation for the respiratory system. The result of long-term exposure to any of those environmental pollutants is lung inflammation (Pryor et al., 1983). Exposure to any of these irritants, which may not affect healthy people, can lead to direct activation of the macrophages and epithelial cells. These irritants can also activate certain inflammatory cells like neutrophils, monocytes, lymphocytes, and eosinophils (MacNee, 2001), which protect the body from certain infections and tumour necrosis factor-a (TNF-a) (Brennan et al., 1995, Drost et al., 1992, Rahman and MacNee, 2000) and cancer-related pro-inflammatory cytokines (interleukins (IL)-1, -6, -8, and -1ß). The cytokine further releases inflammatory cytokines by activating NF-KB responses and producing protein-1 (AP-1) (Hemmeti et al., 2016; Luqing Wei, Liu, Zhenhua, and Guo, 2009) and matrix-metalloprotease (MMPs) to create an amplifying loop. This can trigger a torrent of uncontrolled inflammation which further establishes the pathogenesis that causes lung diseases like asthma, lung cancer, COPD and cystic fibrosis (Chung and Adcock, 2008, Milara and Cortijo, 2012).
In 2013, Ching-Feng Huang et al., studied the anti-inflammatory potential of ATO for the treatment of allergic asthma (AA). For the study, intragastric gavage was used to administer ATO to mice, after which ovalbumin was used for aerosol inhalation to analyse its effects on the bronchoalveolar lavage fluid (BALF) inflammatory cell (Huang et al., 2013). Thompson et al., evaluated the effects of placebo versus ATO when combined with inhaled beclomethasone by smokers with asthma. The study revealed a reduction in the inflammatory mediator’s sputum concentration along with chemokine ligand 7 (CCL7), MMP-8 while transforming growth factor-alpha (TGF) (Thomson et al., 2015). Apart from its ability to inhibit TH1 inflammatory responses, oral ATO administration had some therapeutic effects on TH2 allergic responses and airway hyperresponsiveness. These data indicate that ATO drugs can be used as non-immunosuppressives for treating asthma and other allergic diseases (Huang et al., 2013). Furthermore, Blanquiceth et al., investigated the effects of the intraperitoneal administration of ATO on murine acute allergic asthma. The investigation revealed a reduction in peri-bronchial inflammation, which had a negative correlation with the Regulatory T cells found in the lymph nodes as well as the IL-10 concentration in the lungs with the administration of ATO intraperitoneally. Remarkably, the most obvious impact of ATO was on regulatory T cells, which had reduced after ATO treatment. Regulatory T cells play an essential role in controlling the level of inflammation in allergic patients (Blanquiceth et al., 2016). The murine model of the chronic asthma study investigated the effects of intraperitoneally injected ATO versus dexametazon. The researchers discovered that ATO had beneficial effects on lung histological changes in the model, as compared to dexametazon. Additionally, IL-5 and -4 levels in lung tissues were significantly lower in the ATO group as compared to the placebo. These data confirm the beneficial impact of ATO on changes to the histology of the lung in a chronic murine model of asthma. Similar findings have been discovered by Fırıncı et al., which indicates the capability of ATO to inhibit IL-4 and -5 levels in the lung tissue of the murine model of chronic asthma (Fırıncı et al., 2014).
In addition, lung injury research has indicated the ability of ATO in the treatment. Siempos et al., found that oral ATO was capable of improving alveolar-capillary permeability and haemodynamic parameters in a rabbit induced lung injury model by reducing the ultrafiltration coefficient marker, lowering the protein concentration in BALF, and lowering the increase in mean pulmonary artery pressure (Siempos et al., 2010). Similarly, in another study using a male mice model with inflammatory acute lung injury, ATO was found to modulate inflammation via a reduction in TNF-α, redox markers (superoxide dismutase and catalase), and lipid peroxidation agents (malondialdehyde and hydroperoxides) that play a key role in acute lung injury development (Melo et al., 2013). Furthermore, Ferreira et al., examined the impacts of ATO treatment after CS was simulated (12 cigarettes/day) in mice for five days. It was discovered that lung inflammation can be improved through the prevention of the recruitment of lung leukocytes (mononuclear [MN]), resembling lung histoarchitecture into the lung after ATO treatment. Moreover, ATO can increase monocyte chemoattractant protein-1, resulting in MN reduction. This occurrence may be due to a systemic action by ATO in reducing not only C-C chemokine receptor type 2 expression but also expressions of adhesion molecules, such as vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, thereby preventing migration of the monocyte to the inflammation site (Ferreira et al., 2014). Similar findings were discovered after a group of mice was treated with ATO in conjunction with imipenem after sepsis was stimulated by puncture and caecal ligation. The data indicate that ATO causes a decrease in pro-inflammatory cytokine (TNFα and IL-1β) levels and lung bacterial load in BALF in lung injury (Choudhury et al., 2015).
The ability of ATO as a potential therapy for COPD and pulmonary emphysema was also investigated. Patients with COPD were studied by Mroz et al., following the administration of 40 mg/day of oral ATO. They discovered that oral ATO was able to reduce serum hs-hs-C-reactive protein (CRP) and the influx of inflammatory cells (leukocytes, CD45+ cells, sputum neutrophils). The ATO treatment also led to the downregulation of some of the key genes responsible for leukocyte activation, immune responses, and inflammatory processes. This data has demonstrated the pulmonary-connected anti-inflammatory power of ATO in patients suffering from COPD, with the potential for additional clinical effects (Mroz et al., 2015). ATO effectiveness for pulmonary emphysema was demonstrated not only in animals but also in in vitro experiments.
4.2. Antioxidant activity of atorvastatin in lung diseases
Nitric oxide synthase (NOS) is the enzyme family responsible for the production of nitric oxide (NO). NOS employs arginine and oxygen to stimulate the reaction which forms nitric oxide. NOS comes in three forms: 1) endothelial NOS; 2) neuronal NOS; and 3) inducible NOS (iNOS). Some of the physiological functions of nitric oxide include muscle relaxation, immune modulation, and neuronal activity (Grisham et al., 1999, Liu et al., 2002). As a free radical, nitric oxide can lead to reactive nitrogen species (RNS) and reactive oxygen species (ROS), like superoxide anion and hydroxyl radicals, which can cause oxidative stress to the lungs. When lungs airways are exposed to oxidants and which can be generated either exogenously or endogenously. Furthermore, NOS antioxidant properties include its ability to eliminate oxygen free radicals to prevent reactive oxidant radicals from affecting the cells. However, the overproduction of these RNS and ROS could lead to RNA, DNA, and lipid oxidation as well as protein damage, which can cause lung injury and tissue damage (MacNee, 2001). There is evidence that ATO decreases low- and high-density lipoproteins including very-low-density lipoprotein (VLDL) oxidation through metal ion chelation capacities and free radical scavenging (Aviram et al., 1998), which might be connected to the MVA pathway inhibition, small GTP-binding protein Rac1, and nicotinamide adenine dinucleotide phosphate (NADPH) inactivation (Chen et al., 2012b, Takemoto et al., 2001).
