Abstract
Traditional and complementary medicine constitutes an important, and often underestimated, source of healthcare for multiple diseases including cancer. However, little is known about the ethnomedical knowledge and practices in Northern Africa. The main objective of this study is to identify and analyze the variety of natural products used in Algerian ethnopharmacology for cancer therapy. For this purpose, semi-structured interviews with 225 traditional healers, herbalists and practitioners were realized in twelve locations in Algeria throughout field studies performed from June 2015 to July 2019. Interviews covered popular and vernacular names of the natural product, mode of use and administration, dose, period of treatment, toxicity and side effects among other data. The obtained results reveal the use of 113 medicinal plants (belonging to 53 families and 104 genera), 10 animal species and various products and by-products from different origins such as honey, olive oil, thorns, urine, milk, animal fat and the alkaline water of Zamzam. Basing on the frequency of citation (FC), use reports (UR) and use value (UV), the most used natural products for cancer treatment are honey (FC = 181, UR = 194, UV = 0.65), Nigella sativa L. (FC = 131, UR = 152, UV = 0.54), Aristolochia longa L. (FC = 118, UR = 144, UV = 0.51), Berberis vulgaris L. (FC = 111, UR = 142, UV = 0.51), Curcuma longa L. (FC = 107, UR = 121, UV = 0.43), Trigonella foenum-graecum L. (FC = 102; UR = 119, UV = 0.43), Citrus limon (L.) Obseck (FC = 97, UR = 120, UV = 0.43), Artemisia herba-alba Asso (FC = 92, UR = 115, UV = 0.41) and the holy water ‘Zamzam’ (FC = 110, UR = 110, UV = 0.43) respectively. Mixtures of two or more ingredients were frequently used. The use of Pelophylax saharicus skin’ was reported for the first time for the treatment of visible tumors and skin cancer. This is the first study documenting the traditional uses of various natural products for cancer treatment in Algeria. Our findings are relevant to document the traditional uses of numerous natural products and to provide background basis to search for novel compounds for cancer therapy.
Keywords: Traditional and complementary medicine, Ethnopharmacology, Natural products, Cancer, Algeria
1. Introduction
Cancer constitutes a major public health burden and one of the main causes leading to death in both developing and developed countries (WHO, 2018). In Algeria, the latest statistics of the Global Cancer Observatory (2018) have accounted 29,453 deaths caused by cancer (around 8% of all deaths). The number of new cancer cases has increased also from 28,736 in 2008 to 53,076 in 2018 (Benarba et al., 2014, Bray et al., 2018). The incidence of cancer is increasing in Algeria and the accessibility to health care is difficult due to the insufficient number of oncologists, the serious lack of adapted medical facilities designated for diagnosis and treatment and the incomplete health care framework in the country. Regrettably, the number of cancer centers is insufficient to cover the populations of the whole country (48 departments or wilayas). Therefore, underprivileged families mainly from rural areas and cancer sufferers living away from those cities must travel, in some cases till 1.500 km, via inadequate or even non-existent means of transport to reach these centers. There is also a strong gender bias in cancer treatment as some diseases like breast and cervical cancers are taboo. Besides, the therapeutic efficiency of the conventional treatments is low and often insufficient to eradicate this disease (Jelonek et al., 2017). These methods can even carry different side effects on body tissues where healthy cells are destroyed in the same time as cancerous ones (Durante and Formenti, 2018).
These circumstances, along with the failure of modern medicine in finding effective treatments for numerous diseases including cancer, have promoted the resurgence of traditional medicine (WHO, 2018). Traditional medicinal practitioners use a variety of natural products for their health-care needs, the knowledge of such practices was collected and refined over hundreds or even thousands of years (Yuan et al., 2016).
Moreover, natural products have received an increasing consideration for their potential use as novel cancer therapeutic agents since they hold a variety of important bioactive compounds acting as inhibitors of various stages of tumorigenesis and associated inflammatory processes (Amaral et al., 2019). Currently, more than 60% of anticancer drugs are derived from natural products although medicinal plants are considered the most important source by around 75%. Over 3000 plants have been reported at present worldwide to hold potential anticancer molecules and most of those are yet to be discovered (Rahman et al., 2019). Nigella sativa L., Trigonella foenum-graecum L., Aristolochia spp., Bryonia dioica Jacq., Cassia absus L., Aquilaria malaccensis Lam., Marrubium vulgare L., Lavandula maroccana Murb., Ephedra alata Decne., and Euphorbia resinifera O.Berg were the most used medicinal plants by cancer patients in North African countries mainly in Morocco (Alves-Silva et al., 2017, Bourhia et al., 2019, Kabbaj et al., 2012). However, Arum spp., Artemisia spp., Calotropis procera (Aiton) Dryand., Citrullus colocynthis (L.) Schrad., Nigella sativa L., Pulicaria crispa Sch.Bip., Urtica spp., and Withania somnifera (L.) Dunal were the most used plants species among cancer patients in the Near East countries (Abu-Darwish and Efferth, 2018, Ahmad et al., 2017). These medicinal plant species can provide a promising strategy for complementary medicine and plant-based drugs discovery.
Furthermore, preparations and uses of natural products differ significantly between regions even for the same country (Habtamu et al., 2014). Although Algeria is home of a great plant diversity with 3183 species and numerous endemic and threatened ones due to its climatic and topographic diversity (Ait Abderrahim et al., 2017, Boussaid et al., 2018), only few ethnobotanical and ethnomedicinal studies have been carried out dealing with this diversity and only limited preliminary works have been initiated by (Benarba, 2015, Benarba et al., 2015) then (Benarba and Pandiella, 2018) concerning the exploitation of this diversity for cancer treatment.
With a long history spanning many centuries, wars and colonization which led to the melting of different civilizations (Berber, Greco-Roman, Arab, Ottoman and French), Algeria is characterized as well by its diversified ethnic groups, cultures, languages and beliefs which leads subsequently in turn to diversified traditional medicinal practices. Hence, the traditional uses of natural products in Algeria constitute an important opportunity for focused screening based on their ethnopharmacological and ethnomedicinal utilizations.
Nevertheless, the ancestral knowledge about traditional medicine uses and the associated practices is still not well documented in Algeria. Thus, ethnopharmacological and ethnomedicinal investigations have become more than necessary to disclose locally important natural products and safeguard the traditional medicinal knowledge (Yuan et al., 2016). Also, there is an urgent need to develop a national pharmacopoeia besides national standards and guidelines of collect and uses. Moreover, the collected data are being used as basis in research that needs to be tested in clinical and lab trials.
The present study is an ethnopharmacological investigation aiming to identify and analyze the natural products used in traditional medicines for cancer treatment in Algeria. Another objective of this work is to enrich the communities’ knowledge databases and safeguard the cultural heritage concerning traditional medicines as recognized by the UNESCO (2003). To our knowledge, this is the first ethnopharmacological and ethnomedicinal investigation carried out on cancer in Algeria. Moreover, it will constitute the basis for pharmacological studies on cancer management.
2. Methodology
2.1. Data collection
2.1.1. Study area
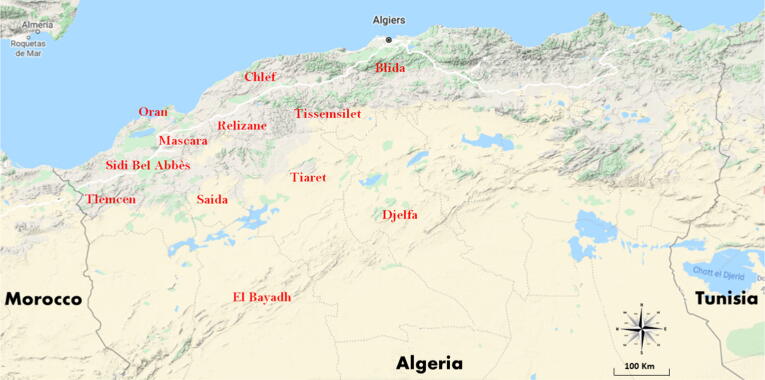
Ethnopharmacological information was collected throughout field studies performed during four years from June 2015 to July 2019. The study was carried out in twelve different locations in Algeria reflecting both, the traditional and the modern regions encompassing both modern Western-style healthcare and traditional, culture-based healthcare (Table 1, Fig. 1). This include the main big cities and the surrounding small villages of each population. The economy of the local populations is based mainly on agricultural activities (animal husbandry, pastoralism, cereal or vegetable farming) and commercial sector, service industries and the other activities are of less importance. The region covered in this study is characterized by a vast ecological and climatic diversity represented by the most characteristically ecosystems of the Algerian territory encompassing the coasts, mountains, forests, arid high plains, steppe and Sahara. These ecosystems shelter a huge faunistic and floristic biodiversity of international importance as defined by the Ramsar Convention. This cross-validation was performed to investigate probable cultural and regional differences within the cancer management used methods by the informants from a part, and in order to obtain representative dataset for the whole West- and Center region of Algeria from another part. Socio-demographic characteristics of the interrogated population are shown in Table 1.
Table 1.
Socio-demographic characteristics of the informants.
| Population |
Gender |
Age |
School level |
|||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Illiterate | Elementary | Secondary | Undergraduate | Graduate | ||
| Blida | 11 | 13 | [21–75] | 2 | 4 | 5 | 6 | 7 |
| Chlef | 9 | 11 | [24–67] | 3 | 3 | 8 | 4 | 2 |
| Djelfa | 10 | 19 | [20–77] | 10 | 9 | 4 | 4 | 2 |
| El Bayadh | 12 | 17 | [23–81] | 11 | 5 | 1 | 6 | 6 |
| Mascara | 9 | 16 | [25–70] | 3 | 8 | 5 | 4 | 5 |
| Oran | 12 | 18 | [21–65] | 2 | 6 | 5 | 4 | 13 |
| Relizane | 10 | 14 | [27–67] | 2 | 6 | 6 | 4 | 6 |
| Saida | 11 | 12 | [25–71] | 3 | 8 | 6 | 3 | 3 |
| Sidi Belabbes | 9 | 13 | [20–65] | 2 | 6 | 4 | 6 | 4 |
| Tiaret | 32 | 41 | [18–84] | 11 | 16 | 19 | 18 | 9 |
| Tissemsilt | 18 | 22 | [20–82] | 13 | 11 | 4 | 6 | 6 |
| Tlemcen | 11 | 14 | [23–64] | 5 | 7 | 3 | 4 | 6 |
| Total | 154 | 210 | [18–84] | 67 | 89 | 70 | 69 | 69 |
Fig. 1.
Geographical location of the study area (Locations names are written with red color).
2.2. Participants
In total, two hundred twenty-five informants were interviewed (n = 225). This includes twenty-one traditional healers (n = 21), seventy-three herbalists (n = 73) and one hundred thirty-one traditional medicine practitioners and rural dwellers most of them were recommended by other locals when the topic came into question (n = 131). The study was conducted in accordance with the requirements of the declarations of Helsinki. Therefore, semi-structured interviews based on note-taking while interviewing the informants (known as guided field walk) were conducted as described by Martin (1995) in collecting the ethnomedicinal data. The interview was conducted without time limit or pressure to allow the informants to answer questions naturally following the International Society of Ethnobiology (ISE) code of ethics.
It should be noted that cancer diagnosis is made by healthcare professionals and specialists in hospitals whereas traditional medicine is used to support the patients and provide care alongside conventional treatments. The study was approved by the scientific committee of the department of Natural and Life Sciences, Ibn Khaldoun University of Tiaret (Algeria). Interviews were performed through the local dialect of the informants and generally took place in public spaces. Informant consent was obtained prior to the interviews to authorize the collection, use and publication of data, then informants were asked to list natural products used to prevent and/or fight cancers and were requested to provide more detailed information about their uses (Albuquerque et al., 2014).
The questions covered during the interviews included, in addition to the sociodemographic information, popular and vernacular names of the species, parts used, mode of preparation and administration, dose, duration of treatment and knowledge about toxicity or side effects among other data. Local names were provided generally in Arabic and/or in Amazigh languages. All informants were asked whether they would be willing to provide a sample or to identify it in photographs if the material was not available.
3. Identification of medicinal species
Collected plant specimens, as pointed out by the informants, were pressed and dried on site then the voucher specimens were identified and conserved in the laboratory at the faculty of Natural and Life Sciences, University of Tiaret (Algeria). The identity of plant and animal species reported by the informants was verified and recognized by specialists from the Department of Natural and Life Sciences and by the available different resources and bibliography (Quézel, 1962-1963, Ozenda, 1977, Kaddem, 1990, Baba Aissa, 1991, Wilson and Reeder, 2005, Frost, 2020). The scientific names of plant species were also confirmed in accordance with the International Index of Plant Name (http://www.ipni.org) and the Plant List database (http://www.theplantlist.org) while animal species were confirmed in accordance with the Animal Diversity Web (https://www.animaldiversity.org).
3.1. Data analysis
The obtained ethnopharmacological data were assigned into 21 cancer type categories, which have been reported by informants. The use report (UR) was assessed by calculating the total uses for the natural product by all informants within each use-category for that product (Prance et al., 1987).
