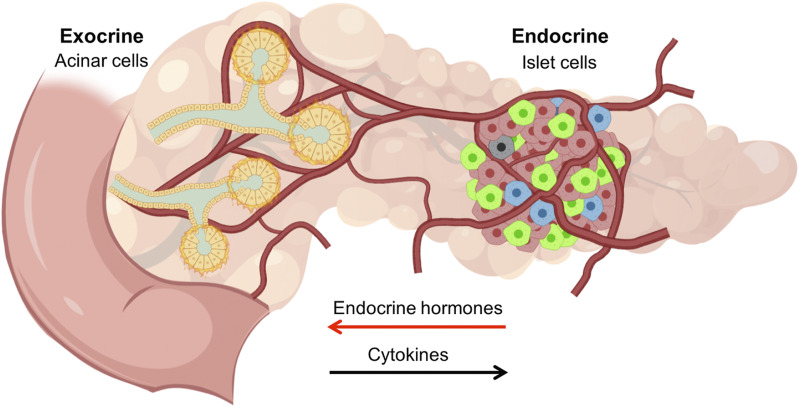
Abstract
The endocrine and exocrine pancreas have been studied separately by endocrinologists and gastroenterologists as two organ systems. The pancreatic islet, consisting of 1–2% mass of the whole pancreas, has long been believed to be regulated independently from the surrounding exocrine tissues. Particularly, islet blood flow has been consistently illustrated as one-way flow from arteriole(s) to venule(s) with no integration of the capillary network between the endocrine and exocrine pancreas. It is likely linked to the long-standing dogma that the rodent islet has a mantle of non–β-cells and that the islet is completely separated from the exocrine compartment. A new model of islet microcirculation is built on the basis of analyses of in vivo blood flow measurements in mice and an in situ three-dimensional structure of the capillary network in mice and humans. The deduced integrated blood flow throughout the entire pancreas suggests direct interactions between islet endocrine cells and surrounding cells as well as the bidirectional blood flow between the endocrine and exocrine pancreas, not necessarily a unidirectional blood flow as in a so-called insuloacinar portal system. In this perspective, we discuss how this conceptual transformation could potentially affect our current understanding of the biology, physiology, and pathogenesis of the islet and pancreas.
Introduction
It should be reasonable to consider that treatment of a disease by targeting either endocrine or exocrine pathogenesis can affect the function of the entire pancreas for better or worse. However, these two parts of the pancreas have been studied and treated separately by physicians in different medical disciplines. The only connection between the two systems proposed to date has been the insuloacinar portal vessels that drain from the islet to exocrine tissues (1,2). This unidirectional blood flow was determined based on two-dimensional (2D) analysis of pancreas images of India ink injection and scanning electron microscopy of corrosion casts with no markers for the islet boundary or arterioles. The rodent islet, in particular, has been seen with a core of β-cells surrounded by non–β-cells in the periphery, specifically in 2D images, which is not observed in three-dimensional (3D) images (3). Indeed, the islet is regarded as a glomerulus-like enclosed microorgan (4), where blood perfusion has been considered to be regulated independently from that of the exocrine pancreas. Therefore, it has been illustrated as being situated between an afferent arteriole and an efferent venule, indicating the one-way traffic of islet blood flow simply from artery to vein, with no integration to the surrounding exocrine capillary network (5–9). Once such a dogma or a gold standard is established, it is considerably difficult to challenge such conventions after they have been widely accepted for many years.
In vivo measurement of pancreatic microcirculation by tracking individual red blood cells in mice has revealed that blood flow is bidirectional and integrated with that of the exocrine pancreas at its entirety, not sporadically or selectively “at multiple locations” (3). This is in line with in situ structural analysis of pancreatic capillaries both in mice and in humans that showed no disruptions of the pancreatic vascular network. The important conceptual difference from the bidirectional pancreatic blood flow is that the insuloacinar portal system includes only unidirectional blood flow from islets to exocrine tissues.
In this perspective, we examined how conceptual changes of pancreatic blood flow may support or refine current notions in the field by revisiting critically debated areas of β-cell/islet biology, exocrine insufficiency and deficiency, and some different types of diabetes, such as islet formation models, small pancreas associated with type 1 diabetes (T1D), cystic fibrosis–related diabetes (CFRD), and type 3c (T3c) diabetes.
