Abstract
Hyperlipidaemia is a well‐established risk factor for cardiovascular diseases and therefore, many animal model have been developed to mimic the human abnormal elevation of blood lipid levels. In parallel, extensive research for the alleviation of ischaemia/reperfusion injury has revealed that hyperlipidaemia is a major co‐morbidity that attenuates the cardioprotective effect of conditioning strategies (preconditioning, postconditioning and remote conditioning) and that of pharmacological interventions by interfering with cardioprotective signalling pathways. In the present review article, we summarize the existing data on animal models of hypercholesterolaemia (total, low density and HDL abnormalities) and hypertriglyceridaemia used in ischaemia/reperfusion injury and protection from it. We also provide recommendations on preclinical animal models to be used for translations of the cardioprotective strategies into clinical practice.
LINKED ARTICLES
This article is part of a themed issue on Risk factors, comorbidities, and comedications in cardioprotection. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.23/issuetoc
Abbreviations
- ApoA‐V
apolipoprotein A‐V
- APOC1
apolipoprotein C1 gene
- APOE
apolipoprotein E gene
- ApoE
apolipoprotein E
- ApoE−/−
deletion of apolipoprotein E gene
- CETP
cholesteryl ester transfer protein
- cp
corpulent
- CRISPR
clustered regularly interspaced short palindromic repeats
- CRISPR/Cas 9
CRISP associated protein 9
- DHC
diet‐induced hypercholesterolaemia
- E3L
ApoE*3‐Leiden transgenic mice
- FHC
familial hypercholesterolaemia
- HCD
high cholesterol diet
- HDL‐c
high density lipoprotein cholesterol
- HFD
high‐fat diet
- I/R
ischaemia reperfusion
- LDL‐c
low density lipoprotein cholesterol
- LDLr
LDL receptor
- LDLr−/−
deletion of LDL receptor
- LpL
lipoprotein lipase
- MI
myocardial infarction
- NZW rabbits
New Zealand White rabbits
- PHHC
Prague hereditary hypercholesterolaemic
- PHT
post‐prandial hypertriglyceridaemic rabbit model
- S1P
sphingosine‐1 phosphate
- SR‐BI
scavenger receptor BI
- TC
total cholesterol
- TGs
triglycerides
- VLDLs
very low density lipoprotein
- VLDL‐c
very low density lipoprotein cholesterol
- WD
Western diets
- WHHL rabbits
Watanabe heritable hyperlipidaemic rabbits
1. INTRODUCTION
Cardiovascular disease is the most common cause of mortality worldwide (Timmis et al., 2018). Hyperlipidaemia is the pathological state characterized by elevated levels of serum cholesterol and triglycerides (TGs) and it is considered as a major risk factor for cardiovascular disease. In fact, hyperlipidaemia is present in most patients with myocardial infarction (ΜI; Osipov, Bianchi, et al., 2009). Chronic elevation of blood cholesterol results in the development of atherosclerosis but also exerts a negative impact on the myocardium by increasing oxidative stress, mitochondrial dysfunction and apoptosis. Moreover, hypercholesterolaemia induces microvascular dysfunction through nitro‐oxidative stress and induction of inflammation, mechanisms which may also account for increased susceptibility of the myocardium to infarction (see for a recent extensive review, Andreadou, Iliodromitis, et al., 2017).
Various animal species have been used to study the effects of diet on cholesterol homeostasis and atherosclerosis including mice (Temel & Rudel, 2007), hamsters (Dillard, Matthan, & Lichtenstein, 2010), guinea pigs (Fernandez & Volek, 2006), rats, rabbits (Badimon, Badimon, & Fuster, 1990; Aliev & Burnstock, 1998), minipigs (Shim, Al‐Mashhadi, Sørensen, & Bentzon, 2016) and farm breed pigs (Badimon, Steele, Badimon, Bowie, & Fuster, 1985; Fuster et al., 1985; Palazón et al., 1998; Vilahur, Padro, & Badimon, 2011). Mice and rats are resistant to spontaneous development of atherosclerosis but both diet and genetic manipulations render these rodents more susceptible to atherosclerosis development, while genetically altered strains respond to cholesterol feeding to a greater extent. Some of these models, mainly mice and rats, have been employed for the investigation of hypelipidaemia's impact on myocardial ischaemia/reperfusion injury (I/R). However, a number of studies (including one in pigs) indicate that the effect of cardioprotective manoeuvres is blunted in these animals (Iliodromitis et al., 2006; Iliodromitis et al., 2010; reviewed in Ferdinandy, Hausenloy, Heusch, Baxter, & Schulz, 2014). In fact, high cholesterol diet (HCD)‐induced hyperlipidaemia was the first co‐morbidity where the loss of cardioprotection was observed in rabbits and rats (Szilvassy et al., 1995).
Several cardioprotective strategies aimed at the attenuation of myocardial I/R injury have been employed with prognostic benefit in some but not all patients (Hausenloy et al., 2017; Heusch, 2013), and therefore, it is of interest to consider whether hyperlipidaemia is one of the co‐morbidities that interferes with cardioprotection (Andreadou, Iliodromitis, et al., 2017). Experimental and clinical studies have shown that hypercholesterolaemia may hamper the cardioprotective signalling of conditioning manoeuvres. Although the effect of hypelipidaemia on the mechanisms of myocardial I/R injury is not fully elucidated, hyperlipideamic animals exhibit deregulation of cardioprotective cascades such as inactivation of the reperfusion injury salvage kinase pathway, modulation of ATP sensitive potassium channels, impaired NO availability and a redistribution of the intracellular localization of connexin 43 in the cardiomyocytes (Görbe et al., 2011; Andreadou, Iliodromitis, et al., 2017). Nowadays, a pressing challenge is to define appropriate models of hyperlipidaemia to improve the translational potential of pharmacological and mechanical interventions related to cardioprotection and reveal the underlying mechanisms (Heusch, 2017).
In this review article, we summarize the current knowledge on the mice, rat and pig models of hyperlipidaemia placing emphasis on those that have been employed for preclinical studies on cardioprotection. We highlight the future perspectives of the use of each model for translational research in terms of infarct size reduction, strategies focusing on the lipid profile and the extent of atherosclerosis development.
2. ANIMAL MODELS FOR HIGH TOTAL CHOLESTEROL AND HIGH LDL CHOLESTEROL
Cholesterol is a component of the cell membrane, and cholesterol metabolites, such as steroid hormones and vitamin D, serve important biological functions in vertebrates. Unbalanced diets containing high levels of fat and carbohydrates, the so‐called Western diets (WD), frequently trigger hypercholesterolaemia. Also, genetic factors such as defects in the apolipoprotein genes or receptors (Fan & Watanabe, 2000) influence susceptibility to diet‐induced hypercholesterolaemia (DHC). In humans, hyperlipidaemia includes hypercholesterolaemia and hypertriglyceridaemia and is classified as primary, also named as familial, and secondary. The manifestation of primary hyperlipidaemia is based on genetic defects of a single gene (monogenic) or of multiple genes (polygenic) and the respective phenotype is accompanied by well‐characterized abnormal lipoprotein patterns. Secondary forms of hyperlipidaemia are acquired along life span following other disorders such as diabetes, nephrotic syndrome, hypothyroidism and prolonged use of drugs like corticosteroids and ß‐adrenoceptor antagonists (beta‐blockers). The main cause of hyperlipidaemia in Western countries includes changes in lifestyle habits and the increased intake of saturated fat. The broad spectrum of lipid dysregulations found in human urges the need for the classification of the animal models of hyperlipidaemia according to their translational resemblance.
Increased circulating total cholesterol (TC) levels or increased cholesterol incorporated in low and very low‐density lipoproteins (LDL and VLDL, respectively) are major risk factors for atherosclerosis. Therefore, most of the animal models that exhibit increased TC or LDL cholesterol (LDL‐c) levels stand also as models of atherosclerosis (Emini Veseli et al., 2017). In contrast, high levels of HDLs are considered to be protective against atherosclerosis (Ference et al., 2017).
2.1. Small animals (mice, rats and rabbits)
Small animals such as mice, rats and rabbits are often used due to ease of breeding, short gestation and life cycle, although they are naturally resistant to develop hypercholesterolaemia and atherosclerosis. While rodents show low expression/absence of cholesteryl ester transfer protein (CETP), rabbits have higher CETP activity, thereby higher LDL and low HDL levels representing a more useful animal model for hypercholesterolaemia research (Tsutsumi, Hagi, & Inoue, 2001).
2.1.1. Diet‐induced hypercholesterolaemia and high LDL cholesterol
Mice and rats
To induce hypercholesterolaemia, a long‐term feeding (several weeks to months) with various diets supplemented with cholesterol (Csont et al., 2002; Giricz et al., 2017) are effectively used together with supportive substances causing lipid metabolism disturbances, for example, cholate (Giricz et al., 2017), coconut oil (Romain et al., 2018), corn starch and sucrose (Romain et al., 2018). Several studies have demonstrated that certain inbred strains of mice, such as C57BL/6, develop atherosclerotic lesions in response to high cholesterol diet (HCD) and fat that contain cholic acid to block cholesterol conversion to bile acids (Roberts & Thompson, 1976). Among the different diets, the typical rodent WD contains high levels of saturated fat (~35 kcal% fat and 21% w/w), such as cocoa butter, palm oil or dairy butter, cholesterol (~0.5 to 1% w/w) and cholic acid (~0.1% to 0.5% w/w). These diets when used for 12 weeks provoke TC and LDL‐c elevation and signs of atherosclerosis (Dietschy, 1998; Zulet, Barber, Garcin, Higueret, & Martínez, 1999).
In contrast, outbreed rat strains (i.e. Sprague–Dawley or Wistar) fed with WD (~45 kcal% fat as hydrogenated coconut oil) or even low‐fat diets (~12 kcal% fat as corn oil) that contain cholesterol (~1% w/w) and cholic acid (0.25–0.5% w/w) exhibit augmented levels of TC and LDL‐c but are resistant to develop atherosclerosis under these conditions, unless a thyroid hormone inhibitor (2‐thiouracil, ~0.5% w/w) is added. Indeed, the normal rat chow supplemented with 4% cholesterol, 1% cholic acid and 0.5% 2‐thiouracil diet over 30 days increase plasma TC, TGs, LDL‐c, VLDL cholesterol (VLDL‐c) with a parallel decrease in HDL cholesterol (HDL‐c; Raja, Saravanakumar, & Sathya, 2012), and 12 weeks of 4% cholesterol, 1% cholic acid and 0.5% 2‐thiouracil induces atherosclerosis (Joris, Zand, Nunnari, Krolikowski, & Majno, 1983). Additionally, in rats, streptozotocin injection in addition to cholesterol and oil‐containing diet induce simultaneous hypercholesterolaemia and hyperglycaemia within a short time period (Adameová et al., 2007). Besides high‐fat diets (HFD), many studies have described dyslipidaemia in rats fed with carbohydrate‐enriched diets and a combination of both (Wong, Chin, Suhaimi, Fairus, & Ima‐Nirwana, 2016).
The Prague hereditary hypercholesterolaemic (PHHC) rat (a strain crossbred from Wistar rats) is used as a model of hypercholesterolaemia induced by dietary cholesterol (Kovár, Tonar, Heczková, & Poledne, 2009). PHHC rats exhibit mild hypercholesterolaemia when fed with standard chow and develop remarkable hypercholesterolaemia (TC > 190 mg·dl−1 [5 mM]) on 2% cholesterol diet. In this strain, most of the cholesterol is found in VLDL particles. Concomitantly, both intermediate‐density lipoprotein cholesterol and LDL‐c concentrations rise without any increase in HDL‐c. PHHC rats do not markedly differ from Wistar rats in the activities of enzymes involved in intravascular remodelling of lipoproteins, LDL catabolism, cholesterol turnover rate and absorption of dietary cholesterol (Kovár et al., 2009). Despite the presence of hypercholesterolaemia, PHHC rats do not develop atherosclerosis even after 6 months on 2% cholesterol diet. Importantly, the crossbreeding experiments documented that the hypercholesterolaemia of PHHC rats is polygenic (Kovár et al., 2009).
In the context of cardioprotection, HCD in mice have shown discrepant results on infarct size depending on the duration of the dietary intervention (Girod, Jones, Sieber, Aw, & Lefer, 1999). The use of WD in rodents mimics the human hyperlipidaemic profile and the increased saturated fat intake observed in humans of the Western countries. Recent developments in commercially available rodent diets have promoted reproducibility among experimental series. Therefore, the protocol of 14 weeks of WD in rodents (Andreadou, Efentakis, et al., 2017; Desmarchelier et al., 2012) is recommended to study the effect of cardioprotective interventions in the presence of DHC. Moreover, many of the above‐discussed rat models of DHC have been used to investigate both non‐pharmacological (ischaemic preconditioning, postconditioning and remote conditioning; Adameova et al., 2007; Kupai et al., 2009; Ma et al., 2012) and pharmacological cardioprotection (Csonka et al., 2014) in terms of reducing infarct size and improving post‐ischaemic recovery of heart function. Most of these studies showed the abrogation of cardioprotection in hyperlipidaemia. However, as indicated above and because of some limitations in the use of small animals in hyperlipidaemia studies, more studies in large animal models would be preferable for cardioprotection studies.
Rabbits
Rabbits are herbivores and therefore do not tend to develop hypercholesterolaemia or atherosclerosis unless they undergo a dietary intervention. New Zealand White (NZW) rabbits are commonly used to study hypercholesterolaemia and atherosclerosis. NZW rabbits under standard laboratory diet have low plasma TC levels and do not develop atherosclerosis. However, cholesterol‐enriched diets (0.2–0.5% cholesterol) and HCD (1.0–1.5% cholesterol) greatly alter the lipid profile of NZW (Emini Veseli et al., 2017). Supplementation of standard diet with 0.2–0.5% cholesterol results in the early development of hypercholesterolaemia (within 6–8 weeks) and atherosclerosis and pronounced atherosclerosis—not affecting the abdominal aorta—is evident after 20–26 weeks.
Several studies have examined both mechanical and pharmacological cardioprotective interventions (Andreadou et al., 2012; Iliodromitis et al., 2006) in short‐term‐fed NZW rabbits and have provided possible explanations for the loss of postconditioning‐induced cardioprotection with hypercholesterolaemia. Thus, this model stands as a well‐characterized mild phenotype of hypercholesterolaemia for use in translational studies. On the other hand, HCDs lead to a massive increase in TC levels (1,500 and 3,000 mg·dl−1 [39 and 77 mM, respectively]; Fan & Watanabe, 2000) without significant translational potential.
Hypercholesterolaemia may exacerbate the I/R injury by altering signalling pathways on the myocardium (Andreadou, Iliodromitis, et al., 2017) and the lipid metabolism. The generation of ROS produces oxidized derivatives of cholesterol, the oxysteroles, which propagate lipid peroxidation and induce cell death and mitochondria dysfunction (Paradis, Leoni, Caccia, Berdeaux, & Morin, 2013). Particularly, oxysterols are involved in the development of atherosclerosis and in the genesis of chronic diseases (Lordan, Mackrill, & O'Brien, 2009).