Frequency of Citation (FC) is calculated as the sum of informants that cite a use for the natural product (Prance et al., 1987).
The use value (UV) is a quantitative method that can be used in order to prove the relative importance of natural product known locally. It was calculated following the adaptation of da Silva et al. (2014) using the following formula:
where UV is the use value of the natural product p mentioned by the informant i; ΣUR ip is the number of uses reports of natural product s mentioned in each event by the informant i; n ip is the number of events in which the informant i cited the natural product p.
The homogeneity on the informants’ knowledge was evaluated by calculating the Informants’ Consensus Factor (FIC) (Andrade-Cetto and Heinrich, 2011) using the formula:
Where Nur is the number of use reports for a particular cancer type and Nt is the number of species cited for the same cancer by all informants. The values of the index range between 0 and 1, where values close to ‘1′ indicate the highest level of consensus.
The Medicinal Importance Index (MI) as a relative importance index of the use of plant species was calculated by dividing the number of use reports cited for a specific cancer by the number of species which have this use (Carrió and Vallès, 2012).
All the statistical analyses were performed using the computing environment R (R Development Core Team, 2013). Continuous data were represented as mean ± standard deviation while frequencies and percentages were calculated for categorical variables.
4. Results
4.1. Sociodemographic features
In the current study, female participants accounted for 58% of the total. In general, the age of the informants is comprised between 18 and 84 years old. Around 18% of the total informants were illiterate whereas 24% of them reached just the primary school. In fact, about 38% of the informants were undergraduate or graduate from the university.
4.2. Plant taxa diversity
Concerning herbal medicine, the obtained results have revealed the use of 113 medicinal plant species, belonging to 104 genera and 53 families, for cancer treatment (Table 2). The reported species were both gymnosperms (4 species, 4%) and angiosperms (109 species, 96%) including both monocotyledonous (12 species, 11%) and dicotyledonous (101 species, 89%) groups. Most of these medicinal plants are herbs (60%) while trees and shrubs represent 20% each.
Table 2.
Medicinal plant species used for cancer management in Algerian traditional medicines.
| Plant family: - Species | Voucher n° | Common name | Local name | FC | UR | Plant part used | Application | Cancer type |
|---|---|---|---|---|---|---|---|---|
| Amaranthaceae | ||||||||
| Atriplex halimus L. | EthTK015 | Sea orache | Gtaf | 92 | 105 | Leaf, flower | Infusion, powder | Thyroid, ovarian |
| Haloxylon scoparium Pomel | EthTK051 | Haloxylon | Remth | 13 | 13 | Aerial parts | Decoction | Liver |
| Spinacia oleracea L. | EthTK017 | Spinach | Salq | 8 | 12 | Root | raw, decoction | Breast, pancreatic, lung, prostate, skin |
| AnacardiaceaePistacia lentiscus L. | EthTK076 | Mastic | Dharw | 18 | 22 | Leaf, fruit | Powder, oil, decoction | Digestive, skin |
| Annonaceae | ||||||||
| Annona cherimola Mill. | EthTK007 | Cherimoya | Cherimoya | 12 | 14 | Fruit, seed | Raw, syrup | Breast, cervical |
| Annona muricata L. | EthTK008 | Sour Sop | Qachta | 12 | 15 | Fruit, seed | Raw, syrup | Breast, bladder, colorectal, prostate |
| Apiaceae | ||||||||
| Ammi visnaga (L.) Lam. | EthTK006 | Visnaga | Noukha | 14 | 14 | Seed | Infusion | Breast |
| Bunium incrassatum (Boiss.) Amo | EthTK021 | Pig Nut | Talghouda | 67 | 73 | Tuber | Powder | Colorectal, thyroid |
| Carum carvi L. | EthTK107 | Caraway | Karwya | 12 | 12 | Seed | Infusion, powder | Uterus |
| Cuminum cyminum L. | EthTK038 | Cumin | Kammun | 9 | 12 | Seed | Decoction, infusion | Lung, neck |
| Daucus sahariensis Murb. | EthTK040 | Saharian Carrot | Zrodya | 7 | 11 | Root, seed | Decoction, infusion | Kidney, digestive, skin |
| Ferula vesceritensis Coss. & Durieu ex Trab. | EthTK045 | Foetida | Hentit | 17 | 21 | Resin | Maceration | Breast, colorectal |
| Foeniculum vulgare Mill. | EthTK047 | Fennel | Besbass | 9 | 11 | Leaf, seed | Infusion, decoction | Digestive, cervical |
| Petroselinum crispum (Mill.) Fuss | EthTK070 | Parsley | Maâdnous | 17 | 23 | Stem, leaf, fruit | Decoction | Bladder, brain, kidney |
| Pimpinella anisum L. | EthTK108 | Anise | Yansoun | 13 | 13 | Fruits, seeds | Infusion, powder | Colorectal |
| Thapsia garganica L. | EthTK098 | Drias Plant | Derias, Bounafaa | 12 | 15 | Root | Decoction | Prostate, skin |
| Arecaceae | ||||||||
| Chamaerops humilis L. | EthTK029 | Fan Palm | Doum | 12 | 15 | Fruit | Raw, powder | Breast, brain, blood |
| Phoenix dactylifera L. | EthTK072 | Date Palm | Tmar | 16 | 20 | Fruit | Raw | Breast, brain, lung |
| Aristolochiaceae | ||||||||
| Aristolochia longa L. | EthTK012 | Snakeroot | Berrostom | 131 | 153 | Root, leaf | Powder | Breast, bone, digestive, prostate, ovary, uterus |
| Asphodelaceae | ||||||||
| Aloe succotrina Lam. | EthTK005 | Socotrine Aloe | Mor-w-sbar | 18 | 24 | Leaf, gel, juice | Decoction, oil, infusion | Breast, colorectal, esophageal, prostate, stomach, skin |
| Asteraceae | ||||||||
| Artemisia campestris L. | EthTK013 | Field wormwood | Tguouffet | 67 | 84 | Aerial parts | Infusion | Digestive |
| Artemisia herba-alba Asso | EthTK014 | white wormwood | Chih | 96 | 119 | Aerial parts | Infusion | Digestive, lung, kidney |
| Carthamus tinctorius L. | EthTK025 | Cartham | Cartam | 4 | 4 | Leaf, seed | Oil | Breast |
| Chamaemelum nobile (L.) All. | EthTK028 | Roman chamomile | Babounj Romani | 6 | 6 | Flower | Infusion | Breast |
| Echinops spinosus L. | EthTK042 | Echinops | Tassekra | 8 | 8 | Root, fruit, seed | Infusion, decoction | Breast |
| Matricaria chamomilla L. | EthTK061 | German Chamomile | Babounj Berri | 14 | 24 | Leaf, flower | Infusion, inhalation | Breast, liver, lung, prostate |
| Matricaria pubescens (Desf.) Sch.Bip. | EthTK062 | Chamomile | Ouezouaza | 9 | 12 | Leaf, flower | Infusion, inhalation | Breast, liver, lung, prostate |
| Inula viscosa (L.) Aiton | EthTK053 | Yellowhead | Magramane | 11 | 13 | Entire plant | Decoction, powder | Breast, bladder, kidney |
| Onopordum macracanthum Schousb. | EthTK067 | Thistle | Chouk lahmar | 8 | 11 | Flower, seed | Decoction, infusion | Colorectal, stomach |
| Saussurea costus (Falc.) Lipsch. | EthTK090 | Costus | Qist el Hindi | 13 | 16 | Rhizome | Decoction, powder | Digestive, prostate |
| Silybum marianum (L.) Gaertn. | EthTK091 | Milk Thistle | Shouk el Jmal | 12 | 18 | Stalk, seed | Juice, decoction | Colorectal, liver, prostate, skin |
| Berberidaceae | ||||||||
| Berberis vulgaris L. | EthTK016 | Barberry | Oud Ghriss | 119 | 142 | Peel, bark | Decoction, powder | Breast, blood, bladder, stomach, skin, ovary |
| Boraginaceae | ||||||||
| Borago officinalis L. | EthTK018 | Borage | Lessane el ferd | 10 | 14 | Stem, leaf, flower | Decoction | Colorectal, cervical, liver, lung, prostate |
| Brassicaceae | ||||||||
| Lepidium sativum L. | EthTK057 | Cress | Horf, hab errchad | 11 | 15 | Leaf, seed | Raw, powder | Breast, digestive, lung, uterus, ovary |
| Brassica oleracea subsp. capitata L. | EthTK020 | Wild Cabbage | Krom lahmar | 14 | 18 | Leaf | Raw, juice, decoction | Breast, cervical, liver |
| Burseraceae | ||||||||
| Boswellia sacra Flueck. | EthTK019 | Frankincense | Loubane | 21 | 24 | Resin | Oil | Breast, colorectal, skin |
| Commiphora myrrha (Nees) Engl. | EthTK034 | Myrrh | El morr | 11 | 16 | Soft sap | Raw, oil | Breast, liver, prostate, skin |
| CactaceaeOpuntia ficus-indica (L.) Mill. | EthTK109 | Prickly pear | Sabbar | 10 | 10 | Fruit | Raw | Bladder |
| Capparaceae | ||||||||
| Capparis spinosa L. | EthTK023 | Caper bush | El Kabbar | 12 | 15 | Root, fruit, flower | Powder, decoction | breast, bone, prostate |
| Costaceae | ||||||||
| Costus arabicus L. | EthTK035 | Spiral Ginger | Qist Al Bahri | 7 | 10 | Rhizome | Infusion, decoction | Breast, lung |
| Cucurbitaceae | ||||||||
| Citrullus colocynthis (L.) Schrad. | EthTK031 | Colocynth | Handhal | 13 | 17 | Stem, leaf, fruit | Decoction, pomade | Breast, colorectal, skin |
| Cucurbita maxima Duchesne | EthTK037 | Winter Squash | Kabouya | 80 | 117 | Fruit | Raw, decoction | Digestive, prostate, skin |
| Ecballium elaterium (L.) A.Rich. | EthTK041 | Squirting Cucumber | Fegous el hmir | 14 | 17 | Fruit | Raw, juice, oil | Breast, digestive, liver |
| Cupressaceae | ||||||||
| Juniperus phoenicea L. | EthTK054 | Phoenicean juniper | Ârâar | 12 | 15 | Leaf, fruit | Infusion, decoction | Colorectal, liver, prostate |
| Tetraclinis articulata (Vahl) Mast. | EthTK097 | Juniper gum | Sandrouss | 13 | 17 | Leaf, fruit | Infusion, decoction | Breast, cervical, liver, ovarian |
| Ephedraceae | ||||||||
| Ephedra alata Decne. | EthTK043 | Ephedra | Elenda, | 92 | 102 | Entire plant | Infusion, decoction | Breast, brain, colorectal, liver, lung |
| Euphorbiaceae | ||||||||
| Euphorbia guyoniana Boiss. & Reut. | EthTK044 | Euphorbia | Lobina | 11 | 14 | Aerial parts | Powder, decoction | Breast, ovarian, prostate |
| Phyllanthus emblica L. | EthTK073 | Emblica | El Amlaj | 7 | 12 | Fruit | Raw, juice, powder | Cervical, lung |
| Fabaceae | ||||||||
| Arachis hypogaea L. | EthTK010 | Peanut | Foul Soudani | 18 | 18 | Seed | Raw | Prostate |