Unidirectional versus Bidirectional Pancreatic Islet Blood Flow
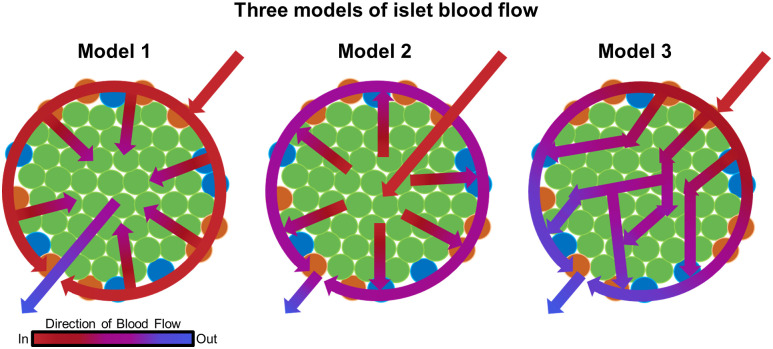
At an international symposium in 1995, researchers presented evidence collected over decades that support three mutually exclusive models of islet microcirculation (10) (Fig. 1). The unique cytoarchitecture of the rodent islet served a fundamental role in determining which cell types were perfused first and which hormones exerted regulatory effects on the others. It was not explicitly stated that complete mantle formation was a prerequisite for the models. However, when determining the hierarchical order of islet blood flow, having non–β-cells forming a complete mantle (or at least an outer layer sufficiently covering its core to be called “a mantle”) was in fact a requirement for models 1 and 2. Otherwise, simultaneous perfusion of all cell types would occur, particularly rendering model 1 unlikely. In mice, α-cells consist of the majority of non–β-cells, comprising up to 20% of the islet cells (11–13). Other cell types such as δ-, pancreatic polypeptide, and ε-cells are in low abundance (14). In the regularly observed diameter range of mouse islets (100–150 μm) (15), theoretically, non–β-cells need to constitute >50% of the entire islet cells to form a complete mantle (3).
Figure 1.
Three models of islet blood flow. Adapted from Dybala et al. (4).
Notably, all models proposed unidirectional blood flow from arteriole(s) to venule(s) but varied in their claims of hierarchical regulatory mechanisms of hormone secretion within the islet as follows: model 1, hormones secreted from non–β-cells (such as glucagon, somatostatin, and pancreatic polypeptide); model 2, insulin secreted from β-cells; and model 3, neither of them. Model 1 was proposed by Fujita (1), who examined arterial injection of India ink to horse and rabbit pancreas, and further supported by subsequent articles from his group that included many other animals (10). The vascular route leading blood into the horse islet was determined as from the center to the periphery, where in this animal, α-cells uniquely form a core of the islet with β-cells in the periphery. In rabbits, which inversely have central β-cells and peripheral α-cells, the author claimed that the vessels are designed to lead blood from the periphery to the center. This directionality was reclaimed in the rat islet by scanning electron microscopy of resin casts (2). It was emphasized that the direction of islet microcirculation should be species dependent because of islet architectural variability (16). Thus, model 1 includes three structurally distinct directions of flow depending on species: core-to-mantle (horse), mantle-to-core (rabbit, rat, and mouse), and mixed (dog, cow, pig, monkey, and human). In addition, the group noted that regardless of species, efferent vessels of the islet drain into capillaries supplying the acinar tissue of the exocrine pancreas, termed the insuloacinar portal system (1,2). Model 2, which is based on studies in rats, proposed that arterial blood perfuses β-cells before non–β-cells via ordered perfusion of the islet core followed by the non–β-cell mantle (10). Islet plasticity among species is also an issue in model 2, particularly human and monkey islets. It was suggested that these islets could be composed of several mantle-core cellular structures or subunits. However, such subunits were not found in 3D (17). Portals consisting of some efferent vessels were similarly observed in model 2. Model 3 proposed a unique gate-controlled polar flow pattern in which blood flows from one “pole” of the islet to the other. External arteriolar sphincters allow blood to initially enter the islet while interior gates, composed of contractile endothelial cells, control the blood flow from pole to pole. Unlike the other models, the order of perfusion of β-cells and non–β-cells is indistinct and dependent on which hemisphere they are positioned.
Our recent model of islet microcirculation in which blood flow is bidirectional and integrated with pancreatic microvasculature suggests that microcirculation is not related to cytoarchitecture (3). We further showed that blood travels through a variety of paths within the islet at different speeds and densities, indicating that the flow is heterogeneous. Furthermore, the various trajectories along with 3D images of blood vessels suggested that the islet capillaries are integrated with those of the exocrine pancreas, contradicting the assumption that microcirculation is confined to a closed structure within islets. Using intravital recordings, the ratio of blood flow from the exocrine to endocrine pancreas was 50.1% ± 1.3% and from the endocrine to exocrine pancreas 49.9% ± 1.3%, supporting bidirectional blood flow. Additionally, bidirectional flow in a nearly 1:1 ratio calls the proposed insuloacinar portal system into question as this proposed model maintained unidirectional flow from islets to acinar tissue. In this new model, islet blood may flow not only to acinar tissue but also from acinar tissue to islets in near-equal proportion. This model implies that islet-secreted products perfuse acinar tissue and that secretions from the acinar pancreas perfuse the islets. This finding has important implications in physically linking the endocrine and exocrine pancreas in light of clinical pathologies where both regions are affected.
