Abstract
Background
Environmental tobacco smoke (ETS) is one of the most toxic environmental exposures and passive smoking is an important general health problem. Children are the most vulnerable group to ETS exposure. This study aimed to compare the salivary total antioxidant capacity (TAC) and lipid peroxidation levels in passive smoking and nonsmoking adolescents aged 12-15 years.
Methods
This descriptive-analytical study was conducted on 80 adolescents aged 12-15 years. The case group included passive smokers and the control group comprised nonsmokers. These groups were age- and sex-matched ones. Unstimulated saliva of both groups was collected using the spitting method. Then, the salivary total antioxidant and lipid peroxidation levels were measured using the ferric-reducing antioxidant power (FRAP) and thiobarbituric acid reactive substances (TBARS) assays, respectively. The independent samples t-test was used for data comparison.
Findings
There was a significant difference in salivary total antioxidant levels between the case group (51.98 ± 88.97 µM) and the control group (174.35 ± 148.15 µM) (P = 0.003). There was no significant difference between the case group (0.97 ± 1.96) and the control group (0.81 ± 0.97) in lipid peroxidation levels (P = 0.542).
Conclusion
It seems that passive smoking can reduce the salivary TAC of adolescents, thereby threatening oral cavity health.
Keywords: Tobacco smoke pollution, Oxidative stress, Antioxidants, Saliva
Introduction
Passive smoking or environmental tobacco smoke (ETS) is a major general health concern.1 There are at least one billion tobacco smokers exposing a minimum of 700 million children to ETS.2 Tobacco smoking accounts for approximately 65%-80% of all tobacco consumption in the world and about 20%-80% of the population are exposed to ETS. According to an epidemiological research (2016), 28.6% of Iranian adolescents are exposed to ETS.3 Both active and passive cigarette smokers are almost equally vulnerable to health risks. It is worth noting that children in the early stages of development are more vulnerable than adults.4 65000 kids die from diseases related to second-hand smoke annually.5 Tobacco exposure during adolescence enhances the rewarding effect of nicotine even after a long period of abstinence.6
Tobacco smoke comprises 4000 chemical compounds many of which are oxidants and produce reactive oxygen species (ROS). Cigarette smoke-induced ROS production can cause conditions of oxidative stress via lipid oxidation, deoxyribonucleic acid (DNA) single-strand breaks, inactivation of certain proteins, and disintegration of biological membranes.7,8
The most common method for oxidative stress assessment is to measure the concentration of lipid peroxidation products. Studies have shown that malondialdehyde (MDA), as a major end product of lipid peroxidation, is a direct predictor of increased levels of oxidative stress.9
Total antioxidant capacity (TAC), including all salivary antioxidants, has clinical importance in the assessment of salivary antioxidants status under normal and pathological conditions and functions as a salivary total antioxidants index.10
According to available evidence, tobacco smoking is associated with increased levels of free radicals and oxidative stress and decreased antioxidant levels. It also reduces salivary total antioxidant, which, in turn, causes oral inflammatory diseases, progression of precancerous changes, and imbalanced condition of the oral cavity.11
The majority of researches have addressed the effects of tobacco smoke on adults’ health, whereas few studies have studied these effects on children and adolescents. Due to the rise in tobacco consumption and the subsequent increase in passive smokers, and given the importance of oral and dental health in people exposed to second-hand smoke, this study compared the salivary total antioxidant levels and lipid peroxidation levels in passive smoking and nonsmoking adolescents aged 12-15 years.
Methods
This descriptive-analytical study included 80 students aged 12-15 years in Zahedan, Iran, out of which 40 passive smoking students were placed in the case group and 40 nonsmoking students in the control group. The inclusion criterion for the case group was passive smokers exposed to tobacco smoke at home with salivary cotinine level ≥ 0.05 ng/ml and for the control group was nonsmokers who had salivary cotinine levels < 0.05 ng/ml.12-14 Each group comprised 20 girls and 20 boys. The groups were similar in terms of the age of the participants.