| Ceratonia siliqua L. | EthTK027 | Carob | Kharroub | 12 | 12 | Fruit | Raw | Lung |
| Glycyrrhiza glabra L. | EthTK049 | Liquorice | Erq-Essous | 21 | 23 | Rhizome, root | Powder | Blood, lung |
| Glycine max (L.) Merr. | EthTK048 | Soybean | Foul el Souya | 4 | 4 | Seed | Oil, decoction | Breast, ovary |
| Phaseolus vulgaris L. | EthTK071 | Bean | Loubya | 3 | 3 | Seed | Raw | Breast |
| Retama raetam (Forssk.) Webb | EthTK082 | Retam | Retem | 12 | 16 | Root, leaf | Powder, decoction | Breast, cervical |
| Senegalia senegal (L.) Britton | EthTK110 | Gum arabic tree | Samgh arabi | 14 | 14 | Sap | Raw | breast |
| Trigonella foenum-graecum L. | EthTK101 | Fenugreek | Helba | 108 | 132 | Seed | Raw, infusion | Breast, brain, digestive, lung |
| Gentianaceae | ||||||||
| Centaurium erythraea Rafn | EthTK026 | Centaury | Mararet el hnech | 22 | 26 | Aerial parts | Powder | Breast, digestive |
| Iridaceae | ||||||||
| Crocus sativus L. | EthTK036 | Saffron crocus | Zaâfrane | 16 | 19 | Flower, pistil | Raw, infusion | Colorectal, kidney, liver, lung, prostate |
| Lamiaceae | ||||||||
| Ajuga iva (L.) Schreb. | EthTK002 | Herb Ivy | Chendgoura | 18 | 18 | Stem, leaf, flower | Powder | Lung |
| Lavandula antineae Maire | EthTK055 | Lavender | Khozama | 16 | 18 | Leaf, flower | Infusion, oil | Neck, urogenital, stomach, skin |
| Marrubium deserti (Noë) Coss. | EthTK060 | Horehound | Djaâda | 67 | 74 | Root, leaf | Decoction, infusion, ingestion | Breast, digestive, ovary |
| Marrubium vulgare L. | EthTK111 | Horehound | Merriwet | 39 | 44 | Stem, leaf | Decoction, infusion, ingestion | Breast, digestive, ovary |
| Mentha spicata L. | EthTK063 | Spearmint | Naânaâ | 16 | 20 | Stem, leaf | Infusion, oil | Breast, digestive, kidney |
| Rosmarinus officinalis L. | EthTK085 | Rosemary | Klil, Halhal, Azir | 15 | 18 | Stem, leaf, flower | Raw, infusion, inhalation | Breast, cervical, digestive, lung |
| Salvia verbenaca L. | EthTK089 | Wild clary | Kassâin raâie el hmam | 17 | 22 | Aerial parts | Infusion, decoction | Breast, blood, colorectal, liver, lung, intestine |
| Thymus vulgaris L. | EthTK100 | Thyme | Zaâtar | 18 | 21 | Entire plant | Infusion | Digestive |
| Origanum floribundum Munby | EthTK068 | Oregano | Zaâtar berri | 24 | 27 | Stem, leaf, flower | Infusion, inhalation | Breast, digestive, kidney, lung |
| Lauraceae | ||||||||
| Cinnamomum verum J.Presl | EthTK030 | Cinnamon | Qarfa | 15 | 17 | Peel, bark | Decoction | Breast, lung |
| Liliaceae | ||||||||
| Allium cepa L. | EthTK003 | Onion | Bsal | 92 | 108 | Bulb | Juice, decoction, | Lung, stomach |
| Allium sativum L. | EthTK004 | Garlic | Thoum | 81 | 103 | Bulb | Juice, decoction | Breast, blood, lung, esophageal, stomach |
| Linaceae | ||||||||
| Linum usitatissimum L. | EthTK058 | Flax | Zerriâat el Kettane | 19 | 25 | Seed | Raw, powder | Breast, blood, digestive, liver, ovary |
| Lycopodiaceae | ||||||||
| Lycopodium clavatum L. | EthTK059 | Clubmoss | Kebrita | 12 | 16 | Leaf, spore | Powder, oil | Brain, colorectal, liver, lung, oral, prostate |
| Lythraceae | ||||||||
| Lawsonia inermis L. | EthTK056 | Henna | Henna | 11 | 13 | Leaf | Powder, paste | Breast, skin |
| Moraceae | ||||||||
| Ficus carica L. | EthTK046 | Fig | Karmous | 13 | 15 | Fruit | Raw | Digestive, lung |
| Moringaceae | ||||||||
| Moringa peregrina (Forssk.) Fiori | EthTK064 | Moringa | Hab el Baan | 5 | 6 | Stem, leaf | Raw, powder, decoction | Colorectal, liver |
| Myrtaceae | ||||||||
| Syzygium aromaticum L. | EthTK095 | Clove | Qronfol | 15 | 19 | Flower | Raw, oil, powder | Liver, lung, oral, thyroid |
| Oleaceae | ||||||||
| Olea europaea L. | EthTK066 | Olive | Zitoune | 35 | 44 | Leaf, fruit | Extraction | Breast, lung |
| PedaliaceaeSesamum indicum L. | EthTK112 | Sesame | Sanouj | 11 | 14 | Seed | Raw, oil, powder | Breast, uterus, prostate |
| Pinaceae | ||||||||
| Pinus halepensis Mill. | EthTK074 | Aleppo Pine | Snawbar | 7 | 11 | Seed | Extraction | Esophageal, stomach |
| Piperaceae | ||||||||
| Piper nigrum L. | EthTK075 | Black Pepper | Felfel k’ḥal | 15 | 19 | Fruit, seed | Powder | Breast, stomach, pancreas |
| Plumbaginaceae | ||||||||
| Plumbago europaea L. | EthTK077 | Plumbago | Siwak erâayen | 9 | 12 | Entire plant | Powder, decoction | Lung, skin |
| Poaceae | ||||||||
| Hordeum vulgare L. | EthTK052 | Barley | Zraâ, Cheêir | 14 | 18 | Seed | Raw, juice, powder | Colorectal, liver, stomach, uterus, prostate |
| Stipa tenacissima L. | EthTK094 | Alfa | Halfa | 3 | 3 | Leaf | Powder | Skin |
| Triticum turgidum L. | EthTK102 | Wheat | Guemh | 19 | 21 | Entire plant, seed | Raw, juice, powder | Breast, colorectal |
| Polygonaceae | ||||||||
| Rheum palmatum L. | EthTK084 | Rhubarb | Rawend | 11 | 12 | Rhizome | Powder | Digestive, oral |
| Punicaceae | ||||||||
| Punica granatum L. | EthTK081 | Pomegranate | Rommane | 54 | 64 | Root, leaf, fruit, peel | Raw, syrup, juice, decoction | Breast, brain, oral, stomach, prostate, colorectal |
| Ranunculaceae | ||||||||
| Nigella sativa L. | EthTK065 | Devil in the bush | Habet el Baraka | 140 | 160 | Seed | Raw, decoction | Breast, blood, colorectal, liver, lung, uterus, skin |
| Rhamnaceae | ||||||||
| Rhamnus alaternus L. | EthTK083 | Buckthorn | Meliless | 12 | 12 | Leaf | Infusion | Breast |
| Ziziphus lotus (L.) Lam. | EthTK106 | Jujube | Sedra | 12 | 18 | Leaf, flower, fruit | Raw, infusion | Breast, colorectal, lung |
| Rosaceae | ||||||||
| Crataegus azarolus L. | EthTK113 | Azarole | Zaârour | 5 | 5 | Leaf, fruit | Infusion, decoction | Colorectal |
| Prunus armeniaca L. | EthTK079 | Apricot | Mechmech | 12 | 16 | Root, leaf, flower, seed | Decoction, powder, oil | Breast, digestive, liver |
| Prunus amygdalus Batsch | EthTK078 | Almond | Louz el mor | 18 | 23 | Root, leaf, seed | Raw, powder, decoction | Breast, bladder, colorectal, oral, uterus |
| Prunus persica (L.) Batsch | EthTK080 | Peach | Khoukh | 80 | 90 | Root, leaf, seed | Raw, powder, decoction | Breast, digestive |
| Rubus fruticosus G.N.Jones | EthTK086 | Blackberry | Allaïak | 8 | 10 | Root, leaf, fruit | Raw, powder, decoction | Colorectal, oral |
| Rubus idaeus L. | EthTK087 | Raspberry | Toute barri | 12 | 14 | Leaf, fruit | Raw, powder, decoction | Colorectal, oral, ovary |
| Rubiaceae | ||||||||
| Coffea canephora Pierre ex A.Froehner | EthTK033 | Robusta coffee | Qahwa | 9 | 11 | Seed | Raw, infusion | Colorectal, oral, prostate |
| Rutaceae | ||||||||
| Citrus limon (L.) Osbeck | EthTK032 | Lemon | Limone | 100 | 123 | Fruit, peel | Juice, infusion | Breast, digestive, lung, liver, skin |
| Ruta chalepensis L. | EthTK088 | Fringed rue | Fidjel | 11 | 14 | Stem, leaf, flower | Decoction | Colorectal, prostate |
| Sapindaceae | ||||||||
| Aesculus hippocastanum L. | EthTK001 | Horse-chestnut | Kastal-hindi | 9 | 11 | Leaf, bark, seed | Powder, decoction | Breast, liver |
| SapotaceaeArgania spinosa (L.) Skeels | EthTK011 | Argan | Argan | 14 | 17 | Seed | Oil | Breast, ovary, skin |
| Solanaceae | ||||||||
| Capsicum annuum L. | EthTK024 | Pepper | Sasafinda | 14 | 19 | Fruit | Raw, decoction | Bladder, digestive, lung, skin |
| Solanum nigrum L. | EthTK092 | Nightshade | Aneb edhib | 17 | 21 | Leaf, fruit | Raw, decoction | Breast, colorectal, cervical |
| Solanum tuberosum L. | EthTK093 | Potato | Batata | 8 | 12 | Tuber | Raw, maceration | Breast, colorectal, cervical, prostate |
| Tamaricaceae | ||||||||
| Tamarix africana Poir. | EthTK096 | African tamarisk | Tarfa | 6 | 6 | Stem, leaf, flower | Decoction, powder | Cervical |
| Theaceae | ||||||||
| Camellia sinensis (L.) Kuntze | EthTK022 | Tea | Chay | 15 | 19 | Leaf | Infusion | Breast, lung, ovary, skin |
| Thymelaeaceae | ||||||||
| Thymelaea microphylla Meisn. | EthTK099 | Thymelaea | Methnan | 13 | 15 | Stem, leaf, flower | Decoction | Cervical, uterus |
| Aquilaria malaccensis Lam. | EthTK009 | Agar wood | Oud | 7 | 11 | Stem, leaf, flower | Oil, decoction | Lung, thyroid |
| Tiliaceae | ||||||||
| Grewia tenax (Forssk.) Fiori | EthTK050 | White Crossberry | Godaym | 26 | 80 | Fruit | Raw | Breast, digestive, liver, lung, prostate, ovary, skin |
| Urticaceae | ||||||||
| Urtica dioica L. | EthTK103 | Nettle | Herrig | 22 | 25 | Entire plant | Infusion, decoction | Breast, bone, stomach, prostate |
| Vitaceae | ||||||||
| Vitis vinifera L. | EthTK104 | Grape vine | Zbib | 23 | 23 | Leaf, fruit | Raw, powder | Breast |
| Zingiberaceae | ||||||||
| Curcuma longa L. | EthTK039 | Turmeric | Curcum | 112 | 133 | Rhizome | Powder, decoction | Breast, bone, lung, digestive, uterus |
| Zingiber officinale Roscoe | EthTK105 | Ginger | Zanjabyl | 88 | 116 | Rhizome | Powder, infusion | Breast, colorectal, liver, lung |
| Zygophyllaceae | ||||||||
| Peganum harmala L. | EthTK069 | Harmel | Harmel | 66 | 87 | Seed | Raw, powder | Breast, brain |
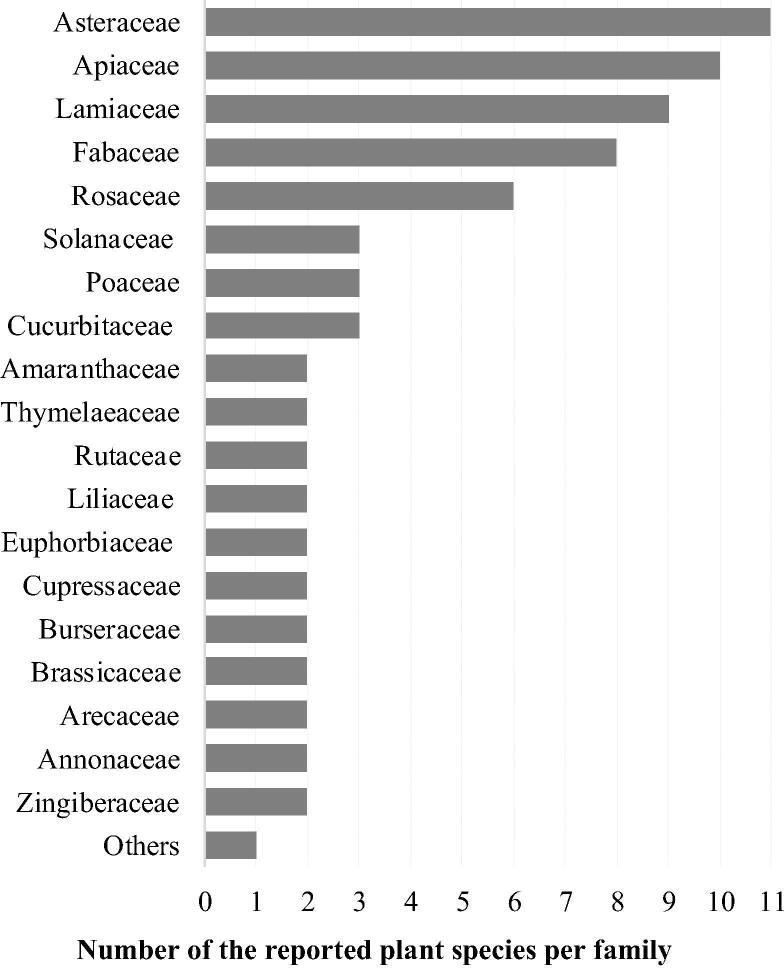
The Asteraceae family was represented by the highest number of species (11 species), followed by the Apiaceae (10 species), Lamiaceae (9 species), Fabaceae (8 species) and Rosaceae (6 species) families respectively. Amaranthaceae, Cucurbitaceae, Poaceae and Solanaceae families were represented by three species each while Annonaceae, Arecaceae, Brassicaceae, Burseraceae, Cupressaceae, Euphorbiaceae, Liliaceae, Rutaceae, Thymelaeaceae and Zingiberaceae families were represented by only two species each. The other families were represented by one species each (Fig. 2).