Islet Formation: Exocrine Pancreas Plays a Pivotal Role during Early Development
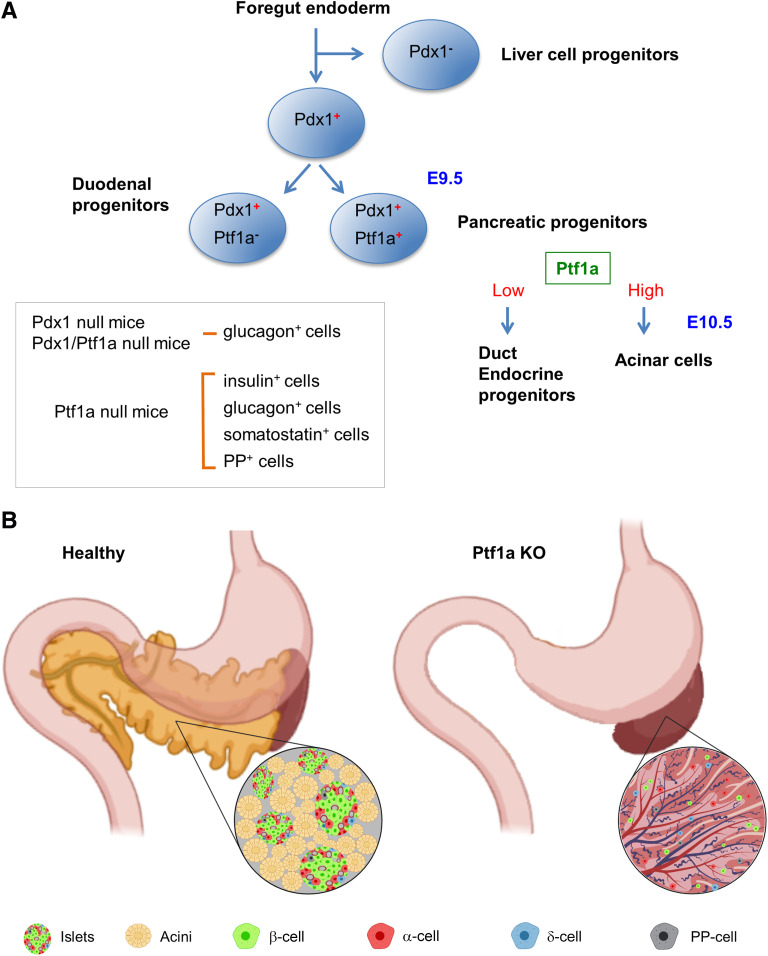
In the foregut endoderm, Pdx1 and Ptf1a play a pivotal role in the early specification of progenitors for liver, duodenum, and pancreas (reviewed in Pan and Wright [18] and Jin and Xiang [19]) (Fig. 2A). Pancreatic multipotent progenitor cells coexpress Pdx1 and Ptf1a between embryonic days 9.5 and 12.5. Deletion of Pdx1 and/or Ptf1a results in pancreas agenesis. Pdx1 null and Pdx1/Ptf1a double-null mice are phenotypically identical with early pancreatic bud formation, which suggests that Pdx1 is required prior to Ptf1a for pancreatic multipotent progenitor cell specification. Pdx1 homozygous deletion allows the formation of the dorsal and ventral pancreatic buds. However, only the dorsal bud undergoes limited branching outgrowth and forms a stunted, irregular epithelial tree that persists in newborns where glucagon-positive cells can be detected. Mice with a homozygous null mutation of the Ptf1a allele died shortly after birth and exhibited a complete absence of exocrine pancreas (20). Interestingly, all four types of endocrine cells (expressing insulin, glucagon, somatostatin, and pancreatic polypeptide) are spared and found individually scattered throughout the spleen, indicating that the exocrine pancreas plays a pivotal role in islet formation and their correct spatial assembly during development (Fig. 2B).
Figure 2.
Islet formation: Exocrine pancreas plays a pivotal role during early development. A: Expression of transcription factors Pdx1 and Ptf1a during early foregut endoderm development. B: In healthy mice (left), islets are distributed throughout the pancreas and integrated with the acinar tissue of the exocrine pancreas. In Ptf1a knockout (KO) mice (right), the pancreas fails to form, and endocrine cells are found randomly scattered throughout the spleen. E, embryonic day; PP, pancreatic polypeptide.