2.1.2. Genetic small animal models of high TC and high LDL
In various rodent strains, dietary cholesterol and fat have been reported to induce disturbances in cholesterol metabolism and aortic lipid accumulation (Dillard et al., 2010; Yin et al., 2012). However, there is a significant diversity of atherosclerotic lesions burden among different mice strains (Paigen, Morrow, Brandon, Mitchell, & Holmes, 1985). Therefore, genetic engineering tools, such as ablation of apolipoprotein E (ApoE; Getz & Reardon, 2006), LDL receptors (LDLr; Girod et al., 1999), or CETP gene mutations (Herrera et al., 1999), have been used to effectively generate animals with “human‐like” cholesterol profile and a predisposition to develop atherosclerotic lesions when fed with a standard diet or exacerbated by HFD/HCD.
Mice
The mouse is characterized by high HDL but low concentrations of VLDL and LDL particles, and the genetic means for promoting hyperlipidaemia involve disturbing this balance. Genetic manipulations in mice disrupt the normal lipoprotein regulation and metabolism and are used for the induction of hypercholesterolaemia and atherosclerosis. Diets containing high levels of cholesterol and fat are frequently used to influence the genetic susceptibility of mouse models to bear hypercholesterolaemia. Most strains of mice are relatively resistant to the development of such phenotypes with changes in the lipoprotein profile, except for C57BL/6 mice.
Mice with deletion of the ApoE gene (ApoE−/−) are widely used due to their propensity to develop atherosclerotic lesions (Meir & Leitersdorf, 2004). This model presents a fivefold to sixfold increase in TC concentrations in comparison to parent C57BL/6 mice and fibrous plaques confined to the aortic root area are apparent after 20 weeks of age (Nakashima, Plump, Raines, Breslow, & Ross, 1994). When fed HFD of HCD, ApoE−/− mice exhibit TC up to 1,160 mg·dl−1 (30 mM) and develop widely distributed atherosclerosis, similar to that seen in humans (Meyrelles, Peotta, Pereira, & Vasquez, 2011). Both models are useful for the investigation of I/R injury in preclinical studies as they reflect mild and severe hypercholesterolaemia/atherosclerosis respectively.
The deletion of LDL receptor (LDLr−/−) mouse is a model bearing a genetic mutation on LDLr and recapitulates the familial hypercholesterolaemia (FHC). When fed on a chow diet, these mice exhibit slightly elevated levels of TC in comparison to the wild types and mild atherosclerotic lesions (Getz & Reardon, 2006). Diet supplementation with cholesterol results in a massive increase of plasma TC and accelerated atherosclerosis. However, the use of this model of hyperlipidaemia is limited for investigation of cardioprotective strategies because infarct size was either increased or decreased upon diet supplementation (Ma et al., 2012). LDLr−/− mice fed for 2 weeks HCD showed increased infarct size but long‐term HCD (12 weeks) reduced infarct size when compared to wild‐type and LDLr−/− mice fed with normal chow (Girod et al., 1999).
Rats
With the establishment technologies for genetic manipulation and the rat genome sequencing, rats are increasingly used in biomedical research. Rats are preferable for the ease in blood collection and dissection of blood vessels over mice and they are lower cost than large animals.
ApoE−/− Sprague–Dawley rats have been generated to effectively mimic hyperlipidaemia and atherosclerotic plaques as seen in humans with decreased HDL‐c and markedly up‐regulated TC and LDL‐c (Zhao et al., 2018). However, there are only few publications using this model (Wei et al., 2015). LDLr−/− rats have also been created by zinc‐finger and clustered regularly interspaced short palindromic repeats associated protein 9 (CRISPR/Cas 9) technology. These rats on normal chow exhibit elevated TC, LDL‐c, TGs and VLDL‐c. Atherosclerotic plaques develop rapidly on WD (Sithu et al., 2017). The difference in lipid profiles between rodents and humans regarding the LDL‐c and HDL‐c distribution is due to the presence of CETP in humans which is responsible for transferring cholesterol ester from HDL particles to VLDL and LDL particles in exchange for TGs (Marotti et al., 1992). A rat which overexpresses the CETP protein has also been developed in a Dahl salt‐sensitive hypertensive strain and this overexpression resulted in profound atherosclerosis. These rats also suffered from MI and decreased survival and thus presented a phenotype which differed from various mouse models (Herrera et al., 1999).
We must mention, however, that rats with genetic mutations have not been used so far for the investigation of I/R injury and further studies must be conducted to compare infarct size with that in wild‐type littermates.
Rabbits
Watanabe heritable hyperlipidaemic (WHHL) rabbits bear a defect on the LDLr and as a result, they stand as an animal model of unprovoked hypercholesterolaemia, with increased levels of LDL‐c and extended atherosclerotic lesions (Fan & Watanabe, 2000). This animal model resembles the FHC seen in humans (Aliev & Burnstock, 1998). Several types of atherosclerotic lesions have been observed in these animals, ranging from early fatty streaks to advanced lesions in the large vessels, including the abdominal aorta. WHHL rabbits are very useful in studying the biological processes of plaque destabilization and thrombogenesis (Shiomi, Koike, & Ito, 2013). However, they are not appropriate models for the investigation of chronic cardioprotective strategies because it is difficult for researchers to distinguish whether the cardioprotective effect is attributed to the attenuation of coronary atherosclerotic lesions development or to other direct effects on the myocardium (Hoshida et al., 1997). Moreover, increased plaque instability with risk of MI may produce high variability in the measurements of infarct size during I/R injury.
High‐fructose and HFD‐fed WHHL rabbits develop early insulin resistance and glucose tolerance and show aortic lesions with a lipid core and calcifications. This model has allowed researchers to investigate the effect of insulin resistance on atherosclerosis lesion formation (Ning et al., 2015). Recently, ApoE−/− rabbits have been generated (Niimi et al., 2016). ApoE−/− rabbits on regular laboratory diet exhibit mild hyperlipidaemia and diet enrichment with cholesterol leads to a drastic increase in TC. ApoE−/− rabbits are more prone to develop atherosclerosis than wild‐type rabbits when fed with a cholesterol diet for 10 weeks (Niimi et al., 2016). However, this rabbit model has not been tested yet in cardioprotection studies. The most recent genetic rabbit model of hypercholesterolaemia has been developed with the CRISPR/Cas9 system and involved the depletion of the LDLr. These LDLr−/− animals 3 months old exhibit a 20‐fold increased plasma TC and a 35‐fold increase of LDL‐c in comparison to wild‐type rabbits. Importantly, they had also elevated TG and reduced HDL‐c levels (Lu et al., 2018). Atherosclerotic lesions were observed in the coronary arteries, but further characterization of this model regarding atherosclerosis progression and MI is required.
2.2. Large animals (diet and genetic) of high TC and high LDL
The lipid profile of larger animals, such as pigs, dogs and nonhuman primates, is more similar to that of humans. However, their routine use in hyperlipidaemia experiments is limited by laboratory facilities, cost and ethical issues. Nevertheless, large animal models provide several key advantages over smaller animals: (a) their cardiovascular system including its neurohumoral regulation resemblances that of humans, (b) when fed with special diet they develop atherosclerosis similar to that in humans and (c) percutaneous interventions can be used in larger animals, providing the opportunity to assess cardioprotective strategies and evaluate pharmacological treatment (Amuzie et al., 2016; Hamamdzic & Wilensky, 2013).
Large animals such as dogs and pigs under normal chow exhibit a high HDL‐c/low LDL‐c profile during their life span, associated with a low cardiovascular risk (Tsutsumi et al., 2001; Yin et al., 2012). Spontaneous atherosclerosis in dog is rare, while it can be found in pigs depending on age and strain. Through dietary interventions (5–10% fat/0.5–1% cholesterol diets) and vascular lesion induction (usually balloon dilatation), the atherosclerotic process can be stimulated. Under a hypercholesterolaemic diet, pigs display TC levels similar to those observed in humans and they develop early‐stage atherosclerotic lesions in the abdominal aorta and coronary arteries within a 50‐day period (Badimon, 2001; Thim et al., 2010; Vilahur et al., 2011). Nonetheless, advanced complex and multiple atherosclerotic plaques development require longer time periods that are associated with increase in weight and size that make the model difficult to use for intervention procedures, surgery and laboratory handling. Consequently, minipig lines such as Göttingen, Yucatan or Ossabaw have been employed to overcome this situation (Kleinert et al., 2018). Ossabaw minipigs exert a metabolic syndrome with insulin resistance, obesity, hypertension and dyslipidaemia (Neeb et al., 2010), and they develop profound coronary atherosclerosis (Trask et al., 2012). DHC in Yucatan miniature pig was characterized by significantly elevated plasma TC, LDL, and VLDL levels, with a proportional reduction in HDL‐c concentrations (Poledne & Jurčíková‐Novotná, 2017). In comparison to DHC, spontaneous homozygous FHC pigs presented increased TC concentrations with a predominant shift to the LDL‐c fraction, which displayed defective LDLr coupling and impaired LDL clearance with concomitantly decreased lecithin cholesterol acyltransferase activity. DHC exhibits a twofold higher TC/apolipoprotein B ratio, indicating less dense LDL particles than FHC. Recently, a minipig model with the same gene mutation, the Bretoncelles Meishan pig, became available (Thim et al., 2010). Likewise, the micro‐minipig (the smallest pig line available for experimental purposes) has been employed as an atherosclerotic model, when fed an HFD/HCD (12–5%, respectively; Hasler‐Rapacz, Nichols, Griggs, Bellinger, & Rapacz, 1994), but its use in I/R studies has not been established, probably due to the low weight of these animals.
Sex differences in humans are becoming increasingly essential for diagnostic and treatment strategies and, therefore, animal models should reflect such distinction. Göttingen minipigs were assessed for sex differences in metabolic parameters during a high cholesterol regimen, demonstrating more severe hypercholesterolaemia and atherosclerosis in female minipigs. Similar results regarding gender differences were later observed in Yucatan and Ossabaw pig lines (Christoffersen, Grand, Golozoubova, Svendsen, & Raun, 2007).
A Yucatan minipig model of FHC was constructed by DNA transposition of human D374Y‐proprotein convertase subtilisin/kexin type 9 gain‐of‐function mutation, displaying severe hypercholesterolaemia due to decreased LDLr levels and LDL catabolism inhibition and, accordingly, associated with rapidly progressive atherosclerosis (Al‐Mashhadi et al., 2013). Bama minipigs were modified by CRISPR/Cas9, targeting ApoE and LDLr and generating biallelic knockout pigs that presented moderately elevated TC, LDL‐c and apoB levels, which were significantly increased after hypercholesterolaemic diet (Fang et al., 2018). To our knowledge, this model has not been tested in I/R injury.
The genetically engineered ExeGen™ consists of a Yucatan minipig model of LDLr deficiency to exert hyperlipidaemia and atherosclerosis. Different study protocols were applied, involving HFD administration and lipid‐reduction pharmacotherapy with β‐hydroxy β‐methylglutaryl‐CoA reductase inhibitor (atorvastatin) or ATP‐citrate lyase inhibitor (bempedoic acid; Amuzie et al., 2016). These pigs displayed weight gain and increased concentrations of TC, LDL‐c and HDL‐c fractions, with the development of atherosclerotic plaques. Both atorvastatin and bempedoic acid attenuated these alterations and reduced Oil Red O lipid staining. In another LDLr knockout model, pigs on standard chow developed atherosclerotic lesions beginning at 6 months of age and atherosclerosis was intensified when a hyperlipidaemic diet was added. In this setting, treatment with pitavastatin failed to modify plasma TC concentration, but achieved a 52% reduction of plaque volume in intravascular ultrasound, that pathologically correlated with early‐stage atherosclerotic lesions (Li et al., 2016). Overall, since pig models with single mutations do not resemble the human hyperlipidaemia, they are discouraged for translational studies but are very helpful for mechanistic studies.
Dog models are less frequently employed due to their lack of atherosclerosis development and dyslipidaemia (Poledne & Jurčíková‐Novotná, 2017). In this species, hypercholesterolaemia is induced through thyroid inhibition and dietary intervention, displaying after high‐fat regimen increased TC, LDL‐c and HDL‐c concentrations, with an irregular atherosclerotic response. This model has been used to test lipid‐lowering drugs (Yin et al., 2012).
A hallmark of vascular disease and early atherosclerosis plaque formation in humans is endothelial dysfunction. This condition, which seems to have important effects in cardiac injury, can be studied in the porcine model of hypercholesterolaemia. As in humans, pigs fed an HFD for a relatively short period of time (10 days) develop endothelial dysfunction amenable for the study of different interventions (Vilahur, Gutiérrez, et al., 2015; Vilahur, Padró, et al., 2015).
A summary of dietary interventions and gene editing for animal models of hypercholesterolaemia and high LDLc is illustrated in Figure 1.
FIGURE 1.
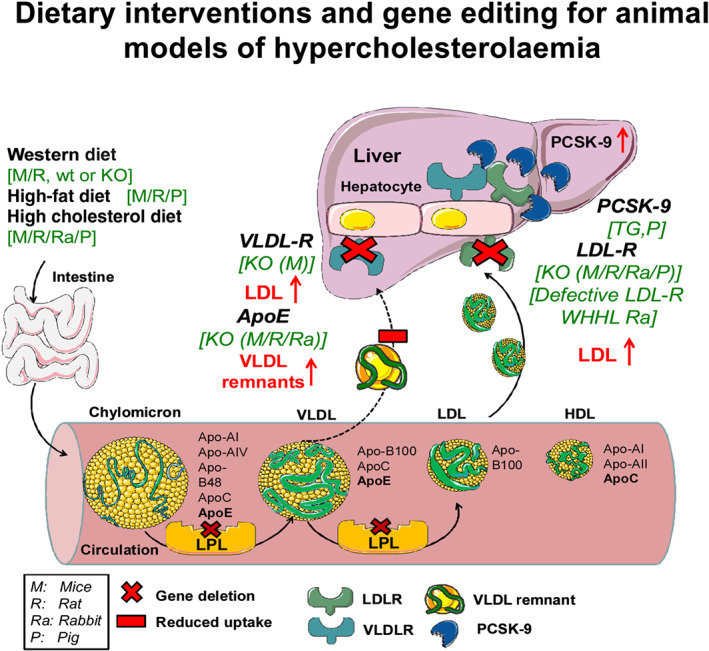
Schematic illustration of lipoprotein metabolism along with some dietary and genetic interventions that lead to hypercholesterolaemia. Dietary interventions include long‐term feeding with various diets supplemented with cholesterol or fats together with supportive substances causing lipid metabolism disturbances. Western type diets are commonly used in rodents (mice and rats) in order to provoke mild hypercholesterolaemia through the increased uptake of fat while high‐fat and high cholesterol diets are employed to produce more intense hypercholesterolaemic phenotypes. Genetic manipulations to provoke hypercholesterolaemia in animal models have been performed in various catabolic steps of lipoproteins. Lipoprotein lipase (LpL) hydrolyses triglycerides in lipoproteins, such as those found in chylomicrons and VLDL, into two free fatty acids and one monoacylglycerol molecule. LpL deletion leads to increased abundance of VLDL and LDL. LDL receptor (LDLR) mediates endocytosis of cholesterol from cholesterol‐rich LDL by recognizing apolipoprotein apoB‐100 and both LDLR and very LDL receptor (VLDLR) recognize lipoproteins baring the apolipoprotein E. The deletion of Ldlr, ApoE and VLDLR gene leads to impaired clearance of chylomicrons, VLDL and LDL. Therefore, these animals develop hypercholesterolaemia to a different degree depending on the animal model used. In parallel, PSCK‐9 is a proprotein convertase involved in the degradation of LDLR from the membranes. Overexpression of PSCK‐9 gene leads to increased binding of the PSCK‐9 protein to the extracellular part of the LDL. In this way, LDLRs are internalized by the cells and degraded. Apo, apolipoprotein; KO; knockout; LDLR, LDL receptor; LPL, lipoprotein lipase; PHHC, Prague hereditary hypercholesterolaemic; PSCK‐9, proprotein convertase subtilisin/kexin type 9; STH, St. Thomas Hospital; TG, transgenic; VLDL, very LDL; VLDLR, VLDL receptor; WHHL, Watanabe heritable hyperlipidaemic rabbits
3. ANIMAL MODELS FOR HYPERTRIGLYCERIDAEMIA
Hypertriglyceridaemia is an independent risk factor for coronary artery disease and several clinical studies have suggested that it is also an independent risk factor for early development of atherosclerosis (Boquist et al., 1999). Dietary TGs are packaged together with cholesterol and lipoproteins (mainly apolipoprotein‐B48) in enterocytes to form chylomicrons. Chylomicrons are carried from lymphatic vessels into the circulation where they are hydrolysed to remnants which are cleared from the circulation via hepatic LDLr (Figure 2). Once in the liver, TGs are released in the form of apoliprotein B‐100 containing VLDL particles. Lipoprotein lipase (LpL) through lipolysis plays an essential role in the formation of remnant particles from TG‐rich lipoproteins (chylomicrons and VLDL) which have been proposed to have a pro‐atherogenic role. TG‐rich lipoproteins–lipolysis leads to the release of potentially toxic oxidized fatty acids that induce vascular inflammation, and, in parallel, remnants per se transform macrophages into foam cells (Miller et al., 2011). TG levels are inversely related to HDL‐cholesterol levels.