In vitro and in vivo studies have revealed that ATO can improve the expression of vascular endothelial cell growth factor (VEGF) in non-small cell lung cancer (NCSCL). These events are mediated by ATO’s antioxidant effects via MVA pathway inducement; then isoprenoid synthases and the NADPH oxidase system deactivate at a cellular level. These processes caused a significant upregulation in CAT activity and GPx, leading to VEGF expression inhibition and a decrease in the generation of ROS (Chen et al., 2012b, Takemoto et al., 2001). It was discovered in rat pulmonary artery endothelial cells that eNOS expression was restored after ATO treatment. This study revealed the potential effects of ATO treatment on monocrotaline-stimulated pulmonary hypertension (Rakotoniaina et al., 2006). Another study found that ATO caused a decrease in bleomycin-induced pulmonary fibrosis in rats by reducing oxidative stress markers and suppressing the inflammatory marker (Ali et al., 2018, Hemmeti et al., 2016, Wei et al., 2011, Wei et al., 2009). In a similar study, ATO had a preventive effect on paraquat-induced pulmonary fibrosis in rats (Khodayar et al., 2014).
4.3. Anti-proliferative activity of atorvastatin in lung diseases
Because of its antiproliferative effects, ATO, as an agent for the treatment of lung cancer has been investigated in various studies (Chen et al., 2013, Chen et al., 2012a, Chen et al., 2012b, Lu et al., 2008). Using A549 cell line, Fan et al., studied the effects of ATO on the treatment of human lung cancer by suppressing cell migration, the epithelial-to-mesenchymal transition process, and actin filament remodelling, and protein sphingosine kinase 1 (SphK1) induced by TGF-β1 (Fan et al., 2016).
In vivo and in vitro studies revealed that when carboplatin is combined with ATO, the expression of RAS protein and EGFR decreases. These two events resulted in proliferation inhibition and an increase in apoptosis (Chen et al., 2012a). In a dose-dependent manner, ATO exerted a significant chemo-preventive effect against lung cancer in COPD patients (Liu et al., 2016). Many studies by Chen et al., have demonstrated that ATO combined with carboplatin or ATO alone is an effective strategy against NSCLCs (Chen et al., 2013, Chen et al., 2012a, Chen et al., 2012b). In a lung study using a mouse model, ATO in combination with polyphenon E caused a decrease in tumour multiplicity and tumour burden lung destruction. It also caused a reduction in tumourigenesis and the growth of lung cancer in H1299 and H460 cells (Lu et al., 2008).
Moreover, it was discovered that ATO can influence tumours, apoptosis, and cell proliferation by inhibiting expressions of the inflammatory marker in smooth muscle cell proliferation of rats (Wang et al., 2016) and human turbinates (Folli et al., 2008), ATO was able to regulate inflammatory gene expression (Folli et al., 2008) by inactivating the RhoA/Rho kinase pathways (Chen et al., 2012a), suppressing protein kinase B (AKT) activity and upregulating the matrix metalloprotein inhibitor, TIMP-1 (Folli et al., 2008). These outcomes were supported by Ghavami et al., after the proliferation of fibroblasts reduced upon treatment with ATO (1 µM); fibroblasts were obtained from healthy turbinates in cultured cells in vitro. The anti-proliferation activity of ATO might help in the treatment of chronic inflammatory respiratory disease (Grisham et al., 1999, Liu et al., 2002).
ATO has also been found to be effective on tuberous sclerosis (TSC). A study by Atochina-Vasserman et al., revealed that cell growth in mouse TSC 2-null was inhibited after ATO treatment (5–10 μM). Additionally, after 10 μM of ATO, a few cellular morphological changes, including TSC2-null cell rounding, were observed. In addition, when rapamycin was combined with ATO, ATP produced an additive growth-inhibitory effect on TSC2-null cells. This finding confirmed the effectiveness of ATO with respect to TSC2-null cell survival in TS (Atochina-Vasserman et al., 2013). A study by Yildirim et al., evaluated the anti-fibrotic effects of ATO in lung fibroblasts and myofibroblasts. The intraperitoneal administration of ATO (20 mg/kg) was evaluated in mice with pulmonary fibrosis for 10 days. The administration of ATO decreased the fibrotic foci, α-smooth muscle actin, lysyl oxidase homolog 2 precursor, and p-sarcoma inducing kinase proteins in the lung. This study revealed that ATO’s anti-fibrotic effect is indicative of fibroblast activation through the inhibition of the differentiation of fibroblasts into myofibroblasts (Yildirim et al., 2018). A similar study revealed that ATO has anti-tumoural properties in NSCLC utilizing cell culture systems and in vivo models. They revealed that the ATO-induced inhibition of oncogenic Cav1 in GLUT3-mediated glucose uptake highlights the potential effects of statins to avoid NSCLC with EGFR-TKI resistance (Ali et al., 2019).
5. Effectiveness of inhaled atorvastatin formulation
From the foregoing, it is obvious that ATO has the potential to treat lung diseases. Therefore, the problems associated with intravenous or oral delivery can be addressed through inhaled administration of ATO to the airways. The evidence of ATO inhaled formulation efficacy is detailed below. The ability of inhaled (intranasal) ATO and curcumin treatment using a MicroSprayer® Aerosolizer attached to a high‐pressure syringe model FMJ‐250 was assessed at three concentration levels (QC1, 2, and 3) in mice lung tissues. ATO intranasal formulation was prepared as ultra‐small lipid nanoparticles by high‐shear homogenization and ultrasonication, containing 5% (w/w) of ATO and 2.5% (w/w) of CUR. Both compounds were dissolved in the lipid molten phase, added to 30 mL of a hot aqueous surfactant solution of 5% (w/v) Tween® 80 and emulsified for 1 min at 24 000 rpm. This information provides a useful tool for supporting the nonclinical biodistribution studies of ATO and curcumin in mice (Silva et al., 2019).
Furthermore, Ribeiro et al., investigated the effects of inhaled ATO (15 min with 1 mg/mL) when administered once a day in a mice model of CS-induced pulmonary emphysema. Interestingly, the most noticeable effect of inhaled ATO was a reduction in ROS formation via a reduction in NADPH oxidase activity that led to a decrease in hydrogen peroxide activity even though superoxide dismutase activity was not transformed. Subsequently, there was also a decrease in catalase enzyme activity (Pinho-Ribeiro et al., 2017). Melo et al., investigated the effect of inhaled administration of ATO on the development of elastase-induced pulmonary emphysema in mice by using porcine pancreatic elastase intranasally. Data suggested that inhaled administration of ATO inhibited the improvement of elastase-induced pulmonary emphysema in mice by recovery the collagen, elastic fibres, and morphology of the pulmonary cells. This inhaled therapeutic effect of ATO was due to reduced nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and MMP-12 (Melo et al., 2018). The efficacy of 1, 5, and 20 mg inhaled and ATO intranasal treatment in mice was also assessed after stimulation with porcine pancreatic elastase (PPE) for up to 64 days. Lung histology and total airway inflammation markers were assessed in BALF. It was discovered that leukocytes in BALF were reduced after ATO treatment (1, 5, and 20 mg) as compared to the PPE group. The number of tissue macrophages in BALF decreased only after 20 mg of ATO. Additionally, the decrease of tissue neutrophil numbers in BALF fell in the A5 mg and A20 mg groups of ATO as compared to the porcine pancreatic elastase group. Finally, the administration of 5 and 20 mg of ATO produced a reduction in Nrf2 and MMP-12 as compared to the PPE group. These data confirm that the intranasal administration of ATO could induce lung tissue repair in emphysematous mice (Melo et al., 2018). These studies did not explore the mechanisms involved, but they did provide some insight into what distinguishes the different types of ATO that could guide therapy in the treatment of chronic lung diseases. The lack of information on the inhaled ATO formulation is a major limitation in these studies.
