Abstract
Three factors, three levels (33) full factorial design was used to develop venlafaxine HCl fast dissolving oral films (FDOFs) to optimize the concentrations of the film forming polymer; hydroxypropyl methylcellulose HPMC (X1), superdisintegrant; sodium starch glycolate SSG, (X2) and glycerol as the film plasticizer (X3). Effects of the three factors on the disintegration time (Y1), swelling index (Y2), and dissolution efficiency at 15 min; DE%15 (Y3) of the prepared FDOFs were evaluated by using statistical models. The optimized film formula was characterized in term of x-ray powder diffraction (XRPD), differential scanning calorimetry (DSC) and morphological characteristics.
Disintegration time was found to increase with the increase in HPMC (X1) concentration, and the shortest disintegration time (21.67 ± 2.08 s) was observed in case of F2 formula (lowest HPMC level and highest glycerol level in absence of SSG). The highest swelling index (3.64 ± 0.59) was observed in case of film formula F1 (medium concentrations of both HPMC and glycerol and highest SSG concentration. The results also indicated that as the concentration of HPMC increased the DE%15 decreased. SSG (X2), with highest value (72.33 ± 1.71%) was recorded for in case of F12 (using 2% HPMC, 5%SSG and 1.5% glycerol). The optimized FDOF formula derived by the statistical models suggested 2% HPMC, 5% SSG, and 1% glycerol.
The data obtained from DSC and XRPD revealed no interaction between drug and FDOT excipients. In addition, XRPD studies proved that the venlafaxine HCl was homogeneously dispersed in the film matrix.
Keywords: Venlafaxine HCl, Fast dissolving oral films, Optimization, In vitro dissolution, Disintegration
1. Introduction
Drug delivery technologies adjust drug release profile, absorption, distribution and elimination for the benefit of improving product efficacy and safety, as well as patient convenience and compliance (Bae and Park, 2020). Oral route of administration is considered as the most common route for drug systemic actions owing to its flexibility, ease of use and painlessness, as well as patient compliance (Nyol and Gupta, 2013).
Fast dissolving films are the most advanced solid dosage form in term of their flexibility. The formulation of fast dissolving buccal film is composed of material such as strip-forming polymers, plasticizers, active pharmaceutical ingredient, sweetening agents, saliva stimulating agents, flavoring agents, coloring agents, surfactant, permeation enhancers, and superdisintegrants (Thakur et al, 2013). These dosage forms are beneficial in patients such as pediatric, geriatrics, bedridden, emetic patients, diarrhoea, sudden episode of allergic attacks, or coughing for those who have an active life style. Administration of oral disintegrating dosage forms offers additional advantage for treatment of patients with psychiatric disorders (Thakur et al, 2013).
The delivery system consists of a very thin oral strip, which is placed on the patient’s tongue or any oral mucosal tissue. The strip is instantly wet by saliva and the film rapidly hydrates and disintegrates and dissolves to release the medication for oromucosal absorption, or with formula modifications, will maintain the quick-dissolving aspects that allow for gastrointestinal absorption to be achieved when swallowed (Zhu et al, 2018).
Also, FDOFs are targeted for mentally ill patients, the developmentally disabled patients, and patients who are uncooperative. FDOFs are also useful when local action is desired such as local anesthetic for toothaches, oral ulcers, cold sores or teething (Liew et al, 2013).
Venlafaxine hydrochloride is a structurally antidepressant for oral administration. It is designated 1-[2-(dimethylamino)-1-(4-methoxyphenyl) ethyl] cyclohexanol hydrochloride or (±)-1-[α-[(dimethyl-amino)methyl]-p-methoxybenzyl] cyclohexanol hydrochloride. Venlafaxine hydrochloride solubility in water is 572 mg/mL. Venlafaxine HCl is an antidepressant of the serotonin-norepinephrine reuptake inhibitor (SNRI) class (Singh and Saadabadi, 2020).
Psychotic patients may spit out oral medication because of its undesirable taste or find injectable dosage form unacceptable or contraindicated. In addition, psychotic patient is agitated or shows difficulty in swallowing. Administration of oral disintegrating dosage forms offers additional advantage for treatment of patients with psychiatric disorders (Danileviciūte et al., 2005). Remeron Sol Tab® orally disintegrating tablets of the antidepressant drug, mirtazapine showed a positive opinion among the patients about the taste of medication (Thyssen et al., 2007). Moreover, Navarro 2010, showed that formulations of antidepressant drugs as oral disintegrating dosage forms can offer convenient alternatives to traditional tablets and may support patient compliance with extended therapy.
Several investigators showed that formulations of venlafaxine HCl as oral disintegrating tablets can offer convenient alternatives to traditional tablets and may support patient compliance with extended therapy (Pathan et al., 2013, Sasidhar et al., 2013, Karimban, 2012).
Formulation of venlafaxine hydrochloride as FDOFs is probable to result in rapid absorption with fast onset of action, and can also improve its bioavailability and help partial evading its first pass effect. In addition, patient compliance and adherence can be improved when the drug is formulated as FDOF due to masking its undesirable taste.
The aim of this study was to formulate and optimize FDOFs containing venlafaxine HCl using 33 full factorial designs, in addition to study the influences of formulation parameters on the film attributes (disintegration, swelling index, and dissolution efficiency; DE%15).
2. Materials and methods
2.1. Materials
Venlafaxine HCL was kindly supplied by (Spimaco, Qassem, KSA). Hydroxypropyl methylcellulose (HPMC) LV E3 was a gift from JRS Pharma Gmbh & Co. (Rosenberg, Germany). Sodium starch glycolate (SSG) was purchased from Sigma-Aldrich, Inc. (Missori, USA). Citric acid was purchased from Avonchim (Cheshire, United Kingdom). Saccharine sodium was purchased from Multi Chem Laboratories (Mumbai, India). Potassium dihydrogen orthophosphate was purchased from Winlab (Leicestershire, United Kingdom). Sodium phosphate dibasic was supplied from Sigma-Aldrich (Missori, USA). Glycerol was purchased from Winlab (Middlesex, United Kingdom). Acetonitrile HPLC grade was purchased from Panreac Quimica S.L.U. (Barcelona, Spain). Other chemicals used are of analytical grade and used as received.
2.2. Experimental design
In this study, factorial design was used to optimize the concentrations of the film forming polymer (HPMC; X1), superdisintegrant (SSG; X2) and the plasticizer (glycerol; X3). Three factors, three levels (33) full factorial design was used to evaluate their effects on fast dissolving oral films disintegration (Y1), swelling index (Y2), and dissolution efficiency at 15 min (Y3). The effect of these formulations variables was investigated for the optimized formula.
Table 1 shows the variables of the factorial design, and Table 2 lists the composition of formulations venlafaxine HCl fast dissolving oral film formulations according to the matrix of formulation.
Table 1.