Fig. 2.
Plant families employed for cancer therapy in Algerian traditional medicines.
Several reported plant species are cultivated and used either for direct consumption or vended commercially such as Brassica oleracea subsp. capitata L., Cucurbita maxima L., Hordeum vulgare L., Phaseolus vulgaris L., Piper nigrum L., Prunus sp. and Triticum turgidum L. among others. However, many other species are grown around the homesteads or along rural infrastructures like Echinops spinosus L., Marrubium vulgare L., Olea europaea L., Onopordum macracanthum Schousb, Thapsia garganica L. and Urtica dioica L. Other species are used for their ornamental values, shade or for feeding human or livestock such as Atriplex halimus L., Ficus carica L. and Juniperus sp. Interestingly, several species are belonging to the Algerian steppe region and Sahara namely Artemisia sp., Ajuga iva (L.) Schreb, Haloxylon scoparium Pomel, Marrubium deserti (Noë) Coss., Origanum floribundum Munby, Retama raetam (Forssk.) Webb, Stipa tenacissima L., Thymelaea microphylla Meisn., Thymus vulgaris L., Peganum harmala L. and Phoenix dactylifera L.
Together with non-native plants, many endemic plant species from Algeria, or Algeria, Morocco and Tunisia, have been reported i.e. Ammi visnaga (L.) Lam., Argania spinosa (L.) Skeels, Artemisia campestris L., Artemisia herba-alba Asso, Atriplex halimus L., Bunium incrassatum (Boiss.) Amo, Carthamus tinctorius L., Citrullus colocynthis (L.) Schrad, Ferula vesceritensis Coss. & Durieu ex Trab., Lavandula antineae Maire, Marrubium deserti (Noë) Coss., Matricaria pubescens Schultz, Origanum floribundum Munby, Petroselinum crispum (Mill.) Fuss, Salvia verbenaca L., Tetraclinis articulata (Vahl) Mast. and Thymelaea microphylla Meisn. The use of exogenous species like Annona sp., Boswellia sacra Flueck, Saussurea costus (Falc.) Lipsh. and Syzygium aromaticum L. was also reported.
4.3. Quantitative ethnopharmacology
On the basis of citation frequency, the most cited plant species by the informants to treat any cancer type were respectively Nigella sativa L. (FC = 131), Aristolochia longa L. (FC = 118), Berberis vulgaris L. (CF = 111), Curcuma longa L. (CF = 107), Trigonella foenum-graecum L. (FC = 102), Citrus limon (L.) Osbeck (CF = 97), Artemisia herba-alba Asso (CF = 92), Ephedra alata Decne (CF = 91), Atriplex halimus L. and Allium cepa L. (CF = 90). Furthermore, on the basis of use reports and use value indices for each species, Nigella sativa L. was the most reported/important plant species (UR = 152, UV = 0.54) followed by Aristolochia longa L. (UR = 144, UV = 0.51), Berberis vulgaris L. (UR = 142, UV = 0.51), Curcuma longa (UR = 121, UV = 0.43), Trigonella foenum-graecum L. (UR = 119, UV = 0.43), Citrus limon Decne (UR = 120, UV = 0.43), Artemisia herba-alba Asso (UR = 115, UV = 0.41) and Allium cepa L. (UR = 107, UV = 0.38). However, the less cited plant species with the lowest use reports were Stipa tenacissima L. and Phaseolus vulgaris L. (CF = 3, UR = 3, UV = 0.01) (Table 2).
4.4. Plant parts and modes of use
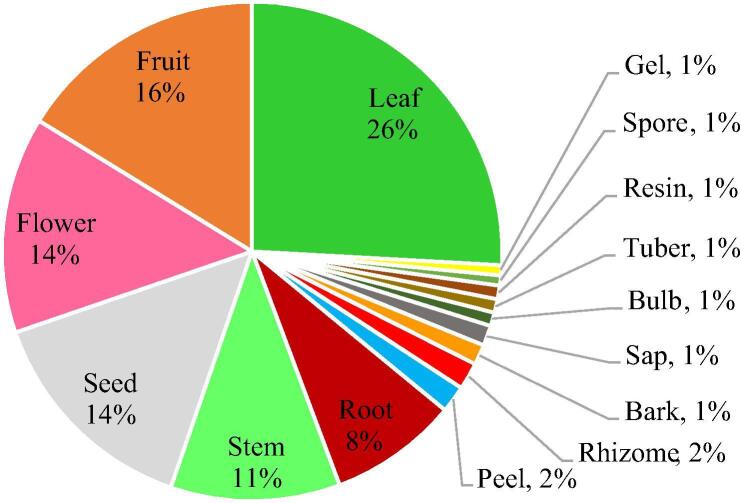
The plants species were used entirely or only the parts supposedly interesting were selected by the informants. Overall, leaves were the most commonly used part by around 26% followed by fruits (15%), flowers (14%) and seeds (14%) as the next most likely plant parts to be used. Stems and roots are used by around 11% and 9% respectively however, the other parts are the least used by the informants (Fig. 3).
Fig. 3.
Frequency of plant parts used for cancer therapy in Algerian traditional medicines.
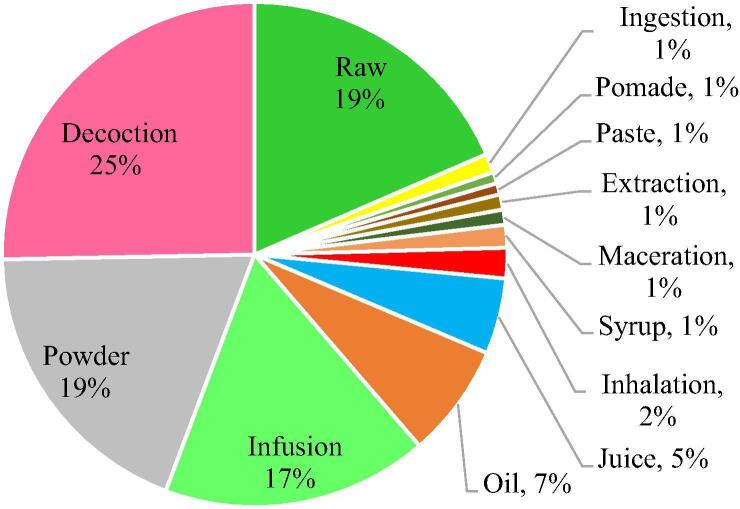
Various methods of use of the medicinal plants’ parts were reported; about 19% of the plants used by the informants were consumed directly as raw material. However, most often, a decoction was prepared (27%) which involved boiling the crushed plant part in water till the volume will be reduced by around the half to extract the juice to be administered orally. Moreover, 18% of the reported plant parts were crushed or powdered while 16% were used as infusion. Herbal infusions take less time to prepare than decoction, but the herbs should stand for 10 min in hot water to steep thoroughly while tightly covered. In other cases, plant part was dried then administered orally as pills. Besides, seeds, if not administered orally, were used for oil extraction (Fig. 4).
Fig. 4.
Frequency of methods of use of medicinal plants parts for cancer therapy in Algerian traditional medicines.
4.5. Animal species diversity
Concerning zootherapy, the use of several species belonging to the animal kingdom and their derived products was reported for cancer treatment in Algerian traditional medicines (Table 3). The consumption of various wild animal species such as Atelerix algirus (FC = 12, UR = 12, UV = 0.04), Cancer pagurus (FC = 28, UR = 28, UV = 0.10), Corallium rubrum (FC = 31, UR = 31, UV = 0.11) and Varanus griseus (FC = 14, UR = 24, UV = 0.05) has been recommended for the treatment of all types of cancer. In addition, the skin of the Sahara frog Pelophylax saharicus was reported for the first time for the treatment of visible tumors and skin cancer via external application (FC = 21, UR = 36, UV = 0.08). Furthermore, some informants have stated even the consumption of the excrement of Hystrix cristata (FC = 12, UR = 19, UV = 0.04) and the use of Atelerix algirus thorns as decoction since they believe on their therapeutical virtues.
Table 3.
Animal species used for cancer management in Algerian traditional medicines.
| Animal family: - Species | Common name | Local name | FC | UR | UV | Part used | Application | Cancer type |
|---|---|---|---|---|---|---|---|---|
| Apidae | ||||||||
| Apis mellifera | Honey bee | Nahl | 181 | 194 | 0.65 | Honey | Raw, infusion | All types |
| Bovidae | ||||||||
| Bos Taurus | Cow | Bakara | 31 | 31 | 0.11 | Colostrum | Raw | All types |
| Capra aegagrus hircus | Goat | Maaza | 43 | 43 | 0.15 | Milk | Beverage | All types |
| 39 | 39 | 0.14 | Butter | Beverage | All types | |||
| Camelidae | ||||||||
| Camelus dromedaries | Camel | Naqa | 53 | 53 | 0.19 | Urine | Beverage | All types |
| 53 | 53 | 0.19 | Milk | Beverage | All types | |||
| Cancridae | ||||||||
| Cancer pagurus | Edible crab | Boujniba | 28 | 28 | 0.10 | Entire animal | Eat | All types |
| Coralliidae | ||||||||
| Corallium rubrum | Red coral | Morjane | 31 | 31 | 0.11 | Entire animal | Eat | All types |
| Erinaceidae | ||||||||
| Atelerix algirus | Algerian hedgehog | Guenfoud | 12 | 18 | 0.04 | Entire animal | Eat | All types |
| 12 | 18 | 0.04 | Thorns | Decoction | All types | |||
| Hystricidae | ||||||||
| Hystrix cristata | Crested porcupine | Dhorbane | 12 | 19 | 0.04 | Excrement | Eat | All types |
| Ranidae | ||||||||
| Pelophylax saharicus | Sahara frog | Jerana | 21 | 36 | 0.08 | Skin | External application | Visible tumors, skin |
| Varanidae | ||||||||
| Varanus griseus | Desert monitor | Werl sahraoui | 14 | 24 | 0.05 | Entire animal | Eat | All types |
4.6. Other natural products
It has been also reported the use and consumption of domestic animal by-products like the fresh cow colostrum (Bos Taurus; FC = 31, UR = 31, UV = 0.11) and goat milk (Capra aegagrus hircus; FC = 43, UR = 43, UV = 0.15). The goat butter was used as well for the preparation of mixtures. It should be noted that, in many cases, milk and urine of Camelus dromedarius were recommended for beverage in mixture mainly by informants living in the Sahara (FC = 53, UR = 53, UV = 0.19).
Remarkably, honey bee was the most cited natural product by the informants in the present study (FC = 181, UR = 194, UV = 0.65). Informants have reported mainly honey produced from Ziziphus lotus flowers but most of them have stated that any pure honey could be of interest. Honey was administered fresh or in infusion, alone or in combination with the other natural products from different origins. The religious influence of many practices resulted in the multiple uses of the holy water ‘Zamzam’ for drink or mixtures as a potential therapeutic agent for the preparation of various (FC = 110, UR = 110, UV = 0.43).
4.7. Monotherapy versus the use of mixtures
Interestingly, many informants have reported the use of mixed plant-based preparations with chemical cancer drugs. It is important to note that the mixture of two or more ingredients was the most common mode of use described among the informants. The storage and consumption of dried medicinal plants was also reported especially for species which are not available throughout the year. The most indicated remedies consisted of mixtures of more than one natural product associated with additives or excipients such as honey, olive oil, milk, butter, vinegar, animal fat or even Zamzam water. These additives are supposed to transport the remedies while diminishing the bitterness and severe taste to make mixtures more palatable.
4.8. Cancers’ category and natural products
In general, the reported animal species and their derived by-products were used for all types of cancers and were not specifically indicated for only a definite type of cancer except for Pelophylax saharicus skin’ which was preconized for external application on cancerous skins and visible tumors. Nevertheless, various aromatic and medicinal plant species were used to manage specific types of cancer. Accordingly, the highest number of plant species was reported to manage breast (65 species), colorectal (60 species), liver (50 species), stomach (45 species), esophageal (41 species) and oral (42 species) cancers while the lowest number of species (11 species) was reported for bone cancer treatment (Table 4).
Table 4.