6. Conclusions
The risk of chronic lung diseases continues to increase with every passing year despite all the therapeutic advancements that have been made in the pharmaceutical industry. There are too many shortcomings in the treatment methods currently available, which is why there is a need for alternative or new treatment methods with better efficacies to combat these diseases.
Atorvastatin (ATO) is one of the drugs from the statin class that can be used as an oral anti-cholesterol drug. A recent discovery points to the fact that ATO is made up of other protective pharmacological properties that have no connections whatsoever to its anti-cholesterol properties, which could serve as a treatment for chronic airway diseases. This explains why the manipulation of ATO molecules that suit different indications, formulation properties, and routes of administration is a realistic solution. There are several benefits to be derived from this manipulation which is considered a better alternative to a brand-new drug molecule. These benefits include: 1) it lowers the cost of manufacturing; 2) it lowers systematic drug concentration after ATO inhalation compared to what is obtainable in oral ATO doses; and 3) it offers a well-established commercial scale of manufacturing, including quality control of active pharmaceutical ingredients. (Apart from the knowledge about systematic metabolism, distribution, safety and pharmacokinetics that it provides, ATO can significantly reduce product development costs, risks, and timelines for investigating drug interactions and systemic toxicity, using new chemical entities.); and 4) the pharmaceutical ingredient properties are active and well-documented.
This review highlights the capabilities that make ATO an effective therapy for treating chronic lung diseases. Research has shown that statins possess more pleiotropic effects than lipid-lowering activities through the modulation of the different signalling pathways governing inflammatory, proliferation, and oxidative stress. Though the protective role of ATO in the treatment of these chronic lung diseases is quite clear, there is still a long way to go when it comes to research on how to use oral statins for clinical trials. The discrepancies can be attributed to the particular ATO delivery routes employed in the trial process, such as inhaled versus oral, which has been considered the limiting factor in the direct assessment of ATO’s beneficial effects as a potential agent for anti-inflammatory treatment. At present, no clinical studies detail the effects of using inhaled ATO. In addition, most of the studies on the reformulation of ATO as a form of inhaled therapy are in their infancy, which means there is a need for further investigations to understand the efficacy, mechanism, and toxicity of ATO actions for the airways. However, there has been an array of in vivo and in vitro studies on ATO’s protective effects and how ATO affects the treatment procedure for chronic inflammatory lung diseases, which is a great start.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The author would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code 19-MED-1-03-0003.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aggarwal S., Gupta G., Chaudhary S. Solubility and dissolution enhancement of poorly aqueous soluble drug atorvastatin calcium using modified gum karaya as carrier: In vitro-In vivo evaluation. Int. J. Drug Deliv. 2012;4(3):341. [Google Scholar]
- Ali A., Levantini E., Fhu C.W., Teo J.T., Clohessy J.G., Goggi J.L. CAV1-GLUT3 signaling is important for cellular energy and can be targeted by Atorvastatin in Non-Small Cell Lung Cancer. Theranostics. 2019;9(21):6157. doi: 10.7150/thno.35805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali E.A., El Sayed M., Daba M.-H.Y., Yassin A.E.A., Badr E.A. The effect of atorvastatin on bleomycin-induced pulmonary fibrosis in rats. Menoufia Med. J. 2018;31(3):1081. [Google Scholar]
- ALKharfy K.M., Kellum J.A., Matzke G.R. Unintended immunomodulation: part II. Effects of pharmacological agents on cytokine activity. Shock. 2000;13(5):346–360. doi: 10.1097/00024382-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Amarenco P. Atorvastatin in prevention of stroke and transient ischaemic attack. Expert Opin. Pharmacother. 2007;8(16):2789–2797. doi: 10.1517/14656566.8.16.2789. [DOI] [PubMed] [Google Scholar]
- Anttila S., Hukkanen J., Hakkola J., Stjernvall T., Beaune P., Edwards R.J. Expression and localization of CYP3A4 and CYP3A5 in human lung. Am. J. Respir. Cell Mol. Biol. 1997;16(3):242–249. doi: 10.1165/ajrcmb.16.3.9070608. [DOI] [PubMed] [Google Scholar]
- Armitage J. The safety of statins in clinical practice. The Lancet. 2007;370(9601):1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- Athanazio R. Airway disease: similarities and differences between asthma, COPD and bronchiectasis. Clinics. 2012;67(11):1335–1343. doi: 10.6061/clinics/2012(11)19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atochina-Vasserman E.N., Goncharov D.A., Volgina A.V., Milavec M., James M.L., Krymskaya V.P. Statins in Lymphangioleiomyomatosis. Simvastatin and atorvastatin induce differential effects on tuberous sclerosis complex 2–null cell growth and signaling. Am. J. Respir. Cell Mol. Biol. 2013;49(5):704–709. doi: 10.1165/rcmb.2013-0203RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram M., Rosenblat M., Bisgaier C.L., Newton R.S. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis. 1998;138(2):271–280. doi: 10.1016/s0021-9150(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Barker A.F. Bronchiectasis. N. Engl. J. Med. 2002;346(18):1383–1393. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. Future treatments for chronic obstructive pulmonary disease and its comorbidities. Proc. Am. Thoracic Soc. 2008;5(8):857–864. doi: 10.1513/pats.200807-069TH. [DOI] [PubMed] [Google Scholar]
- Barnes P.J., Ito K., Adcock I.M. Corticosteroid resistance in chronic obstructive pulmonary disease: inactivation of histone deacetylase. The Lancet. 2004;363(9410):731–733. doi: 10.1016/S0140-6736(04)15650-X. [DOI] [PubMed] [Google Scholar]
- Bernini F., Poli A., Paoletti R. Safety of HMG-CoA reductase inhibitors: focus on atorvastatin. Cardiovasc. Drugs Ther. 2001;15(3):211–218. doi: 10.1023/a:1011908004965. [DOI] [PubMed] [Google Scholar]
- Blamoun A., Batty G., DeBari V., Rashid A., Sheikh M., Khan M. Statins may reduce episodes of exacerbation and the requirement for intubation in patients with COPD: evidence from a retrospective cohort study. Int. J. Clin. Pract. 2008;62(9):1373–1378. doi: 10.1111/j.1742-1241.2008.01731.x. [DOI] [PubMed] [Google Scholar]
- Blanquiceth Y., Rodríguez-Perea A.L., Tabares Guevara J.H., Correa L.A., Sánchez M.D., Ramírez-Pineda J.R., Velilla P.A. Increase of frequency and modulation of phenotype of regulatory T cells by atorvastatin is associated with decreased lung inflammatory cell infiltration in a murine model of acute allergic asthma. Front. Immunol. 2016;7:620. doi: 10.3389/fimmu.2016.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquillon C. Drug transporters in the lung—do they play a role in the biopharmaceutics of inhaled drugs? J. Pharm. Sci. 2010;99(5):2240–2255. doi: 10.1002/jps.21995. [DOI] [PubMed] [Google Scholar]