The Variables for venlafaxine HCl FDOF formulae in 33 factorial design.
| Independent Variables (Factors) | |||
|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | |
| X1: Hyroxypropylmethylcellulose: (HPMC E3)(%) | 2 | 3.5 | 5 |
| X2: Sodium Starch Glycolate: (SSG) (%) | 0 | 2.5 | 5 |
| X3: Glycerol (%) | 0 | 1.5 | 3 |
| Dependent Variable (Response): | |||
| Y1: Disintegration Time (sec) | |||
| Y2: Swelling Index (%) | |||
| Y3: % Dissolution Efficiency (DE% 15) After 15 Min |
Table 2.
Composition of different formula of fast dissolving oral films (FDOFs).
| Ingredients (%W/W) |
||||||
|---|---|---|---|---|---|---|
| Formulation | HPMC % | SSG % | Glycerol % | Saccharine Sodium % | Citric Acid % | Venlafaxine HCl % |
| F1 | 3.5 | 5 | 1.5 | 1 | 0.5 | 2.5 |
| F2 | 2 | 0 | 3 | 1 | 0.5 | 2.5 |
| F3 | 5 | 5 | 0 | 1 | 0.5 | 2.5 |
| F4 | 2 | 2.5 | 0 | 1 | 0.5 | 2.5 |
| F5 | 5 | 2.5 | 3 | 1 | 0.5 | 2.5 |
| F6 | 2 | 2.5 | 1.5 | 1 | 0.5 | 2.5 |
| F7 | 5 | 0 | 3 | 1 | 0.5 | 2.5 |
| F8 | 2 | 5 | 3 | 1 | 0.5 | 2.5 |
| F9 | 3.5 | 2.5 | 1.5 | 1 | 0.5 | 2.5 |
| F10 | 3.5 | 0 | 3 | 1 | 0.5 | 2.5 |
| F11 | 5 | 5 | 1.5 | 1 | 0.5 | 2.5 |
| F12 | 2 | 5 | 1.5 | 1 | 0.5 | 2.5 |
| F13 | 5 | 2.5 | 0 | 1 | 0.5 | 2.5 |
| F14 | 5 | 0 | 0 | 1 | 0.5 | 2.5 |
| F15 | 2 | 2.5 | 3 | 1 | 0.5 | 2.5 |
| F16 | 3.5 | 2.5 | 0 | 1 | 0.5 | 2.5 |
| F17 | 3.5 | 2.5 | 3 | 1 | 0.5 | 2.5 |
| F18 | 5 | 5 | 3 | 1 | 0.5 | 2.5 |
| F19 | 5 | 2.5 | 1.5 | 1 | 0.5 | 2.5 |
| F20 | 5 | 0 | 1.5 | 1 | 0.5 | 2.5 |
| F21 | 3.5 | 5 | 3 | 1 | 0.5 | 2.5 |
| F22 | 2 | 0 | 1.5 | 1 | 0.5 | 2.5 |
| F23 | 2 | 0 | 0 | 1 | 0.5 | 2.5 |
| F24 | 3.5 | 0 | 0 | 1 | 0.5 | 2.5 |
| F25 | 2 | 5 | 0 | 1 | 0.5 | 2.5 |
| F26 | 3.5 | 5 | 0 | 1 | 0.5 | 2.5 |
| F27 | 3.5 | 0 | 1.5 | 1 | 0.5 | 2.5 |
2.3. Manufacture of FDOFs
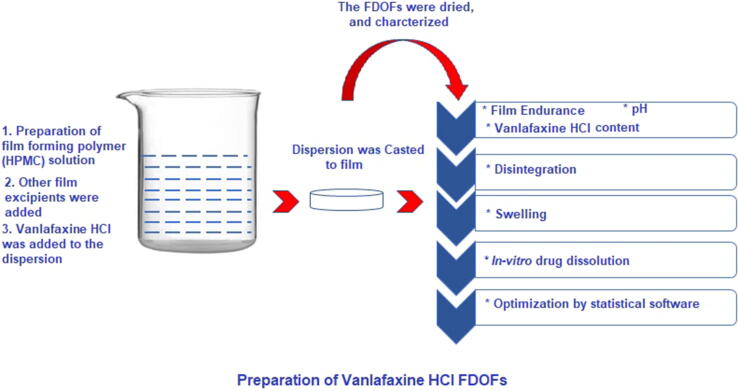
Solvent casting method was used to prepare Venlafaxine HCl FDOFs as shown in Fig. 1. For each formulation the plasticizer (glycerol) and sweetening agent (saccharine sodium) were weighed and added in beaker with the addition of a portion distilled water. The required amount of polymer was then dispersed over the contents of the beaker with the addition of more water. The polymer was left to swell for one hour. Then the solution was stirred with magnetic stirrer until polymer was completely dissolved. Thereafter, the remainder of excipients were added with the addition of the remaining portion of distilled water. Finally, the drug was added to solution and mixed thoroughly with magnetic stirrer. The polymeric solution was poured into a clean and dry circular Teflon coated plate (area = 2 cm2), in a way that each film contains 25 mg of drug. The films were dried in a universal oven (Mermmert U - Schwabach, Germany) at 34 °C for 24 h, then they were carefully removed. The films were stored in aluminum foil at room temperature in a desiccator.
Fig. 1.
Preparation of Venlafaxine HCl FDOFs.
2.4. UPLC assay of venlafaxine HCl
UPLC analysis was used to assess venlafaxine HCl stability during film preparation. The study employed with a highly sensitive UHPLC system (Ultimate 3000® binary solvent manager) equipped with automatic sampler and a Photodiode Array (PDA) eλ detector obtained from Thermo scientific, Bedford, MA, USA. The separation was achieved by reverse-phase isocratic elution using a mobile phase consisting of acetonitrile and 0.1% acetic acid in water (70/30 %V/V) delivered at a flow rate of 0.2 ml/min through an Acquity® UHPLC column HSS C18 (2.1 × 50 mm, 1.7 μm). The column temperature was maintained at 40 °C. The total run time was 2 min, where the venlafaxine peak was eluted at 1.163 min. Freshly prepared mobile phase was filtered outside using a 0.22 µm filter paper and degassed in the UHPLC system continuously by an online degasser. The detector wavelength was set at 226 nm and the injection volume was 2.0 µl.
2.5. Evaluation of venlafaxine HCl fast dissolving oral films (FDOFs)
2.5.1. Film thickness
A micrometer screw gauge Fowler Electronic Coolant Resistant (Fowler High Precision Micrometer, Inc. - Massachusetts, USA) was used to measure the film thickness. In order to obtain uniformity of film, thickness will be measured at 5 different locations of the whole intact film. Three films were used in this test (Bhyan et al, 2011).