General inclusion criteria were: no systemic diseases, no consumption of medications including immunosuppressive drugs, vitamin supplements, and nonsteroidal anti-inflammatory drugs (NSAIDs) in the past three months, no periodontitis with the attachment loss of equal or more than 5 ml, and no consumption of tobacco and alcohol.10,14
This study was approved by the Ethics Committee of Zahedan University of Medical Sciences (code: IR.ZAUMS.REC.1395.266). Firstly, the research objective was explained to all the participants and then their informed written consent was obtained. After completing the demographic questionnaire, the saliva of participants was sampled under identical conditions at room temperature between 8-10 A.M. The participants were asked to avoid eating, drinking, and brushing their teeth 90 minutes before the samples were taken.
Before saliva sampling, the participants were asked to wash their mouths with physiological serum. Then, unstimulated saliva was collected in five minutes using the spitting method. To this end, the participants were asked to spit into the collecting test tubes once every minute for 5 minutes.15 The saliva samples were then centrifuged at 2000 revolutions per minute (rpm) and kept at -70 °C until the completion of the sampling process and the beginning of the biochemical tests.
In the current study, cotinine level of saliva was assessed by laboratory cotinine kit and the enzyme-linked immunosorbent assay (ELISA) technique.16
In this study, the salivary TAC was measured using the ferric-reducing antioxidant power (FRAP) method. In this method, the saliva is exposed to Fe 3+ and ferric reduction by salivary antioxidants takes place because of their activity.
The reaction produced a blue color that was assessed against the FeSO4 standard curve at 593 nm using a spectrophotometer. To this end, the FRAP solution was first prepared as follows:
25 ml acetate buffer (300 mM), 2.5 ml 2,4,6-Tris(2-pyridyl)-1,3,5-triazine (TPTZ) solution (10 mM) in hydrochloric acid (40 mM), and 2.5 ml FeCl3.6H2O solution (20 mM) were mixed. Moreover, 20 mM FeSO4.7H2O standard solution, which included 0.278 g of FeSO4.7H2O in 50 ml, was prepared at 200-1400 μM concentrations.
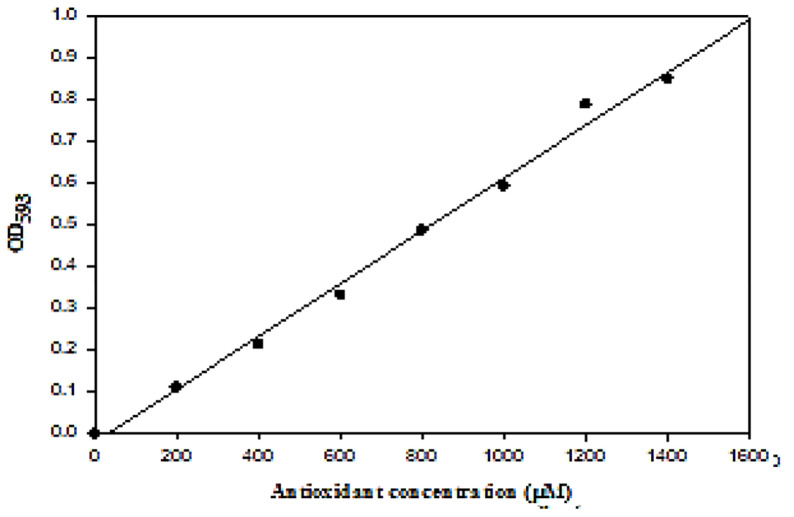
After the preparation of the FRAP working solution, 1.5 ml of it was heated to 30 °C. Then, 50 μl of the saliva was added to this solution to initiate the reaction. Absorbance of the samples and the standard solution was measured at the wavelength of 593 nm and 30 °C for 4 minutes. The standard curve was drawn based on the absorbance of the standard solution (Figure 1). The antioxidant concentration of the sample was obtained by comparing its absorbance with that of the standard solution on the standard curve.10
Figure 1.