- Bradbury P., Traini D., Ammit A.J., Young P.M., Ong H.X. Repurposing of statins via inhalation to treat lung inflammatory conditions. Adv. Drug Deliv. Rev. 2018;133:93–106. doi: 10.1016/j.addr.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Braganza G., Chaudhuri R., McSharry C., Weir C.J., Donnelly I., Jolly L. Effects of short-term treatment with atorvastatin in smokers with asthma-a randomized controlled trial. BMC Pulmonary Med. 2011;11(1):16. doi: 10.1186/1471-2466-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F., Maini R., Feldmann M. Cytokine expression in chronic inflammatory disease. Br. Med. Bull. 1995;51(2):368–384. doi: 10.1093/oxfordjournals.bmb.a072967. [DOI] [PubMed] [Google Scholar]
- Castaño G., Mas R., Fernández L., Illnait J., Mesa M., Alvarez E., Lezcay M. Comparison of the efficacy and tolerability of policosanol with atorvastatin in elderly patients with type II hypercholesterolaemia. Drugs Aging. 2003;20(2):153–163. doi: 10.2165/00002512-200320020-00006. [DOI] [PubMed] [Google Scholar]
- Chen J., Bi H., Hou J., Zhang X., Zhang C., Yue L. Atorvastatin overcomes gefitinib resistance in KRAS mutant human non-small cell lung carcinoma cells. Cell Death Dis. 2013;4(9) doi: 10.1038/cddis.2013.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lan T., Hou J., Zhang J., An Y., Tie L. Atorvastatin sensitizes human non-small cell lung carcinomas to carboplatin via suppression of AKT activation and upregulation of TIMP-1. Int. J. Biochem. Cell Biol. 2012;44(5):759–769. doi: 10.1016/j.biocel.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Chen J., Liu B., Yuan J., Yang J., Zhang J., An Y. Atorvastatin reduces vascular endothelial growth factor (VEGF) expression in human non-small cell lung carcinomas (NSCLCs) via inhibition of reactive oxygen species (ROS) production. Mol. Oncol. 2012;6(1):62–72. doi: 10.1016/j.molonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary A., Rana A.C., Aggarwal G., Kumar V., Zakir F. Development and characterization of an atorvastatin solid dispersion formulation using skimmed milk for improved oral bioavailability. Acta Pharm. Sin. B. 2012;2(4):421–428. [Google Scholar]
- Choudhury S., Kandasamy K., Maruti B.S., Addison M.P., Kasa J.K., Darzi S.A. Atorvastatin along with imipenem attenuates acute lung injury in sepsis through decrease in inflammatory mediators and bacterial load. Eur. J. Pharmacol. 2015;765:447–456. doi: 10.1016/j.ejphar.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Chung K., Adcock I. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur. Respir. J. 2008;31(6):1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- Collaboration, C.T.T., 2015. Efficacy and safety of LDL‐lowering therapy among men and women: meta‐analysis of individual data from 174,000 participants in 27 randomised trials. [DOI] [PubMed]
- Corsini A., Bellosta S., Baetta R., Fumagalli R., Paoletti R., Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol. Ther. 1999;84(3):413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Anti-inflammatory agents: present and future. Cell. 2010;140(6):935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost, E., Selby, C., Lannan, S., Lowe, G., MacNee, W., 1992. Changes in neutrophil deformability following in vitro smoke exposure: mechanism and protection. [DOI] [PubMed]
- Emruzi Z., Babaheidarian P., Arshad M., Stockinger H., Ahangari G. Immune modulatory effects of hypercholesterolemia: can atorvastatin convert the detrimental effect of hypercholesterolemia on the immune system? Iran. J. Allergy Asthma Immunol. 2018:1–13. doi: 10.18502/ijaai.v18i5.1925. [DOI] [PubMed] [Google Scholar]
- Endo A. Paper presented at the International Congress Series. 2004. The origin of the statins. [Google Scholar]
- Endo A., Kuroda M., Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogensis produced by Penicillium citrinum. J. Antibiotics. 1976;29(12):1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- Endo A., Tsujita Y., Kuroda M., Tanzawa K. Inhibition of Cholesterol Synthesis in vitro and in vivo by ML-236A and ML-236B, Competitive Inhibitors of 3-Hydroxy-3-methylglutaryl-Coenzyme A Reductase. Eur. J. Biochem. 1977;77(1):31–36. doi: 10.1111/j.1432-1033.1977.tb11637.x. [DOI] [PubMed] [Google Scholar]
- Fahimi F., Salamzadeh J., Jamaati H., Sohrabi S., Fakharian A., Mohammadtaheri Z. Do statins improve lung function in asthmatic patients? A randomized and double-blind trial. Iran. J. Pharm. Sci. 2009;5(1):13–20. [Google Scholar]
- Fan Z., Jiang H., Wang Z., Qu J. Atorvastatin partially inhibits the epithelial-mesenchymal transition in A549 cells induced by TGF-β1 by attenuating the upregulation of SphK1. Oncol. Rep. 2016;36(2):1016–1022. doi: 10.3892/or.2016.4897. [DOI] [PubMed] [Google Scholar]
- Ferreira T.S., Lanzetti M., Barroso M.V., Rueff-Barroso C.R., Benjamim C.F., de Brito-Gitirana L. Oxidative stress and inflammation are differentially affected by atorvastatin, pravastatin, rosuvastatin, and simvastatin on lungs from mice exposed to cigarette smoke. Inflammation. 2014;37(5):1355–1365. doi: 10.1007/s10753-014-9860-y. [DOI] [PubMed] [Google Scholar]
- Fırıncı F., Karaman M., Cilaker-Mıcılı S., Bagrıyanık A., Uzuner N., Karaman Ö. The effect of atorvastatin on lung histopathology in a murine model of chronic asthma. Allergol. Immunopathol. 2014;42(4):355–361. doi: 10.1016/j.aller.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Fogari R., Derosa G., Lazzari P., Zoppi A., Fogari E., Rinaldi A., Mugellini A. Effect of amlodipine–atorvastatin combination on fibrinolysis in hypertensive hypercholesterolemic patients with insulin resistance. Am. J. Hypertens. 2004;17(9):823–827. doi: 10.1016/j.amjhyper.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Folli C., Descalzi D., Bertolini S., Riccio A.M., Scordamaglia F., Gamalero C., Canonica G.W. Effect of statins on fibroblasts from human nasal polyps and turbinates. Eur. Ann. Allergy.. Clin. Immunol. 2008;40(3):84. [PubMed] [Google Scholar]
- Gazzerro P., Proto M.C., Gangemi G., Malfitano A.M., Ciaglia E., Pisanti S. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol. Rev. 2012;64(1):102–146. doi: 10.1124/pr.111.004994. [DOI] [PubMed] [Google Scholar]
- Gee M., Hasson N.K., Hahn T., Ryono R. Effects of a tablet-splitting program in patients taking HMG-CoA reductase inhibitors: analysis of clinical effects, patient satisfaction, compliance, and cost avoidance. J. Managed Care Pharmacy. 2002;8(6):453–458. doi: 10.18553/jmcp.2002.8.6.453. [DOI] [PubMed] [Google Scholar]
- Ghobadi H., Lari S.M., Pourfarzi F., Mahmoudpour A., Ghanei M. The effects of atorvastatin on mustard-gas-exposed patients with chronic obstructive pulmonary disease: a randomized controlled trial. J. Res. Med. Sci.: Off. J. Isfahan Univ. Med. Sci. 2014;19(2):99. [PMC free article] [PubMed] [Google Scholar]
- Grisham M.B., Jourd’Heuil D., Wink D.A. I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am. J. Physiol.-Gastrointestinal Liver Physiol. 1999;276(2):G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- Grommes J., Vijayan S., Drechsler M., Hartwig H., Morgelin M., Dembinski R. Simvastatin reduces endotoxin-induced acute lung injury by decreasing neutrophil recruitment and radical formation. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra S., Sherrill D.L., Venker C., Ceccato C.M., Halonen M., Martinez F.D. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax. 2009;64(10):894–900. doi: 10.1136/thx.2008.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guideline, I.H.T., 2003. Stability testing of new drug substances and products. Q1A (R2), current step, 4.