FIGURE 2.
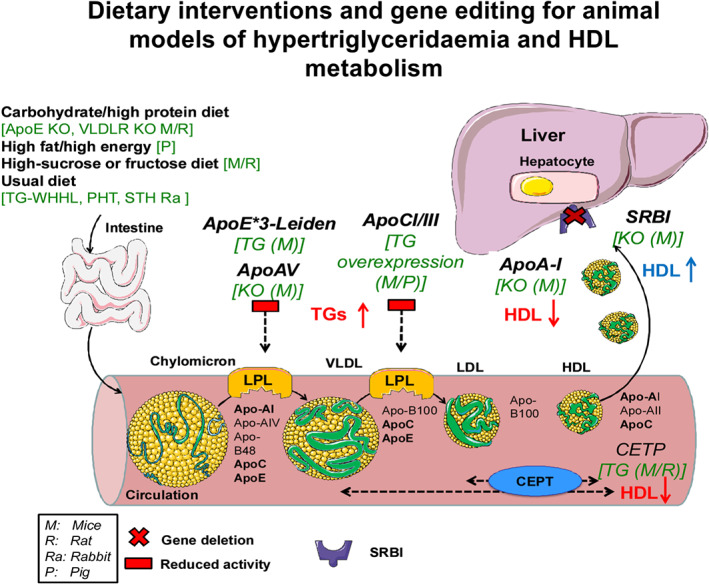
Schematic illustration of lipoprotein metabolism together with some dietary and genetic interventions that lead to hypertriglyceridaemia and disturbed HDL metabolism. Similar to the induction of hypercholesterolaemia, different types of diets are used to introduce mice to hypertriglyceridaemia and they mostly contain carbohydrates and sucrose instead of fat content. ApoE*3‐Leiden mice regulate the expression of APOE and APOC1 apolipoproteins, present a moderate hyperlipidaemia uptake, and lead to a significant increase of plasma TG levels. Apolipoprotein A‐V (ApoA‐V) and apolipoprotein C‐III (APOCIII) have opposing effects on plasma TG metabolism and they act as exchangeable constituents of VLDL and HDL and they are activating LpL. Therefore, ApoA‐V knockout mice and mice with overexpression of APOCIII, APOCI have decreased LpL enzyme activity and up‐regulated triglycerides. Regarding HDL metabolism, ApoA‐I null mice and the CETP transgenic mice bearing the human or cynomologus monkey CETP protein have reduced plasma HDL‐c levels, while deletion of the scavenger receptor BI (SR‐BI) results in an increase in plasma HDL‐c. ApoE‐3*L, ApolipoproteinE*3‐Leiden mice, apolipoprotein; CETP, cholesteryl ester transfer protein; KO, knockout; LPL, lipoprotein lipase; PHHC, Prague hereditary hypercholesterolaemic; SR‐BI, scavenger receptor BI; STH, St. Thomas Hospital; TG, transgenic; TGs, triglycerides; WHHL, Watanabe heritable hyperlipidaemic rabbits
3.1. Small animals
As in humans, rabbit apoB‐48 is only present in dietary‐derived chylomicrons but not in liver‐derived VLDLs whereas, in the mouse, apoB‐48 is also produced in the liver. Hence, mouse VLDLs contain both apoB‐48 and apoB‐100. Furthermore, rodents have a soluble form of hepatic lipase while humans have the membrane‐bound type to perform the clearance of remnant lipoproteins (de Silva, Más‐Oliva, Taylor, & Mahley, 1994).
3.1.1. Diet‐induced hypertriglyceridaemia
Mice
ApoE/LDLr−/− mice fed a low carbohydrate/high protein diet have doubled TG levels and marginally increased TC after 10 weeks displaying higher plaque burden than ApoE/LDLr−/− mice fed regular chow (Kostogrys et al., 2012). Likewise, fructose supplemented LDLr−/− or C57Bl/6 mice fed standard or WD increased TG levels twofold as compared to matched controls (Mastrocola et al., 2016). Interestingly, infarct size was 50% larger in C57Bl/6 mice exposed to a high‐fructose diet as compared to the standard diet (Mastrocola et al., 2016).
Rats
Overfeeding rats with sucrose or fructose induces hypertriglyceridaemia. Whereas sucrose favours hepatic fatty acid esterification and VLDL synthesis, fructose deteriorates VLDL–TGs catabolism (Hirano, Mamo, Poapst, Kuksis, & Steiner, 1989). Wistar rats fed a high‐sucrose (68%) or fructose (60%) diets have a fourfold increase in TG levels after 3 and 8 weeks respectively (Hirano et al., 1989; Kawashima et al., 2018). The protective effects of ischaemic preconditioning were abrogated in fructose fed‐hypertriglyceridaemic rats (Babbar, Mahadevan, & Balakumar, 2013).
Rabbits
Homozygous inbreeding of WHHL rabbits provided hypercholesterolaemic rabbit colonies presenting TG levels between 150 and 1,500 mg·dl−1 (1.69 to 16.9 mM; TG–WHHL; Zhang, Jin, Liu, Liu, & Ito, 2009). Crossbreed between TG–WHHL rabbits and Japanese White rabbits resulted in a post‐prandial hypertriglyceridaemic rabbit model (PHT) that exhibits remarkably high levels of serum TGs after feeding a standard diet (Kawai et al., 2006). However, both TG–WHHL and PHT rabbits develop insulin resistance and obesity and therefore also serve as a model of metabolic syndrome. Long‐term treatment with probucol significantly ameliorates myocardial injury in TG–WHHL rabbits (Hoshida et al., 1997). The PHT rabbits have not been tested in I/R studies so far.
3.1.2. Genetic animal models of hypertriglyceridaemia
Mice
APOE*3‐Leiden transgenic (E3L) mice are generated by introducing into C57Bl/6 mice a human APOE*3‐Leiden gene construct, consisting of APOE*3‐Leiden and apolipoprotein C1 (APOC1) genes and a promoter element that regulates the expression of APOE and APOC1 genes. As compared with ApoE−/− and LDLr−/− mice, E3L mice present a mouse model of moderate hyperlipidaemia (cholesterol levels on chow are about 77 mg·dl−1 [2 mM] and do not exceed 970 mg·dl−1 [25 mM] on an HFD; Zadelaar et al., 2007). Yet the presence of the APOC1 gene lowers lipolysis and VLDL uptake through both the LDLr and LDL receptor‐related protein, leading to a significant increase of plasma TG levels on a regular chow diet and a prominent increase under an HFD. E3L mice develop atherosclerotic lesions with all the characteristics of human vascular pathology, varying from fatty streaks to mild, moderate and severe plaques.
Apolipoprotein A‐V (ApoA‐V) and apolipoprotein C‐III have opposing effects on plasma TG metabolism since they act as exchangeable constituents of VLDL and HDL (Qu et al., 2007). ApoA‐V accelerates TG‐rich lipoprotein catabolism by activating LpL. ApoA‐V knockout mice display four times higher TG levels than their wild‐type littermates, whereas mice overexpressing human ApoA‐V gene display plasma TG levels one third of those of the control littermates (Pennacchio et al., 2001). In contrast, APOCI or APOCIII overexpressing mice have elevated plasma TG levels because of low LpL catalytic activity (Jong et al., 1997). Indeed, transgenic APOCIII production results in hypertriglyceridaemia due to the delayed catabolism of VLDL and chylomicrons (Ito, Azrolan, O'Connell, Walsh, & Breslow, 1990). ApoB‐100 overexpressing mice show hypertriglyceridaemia, however if these mice are fed an HCD they show both hypercholesterolaemic and hypertriglyceridaemic phenotype (Csont et al., 2007).
VLDLr−/− mice present twofold higher TG levels than their wild type (316 mg·dl−1 [3.6 mM] vs. 141 mg·dl−1 [1.6 mM]) and the increase in TG is exacerbated (544 mg·dl−1 [6 mM)]) when VLDLr−/− mice are crossed with heterozygous LpL‐deficient and human apoB transgenic mice (Yagyu et al., 2002). VLDLr−/− mice have smaller infarct size probably due to protection from cardiac lipotoxicity (Perman et al., 2011) and therefore are not suitable to study cardioprotective interventions in I/R injury.
Rats
The rats that are homozygous for the corpulent gene (cp/cp) become obese, insulin resistant and hypertriglyceridaemic. Hyperlipidaemia in the cp/cp rat is primarily due to hepatic hypersecretion of VLDL. Thus, with a regular diet, hyperlipidaemia is essentially confined to TG‐rich fractions, and elevations of cholesterol are generally marginal. Among the different cp strains, the JCR:LA‐cp rats display hyperlipidaemia associated with apoB‐48‐containing particles after oral fat loading (60% fat), particularly in the early post‐prandial phase. This pattern likely contributes to atherogenesis by facilitating or exacerbating the saturation of LpL activity (Mangat et al., 2007). Hypertension (140–150 mm Hg) in Prague hereditary hypertriglyceridaemic rats (Vrána & Kazdová, 1990) develops in parallel with the increase of plasma TGs.
So far, severe myocardial I/R injury has been reported in hypertriglyceridaemic rats (Babbar et al., 2013). Moreover, ischaemic postconditioning was markedly impaired in hypertriglyceridaemic rat hearts as compared to matched controls. However, pharmacological reduction of TGs using 8‐week treatment with fenofibrate restored the ischaemic postconditioning effect (Babbar et al., 2013).
Rabbits
The St. Thomas Hospital rabbit displays a Mendelian form of hypertriglyceridaemia accompanied by accelerated atherosclerosis (Beaty et al., 1992).
3.2. Large animal models for hypertriglyceridaemia
Göttingen minipigs under a dietary intervention consisting of HFD/high energy diet or low‐fat/low energy diet (i.e. energy derived from fat was equivalent to 55% and 13%, respectively) evidenced that TG levels were significantly elevated in the HFD/high energy diet group, without influence on TC, glucose and insulin concentrations (Johansen, Hansen, Richelsen, & Malmlöf, 2001). In this model, the increased TG levels were associated with an increase in the HDL‐c fraction,however the LDL‐c and the VLDL‐c cholesterol fractions were not reported (Johansen et al., 2001). In Yucatan minipigs, hypercholesterolaemic chow induced an increase of TC levels as well as of LDL‐c and HDL‐c. The levels of TG, however, remained unchanged (Osipov, Robich, et al., 2009).
Transgenic miniature pig with human APOCIII overexpression had a significant 2.5‐fold increase in plasma TG concentration, associated with impaired TG catabolism and a reduced LpL activity (Wei et al., 2012).
So far, severe myocardial I/R injury has been reported in hypertriglyceridaemic Yucatan minipigs hearts as compared to the hearts of the respective control animals (Osipov, Robich, et al., 2009). In line with the rat data, ischaemic postconditioning significantly improved endothelial function and decreased area of no reflow and necrosis in minipigs fed a regular chow, whereas in those fed a hypercholesterolaemic‐enriched diet (2% cholesterol and 15% lard by weight) for 4 weeks did not improve endothelial function or reduced area of no reflow and necrosis (Zhao, Yang, You, Cui, & Gao, 2007).
4. ANIMAL MODELS FOR HDL METABOLISM
HDL‐c exerts cardio/vascular‐protective properties by taking part in reverse cholesterol transport, from cholesterol efflux (Vaidya et al., 2019), lipoprotein remodelling to hepatic lipid uptake and clearance (Favari et al., 2015). Accordingly, HDL levels have been inversely related to cardiovascular risk and atherosclerosis development. The HDL fraction exerts direct cardioprotective effects, reduces infarct size, and improves cardiac performance (Theilmeier et al., 2006; Vilahur, Gutiérrez, et al., 2015). However, this relationship seems to be more complex than originally thought due to, at least in part, the complexity of HDL particles with changes in composition that may result in a shift in HDL functions in the presence of different cardiovascular risk factors (Padró et al., 2017; Woudberg et al., 2017). Therefore, multiple experimental models have been developed to study HDL, most of them targeting different steps of the metabolism of HDL particles.
4.1. Small animals (mice, rats and rabbits)
Multiple ex vivo and in vivo studies conducted in small animals have proven that the cardiovascular benefit of HDL goes beyond its role on the reverse cholesterol transport. Indeed, HDL presents multiple benefits including antioxidant, anti‐inflammatory and anti‐thrombotic properties (Keul et al., 2019; Woudberg et al., 2017). Genetic animal models, diet‐induced animal models and native or reconstituted HDL‐c have been used to explore the cardioprotective effect of HDL (see Table 3).
TABLE 3.