Total antioxidant capacity (TAC) concentration of unstimulated saliva
Moreover, MDA level as a product of the lipid peroxidation was measured using the thiobarbituric acid reactive substances (TBARS) assay. The required solutions for MDA measurement, 1% phosphoric acid and 0.6% thiobarbituric acid, were prepared. First, a test tube was marked as the blank tube (B). There was one standard tube for each standard and one test tube (T) for each sample. The blank tube contained 3 ml of 1% phosphoric acid, 1 ml of thiobarbituric acid, and 0.5 ml of phosphate-buffered saline (PBS). The blank tube was used to zero out the spectrophotometer. The standard tubes contained 3 ml of 1% phosphoric acid, 1 ml of thiobarbituric acid, and 0.5 ml of the standard solution. The test tubes contained 3 ml of 1% phosphoric acid, 1 ml of thiobarbituric acid, and 0.5 ml of the saliva sample. After preparing the tubes, they were placed in a boiling water bath (100 ˚C) for 45 minutes.9
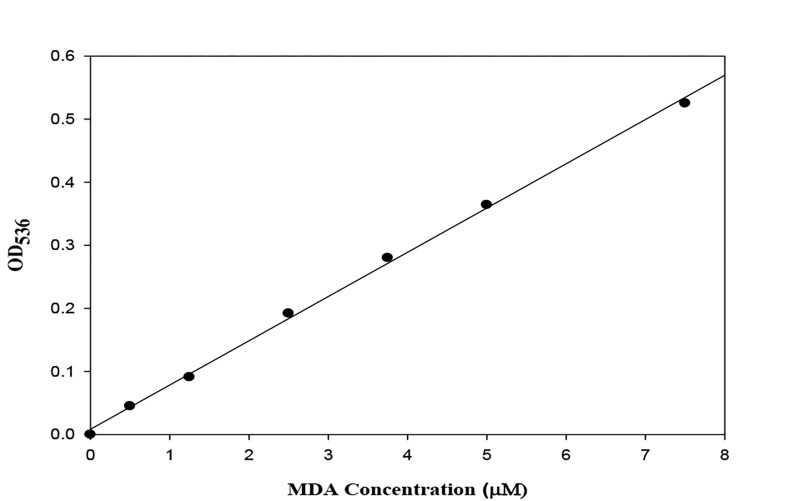
The tubes were removed from the water bath, cooled, and centrifuged at 3000 rpm for 10 minutes to obtain transparent supernatants. The blank tube was used to zero out the spectrophotometer and absorbance of the samples was read at 536 nm. Using the concentrations and absorbance values, the standard curves were drawn to calculate the MDA concentrations of the samples on them (Figure 2). Finally, the independent samples t-test was used to compare the findings.
Figure 2.
Malondialdehyde (MDA) concentration of unstimulated saliva
Results
In this study, 80 students including 40 (20 boys and 20 girls) nonsmoker and 40 (20 boys and 20 girls) passive smokers were enrolled. The mean age of the participants was 13.60 ± 1.07 years in the case group and 13.50 ± 1.13 years in the control group (P > 0.05). In addition, salivary cotinine levels had no significant difference between nonsmoker boys (0.014 ± 0.010 ng/ml) and girls (0.017 ± 0.014 ng/ml) as well as passive smoker boys (0.12 ± 0.34 ng/ml) and girls (0.15 ± 0.37 ng/ml) (P > 0.05) (Table 1).
Table 1.
Salivary cotinine levels in two studied groups based on gender
| Group | Saliva cotinine (ng/ml) | P | |
|---|---|---|---|
| (mean ± SD) | |||
| Nonsmokers | Boys | 0.014 ± 0.010 | 0.860 |
| Girls | 0.017 ± 0.014 | ||
| Passive smokers | Boys | 0.120 ± 0.340 | 0.800 |
| Girls | 0.150 ± 0.370 |
SD: Standard deviation
The Kolmogorov-Smirnov test (K-S test) revealed the normality of samples in both groups. As a result, the independent t-test was used for data analysis.