- Gullestad L., Aukrust P., Ueland T., Espevik T., Yee G., Vagelos R. Effect of high-versus low-dose angiotensin converting enzyme inhibition on cytokine levels in chronic heart failure. J. Am. Coll. Cardiol. 1999;34(7):2061–2067. doi: 10.1016/s0735-1097(99)00495-7. [DOI] [PubMed] [Google Scholar]
- Hemmeti A.A., Zerafatfard M.R., Goudarzi M., Khodayar M.J., Rezaie A., Nooshabadi M.R.R., Kiani M. Ameliorative effects of atorvastatin on bleomycin-induced pulmonary fibrosis in rats. Jundishapur J. Nat. Pharm. Prod. 2016;11(4) [Google Scholar]
- Hermann M., Bogsrud M.P., Molden E., Åsberg A., Mohebi B.U., Ose L., Retterstøl K. Exposure of atorvastatin is unchanged but lactone and acid metabolites are increased several-fold in patients with atorvastatin-induced myopathy. Clin. Pharmacol. Ther. 2006;79(6):532–539. doi: 10.1016/j.clpt.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Hoffart E., Ghebreghiorghis L., Nussler A., Thasler W., Weiss T., Schwab M., Burk O. Effects of atorvastatin metabolites on induction of drug-metabolizing enzymes and membrane transporters through human pregnane X receptor. Br. J. Pharmacol. 2012;165(5):1595–1608. doi: 10.1111/j.1476-5381.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman W., Alberts A., Anderson P., Chen J., Smith R., Willard A. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 4. Side-chain ester derivatives of mevinolin. J. Med. Chem. 1986;29(5):849–852. doi: 10.1021/jm00155a040. [DOI] [PubMed] [Google Scholar]
- Hothersall E., McSharry C., Thomson N. Potential therapeutic role for statins in respiratory disease. Thorax. 2006;61(8):729–734. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothersall, E.J., 2008. Effect of atorvastatin on asthma control and airway inflammation: a randomised controlled trial. University of Glasgow, Retrieved from http://europepmc.org/theses/ETH/495335 EThOS database.
- Hothersall E.J., Chaudhuri R., McSharry C., Donnelly I., Lafferty J., McMahon A.D. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. 2008;63(12):1070–1075. doi: 10.1136/thx.2008.100198. [DOI] [PubMed] [Google Scholar]
- Hovenberg H., Davies J., Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem. J. 1996;318:319–324. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-F., Peng H.-J., Wu C.-C., Lo W.-T., Shih Y.-L., Wu T.-C. Effect of oral administration with pravastatin and atorvastatin on airway hyperresponsiveness and allergic reactions in asthmatic mice. Ann. Allergy Asthma Immunol. 2013;110(1):11–17. doi: 10.1016/j.anai.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Irngartinger M., Camuglia V., Damm M., Goede J., Frijlink H. Pulmonary delivery of therapeutic peptides via dry powder inhalation: effects of micronisation and manufacturing. Eur. J. Pharm. Biopharm. 2004;58(1):7–14. doi: 10.1016/j.ejpb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Ishigami M., Honda T., Takasaki W., Ikeda T., Komai T., Ito K., Sugiyama Y. A comparison of the effects of 3-hydroxy-3-methylglutaryl-coenzyme a (HMG-CoA) reductase inhibitors on the CYP3A4-dependent oxidation of mexazolam in vitro. Drug Metab. Dispos. 2001;29(3):282–288. [PubMed] [Google Scholar]
- Jacobsen W., Kuhn B., Soldner A., Kirchner G., Sewing K.-F., Kollman P.A. Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab. Dispos. 2000;28(11):1369–1378. [PubMed] [Google Scholar]
- James A., Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur. Respir. J. 2007;30(1):134–155. doi: 10.1183/09031936.00146905. [DOI] [PubMed] [Google Scholar]
- Jialal I., Stein D., Balis D., Grundy S., Adams-Huet B., Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103(15):1933–1935. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- Keddissi J.I., Younis W.G., Chbeir E.A., Daher N.N., Dernaika T.A., Kinasewitz G.T. The use of statins and lung function in current and former smokers. Chest. 2007;132(6):1764–1771. doi: 10.1378/chest.07-0298. [DOI] [PubMed] [Google Scholar]
- Khan F.N., Dehghan M.H.G. Enhanced bioavailability of atorvastatin calcium from stabilized gastric resident formulation. AAPS PharmSciTech. 2011;12(4):1077–1086. doi: 10.1208/s12249-011-9673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodayar M.J., Kiani M., Hemmati A.A., Rezaie A., Zerafatfard M.R., Nooshabadi M.R.R., Goudarzi M. The preventive effect of atorvastatin on paraquat-induced pulmonary fibrosis in the rats. Adv. Pharm. Bull. 2014;4(4):345. doi: 10.5681/apb.2014.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-S., Kim M.-S., Park H.J., Jin S.-J., Lee S., Hwang S.-J. Physicochemical properties and oral bioavailability of amorphous atorvastatin hemi-calcium using spray-drying and SAS process. Int. J. Pharm. 2008;359(1–2):211–219. doi: 10.1016/j.ijpharm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Kim W.D. Lung mucus: a clinician's view. Eur. Respir. J. 1997;10(8):1914–1917. doi: 10.1183/09031936.97.10081914. [DOI] [PubMed] [Google Scholar]
- Kirkham S., Sheehan J., Knight D., Richardson P., Thornton D. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommanaboyina B., Rhodes C. Trends in stability testing, with emphasis on stability during distribution and storage. Drug Dev. Ind. Pharm. 1999;25(7):857–868. doi: 10.1081/ddc-100102246. [DOI] [PubMed] [Google Scholar]
- Lahousse L., Loth D.W., Joos G.F., Hofman A., Leufkens H.G., Brusselle G.G., Stricker B.H. Statins, systemic inflammation and risk of death in COPD: the Rotterdam study. Pulm. Pharmacol. Ther. 2013;26(2):212–217. doi: 10.1016/j.pupt.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Lau Y.Y., Okochi H., Huang Y., Benet L.Z. Pharmacokinetics of atorvastatin and its hydroxy metabolites in rats and the effects of concomitant rifampicin single doses: relevance of first-pass effect from hepatic uptake transporters, and intestinal and hepatic metabolism. Drug Metab. Dispos. 2006;34(7):1175–1181. doi: 10.1124/dmd.105.009076. [DOI] [PubMed] [Google Scholar]
