Abstract
Targeting T cell receptor β-chain constant region 1 (TRBC1) CAR-T could specifically kill TRBC1+ T-cell malignancies. However, over-expressed CARs on anti-TRBC1 CAR transduced TRBC1+ T cells (CAR-C1) bound to autologous TRBC1, masking TRBC1 from identification by other anti-TRBC1 CAR-T, and moreover only the remaining unoccupied CARs recognized TRBC1+ cells, considerably reducing therapeutic potency of CAR-C1. In addition, co-culture of anti-TRBC1 CAR-T and TRBC1+ cells could promote exhaustion and terminal differentiation of CAR-T. These findings provide a rationale for pre-depleting TRBC1+ T cells before anti-TRBC1 CAR-T manufacturing.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12943-020-01282-7.
Keywords: T cell receptor β-chain constant region 1, CAR-T, T-cell malignancy
Background
Chimeric antigen receptor (CAR) T cells showed remarkable efficacy for the treatment of B-cell malignancies and have been approved by the US Food and Drug Administration for the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) and diffuse large B-cell lymphoma (DLBCL) [1, 2]. However, the development of CAR-T cells against T-cell malignancies seems more challenging due to the similarities between the normal, malignant and therapeutic T cells, which could result into CAR-T cell fratricide, T cell aplasia, and contamination of CAR-T cell products with malignant T cells [3, 4].
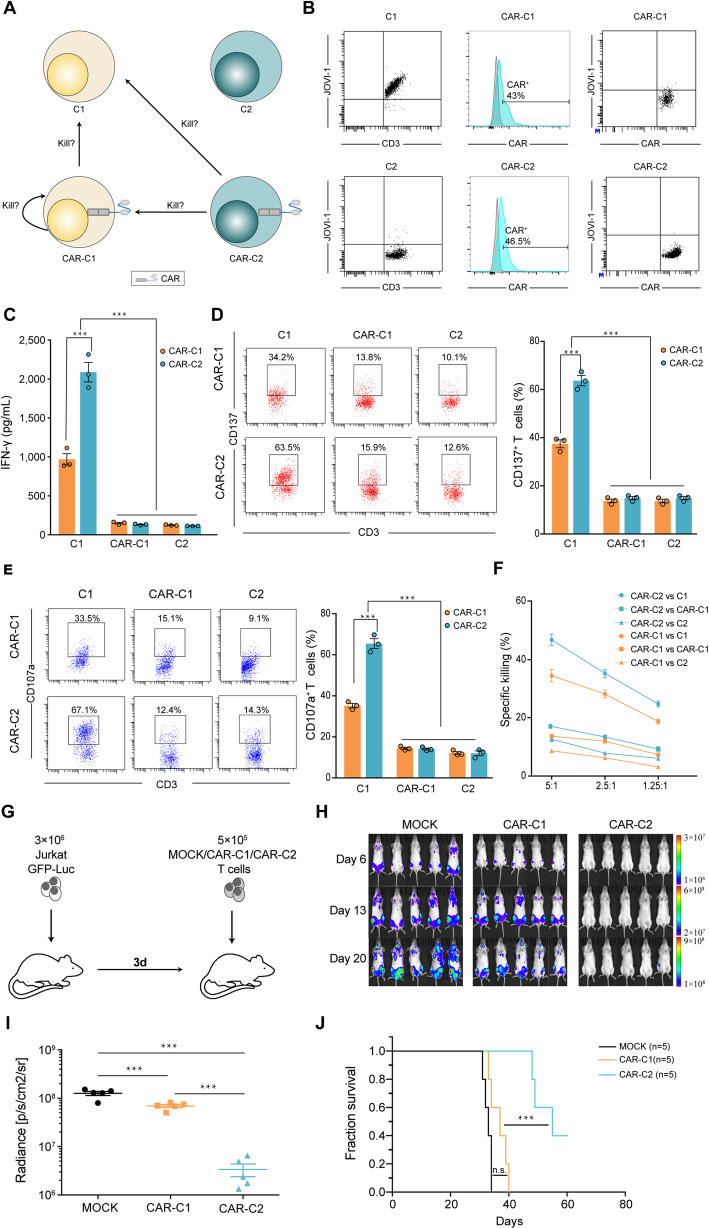
An innovative treatment option for T-cell malignancy was proposed that targeting T cell receptor β-chain constant region 1 (TRBC1) CAR-T could specifically identify and kill TRBC1+ T-cell malignancies, since either TRBC1 or TRBC2 is mutually exclusively expressed in T cells and moreover proportion of TRBC1+ T cells varies between 25 and 47% in healthy individuals, but malignant T cells are clonally TRBC1 positive or negative [5, 6]. Thus, anti-TRBC1 CAR-T cells could specifically kill TRBC1+ malignant T cells while sparing TRBC2+ normal T cells. However, anti-TRBC1 CAR gene could probably be inadvertently transferred into TRBC1+ malignant T cells during CAR-T cell manufacturing, and its product could in cis bind to autologous TRBC1 on the surface of malignant T cells, which could result into masking TRBC1 from identification by and mediating resistance to anti-TRBC1 CAR-T and meanwhile weaken effector function of anti-TRBC1 CAR transduced TRBC1+ cells. Following transduction of T cells with lentivirus encoding anti-TRBC1 CAR, all T cells could be categorized into TRBC1+ cells (C1), TRBC2+ cells (C2), anti-TRBC1 CAR transduced C1 cells (CAR-C1) and anti-TRBC1 CAR transduced C2 cells (CAR-C2) (Fig. 1a). Thus, it is interesting to evaluate whether both C1 and CAR-C1 could be identified and killed by CAR-C1 and CAR-C2 (Fig. 1a).