K-S test result for investigating normality of the variables is shown in table 2.
Table 2.
The Kolmogorov-Smirnov test (K-S test) results for evaluation of variables’ normality
| Variable | N | P |
|---|---|---|
| TAC of control group | 40 | 0.327 |
| TAC of case group | 40 | 0.372 |
| MDA of control group | 40 | 0.315 |
| MDA of case group | 40 | 0.306 |
TAC: Total antioxidant capacity; MDA: Malondialdehyde
The K-S test results for TAC of control group and TAC of case group were P = 0.327 and P = 0.372, respectively. Also the results of this test for MDA of control group and MDA of case group were P = 0.315 and P = 0.306, respectively (Table 2).
In this study, the salivary total antioxidant levels in the case group (51.98 ± 88.97 µM) were significantly lower than the control group (174.35 ± 148.15 µM, P = 0.003). Although the lipid peroxidation levels in the case group (0.97 ± 1.96 µM) were higher than the control (0.81 ± 0.97 µM), this difference was not significant (P = 0.542) (Table 3).
Table 3.
Comparison of salivary total antioxidant capacity (TAC) and malondialdehyde (MDA) levels in passive smokers and nonsmokers
| Salivary marker | Group |
P | Independent t | |
|---|---|---|---|---|
| Passive smokers (mean ± SD) | Nonsmokers (mean ± SD) | |||
| TAC (µM) | 51.98 ± 88.97 | 174.35 ± 148.15 | 0.003 | 3.10 |
| Lipid peroxidation (µM) | 0.97 ± 1.96 | 0.81 ± 0.97 | 0.542 | -0.45 |
TAC: Total antioxidant capacity; SD: Standard deviation
There was no significant difference between the control girls and boys in levels of salivary TAC (P = 0.565), whereas the levels of salivary TAC were significantly lower in passive smoking boys than in nonsmoking boys (P = 0.039). The salivary lipid peroxidation level was slightly higher in passive smoking boys than in the nonsmoking boys, that this difference was not significant (P = 0.658) (Table 4).
Table 4.
Comparison of salivary total antioxidant capacity (TAC) and malondialdehyde (MDA) levels in passive smokers and nonsmokers based on gender
| Studied groups | Salivary marker |
|
|---|---|---|
| Salivary TAC (µM) (mean ± SD) | MDA (µM) (mean ± SD) | |
| Case girls (n = 20) | 0.66 ± 0.48 | 43.75 ± 66.61 |
| Control girls (n = 20) | 0.64 ± 0.71 | 131.15 ± 162.11 |
| P | 0.926 | 0.035 |
| Case boys (n = 20) | 1.27 ± 2.37 | 60.21 ± 108.01 |
| Control boys (n = 20) | 0.97 ± 1.17 | 165.16 ± 188.43 |
| P | 0.658 | 0.039 |
| Control girls (n = 20) | 0.64 ± 0.71 | 131.15 ± 162.11 |
| Control boys (n = 20) | 0.97 ± 1.17 | 165.16 ± 188.43 |
| P | 0.299 | 0.544 |
| Case girls (n = 20) | 0.66 ± 0.48 | 43.75 ± 66.61 |
| Case boys (n = 20) | 1.27 ± 2.73 | 60.21 ± 108.01 |
| P | 0.338 | 0.565 |
TAC: Total antioxidant capacity; MDA: Malondialdehyde; SD: Standard deviation
The levels of TAC in passive smoking girls were significantly lower compared to the nonsmoking girls (P = 0.035), whereas there was no significant difference between passive smoking girls and control boys in lipid peroxidation levels (P = 0.338) (Table 4).