Informants' Consensus Factor (FIC) and Medicinal Importance Index (MI) of medicinal plants used for cancer management in Algerian traditional medicines.
| Cancer | Use reports | Plant species | FIC | IMI |
|---|---|---|---|---|
| Breast | 678 | 65 | 0.91 | 10.43 |
| Colorectal | 565 | 60 | 0.90 | 9.42 |
| Stomach | 401 | 45 | 0.89 | 8.91 |
| Liver | 355 | 50 | 0.86 | 7.10 |
| Esophageal | 133 | 41 | 0.70 | 3.24 |
| Oral | 116 | 42 | 0.64 | 2.76 |
| Lung | 356 | 39 | 0.89 | 9.13 |
| Pancreas | 84 | 35 | 0.59 | 2.40 |
| Intestine | 114 | 40 | 0.65 | 2.85 |
| Prostate | 99 | 33 | 0.67 | 3.00 |
| Skin | 120 | 29 | 0.76 | 4.14 |
| Blood | 88 | 26 | 0.71 | 3.38 |
| Brain | 69 | 22 | 0.69 | 3.14 |
| Ovary | 70 | 18 | 0.75 | 3.89 |
| Cervical | 73 | 22 | 0.71 | 3.32 |
| Kidney | 71 | 16 | 0.79 | 4.44 |
| Thyroid | 99 | 16 | 0.85 | 6.19 |
| Uterus | 53 | 16 | 0.71 | 3.31 |
| Bladder | 52 | 12 | 0.78 | 4.33 |
| Bone | 29 | 11 | 0.64 | 2.64 |
| Neck | 23 | 12 | 0.50 | 1.92 |
The consistency of the use-reports within each cancer category was evaluated numerically using the informants’ consensus factor (CIF). There was a great agreement amongst informants about ethnomedicinal utilizations of plant species for specific cancer with FIC values ranging between 0.50 and 0.91 revealing high homogeneity in the reported cancer types (Table 4). The obtained higher values reflect the important number of use reports for each particular cancer type i.e. breast (FIC = 0.91, UR = 675), colorectal (FIC = 0.90, UR = 563), stomach (FIC = 0.89, UR = 393), liver (FIC = 0.86, UR = 352), lung (FIC = 0.89, UR = 346) and esophageal (FIC = 0.70, UR = 133). However, the medicinal importance index values ranged from 1.92 to 10.38; the higher values were recorded respectively for breast cancer (IMI = 10.38), colorectal (IMI = 9.38), lung (IMI = 8.87), stomach (IMI = 8.73), liver (IMI = 7.04). However, the lower values were recorded for oral (IMI = 2.76), bone (IMI = 2.45), pancreas (IMI = 2.37) and neck cancer (IMI = 1.92) (Table 4).
4.9. Dosage
Dosage varied significantly between one informant and another which made difficult establish standards or guidelines of uses. Usually, the prescribed dosages are undertaken by hand palm, tablespoon, little finger index, coffee cup or glass measures or weight. The commonly described dose by informants for decoction and infusion was a glass measure (100 to 150 mL) which could be mixed with honey but not with sugar and administered orally two to three times per day before breakfast or after lunch and dinner. For mixtures and preparations, ingredients are ground and crushed then well mixed with some additives (honey, olive oil, butter, milk, water…) in order to obtain a paste. This paste is designed for the formation of tablets and pills or it is stored in hermetically closed glass jars. For certain preparations, informants recommend that the mixture should be combined with additives just before consumption. Patients usually take one to three tablet or tablespoon three times per day.
5. Discussion
Algerian populations hold enormous traditional knowledge and practices on natural products that are being used for the treatment and management of various ailments including cancer (Azzi et al., 2012, Chermat and Gharzouli, 2015). This precious knowledge was handed down through apprenticeship from earlier generations to descendants and through the intermingling of diverse ethnicities and civilizations in North Africa (Bouasla and Bouasla, 2017, Bouzabata and Mahomoodally, 2019).
In the present study, 128 natural products have been recognized traditionally used for cancer treatment; 113 medicinal plant species were reported along with 10 animal species besides various products and by-products such as honey, olive oil, thorns, urine, milk, animal fat and even the holy water ‘Zamzam’ (Table 2, Table 3).
Besides, the use of natural products for cancer treatment might be explained by the failure of the standard cancer therapy, or because of the inaccessibility to a standard treatment (Ahmad et al., 2017). In fact, some natural products from various sources are known to have the ability to enhance several physiological and molecular pathways which are required for cancer treatment (Sharifi-Rad et al., 2019). In addition, it is well known that drought and temperature changes alter the metabolic composition of plants and increase the metabolites related to abiotic stress tolerance (Chorfi and Taïbi, 2011, Taïbi et al., 2018, Taïbi et al., 2017). Consequently, the abiotic stress occurring under arid conditions increase the amount of antioxidant compounds in plants (Taïbi et al., 2016) which might explain partially some of the alleged anticancer properties of the reported medicinal plants belonging to steppe and Sahara.
5.1. Plant taxa diversity
The diversity of plant taxa inventoried throughout the present study for cancer management in Algeria, represented by 113 plant species distributed within 104 genera and 53 families, demonstrates the importance of the local populational knowledge in the use of traditional medicines. Previous ethnobotanical studies carried out in Algeria, for not specific ailments, have reported 58 plant species (50 genera and 27 families) in the region of M’sila (east Algeria) (Boudjelal et al., 2013), 41 plant species (37 genera and 24 families) in the region of Hodna (east Algeria) (Sarri et al., 2015), 141 plant species (125 genera and 54 families) in the region of Mascara (north west Algeria) (Benarba et al., 2015), 98 species (90 genera and 48 families) in the region of Tizi-ouzou (north center Algeria) (Meddour and Meddour-Sahar, 2016) and 90 species (85 genera and 42 families) in the region of Skikda (north east Algeria) (Bouasla and Bouasla, 2017).
The most represented families were Asteraceae (11 species, 20%), Lamiaceae (10 species, 19%), Apiaceae (9 species, 17%) and Fabaceae (8 species, 15%) respectively. The predominance of these plant families in the medicinal flora is well established in Algeria (Benarba, 2015, Meddour and Meddour-Sahar, 2016, Sarri et al., 2015, Sarri et al., 2014) and in the whole Mediterranean region (González-Tejero et al., 2008). Probable reason for the high number of anticancer plant species reported in these families could be due to the presence of special and effective bioactive ingredients holding potential biological activities, e.g. flavonoids, sesquiterpenes lactones, tricyclic sesquiterpenes and germacranolide sesquiterpene lactones in the Asteraceae family (Babaei et al., 2018, Shoaib et al., 2017), and terpenoids, flavonoids, coumarins, polyacetylenes, steroids, sesquiterpenes and flavonols in the Apiaceae family (Moazzami Farida et al., 2018). A lot of bioactive compounds with proven therapeutic activity are present in the Lamiaceae family such as arbutin (Rychlinska and Nowak, 2012), apigenin and naringenin (Stacks, 2015), luteolin (López-Lázaro, 2009), hesperidin (Lee et al., 2010) and rutin (Chua, 2013). Some other bioingredients are specific to this family such as clerodendranoic acid (Zheng et al., 2012) and carnosic acid (Birtić et al., 2015).
5.2. Quantitative ethnopharmacology
Quantitative ethnopharmacology analysis has demonstrated that Nigella sativa L. (UR = 152, UV = 0.54), Aristolochia longa L. (UR = 144, UV = 0.51), Berberis vulgaris L. (UR = 142, UV = 0.51), Curcuma longa L. (UR = 121, UV = 0.43), Trigonella foenum-graecum L. (UR = 119, UV = 0.43), Citrus limon L. (UR = 120, UV = 0.43), Artemisia herba-alba Asso (UR = 115, UV = 0.41) and Allium cepa L. (UR = 107, UV = 0.38) were the most reported plant species for the management of cancer in Algeria. Interestingly, despite the fact that these species are frequently cited for the treatment of various ailments worldwide including Algeria, they have been reported at topmost species for cancer management in the neighboring countries from North Africa according to the review achieved by Alves-Silva et al. (2017). Remarkably, even though the inventoried species were frequently reported in previous studies carried out in Algeria for multiple uses, only Atriplex halimus L., Berberis vulgaris L., Prunus persica (L.) Batsch and Vitis vinifera L. that have been reported for cancer management by Benarba et al. (2015) in addition to Allium cepa L. and Pistacia lentiscus L. mentioned by Bouasla and Bouasla (2017). In another study, Benarba (2015) has stated only Berberis vulgaris L., Aristolochia longa L., Atriplex halimus L., Glycyrrhiza glabra L., Nigella sativa L., Pimpinella anisum L., Allium sativum L., Thymus vulgaris L. and Artemisia herba-alba Asso for the management of breast cancer.
5.3. Plant forms, plant parts and modes of use
The medicinal plants reported in this study are not all locally cultivated and most of them grow naturally in the wild. Furthermore, many species pass the borders from other countries without proper inspection and quality control verification. Other species are grown around the homesteads or along roads borders and rural infrastructures which makes them susceptible to pollution and toxicity problems. The most common plants’ life-forms used by the traditional healers for the preparation of ethnomedicines in treating cancers were herbs (60%), shrubs (20%) and trees (20%). The use of herbs could be due to their higher abundance, easy collection and therapeutic efficacy (Ahmad et al., 2009). However, the use of shrub and tree life-forms in herbal medicine may be due to their accessibility throughout the year (Khan et al., 2013). In addition, the most common used plant parts among informants were leaves (26% plants, Fig. 2). Fortunately, the use of these parts does not threaten the life cycle of the plants (Bhat et al., 2013). However, the subsequent frequent harvested plant parts were fruits (15%), flowers (14%), seeds (14%) and stems (11%). The studies conducted by Panghal et al., 2010, Ghasemi Pirbalouti et al., 2012, Dolatkhahi et al., 2014 have reported similarly the predominant use of plant leaves followed by flowers, fruits and stems. Moreover, roots harvest was also reported in this study (9%). This is possibly due to their richness with bioactive components (Adnan et al., 2014). The unsustainability of roots harvesting is well known by conservationists who labeled those used medicinal plants as highly threatened (Maroyi, 2013).
Furthermore, the most common mode of preparation of plants parts was decoction (27%), followed by direct consumption (19%), powdering (18%) and infusion (16%). Similar findings were reported by Rajaei and Mohamadi (2012). The mentioned methods of preparation could be suitable for some plants but not for the others. In fact, the boiling procedure reported by the informants can cause severe degradation of the therapeutic components in some medicinal plants. In addition, the suitable dosage required to have the expected benefits is not clear throughout this study. This concern has been raised also by several researchers indicating that the fixed doses to be administered by patients in traditional medicine is still not yet well-defined (Jaradat et al., 2016). Therefore, further studies are needed to determine the concentration of active ingredients with respect to their method of preparation since categorization of finished natural products into dosage forms may aid as well to establish specific procedures for quality control and stability testing.
5.4. Animal species diversity
Likewise, zootherapy based on the use of animals or animal derived products has been practiced in traditional medicine of various cultures at the same extent as phytotherapy (Alves et al., 2011). However, research on animals remains neglected in comparison to medicinal plants that are used in larger quantities with great specific variation (Alves and Rosa, 2007). Recently, animal products are increasingly used as raw materials in the preparation of modern medicines and herbal preparations (Alves and Rosa, 2005). In this study 10 animal species have been reported for the treatment of cancer. With regards to their habitat type, 7 animal species are terrestrials, 2 are aquatic species and 1 amphibian. In fact, the use of zootherapy in traditional medicine is related to the faunal composition and the relative abundance of animal species in the given region in which the people live (Alves et al., 2011, Alves and Rosa, 2007). Most animal species used in Algerian traditional medicines are native to arid and semi-arid regions with the exception of Cancer pagurus and Corallium rubrum. The use of Pelophylax saharicus skin’ in traditional medicine to treat visible tumors is reported for the first time throughout the present study. Interestingly, several natural products secreted from frog skins have been reported as novel and potent antimicrobial and anticancer peptide agents; Kang et al. (2012) have described Brevinin as potentially useful new anticancer peptide agent against several tumor cell lines namely skin (SK-MEL-2), lung (A549), stomach (MKN45), colon (HCT116), kidney (A498), prostate (PC-3) and ovary (SK-OV-3). Later, Hassanvand Jamadi et al. (2019) have stated the significant anticancer activity of Brevinin in vitro on human breast (MCF-7) and lung (A549) cancer cells with less cytotoxic effects on human red blood cells. By the same, Temporin is another natural frog product characterized by its cytotoxic effect on MCF-7 breast cancer cell line and capable to induce cell death (Shaheen et al., 2018).
5.5. Other natural products
In addition, the reported animal derived products consisted mainly in honey, milk, butter, thorns and camel urine. Some of these products have been the subject of several studies which demonstrated their health benefits. For instance, honey holds several compounds with anti-cancer activity such as phenolic acids (caffeic acid and gallic acid) and flavonoids (catechin, kaempferol and quercetin among others) (Waheed et al., 2019). It was reported to have antimutagenic (Saxena et al., 2012), tumor necrosis factor inhibiting (Ahmed and Othman, 2013, Can et al., 2015), antioxidant (Almasaudi et al., 2016), apoptotic, immunomodulatory, antiproliferative (Jaganathan et al., 2015), anticarcinogenic action (Subramanian et al., 2016), anti-inflammatory and estrogenic effects (Porcza et al., 2016). Local honey of Ziziphus lotus which is the most cited in this study was recently characterized for the first time by Zerrouk et al. (2018). However, despite that Cheng et al. (2019) have recently recognized the anticancer effect Ziziphus jujube honey’ on hepatocellular carcinoma, there is no published data about the anticancer activity of Ziziphus lotus honey to the knowledge of the authors.