2.5.2. Folding endurance
Folding endurance is another procedure to estimate the mechanical properties of a film. It is measured by repeatedly folding a film at the same point until it breaks. Folding endurance value is number of times the film is folded without breaking. Three films of each formula were used in this test (Irfan et al, 2016).
2.5.3. Film pH
Three films of each formulation were dissolved in beaker containing 5 ml of distilled water. The solution was measured by pH meter (Mettler Toledo - Greifensee, Switzerland). A mean of three readings and standard deviation was recorded (Sharma et al, 2015).
2.5.4. Film moisture content
Moisture content tests were preformed to ensure dryness. The prepared films were initially weighed and located in the desiccators containing calcium chloride. After 3 days the films were reweighed to obtain the percentage of moisture loss (Patil et al., 2014). Three films of each formula were used in this test.
2.5.5. Drug content
Venlafaxine HCl-loaded FDOF was placed in 100 ml volumetric flask, then dissolved in 50 ml phosphate buffer (pH 6.8) using magnetic stirrer. The volume was completed with phosphate buffer (pH 6.8). One ml of the solution was further diluted to 10 ml with phosphate buffer solution (pH 6.8) and the absorbance was measured at 225 nm using UV spectrophotometer (Biochrom Libra S22, Biochrom Ltd, Cambridge England) against a suitable blank prepared from non-medicated film. Drug content uniformity was conducted in triplicate (Bhyan et al, 2011‘). The films were accepted in terms of drug content when the range for drug content is between 85 and 115% (Sharma et al, 2015). A mean of three readings and standard deviation was recorded. Three films of each formula were used in this test.
2.5.6. Swelling index
The studies of swelling index of the film were conducted in 6.8 pH phosphate buffer solution. A film was weighed and placed in a pre-weighed stainless steel wire sieve.
The mesh containing the film was submerged into 15 ml of phosphate buffer 6.8 pH contained in a petri dish. The increase in film weight was determined at each interval (1 min) until a constant weight was observed. Three films of each formula were used in this test (Sharma et al, 2015. The degree of swelling was calculated using the formula:
where SI = swelling index, Wt. = weight of the film at time “t”, and WO = weight of the film at t = 0.
2.5.7. In-vitro disintegration
Disintegration of venlafaxine HCl-loaded FDOFs was performed using disintegration tester (PTZ-S Pharma Test Apparatebau AG, Hainburg, Germany). Three films of each formula were tested. One film of each formula was placed in one of the six tubes of the basket. Then the apparatus was operated using phosphate buffer pH 6.8 as disintegration medium and maintained at 37 °C ± 2 °C. The disintegration time was recorded (Bhyan et al, 2011).
2.5.8. In-vitro dissolution
In-vitro dissolution test of venlafaxine HCl-loaded oral films was performed using USP dissolution apparatus (Type II) (apparatus Pharma Test Apparatebau PT-DT70 - Hainburg, Germany). The test was carried out at 37 °C ± 0.5 °C with stirring speed of 100 rpm in 900 ml of phosphate buffer (pH 6.8). Samples were withdrawn at predetermined time intervals (2, 5, 10, 15, 20, 30, 45 and 60 min) and replaced with the same volume of fresh buffer, in which sink conditions were maintained during dissolution. The absorbance was determined at 225 nm using UV–visible spectrophotometer (Biochrom Libra S22, Biochrom Ltd -Cambridge England) against a blank made of non-medicated films at the same conditions. The amount dissolved from each film was calculated. Three films of each formula were tested (Arya et al, 2010).
2.5.9. Differential scanning calorimetry (DSC)
The DSC scans were recorded for venlafaxine HCl FDOFs compared to that of the individual components and physical mixture, to determine any physical or chemical interaction between the drug and the excipients used in the preparation of FDOFs. The samples (3–5 mg) were hermetically sealed in aluminum pans and heated at a constant rate of 10 °C/min, over a temperature range of 25–350 °C. Thermograms of the samples were obtained using differential scanning calorimetry (DSC-60 - Shimadzu, Japan). Thermal analysis data was recorded using a TA 50I PC system with Shimadzu software programs. To calibrate the DSC temperature and enthalpy scale, indium was used as a standard. Nitrogen gas was used as purging gas at rate of 40 ml/min (Auda et al, 2014). The samples that were used were venlafaxine HCl, HPMC, SSG, saccharine sodium, citric acid, and optimized FDOF.
2.5.10. X-ray diffraction (XRPD)
The X-ray diffraction patterns on powder and medicated films (free of plasticizers) were obtained using a (PANalytical Philips 1700 series diffractometer - Almelo, Netherlands). The apparatus is equipped with a curved graphite crystal monochromater, automatic divergence slite and automatic controller PW/1710. The target used was CuK a radiation operating at 40 Kn and 30 mA (1Ka = 1.54 18 A). The system was calibrated using silicon disc and/or powder (d111 = 3.1355 a) as an external standard. The diffraction pattern was achieved using continuous scan mode with 2q° ranging from 40° to 60° (Abou Obaid et al, 2020).
2.5.11. Scanning electron microscopy (SEM)
Surface morphology of venlafaxine HCl optimized FDOF formula was investigated by using scanning electron microscopy (SEM). The film sample was attached on the stubs by adhesive carbon tape (SPI Supplies, West Chester, PA, USA), and then sputter-coated with a thin gold palladium layer under an argon atmosphere using a gold sputter module in a high-vacuum evaporator. Coated samples were then scanned and photomicrographs were taken with an SEM (Zeiss EVO LS10; Cambridge, UK).
2.6. Statistical analysis
All results were expressed as mean values, standard deviation (SD), unless otherwise specified elsewhere. One-way analysis of variance (ANOVA) using a statistical package (Statgraphics Plus, version 5, Warrenton - VA, USA). Statistical difference yielding (p > 0.05) was significant.
3. Results and discussion
The drug content of some selected venlafaxine HCl FDOF formulations (F4, F5, F8, F9 and F10) was determined by UPLC analysis to check stability of the drug during film preparation. The results showed that venlafaxine HCl content in the prepared FDOFs ranged from 91 to 100%, indicating a good stability of the drug during the preparation and casting of film formula for 24 h. Moreover, the presence of citric acid exhibited neither stabilizing nor degrading effect on venlafaxine HCl during film preparation.
3.1. Evaluation of film properties
Venlafaxine HCl content of the prepared FDOFs was ranged from 87.04% ± 0.99 to 99.36% ± 0.43, Table 3, which is complying with the pharmacopoeial guidelines (USP30-NF25). Venlafaxine HCl FDOFs formulated exhibited a thickness from 0.20 to 0.35 mm, folding endurance from 1 to 12 folds, pH levels from 2.9 ± 0.03 to 4.28 ± 0.01, and moisture content from 0.66 to 5.69. The extent of moisture uptake depends upon the concentration of hygroscopic excipient present. In addition, the obtained data indicated that increasing HPMC and glycerol concentration led to an increase in the moisture uptake by the films (Singh et al, 2013). Also, the amount of film forming polymer affected film, which is in accordance with data obtained by Bhupinder and Sarita (2012). Higher folding endurance value depicts the more mechanical strength of a film. A direct relation exists between mechanical strength and folding endurance of films. As mechanical strength is governed by plasticizer concentration so it is clearly evident that plasticizer concentration also directly affects folding endurance value (Irfan et al., 2016). The surface pH of oral strip is calculated in order to examine the risk of any adverse effect in-vivo. The slightly acidic nature of the prepared FDOFs is due to the use of citric acid a salivary stimulating agent (Bala et al., 2013).