Discussion
In the present research, which was conducted to determine salivary TAC and MDA levels in 12-15-year-old passive smokers and nonsmokers, levels of salivary lipid peroxidation in the two groups were not significantly different (P = 0.542).
Mottalebnejad et al. also reported no significant difference between passive smoking and smoking adolescents, aged 12-15 years, in salivary lipid peroxidation levels (P = 0.176).14
In contrast, Demirtas et al. reported a conspicuously higher salivary MDA levels in smoking as well as passive smoking people than in nonsmoking individuals (P < 0.050).17 Yildirim et al. investigated the salivary oxidative stress in passive smoker and nonsmoker Turkish preschool children and unlike the current study found that salivary oxidative stress level in passive smokers was significantly higher (P < 0.001) and indirect cigarette smoke was a powerful oxidant for kids.18 The differences between the age groups under consideration (aged 20-45 years in Demirtas et al.17 and preschool children in Yildirim et al.18 studies compared to 12-15-year-olds in our research) justify this difference.
The free radicals-induced lipid peroxidation causes marked changes in cell membranes. Lipid membrane peroxidation is associated with the pathogenesis of many degenerative diseases such as atherosclerosis, cancer, and diabetes. Relatively high levels of antioxidants can greatly inhibit lipid peroxidation, protein and carbohydrate oxidation, and oxidative DNA damage.14
Tobacco smoking is injurious for internal body environment and oral health. Passive smokers also suffer from smoke. Saliva, as the first biological fluid encountering cigarette smoke, contains antioxidant defense system to decline the toxic effects of tobacco smoke.19
In the present study, the salivary total antioxidant level was significantly lower in the passive smoking group than in the active smokers (P = 0.003), which is consistent with Azadbakht et al.’s research.19 In their study, uric acid level, the important salivary antioxidant, was decreased significantly in passive smoking subjects.
Falsafi et al. found that the salivary total antioxidant level and vitamin C were conspicuously lower in tobacco smokers.20
Ghadimi et al. reported that the volume of saliva and changes in pH significantly declined in the smoking group compared to the nonsmoking group. They also noticed that three antioxidative enzymes [peroxidase, superoxide dismutase (SOD), and catalase] decreased in tobacco smokers.21
Mahrous et al. compared TAC levels in smokers, passive smokers, and nonsmokers and found that the levels of TAC were lower in smoking individuals than in passive smoking and nonsmoking people. In contrast, in their study, TAC levels did not differ in passive smoking and nonsmoking people although they assessed blood TAC in studied groups.22 Mottalebnejad et al. similarly found that the salivary levels of TAC in passive smokers were significantly lower compared to the control group (P = 0.023).14
Bakhtiari et al. investigated the effect of tobacco smoke on salivary TAC in 30 smoking and 30 nonsmoking people and found that it was significantly higher in the latter group (P < 0.001).23
Arinola et al. investigated the antioxidant level in smokers, nonsmokers, and passive smokers and found that smokers and passive smokers had equal antioxidant levels.24 As a result, active smoking and passive smoking have similar adverse effects on the body’s antioxidant system.
Kanehira et al. found a higher salivary level of antioxidants in tobacco smokers; however, the nonsmoking people showed more salivary antioxidant activity.25
These studies are consistent with those of the present one and indicate that tobacco smoke considerably reduces salivary TAC that, in turn, causes an imbalance in the oxidant/antioxidant system in active nonsmokers. However, there are some conflicting results in this regard.
Ugochukwu et al. found that the total antioxidant status was lower in current tobacco smokers than in nonsmokers and MDA level was higher in current smokers.26 In the present study, results from investigating levels of salivary TAC, as the overall outcome of antioxidants, were similar to this study.26
Buduneli et al. with comparison of smokers and nonsmokers did not find any significant between-group difference in salivary TAC.27 However, the present study, which was conducted on a larger sample size and active nonsmokers, justifies this difference.