Experimental models used to define the role of HDL metabolism on cardioprotection
| Animal model | Ischaemia/reperfusion protocol | Treatment | Key findings | Reference |
|---|---|---|---|---|
| Mice | ||||
| C57BL/6 mice on normal and HFD | 30 min ischaemia/1 hr reperfusion | rHDL (25‐mg ApoA1/ml) delivered during reperfusion | Reduction in infarct size in mice on normal and HFD | Heywood et al., 2017 |
| C57Bl6/N mice | 30 min ischaemia/24 hr reperfusion | HDL administered intravenously for 21 days prior to ischaemia | Reduction in infarct size | Theilmeier et al., 2006 |
| C57BL/6 mice | 35 min ischaemia/45 min reperfusion | rHDL (100, 200, and 400 μg·ml−1) perfused for 7 min at onset of reperfusion | Dose‐dependent reduction in infarct size | Frias et al., 2013 |
| C57BL/6 mice | 45 min ischaemia/24 hr reperfusion | rHDL enriched with S1P infused 1 min before reperfusion | Reduction in infarct size | Brulhart‐Meynet et al., 2015 |
| ApoA1−/− C57BL/6 mice | 30 min ischaemia/3 hr reperfusion | — | Increase in infarct size in ApoA1−/− mice | Dadabayev et al., 2014 |
| Rats | ||||
| Healthy male Sprague–Dawley rats | 15 min ischaemia/20 min reperfusion | Physiological concentrations of HDL infused during I/R | Reduction in the incidence of arrhythmias | Mochizuki et al., 1991 |
| Healthy male Sprague–Dawley rats | 20 min ischaemia/30 min reperfusion | HDL (0.5 and 1.0 mg·ml−1) perfused 10 min prior to ischaemia | Improved post‐ischaemic left ventricular functional recovery at reperfusion | Calabresi et al., 2003 |
| Healthy male Sprague–Dawley rats | 20 min ischaemia/30 min reperfusion | Synthetic HDL (0.5, 1.0, and 2.0 mg·ml−1) perfused 10 min prior to ischaemia | Dose‐dependent improved left ventricular functional recovery | Rossoni et al., 2004 |
| Healthy male Sprague–Dawley rats | 20 min ischaemia/30 min reperfusion | Synthetic HDL (1.0 mg·ml−1) perfused 10 min prior to ischaemia | Improvement in post‐ischaemic left ventricular developed pressure recovery and attenuation of coronary perfusion pressure elevation | Gomaraschi et al., 2008 |
| Healthy male Wistar rats | Rat coronary artery ligation | rHDL was infused 10 min before permanent ligation | Reduction of fibrosis in ligated hearts | Kiya et al., 2009 |
| Healthy male Sprague–Dawley rats | 30 min ischaemia/3 hr reperfusion | Synthetic HDL infused 10 min before reperfusion | Reduction in creatine kinase, TNF‐α, and IL‐6 in cardiac tissue | Gu et al., 2007 |
| Healthy male Wistar rats | 5 min ischaemia/3 min reperfusion | rHDL was infused 10 min before occlusion | Suppression of reperfusion‐induced arrhythmias | Imaizumi et al., 2008 |
| Healthy male Wistar rats | 35 min ischaemia/90 min reperfusion | HDL from healthy and MI patients infused during first 7 min of reperfusion | HDL isolated from MI patients failed to reduce the infarct size | Soares et al., 2019 |
| Rabbits | ||||
| Healthy Male New Zealand White rabbits | 30 min ischaemia/1 hr reperfusion ApoA1‐Milano | Hearts were treated during the 10‐min pretreatment and the 60 min of reperfusion with ApoA1‐Milano | Reduction of tissue lipid hydroperoxides in hearts subjected to global ischaemia | Marchesi et al., 2008 |
| Healthy Male New Zealand White rabbits | 30 min ischaemia/4 hr reperfusion | 100 mg·kg−1 synthetic ApoA1‐Milano (ETC‐216) 1 day before and at the time of the surgical procedure | Reduction in infarct size |
Marchesi et al., 2004 Marchesi et al., 2008 |
| Pigs | ||||
| Young healthy pigs | 60‐min closed‐chest coronary balloon occlusion followed by reperfusion for 3 days when cardiac magnetic resonance was performed | Isolation of HDL particles from hypercholesterolaemic and non‐hypercholesterolaemic pigs. 4 days and 1 day before the induction of ischaemia, two intravenous infusions of 15 mg·kg−1 HDL particles were performed | HDL particles from hypercholesterolaemic pigs are not cardioprotective | Vilahur, Gutiérrez, et al., 2015 |
4.1.1. Non‐genetic animal models for HDL
Following on the seminal work from Badimon et al., in the early 90s where plasma HDL isolated from healthy rabbits and injected into rabbits with a cholesterol‐enriched diet resulted in the inhibition of atherosclerosis progression and the induction of regression of existing atherosclerosis (Badimon, Badimon, Galvez, Dische, & Fuster, 1989; Badimon et al., 1990), several preclinical studies exploring the role of HDL in cardioprotection have been conducted in mice and rats. The cardioprotective effect of HDL has been tested following the administration of different types of HDL. First, human plasma HDL, isolated from healthy individuals, reduced ventricular arrhythmias and infarct size in a dose‐dependent manner when given prior to or after an ischaemic insult to an isolated rat or mouse heart model (Calabresi et al., 2003; Frias et al., 2013; Mochizuki et al., 1991) or in vivo (Theilmeier et al., 2006). On the contrary, plasma HDL isolated from patients with MI failed to reduce the infarct size in isolated rat hearts (Soares et al., 2019). The species origin of the isolated HDL to be used for cardioprotective studies in rats and mice does not seem to affect its cardioprotective effect as both mice and human HDL seem to activate the cardioprotective signalling pathways in a similar manner (Pedretti et al., 2019). The use of synthetic or reconstituted HDL particles has been developed with the aim to facilitate its clinical translation compared to native HDL. Originally made of purified or recombinant ApoA‐I and phospholipids (usually phosphatidylcholine), their protective effect against I/R has been shown in both rats and mice, although it is less pronounced than that of native HDL (Calabresi et al., 2003; Frias et al., 2013; Imaizumi et al., 2008). If the concentration of ApoA‐I is critical for cardioprotection (Gu, Shi, & Wu, 2007), the addition of sphingosine‐1 phosphate (S1P) to the composition of reconstituted HDL improves the cardioprotective effect of reconstituted HDL to the equivalent of native HDL (Brulhart‐Meynet et al., 2015). In fact, S1P is important for the cardioprotection by HDL. HDL bound‐S1P predicts the severity of coronary atherosclerosis in humans (Sattler et al., 2014) and HDL reduces the risk of periprocedural infarction in patients undergoing percutaneous coronary intervention (Sattler et al., 2009). S1P is known to be cardioprotective by triggering preconditioning mechanisms (reviewed in Karliner, 2013) and S1P−/− mice exhibit no reduction of infarct size when undergoing an ischaemic preconditioning protocol (Keul et al., 2016). Other alternatives in the composition of reconstituted HDL consider the substitution of ApoA‐I by ApoA‐I Milano (Marchesi et al., 2008) or other ApoA‐I variants such as 37pA (Gomaraschi et al., 2008).
4.1.2. Genetic animal models for HDL
Multiple genetically modified animal models have been developed with the aim to enlighten the physiology of HDL particles. These models all target different steps of the HDL particle homeostasis.
Mice with genetic deletion of ApoA‐I, phospholipid transfer protein, ATP binding cassette transporter A1, or lecithin cholesterol acyltransferase have a decrease in plasma HDL‐c. The deletion of scavenger receptor BI (SR‐BI) results in an increase in plasma HDL‐c. Other plasma lipoproteins levels such as LDL‐c and VLDL‐c are also affected in these models (also reviewed by Hoekstra & Van Eck, 2015).
ApoA‐I null and heterozygous mice have plasma HDL‐c levels reduced by approximately 80% and 50%, respectively, compared to wild‐type mice. When subjected to in vivo ischaemia, these mice have a 52% and 125% increase in infarct size compared to wild type (Dadabayev et al., 2014). In a SR‐BI/ApoE double knockout mouse model, spontaneous MI and early death occur under a standard chow diet (Braun et al., 2002). Similarly, SRB‐I/LDL receptor double knockout mice fed with a WD containing 0.5% cholesterol develop atherosclerosis, ischaemic heart disease, and early death (Liao et al., 2017). Although the generation of these genetically modified mice greatly contributes to the understanding of the metabolism and function of HDL, they may not represent an ideal model to mimic the clinical setting for cardioprotective studies due to the complexity of HDL metabolism that these models may fail to reproduce.
A model that exhibits decreased HDL‐c and a variable degree of atherosclerosis along with a small increase in VLDL‐c and LDL‐c on the basal (chow) diet is the CETP transgenic mice bearing the human or cynomologus monkey CETP protein (Jiang et al., 1993). The increase in VLDL‐c and LDL‐c is exacerbated upon an HFD regimen, reaching values of TC of 250 mg·dl−1 versus 163 mg·dl−1 of non‐transgenic mice. With the use of these transgenic mice, CETP activity was found inversely associated with ApoA‐I and positively associated with apoB (Marotti et al., 1992).
4.2. Large animal models for HDL
Pig models have been used to investigate HDL effects on lipid profile and atherosclerosis. Farm pigs were fed an HCD for 8 weeks, 16 weeks or standard diet (control). Eight‐ and 16‐week HCD induced 10‐fold higher TC and LDL values than in control animals. The resulting HDL‐c concentration between 20 and 80 mg·dl−1 (0.5 to 2 mM) correlated inversely with the number and degree of atherosclerotic lesions (Pelosi et al., 2014). The cardioprotection associated with the administration of native HDL particles against I/R disappears when infused HDL particles are formed under hypercholesterolaemic conditions highlighting the negative impact of hypercholesterolaemia on HDL‐related cardioprotective effects (Vilahur, Gutiérrez, et al., 2015). Again, S1P is important here, since S1P loading of HDL restores the vascular protective effects of HDL from patients with coronary artery disease (Sattler et al., 2015).
A summary of dietary interventions and gene editing for animal models of hypertriglyceridaemia and HDL metabolism is illustrated in Figure 2.
5. CONCLUSIONS AND RECOMMENDATIONS
Despite the fact that various animal models of different types of hyperlipidaemia exist, only some of them have been employed for studying myocardial I/R injury and cardioprotection. An ideal animal model for studying hyperlipidaemia and cardioprotection would be affordable and mimic adequately the human phenotype including the gradual development of atherosclerosis and the direct effect of hyperlipidaemia on the myocardium and other tissues. However, several limitations regarding the lipidaemic profile of different animal species in comparison to that measured in humans should be taken into consideration. The animal models of hypercholesterolaemia with a brief description of their phenotypes are illustrated in Table 1. Models with HFD/HCDs and genetic modifications have been suggested for cardioprotection (Table 2) and exogenous supplementation with lipoprotein particles in I/R injury has proven to be very useful for mechanistic studies (Table 3). We must mention, however, that due to reproducibility issues of lipid levels especially in the diet‐induced hyperlipidaemia models, appropriate time and age‐matched controls are needed. Moreover, in most of the hyperlipidaemic models, confounding effect of obesity and insulin resistance should be taken into account (see Table 1). Only a few animal species (mainly the large animals) possess the lipid profile similar to humans but studies on myocardial I/R injury are scarce. Therefore, further studies in these animal models are required to gather more data on the effect of hyperlipidaemia on I/R injury and cardioprotection for discovery and validation of effective cardioprotective drug targets and translating these findings into the clinical arena.
TABLE 1.