Table 3.
Properties of venlafaxine HCl FDOF formulations.
| Formulation | Drug content (mg) ± SD | Thickness (mm) | Folding endurance | pH ± SD | Moisture content |
|---|---|---|---|---|---|
| F1 | 23.95 ± 1.63 | 0.31 | 1 | 4.26 ± 0.04 | 4.64 |
| F2 | 23.65 ± 0.83 | 0.28 | 4 | 2.9 0 ± 0.02 | 0.66 |
| F3 | 24.32 ± 1.38 | 0.35 | 1 | 4.20 ± 0.11 | 2.42 |
| F4 | 21.76 ± 0.99 | 0.24 | 1 | 3.79 ± 0.04 | 3.01 |
| F5 | 24.23 ± 1.60 | 0.30 | 12 | 3.9 0 ± 0.05 | 5.69 |
| F6 | 23.24 ± 0.59 | 0.26 | 3 | 3.79 ± 0.01 | 2.85 |
| F7 | 23.71 ± 0.56 | 0.30 | 3 | 2.99 ± 0.01 | 2.79 |
| F8 | 23.74 ± 0.16 | 0.30 | 4 | 4.23 ± 0.00 | 3.67 |
| F9 | 22.11 ± 0.50 | 0.26 | 2 | 3.85 ± 0.02 | 2.33 |
| F10 | 22.85 ± 0.27 | 0.28 | 3 | 2.92 ± 0.01 | 2.40 |
| F11 | 23.24 ± 0.68 | 0.32 | 2 | 4.17 ± 0.01 | 2.34 |
| F12 | 23.18 ± 0.44 | 0.25 | 2 | 4.17 ± 0.01 | 2.51 |
| F13 | 21.76 ± 0.99 | 0.27 | 1 | 3.79 ± 0.01 | 2.16 |
| F14 | 23.71 ± 1.69 | 0.24 | 1 | 2.98 ± 0.00 | 0.69 |
| F15 | 23.66 ± 1.13 | 0.24 | 1 | 3.83 ± 0.01 | 1.84 |
| F16 | 22.84 ± 0.59 | 0.27 | 1 | 3.76 ± 0.01 | 1.22 |
| F17 | 23.08 ± 0.38 | 0.30 | 7 | 3.88 ± 0.01 | 2.04 |
| F18 | 23.09 ± 0.64 | 0.35 | 5 | 4.23 ± 0.02 | 4.62 |
| F19 | 22.66 ± 1.54 | 0.28 | 1 | 3.81 ± 0.02 | 2.89 |
| F20 | 23.94 ± 0.24 | 0.26 | 3 | 3.06 ± 0.01 | 1.86 |
| F21 | 22.94 ± 1.59 | 0.26 | 3 | 4.28 ± 0.01 | 3.98 |
| F22 | 24.67 ± 0.30 | 0.26 | 3 | 2.93 ± 0.01 | 1.89 |
| F23 | 23.47 ± 0.76 | 0.26 | 1 | 2.9 ± 0.03 | 3.40 |
| F24 | 22.26 ± 0.61 | 0.20 | 1 | 2.96 ± 0.02 | 2.46 |
| F25 | 23.41 ± 2.41 | 0.24 | 1 | 4.16 ± 0.02 | 1.46 |
| F26 | 23.72 ± 1.23 | 0.25 | 1 | 4.19 ± 0.03 | 1.05 |
| F27 | 24.84 ± 0.43 | 0.23 | 4 | 2.96 ± 0.02 | 1.99 |
3.2. Effect of independent formulation parameters on disintegration time
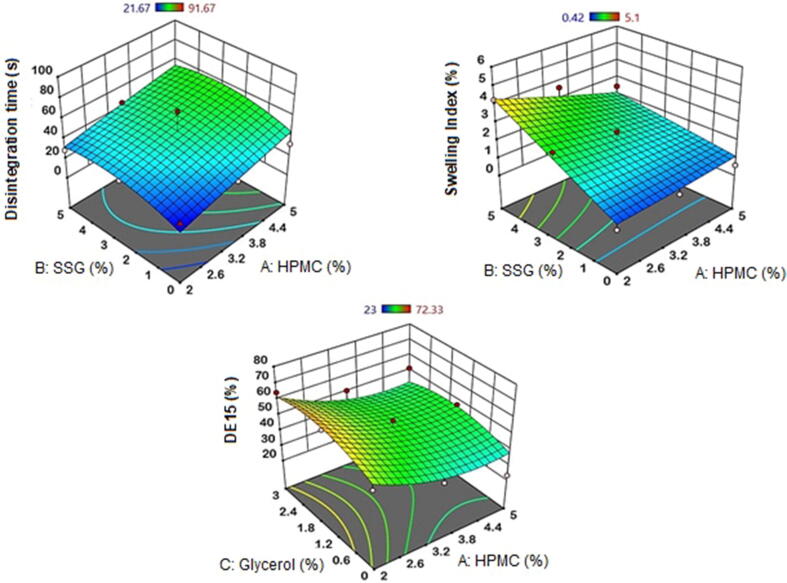
The disintegration time of FDOFs is one the crucial independent factors that should be optimized during the development of film formulations. The analysis of variance for disintegration time of venlafaxine HCl FDOFs can be seen in Table 4. HMPC (X1) exerted a significant (p = 0.0006 and sum of squares is 2599.45) agonistic action on film disintegration time; as the concentration of HPMC increases, the disintegration time was prolonged, and the, which indicates significance. In contrast, SSG (X2) exhibited a clear antagonistic, but insignificant (p > 0.05) effect on the FDOF disintegration time. Moreover, slight effect was exerted by glycerol (X3) on the films disintegration time at higher glycerol levels, but also this effect is not significant. It is visibly clear that high concentrations of film plasticizer; glycerol (X3) demonstrated an antagonistic action on the effects of both HPMC and SSG on film disintegration time. It means that increasing glycerol level shortened film disintegration time. This can be seen in the response surface plot, Fig. 2. Disintegration time was found to be longer with the increase in HPMC (X1) concentration. At higher concentrations of SSG (X2), a prolonged disintegration time was observed. This could be attributed to the increased microviscosity of the FDOFs on using high concentration of the superdisintegrant (Zhang et al., 2015). Glycerol (X3) on the other hand has the opposite effect. The disintegration time was shorter, with increase in glycerol concentration up to 1.5%, after which the disintegration time became longer. As the concentration of plasticizer was increased from low level to high level, the disintegration time was found to be prolonged. Plasticizer molecules act by inserting themselves between the polymer strands and break the polymer–polymer interactions and increases the molecular mobility of the polymer strands, resulting in prolonged disintegration time (Zhang et al., 2015).