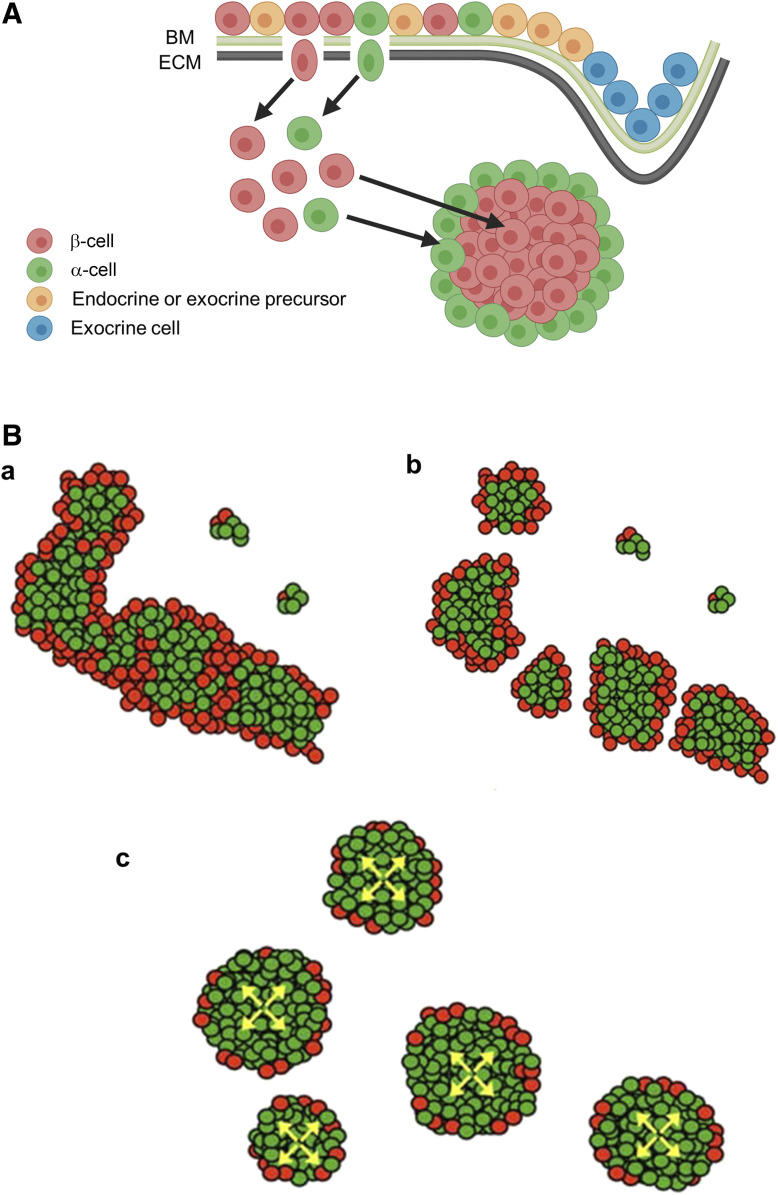
The prevailing model of islet formation has been based on epithelial-to-mesenchymal transition (EMT) and subsequent islet cell aggregation (21) (Fig. 3A). As progenitor cells differentiate, they undergo EMT and islet precursors migrate through the basal membrane (BM) and extracellular matrix (ECM) that separate epithelium and mesenchyme. This model was deduced from in vitro studies of isolated islets that showed reaggregation of dissociated single islet cells (22,23). As the epithelium branches into the mesenchyme, the exocrine precursors localize to the distal ends of the pancreatic ductal epithelium. The endocrine cell migration occurs via degradation of both the BM and ECM molecules. After islet cell formation, islet cell-ECM interactions and cell-cell interactions coordinate cell aggregation and sorting. Altogether, it may represent an “islet-intrinsic” concept that was applied to early islet development, without a role for the exocrine pancreatic tissue.
Figure 3.
Two distinct models of islet formation. A: Islet aggregation model. Endocrine and exocrine precursor cells line the BM and ECM of the epithelium in the developing pancreas. Endocrine (islet) precursors pass through the BM and ECM before reaggregating into islets in the mesenchyme. B: Fission model of islet formation during fetal and neonatal development. a: Endocrine cells (β-cells in red, α-cells in green) proliferate contiguously, forming branching cord-like structures. b: Islet formation progresses with fission of branched cords. Note the random distribution of islet size. c: Further expansion of β-cells within the newly formed islets leads to the observed α-cell proportion of 5–10%. Adapted from Miller et al. (24).
More recent studies from us and others have challenged this cell aggregation model. We observed that endocrine cells proliferate contiguously, forming branched cord-like structures starting in embryos (24) (Fig. 3B). Further, in the neonatal pancreas, long stretches of interconnected islets are located along large blood vessels. α-cells span the elongated islet-like structures, which we hypothesized represent sites of fission and facilitate the eventual formation of discrete islets. Our model proposes that islet formation occurs by a process of fission following contiguous endocrine cell proliferation rather than by local aggregation or fusion of isolated β-cells and islets. A gradual increase in the ratio of β-cells within an islet allows formation of larger, spherical-shaped islets. Fission may be random, which results in variable islet sizes in the adult pancreas. Another model of islet formation has been proposed by mapping changes in gene expression during early pancreas development (25). In this model, differentiated endocrine progenitors migrate in cohesion and form peninsula-like islet precursors. α-cells precede the development of β-cells; therefore, nascent peninsulas have a bilayered structure with β-cells between epithelial cords and α-cells on the exterior of peninsula. An in silico version of this model showed that islets were vascularized throughout the entire development process, although blood flow appears to be unidirectional. A parallel in vitro model from the same study showed that similar peninsular islet precursors also formed from spheroid clusters of endocrine progenitors.