Fig. 1.
Effector functions of TRBC1+ and TRBC2+ cells genetically engineered with anti-TRBC1 CAR. a The categories and relationship of T cells following transduction with anti-TRBC1 CAR. TRBC1+ cells, C1; TRBC2+ cells, C2; anti-TRBC1 CAR transduced TRBC1+ cells, CAR-C1; anti-TRBC1 CAR transduced TRBC2+ cells, CAR-C2. b TRBC1 expression and CAR transduction efficacy of TRBC1-sorted and TRBC1-depleted T cells as well as CAR and TRBC1 expression of CAR-C1 and CAR-C2 analyzed by flow cytometry. c IFN-γ secretion by CAR-C1 and CAR-C2 against C1, CAR-C1 or C2 after 24-h co-culture. d-e Left, representive FACS profile of CD137 and C107a expression on CAR-C1 and CAR-C2 co-cultured with C1, CAR-C1 or C2. Right, percentages of CD137- and C107a-positive CAR-C1 and CAR-C2 following co-culture with C1, CAR-C1 or C2. f Cytotoxic activities of CAR-C1 and CAR-C2 against C1, CAR-C1 or C2 were examined by standard CFSE-based cytotoxity assays at several effector/target (E/T) ratios. g Scheme of the xenograft model. NOG mice (n = 5/group) were IV injected with 3 × 106 Luc/GFP–expressing Jurkat cells followed 3 days after by a single IV injection of 5 × 105 MOCK, CAR-C1 or CAR-C2. h IVIS imaging of tumor burden monitored by BLI at the indicated time points following MOCK, CAR-C1 or CAR-C2 T cell injection (day 0). i Radiance of individual mice at day 20 following MOCK, CAR-C1 or CAR-C2 T cell injection. n = 5 mice per group. j Kaplan-Meier survival curve of mice injected with mock, CAR-C1 or CAR-C2 T cells. ***P < 0.001 and n.s., not significant
Results and discussions
To evaluate whether C1 and CAR-C1 could be identified and killed by CAR-C1 and CAR-C2, we first sorted donor T cells into TRBC1+ and TRBC1− (designated as C2) fractions using magnetic beads. A portion of C1 or C2 were used as target cells and other C1 and C2 from the same donor were genetically engineered with anti-TRBC1 CAR to obtain CAR-C1 and CAR-C2 as effect cells. We confirmed that transduction efficacy of anti-TRBC1 CAR was similar on C1 and C2, and moreover TRBC1 was not detected on CAR-C1 through flow cytometry (Fig. 1b). Since primed T cells could increase CD137 expression and IFN-γ secretion, and moreover cytotoxic T cells could express CD107 and mediated killing of target cells, these markers could be used to detect activation and cytolytic activity of T cells. We found that CAR-C2 than CAR-C1 showed higher level of IFN-γ production and CD137 expression when co-cultured with C1 but not CAR-C1 or C2 (Fig. 1c and d). In flow cytometry–based cytotoxicity assays, CAR-C2 and CAR-C1 both specifically killed C1 but not CAR-C1 or C2, more so in CAR-C2 than CAR-C1 (Fig. 1e and f).