Table 4.
Analysis of variance for disintegration time, swelling index and %DE15 of venlafaxine HCl fast dissolving oral films.
| Source | Disintegration (sec) |
Swelling index |
%DE15 |
|||
|---|---|---|---|---|---|---|
| Sum of Squares | P-Value | Sum of Squares | P-Value | Sum of Squares | P-Value | |
| X1: HPMC | 2599.45 | 0.0006 | 3.6468 | 0.0017 | 1488.21 | 0.0005 |
| X2: SSG | 570.319 | 0.0655 | 22.9322 | 0.0001 | 46.5612 | 0.4588 |
| X3: Glycerol | 274.795 | 0.1896 | 0.532512 | 0.1736 | 205.369 | 0.1298 |
| X12 | 1.71735 | 0.9152 | 0.013254 | 0.8254 | 195.739 | 0.1386 |
| X1X2 | 8.31668 | 0.8149 | 4.90497 | 0.0005 | 85.7071 | 0.3182 |
| X1X3 | 1.5552 | 0.9193 | 0.515845 | 0.1802 | 5.41363 | 0.7991 |
| X22 | 443.072 | 0.1008 | 0.000308167 | 0.9731 | 5.98002 | 0.7892 |
| X2X3 | 112.119 | 0.3949 | 0.0278403 | 0.7493 | 270.275 | 0.0854 |
| X32 | 375.25 | 0.1287 | 0.365067 | 0.2558 | 184.815 | 0.1494 |
Fig. 2.
Response surface plots estimating the effect of indepe.
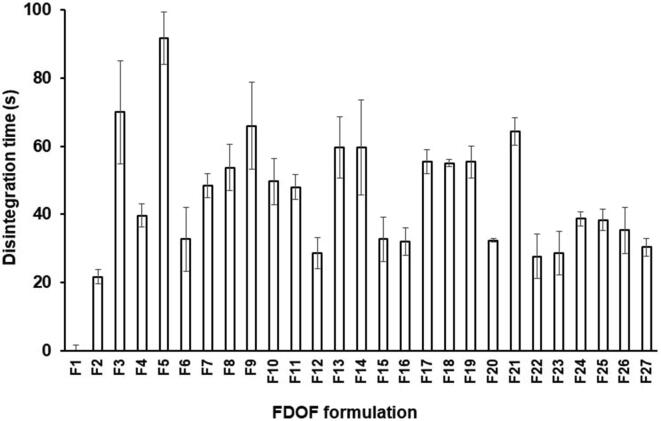
Fig. 3 illustrates the disintegration time of all venlafaxine HCl FDOFs. In case of using low level of glycerol (0%), FDOFs formulations F3, F13, F14, F5, F7, F8, F 9 F10, F17, F18, F19 and F21 (which contain 5% HPMC, highest concentration of HPMC and varying concentrations of SSG and glycerol) exhibited long disintegration times compared to other formulations. This might be due to the increased viscosity of these formulations by adding high HPMC levels (Gurdale et al., 2014). Aldawsari and Badr-Eldin (2020) observed that a slow disintegration rate of the antidepressant drug (dapoxetine HCl) up on increasing HPMC E5 level in the film formulation. They ascribed this finding to the formation of viscous gel layer upon contacting medium at higher polymer levels with subsequent retardation of more penetration of fluids. Also, FDOF formulations F1 and F11, that contain higher concentrations of HPMC, 5% SSG, showed moderate disintegration times when compared to the previous formulations; which ranged from 45.0 0 ± 1.63 and 48.00 ± 3.61 s. This can be attributed to the increased disintegrating effect of SSG at lower glycerol concentrations (Gorle and Patil, 2017). Raju et al. 2013, utilized HPMC as a film forming polymer for the preparation of of loratidine FDOFs, and they observed that film disintegration and in vitro drug release from FDOFs principally was controlled by polymer concentration, which affected the viscosity of film casting solution.
Fig. 3.
Disintegration time of different venlafaxine HCl FDOFs.
3.3. Effect of independent formulation parameters on the swelling index
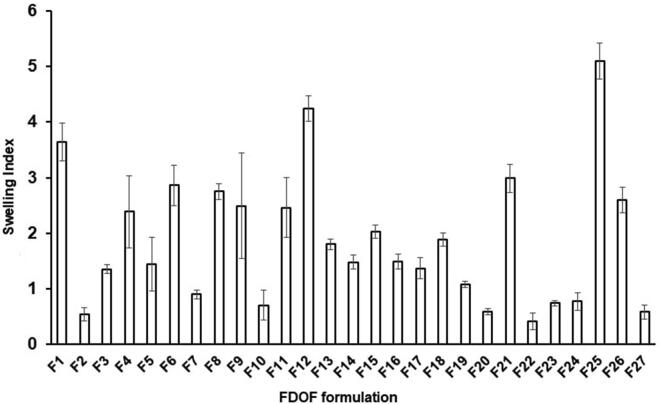
The effect of different independent variables on the swelling index of venlafaxine HCl formulations are displayed in Fig. 4. The analysis of variance for swelling index of venlafaxine HCl FDOFs, Table 4, showed that the most significant followed by the interaction between HPMC and SSG and lastly HPMC alone. As the concentration of SSG increases swelling index also increases, since the p-value is 0.0007 and sum of squares is 22.9322. The interactive effect of X1X2 is also significant. When higher concentrations of polymer and superdisintegrant are combined swelling index tends to decrease, with a p-value of 0.005 and sum of squares is 4.90497. X1 also has a significant effect on swelling index but to a lesser degree. As HPMC concentration increases swelling index decreases, with a p-value is 0.0017 and sum of squares is 3.6468. Slight effect was exerted by glycerol (X3) on the films’ swelling index at higher glycerol levels, but this effect is not significant. The increase in glycerol concentration did reduce the swelling index of the prepared FDOFs (p = 0.174).
Fig. 4.
Swelling index values of different venlafaxine HCl FDO.