- Lea A.P., McTavish D. Atorvastatin. Drugs. 1997;53(5):828–847. doi: 10.2165/00003495-199753050-00011. [DOI] [PubMed] [Google Scholar]
- Lee T.J., Holtz W., Smith R., Alberts A., Gilfillan J. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 8. Side chain ether analogs of lovastatin. J. Med. Chem. 1991;34(8):2474–2477. doi: 10.1021/jm00112a024. [DOI] [PubMed] [Google Scholar]
- Lennernäs H. Clinical pharmacokinetics of atorvastatin. Clin. Pharmacokinet. 2003;42(13):1141–1160. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- Li J., Li J.J., He J.G., Nan J.I., Guo Y.I., Xiong C.M. Atorvastatin decreases C-reactive protein-induced inflammatory response in pulmonary artery smooth muscle cells by inhibiting nuclear factor-κB pathway. Cardiovasc. Therap. 2010;28(1):8–14. doi: 10.1111/j.1755-5922.2009.00103.x. [DOI] [PubMed] [Google Scholar]
- Liu B., Gao H.M., Wang J.Y., Jeohn G.H., Cooper C.L., Hong J.S. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann. New York Acad. Sci. 2002;962(1):318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Liu J.-C., Yang T.-Y., Hsu Y.-P., Hao W.-R., Kao P.-F., Sung L.-C. Statins dose-dependently exert a chemopreventive effect against lung cancer in COPD patients: a population-based cohort study. Oncotarget. 2016;7(37):59618. doi: 10.18632/oncotarget.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livraghi A., Randell S.H. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol. Pathol. 2007;35(1):116–129. doi: 10.1080/01926230601060025. [DOI] [PubMed] [Google Scholar]
- Lu G., Xiao H., You H., Lin Y., Jin H., Snagaski B., Yang C.S. Synergistic inhibition of lung tumorigenesis by a combination of green tea polyphenols and atorvastatin. Clin. Cancer Res. 2008;14(15):4981–4988. doi: 10.1158/1078-0432.CCR-07-1860. [DOI] [PubMed] [Google Scholar]
- Lundgren J.D., Shelhamer J.H. Pathogenesis of airway mucus hypersecretion. J. Allergy Clin. Immunol. 1990;85(2):399–417. doi: 10.1016/0091-6749(90)90147-v. [DOI] [PubMed] [Google Scholar]
- MacNee W. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 2001;429(1):195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- Mandal P., Chalmers J.D., Graham C., Harley C., Sidhu M.K., Doherty C. Atorvastatin as a stable treatment in bronchiectasis: a randomised controlled trial. Lancet Respiratory Med. 2014;2(6):455–463. doi: 10.1016/S2213-2600(14)70050-5. [DOI] [PubMed] [Google Scholar]
- Manoj K., Jain N., Madhu S. Myopathy in patients taking atorvastatin: a pilot study. Indian J. Endocrinol. Metab. 2017;21(4):504. doi: 10.4103/ijem.IJEM_79_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin L., Colombo P., Bebawy M., Young P.M., Traini D. Chronic obstructive pulmonary disease: patho-physiology, current methods of treatment and the potential for simvastatin in disease management. Expert Opinion Drug Deliv. 2011;8(9):1205–1220. doi: 10.1517/17425247.2011.588697. [DOI] [PubMed] [Google Scholar]
- Marin L., Traini D., Bebawy M., Colombo P., Buttini F., Haghi M. Multiple dosing of simvastatin inhibits airway mucus production of epithelial cells: implications in the treatment of chronic obstructive airway pathologies. Eur. J. Pharm. Biopharm. 2013;84(3):566–572. doi: 10.1016/j.ejpb.2013.01.021. [DOI] [PubMed] [Google Scholar]
- McAuley D.F., O’Kane C.M., Craig T.R., Shyamsundar M., Herwald H., Dib K. Simvastatin decreases the level of heparin-binding protein in patients with acute lung injury. BMC Pulmonary Med. 2013;13(1):47. doi: 10.1186/1471-2466-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo A.C., Cattani-Cavalieri I., Barroso M.V., Quesnot N., Gitirana L.B., Lanzetti M., Valença S.S. Atorvastatin dose-dependently promotes mouse lung repair after emphysema induced by elastase. Biomed. Pharmacother. 2018;102:160–168. doi: 10.1016/j.biopha.2018.03.067. [DOI] [PubMed] [Google Scholar]
- Melo A.C., Valença S.S., Gitirana L.B., Santos J.C., Ribeiro M.L., Machado M.N. Redox markers and inflammation are differentially affected by atorvastatin, pravastatin or simvastatin administered before endotoxin-induced acute lung injury. Int. Immunopharmacol. 2013;17(1):57–64. doi: 10.1016/j.intimp.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Merx M.W., Weber C. Statins in the intensive care unit. Curr. Opin. Critical Care. 2006;12(4):309–314. doi: 10.1097/01.ccx.0000235207.00322.96. [DOI] [PubMed] [Google Scholar]
- Milara J., Cortijo J. Tobacco, inflammation, and respiratory tract cancer. Curr. Pharm. Des. 2012;18(26):3901–3938. doi: 10.2174/138161212802083743. [DOI] [PubMed] [Google Scholar]
- Moini A., Azimi G., Farivar A. Evaluation of atorvastatin for the treatment of patients with asthma: a double-blind randomized clinical trial. Allergy Asthma Immunol. Res. 2012;4(5):290–294. doi: 10.4168/aair.2012.4.5.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroz R., Lisowski P., Tycinska A., Bierla J., Trzeciak P., Minarowski L. Anti-inflammatory effects of atorvastatin treatment in chronic obstructive pulmonary disease. A controlled pilot study. J. Physiol. Pharmacol. 2015;66(1):111–128. [PubMed] [Google Scholar]
- Murray T.S., Egan M., Kazmierczak B.I. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr. Opin. Pediatr. 2007;19(1):83–88. doi: 10.1097/MOP.0b013e3280123a5d. [DOI] [PubMed] [Google Scholar]
- Nováková L., Šatínský D., Solich P. HPLC methods for the determination of simvastatin and atorvastatin. TrAC, Trends Anal. Chem. 2008;27(4):352–367. [Google Scholar]
- Oliveira M.A., Yoshida M.I., Belinelo V.J., Valotto R.S. Degradation kinetics of atorvastatin under stress conditions and chemical analysis by HPLC. Molecules. 2013;18(2):1447–1456. doi: 10.3390/molecules18021447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-E., Kim K.-B., Bae S., Moon B.-S., Liu K.-H., Shin J.-G. Contribution of cytochrome P450 3A4 and 3A5 to the metabolism of atorvastatin. Xenobiotica. 2008;38(9):1240–1251. doi: 10.1080/00498250802334391. [DOI] [PubMed] [Google Scholar]