Besides, consumption of milk or dairy products (cheese, butter…) is correlated with a reduced risk of numerous types of cancer (Jeyaraman et al., 2019). These properties are attributed to the variety of components such as fat compounds, conjugated linoleic acid, and proteins such as casein and vitamins (A, B, C, D) which have been proved to have protective and/or anticarcinogenic properties (Davoodi et al., 2013). The use of camel urine by Muslims comes from the fact that prophet of Islam Mohamad ‘Peace Be Upon Him’ advised its use in the treatment of various ailments (Alebie et al., 2017).
In fact, informants have recorded the wide use of the holy water of Zamzam, from Zamzam well in Saudi Arabia, as narrated in holy books of various religions including the Torah, the Bible, and the Quran (Khalid et al., 2014). Zamzam water is a naturally hard alkaline type of water (average pH of 8) with unique physicochemical properties that are different from any other water (Al Doghaither et al., 2016). Analysis revealed that Zamzam water holds high concentrations of minerals such as calcium, magnesium, sodium, potassium and fluoride. It contains also arsenic and nitrate below the level of human consumption danger but above the standard limit of WHO (Shomar, 2012). The healing properties of Zamzam water may be attributed in part to its alkaline nature which reduces oxidative stress (Nassini et al., 2010) and helps get rid of mercury, acidic wastes and other toxins in the body, as acidic body tends to hold onto (heavy) metals (Shomar, 2012). Moreover, numerous researchers have focused on the relationship of pH and cancer who cannot survive in alkaline medium (Estrella et al., 2013, Robey et al., 2009). However, other studies have demonstrated that the proliferation of cancer cells is inhibited under certain doses of some toxic minerals such as selenium, arsenic, and lithium (Wang et al., 2011). Omar et al. (2017) reported that Zamzam water has positive effect in cancer therapy and combats blood cancer by targeting specific proteins.
5.6. Monotherapy versus the use of mixtures
Otherwise, both monotherapy and concoctions based on the combination of two or more natural products were reported among informants. Interestingly, the informants reported their uses of mixed traditional-based preparations with chemical cancer drugs. The use of multiple medicinal therapies through mixing two or more natural products even combined with chemical drugs may increase the effectiveness of such medicines (Ait Abderrahim et al., 2019). Traditional healers may add also different accessory additives in their formulas to enhance the healing efficiency of the mixture, improve the taste and reduce pain and adverse effects (Bayat Mokhtari et al., 2017). Interestingly, the majority of natural products and accessory additives recorded throughout this study are mentioned in the Holy Quran, such as honey, Allium cepa L., Allium sativum L., Ficus carica L., Olea europaea L., Phoenix dactylifera L., Punica granatum L., Triticum turgidum L. and Vitis vinifera L., or by the Prophet Mohamad (PBUH) like Citrullus colocynthis (L.) Schrad, Cuminum cyminum L., Nigella sativa L., Thymus vulgaris L., Trigonella foenum-graecum L., Zingiber officinale Roscoe and the holy water Zamzam. These natural products have been exploited since long time for food or treatment purposes and their applications have demonstrated considerable benefits in cancer treatment (Ahmad et al., 2017). Several active substances with anticancer effects have been purified from these species such as Quercetin isolated from Allium sp. (Ranelletti et al., 2000), Apigenin from Petroselinum crispum, Allium sativum, Brassica oleracea Subsp. capitata L., Capsicum annuum L. (Manach et al., 2004), Genistein from Glycine max L. and Phaseolus vulgaris L. (Rossi et al., 2006), resveratrol isolated from Vitis vinifera L. (Singh et al., 2013), alliin and allicin from Allium sativum L. (Borlinghaus et al., 2014), Oleuropein/ omega-3 fatty acids from Olea europaea L. (Bulotta et al., 2014), Curcumin from Curcuma longa L. (Prasad et al., 2014), thymoquinone from Nigella sativa L. (Kortüm et al., 2015) and Kaempferol from Camellia sinensis L. (Gutiérrez-del-Río et al., 2016). The increasing interest in traditional Arabic and Islamic medicine is stimulated by the fact that these natural products are safe, cost-effective, and exhibit a wide spectrum of biological activities including stimulation of the immune system, antioxidant, anti-mutagenic, anti-inflammatory, antiviral, and anti-cancer effects (Ahmad et al., 2017, Saad and Said, 2011). In addition, Islamic medicinal practices generate specific cultural behaviors that aim to preserve health through prevention rather than therapeutic medicine since cancers evolve over a long period of time and biocomponents able to inhibit or delay one or more of its stages may affect the overall development of the disease (Ahmad et al., 2017).
5.7. Cancers’ category and natural products
The consistency of the use-reports per cancer category was evaluated by the informants’ consensus factor (CIF). FIC values ranged from 0.50 to 0.90 revealing high homogeneity in the reported cancer types mainly for breast, colorectal, lung and stomach cancers. These values are higher in comparison to those reported by Benarba et al. (2015) ranging from 0.13 to 0.66 and those of Bouasla and Bouasla (2017) from 0.09 to 0.66. It should be noted here that these cited studies are not designed for specific ailment such as cancer which is the case in our study. Andrade-Cetto and Heinrich (2011) have stated that higher values of the index are obtained when the use of inventoried plant species is reported by a high proportion of informants for the treatment of a particular cancer, whereas low values indicate that informants disagree over which plant to use. As an indicator of the relative medicinal importance of the inventoried plant species (Table 4), the Medicinal Importance index ranged from 1.92 for neck cancer to 10.43 for breast cancer. These values are in the same range of those reported by Baydoun et al. (2015) carrying ethnopharmacological in Lebanon (from 2.66 to 16.24) but higher than those of Carrió and Vallès (2012) in Balearic Islands (Spain). This could be attributed to the high number of informants engaged in this study in comparison to the others mentioned studies from apart, and to the low number of disorders (cancer types) in our study which are healed nearly by the same number of plant species from another part (Baydoun et al., 2015).
5.8. Toxicity and side effects
It should be noted that traditional medicines are not always free of side effects due to the presence of various pharmacological substances which may induce problems through inappropriate dosage and method of administration (Izzo et al., 2016). Likewise, it is relevant to mention that our informants recognized as toxic some plant species such as Aristolochia longa L., Berberis vulgaris L. and Peganum harmala L. although some had local medicinal uses but not all the toxic plants. It is important to note that among the reported plants, Aristolochia longa L., Artemisia herba-alba Asso, Crocus sativus L., Cuminum cyminum L., Echinops spinosus L., Ephedra alata L., Ferula vesceritensis L., Haloxylon scoparium Pomel, Juniperus phoenicea L., Lawsonia inermis L., Lepidium sativum L., Mentha spicata L., Pistacia lentiscus L., Retama raetam i(Forssk.) Webb, Rosmarinus officinalis L., Tamarix Africana Poir., Thymelaea microphylla Meisn. are considered all as toxic at higher doses and are not recommended to pregnant women (Hammiche et al., 2013). Besides, the harmful use of Ruta chalepensis L. on spermatozoids motility as well as its embryotoxic effect have been also described (Gonzales et al., 2007, Zeichen de Sa et al., 2000). As well, human consumption of cyanogenic plants like Prunus sp. rich with glycosides, cyanogen and amygdaline at higher doses might cause weakness, nausea, vomiting, diarrhea and spasms followed by terminal coma and death due to cyanide poisoning (Chaouali et al., 2013). Also, Thapsia garganica L. is a poisonous plant rich with histamine-releasing substances such as lactones sesquiterpenes (thapsigargine and thapsigarginine). Exposure to its molecules might induce vomiting, violent diarrhea, digestive mucous inflammation, salivary secretion, nervous disorders and gastroenteritis which leads lastly to death (Hammiche et al., 2013). Cucurbitacines and glycosides present in Citrullus colocynthis (L.) Schrad. and Ecballium elaterium L. might cause severe cutaneous and digestive adverse reactions that should not be ignored (De Smet, 1997, Raikhlin-Eisenkraft and Bentur, 2000).
In general, in comparison to synthetic drugs, ethnomedicines are also efficient and better tolerant nevertheless, patients must have enough information about their formulation methods, precise dosages, toxicity and side effects. Actually, the use of traditional medicinal knowledge by native populations is not only valuable for conservation of cultural traditions and resources but also useful for the population’s healthcare and drug discovery (Orhan, 2014).
6. Conclusion
Cancer remains one of the major causes of death worldwide. Ethnopharmacological studies provide significant sources of knowledge since the popular uses of many natural products may be linked to the presence of bioactive compounds and anticancer properties.
This is the first field-study documenting the uses of natural products for traditional cancer therapy in Algeria. The obtained results reveal the use of 113 medicinal plants species (belonging to 53 families and 104 genera), 10 animal species along with various products and by-products such as honey, olive oil, thorns, urine, milk, animal fat and alkaline water of Zamzam for cancer treatment by Algerian local populations.
Quantitative ethnopharmacological analyses have demonstrated that honey, Nigella sativa L., Aristolochia longa L., Berberis vulgaris L., Curcuma longa L., Trigonella foenum-graecum L., Citrus limon L., Artemisia herba-alba Asso and the holy water ‘Zamzam’ were the most used natural products.
Interestingly, we report for the first time the use of Pelophylax saharicus skin’ for the treatment of visible tumors and skin cancer. Furthermore, the obtained data describe new therapeutic uses for Allium cepa L., Allium sativum L., Aristolochia longa L., Artemisia herba-alba Asso, Atriplex halimus L., Berberis vulgaris L., Glycyrrhiza glabra L., Nigella sativa L., Pimpinella anisum L., Pistacia lentiscus L., Prunus persica (L.) Batsch, Thymus vulgaris L. and Vitis vinifera L. However, the remaining 100 medicinal plants, including several endemic species, are documented for the first time for their traditional uses in cancer management in Algeria.
Further in vitro and in vivo studies should be carried out to validate the popular uses of the reported natural products. Besides, associating traditional medicine and conventional treatments could be of great interest as a promising therapeutic approach for cancer treatment. Finally, it is necessary to develop a parallel strategy to preserve biodiversity and to protect it from over exploitation.
Author contributions
TK designed study, analyzed the data, interpreted the results and wrote manuscript; All authors carried out field studies, identified natural products, collected, prepared and revised data, read and approved the manuscript.
Declaration of Competing Interest
The authors declare that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
Acknowledgements
Authors would like to acknowledge the local community in general and informants in particular for their valuable information and support.
Funding
This report was financed solely by the authors own resources, without financial help of any institution.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abu-Darwish M.S., Efferth T. Medicinal Plants from Near East for Cancer Therapy. Front. Pharmacol. 2018;9(56) doi: 10.3389/fphar.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan M., Ullah I., Tariq A., Murad W., Azizullah A., Khan A.L., Ali N. Ethnomedicine use in the war affected region of northwest Pakistan. J. Ethnobiol. Ethnomed. 2014;10:16. doi: 10.1186/1746-4269-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad H., Khan S.M., Ghafoor S., Ali N. Ethnobotanical Study of Upper Siran. J. Herbs Spices Med. Plants. 2009;15(1):86–97. [Google Scholar]
- Ahmad R., Ahmad N., Naqvi A.A., Shehzad A., Al-Ghamdi M.S. Role of traditional Islamic and Arabic plants in cancer therapy. J. Tradition. Complement. Med. 2017;7(2):195–204. doi: 10.1016/j.jtcme.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Othman N.H. Honey as a potential natural anticancer agent: a review of its mechanisms. Evid. Based Complement Alternat. Med. 2013;2013:829070. doi: 10.1155/2013/829070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait Abderrahim L., Taïbi K., Ait Abderrahim C. Assessment of the Antimicrobial and Antioxidant Activities of Ziziphus lotus and Peganum harmala. Iranian J. Sci. Technol., Trans. A: Sci. 2017;43(2):409–414. [Google Scholar]
- Ait Abderrahim L., Taïbi K., Ait Abderrahim N., Boussaid M., Rios-Navarro C., Ruiz-Saurí A. Euphorbia honey and garlic: Biological activity and burn wound recovery. Burns. 2019;45(7):1695–1706. doi: 10.1016/j.burns.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Al Doghaither H.A., Al-Ghafari A.B., Rahimulddin S.A., Al Zahrani S.M., Omar A.S., Omar U.M. Evaluation of the potential anticancer activity of zamzam water in human colon cancer cell line. Cancer Oncol. Res. 2016;4(3):33–41. [Google Scholar]
- Albuquerque U.P., Ramos M.A., de Lucena R.F.P., Alencar N.L. Methods and techniques used to collect ethnobiological data, Methods and techniques in Ethnobiology and Ethnoecology. Springer. 2014:15–37. [Google Scholar]
- Alebie G., Yohannes S., Worku A. Therapeutic applications of camel’s milk and urine against cancer: current development efforts and future perspectives. J. Cancer Sci. Therapy. 2017;9:468–478. [Google Scholar]
- Almasaudi S.B., El-Shitany N.A., Abbas A.T., Abdel-dayem U.A., Ali S.S., Al Jaouni S.K., Harakeh S. Oxidative medicine and cellular longevity 2016. 2016. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves R.R.N., Barbosa J.A.A., Santos S.L.D.X., Souto W.M.S., Barboza R.R.D. Animal-based remedies as complementary medicines in the semi-arid region of northeastern Brazil. Evid. Based Complement Alternat. Med. 2011;2011:179876. doi: 10.1093/ecam/nep134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves R.R.N., Rosa I.L. Why study the use of animal products in traditional medicines? J. Ethnobiol. Ethnomed. 2005;1:5. doi: 10.1186/1746-4269-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves R.R.N., Rosa I.M.L. Biodiversity, traditional medicine and public health: where do they meet? J. Ethnobiol. Ethnomed. 2007;3(1):14. doi: 10.1186/1746-4269-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Silva J.M., Romane A., Efferth T., Salgueiro L. North African Medicinal Plants Traditionally Used in Cancer Therapy. Front. Pharmacol. 2017;8:383. doi: 10.3389/fphar.2017.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral R., dos Santos S., Andrade L., Severino P., Carvalho A.J.C.O. Natural Products as Treatment against Cancer: A Historical and Current Vision. Clin. Oncol. 2019;4(5):1562. [Google Scholar]
- Andrade-Cetto A., Heinrich M. From the field into the lab: useful approaches to selecting species based on local knowledge. Front. Pharmacol. 2011;2:20. doi: 10.3389/fphar.2011.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi R., Djaziri R., Lahfa F.B., Sekkal F.Z., Benmehdi H., Belkacem N. Ethnopharmacological survey of medicinal plants used in the traditional treatment of diabetes mellitus in the North Western and South Western Algeria. J. Med. Plant Res. 2012;6(10):2041–2050. [Google Scholar]
- Baba Aissa, F., 1991. Medicinal plants in Algeria. Identification, description of active ingredient properties and traditional use of common plants in Algeria. Bouchène and Ad. Diwan, Algiers, pp. 1–181.