The response surface plot estimating the effect of HPMC and SSG on the swelling index of venlafaxine HCl FDOFs at the middle concentrations of glycerol (Fig. 2) showed that increasing the concentration of HPMC resulted in decreased swelling index at 0% glycerol. Moreover, Zhang et al. (2018) showed that using high concentration of film forming polymer (HPMC) resulted in increased viscosity of polymer solution and casted FDOFs, which may delay film swelling. In addition, as the concentration of SSG increases, swelling index was also increased. Superdisintegrants in FDOFs showed high swelling index due to their ability to absorb more water (Jahangir et al., 2014). It indicates that the drug dissolution from the formulation increases with increase in concentration of superdisintegrant. Sodium starch glycolate; SSG (Explotab®) showed its better disintegrating property when it is used in 2–10% w/w concentration (Mostafa et al., 2014). SSG swells 7–12 folds in less than 30 s, by wicking action (porosity and capillary mechanism) (Deepak et al., 2014). Also, there is a correlation between swelling index and disintegration time. As the swelling index increases, the fast dissolving film will be able to absorb more quantity of liquid and therefore is able to breakdown quickly and thus have shorter disintegration time, this finding is not applied at high concentrations of superdidintegrant. As the concentration of superdisintegrant increases gelling occurs and disintegration time is prolonged (Goel et al., 2008).
3.4. Effect of independent formulation parameters on %DE15
The effect of independant variables on the dissolution efficiency at 15 min (DE%15) of venlafaxine HCl formulations are determined in Fig. 5, and the analysis of variance for DE%15 of venlafaxine HCl FDOFs can be seen in Table 4.The results indicate that as the concentration of HPMC increases the DE%15 decreased with the p-value of 0.0005 and the sum of squares is 1488.21. SSG (X2) and Glycerol (X3) exerted slight effect on DE%15 of the FDOFs, but this effect is not significant (p > 0.05).
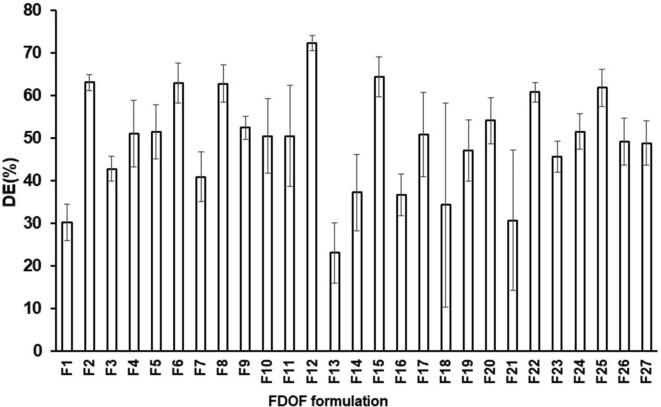
Fig. 5.
Dissolution efficiency after 15 min (?5) of differen.
Response surface 3D plot, Fig. 2 reveals that DE%15 significantly decreases with the increase in HPMC (X1) concentration (p = 0.005). Increasing the concentration of SSG resulted in a slight and insignificant decrease in DE%15 (p = 0.459). Glycerol (X3) on the other hand has the opposite effect. The DE%15 increased with increase in the concentration of glycerol up to 1.5% concentration, after which the DE%15 decreased. This could be explained on the basis that at higher glycerol levels, an increase in viscosity of the film prevails. However, the effect of glycerol on DE%15 is not significant (p = 0.1298).
Highest %DE15 values were recorded in case of FDOF formulations containing the lowest HPLC concentrations; F2 (2% HPMC, 0% SSG and 3% glycerol), F6 (2% HPMC, 2.5% SSG and 1.5% glycerol), F8 (2% HPMC, 5% SSG and 3% glycerol), F12 (2% HPMC, 5% SSG and 1.5% glycerol), F15 (2% HPMC, 2.5% SSG and 3% glycerol) and F25 (2% HPMC, 5% SSG and 0% glycerol). The recorded %DE15 values for these formulations were 63.03 ± 1.95, 62.95 ± 4.66, 62.76 ± 4.42, 72.33 ± 1.71, 64.38 ± 4.69 and 61.82 ± 4.39, respectively. In contrast, lowest values for %DE15 were observed in case of FDOF formulae F1 (3.5% HPMC, 5% SSG and 1.5% glycerol), F13 (5% HPMC, 2.5% SSG and 0% glycerol) and F21(3.5% HPMC, 5% SSG and 3% glycerol), for in which the calculated %DE15 values were 30.20 ± 4.30, 23.00 ± 7.17 and 30.63 ± 6.5, respectively. These formulations contain medium or high levels of HPMC.
Upon contact with the dissolution, the viscous nature of the film forming polymer (HPMC) causes a creation of thick matrix gel fluid, which delays the drug release from the films (Kumari et al., 2014). Also, as the concentration of the polymer increased, the drug release was found to be decreased due to the increase in the time required for wetting and dissolving the drug molecules present in the polymer matrices (Prabhu et al., 2011). Prabhu et al. (2011) proposed that the drug release mechanism from the HPMC matrix may imply penetration of water molecules along with polymer relaxation. This can result in the formation of a viscous rubbery region or a gel layer, which governs drug release by resisting drug diffusion or for matrix erosion. Increasing film forming polymer concentration can result in viscosity of such gel layer, and slow drug release rate. The effect of SSG on DE%15 in our study was more prominent at higher concentrations of glycerol. SSG has an antagonistic effect on DE%15. As the concentration of SSG increases DE%15 decreases, however its effect remains insignificant.
3.5. Optimized formula of venlafaxine hydrochloride FDOFs
Based on the modeling made by statistical program, and desirability factor equal to 95%, the following factors were suggested by the software for the preparation of the optimal formulation: 2% HPMC, 5% SSG, and 1% glycerol.
The resultant experimental values of the responses were quantitatively compared with that of predicted values to calculate the percentage prediction error; Table 5. From the practical results, it was observed that the optimized FDOF formula (F12) showed short disintegration time 33.67 ± 2.08 s, swelling index of 3.58 ± 0.50, and 72.33% DE%15. This formulation is considered to be the best film formulation compared with the optimized formula obtained by 33 full factorial design. These differences in the film’s responses were not significantly deviated from the predicted response values of the optimized formula (predicted disintegration time is 30.72 s; swelling index is 4.4 and DE%15 is 65.15%).
Table 5.
