On behalf of the EU‐CARDIOPROTECTION COST Action (CA16225 – www.cardioprotection.eu), in this Themed Issue “Risk factors, co‐morbidities, and co‐medications in cardioprotection,” we provide an up‐to‐date account on the influence of cardiovascular risk factors on cardioprotection. In particular, the Themed Issue focusses on co‐morbidities and their co‐medications on acute myocardial ischemia/reperfusion injury, cardioprotection, and their importance in the development of new cardioprotective therapies from preclinical to clinical studies.
Since the original observations in the mid‐nineties showing that hypercholesterolaemia attenuated the effect of preconditioning in rabbits (Szilvassy et al., 1995) and rats (Ferdinandy et al., 1997), it has become evident that the cardioprotective signalling is markedly affected by risk factors such as aging, sex and environmental factors, as well as by co‐morbidities and their medications (Ferdinandy, Schulz, & Baxter, 2007; Ferdinandy, Hausenloy, Heusch, Baxter, & Schulz, 2014; Ruiz‐Meana et al., 2020; Perrino et al., 2020; Figure 1) and that there are large numbers of non‐responders to cardioprotective stimuli (Schreckenberg et al., 2020). Moreover, in spite of a number of preclinical studies showing robust cardioprotection by a variety of cardioprotective interventions, their clinical translation is still lacking, as shown by the number of large clinical trials with neutral outcome for efficacy of these cardioprotective interventions (Davidson et al., 2019; Ferdinandy et al., 2014; Hausenloy et al., 2017).
FIGURE 1.
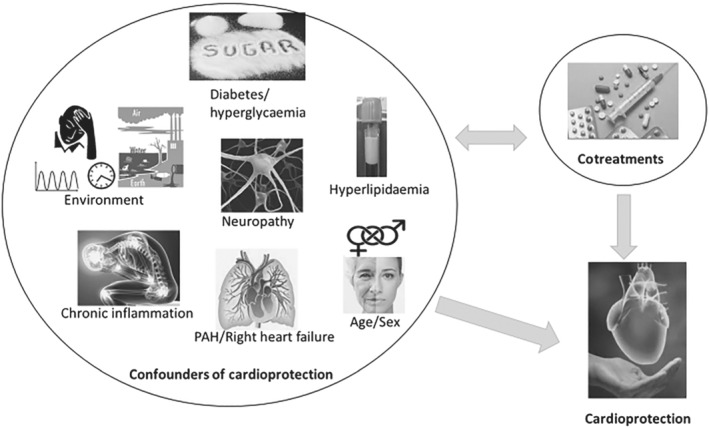
Summary of confounding factors for cardioprotection
Accordingly, in this Themed Issue, Kleinbongard, Bøtker, Ovize, Hausenloy, and Heusch (2020) critically reviewed the fact that translation of cardioprotection from robust experimental evidence to beneficial clinical outcome for patients has been largely disappointing. They analysed the evidence for confounders of cardioprotection in patients with acute myocardial infarction and in patients undergoing cardiovascular surgery. They showed that there is solid experimental evidence for such confounding of cardioprotection by single co‐morbidities and co‐medications, but that the clinical evidence from retrospective analyses of the limited number of clinical data is less robust.
Papers in this Themed Issue provide updates on well‐known risk factors and co‐morbidities interfering with cardioprotection. Ruiz‐Meana et al. (2020) review the effects of age and sex on the susceptibility and outcome of ischaemic heart disease. Andreadou et al. (2020) summarize the existing data on animal models of hypercholesterolaemia (total, low density, and HDL abnormalities) and hypertriglyceridaemia used in ischaemia/reperfusion injury and cardioprotection and provide recommendations on animal models of translational value. Penna et al. (2020) review the effect of diabetes and hyperglycaemia on cardioprotection. They overview the available animal models of diabetes and hyperglycaemia investigating acute myocardial ischaemia/reperfusion injury and cardioprotection. Moreover, they highlight the effects of anti‐hyperglycaemic agents on acute myocardial ischaemia/reperfusion injury and cardioprotection.
Other papers in this Themed Issue give overviews of less studied confounding factors of cardioprotection. Bencsik et al. (2020) review the effect of sensory neuropathy of diverse aetiology (including diabetes and hyperglycaemia) on cardioprotection and the mechanisms by which neuropathies may interfere with ischaemic heart disease and cardioprotective signalling. They also suggest future therapeutic options targeting both ischaemic heart and sensory neuropathy. Lazou et al. (2020) review the effect of major chronic inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, psoriasis and inflammatory bowel disease on cardioprotection. They give recommendations to evaluate common molecular targets of inflammatory and cardiovascular disease to design therapies to alleviate inflammatory disease‐related cardiovascular diseases. In line with this, Chen et al. (2020) showed that a novel chalcone derivative exhibits atheroprotective effects by reducing inflammation‐induced endothelial dysfunction.