- Babaei G., Aliarab A., Abroon S., Rasmi Y., Aziz S.G.-G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018;106:239–246. doi: 10.1016/j.biopha.2018.06.131. [DOI] [PubMed] [Google Scholar]
- Bayat Mokhtari R., Homayouni T.S., Baluch N., Morgatskaya E., Kumar S., Das B., Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun S., Chalak L., Dalleh H., Arnold N. Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J. Ethnopharmacol. 2015;173:139–156. doi: 10.1016/j.jep.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Benarba B. Use of medicinal plants by breast cancer patients in Algeria. EXCLI J. 2015;14:1164–1166. doi: 10.17179/excli2015-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarba, B., Belabid, L., Righi, K., Bekkar, A.A., Elouissi, M., Khaldi, A., Hamimed, A., 2015. Ethnobotanical study of medicinal plants used by traditional healers in Mascara (North West of Algeria). J. Ethnopharmacol. 175, 626–637. [DOI] [PubMed]
- Benarba B., Meddah B., Hamdani H. Cancer incidence in North West Algeria (Mascara) 2000–2010: results from a population-based cancer registry. EXCLI J. 2014;13:709–723. [PMC free article] [PubMed] [Google Scholar]
- Benarba B., Pandiella A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed. Pharmacother. 2018;107:408–423. doi: 10.1016/j.biopha.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Bhat J.A., Kumar M., Bussmann R.W. Ecological status and traditional knowledge of medicinal plants in Kedarnath Wildlife Sanctuary of Garhwal Himalaya, India. J. Ethnobiol. Ethnomed. 2013;9(1):1. doi: 10.1186/1746-4269-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtić S., Dussort P., Pierre F.-X., Bily A.C., Roller M. Carnosic acid. Phytochemistry. 2015;115:9–19. doi: 10.1016/j.phytochem.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Borlinghaus J., Albrecht F., Gruhlke M.C.H., Nwachukwu I.D., Slusarenko A.J. Allicin: chemistry and biological properties. Molecules. 2014;19(8):12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouasla A., Bouasla I. Ethnobotanical survey of medicinal plants in northeastern of Algeria. Phytomedicine. 2017;36:68–81. doi: 10.1016/j.phymed.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Boudjelal A., Henchiri C., Sari M., Sarri D., Hendel N., Benkhaled A., Ruberto G. Herbalists and wild medicinal plants in M'Sila (North Algeria): An ethnopharmacology survey. J. Ethnopharmacol. 2013;148(2):395–402. doi: 10.1016/j.jep.2013.03.082. [DOI] [PubMed] [Google Scholar]
- Bourhia, M., Abdelaziz Shahat, A., Mohammed Almarfadi, O., Ali Naser, F., Mostafa Abdelmageed, W., Ait Haj Said, A., El Gueddari, F., Naamane, A., Benbacer, L., Khlil, N., 2019. Ethnopharmacological Survey of Herbal Remedies Used for the Treatment of Cancer in the Greater Casablanca-Morocco. Evid.-Based Complement. Alternat. Med. 2019, 1613457. [DOI] [PMC free article] [PubMed]
- Boussaid M., Taïbi K., Ait Abderrahim L., Ennajah A. Genetic diversity of Ziziphus lotus natural populations from Algeria based on fruit morphological markers. Arid Land Res. Manage. 2018;32(2):184–197. [Google Scholar]
- Bouzabata A., Mahomoodally M.F. A quantitative documentation of traditionally-used medicinal plants from Northeastern Algeria: Interactions of beliefs among healers and diabetic patients. J. Herbal Med. 2019;100318 [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Bulotta S., Celano M., Lepore S.M., Montalcini T., Pujia A., Russo D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014;12:219. doi: 10.1186/s12967-014-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can Z., Yildiz O., Sahin H., Akyuz Turumtay E., Silici S., Kolayli S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015;180:133–141. doi: 10.1016/j.foodchem.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Carrió E., Vallès J. Ethnobotany of medicinal plants used in Eastern Mallorca (Balearic Islands, Mediterranean Sea) J. Ethnopharmacol. 2012;141(3):1021–1040. doi: 10.1016/j.jep.2012.03.049. [DOI] [PubMed] [Google Scholar]
- Chaouali N., Gana I., Dorra A., Khelifi F., Nouioui A., Masri W., Belwaer I., Ghorbel H., Hedhili A. Potential Toxic Levels of Cyanide in Almonds (Prunus amygdalus), Apricot Kernels (Prunus armeniaca), and Almond Syrup. ISRN Toxicol. 2013;2013:610648. doi: 10.1155/2013/610648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N., Zhao H., Chen S., He Q., Cao W. Jujube honey induces apoptosis in human hepatocellular carcinoma HepG2 cell via DNA damage, p53 expression, and caspase activation. J. Food Biochem. 2019;43(11) doi: 10.1111/jfbc.12998. [DOI] [PubMed] [Google Scholar]
- Chermat S., Gharzouli R. Ethnobotanical Study of Medicinal Flora in the North East of Algeria - An Empirical Knowledge in Djebel Zdimm (Setif) J. Mater. Sci. Eng. A. 2015;1–2:50–59. [Google Scholar]
- Chorfi A., Taïbi K. Biochemical Screening for Osmotic Adjustment of Wheat Genotypes under Drought Stress. Tropicultura. 2011;29(2):82–87. [Google Scholar]
- Chua L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013;150(3):805–817. doi: 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- da Silva, V.A., do Nascimento, V.T., Soldati, G.T., Medeiros, M.F.T., Albuquerque, U.P., 2014. Techniques for analysis of quantitative ethnobiological data: use of indices, Methods Tech. Ethnobiol. Ethnoecol. Springer, pp. 379–395.
- Davoodi H., Esmaeili S., Mortazavian A.M. Effects of Milk and Milk Products Consumption on Cancer: A Review. Compr. Rev. Food Sci. Food Saf. 2013;12(3):249–264. [Google Scholar]
- De Smet P.A.G.M. Citrullus Colocynthis. In: De Smet P.A.G.M., Keller K., Hänsel R., Chandler R.F., editors. Adverse Effects of Herbal Drugs. Springer; Berlin Heidelberg, Berlin, Heidelberg: 1997. p. 391. [Google Scholar]
- Dolatkhahi M., Dolatkhahi A., Nejad J.B. Ethnobotanical study of medicinal plants used in Arjan - Parishan protected area in Fars Province of Iran. Avicenna J. Phytomed. 2014;4(6):402–412. [PMC free article] [PubMed] [Google Scholar]
- Durante M., Formenti S.C. Radiation-Induced Chromosomal Aberrations and Immunotherapy: Micronuclei, Cytosolic DNA, and Interferon-Production Pathway. Front. Oncol. 2018;8:192. doi: 10.3389/fonc.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrella V., Chen T., Lloyd M., Wojtkowiak J., Cornnell H.H., Ibrahim-Hashim A., Bailey K., Balagurunathan Y., Rothberg J.M., Sloane B.F., Johnson J., Gatenby R.A., Gillies R.J. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73(5):1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, D.R., 2020. Amphibian Species of the World: an online reference. Version 6.0 (05/04/2020). Electronic Database accessible at https://amphibiansoftheworld.amnh.org/index.php American Museum of Natural History, New York, USA.
- Ghasemi Pirbalouti A., Momeni M., Bahmani M. Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan Districts, Ilam Province, Iran. Afr. J. Tradit. Complement. Altern. Med. 2012;10(2):368–385. doi: 10.4314/ajtcam.v10i2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales J., Benavides V., Rojas R., Pino J. Efecto embriotóxico y teratogénico de Ruta chalepensis L. «ruda», en ratón (Mus musculus) %J. Revista Peruana de Biología. 2007;13:223–226. [Google Scholar]
- González-Tejero M.R., Casares-Porcel M., Sánchez-Rojas C.P., Ramiro-Gutiérrez J.M., Molero-Mesa J., Pieroni A., Giusti M.E., Censorii E., de Pasquale C., Della A., Paraskeva-Hadijchambi D., Hadjichambis A., Houmani Z., El-Demerdash M., El-Zayat M., Hmamouchi M., ElJohrig S. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008;116(2):341–357. doi: 10.1016/j.jep.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-del-Río I., Villar C.J., Lombó F. Therapeutic uses of Kampferol: Anticancer and antiinflammatory activity. Biosynthesis, Food Sources Therapeutic Uses. 2016:71. [Google Scholar]
- Habtamu A., Mulatu O., Tsdeke L. Traditional medicinal plants utilization, management and threats in Hadiya Zone Ethiopia. J. Med. Plants Stud. 2014;2(2):16. [Google Scholar]
- Hammiche V., Merad R., Azzouz M. Plantes toxiques à usage médicinal du pourtour méditerranéen. In: Spiegler V., editor. Springer. Paris; 2013. [Google Scholar]
- Hassanvand Jamadi R., Yaghoubi H., Sadeghizadeh M. Brevinin-2R and Derivatives as Potential Anticancer Peptides: Synthesis, Purification, Characterization and Biological Activities. Int. J. Pept. Res. Ther. 2019;25(1):151–160. [Google Scholar]
- IPNI (2020). International Plant Names Index. Published on the Internet http://www.ipni.org, The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens.
- Izzo A.A., Hoon-Kim S., Radhakrishnan R., Williamson E.M. A Critical Approach to Evaluating Clinical Efficacy, Adverse Events and Drug Interactions of Herbal Remedies. Phytother. Res. 2016;30(5):691–700. doi: 10.1002/ptr.5591. [DOI] [PubMed] [Google Scholar]
- Jaganathan S.K., Balaji A., Vellayappan M.V., Asokan M.K., Subramanian A.P., John A.A., Supriyanto E., Razak S.I., Marvibaigi M. A review on antiproliferative and apoptotic activities of natural honey. Anti-Cancer Agents Med. Chem. 2015;15(1):48–56. doi: 10.2174/1871520614666140722084747. [DOI] [PubMed] [Google Scholar]
- Jaradat N.A., Al-Ramahi R., Zaid A.N., Ayesh O.I., Eid A.M. Ethnopharmacological survey of herbal remedies used for treatment of various types of cancer and their methods of preparations in the West Bank-Palestine. BMC Complement. Altern. Med. 2016;16:93. doi: 10.1186/s12906-016-1070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelonek K., Pietrowska M., Widlak P. Systemic effects of ionizing radiation at the proteome and metabolome levels in the blood of cancer patients treated with radiotherapy: the influence of inflammation and radiation toxicity. Int. J. Radiat Biol. 2017;93(7):683–696. doi: 10.1080/09553002.2017.1304590. [DOI] [PubMed] [Google Scholar]
- Jeyaraman M.M., Abou-Setta A.M., Grant L., Farshidfar F., Copstein L., Lys J., Gottschalk T., Desautels D., Czaykowski P., Pitz M., Zarychanski R. Dairy product consumption and development of cancer: an overview of reviews. BMJ Open. 2019;9(1) doi: 10.1136/bmjopen-2018-023625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj F., Meddah B., Cherrah Y., Faouzi E. Ethnopharmacological profile of traditional plants used in Morocco by cancer patients as herbal therapeutics. Phytopharmacology. 2012;2(2):243–256. [Google Scholar]
- Kaddem, S.E., 1990. Les plantes médicinales en Algérie, Ed. Bouchène, Oued Zenati, Algérie.