Venlafaxine HCl FDOF optimized formula composition, predicted and observed responses.
| Optimized formula composition | Response (Y) |
||
|---|---|---|---|
| Predicted | Observed | ||
| HPMC (X1): 2% w/w | Disintegration time (Y1) | 30.72 s | 33.67 ± 2.08 |
| SSG (X2): 5% w/w | Swelling index: (Y2) | 4.40 | 3.58 ± 0.50 |
| Glycerol (X1): 1% w/w | DE%15 (Y3) | 65.16% | 72.33 ± 3.17 |
3.6. Characterization of venlafaxine HCl optimized FDOF formula
3.6.1. Differential scanning calorimetry (DSC)
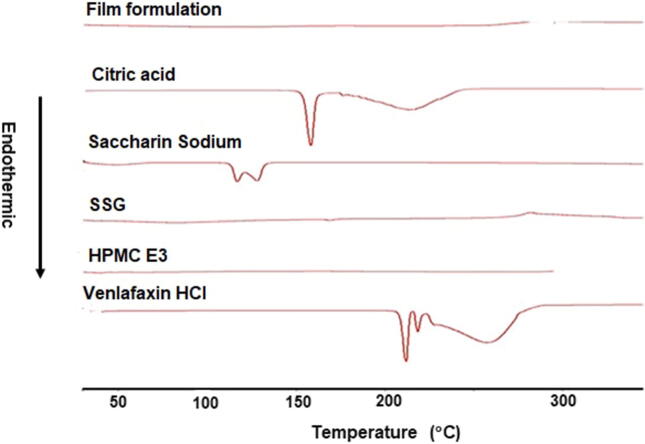
Fig. 6 represents the DSC thermograms of pure materials, including venlafaxine HCl, HPMC, SSG, saccharine sodium, citric acid, and film. The pure drug exhibited an endothermic peak at 212.04 °C. HPMC and SSG did not display any thermal event in the examined temperature range. Citric acid displayed an endothermic peak at 158 °C corresponding to its melting point, and saccharine sodium showed an endothermic peak at 116.6 °C. The optimized film formula showed the disappearance of characteristic peak of venlafaxine HCl, which might be due to homogenous dispersion of the drug in the film forming excipients. Similarly, Bala et al. (2013) concluded that the melting endothermic peak of clobazam was not observed in the drug loaded PVA orally dissolving film. This indicates that clobazam was uniformly dispersed and present in an amorphous state in the polymeric matrix.
Fig. 6.
DSC thermograms of venlafaxine HCl FDOF optimized form.
3.6.2. X-ray powder diffraction
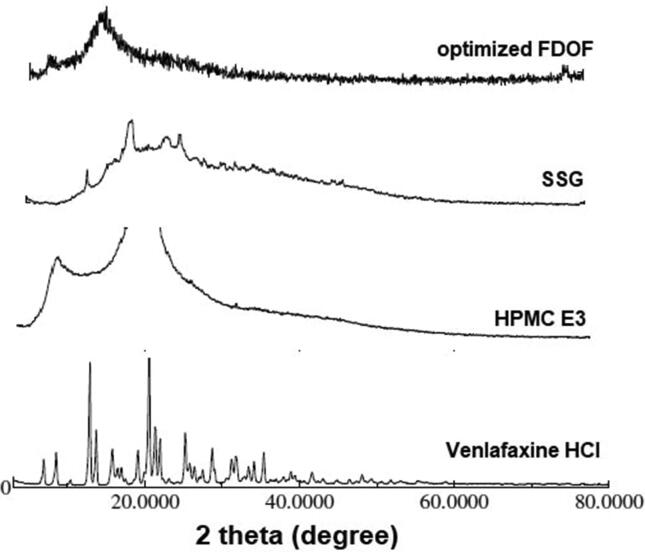
To get further evidence on the solid state changes, x-ray diffraction spectra were carried out on venlafaxine HCl, HPMC, SSG, and the optimized FDOF formulation. The presence of numerous distinct peaks in the x-ray diffraction spectrum of venlafaxine HCl indicates that the drug is present as a crystalline material with characteristic diffraction peaks appearing at diffraction angles of 2θ at 12.8 Å, 13.7 Å, 20.5 Å, 21.3 Å, 21.9 Å, and 25.2 Å, Fig. 7. HPMC showed characteristic peak at 8.5 Å and 20.5 Å, and SSG showed characteristic diffraction peak at 17.4 Å. X-ray diffraction spectrum of the venlafaxine HCl FDOF formula revealed the absence of any characteristic diffraction peak, indicating that the drug completely dispersed in the film matrix. This corresponds with the results found from DSC testing in which there is a disappearance of characteristic peaks of the drug, polymer, and superdisintegrant in the film formulation. Thus the drug is homogenously dissolved in the polymer matrix. These findings are in agreement with (Dinge and Nagarsenker, 2008), who stated that both the components selected for the investigation using XRPD confirmed their potential in improving triclosan solubility which would be useful for the successful development of fast dissolving films that can quickly release the drug in solubilized form.
Fig. 7.
X-ray powder diffraction pattern of venlafaxine HCl,
3.6.3. Film surface morphology and structure
The SEM image showing morphology and surface of venlafaxine HCl optimized FDOF formula is illustrated in Fig. 8. The image revealed smooth slightly porous surface, indicating complete solubility of the drug in the polymeric film matrix distribution of drug particles (Bala et al., 2014).
Fig. 8.
Scanning Electron microscopic (SEM) image of venlafaxi.
4. Conclusion
The results showed that formulation parameters (film forming polymer; HPMC, superdisintegrant; SSG and plasticizer; glycerol) noticeably affected the essential attributes of Venlafaxine HCl-loaded FDOFs, as swelling and disintegration as well as in vitro drug dissolution. The optimized FDOF formulation was derived from statistical program based on using lowest concentration of HPMC, highest level of SSG and lowest level of plasticizer as well. From this investigation, it can be concluded that oral thin film formulation can be a potential novel drug dosage form for pediatric, geriatric and also for general population. Hence fast dissolving oral films of venlafaxine HCl were found to be suitable for eliciting better therapeutic effect in the treatment of depression.