Animal models of hypercholesterolaemia, values of lipid levels and a brief description of the phenotype
| Animal model | Protocol | TC mg dl−1 (mM) | LDL‐c mg dl−1 (mM) | HDL‐c mg dl−1 (mM) | VLDL mg dl−1 (mM) | TGs mg dl−1 (mM) | Brief description of phenotype | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mice | ||||||||||
| C57BL/6J | Normal chow | 75–110 (1.9–2.9) | 10 (0.26) | 50 (1.3) | 20 (0.5) | 65 (0.8) | Normal lipidaemic profile | Jang et al., 2012; Andreadou, Efentakis, et al., 2017 | ||
| C57BL/6 N | Adult mice 9 weeks on WD | 181 ± 5 (4.7 ± 0.1) | N/A | 123 ± 3 (3.2 ± 0.07) | N/A | 78 ± 4 (0.9 ± 0.04) |
Mild hypercholesterolaemia Increased body weight, hyperglycαemia, and hyperinsulinαemia |
Desmarchelier et al., 2012 | ||
| C57BL/6J | Adult mice 14 weeks on WD (TD88137) | 233 ± 43 (6.0 ± 1.1) | N/A | N/A | N/A | 37 ± 9 (0.4 ± 0.1) |
Mild hypercholesterolaemia Model of metabolic syndrome Increased body weight and hyperglycαemia |
Andreadou, Efentakis, et al., 2017 | ||
| C57BL/6J | Adult mice 2 weeks on HCD | 144 ± 9 (3.7 ± 0.2) | N/A | N/A | N/A | 25 ± 17 (0.3 ± 0.2) |
Hypercholesterolaemia with unstable triglyceride levels Body weight changes and glucose tolerance were not reported |
Girod et al., 1999 | ||
| C57BL/6J | Adult mice 12 weeks on HCD | 295 ± 18 (7.6 ± 0.5) | N/A | N/A | N/A | 284 ± 105 (3.2 ± 1.2) |
Hypercholesterolaemia and hypertriglyceridaemia Body weight changes and glucose tolerance were not reported |
Girod et al., 1999 | ||
| ApoE−/− |
Normal chow 4–6 months old |
400–600 (10.3–15.5) 460 ± 50 (11.9 ± 1.3) |
140 (3.6) | 50 (1.3) | 200 (5.2) |
110 (1.2) 115 ± 35 (1.3 ± 0.4) |
Mild hypercholesterolaemia and atherosclerotic lesions on the aortic sinus Moderate increase in body weight Glucose levels were not reported |
Meyrelles et al., 2011 Jiang, Jones, Husband, & Dusting, 2003 |
||
| APOE−/− |
4–6 months old 2 weeks on HCD 10 weeks old on WD for 20 weeks |
1,048 ± 46 (27.1 ± 1.2) 567 ± 265 (14.7 ± 6.8) |
N/A N/A |
224 ± 12 (5.8 ± 0.3) 54.1 ± 25.4 (1.4 ± 0.7) |
822.4 ± 38.6 (21.3 ± 1.0) N/A |
824 ± 35 (3.9 ± 0.4) 96.4 ± 29.6 (1.1 ± 0.3) |
Severe hypercholesterolaemia and extended atherosclerosis Increased body weight and glucose intolerance |
Meyrelles et al., 2011 |
||
| LDLR−/− |
Female phenotype at 16 weeks on standard chow Male phenotype at late stages on standard chow |
250 ± 8 (6.5 ± 0.2) 358 ± 13 (9.3 ± 0.3) |
168 ± 5 (4.3 ± 0.1) N/A |
69 ± 3 mg·dl−1 (1.7 ± 0.07) N/A |
13 ± 1 (0.34 ± 0.03) N/A |
124 ± 8 (1.4 ± 0.09) 495 ± 17 (5.6 ± 0.2) |
Moderate elevation of TC Body weight and glucose levels are similar to Ldlr + / + animals (Ngai et al., 2010) At late adulthood, this model presents with hypertriglyceridaemia |
Yin et al., 2012 Girod et al., 1999 |
||
| LDLR−/− | Female mice 16 weeks old on WD (TD88137) | 1.677 ± 98 (43.4 ± 2.5) | 761 ± 57 (19.7 ± 1.5) | 12 ± 2 mg·dl−1 (0.3 ± 0.05) | 904 ± 47 (23.4 ± 1.2) | 404 ± 35 (4.6 ± 0.4) |
Extreme elevation in TC, LDL‐c, VLDL. Increased TGs and decreased HDL levels Body weight changes and glucose tolerance were not reported |
Yin et al., 2012 |
||
| LDLR−/− |
Adult mice 2 weeks on HCD Adult mice 12 weeks on HCD |
2,479 ± 213 (64.1 ± 5.5) 3,180 ± 70 (82.2 ± 1.8) |
N/A N/A |
N/A N/A |
N/A N/A |
5,397 ± 784 (60.9 ± 8.9) 4,964 ± 735 (56.0 ± 8.3) |
Severe hypercholesterolaemia and hypertriglyceridaemia Body weight and glucose levels are similar to Ldlr + / + animals (Ngai et al., 2010) |
Girod et al., 1999 | ||
| ApoA‐V−/− | Adult mice on standard chow | Unchanged compared to wild‐type‐mixed 129Sv/C57BL6 strain controls littermates | N/A | Similar to wild‐type littermates | Increased in compared to wild‐type littermates | 153 ± 77 (1.7 ± 0.9) |
Four times higher TG levels than their wild‐type littermates. This is a mouse model of hypertriglyceridaemia No changes in body weight and glucose levels |
Qu et al., 2007; Pennacchio et al., 2001 | ||
| APO‐Leiden 3 |
Adult male on chow diet Adult female on chow diet |
128 ± 4 (3.3 ± 0.1) 120 ± 8 (3.1 ± 0.2) |
N/A | N/A | N/A |
213 ± 8.9 (2.4 ± 0.1) 230 ± 27 (2.6 ± 0.3) |
Moderate hyperlipidaemia Significant raise of plasma TG levels on a regular chow diet and a prominent raise under an HFD. No changes in body weight and glucose levels |
Bijland et al., 2009 | ||
| APO‐Leiden 3 | 2 months old mice atherogenic diet (containing 40.5% sucrose, 15% cocoa butter, 1% cholesterol, and 0.5% sodium cholate) for 2 or 4 months | 93 ± 23 (2.4 ± 0.6) | N/A | N/A | N/A | N/A |
Increase in VLDL and LDL fractions while HDL is very low when compared to the non‐transgenic control. After 4 months, lesions had developed which were advanced at the aortic arch Increased body weight and glucose intolerance |
Leppänen, Luoma, Hofker, Havekes, & Ylä‐Herttuala, 1998 | ||
| Rats | ||||||||||
| Wistar | Male on standard diet |
70 ± 3.9 (1.8 ± 0.1) 65 ± 8 (1.7 ± 0.2) 83.6 ± 7.4 (2.2 ± 0.2) |
9.3 ± 3.9 (0.24 ± 0.10) N/A 13.2 ± 2.6 (0.34 ± 0.06) |
28 ± 4.2 (0.7 ± 0.1) N/A 49.8 ± 5.8 (1.3 ± 0.2) |
10.8 ± (0.28 ± 0.05) N/A 22.4 ± 3.11 (0.58 ± 0.08) |
125 ± 10 (1.4 ± 0.1) 106 ± 18 (1.2 ± 0.2) 59.6 ± 5.36 (0.7 ± 0.06) |
Normal lipidaemic profile |
Ferko et al., 2018 Kovár et al., 2009 Raja et al., 2012 |
||
| Wistar | Male adult rats HFD (5% palm oil) for 3 weeks | 77 ± 10 (1.98 ± 0.26) | N/A | N/A | N/A | 109.8 ± 37 (1.24 ± 0.42) |
Marginally elevated TC Changes in body weight or glucose tolerance were not reported |
Kovár et al., 2009 | ||
| Wistar |
Male adult rats High‐fat–high cholesterol diet (5% palm oil, 1% cholesterol) for 3 weeks |
90.5 ± 16 (2.4 ± 0.4) | N/A | N/A | N/A | 159 ± 35 (1.79 ± 0.39) |
Elevated TC Changes in body weight or glucose tolerance were not reported |
Kovár et al., 2009 | ||
| Wistar |
Male with insulin resistance High‐fat–high cholesterol diet for 5 days |
116 ± 23 (3.0 ± 0.6) | 53.4 ± 15.1 (1.38 ± 0.39) | 33 ± 6 (0.86 ± 0.15) | 24.3 ± 5.2 (0.63 ± 0.13) | 490 ± 42 (5.5 ± 0.5) |
Hypercholesterolaemia Hyperinsulinaemia and hyperglycaemia |
Adameová et al., 2007; Ferko et al., 2018 | ||
| Wistar | Male 7 weeks old supplemented with CCT diet (4% cholesterol, 1% cholic acid, and 0.5% 2‐thiouracil) for 30 days | 198 ± 11 (5.1 ± 0.3) | 34.2 ± 4.6 (0.9 ± 0.1) | 26 ± 3 (0.7 ± 0.07) | 125.7 ± 5.0 (3.26 ± 0.13) | 169 ± 4 (1.9 ± 0.04) |
Increased plasma TC, TG, LDL‐C, VLDL‐C, decreased HDL. Marked atherosclerosis Increased body weight and blood pressure. Glucose tolerance was not reported |
Raja et al., 2012 | ||
| James C Russell corpulent (JCR:LA‐cp) rat | Adult rats on normal chow | 159 ± 12 (4.12 ± 0.31) | 17.8 ± 7 (0.46 ± 0.18) | 84 ± 7.3 (2.16 ± 0.19) | N/A | 337 ± 42 (3.80 ± 0.47) |
Male cp/cp rats are characterized by hyperleptinaemia, hyperinsulinaemia and hyperphagia along with the marked hypertriglyceridaemia Increased body weight Glucose tolerance was not reported |
Reimer & Russell, 2008 | ||
| Prague hereditary hypercholesterolaemic (PHHC) rat | Male adult rats fed on chow diet | 97 ± 8 (2.5 ± 0.2) | N/A | N/A | N/A | 81.5 ± 18.6 (0.92 ± 0.21) |
Relatively higher HDL‐c Glucose levels similar to Wistar rats |
Kovár et al., 2009 | ||
| PHHC rat | Male adult rats HFD (5% palm oil) for 3 weeks | 104 ± 12 (2.7 ± 0.3) | N/A | N/A | N/A | 84 ± 34 (0.95 ± 0.38) |
Slight exacerbation of the normal fed phenotype Changes in body weight or glucose tolerance were not reported |
Kovár et al., 2009 | ||
| PHHC rat |
Male adult rats High‐fat–high cholesterol diet (5% palm oil,1% cholesterol) for 3 weeks |
164 ± 9 (4.2 ± 0.2) | N/A | N/A | N/A | 125.8 ± 42 (1.42 ± 0.47) |
Most of the cholesterol in PHHC rats on cholesterol diet is carried out inVLDL and IDL. Similar to the dys‐betalipoproteinaemia (hyperlipoproteinaemia type III) in humans. Changes in body weight or glucose tolerance were not reported |
Kovár et al., 2009 | ||
| Sprague–Dawley | Adult rats 64 weeks old | Liver: 5.39 ± 0.14 mg·g−1 tissue | 94 ± 16 (2.4 ± 0.4) | N/A | 10 ± 1 (0.26 ± 0.03) | Liver: 13.12 ± 1.35 mg·g−1 tissue | Normal lipidaemic profile | Sithu et al., 2017 | ||
| Sprague–Dawley ApoE−/− | On normal chow 24 weeks old | Up‐regulation compared to wild‐type littermates | Up‐regulation compared to wild‐type littermates | Decreased | Elevated | Not significantly elevated |
Females and males are resistant to developing hyperlipidaemia Body weight is slightly increased. Glucose tolerance Severe hyperlipidaemia and redistribution of cholesterol. The diet exacerbates the phenotype and accelerates atherosclerosis development. |
Zhao et al., 2018 | ||
| Sprague–Dawley ApoE−/− | On WD 24 weeks old | Up‐regulation compared to wild‐type littermates | Up‐regulation compared to wild‐type littermates | Decreased | Elevated | Not significantly elevated | Zhao et al., 2018 | |||
| Sprague–Dawley LDLR−/− |
64 weeks old rats on normal chow 64 weeks old WD for 52 weeks (from the 12th week of age) |
Liver: 10.38 ± 2.17 mg·g−1 tissue Liver: 21.11 ± 3.40 mg·g−1 tissue |
168 ± 8 (4.3 ± 0.2) 139 ± 12 (3.6 ± 0.3) |
N/A N/A |
178 ± 26 (4.61 ± 0.67) 29 ± 6 (0.75 ± 0.16) |
Liver: 25.76 ± 5.72 mg·g−1 tissue Liver: 7. 76 ± 0.72 mg·g−1 tissue |
Moderate increase in LDL. Atherosclerotic lesions developed only on HCD Increased body weight and glucose intolerance. Model of atherogenesis, particularly in the context of diabetes and obesity |
Sithu et al., 2017 | ||
| Sprague–Dawley LDLR−/− | WD 24 weeks old | Up‐regulation compared to wild‐type littermates | Up‐regulation compared to wild‐type littermates | Unaltered compared to wild type | Elevated compared to wild type | Dramatic elevation of TGs |
Severe hypelipidaemia. The only model of genetic manipulation in rats that shows a predisposition for elevated TGs. No changes in body weight and glucose tolerance The diet exacerbates the phenotype and accelerates atherosclerosis development. |
Zhao et al., 2018 | ||
| Rabbits | ||||||||||
| New Zealand White rabbit | Standard diet | 29 ± 1 (0.7 ± 0.03) | 8 ± 0.4 (0.2 ± 0.01) | 18 ± 1 (0.5 ± 0.02) | 4 ± 0.3 (0.1 ± 0.01) | 47 ± 5 (0.53 ± 0.06) | Normal lipidaemic profile | Yin et al., 2012 | ||
| New Zealand White rabbit |
Male 0.5% Cholesterol for unspecified period of time |
811 ± 48 (21.0 ± 1.2) | 317 ± 24 ± 0.6) | 44 ± 4 (1.1 ± 0.1) | 439 ± 26 (11.4 ± 0.7) | 48 ± 4 (0.54 ± 0.05) |
Hyperlipidaemia that is mainly attributed to a redistribution of cholesterol in favour of VLDL particles Body weight and glucose tolerance were not reported |
Yin et al., 2012 | ||
| New Zealand White rabbit | Adult male rabbits 8 weeks with cholesterol enriched diet* | 1,402 ± 125 (36.3 ± 3.2) | N/A | N/A | N/A | N/A |
Hypercholesterolaemia with atherosclerosis evident in the ascending aorta, the ostia of the coronaries, and the epicardial vessels Increased body weight Glucose tolerance was not reported |
Kremastinos et al., 2000 | ||
| New Zealand White rabbit | Adult male rabbits 6 weeks with cholesterol enriched diet* | 912 ± 90 (23.6 ± 2.3) | N/A | N/A | N/A | N/A |
Hypercholesterolaemia and early signs of atherogenesis Increased body weight glucose tolerance was not reported A suitable model for prevention strategies from atherosclerosis |
Iliodromitis et al., 2006 | ||
| Watanabe hereditary hypercholesterolaemic (WHHL) rabbit |
Young male rabbits Adults male rabbits |
698 ± 31 (18.1 ± 0.8) 650–950 (16.8 ± 24.6) |
Ν/Α Ν/Α |
N/A | N/A |
189 ± 24 (2.1 ± 0.3) 652.2 ± 138.1 (7.4 ± 1.6) |
Pronounced hypercholesterolaemia Hypertriglyceridaemia/severe coronary atherosclerosis No change in body weight and glucose tolerance |
Hoshida et al., 1997; Aliev & Burnstock, 1998 | ||
| ApoE−/− rabbit | Rabbits were fed the standard chow | 156 ± 59 (4.0 ± 1.5) | N/A | 17 ± 6 (0.4 ± 0.15) | N/A | Increased but with marked variations. |
Model of hyperlipidaemia due to a sixfold increase in TC. HDL‐c was not remarkably changed No changes in body weight and increased serum glucose levels (Beierfuß et al., 2017 ) |
Niimi et al., 2016 | ||
| ApoE−/− rabbit | 0.3% cholesterol for 2 weeks | 1,070 ± 61 (27.7 ± 1.6) | N/A | 15 ± 1 (0.4 ± 0.02) | N/A | 132 ± 58 (1.5 ± 0.7) |
Suitable model of mixed hypertriglyceridaemia and severe hypercholesterolaemia HDL‐c was not remarkably changed Body weight changes and glucose tolerance were not reported |
Niimi et al., 2016 | ||
| Pigs | ||||||||||
| Ossabaw pig | High‐fat/cholesterol diet for 43 weeks | 338 ± 28 (8.7 ± 0.7) | 255 ± 29 (6.6 ± 0.7) | 75 ± 9 (1.9 ± 0.2) | N/A | 41 ± 7 (0.46 ± 0.08) |
Hyperlipidaemia with a minor increase in triglycerides, native atheroma, and greater elevations in metabolic features of metabolic syndrome compared to Yucatan pig Increased body weight and glucose intolerance |
Neeb et al., 2010 Trask et al., 2012 |
||
| Yucatan pig | High‐fat/cholesterol diet for 43 weeks | 420 ± 72 (10.9 ± 1.9) | 351 ± 76 (9.0 ± 1.9) | 60 ± 10 (1.6 ± 0.3) | N/A | 41 ± 6 (0.46 ± 0.07) |
Hyperlipidaemia without increase in triglycerides, less native atheroma than the Ossabaw pig Increased body weight and normal glucose tolerance |
Neeb et al., 2010 | ||
| Pigs | Female, fixed crossbreeding, Cholesterol‐rich diet (2% cholesterol; 1% cholic acid; 20% beef tallow) for 100 days | Increased | Increased | Increased | Increased | Unaltered |
Diet‐induced hyperlipidaemia in smaller period of time Body weight changes and glucose tolerance were not reported |
Casani, Sanchez‐Gomez, Vilahur, & Badimon, 2005 | ||
| Yucatan minipigs with transposition of human D374Y‐PCSK9 gene expression | Phenotype had developed after 8 weeks |
Increased ~770 (~20) |
Increased | Decreased compared to wild type | N/A | Unaltered on standard diet Increased on HFD |
Hypercholesterolaemia and spontaneous development of progressive atherosclerotic lesions No change in body weight and glucose tolerance |
Al‐Mashhadi et al., 2013 | ||
| ApoE−/− Bama miniature pigs | 8 weeks old | 99.4 ± 21.3 (2.57 ± 0.55) | Increased compared to wild type | Increased compared to wild type | N/A | Similar to the wild type |
Moderate, yet consistently, elevated serum cholesterol levels Body weight changes and glucose tolerance were not reported |
Fang et al., 2018 | ||
| ApoE−/− Bama miniature pigs |
3‐month‐old pigs High‐fat/cholesterol diet for 3 months |
~1,550 (~40) | Increased compared to wild type | Increased compared to wild type | N/A | ~133 (~1.5) |
Severe hypercholesterolaemia and progressive atherosclerotic lesions Body weight changes and glucose tolerance were not reported |
Fang et al., 2018 | ||
| Göttingen minipigs |
Female 9–10 months old 5 weeks on HFD (55% in fat) |
79 ± 6.6 (2.03 ± 0.17) Unchanged compared to low‐fat diet |
N/A | 39.9 ± 2.7 (1.03 ± 0.07) | N/A |
21.3 ± 2.7 (0.24 ± 0.03) Increased in comparison to low‐fat diet |
These pigs mimic the metabolic syndrome in humans. They exhibit hypertriglyceridaemia. Increased body weight and glucose levels |
Johansen et al., 2001 | ||
| APOCIII transgenic miniature pig | Standard diet | Similar to wild type | Similar to wild type | Similar to wild type | Similar to wild type | 94.9 ± 10.4 (1.1 ± 0.1) |
Model of hypertriglyceridaemia without any other abnormality in lipids. Body weight changes and glucose tolerance were not reported Low survival rates of the pigs may hamper their use |
Wei et al., 2012 | ||
| Farm pigs | HCD for 8 or 16 weeks | 10‐fold increase compared to normal fed | 10‐fold increase compared to normal fed | 20–80 (0.5–2) | N/A | N/A |
HDL‐c levels were inversely associated with the development of atherosclerotic lesions Body weight changes and glucose tolerance were not reported |
Pelosi et al., 2014 | ||
Note. Mice consume a low‐fat diet and this forms the basis for the formulation of the standard mouse or rodent chow containing 4% to 6% fat (fat weight per weight of diet) and a cholesterol content <0.02% (w/w). Western diet (WD) for mice consists of 21% milk fat and 0.2% cholesterol (0.15% added, 0.05% from milk fat). High cholesterol diet (HCD) for mice contains 1.25% cholesterol and 15.8% fat. Cholesterol‐enriched diet in rabbits consists of 1 g–2 g·kg−1 (0.1–0.2%) of normal chow and 6% corn oil.