We next evaluated the anti-tumour activity of CAR-C1 and CAR-C2 in vivo using Luc-expressing Jurkat T-ALL cells. NOG mice were transplanted with 3 × 106 Luc-expressing Jurkat cells 3 days before IV infusion of 5 × 105 CAR-C1, CAR-C2 or MOCK T cells (Fig. 1g). Consistent with the in vitro observation, CAR-C1 induced transient tumour regression, but tumours re-progressed rapidly. In contrast, mice treated with an equal number of CAR-C2 exhibited significantly higher ani-tumour ability with significantly prolonged survival (P < 0.001) (Fig. 1h-j).
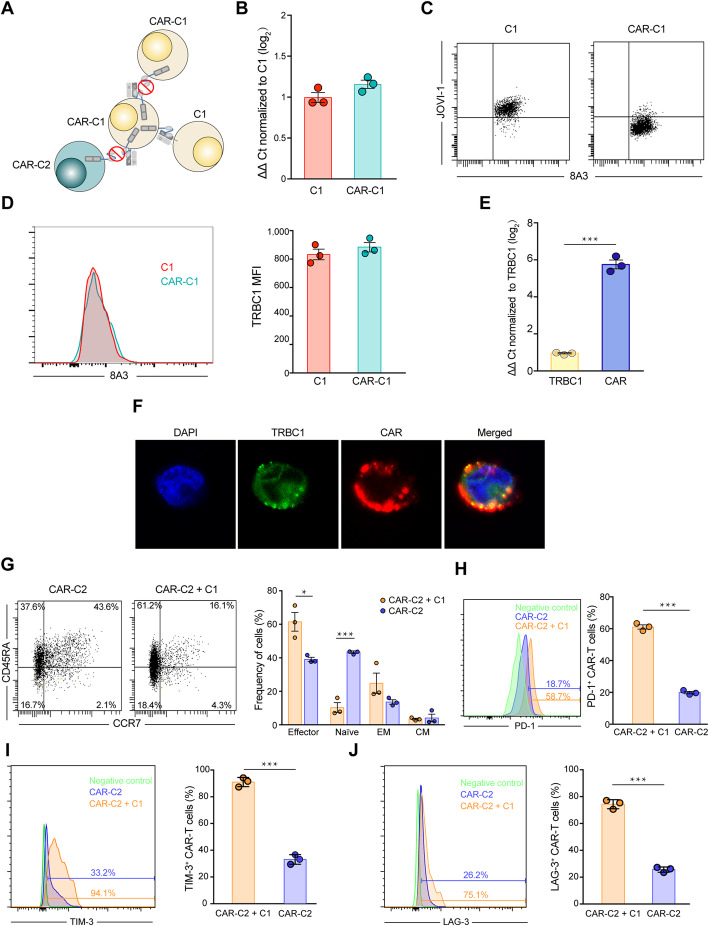
To investigate why CAR-C1 than CAR-C2 demonstrated lesser killing ability against C1 and moreover neither of them could identify and kill CAR-C1, we hypothesize that since expression abundance of anti-TRBC1 CAR is significantly higher than TRBC1 on CAR-C1, a proportion of CARs in cis bind to autologous TRBC1 on CAR-C1, masking TRBC1 from identification by other anti-TRBC1 CAR-T, and meanwhile only the remaining unoccupied CARs identify C1, weakening effector function of CAR-C1 (Fig. 2a).