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/171), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abou Obaid N.I., Al-Jenoobi F.I., Ibrahim M.A., Alam M.A. Losartan potassium sustained release pellets with improved in vitro and in vivo performance. Pharm. Dev. Technol. 2020;28:1–12. doi: 10.1080/10837450.2020.1782934. [DOI] [PubMed] [Google Scholar]
- Aldawsari H.M., Badr-Eldin S.M. Enhanced pharmacokinetic performance of dapoxetine hydrochloride via the formulation of instantly-dissolving buccal films with acidic pH modifier and hydrophilic cyclodextrin: Factorial analysis, in vitro and in vivo assessment. J. Adv. Res. 2020;24:281–290. doi: 10.1016/j.jare.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya A., Chandra A., Sharma V., Pathak K. Fast dissolving oral films: an innovative drug delivery system and dosage form. Int. J. Chem. Tech. Res. 2010;2(1):576–583. [Google Scholar]
- Auda S.H., Elbadry M.A., Ibrahim M.A. Design formulation and characterization of fast dissolving films containing dextromethorphan. Dig. J. Nanomat. Bios. 2014;9(1):133–141. [Google Scholar]
- Bae Y.H., Park K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv. Drug Deliv. Rev. 2020 doi: 10.1016/j.addr.2020.06.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala R., Pawar P., Khanna S., Arora S. Orally dissolving strips: a new approach to oral drug delivery system. Int. J. Pharm. Invest. 2013;3(2):67–76. doi: 10.4103/2230-973X.114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala R., Khanna S., Pawar P. Design optimization and in vitro-in vivo evaluation of orally dissolving strips of clobazam. J. Drug Deliv. 2014;2014:1–15. doi: 10.1155/2014/392783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhupinder B., Sarita J. Formulation and evaluation of fast dissolving sublingual films of Rizatriptan Benzoate. Int. J. Drug. Dev. Res. 2012;4(1):133–143. [Google Scholar]
- Bhyan B., Jangra S., Kaur M., Singh H. Orally fast dissolving films: innovations in formulation and technology. Int. J. Pharm. Sci. Rev. Res. 2011;9(2):9–15. [Google Scholar]
- Danileviciūte V., Adomaitiene V., Sveikata A., Maciulaitis R., Kadusevicius E. Compliance in psychiatry: results of a survey of depressed patients using orally disintegrating tablet. Medicina (Kaunas) 2005;42(12):1006–1012. [PubMed] [Google Scholar]
- Deepak H., Aggarwal G., Kumar S.H. Development of fast dissolving oral films and tablets of cinnarizine: effect of superdisintegrants. Int. J. Pharm. Pharm. Sci. 2014;6(2):186–191. [Google Scholar]
- Dinge A., Nagarsenker M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS Pharm SciTech. 2008;9(2):349–356. doi: 10.1208/s12249-008-9047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa H.F., Ibrahim M.A., Sakr A. Dextromethorphan HBr orally disintegrating tablets: development and optimization using different formulation variables. Pharm. Ind. 2014;8:1300–1311. [Google Scholar]
- Goel H., Rai P., Rana V., Tiwary A.K. Orally disintegrating systems: innovations in formulation and technology. Rece. Pat. Drug Deliv. Formul. 2008;3:258–274. doi: 10.2174/187221108786241660. [DOI] [PubMed] [Google Scholar]
- Gorle A., Patil G. Design, development and evaluation of fast dissolving film of amlodipine besylate. Int. J. Chem. Tech. Res. 2017;10(4):334–344. [Google Scholar]
- Gurdale M.S., Lade M.S., Payghan S.A., Disouza J.I. Fast dissolving HPMC E5 based oral film for rapid absorption of Metoprolol Tartrate. Eur. J. Pharm. Med. Res. 2014;1(1):75–91. [Google Scholar]
- Irfan M., Rabel S., Bukhtar Q., Qadir M.I., Jabeen F., Khan A. Orally disintegrating films: a modern expansion in drug delivery system. Saudi Pharm. J. 2016;24(5):537–546. doi: 10.1016/j.jsps.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangir M.A., Saleem M.A., Patel H.R., Kazmi I., Muheem A., Ahmad K. Formulation and evaluation of oral fast dissolving films of levocetirizine. World J. Pharm. Pharm. Sci. 2014;3(9):1310–1323. [Google Scholar]
- Karimban J.A. Formulation and evaluation of immediate release venlafaxine HCl tablets: comparative study of super disintegrant and diluents. Int. Res. J. Pharm. 2012;3(4):324–329. [Google Scholar]
- Kumari A., Kaza R., Sumathi P. Design and evaluation of losartan potassium fast dissolving films. Int. J. Innov. Pharm. Res. 2014;5(4):431–439. [Google Scholar]
- Liew K.B., Peh K.K., Tan Y.T. Orally disintegrating dosage forms: breakthrough solution for non-compliance. Int. J. Pharm. Pharm. Sci. 2013;5(4):4–8. [Google Scholar]
- Nyol S., Gupta M.M. Immediate drug release dosage form: a review. J. Drug Deliv. Ther. 2013;3(2):155–161. [Google Scholar]
- Pathan I.B., Shingare P.R., Kurumkar P. Formulation design and optimization of novel mouth dissolving tablets for venlafaxine hydrochloride using sublimation technique. J. Pharm. Res. 2013;6(6):593–598. [Google Scholar]
- Patil P.C., Shrivastava S.K., Vaidehi S., Ashwini P. Oral Fast Dissolving drug delivery system: a modern approach for patient compliance. Int. J. Drug. Reg. Affairs. 2014;2(2):49–60. [Google Scholar]
- Prabhu P., Malli R., Koland M., Vijaynarayana K., D'Souza U., Harish N.M. Formulation and evaluation of fast dissolving films of levocitirizine di hydrochloride. Int. J. Pharm. Invest. 2011;1(2):99–104. doi: 10.4103/2230-973X.82417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju P.N., Kumar M.S., Reddy C.M., Ravishankar K. Formulation and evaluation of fast dissolving films of loratidine by solvent casting method. Pharma. Innov. 2013;2(2):31–35. [Google Scholar]
- Sasidhar R.L.C., Vidyadhara S., Nagaraju R., Raj K.S., Wilwin E. Formulation and evaluation of venlafaxine hydrochloride mouth dissolving tablets by effervescent technique. J. Pharm. Res. 2013;1(3):258–263. [Google Scholar]
- Sharma D., Kaur D., Verma S., Singh D., Singh M., Singh G. Fast dissolving oral films technology: a recent trend for an innovative oral drug delivery system. Int. J. Drug Deliv. 2015;7(2):60–75. [Google Scholar]
- Singh, D., Saadabadi, A., 2020. Venlafaxine. NCBI Bookshelf, StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL), 2020.
- Singh H., Kaur M., Verma H. Optimization and evaluation of desloratadine oral strip: an innovation in paediatric medication. Sci. World J. 2013;13:1–9. doi: 10.1155/2013/395681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur N., Bansal M., Sharma N., Yadav G., Khare P. Overview “a novel approach of fast dissolving films and their patients”. Adv. Biol. Res. 2013;7(2):50–58. [Google Scholar]
- Thyssen A., Remmerie B., D'Hoore P., Kushner S., Mannaert E. Rapidly disintegrating risperidone in subjects with schizophrenia or schizoaffective disorder: a summary of ten phase I clinical trials assessing taste, tablet disintegration time, bioequivalence, and tolerability. Clin. Ther. 2007;29(2):290–304. doi: 10.1016/j.clinthera.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Zhang H., Han M.G., Wang Y., Zhang J., Han Z.M., Li S.J. Development of oral fast-disintegrating levothyroxine films for management of hypothyroidism in pediatrics. Trop. J. Pharm. Res. 2015;14(10):1755–1762. [Google Scholar]
- Zhu Z., You X., Huang K., Raza F., Lu X., Chen Y. Effect of taste masking technology on fast dissolving oral film: dissolution rate and bioavailability. Nanotechnol. 2018;29(30) doi: 10.1088/1361-6528/aac010. [DOI] [PubMed] [Google Scholar]
- Zhang L., Aloia M., Pielecha-Safira B., Lin H., Rajai P.M., Kunnath K. Impact of super-disintegrants and film thickness on disintegration time of strip films loaded with poorly watersoluble drug microparticles. J Pharm Sci. 2018;107(8):2107–2118. doi: 10.1016/j.xphs.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]