- Pathak N.N., Balaganur V., Lingaraju M.C., More A.S., Kant V., Kumar D. Antihyperalgesic and anti-inflammatory effects of atorvastatin in chronic constriction injury-induced neuropathic pain in rats. Inflammation. 2013;36(6):1468–1478. doi: 10.1007/s10753-013-9688-x. [DOI] [PubMed] [Google Scholar]
- Pilcer G., Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010;392(1):1–19. doi: 10.1016/j.ijpharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Pinho-Ribeiro V., Melo A.C., Kennedy-Feitosa E., Graca-Reis A., Barroso M.V., Cattani-Cavalieri I. Atorvastatin and simvastatin promoted mouse lung repair after cigarette smoke-induced emphysema. Inflammation. 2017;40(3):965–979. doi: 10.1007/s10753-017-0541-5. [DOI] [PubMed] [Google Scholar]
- Poli A. Atorvastatin. Drugs. 2007;67(1):3–15. doi: 10.2165/00003495-200767001-00002. [DOI] [PubMed] [Google Scholar]
- Pryor W.A., Prier D.G., Church D.F. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ. Health Perspect. 1983;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I., MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000;16(3):534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- Rakotoniaina Z., Guerard P., Lirussi F., Goirand F., Rochette L., Dumas M., Bardou M. The protective effect of HMG-CoA reductase inhibitors against monocrotaline-induced pulmonary hypertension in the rat might not be a class effect: comparison of pravastatin and atorvastatin. Naunyn-Schmiedeberg's Arch. Pharmacol. 2006;374(3):195–206. doi: 10.1007/s00210-006-0112-z. [DOI] [PubMed] [Google Scholar]
- Reid C.J., Gould S., Harris A. Developmental expression of mucin genes in the human respiratory tract. Am. J. Respir. Cell Mol. Biol. 1997;17(5):592–598. doi: 10.1165/ajrcmb.17.5.2798. [DOI] [PubMed] [Google Scholar]
- Rezaie-Majd A., Maca T., Bucek R.A., Valent P., Muller M.R., Husslein P. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol. 2002;22(7):1194–1199. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- Rodde M.S., Divase G.T., Devkar T.B., Tekade A.R. Solubility and bioavailability enhancement of poorly aqueous soluble atorvastatin: in vitro, ex vivo, and in vivo studies. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/463895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe K., Fukuda N., Fukuda Y., Wakayama K., Nada T., Morishita S. Comparisons of short-and intermediate-term effects of pitavastatin versus atorvastatin on lipid profiles, fibrinolytic parameter, and endothelial function. Int. J. Cardiol. 2008;125(1):136–138. doi: 10.1016/j.ijcard.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Shah D., Bhatt K., Mehta R., Baldania S., Gandhi T. Stability indicating RP-HPLC estimation of atorvastatin calcium and amlodipine besylate in pharmaceutical formulations. Indian J. Pharm. Sci. 2008;70(6):754. doi: 10.4103/0250-474X.49117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsuddin M.F., Ansari S.H., Ali J. Atorvastatin solid dispersion for bioavailability enhancement. J. Adv. Pharm. Technol. Res. 2016;7(1):22. doi: 10.4103/2231-4040.169873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L., Jia S.-S., Jiang H.-M., Wang H., Xu W.-H., Lv C.-J. Simvastatin downregulates expression of TGF-βRII and inhibits proliferation of A549 cells via ERK. Tumor Biol. 2015:1–6. doi: 10.1007/s13277-015-3134-7. [DOI] [PubMed] [Google Scholar]
- Sherikar O., Mehta P. Comprehensive assessment of degradation behavior of aspirin and atorvastatin singly and in combination by using a validated RP-HPLC method. Sci. Pharm. 2012;81(1):195–210. doi: 10.3797/scipharm.1210-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siempos I.I., Maniatis N.A., Kopterides P., Magkou C., Glynos C., Roussos C., Armaganidis A. Pretreatment with atorvastatin attenuates lung injury caused by high-stretch mechanical ventilation in an isolated rabbit lung model. Crit. Care Med. 2010;38(5):1321–1328. doi: 10.1097/CCM.0b013e3181d9dad6. [DOI] [PubMed] [Google Scholar]
- Silva J., Basso J., Sousa J., Fortuna A., Vitorino C. Development and full validation of an HPLC methodology to quantify atorvastatin and curcumin after their intranasal co-delivery to mice. Biomed. Chromatogr. 2019;33(10) doi: 10.1002/bmc.4621. [DOI] [PubMed] [Google Scholar]
- Souza-Costa D.C., Sandrim V.C., Lopes L.F., Gerlach R.F., Rego E.M., Tanus-Santos J.E. Anti-inflammatory effects of atorvastatin: modulation by the T-786C polymorphism in the endothelial nitric oxide synthase gene. Atherosclerosis. 2007;193(2):438–444. doi: 10.1016/j.atherosclerosis.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Sparks D.L., Sabbagh M.N., Connor D.J., Lopez J., Launer L.J., Petanceska S. Atorvastatin therapy lowers circulating cholesterol but not free radical activity in advance of identifiable clinical benefit in the treatment of mild-to-moderate AD. Curr. Alzheimer Res. 2005;2(3):343–353. doi: 10.2174/1567205054367900. [DOI] [PubMed] [Google Scholar]
- Srinivasa Rao K., Prasad T., Mohanta G., Manna P. An overview of statins as hypolipidemic drugs. IJPSDR. 2011;3(3):178–183. [Google Scholar]
- Szilasi M., Dolinay T., Nemes Z., Strausz J. Pathology of chronic obstructive pulmonary disease. Pathol. Oncol. Res. 2006;12(1):52–60. doi: 10.1007/BF02893433. [DOI] [PubMed] [Google Scholar]
- Takemoto M., Node K., Nakagami H., Liao Y., Grimm M., Takemoto Y. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J. Clin. Investig. 2001;108(10):1429–1437. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani S., Mobarrez F., Antovic A., Santesson P., Lins P.-E., Adamson U. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thromb. Res. 2010;126(3):e225–e231. doi: 10.1016/j.thromres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Thomson N.C., Charron C.E., Chaudhuri R., Spears M., Ito K., McSharry C. Atorvastatin in combination with inhaled beclometasone modulates inflammatory sputum mediators in smokers with asthma. Pulm. Pharmacol. Ther. 2015;31:1–8. doi: 10.1016/j.pupt.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Tobert J.A. Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003;2(7):517–526. doi: 10.1038/nrd1112. [DOI] [PubMed] [Google Scholar]