Taken together, a crucial role of the exocrine pancreas that defines islet cell assembly and these new models of islet formation highlight the importance of a physical link between the endocrine and exocrine pancreas during early pancreas development through the fully integrated pancreatic microcirculation. Indeed, islet vascularization is necessary in developing islets for normal pancreatic function, as absence of vascular endothelial growth factor A (VEGF-A) in the developing pancreas leads to reduced vascularization and fenestration of islet capillaries (26). In the adult islet, however, VEGF-A is not required for islet survival, and adult islets maintain their architectural arrangement and β-cell mass despite a loss of vascularization (27). Besides endothelial-centric approaches, studies by the Lammert group have elegantly shown that the epithelial biomechanical properties, such as cell cortex tension and adhesion to blood capillary, determine the location of islet vasculature (28). Our recent study of in vivo mouse islet microcirculation provided evidence of vascular reciprocity between endocrine and exocrine pancreas (3). This bidirectional exchange of blood between exocrine and endocrine compartments in the developing pancreas may provide a mechanism for exchange of necessary factors regulating normal islet development.
Small Pancreas in Patients with T1D: Lack of Insulin May Affect Exocrine Pancreatic Volume as a Growth Factor
Studies have shown that children, adolescents, and adults with T1D have reduced pancreatic volume and exocrine function (29–32). The integrated blood flow between endocrine and exocrine pancreas may contribute to this reduced pancreas size in T1D as a result of the role of insulin serving as a growth factor for acinar cells that express receptors for insulin, IGF-I and IGF-II (33). It was observed decades ago that juxtainsular acini positioned closest to islet cells appeared to be a halo around an islet. Cells in the halos tended to be larger, had more nuclei, and contained more zymogen granules compared with those farther away from the islets (34). In in vitro and in vivo studies of insulin action on the acinar tissue, it was found that insulin enhances protein synthesis, increases sensitivity to cholecystokinin, and increases binding of IGF-II, stimulating acinar cell growth (33,35). Furthermore, it was shown that insulin stimulated cell growth in rat acinar cells, measured by an increase in DNA and protein, in a dose-dependent manner (36). A recent study by Egozi et al. (37) has shown that islet-derived cholecystokinin induces such zonated acinar cells in diabetic mice upregulating trypsin genes and mTOR activity, where trypsin may, in turn, contribute to islet expansion as a growth factor.
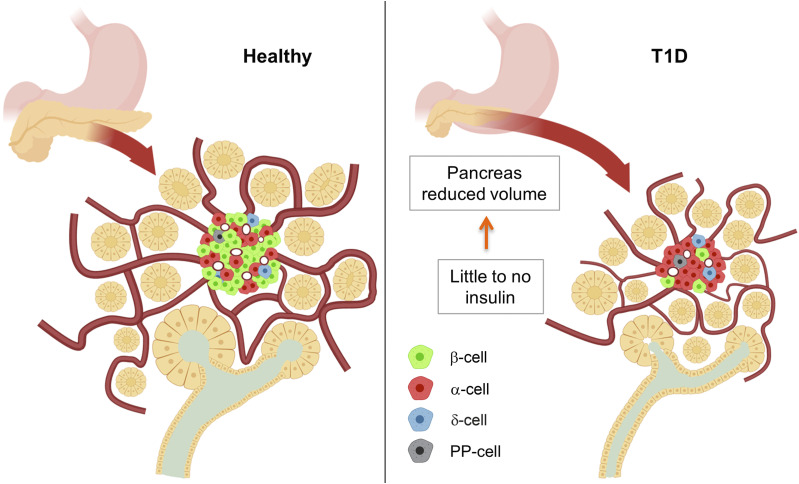
Lack of insulin in T1D may contribute to the observed reduced pancreatic volume and exocrine insufficiency, especially considering recent insight into the vascular connection between exocrine and endocrine pancreas (Fig. 4). Reduced pancreatic volume is observed in patients with both recent-onset and long-duration T1D, which may indicate that pancreatic volume reduction is the result of an acute loss of islet hormone sensing via bidirectional blood flow rather than through long-term inflammation (38). Since the acinar tissue would see higher concentrations of insulin than the rest of the body in this integrated blood circulation model, a lack of insulin in patients with T1D, as a growth factor for the acinar cell, may result in a smaller pancreas and subsequently deteriorated exocrine function. Of note, no reduction of pancreas weight was observed in three mouse models of diabetes: 1) female NOD mice (autoimmune β-cell destruction); 2) male Akita mice (misfolded mutant proinsulin-induced toxicity); and 3) NOD.Cg-PrkdcscidIL2rgtm1WjlSz (NSG) mice expressing diphtheria toxin (39).
Figure 4.
Small pancreas in patients with T1D. Lack of insulin may affect exocrine pancreatic volume as a growth factor. Functional islets in healthy patients (left) are integrated with the exocrine pancreas, providing sufficient insulin for normal growth and function. In patients with T1D (right), β-cells are lost, and insulin perfusion in the exocrine tissue is reduced with decreased exocrine and whole-pancreas volume. PP, pancreatic polypeptide.
The β-Cell Extrinsic Hypothesis for CFRD: Exocrine Inflammation Directly Affects the β-Cell/Islet Function
Cystic fibrosis (CF) is a disease that affects the lungs and digestive system as a result of autosomal recessive mutations in the CF transmembrane conductance regulator (CFTR) gene that lead to impaired ion transport and accumulation of thick mucus (40). CFRD is a type of diabetes that affects 35–50% of patients with CF and shares some similarities with both T1D and T2D (41). Current understanding of CFRD pathogenesis and pathophysiology is incomplete. Two hypotheses regarding the role of CFTR in insulin insufficiency have been proposed: β-cell intrinsic and β-cell extrinsic.