According to epidemiological data, environmental risk factors (air pollution and noise) are associated with higher risk for cardiovascular, metabolic, and mental diseases, including hypertension, heart failure, myocardial infarction, diabetes, arrhythmia, stroke, depression, and anxiety disorders. Li et al. (2020) provide an overview on the effects of the external exposome comprising risk factors/exposures on cardiovascular health with a focus on dysregulation of stress hormones, mitochondrial function, redox balance and inflammation, with special emphasis on the circadian clock. They assess the effects of circadian clock dysregulation on cardiovascular health and the potential of environment‐specific preventive strategies including “chrono” therapy for cardioprotection.
Boengler, Schlüter, Schermuly, and Schulz (2020) highlight an interesting issue of cardioprotection, that is, protection of the right heart. Whereas the molecular mechanisms of conditioning in the left ventricle are well characterized, cardioprotection of the right ventricle is much less studied. Similar to what is known for the left ventricle, a dysregulation in signalling pathways seems to play a role in ischaemia/reperfusion injury of the healthy and failing right ventricle and in the ability/inability of the right ventricle to respond to a conditioning stimulus. They highlight that, although similar molecular mechanisms mediate ischaemic and pharmacological preconditioning in the left and right ventricles, the two ventricles seem to respond differently, for example, to exercise‐induced preconditioning.
In conclusion, papers in this Themed Issue underline the importance of careful preclinical assessment of novel cardioprotective therapies is necessary in clinically relevant animal models of different risk factors and co‐morbidities and their medications to gather more knowledge on individual responses to cardioprotective therapies, thereby allowing better planning of clinical studies for cardioprotection in different patient populations.
CONFLICT OF INTEREST
P.F. is the founder and CEO of Pharmahungary Group, a group of R&D companies. The rest of the authors declare no conflict of interest.
ACKNOWLEDGEMENTS
P.F. was supported by the National Research, Development and Innovation Office of Hungary (Research Excellence Program ‐ TKP, National Heart Program NVKP 16‐1‐2016‐0017) and by the Higher Education Institutional Excellence Program of the Ministry of Human Capacities in Hungary, within the framework of the Therapeutic Development thematic program of the Semmelweis University. D.H. was supported by the British Heart Foundation (CS/14/3/31002), the Duke‐NUS Signature Research Programme funded by the Ministry of Health, Singapore Ministry of Health's National Medical Research Council under its Clinician Scientist‐Senior Investigator scheme (NMRC/CSA‐SI/0011/2017), Centre Grant, and Collaborative Centre Grant scheme (NMRC/CGAug16C006). This article is based upon work from COST Action EU‐CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
Contributor Information
Rainer Schulz, Email: rainer.schulz@physiologie.med.uni-giessen.de.
Péter Ferdinandy, Email: peter.ferdinandy@pharmahungary.com.
REFERENCES
- Andreadou, I. , Schulz, R. , Badimon, L. , Adameová, A. , Kleinbongard, P. , Lecour, S. , … Ferdinandy, P. (2020). Hyperlipidaemia and cardioprotection: Animal models for translational studies. British Journal of Pharmacology, 177, 5287–5311. 10.1111/bph.14931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencsik, P. , Gömöri, K. , Szabados, T. , Sántha, P. , Helyes, Z. , Jancsó, G. , … Görbe, A. (2020). Myocardial ischaemia reperfusion injury and cardioprotection in the presence of sensory neuropathy: Therapeutic options. British Journal of Pharmacology, 177, 5336‐5356. 10.1111/bph.15021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler, K. , Schlüter, K. D. , Schermuly, R. T. , & Schulz, R. (2020). Cardioprotection in right heart failure. British Journal of Pharmacology, 177, 5413–5431. 10.1111/bph.14992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. W. , Tsai, M. C. , Chern, C. Y. , Tsao, T. P. , Lin, F. Y. , Chen, S. J. , … Lin, C. S. (2020). A chalcone derivative, 1m‐6, exhibits atheroprotective effects by increasing cholesterol efflux and reducing inflammation‐induced endothelial dysfunction. British Journal of Pharmacology, 177, 5375–5392. 10.1111/bph.15175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, S. M. , Ferdinandy, P. , Andreadou, I. , Bøtker, H. E. , Heusch, G. , Ibáñez, B. , … Cardioprotection Cost Action (CA16225) . (2019). Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. Journal of the American College of Cardiology, 73, 89–99. 10.1016/j.jacc.2018.09.086 [DOI] [PubMed] [Google Scholar]