Inconsistent with the present study, Aycicek et al. with investigation of oxidative stress levels in passive smokers and nonsmokers found that, despite increased antioxidant levels in passive smokers, lipid peroxidation significantly decreased in this group.28 Increased TAC in passive smokers in Aycicek et al.’s research was same to the present findings.28
Similarly, Motalebnejad et al. conducted an experimental study on 18 rats, aged 7-11 months, and found that the TAC level was conspicuously higher in the passive smoker group than in the nonsmoker group on the 0th and 15th days.7
The conflict between these results and those of the present study can be due to the differences in race, genetic diversity, diet, type of antioxidant, and sample size. In general, inhaling second-hand smoke can be as harmful as active smoking.
Since children have smaller bronchial tubes and their immune system is less developed, they are more vulnerable to digestive and respiratory complications if exposed to cigarette smoke. Rapid breathing in children causes a greater intake of harmful chemicals than in adults per kilogram of body weight.7,29
Preston et al. investigated 512 children aged 2-12 years and concluded that even brief exposure to tobacco smoke could considerably reduce their plasma level of vitamin C.30
Torun et al. showed that the condition of the oxidant/antioxidant system in children exposed to tobacco smoke shifted toward the pre-oxidation state. Therefore, the oxidant system of the body was disrupted before the involvement of the antioxidative system.31
Tobacco smoke induces oxidative stress by weakening the antioxidant defense system as well as producing reactive oxygen radicals.32
Reduced salivary TAC in passive tobacco smokers can cause an imbalance in the oxidant/antioxidant system of the body. This will increase oxidative stress. An increase in oxidative stress has a marked effect on health status and is associated with many diseases such as atherosclerosis, diabetes, cancer, and cerebrovascular diseases. Moreover, conspicuous exposure to tobacco smoke in childhood is associated with a high risk of mortality caused by chronic obstructive pulmonary disease (COPD). Consequently, protecting children from exposure to tobacco smoke is essential to reduce mortality and morbidity rates during their lifetime.30-32
Conclusion
The present study investigated the effect of passive smoking on the salivary levels of oxidative and antioxidative enzymes in children and adolescents as compared to their nonsmoking peers. In contrast to the majority of studies conducted on the effect of passive smoking on the antioxidant system of adults, there are few similar studies on children and adolescents. Our study did not show any significant difference between passive smoking and nonsmoking people in salivary lipid peroxidation levels. However, the passive smoking condition conspicuously decreased salivary antioxidant capacity. This finding indicates an imbalanced oxidant-antioxidant system under the passive smoking scenario. It seems that the greater use of antioxidants to reduce oxidative stress and maintain the balance in the oxidant-antioxidant system can reduce the level of TAC. Reduced level of salivary TAC in children exposed to tobacco smoke can endanger their general and oral cavity health. Passive smoking can cause serious diseases in children and is associated with high mortality and morbidity. Results of the present research showed that children should not be exposed to tobacco smoke.
Acknowledgments
The authors would like to express their gratitude to Zahedan University of Medical Sciences for financially supporting this research project.
Conflicts of Interest
The authors have no conflict of interest.
Authors’ Contribution
Designed and performed experiments, analyzed data and co-wrote the paper: FN and MS; performed experiments: SS.