In the β-cell intrinsic hypothesis, it is granted that CFTR is expressed in β-cells, and thus its mutation impairs insulin secretion, leading to intrinsic islet dysfunction. CFTR is believed to have a role in insulin granular priming and exocytosis of the primed granules for insulin release (42). The priming of storage granules is a necessary step that involves acidification of the granular lumen via chloride ions flowing through the chloride transporter CLC3. The chloride ions enter the cell by the chloride transporter anosmin 1 (ANO1). An intracellular rise in cAMP levels is stimulated by incretins, such as GLP-1, leading to cAMP activation of the CFTR channel. It is suggested that ANO1 may cooperate with CFTR in granular priming in both human and mouse β-cells (43). In contrast, the β-cell extrinsic hypothesis proposes that CFRD results from CF-induced pancreatitis and reduced β-cell mass rather than dysfunction of CFTR within the β-cell. A recent study showed that β-cell–specific deletion of CFTR in mice does not alter glucose tolerance or in vitro β-cell function (44). In human islets, CFTR protein and electrical activity were not detected. It is noteworthy that the authors reported minimal CFTR transcripts by analyzing published gene expression data sets based on single-cell RNA sequencing of FACS-sorted human islet cells: β-cells 5%, α-cells 3%, and δ-cells 4%. This is further confirmed by a study of in situ CFTR mRNA expression (β-cells <1%) as well as of protein expression (negative) in human pancreatic tissues (44).
The CFTR protein is highly expressed in the pancreatic duct epithelia and plays a central role in ductal anions (HCO3− and Cl−) and fluid secretion (45). A resulting increase of alkaline fluid allows the highly concentrated proteins secreted from acinar cells to remain soluble. This is compromised in patients with CF, and such deficiencies begin in utero (46). From birth onward, proteins such as immune reactive trypsinogen can be detected in blood, which is used as a marker for the neonatal screening test for CF during an asymptomatic period, suggesting pancreatic involvement in the early life of patients with CF. The lack of CFTR expression in most human β-cells suggests that impairment of duct cell functions may trigger pancreatic inflammation, as CFRD has an unusual phenotype accompanied by insulin resistance, particularly during acute pulmonary exacerbations (47). Our new model of integrated pancreatic islet blood flow supports the currently accepted β-cell extrinsic hypothesis (3). The close interaction and communication between the exocrine and endocrine cells are important in understanding the pathogenesis of CFRD, where exocrine-derived acute phase inflammatory factors such as IL-6 may directly affect β-cell/islet function and development of CFRD (48) (Fig. 5).
Figure 5.
Exocrine inflammation directly affects β-cell/islet function through the integrated pancreatic blood flow. Normal function of the exocrine pancreas is disrupted in CFRD and T3c diabetes, leading to β-cell/islet dysfunction. Inflammatory cytokines associated with exocrine pancreatic disease, such as IL-6 in CFRD, travel throughout the pancreas to the islets, eventually leading to progressive endocrine hormone deficiency. Normal bidirectional blood flow between endocrine and exocrine pancreas facilitates the exchange of cytokines central to the pathogenesis of CFRD and T3c diabetes.
T3c Diabetes: The Integrated Pancreatic Blood Flow Physically Connects the Exocrine and Endocrine Pancreas as a Single Organ
When diabetes develops secondary to a disease of the exocrine pancreas, it is collectively referred to as T3c diabetes (49). It is characterized by persistent hyperglycemia due to various exocrine pancreatic pathologies, including acute and chronic pancreatitis, pancreatic cancer, hemochromatosis, CF, and previous pancreatic surgery (50). T3c diabetes encompasses a range of pathophysiologies commonly driven by β-cell dysfunction and insulin resistance. T3c diabetes is most commonly a comorbidity of pancreatitis, with prevalence estimates ranging from 25% to 80%. Among these etiologies, there exists heterogeneity in the contribution of β-cell loss and insulin resistance that lead to the diabetic phenotype (51). In addition, pancreatic fibrosis as a result of persistent inflammation could reduce the functional capacity of residual islets by damaging the vascular network. The fibroinflammatory environment increases the concentration of cytokines over the course of the pancreatitis, which eventually mediates β-cell dysfunction and contributes to progressive insulin deficiency (Fig. 5).
A recent retrospective cohort study reported that in 559 patients with diabetes following pancreatic disease (out of over 2 million people in England), only 2.7% of them were classified as having T3c diabetes (52). They were most commonly diagnosed as having T2D (87.8%). It is suggested that with clinician awareness of underlying or any history of pancreatic disease, these patients might have benefited from more tailored management, including a choice of antihyperglycemic therapy and consideration of malabsorption requiring pancreatic enzyme and vitamin D prescription. In fact, they showed significantly worse glycemic control and a greater need for insulin compared with patients with T2D.