- Tong P., Zografi G. Effects of water vapor absorption on the physical and chemical stability of amorphous sodium indomethacin. AAPS PharmSciTech. 2004;5(2):9–16. doi: 10.1208/pt050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse S.M., Charland S.L., Stanek E., Herrera V., Goldfarb S., Litonjua A.A. Statin use in asthmatics on inhaled corticosteroids is associated with decreased risk of emergency department visits. Curr. Med. Res. Opin. 2014;30(4):685–693. doi: 10.1185/03007995.2013.865599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulbah A.S., Ong H.X., Colombo P., Young P.M., Traini D. Could simvastatin be considered as a potential therapy for chronic lung diseases? A debate on the pros and cons. Expert Opin. Drug Deliv. 2016;13(10):1407–1420. doi: 10.1080/17425247.2016.1193150. [DOI] [PubMed] [Google Scholar]
- Tulbah A.S., Ong H.X., Lee W.-H., Colombo P., Young P.M., Traini D. Biological effects of simvastatin formulated as pMDI on pulmonary epithelial cells. Pharm. Res. 2015:1–10. doi: 10.1007/s11095-015-1766-3. [DOI] [PubMed] [Google Scholar]
- Tulbah A.S., Ong H.X., Morgan L., Colombo P., Young P.M., Traini D. Dry powder formulation of simvastatin. Expert opinion on drug delivery. 2015;12(6):857–868. doi: 10.1517/17425247.2015.963054. [DOI] [PubMed] [Google Scholar]
- Van Linthout S., Riad A., Dhayat N., Spillmann F., Du J., Dhayat S. Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia. 2007;50(9):1977–1986. doi: 10.1007/s00125-007-0719-8. [DOI] [PubMed] [Google Scholar]
- Vaughan C.J., Murphy M.B., Buckley B.M. Statins do more than just lower cholesterol. The Lancet. 1996;348(9034):1079–1082. doi: 10.1016/S0140-6736(96)05190-2. [DOI] [PubMed] [Google Scholar]
- Vukkum P., Babu J., Muralikrishna R. Stress degradation behavior of atorvastatin calcium and development of a suitable stability-indicating LC method for the determination of atorvastatin, its related impurities, and its degradation products. Sci. Pharm. 2012;81(1):93–114. doi: 10.3797/scipharm.1208-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelee H.A., Takimoto C.H., Lopez-Anaya A., Chu Q., Middleton G., Dunlop D. The effect of bexarotene on atorvastatin pharmacokinetics: results from a phase I trial of bexarotene plus chemotherapy in patients with advanced non-small cell lung cancer. Cancer Chemother. Pharmacol. 2012;69(2):563–571. doi: 10.1007/s00280-011-1772-z. [DOI] [PubMed] [Google Scholar]
- Walsh, G.M., 2008. Statins as emerging treatments for asthma and chronic obstructive pulmonary disease. [DOI] [PubMed]
- Wang Q., Guo Y.-Z., Zhang Y.-T., Xue J.-J., Chen Z.-C., Cheng S.-Y. The effects and mechanism of atorvastatin on pulmonary hypertension due to left heart disease. PLoS ONE. 2016;11(7) doi: 10.1371/journal.pone.0157171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B., Liu Y. Clinical significance and efficacy analysis of atorvastatin in the treatment of patients with cerebral infarction and aspiration pneumonia. Exp. Therap. Med. 2018;16(6):5144–5148. doi: 10.3892/etm.2018.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Dong Y., Li Z. Effect of atorvastatin on MMP-9 and TIMP-1 levels in bronchoalveolar lavage fluid and serum of rats with bleomycin-induced pulmonary fibrosis. Zhejiang da xue xue bao Yi xue ban = J. Zhejiang Univ. Med. Sci. 2011;40(1):64–70. doi: 10.3785/j.issn.1008-9292.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Wei L., Liu B., Zhenhua L., Guo W. Atorvastatin alleviates pulmonary fibrosis-induced by bleomycin in rats. Basic Clin. Med. 2009;29(11):1198–1202. [Google Scholar]
- Williams O.W., Sharafkhaneh A., Kim V., Dickey B.F., Evans C.M. Airway mucus: from production to secretion. Am. J. Respir. Cell Mol. Biol. 2006;34(5):527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt T.J., Bloomfield H.E., MacDonald R., Nelson D., Rutks I., Ho M. Effectiveness of statin therapy in adults with coronary heart disease. Arch. Intern. Med. 2004;164(13):1427–1436. doi: 10.1001/archinte.164.13.1427. [DOI] [PubMed] [Google Scholar]
- Yang Y., Tsifansky M.D., Shin S., Lin Q., Yeo Y. Mannitol-Guided delivery of ciprofloxacin in artificial cystic fibrosis mucus model. Biotechnol. Bioeng. 2011;108(6):1441–1449. doi: 10.1002/bit.23046. [DOI] [PubMed] [Google Scholar]
- Yeganeh B., Wiechec E., Ande S.R., Sharma P., Moghadam A.R., Post M. Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease. Pharmacol. Ther. 2014;143(1):87–110. doi: 10.1016/j.pharmthera.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M., Kayalar O., Atahan E., Oztay F. Eur Respiratory Soc. 2018. Anti-fibrotic effect of Atorvastatin on the lung fibroblasts and myofibroblasts. [Google Scholar]
- Yu X., Pan Y., Ma H., Li W. Simvastatin inhibits proliferation and induces apoptosis in human lung cancer cells. Oncol. Res. Featuring Preclinical Clin. Cancer Therap. 2013;20(8):351–357. doi: 10.3727/096504013X13657689382897. [DOI] [PubMed] [Google Scholar]
- Zaheer Z., Farooqui M., Nikalje A.M.A. Stability-indicating high performance liquid chromatographic determination of atorvastatin calcium in pharmaceutical dosage form. African J. Pharm. Pharmacol. 2008;2(10):204–210. [Google Scholar]
- Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154(1):69–75. doi: 10.1111/imm.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki A.A., Oldham J., Wilson M., Fortenko O., Goyal V., Last M. Statin use and asthma control in patients with severe asthma. BMJOpen. 2013;3(8) doi: 10.1136/bmjopen-2013-003314. [DOI] [PMC free article] [PubMed] [Google Scholar]