- Kang S.-J., Ji H.-Y., Lee B.-J. Anticancer activity of undecapeptide analogues derived from antimicrobial peptide, Brevinin-1EMa. Arch. Pharmacal. Res. 2012;35(5):791–799. doi: 10.1007/s12272-012-0505-0. [DOI] [PubMed] [Google Scholar]
- Khalid N., Ahmad A., Khalid S., Ahmed A., Irfan M. Mineral Composition and Health Functionality of Zamzam Water: A Review. Int. J. Food Prop. 2014;17(3):661–677. [Google Scholar]
- Khan S.M., Page S., Ahmad H., Shaheen H., Ullah Z., Ahmad M., Harper D.M. Medicinal flora and ethnoecological knowledge in the Naran Valley, Western Himalaya, Pakistan. J. Ethnobiol. Ethnomed. 2013;9(1):4. doi: 10.1186/1746-4269-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortüm B., Campregher C., Lang M., Khare V., Pinter M., Evstatiev R., Schmid G., Mittlböck M., Scharl T., Kucherlapati M.H., Edelmann W., Gasche C. Mesalazine and thymoquinone attenuate intestinal tumour development in Msh2(loxP/loxP) Villin-Cre mice. Gut. 2015;64(12):1905–1912. doi: 10.1136/gutjnl-2014-307663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-H., Yeh M.-H., Kao S.-T., Hung C.-M., Liu C.-J., Huang Y.-Y., Yeh C.-C. The inhibitory effect of hesperidin on tumor cell invasiveness occurs via suppression of activator protein 1 and nuclear factor-kappaB in human hepatocellular carcinoma cells. Toxicol. Lett. 2010;194(1):42–49. doi: 10.1016/j.toxlet.2010.01.021. [DOI] [PubMed] [Google Scholar]
- López-Lázaro M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev. Med. Chem. 2009;9(1):31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Maroyi A. Traditional use of medicinal plants in south-central Zimbabwe: review and perspectives. J. Ethnobiol. Ethnomed. 2013;9(1):31. doi: 10.1186/1746-4269-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. Chapman et Hall; London: 1995. Ethnobotany-A manual of methods. [Google Scholar]
- Meddour R., Meddour-Sahar O. Medicinal plants and their traditional uses in Kabylia (Tizi Ouzou, Algeria) Arabian J. Med. Aromatic Plants. 2016;1(2):137–151. [Google Scholar]
- Moazzami Farida, S.H., Ghorbani*, A., Ajani, Y., Sadr, M., Mozaffarian, V., 2018. Ethnobotanical Applications and Their Correspondence with Phylogeny in Apiaceae-Apioideae. Res. J. Pharmacognosy 5(3), 79–97.
- Myers, P., R. Espinosa, C. S. Parr, T. Jones, G. S. Hammond, and T. A. Dewey. 2020. The Animal Diversity Web (online). Accessed at https://animaldiversity.org.
- Nassini, R., Andr, egrave, Eunice, Gazzieri, D., Siena, G.D., Zanasi, A., Geppetti, P., Materazzi, S., 2010. A Bicarbonate-Alkaline Mineral Water Protects from Ethanol-Induced Hemorrhagic Gastric Lesions in Mice. Biolog. Pharmaceut. Bull. 33, 8, 1319–1323. [DOI] [PubMed]
- Omar U.M., Al Doghaither H.A., Rahimulddin S.A., Al Zahrani S.M., Al-Ghafari A.B. In Vitro Cytotoxic and Anticancer Effects of Zamzam Water in Human Lung Cancer (A594) Cell Line. Malays. J. Med. Sci. 2017;24(3):15–25. doi: 10.21315/mjms2017.24.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan I.E. Pharmacognosy: Science of natural products in drug discovery. Bioimpacts. 2014;4(3):109–110. doi: 10.15171/bi.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozenda, P., 1977. Flore du Sahara. Ed CNRS, Paris, p. 622.
- Panghal, M., Arya, V., Yadav, S., Kumar, S., Yadav, J.P., 2010. Indigenous knowledge of medicinal plants used by Saperas community of Khetawas, Jhajjar District, Haryana, India. J. Ethnobiol. Ethnomed. 6, 1, 4. Pharmacognosy Research 2, 2, 31–35. [DOI] [PMC free article] [PubMed]
- Porcza L.M., Simms C., Chopra M. Honey and Cancer: Current Status and Future Directions. Diseases. 2016;4(4):30. doi: 10.3390/diseases4040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prance G.T., Balée W., Boom B.M., Carneiro R.L. Quantitative Ethnobotany and the Case for Conservation in Ammonia*. Conserv. Biol. 1987;1(4):296–310. [Google Scholar]
- Prasad S., Tyagi A.K., Aggarwal B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat. 2014;46(1):2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quézel, P., Santa, S., 1962–1963. Nouvelle Flore de l'Algérie et des Régions Désertiques Méridionales. Paris, 2 Tomes, Centre National de la Recherche Scientifique.
- R Development Core Team, R.T., 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.r-project.org.
- Rahman M.M., Hossain A.S.M.S., Mostofa M.G., Khan M.A., Ali R., Mosaddik A., Sadik M.G., Alam A.H.M.K. Evaluation of anti-ROS and anticancer properties of Tabebuia pallida L. Leaves. Clin. Phytosci. 2019;5(1):17. [Google Scholar]
- Raikhlin-Eisenkraft B., Bentur Y. Ecbalium elaterium (squirting cucumber)–remedy or poison? J. Toxicol. Clin. Toxicol. 2000;38(3):305–308. doi: 10.1081/clt-100100936. [DOI] [PubMed] [Google Scholar]
- Rajaei P., Mohamadi N. Ethnobotanical study of medicinal plants of hezar mountain allocated in South East of iran. Iran J. Pharm. Res. 2012;11(4):1153–1167. [PMC free article] [PubMed] [Google Scholar]
- Ranelletti F.O., Maggiano N., Serra F.G., Ricci R., Larocca L.M., Lanza P., Scambia G., Fattorossi A., Capelli A., Piantelli M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer. 2000;85(3):438–445. [PubMed] [Google Scholar]
- Robey I.F., Baggett B.K., Kirkpatrick N.D., Roe D.J., Dosescu J., Sloane B.F., Hashim A.I., Morse D.L., Raghunand N., Gatenby R.A., Gillies R.J. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69(6):2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M., Negri E., Talamini R., Bosetti C., Parpinel M., Gnagnarella P., Franceschi S., Dal Maso L., Montella M., Giacosa A., La Vecchia C. Flavonoids and Colorectal Cancer in Italy. Cancer Epidemiol. Biomark. Prev. 2006;15(8):1555. doi: 10.1158/1055-9965.EPI-06-0017. [DOI] [PubMed] [Google Scholar]
- Rychlinska I., Nowak S. Quantitative determination of arbutin and hydroquinone in different plant materials by HPLC. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2012;40(2):109–113. [Google Scholar]
- Saad B., Said O. Wiley; Hoboken, N.J.: 2011. Greco-Arab and Islamic Herbal Medicine : Traditional System, Ethics, Safety, Efficacy, and Regulatory Issues. [Google Scholar]
- Sarri M., Boudjelal A., Hendel N., Sarri D., Benkhaled A. Flora and ethnobotany of medicinal plants in the southeast of the capital of Hodna (Algeria) Arabian J. Med. Aromatic Plants. 2015;1(1):2015. [Google Scholar]
- Sarri M., Mouyet F.Z., Benziane M., Cheriet A. Traditional use of medicinal plants in a city at steppic character (M’sila, Algeria) J. Pharm. Pharmacognosy Res. 2014;2(2):31–35. [Google Scholar]
- Saxena S., Gautam S., Maru G., Kawle D., Sharma A. Suppression of error prone pathway is responsible for antimutagenic activity of honey. Food Chem. Toxicol. 2012;50(3):625–633. doi: 10.1016/j.fct.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Shaheen F., Nadeem-ul-Haque M., Ahmed A., Simjee S.U., Ganesan A., Jabeen A., Shah Z.A., Choudhary M.I. Synthesis of breast cancer targeting conjugate of temporin-SHa analog and its effect on pro- and anti-apoptotic protein expression in MCF-7 cells. Peptides. 2018;106:68–82. doi: 10.1016/j.peptides.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Sharifi-Rad J., Ozleyen A., Boyunegmez Tumer T., Oluwaseun Adetunji C., El Omari N., Balahbib A., Taheri Y., Bouyahya A., Martorell M., Martins N., Cho W.C. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules. 2019;9(11):679. doi: 10.3390/biom9110679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M., Shah I., Ali N., Adhikari A., Tahir M.N., Shah S.W.A., Ishtiaq S., Khan J., Khan S., Umer M.N. Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement. Altern. Med. 2017;17(1):27. doi: 10.1186/s12906-016-1517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomar B. Zamzam water: Concentration of trace elements and other characteristics. Chemosphere. 2012;86(6):600–605. doi: 10.1016/j.chemosphere.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Singh C.K., George J., Ahmad N. Resveratrol-based combinatorial strategies for cancer management. Ann. N. Y. Acad. Sci. 2013;1290(1):113–121. doi: 10.1111/nyas.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacks N.M. Nova Science Publishers; Incorporated: 2015. Apigenin and Naringenin: Natural Sources, Pharmacology and Role in Cancer Prevention. [Google Scholar]
- Subramanian A.P., John A.A., Vellayappan M.V., Balaji A., Jaganathan S.K., Mandal M., Supriyanto E. Honey and its Phytochemicals: Plausible Agents in Combating Colon Cancer through its Diversified Actions. J. Food Biochem. 2016;40(4):613–629. [Google Scholar]
- Taïbi K., Del Campo A.D., Vilagrosa A., Bellés J.M., López-Gresa M.P., López-Nicolás J.M., Mulet J.M. Distinctive physiological and molecular responses to cold stress among cold-tolerant and cold-sensitive Pinus halepensis seed sources. BMC Plant Biol. 2018;18(1):236. doi: 10.1186/s12870-018-1464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taïbi K., del Campo A.D., Vilagrosa A., Bellés J.M., López-Gresa M.P., Pla D., Calvete J.J., López-Nicolás J.M., Mulet J.M. Drought Tolerance in Pinus halepensis Seed Sources As Identified by Distinctive Physiological and Molecular. Markers. 2017;8(1202) doi: 10.3389/fpls.2017.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taïbi K., Taïbi F., Ait Abderrahim L., Ennajah A., Belkhodja M., Mulet J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016;105:306–312. [Google Scholar]
- The Plant List (2013). Version 1.1. Published on the Internet; http://www.theplantlist.org.
- Waheed M., Hussain M.B., Javed A., Mushtaq Z., Hassan S., Shariati M.A., Khan M.U., Majeed M., Nigam M., Mishra A.P., Heydari M. Honey and cancer: A mechanistic review. Clin. Nutr. 2019;38(6):2499–2503. doi: 10.1016/j.clnu.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang Y., Yang L., Cai B., Li J., Zhou Y., Yin L., Yang L., Yang B.F., Lu Y.J. Arsenic trioxide induces the apoptosis of human breast cancer MCF-7 cells through activation of caspase-3 and inhibition of HERG channels. Exp. Ther. Med. 2011;2(3):481–486. doi: 10.3892/etm.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2018. Global Health Observatory. who.int/gho/database/en/, in: Geneva, S.W.H.O. (Ed.).
- Wilson D.E., Reeder D.M. (3rd Ed), Johns Hopkins University Press; 2005. Mammal Species of the World. A Taxonomic and Geographic Reference. [Google Scholar]
- Yuan H., Ma Q., Ye L., Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules. 2016;21(5):559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichen de Sa R., Rey A., Argañaraz E., Bindstein E. Perinatal toxicology of Ruta chalepensis (Rutaceae) in mice. J. Ethnopharmacol. 2000;69(2):93–98. doi: 10.1016/s0378-8741(98)00232-3. [DOI] [PubMed] [Google Scholar]
- Zerrouk S., Seijo M.C., Escuredo O., Rodríguez-Flores M.S. Characterization of Ziziphus lotus (jujube) honey produced in Algeria. J. Apic. Res. 2018;57(1):166–174. [Google Scholar]
- Zheng Q., Sun Z., Zhang X., Yuan J., Wu H., Yang J., Xu X. Clerodendranoic acid, a new phenolic acid from Clerodendranthus spicatus. Molecules. 2012;17(11):13656–13661. doi: 10.3390/molecules171113656. [DOI] [PMC free article] [PubMed] [Google Scholar]