TABLE 2.
Animal models of hypercholesterolaemia used in cardioprotection
| Animal model | Ischaemia/reperfusion protocol | Key findings | Reference |
|---|---|---|---|
| Mice | |||
| C57BL/6J WD for 14 weeks | 30 min ischaemia/2 hr reperfusion | Infarct size comparable to the mice fed on normal chow | Andreadou, Efentakis, et al., 2017 |
| C57BL/6J short‐(2 weeks) and long‐term (12 weeks) HCD | 30 min ischaemia/2 hr reperfusion | Short‐term HCD leads to similar infarct size in comparison to normal fed mice while 12 weeks diet decreases infarct size | Girod et al., 1999 |
| ApoE−/− on normal chow | 30 min ischaemia/2 hr reperfusion | Infarct size comparable to wild‐type littermates | Efentakis et al., 2017 |
| ApoE−/− HCD (2 weeks) | 30 min ischaemia/24 hr reperfusion | Increased myocardial infarct size in comparison to normal fed ApoE−/− | Scalia et al., 2001 |
| LDLR−/− on normal chow | 30 min ischaemia/2 hr reperfusion | This genetic mutation reduces infarct size in comparison with WT littermates | Girod et al., 1999 |
| LDLR−/− short‐(2 weeks) and long‐term (12 weeks) HCD | 30 min ischaemia/2 hr reperfusion | Short‐term diet increases infarct size while long‐term HCD attenuates myocardial necrosis | Girod et al., 1999 |
| Rats | |||
| Wistar rats HCD for 12 weeks | 30 min ischaemia/2 hr reperfusion | Infarct size comparable to normal healthy rats. Preconditioning and postconditioning‐mediated cardioprotection was abolished | Kupai et al., 2009 |
|
Wistar rats with insulin resistance High‐fat/cholesterol diet for 5 days |
30 min ischaemia/2 hr reperfusion | High‐fat/cholesterol diet did not affect infarct size | Adameová et al., 2007 Ferko et al., 2018 |
| Rabbits | |||
| New Zealand White rabbits cholesterol‐enriched diet for 6–8 weeks | 30 min ischaemia/3 hr reperfusion | Similar or slightly elevated infarct size compared to normal fed New Zealand White rabbits | Iliodromitis et al., 2006; Kremastinos et al., 2000; Andreadou et al., 2012 |
| Watanabe hereditary hypercholesterolaemic (WHHL) rabbit on standard laboratory diet | 30 min ischaemia/24 hr reperfusion | Infarct size in WHHL rabbit is greater than age‐matched New Zealand White rabbits | Hoshida et al., 1997 |
| Pigs | |||
|
Yucatan minipig 20 weeks old High‐fat/cholesterol diet for 1 month |
60 min ischaemia/2 hr reperfusion | Infarct size was higher in comparison to non‐hypecholesterolaemic animals | Osipov, Robich, et al., 2009 |
| Young healthy pigs HCD (20% beef tallow, 2% cholesterol, and 1% cholic acid) during 10 days | 90 min of ischaemia followed by 21 days of reperfusion | Infarct size was ~44% larger in comparison to non‐hypercholesterolaemic animals | Vilahur, Casani, Juan‐Babot, Guerra, & Badimon, 2012 |
Note. Infarct size has been measured by triphenyl tetrazolium chloride (TTC) staining.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
CONFLICT OF INTEREST
P.F. is a founder and CEO of Pharmahungary Group, a group of R&D companies.
ACKNOWLEDGEMENTS
This article is based upon work from COST Action EU‐CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). I.A. and P.E.N. were supported by Kleon Tsetis Foundation Scientific Research & Culture. P.F. was supported by the National Research, Development and Innovation Office of Hungary (Grant OTKA KH_17 125570), the National Heart Program NVKP (Grant 16‐1‐2016‐0017), and by the Higher Education Institutional Excellence Program of the Ministry of Human Capacities in Hungary, within the framework of the Therapeutic Development thematic program of the Semmelweis University. G.H. and P.K. are supported by the German Research Foundation (Grant SFB 1116 B8). L.B. and G.V. are supported by Plan Nacional de Salud (PNS) (Grants SAF2016‐76819‐R to L.B. and PGC2018‐094025‐B‐I00 to G.V.) and from the Spanish Ministry of Science and Innovation and funds FEDER “Una Manera de Hacer Europa.”
Andreadou I, Schulz R, Badimon L, et al. Hyperlipidaemia and cardioprotection: Animal models for translational studies. Br J Pharmacol. 2020;177:5287–5311. 10.1111/bph.14931
This article is part of a themed issue entitled “Risk factors, co‐morbidities, and co‐medications in cardioprotection” co‐edited by Peter Ferdinandy, Derek Hausenloy, Rainer Schulz, and Ioanna Andreadou.
Contributor Information
Ioanna Andreadou, Email: jandread@pharm.uoa.gr.
Péter Ferdinandy, Email: peter.ferdinandy@pharmahungary.com.
REFERENCES
- Adameová, A. , Kuzelová, M. , Andelová, E. , Faberová, V. , Pancza, D. , Svec, P. , … Ravingerová, T. (2007). Hypercholesterolemia abrogates an increased resistance of diabetic rat hearts to ischemia‐reperfusion injury. Molecular and Cellular Biochemistry, 295, 129–136. 10.1007/s11010-006-9282-8 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Keely, E. A. , Mathie, A. , Peter, J. A. , Veale, E. L. , Armstrong, J. H. , … GTP collaborators (2019). The concise guide to pharmacology 2019/2020: Introduction and other protein target. British Journal of Pharmacology, 176, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev, G. , & Burnstock, G. (1998). Watanabe rabbits with heritable hypercholesterolaemia: A model of atherosclerosis. Histology and Histopathology, 13, 797–817. 10.14670/HH-13.797 [DOI] [PubMed] [Google Scholar]
- Al‐Mashhadi, R. H. , Sørensen, C. B. , Kragh, P. M. , Christoffersen, C. , Mortensen, M. B. , Tolbod, L. P. , … Bentzon, J. F. (2013). Familial hypercholesterolemia and atherosclerosis in cloned minipigs created by DNA transposition of a human PCSK9 gain‐of‐function mutant. Science Translational Medicine, 5, 1–11, 166ra1. 10.1126/scitranslmed.3004853 [DOI] [PubMed] [Google Scholar]
- Amuzie, C. , Swart, J. R. , Rogers, C. S. , Vihtelic, T. , Denham, S. , & Mais, D. E. (2016). A translational model for diet‐related atherosclerosis: Effect of statins on hypercholesterolemia and atherosclerosis in a minipig. Toxicologic Pathology, 44, 442–449. 10.1177/0192623315622304 [DOI] [PubMed] [Google Scholar]
- Andreadou, I. , Efentakis, P. , Balafas, E. , Togliatto, G. , Davos, C. H. , Varela, A. , … Iliodromitis, E. K. (2017). Empagliflozin limits myocardial infarction in vivo and cell death in vitro: Role of STAT3, mitochondria, and redox aspects. Frontiers in Physiology, 8, 1–13, 1077. 10.3389/fphys.2017.01077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadou, I. , Farmakis, D. , Prokovas, E. , Sigala, F. , Zoga, A. , Spyridaki, K. , … Iliodromitis, E. K. (2012). Short‐term statin administration in hypercholesterolaemic rabbits resistant to postconditioning: Effects on infarct size, endothelial nitric oxide synthase, and nitro‐oxidative stress. Cardiovascular Research, 94, 501–509. 10.1093/cvr/cvs121 [DOI] [PubMed] [Google Scholar]
- Andreadou, I. , Iliodromitis, E. K. , Lazou, A. , Görbe, A. , Giricz, Z. , Schulz, R. , & Ferdinandy, P. (2017). Effect of hypercholesterolaemia on myocardial function, ischaemia‐reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. British Journal of Pharmacology, 174, 1555–1569. 10.1111/bph.13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbar, L. , Mahadevan, N. , & Balakumar, P. (2013). Fenofibrate attenuates impaired ischemic preconditioning‐mediated cardioprotection in the fructose‐fed hypertriglyceridemic rat heart. Naunyn Schmiedebergs Archives of Pharmacology, 386, 319–329. 10.1007/s00210-012-0830-3 [DOI] [PubMed] [Google Scholar]
- Badimon, J. J. , Badimon, L. , & Fuster, V. (1990). Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol‐fed rabbit. The Journal of Clinical Investigation, 85, 1234–1241. 10.1172/JCI114558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon, J. J. , Badimon, L. , Galvez, A. , Dische, R. , & Fuster, V. (1989). High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol‐fed rabbits. Laboratory Investigation, 60, 455–461. [PubMed] [Google Scholar]
- Badimon, L. (2001). Atherosclerosis and thrombosis: Lessons from animal models. Thrombosis and Haemostasis, 86, 356–365. [PubMed] [Google Scholar]
- Badimon, L. , Steele, P. , Badimon, J. J. , Bowie, E. J. , & Fuster, V. (1985). Aortic atherosclerosis in pigs with heterozygous von Willebrand disease. Comparison with homozygous von Willebrand and normal pigs. Arteriosclerosis, 5, 366–370. 10.1161/01.ATV.5.4.366 [DOI] [PubMed] [Google Scholar]
- Beaty, T. H. , Prenger, V. L. , Virgil, D. G. , Lewis, B. , Kwiterovich, P. O. , & Bachorik, P. S. (1992). A genetic model for control of hypertriglyceridemia and apolipoprotein B levels in the Johns Hopkins colony of St. Thomas Hospital rabbits. Genetics, 132, 1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierfuß, A. , Dietrich, H. , Kremser, C. , Hunjadi, M. , Ritsch, A. , Rülicke, T. , … Mern, D. S. (2017). Knockout of apolipoprotein E in rabbit promotes premature intervertebral disc degeneration: A new in vivo model for therapeutic approaches of spinal disc disorders. PLoS ONE, 12, 1–18, e0187564 10.1371/journal.pone.0187564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijland, S. , van den Berg, S. A. A. , Voshol, P. J. , van den Hoek, A. M. , Princen, H. M. G. , Havekes, L. M. , … Willems van Dijk, K. (2009). CETP does not affect triglyceride production or clearance in APOE*3‐Leiden mice. Journal of Lipid Research, 51(1), 97–102. 10.1194/jlr.M900186-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquist, S. , Ruotolo, G. , Tang, R. , Björkegren, J. , Bond, M. G. , de Faire, U. , … Hamsten, A. (1999). Alimentary lipemia, postprandial triglyceride‐rich lipoproteins, and common carotid intima‐media thickness in healthy, middle‐aged men. Circulation, 100, 723–728. 10.1161/01.cir.100.7.723 [DOI] [PubMed] [Google Scholar]
- Braun, A. , Trigatti, B. L. , Post, M. J. , Sato, K. , Simons, M. , Edelberg, J. M. , … Krieger, M. (2002). Loss of SR‐BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E‐deficient mice. Circulation Research, 90, 270–276. 10.1161/hh0302.104462 [DOI] [PubMed] [Google Scholar]
- Brulhart‐Meynet, M.‐C. , Braunersreuther, V. , Brinck, J. , Montecucco, F. , Prost, J.‐C. , Thomas, A. , … Frias, M. A. (2015). Improving reconstituted HDL composition for efficient post‐ischemic reduction of ischemia reperfusion injury. PLoS ONE, 10, 1–16, e0119664 10.1371/journal.pone.0119664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi, L. , Rossoni, G. , Gomaraschi, M. , Sisto, F. , Berti, F. , & Franceschini, G. (2003). High‐density lipoproteins protect isolated rat hearts from ischemia‐reperfusion injury by reducing cardiac tumor necrosis factor‐α content and enhancing prostaglandin release. Circulation Research, 92, 330–337. 10.1161/01.res.0000054201.60308.1a [DOI] [PubMed] [Google Scholar]
- Casani, L. , Sanchez‐Gomez, S. , Vilahur, G. , & Badimon, L. (2005). Pravastatin reduces thrombogenicity by mechanisms beyond plasma cholesterol lowering. Thrombosis and Haemostasis, 94, 1035–1041. 10.1160/TH05-04-0245 [DOI] [PubMed] [Google Scholar]
- Christoffersen, B. O. , Grand, N. , Golozoubova, V. , Svendsen, O. , & Raun, K. (2007). Gender‐associated differences in metabolic syndrome‐related parameters in Göttingen minipigs. Comparative Medicine, 57, 493–504. [PubMed] [Google Scholar]
- Csonka, C. , Kupai, K. , Bencsik, P. , Görbe, A. , Pálóczi, J. , Zvara, A. , … Ferdinandy, P. (2014). Cholesterol‐enriched diet inhibits cardioprotection by ATP‐sensitive K+ channel activators cromakalim and diazoxide. American Journal of Physiology. Heart and Circulatory Physiology, 306, H405–H413. 10.1152/ajpheart.00257.2013 [DOI] [PubMed] [Google Scholar]
- Csont, T. , Balogh, G. , Csonka, C. , Boros, I. , Horváth, I. , Vigh, L. , & Ferdinandy, P. (2002). Hyperlipidemia induced by high cholesterol diet inhibits heat shock response in rat hearts. Biochemical and Biophysical Research Communications, 290, 1535–1538. 10.1006/bbrc.2002.6377 [DOI] [PubMed] [Google Scholar]
- Csont, T. , Bereczki, E. , Bencsik, P. , Fodor, G. , Görbe, A. , Zvara, A. , … Ferdinandy, P. (2007). Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB‐100 transgenic mice. Cardiovascular Research, 76, 100–109. 10.1016/j.cardiores.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Dadabayev, A. R. , Yin, G. , Latchoumycandane, C. , McIntyre, T. M. , Lesnefsky, E. J. , & Penn, M. S. (2014). Apolipoprotein A1 regulates coenzyme Q10 absorption, mitochondrial function, and infarct size in a mouse model of myocardial infarction. The Journal of Nutrition, 144, 1030–1036. 10.3945/jn.113.184291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva, H. V. , Más‐Oliva, J. , Taylor, J. M. , & Mahley, R. W. (1994). Identification of apolipoprotein B‐100 low density lipoproteins, apolipoprotein B‐48 remnants, and apolipoprotein E‐rich high density lipoproteins in the mouse. Journal of Lipid Research, 35, 1297–1310. [PubMed] [Google Scholar]