- Ferdinandy, P. , Hausenloy, D. J. , Heusch, G. , Baxter, G. F. , & Schulz, R. (2014). Interaction of risk factors, co‐morbidities, and co‐medications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacological Reviews, 66, 1142–1174. 10.1124/pr.113.008300 [DOI] [PubMed] [Google Scholar]
- Ferdinandy, P. , Schulz, R. , & Baxter, G. F. (2007). Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacological Reviews, 59, 418–458. 10.1124/pr.107.06002 [DOI] [PubMed] [Google Scholar]
- Ferdinandy, P. , Szilvássy, Z. , Horváth, L. I. , Csonka, C. , Nagy, E. , Szentgyörgyi, R. , … Dux, L. (1997). Loss of pacing‐induced preconditioning in rat hearts: Role of nitric oxide and cholesterol‐enriched diet. Journal of Molecular and Cellular Cardiology, 29, 3321–3333. 10.1006/jmcc.1997.0557 [DOI] [PubMed] [Google Scholar]
- Hausenloy, D. J. , Garcia Dorado, D. , Bøtker, H. E. , Davidson, S. M. , Downey, J. , Engel, F. B. , … Ferdinandy, P. (2017). Novel targets and future strategies for acute cardioprotection: Position paper of the European Society of Cardiology Working Group on cellular biology of the heart. Cardiovascular Research, 113, 564–585. 10.1093/cvr/cvx049 [DOI] [PubMed] [Google Scholar]
- Kleinbongard, P. , Bøtker, H. E. , Ovize, M. , Hausenloy, D. J. , & Heusch, G. (2020). Co‐morbidities and co‐medications as confounders of cardioprotection—Does it matter in the clinical setting? British Journal of Pharmacology, 177, 5252–5269. 10.1111/bph.14839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazou, A. , Ikonomidis, I. , Bartekova, M. , Benedek, T. , Makavos, G. , Palioura, D. , … Andreadou, I. (2020). Chronic inflammatory diseases, myocardial function and cardioprotection. British Journal of Pharmacology, 177, 5357–5374. 10.1111/bph.14975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Kilgallen, A. B. , Münzel, T. , Wolf, E. , Lecour, S. , Schulz, R. , … Van Laake, L. W. (2020). Influence of mental stress and environmental toxins on circadian clocks: Implications for redox regulation of the heart and cardioprotection. British Journal of Pharmacology, 177, 5393–5412. 10.1111/bph.14949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna, C. , Andreadou, I. , Aragno, M. , Beauloye, C. , Bertrand, L. , Lazou, A. , … Hausenloy, D. J. (2020). Effect of hyperglycaemia and diabetes on acute myocardial ischaemia‐reperfusion injury and cardioprotection by ischaemic conditioning protocols. British Journal of Pharmacology, 177, 5312–5335. 10.1111/bph.14993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino, C. , Ferdinandy, P. , Bøtker, H. E. , Brundel, B. J. J. M. , Collins, P. , Davidson, S. M. , … Ytrehus, K. (2020). Improving translational research in sex‐specific effects of comorbidities and risk factors in ischemic heart disease and cardioprotection: Position paper and recommendations of the ESC working group on cellular biology of the heart. Cardiovascular Research. 10.1093/cvr/cvaa155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Meana, M. , Boengler, K. , Garcia‐Dorado, D. , Hausenloy, D. J. , Kaambre, T. , Kararigas, G. , … Ytrehus, K. (2020). Ageing, sex, and cardioprotection. British Journal of Pharmacology, 177, 5270–5286. 10.1111/bph.14951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Meana, M. , Bou‐Teen, D. , Ferdinandy, P. , Gyongyosi, M. , Pesce, M. , Perrino, C. , … Madonna, R. (2020). Cardiomyocyte ageing and cardioprotection: Consensus document from the ESC working groups cell biology of the heart and myocardial function. Cardiovascular Research, 166, 1835–1849. 10.1093/cvr/cvaa132 [DOI] [PubMed] [Google Scholar]
- Schreckenberg, R. , Klein, J. , Kutsche, H. S. , Schulz, R. , Gömöri, K. , Bencsik, P. , … Schlüter, K.‐D. (2020). Ischaemic post‐conditioning in rats: Responder and non‐responder differ in transcriptome of mitochondrial proteins. Journal of Cellular and Molecular Medicine, 24, 5528–5541. 10.1111/jcmm.15209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy, Z. , Ferdinandy, P. , Szilvassy, J. , Nagy, I. , Karcsu, S. , Lonovics, J. , … Koltai, M. (1995). The loss of pacing‐induced preconditioning in atherosclerotic rabbits: Role of hypercholesterolaemia. Journal of Molecular and Cellular Cardiology, 27, 2559–2569. 10.1006/jmcc.1995.0043 [DOI] [PubMed] [Google Scholar]