REFERENCES
- 1.World Health Organization. Global Health Observatory (GHO) data: Second-hand smoke [Online]. 2019. Available from: URL: https://www.who.int/gho/phe/secondhand_smoke/en/
- 2.Hwang SH, Hwang JH, Moon JS, Lee DH. Environmental tobacco smoke and children's health. Korean J Pediatr. 2012;55(2):35–41. doi: 10.3345/kjp.2012.55.2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salimzadeh H, Najafipour H, Mirzaiepour F, Navadeh S, Shadkam-Farrokhi M, Mirzazadeh A. Prevalence of active and passive smoking among adult population: Findings of a Population-Based Survey in Kerman (KERCADRS), Iran. Addict Health. 2016;8(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- 4.Oberg M, Jaakkola MS, Pruss-Ustun A, Schweizer C, Woodward A. Second hand smoke: Assessing the burden of disease at national and local levels: Environmental burden of disease series, No. 18. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 5.World Health Organization. WHO report on the global tobacco epidemic 2019 [Online]. 2019. Available from: URL: https://www.who.int/tobacco/global_report/en/
- 6.Salmanzadeh H, Ahmadi-Soleimani SM, Pachenari N, Azadi M, Halliwell RF, Rubino T, et al. Adolescent drug exposure: A review of evidence for the development of persistent changes in brain function. Brain Res Bull. 2020;156:105–17. doi: 10.1016/j.brainresbull.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Motalebnejad M, Pouramir M, Moghadamnia AA, Ghasemi L, Soleimani L. The effect of passive smoking on total antioxidant capacity of serum and saliva in rats. J Dent Sch. 2020;31(2):117–24. [Google Scholar]
- 8.Zalata A, Yahia S, El-Bakary A, Elsheikha HM. Increased DNA damage in children caused by passive smoking as assessed by comet assay and oxidative stress. Mutat Res. 2007;629(2):140–7. doi: 10.1016/j.mrgentox.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Shirzaiy M, Dalirsani Z. The effect of glycemic control on salivary lipid peroxidation in type II diabetic patients. Diabetes Metab Syndr. 2019;13(3):1991–4. doi: 10.1016/j.dsx.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Shirzaiy M, Aiub Rigi Ladiz M, Dalirsani Z, Dehghan Haghighi J, Nakhaii A. Evaluation of salivary total antioxidant capacity in smokers with severe chronic periodontitis. Int J High Risk Behav Addict. 2017;6(3):e59486. [Google Scholar]
- 11.Greabu M, Totan A, Battino M, Mohora M, Didilescu A, Totan C, et al. Cigarette smoke effect on total salivary antioxidant capacity, salivary glutathione peroxidase and gamma-glutamyltransferase activity. Biofactors. 2008;33(2):129–36. doi: 10.1002/biof.5520330205. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Curtis LM, Khiani S, Moy J, Shalowitz MU, Sharp L, et al. A community-based study of tobacco smoke exposure among inner-city children with asthma in Chicago. J Allergy Clin Immunol. 2008;122(4):754–9. doi: 10.1016/j.jaci.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13(12) doi: 10.3390/ijerph13121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mottalebnejad M, Pouramir M, Jenabian N, Ranjbar Omrani M, Bijani A, Yarmand F. Evaluating the association between passive smoking with total antioxidant capacity and salivary lipid peroxidation levels in 12 to 15 year old adolescents. J Res Dent Sci. 2014;11(1):40–4. [Google Scholar]
- 15.Delpisheh A, Kelly Y, Brabin BJ. Passive cigarette smoke exposure in primary school children in Liverpool. Public Health. 2006;120(1):65–9. doi: 10.1016/j.puhe.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Nosratzehi T, Arbabi-Kalati F, Alijani E, Tajdari H. Comparison of cotinine salivary levels in hookah smokers, passive smokers, and non-smokers. Addict Health. 2015;7(3-4):184–91. [PMC free article] [PubMed] [Google Scholar]