- Desmarchelier, C. , Dahlhoff, C. , Keller, S. , Sailer, M. , Jahreis, G. , & Daniel, H. (2012). C57Bl/6 N mice on a western diet display reduced intestinal and hepatic cholesterol levels despite a plasma hypercholesterolemia. BMC Genomics, 13, 1–16, 84. 10.1186/1471-2164-13-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy, J. M. (1998). Dietary fatty acids and the regulation of plasma low density lipoprotein cholesterol concentrations. The Journal of Nutrition, 128, 444S–448S. 10.1093/jn/128.2.444S [DOI] [PubMed] [Google Scholar]
- Dillard, A. , Matthan, N. R. , & Lichtenstein, A. H. (2010). Use of hamster as a model to study diet‐induced atherosclerosis. Nutrition & Metabolism (London), 7, 1–12, 89. 10.1186/1743-7075-7-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumouchtsis, E. K. , Tzani, A. , Doulamis, I. P. , Konstantopoulos, P. , Laskarina‐Maria, K. , Agrogiannis, G. , … Perrea, D. N. (2017). Effect of saffron on metabolic profile and retina in apolipoprotein E–knockout mice fed a high‐fat diet. Journal of Dietary Supplements, 15, 471–481. 10.1080/19390211.2017.1356417 [DOI] [PubMed] [Google Scholar]
- Efentakis, P. , Rizakou, A. , Christodoulou, E. , Chatzianastasiou, A. , López, M. G. , León, R. , & Andreadou, I. (2017). Saffron (Crocus sativus) intake provides nutritional preconditioning against myocardial ischemia‐reperfusion injury in wild type and ApoE(−/−)mice: Involvement of Nrf2 activation. Nutrition Metabolism and Cardiovascular Diseases, 27, 919–929. 10.1016/j.numecd.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Emini Veseli, B. , Perrotta, P. , De Meyer, G. R. A. , Roth, L. , Van der Donckt, C. , Martinet, W. , & De Meyer, G. R. Y. (2017). Animal models of atherosclerosis. European Journal of Pharmacology, 816, 3–13. 10.1016/j.ejphar.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Fan, J. , & Watanabe, T. (2000). Cholesterol‐fed and transgenic rabbit models for the study of atherosclerosis. Journal of Atherosclerosis and Thrombosis, 7, 26–32. 10.5551/jat1994.7.26 [DOI] [PubMed] [Google Scholar]
- Fang, B. , Ren, X. , Wang, Y. , Li, Z. , Zhao, L. , Zhang, M. , … Dai, Y. (2018). Apolipoprotein E deficiency accelerates atherosclerosis development in miniature pigs. Disease Models & Mechanisms, 11, 1–8, dmm036632. 10.1242/dmm.036632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favari, E. , Chroni, A. , Tietge, U. J. F. , Zanotti, I. , Escolà‐Gil, J. C. , & Bernini, F. (2015). Cholesterol efflux and reverse cholesterol transport. Handbook of Experimental Pharmacology, 224, 181–206. 10.1007/978-3-319-09665-0_4 [DOI] [PubMed] [Google Scholar]
- Ferdinandy, P. , Hausenloy, D. J. , Heusch, G. , Baxter, G. F. , & Schulz, R. (2014). Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacological Reviews, 66, 1142–1174. 10.1124/pr.113.008300 [DOI] [PubMed] [Google Scholar]
- Ference, B. A. , Ginsberg, H. N. , Graham, I. , Ray, K. K. , Packard, C. J. , Bruckert, E. , … Catapano, A. L. (2017). Low‐density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. European Heart Journal, 38, 2459–2472. 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferko, M. , Farkasova, V. , Jasova, M. , Kancirova, I. , Ravingerova, T. , Duris Adameova, A. , … Waczulikova, I. (2018). Hypercholesterolemia antagonized heart adaptation and functional remodeling of the mitochondria observed in acute diabetes mellitus subjected to ischemia/reperfusion injury. Journal of Physiology and Pharmacology, 69, 685–697, 5. 10.26402/jpp.2018.5.03 [DOI] [PubMed] [Google Scholar]
- Fernandez, M. L. , & Volek, J. S. (2006). Guinea pigs: A suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutrition & Metabolism (London), 3, 1–6, 17. 10.1186/1743-7075-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias, M. A. , Pedretti, S. , Hacking, D. , Somers, S. , Lacerda, L. , Opie, L. H. , … Lecour, S. (2013). HDL protects against ischemia reperfusion injury by preserving mitochondrial integrity. Atherosclerosis, 228, 110–116. 10.1016/j.atherosclerosis.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Fuster, V. , Lie, J. T. , Badimon, L. , Rosemark, J. A. , Badimon, J. J. , & Bowie, E. J. (1985). Spontaneous and diet‐induced coronary atherosclerosis in normal swine and swine with von Willebrand disease. Arteriosclerosis, 5, 67–73. 10.1161/01.atv.5.1.67 [DOI] [PubMed] [Google Scholar]
- Getz, G. S. , & Reardon, C. A. (2006). Diet and murine atherosclerosis. Arteriosclerosis, Thrombosis and Vascular Biology, 26, 242–249. 10.1161/01.ATV.0000201071.49029.17 [DOI] [PubMed] [Google Scholar]
- Giricz, Z. , Koncsos, G. , Rajtík, T. , Varga, Z. V. , Baranyai, T. , Csonka, C. , … Ferdinandy, P. (2017). Hypercholesterolemia downregulates autophagy in the rat heart. Lipids in Health and Disease, 16, 1–8, 60. 10.1186/s12944-017-0455-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod, W. G. , Jones, S. P. , Sieber, N. , Aw, T. Y. , & Lefer, D. J. (1999). Effects of hypercholesterolemia on myocardial ischemia‐reperfusion injury in LDL receptor‐deficient mice. Arteriosclerosis, Thrombosis and Vascular Biology, 19, 2776–2781. 10.1161/01.atv.19.11.2776 [DOI] [PubMed] [Google Scholar]
- Gomaraschi, M. , Calabresi, L. , Rossoni, G. , Iametti, S. , Franceschini, G. , Stonik, J. A. , & Remaley, A. T. (2008). Anti‐inflammatory and cardioprotective activities of synthetic high‐density lipoprotein containing apolipoprotein A‐I mimetic peptides. The Journal of Pharmacology and Experimental Therapeutics, 324, 776–783. 10.1124/jpet.107.129411 [DOI] [PubMed] [Google Scholar]
- Görbe, A. , Varga, Z. V. , Kupai, K. , Bencsik, P. , Kocsis, G. F. , Csont, T. , … Ferdinandy, P. (2011). Cholesterol diet leads to attenuation of ischemic preconditioning‐induced cardiac protection: The role of connexin 43. American Journal of Physiology. Heart and Circulatory Physiology, 300, H1907–H1913. 10.1152/ajpheart.01242.2010 [DOI] [PubMed] [Google Scholar]
- Gu, S.‐S. , Shi, N. , & Wu, M.‐P. (2007). The protective effect of apolipoproteinA‐I on myocardial ischemia‐reperfusion injury in rats. Life Sciences, 81, 702–709. 10.1016/j.lfs.2007.06.021 [DOI] [PubMed] [Google Scholar]
- Hamamdzic, D. , & Wilensky, R. L. (2013). Porcine models of accelerated coronary atherosclerosis: Role of diabetes mellitus and hypercholesterolemia. Journal of Diabetes Research, 2013, 1–7, 761415. 10.1155/2013/761415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler‐Rapacz, J. O. , Nichols, T. C. , Griggs, T. R. , Bellinger, D. A. , & Rapacz, J. (1994). Familial and diet‐induced hypercholesterolemia in swine. Lipid, ApoB, and ApoA‐I concentrations and distributions in plasma and lipoprotein subfractions. Arteriosclerosis and Thrombosis, 14, 923–930. 10.1161/01.ATV.14.6.923 [DOI] [PubMed] [Google Scholar]
- Hausenloy, D. J. , Garcia‐Dorado, D. , Bøtker, H. E. , Davidson, S. M. , Downey, J. , Engel, F. B. , … Ferdinandy, P. (2017). Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovascular Research, 113, 564–585. 10.1093/cvr/cvx049 [DOI] [PubMed] [Google Scholar]
- Herrera, V. L. M. , Makrides, S. C. , Xie, H. X. , Adari, H. , Krauss, R. M. , Ryan, U. S. , & Ruiz‐Opazo, N. (1999). Spontaneous combined hyperlipidemia, coronary heart disease and decreased survival in Dahl salt‐sensitive hypertensive rats transgenic for human cholesteryl ester transfer protein. Nature Medicine, 5, 1383–1389. 10.1038/70956 [DOI] [PubMed] [Google Scholar]
- Heusch, G. (2013). Cardioprotection: Chances and challenges of its translation to the clinic. The Lancet, 381, 166–175. 10.1016/S0140-6736(12)60916-7 [DOI] [PubMed] [Google Scholar]
- Heusch, G. (2017). Critical issues for the translation of cardioprotection. Circulation Research, 120, 1477–1486. 10.1161/CIRCRESAHA.117.310820 [DOI] [PubMed] [Google Scholar]
- Heywood, S. E. , Richart, A. L. , Henstridge, D. C. , Alt, K. , Kiriazis, H. , Zammit, C. , … Kingwell, B. A. (2017). High‐density lipoprotein delivered after myocardial infarction increases cardiac glucose uptake and function in mice. Science Translational Medicine, 9(411), 1–10, eaam6084. 10.1126/scitranslmed.aam6084 [DOI] [PubMed] [Google Scholar]
- Hirano, T. , Mamo, J. C. , Poapst, M. E. , Kuksis, A. , & Steiner, G. (1989). Impaired very low‐density lipoprotein‐triglyceride catabolism in acute and chronic fructose‐fed rats. American Journal of Physiology, 256, 559–565. [DOI] [PubMed] [Google Scholar]
- Hoekstra, M. , &, Van Eck, M. (2015). Mouse models of disturbed HDL metabolism. Handbook of Experimental Pharmacology , 224, 301–336. 10.1007/978-3-319-09665-0_9 [DOI] [PubMed] [Google Scholar]
- Hoshida, S. , Yamashita, N. , Igarashi, J. , Aoki, K. , Kuzuya, T. , & Hori, M. (1997). Long‐term probucol treatment reverses the severity of myocardial injury in Watanabe heritable hyperlipidemic rabbits. Arteriosclerosis, Thrombosis and Vascular Biology, 17, 2801–2807. 10.1161/01.ATV.17.11.2801 [DOI] [PubMed] [Google Scholar]
- Iliodromitis, E. K. , Andreadou, I. , Prokovas, E. , Zoga, A. , Farmakis, D. , Fotopoulou, T. , … Kremastinos, D. T. (2010). Simvastatin in contrast to postconditioning reduces infarct size in hyperlipidemic rabbits: Possible role of oxidative/nitrosative stress. Basic Research in Cardiology, 105, 193–203. 10.1007/s00395-009-0078-3 [DOI] [PubMed] [Google Scholar]
- Iliodromitis, E. K. , Zoga, A. , Vrettou, A. , Andreadou, I. , Paraskevaidis, I. A. , Kaklamanis, L. , & Kremastinos, D. T. (2006). The effectiveness of postconditioning and preconditioning on infarct size in hypercholesterolemic and normal anesthetized rabbits. Atherosclerosis, 188, 356–362. 10.1016/j.atherosclerosis.2005.11.023 [DOI] [PubMed] [Google Scholar]
- Imaizumi, S. , Miura, S. , Nakamura, K. , Kiya, Y. , Uehara, Y. , Zhang, B. , … Saku, K. (2008). Antiarrhythmogenic effect of reconstituted high‐density lipoprotein against ischemia/reperfusion in rats. Journal of the American College of Cardiology, 51, 1604–1612. 10.1016/j.jacc.2007.12.040 [DOI] [PubMed] [Google Scholar]
- Ito, Y. , Azrolan, N. , O'Connell, A. , Walsh, A. , & Breslow, J. (1990). Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science, 249, 790–793. 10.1126/science.2167514 [DOI] [PubMed] [Google Scholar]
- Jang, H.‐H. , Park, M.‐Y. , Kim, H.‐W. , Lee, Y.‐M. , Hwang, K.‐A. , Park, J.‐H. , … Kwon, O. (2012). Black rice (Oryza sativa L.) extract attenuates hepatic steatosis in C57BL/6 J mice fed a high‐fat diet via fatty acid oxidation. Nutrition & Metabolism, 9, 1–11, 27. 10.1186/1743-7075-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, F. , Jones, G. T. , Husband, A. J. , & Dusting, G. J. (2003). Cardiovascular protective effects of synthetic isoflavone derivatives in apolipoprotein E‐deficient mice. Journal of Vascular Research, 40, 276–284. 10.1159/000071891 [DOI] [PubMed] [Google Scholar]
- Jiang, X. C. , Masucci‐Magoulas, L. , Mar, J. , Lin, M. , Walsh, A. , Breslow, J. L. , & Tall, A. (1993). Down‐regulation of mRNA for the low density lipoprotein receptor in transgenic mice containing the gene for human cholesteryl ester transfer protein. Mechanism to explain accumulation of lipoprotein B particles. Journal of Biological Chemistry, 268, 27406–27412. [PubMed] [Google Scholar]
- Johansen, T. , Hansen, H. S. , Richelsen, B. , & Malmlöf, R. (2001). The obese Göttingen minipig as a model of the metabolic syndrome: Dietary effects on obesity, insulin sensitivity, and growth hormone profile. Comparative Medicine, 51, 150–155. [PubMed] [Google Scholar]
- Jong, M. C. , van Ree, J. H. , Dahlmans, V. E. H. , Frants, R. R. , Hofker, M. H. , & Havekes, L. M. (1997). Reduced very‐low‐density lipoprotein fractional catabolic rate in apolipoprotein C1‐deficient mice. Biochemical Journal, 321, 445–450. 10.1042/bj3210445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris, I. , Zand, T. , Nunnari, J. J. , Krolikowski, F. J. , & Majno, G. (1983). Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. The Americal Journal of Pathology, 113, 341–358. [PMC free article] [PubMed] [Google Scholar]
- Karliner, J. S. (2013). Sphingosine kinase and sphingosine 1‐phosphate in the heart: A decade of progress. Biochimica et Biophysica Acta, 1831, 203–212. 10.1016/j.bbalip.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, T. , Ito, T. , Ohwada, K. , Mera, Y. , Matsushita, M. , & Tomoike, H. (2006). Hereditary postprandial hypertriglyceridemic rabbit exhibits insulin resistance and central obesity: A novel model of metabolic syndrome. Arteriosclerosis Thrombosis and Vascular Biology, 26, 2752–2757. 10.1161/01.ATV.0000245808.12493.40 [DOI] [PubMed] [Google Scholar]