Discussion
The integrated pancreatic blood flow implies that the islet is not an enclosed microorgan and that islet microcirculation has no relation to islet cytoarchitecture, which explains its well-known variability throughout species (16). It has also not been fully appreciated that the endocrine and exocrine pancreas are physically and thus functionally linked. It all started with the notion of core-mantle arrangement of β- and non–β-cells in 2D views of islets, mainly in rodents but also in other animals such as rabbits and horses (1,2,15). We propose that it is attributed to the visual and conceptual perceptions of human brain, which is well explained by several Gestalt principles (4). These principles describe the capability of our brain to fill any missing information and generate whole forms from lines, shapes, and curves and reduce complexity to simplified concepts and structures. The unique islet architecture naturally led researchers to seek functional implications (9). Widely, on many occasions, dogmas, gold standards, and even simple common practices are hard to question. In fact, in a later study using advanced intravital imaging, these original three models of microcirculation were still believed to be the gold standard of islet blood flow (53).
The recent advancement of confocal microscopy and tissue clearing techniques has made 3D imaging of thick tissues possible, which is pivotal to studying network structures such as vasculature. Interpretation of past islet architecture studies may have been influenced by historical approaches to islet imaging in addition to visual interpretation by the human brain. As a result, rigorous assessment of experimental results in relation to structural and visual observations is critical in the process of developing novel hypotheses.
Article Information
Acknowledgments. Figures (except Figs. 1 and 3B) were created using BioRender.com.
Funding. The study was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health grants DK-117192, and DK-020595 to The University of Chicago Diabetes Research and Training Center (Physiology Core), and a gift from the Kovler Family Foundation to M.H. Micro-Metcalf Awards to L.R.G., M.E.R., and Y.Y.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.H. conceived the idea and designed the study. M.P.D., L.R.G., M.E.R., Y.Y., and M.H. wrote the manuscript. M.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Fujita T. Insulo-acinar portal system in the horse pancreas. Arch Histol Jpn 1973;35:161–171 [DOI] [PubMed] [Google Scholar]
- 2.Murakami T, Fujita T. Microcirculation of the rat pancreas, with special reference to the insulo-acinar portal and insulo-venous drainage systems: a further scanning electron microscope study of corrosion casts. Arch Histol Cytol 1992;55:453–476 [DOI] [PubMed] [Google Scholar]
- 3.Dybala MP, Kuznetsov A, Motobu M, et al. Integrated pancreatic blood flow: bidirectional microcirculation between endocrine and exocrine pancreas. Diabetes 2020;69:1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dybala MP, Butterfield JK, Hendren-Santiago BK, Hara M. Pancreatic islets and Gestalt principles. Diabetes 2020;69:1864–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 1982;31:883–889 [DOI] [PubMed] [Google Scholar]
- 6.Eberhard D, Kragl M, Lammert E. ‘Giving and taking’: endothelial and beta-cells in the islets of Langerhans. Trends Endocrinol Metab 2010;21:457–463 [DOI] [PubMed] [Google Scholar]
- 7.Cleaver O, Dor Y. Vascular instruction of pancreas development. Development 2012;139:2833–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pénicaud L. Autonomic nervous system and pancreatic islet blood flow. Biochimie 2017;143:29–32 [DOI] [PubMed] [Google Scholar]
- 9.Jansson L, Carlsson PO. Pancreatic blood flow with special emphasis on blood perfusion of the islets of Langerhans. Compr Physiol 2019;9:799–837 [DOI] [PubMed] [Google Scholar]
- 10.Brunicardi FC, Stagner J, Bonner-Weir S, et al.; Long Beach Veterans Administration Regional Medical Education Center Symposium . Microcirculation of the islets of langerhans. Diabetes 1996;45:385–392 [DOI] [PubMed] [Google Scholar]
- 11.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–1097 [DOI] [PubMed] [Google Scholar]
- 12.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: a comparative study. Islets 2009;1:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Silva Xavier G. The cells of the islets of langerhans. J Clin Med 2018;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilimnik G, Kim A, Jo J, Miller K, Hara M. Quantification of pancreatic islet distribution in situ in mice. Am J Physiol Endocrinol Metab 2009;297:E1331–E1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets 2010;2:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dybala MP, Hara M. Heterogeneity of the human pancreatic islet. Diabetes 2019;68:1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 2011;240:530–565 [DOI] [PubMed] [Google Scholar]
- 19.Jin K, Xiang M. Transcription factor Ptf1a in development, diseases and reprogramming. Cell Mol Life Sci 2019;76:921–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krapp A, Knöfler M, Ledermann B, et al. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev 1998;12:3752–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev 2001;15:111–127 [DOI] [PubMed] [Google Scholar]
- 22.Rouiller DG, Cirulli V, Halban PA. Uvomorulin mediates calcium-dependent aggregation of islet cells, whereas calcium-independent cell adhesion molecules distinguish between islet cell types. Dev Biol 1991;148:233–242 [DOI] [PubMed] [Google Scholar]