- 17.Demirtas M, Senel U, Yuksel S, Yuksel M. A comparison of the generation of free radicals in saliva of active and passive smokers. Turk J Med Sci. 2014;44(2):208–11. [PubMed] [Google Scholar]
- 18.Yildirim F, Sermetow K, Aycicek A, Kocyigit A, Erel O. Increased oxidative stress in preschool children exposed to passive smoking. J Pediatr (Rio J) 2011;87(6):523–8. doi: 10.2223/JPED.2139. [DOI] [PubMed] [Google Scholar]
- 19.Azadbakht M, Sariri R, Soltani FM, Ghafoori H, Aghamaali MR, Erfani Karimzadeh Toosi A. Salivary Antioxidant Power of Passive Smokers. J Nanomedine Biotherapeutic Discov. 2016;6:142. [Google Scholar]
- 20.Falsafi P, Nasrabadi E, Nasrabadi H, Khiyavi R, Eslami H. Comparison of total antioxidant capacity and vitamin C in smokers and non-smokers. Biomed Pharmacol J. 2016;9(1):299–304. [Google Scholar]
- 21.Ghadimi A, Sariri R, Aryapour H, Erfani A, Nosratabadi F. variations in biological activity of salivary enzymes of smokers. Journal of Molecular and Cellular Research (Iranian Journal of Biology) 2014;27(1):125–35. [Google Scholar]
- 22.Mahrous MM, El-Barrany UM, Ismail MME-D, Gaballah IF, Rashed LA. Blood biomarkers of nicotine-induced toxicity in healthy males. Egypt J Forensic Sci. 2019;9(1):28. [Google Scholar]
- 23.Bakhtiari S, Azimi S, Mehdipour M, Amini S, Elmi Z, Namazi Z. Effect of cigarette smoke on salivary total antioxidant capacity. J Dent Res Dent Clin Dent Prospects. 2015;9(4):281–4. doi: 10.15171/joddd.2015.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arinola G, Akinosun O, Olaniyi J. Passive and active cigarette smoking: Effects on the levels of antioxidant vitamin, immunoglobulin classes and acute phase reactants. Afr J Biotechnol. 2011;10:6130–2. [Google Scholar]
- 25.Kanehira T, Shibata K, Kashiwazaki H, Inoue N, Morita M. Comparison of antioxidant enzymes in saliva of elderly smokers and non-smokers. Gerodontology. 2006;23(1):38–42. doi: 10.1111/j.1741-2358.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 26.Ugochukwu LO, Anyadike N, Chidimma O, Dioka CE, Meludu SC. Evaluation of total antioxidant status, superoxide dismutase and malondialdehyde in apparently healthy active tobacco smokers in Nnewi Metropolis, South-East, Nigeria. Journal of Scientific and Innovative Research. 2017;6(3):105–11. [Google Scholar]
- 27.Buduneli N, Kardesler L, Isik H, Willis CS, Hawkins SI, Kinane DF, et al. Effects of smoking and gingival inflammation on salivary antioxidant capacity. J Clin Periodontol. 2006;33(3):159–64. doi: 10.1111/j.1600-051X.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 28.Aycicek A, Erel O, Kocyigit A. Decreased total antioxidant capacity and increased oxidative stress in passive smoker infants and their mothers. Pediatr Int. 2005;47(6):635–9. doi: 10.1111/j.1442-200x.2005.02137.x. [DOI] [PubMed] [Google Scholar]
- 29.Dede C, Cinar N. Environmental tobacco smoke and children's health. Iran J Pediatr. 2016;26(5):e5935. doi: 10.5812/ijp.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preston AM, Rodriguez C, Rivera CE, Sahai H. Influence of environmental tobacco smoke on vitamin C status in children. Am J Clin Nutr. 2003;77(1):167–72. doi: 10.1093/ajcn/77.1.167. [DOI] [PubMed] [Google Scholar]
- 31.Torun E, Kahraman FU, Goksu AZ, Vahapoglu A, Cakin ZE. Serum catalase, thiol and myeloperoxidase levels in children passively exposed to cigarette smoke. Ital J Pediatr. 2019;45(1):59. doi: 10.1186/s13052-019-0652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diver WR, Jacobs EJ, Gapstur SM. secondhand smoke exposure in childhood and adulthood in relation to adult mortality among never smokers. Am J Prev Med. 2018;55(3):345–52. doi: 10.1016/j.amepre.2018.05.005. [DOI] [PubMed] [Google Scholar]