Fig. 2.
The cause for undetected TRBC1 and lesser effector function of CAR-C1. a Due to higher expression level of CAR than TRBC1 on CAR-C1, some CARs in cis bind to autologous TRBC1 on CAR-C1, resulting into masking TRBC1 from identification by other anti-TRBC1 CAR-T and meanwhile occupying these CARs, and thus only the remaining unoccupied CARs target TRBC1. b TRBC1 mRNA expression is maintained in CAR-C1 as compared to C1, as determined by qRT-PCR (ΔΔ Ct normalized to C1). c TRBC1 on C1 is detectable using both mAb 8A3 targeting TCRβ-chain constant region and mAb JOVI-1 from which the anti-TRBC1 CAR was derived, but TRBC1 on CAR-C1 cells is only recognized by mAb 8A3. d Left, representive FACS profile of TRBC1 expression on CAR-C1 and C1. Right, MFI of TRBC1 on CAR-C1 and C1. e Expression level of CAR was significantly higher than TRBC1 on CAR-C1 determined by qRT-PCR analysis (ΔΔ Ct normalized to TRBC1). f Confocal imaging of CAR-C1 using FITC-conjugated anti-TRBC1 antibody (green), TRITIK-conjugated anti-FLAG antibody (red), and DAPI (blue). Scale bars, 5 μm. g Left, representive FACS profile of CD45RA and CCR7 expression on CAR-C2 after 6-day culture alone or co-culture with C1. Right, percentages of naïve (CD45RA+ CCR7+), effector (CD45RA+ CCR7−), effector memory (CD45RA− CCR7−) and central memory (CD45RA− CCR7+) CAR-C2 cells. h-j Left, representive FACS profile of PD-1 (h), TIM-3 (i) and LAG-3 (j) expression on CAR-C2 after 6-day culture alone or co-culture with C1. Right, percentage of PD-1 (h), TIM-3 (i) and LAG-3 (j) positive CAR-C2. *P < 0.05, ***P < 0.001. Data are representative of three independent experiments
We first found that TRBC1 mRNA expression was preserved in CAR-C1 as compared to C1 determined by qRT-PCR analysis (Fig. 2b). We further confirmed via flow cytometry that TRBC1 on CAR-C1 was detectable by anti-TRBC monoclonal antibody (mAb) 8A3 targeting not the same epitope recognized by mAb JOVI-1 from which the anti-TRBC1 CAR was derived (Fig. 2c), and moreover expression level of TRBC1 protein was similar on CAR-C1 and C1 (Fig. 2d). Meanwhile, qRT-PCR analysis demonstrated that expression level of CAR was significantly higher than TRBC1 in CAR-C1 and moreover confocal microscopy further confirmed that colocalization of anti-TRBC1 CAR and TRBC1 on the cell surface of CAR-C1 (Fig. 2e and f). These findings supported that TRBC1 molecules were still expressed on the surface of CAR-C1 but in cis bound by a proportion of anti-TRBC1 CARs, masking TRBC1 from identification by other anti-TRBC1 CAR-T, and meanwhile only the remaining unoccupied CARs identified C1, weakening effector function of CAR-C1.
In addition, contaminating TRBC1+ malignant cells during anti-TRBC1 CAR-T manufacturing not only produced CAR-C1 which was resistant to anti-TRBC1 CAR-T and had lesser killing ability, but were expected to accelerate exhaustion and terminal differentiation of anti-TRBC1 CAR-T with limited in vivo persistence due to continuous (tonic) ligand-driven CAR stimulation [7, 8]. Co-culture of CAR-C2 with C1 in a 2:1 ratio (physiological condition) for 6 days revealed lower and higher percent of naïve and effect CAR-C2 cells, respectively, compared to solo culture of CAR-C2 (Fig. 2g). In addition, the co-culture of CAR-C2 and C1 exhibited increasing expression of PD-1, TIM-3 and LAG-3 in CAR-C2 (Fig. 2h-j). These findings suggested that compared with unfractionated T cells, TRBC1-depleted T cells genetically engineered with anti-TRBC1 CAR not only avoided resistance to anti-TRBC1 CAR-T, but reduced exhaustion and terminal differentiation.