- 23.Esni F, Täljedal IB, Perl AK, Cremer H, Christofori G, Semb H. Neural cell adhesion molecule (N-CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets. J Cell Biol 1999;144:325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller K, Kim A, Kilimnik G, et al. Islet formation during the neonatal development in mice. PLoS One 2009;4:e7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharon N, Chawla R, Mueller J, et al. A peninsular structure coordinates asynchronous differentiation with morphogenesis to generate pancreatic islets. Cell 2019;176:790–804.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lammert E, Gu G, McLaughlin M, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Boil 2003;13:1070–1074 [DOI] [PubMed] [Google Scholar]
- 27.Reinert RB, Brissova M, Shostak A, et al. Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes 2013;62:4154–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kragl M, Schubert R, Karsjens H, et al. The biomechanical properties of an epithelial tissue determine the location of its vasculature. Nat Commun 2016;7:13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Campbell-Thompson M, Wasserfall CH, et al. Serum trypsinogen levels in type 1 diabetes. Diabetes Care 2017;40:577–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams AJ, Thrower SL, Sequeiros IM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab 2012;97:E2109–E2113 [DOI] [PubMed] [Google Scholar]
- 31.Gaglia JL, Guimaraes AR, Harisinghani M, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest 2011;121:442–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Augustine P, Gent R, Louise J, et al.; ENDIA Study Group . Pancreas size and exocrine function is decreased in young children with recent-onset Type 1 diabetes. Diabet Med 2020;37:1340–1343 [DOI] [PubMed] [Google Scholar]
- 33.Williams JA, Goldfine ID. The insulin-pancreatic acinar axis. Diabetes 1985;34:980–986 [DOI] [PubMed] [Google Scholar]
- 34.Hellman B, Wallgren A, Petersson B. Cytological characteristics of the exocrine pancreatic cells with regard to their position in relation to the islets of Langerhans. A study in normal and obese-hyperglycaemic mice. Acta Endocrinol (Copenh) 1962;39:465–473 [DOI] [PubMed] [Google Scholar]
- 35.Adler G, Kern HF. Regulation of exocrine pancreatic secretory process by insulin in vivo. Horm Metab Res 1975;7:290–296 [DOI] [PubMed] [Google Scholar]
- 36.Mössner J, Logsdon CD, Goldfine ID, Williams JA. Do insulin and the insulin like growth factors (IGFs) stimulate growth of the exocrine pancreas? Gut 1987;28(Suppl.):51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egozi A, Bahar Halpern K, Farack L, Rotem H, Itzkovitz S. Zonation of pancreatic acinar cells in diabetic mice. Cell Rep 2020;32:108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell-Thompson M. Organ donor specimens: what can they tell us about type 1 diabetes? Pediatr Diabetes 2015;16:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright JJ, Saunders DC, Dai C, et al. Decreased pancreatic acinar cell number in type 1 diabetes. Diabetologia 2020;63:1418–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies JC, Alton EW, Bush A. Cystic fibrosis. BMJ 2007;335:1255–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayani K, Mohammed R, Mohiaddin H. Cystic fibrosis-related diabetes. Front Endocrinol (Lausanne) 2018;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barg S, Huang P, Eliasson L, et al. Priming of insulin granules for exocytosis by granular Cl(-) uptake and acidification. J Cell Sci 2001;114:2145–2154 [DOI] [PubMed] [Google Scholar]
- 43.Edlund A, Esguerra JL, Wendt A, Flodström-Tullberg M, Eliasson L. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med 2014;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hart NJ, Aramandla R, Poffenberger G, et al. Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight 2018;3:e98240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White MG, Maheshwari RR, Anderson SJ, et al. In situ analysis reveals that CFTR is expressed in only a small minority of β-cells in normal adult human pancreas. J Clin Endocrinol Metab 2020;105:dgz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pallagi P, Hegyi P, Rakonczay Z Jr. The physiology and pathophysiology of pancreatic ductal secretion: the background for clinicians. Pancreas 2015;44:1211–1233 [DOI] [PubMed] [Google Scholar]
- 47.Castellani C, Assael BM. Cystic fibrosis: a clinical view. Cell Mol Life Sci 2017;74:129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun X, Yi Y, Xie W, et al. CFTR influences beta cell function and insulin secretion through non-cell autonomous exocrine-derived factors. Endocrinology 2017;158:3325–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev 2012;28:338–342 [DOI] [PubMed] [Google Scholar]
- 50.Hart PA, Bellin MD, Andersen DK, et al.; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer(CPDPC) . Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol 2016;1:226–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gudipaty L, Rickels MR. Pancreatogenic (type 3c) diabetes. Pancreapedia. Prairie Village, KS, American Pancreatic Association, 2015 [Google Scholar]
- 52.Woodmansey C, McGovern AP, McCullough KA, et al. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (type 3c): a retrospective cohort study. Diabetes Care 2017;40:1486–1493 [DOI] [PubMed] [Google Scholar]
- 53.Nyman LR, Wells KS, Head WS, et al. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest 2008;118:3790–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]