Conclusions
Although anti-TRBC1 CAR-T appeared a promising approach for T-cell malignancy, unfractioned T cells transduced to express anti-TRBC1 CAR could not only produce CAR-C1 cells which had lesser killing ability against TRBC1+ malignant T cells and moreover were resistant to anti-TRBC1 CAR-T, but contaminate TRBC1+ cells which promoted exhaustion and terminal differentiation of anti-TRBC1 CAR-T. Therefore, it was necessary to pre-deplete TRBC1+ T cells, even if allogeneic T cells were used for anti-TRBC1 CAR-T manufacturing for patients without sufficient autologous T cells.
Supplementary Information
Acknowledgements
We thank Dr. Huirong Ding at the Department of Central Laboratory, Key laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing) for her assistance of flow cytometry experiments.
Abbreviations
- CAR
Chimeric antigen receptor
- B-ALL
B-cell acute lymphoblastic leukemia
- DLBCL
diffuse large B-cell lymphoma
- TRBC1
T cell receptor β-chain constant region 1
- C1
TRBC1+ cells
- C2
TRBC2+ cells
- CAR-C1
anti-TRBC1 CAR transduced C1 cells
- CAR-C2
anti-TRBC1 CAR transduced C2 cells
Authors’ contributions
Z.M.L, W.J.Y, B.T.Y and C.T.Z designed the research; H. P, C.T.Z, Y. L, L.Y.S, S.C.L and N.J.L conducted experiments; C.T.Z, Z.M.L, H. P and Z.N.R analyzed data; and C.T.Z, Z.M.L and H. P wrote the paper. The author(s) read and approved the final manuscript.
Funding
This work was supported by National Key R&D Program of China (Grant No 2018YFC0910700); Natural Science Foundation of China [Grant No 81972880, Grant No 82003246, Grant No 81760525]; Beijing Natural Science Foundation (Grant No 7171001, Grant No 7182029); Beijing Municipal Science & Technology Commission (Grant No Z171100001017136); Capital’s Funds for Health Improvement and Research (Grant No 2020–4-1028); Open Project funded by Key laboratory of Carcinogenesis and Translational Research, Ministry of Education/Beijing (Grant No 2019 Open Project-4).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Peking University School of Oncology, China.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chaoting Zhang and Heyilimu Palashati contributed equally to this work.
Contributor Information
Bentong Yu, Email: yubentong@126.com.
Wenjun Yang, Email: ywj007@yeah.net.
Zheming Lu, Email: luzheming@bjmu.edu.cn.
References
- 1.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper ML, Choi J, Staser K, Ritchey JK, Devenport JM, Eckardt K, Rettig MP, Wang B, Eissenberg LG, Ghobadi A, et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018. [DOI] [PMC free article] [PubMed]
- 4.Alcantara M, Tesio M, June CH, Houot R. CAR T-cells for T-cell malignancies: challenges in distinguishing between therapeutic, normal, and neoplastic T-cells. Leukemia. 2018. [DOI] [PMC free article] [PubMed]
- 5.Sims JE, Tunnacliffe A, Smith WJ, Rabbitts TH. Complexity of human T-cell antigen receptor beta-chain constant- and variable-region genes. Nature. 1984;312:541–545. doi: 10.1038/312541a0. [DOI] [PubMed] [Google Scholar]
- 6.Maciocia PM, Wawrzyniecka PA, Philip B, Ricciardelli I, Akarca AU, Onuoha SC, Legut M, Cole DK, Sewell AK, Gritti G, et al. Targeting the T cell receptor beta-chain constant region for immunotherapy of T cell malignancies. Nat Med. 2017;23:1416–1423. doi: 10.1038/nm.4444. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scherer LD, Brenner MK, Mamonkin M. Chimeric antigen receptors for T-cell malignancies. Front Oncol. 2019;9:126. doi: 10.3389/fonc.2019.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.