- Kawashima, Y. , Eguchi, Y. , Yamazaki, T. , Karahashi, M. , Kawai, H. , & Kudo, N. (2018). Reduction in secretion of very low density lipoprotein‐triacylglycerol by a matrix metalloproteinase inhibitor in a rat model of diet‐induced hypertriglyceridemia. The Journal of Pharmacology and Experimental Therapeutics, 366, 194–204. 10.1124/jpet.117.246165 [DOI] [PubMed] [Google Scholar]
- Keul, P. , Polzin, A. , Kaiser, K. , Gräler, M. , Dannenberg, L. , Daum, G. , … Levkau, B. (2019). Potent anti‐inflammatory properties of HDL in vascular smooth muscle cells mediated by HDL‐S1P and their impairment in coronary artery disease due to lower HDL‐S1P: A new aspect of HDL dysfunction and its therapy. The FASEB Journal, 33, 1482–1495. 10.1096/fj.201801245R [DOI] [PubMed] [Google Scholar]
- Keul, P. , van Borren, M. M. G. J. , Ghanem, A. , Müller, F. U. , Baartscheer, A. , Verkerk, A. O. , … Levkau, B. (2016). Sphingosine‐1‐phosphate receptor 1 regulates cardiac function by modulating Ca2+ sensitivity and Na+/H+ exchange and mediates protection by ischemic preconditioning. Journal of the American Heart Association, 5, 1–20, 5. 10.1161/JAHA.116.003393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiya, Y. , Miura, S.‐I. , Imaizumi, S. , Uehara, Y. , Matsuo, Y. , Abe, S. , … Saku, K. (2009). Reconstituted high‐density lipoprotein attenuates postinfarction left ventricular remodeling in rats. Atherosclerosis, 203, 137–144. 10.1016/j.atherosclerosis.2008.05.056 [DOI] [PubMed] [Google Scholar]
- Kleinert, M. , Clemmensen, C. , Hofmann, S. M. , Moore, M. C. , Renner, S. , Woods, S. C. , … Tschöp, M. H. (2018). Animal models of obesity and diabetes mellitus. Nature Reviews Endocrinology, 14, 140–162. 10.1038/nrendo.2017.161 [DOI] [PubMed] [Google Scholar]
- Kostogrys, R. B. , Franczyk‐Żarów, M. , Maślak, E. , Gajda, M. , Mateuszuk, L. , Jackson, C. L. , & Clopicki, S. (2012). Low carbohydrate, high protein diet promotes atherosclerosis in apolipoprotein E/low‐density lipoprotein receptor double knockout mice (apoE/LDLR(−/−)). Atherosclerosis, 223, 327–331. 10.1016/j.atherosclerosis.2012.05.024 [DOI] [PubMed] [Google Scholar]
- Kovár, J. , Tonar, Z. , Heczková, M. , & Poledne, R. (2009). Prague hereditary hypercholesterolemic (PHHC) rat—A model of polygenic hypercholesterolemia. Physiological Research, 58(Suppl 2), S95–S99. [DOI] [PubMed] [Google Scholar]
- Kremastinos, D. T. , Bofilis, E. , Karavolias, G. K. , Papalois, A. , Kaklamanis, L. , & Iliodromitis, E. K. (2000). Preconditioning limits myocardial infarct size in hypercholesterolemic rabbits. Atherosclerosis, 150, 81–89. 10.1016/s0021-9150(99)00389-5 [DOI] [PubMed] [Google Scholar]
- Kupai, K. , Csonka, C. , Fekete, V. , Odendaal, L. , van Rooyen, J. , Marais, D. W. , … Ferdinandy, P. (2009). Cholesterol diet‐induced hyperlipidemia impairs the cardioprotective effect of postconditioning: Role of peroxynitrite. American Journal of Physiology Heart and Circulatory Physiology, 297, H1729–H1735. 10.1152/ajpheart.00484.2009 [DOI] [PubMed] [Google Scholar]
- Leppänen, P. , Luoma, J. S. , Hofker, M. H. , Havekes, L. M. , & Ylä‐Herttuala, S. (1998). Characterization of atherosclerotic lesions in apo E3‐leiden transgenic mice. Atherosclerosis, 136, 147–152. 10.1016/s0021-9150(97)00196-2 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Fuchimoto, D. , Sudo, M. , Haruta, H. , Lin, Q.‐F. , Takayama, T. , … Onishi, A. (2016). Development of human‐like advanced coronary plaques in low‐density lipoprotein receptor knockout pigs and justification for statin treatment before formation of atherosclerotic plaques. Journal of the American Heart Association, 5, 1–15, e002779 10.1161/JAHA.115.002779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, J. , Guo, X. , Wang, M. , Dong, C. , Gao, M. , Wang, H. , … Liu, G. (2017). Scavenger receptor class B type 1 deletion led to coronary atherosclerosis and ischemic heart disease in low‐density lipoprotein receptor knockout mice on modified Western‐type diet. Journal of Atherosclerosis and Thrombosis, 24, 133–146. 10.5551/jat.33019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordan, S. , Mackrill, J. J. , & O'Brien, N. M. (2009). Oxysterols and mechanisms of apoptotic signaling: Implications in the pathology of degenerative diseases. TheJournal of Nutritional Biochemistry, 20, 321–336. 10.1016/j.jnutbio.2009.01.001 [DOI] [PubMed] [Google Scholar]
- Lu, R. , Yuan, T. , Wang, Y. , Zhang, T. , Yuan, Y. , Wu, D. , … Cheng, Y. (2018). Spontaneous severe hypercholesterolemia and atherosclerosis lesions in rabbits with deficiency of low‐density lipoprotein receptor (LDLR) on exon 7. eBioMedicine, 36, 29–38. 10.1016/j.ebiom.2018.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Wang, W. , Zhang, J. , Lu, Y. , Wu, W. , Yan, H. , & Wang, Y. (2012). Hyperlipidemia and atherosclerotic lesion development in Ldlr‐deficient mice on a long‐term high‐fat diet. PLoS ONE, 7, 1–8, e35835 10.1371/journal.pone.0035835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangat, R. , Su, J. , Scott, P. G. , Russell, J. C. , Vine, D. F. , & Proctor, S. D. (2007). Chylomicron and apoB48 metabolism in the JCR: LA corpulent rat, a model for the metabolic syndrome. Biochemical Society Transactions, 35, 477–481. 10.1042/BST0350477 [DOI] [PubMed] [Google Scholar]
- Marchesi, M. , Booth, E. A. , Davis, T. , Bisgaier, C. L. , & Lucchesi, B. R. (2004). Apolipoprotein A‐IMilano and 1‐palmitoyl‐2‐oleoyl phosphatidylcholine complex (ETC‐216) protects the in vivo rabbit heart from regional ischemia‐reperfusion injury. The Journal of Pharmacology and Experimental Therapeutics, 311, 1023–1031. 10.1124/jpet.104.070789 [DOI] [PubMed] [Google Scholar]
- Marchesi, M. , Booth, E. A. , Rossoni, G. , García, R. A. , Hill, K. R. , Sirtori, C. R. , … Lucchesi, B. R. (2008). Apolipoprotein A‐IMilano/POPC complex attenuates post‐ischemic ventricular dysfunction in the isolated rabbit heart. Atherosclerosis, 197, 572–578. 10.1016/j.atherosclerosis.2007.08.028 [DOI] [PubMed] [Google Scholar]
- Marotti, K. R. , Castle, C. K. , Murray, R. W. , Rehberg, E. F. , Polites, H. G. , & Melchior, G. W. (1992). The role of cholesteryl ester transfer protein in primate apolipoprotein A‐I metabolism. Insights from studies with transgenic mice. Arteriosclerosis and Thrombosis, 12, 736–744. 10.1161/01.ATV.12.6.736 [DOI] [PubMed] [Google Scholar]
- Mastrocola, R. , Collino, M. , Penna, C. , Nigro, D. , Chiazza, F. , Fracasso, V. , … Aragno, M. (2016). Maladaptive modulations of NLRP3 inflammasome and cardioprotective pathways are involved in diet‐induced exacerbation of myocardial ischemia/reperfusion injury in mice. Oxidative Medicine and Cellular Longevity, 2016, 1–12, 3480637 10.1155/2016/3480637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir, K. S. , & Leitersdorf, E. (2004). Atherosclerosis in the apolipoprotein‐E‐deficient mouse: A decade of progress. Arteriosclerosis, Thrombosis and Vascular Biology, 24, 1006–1014. 10.1161/01.ATV.0000128849.12617.f4 [DOI] [PubMed] [Google Scholar]
- Meyrelles, S. S. , Peotta, V. A. , Pereira, T. M. , & Vasquez, E. C. (2011). Endothelial dysfunction in the apolipoprotein E‐deficient mouse: Insights into the influence of diet, gender and aging. Lipids in Health and Disease, 10, 1–19, 211. 10.1186/1476-511X-10-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. , Stone, N. J. , Ballantyne, C. , Bittner, V. , Criqui, M. H. , Ginsberg, H. N. , … Pennathur, S. (2011). Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation, 123, 2292–2333. 10.1161/CIR.0b013e3182160726 [DOI] [PubMed] [Google Scholar]
- Mochizuki, S. , Okumura, M. , Tanaka, F. , Sato, T. , Kagami, A. , Tada, N. , & Nagano, M. (1991). Ischemia‐reperfusion arrhythmias and lipids: Effect of human high‐ and low‐density lipoproteins on reperfusion arrhythmias. Cardiovascular Drugs and Therapy, 5(Suppl 2), 269–276. [DOI] [PubMed] [Google Scholar]
- Nakashima, Y. , Plump, A. S. , Raines, E. W. , Breslow, J. L. , & Ross, R. (1994). ApoE‐deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arteriosclerosis and Thrombosis, 14, 133–140. 10.1161/01.atv.14.1.133 [DOI] [PubMed] [Google Scholar]
- Neeb, Z. P. , Edwards, J. M. , Alloosh, M. , Long, X. , Mokelke, E. A. , & Sturek, M. (2010). Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comparative Medicine, 60, 300–315. [PMC free article] [PubMed] [Google Scholar]
- Ngai, Y. F. , Quong, W. L. , Glier, M. B. , Glavas, M. M. , Babich, S. L. , Innis, S. M. , … Gibson, W. T. (2010). Ldlr−/− mice display decreased susceptibility to Western‐type diet‐induced obesity due to increased thermogenesis. Endocrinology, 151, 5226–5236. 10.1210/en.2010-0496 [DOI] [PubMed] [Google Scholar]
- Niimi, M. , Yang, D. , Kitajima, S. , Ning, B. , Wang, C. , Li, S. , … Fan, J. (2016). ApoE knockout rabbits: A novel model for the study of human hyperlipidemia. Atherosclerosis, 245, 187–193. 10.1016/j.atherosclerosis.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Ning, B. , Wang, X. , Yu, Y. , Waqar, A. B. , Yu, Q. , Koike, T. , … Fan, J. (2015). High‐fructose and high‐fat diet‐induced insulin resistance enhances atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Nutrition & Metabolism (London), 12, 1–11, 30. 10.1186/s12986-015-0024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipov, R. M. , Bianchi, C. , Feng, J. , Clements, R. T. , Liu, Y. , Robich, M. P. , … Sellke, F. W. (2009). Effect of hypercholesterolemia on myocardial necrosis and apoptosis in the setting of ischemia‐reperfusion. Circulation, 120, S22–S30. 10.1161/CIRCULATIONAHA.108.842724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipov, R. M. , Robich, M. P. , Feng, J. , Clements, R. T. , Liu, Y. , Glazer, H. P. , … Sellke, F. W. (2009). Effect of thrombin fragment (TP508) on myocardial ischemia‐reperfusion injury in hypercholesterolemic pigs. Journal of Applied Physiology (Bethesda, MD: 1985), 106, 1993–2001. 10.1152/japplphysiol.00071.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padró, T. , Cubedo, J. , Camino, S. , Béjar, M. T. , Ben‐Aicha, S. , Mendieta, G. , … Vilahur, G. (2017). Detrimental effect of hypercholesterolemia on high‐density lipoprotein particle remodeling in pigs. Journal of the American College of Cardiology, 70, 165–178. 10.1016/j.jacc.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Paigen, B. , Morrow, A. , Brandon, C. , Mitchell, D. , & Holmes, P. (1985). Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis, 57, 65–73. 10.1016/0021-9150(85)90138-8 [DOI] [PubMed] [Google Scholar]
- Palazón, C. P. , Alfón, J. , Gaffney, P. , Berrozpe, M. , Royo, T. , & Badimon, L. (1998). Effects of reducing LDL and increasing HDL with gemfibrozil in experimental coronary lesion development and thrombotic risk. Atherosclerosis, 136, 333–345. Erratum in: Atherosclerosis,139, 415. 10.1016/s0021-9150(97)00236-0 [DOI] [PubMed] [Google Scholar]
- Paradis, S. , Leoni, V. , Caccia, C. , Berdeaux, A. , & Morin, D. (2013). Cardioprotection by the TSPO ligand 4′‐chlorodiazepam is associated with inhibition of mitochondrial accumulation of cholesterol at reperfusion. Cardiovascular Research, 98, 420–427. 10.1093/cvr/cvt079 [DOI] [PubMed] [Google Scholar]
- Pedretti, S. , Brulhart‐Meynet, M.‐C. , Montecucco, F. , Lecour, S. , James, R. W. , & Frias, M. A. (2019). HDL protects against myocardial ischemia reperfusion injury via miR‐34b and miR‐337 expression which requires STAT3. PLoS ONE, 14, 1–19, e0218432 10.1371/journal.pone.0218432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi, G. , Puntoni, M. , Viglione, F. , Bernini, F. , Trivella, M. G. , & Parodi, O. (2014). HDL‐mediated atheroprotection in a high cholesterol diet swine model by quantitative (immuno)histology coronary profiling. Atherosclerosis, 235, 84–191, e103 10.1016/j.atherosclerosis.2014.05.277 24819747 [DOI] [Google Scholar]
- Pennacchio, L. A. , Olivier, M. , Hubacek, J. A. , Cohen, J. C. , Cox, D. R. , Fruchart, J. C. , … Robin, E. M. (2001). An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science, 294, 169–173. 10.1126/science.1064852 [DOI] [PubMed] [Google Scholar]
- Perman, J. C. , Boström, P. , Lindbom, M. , Lidberg, U. , StÅhlman, M. , Hägg, D. , … Boren, J. (2011). The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. The Journal of Clinical Investigation, 121, 2625–2640. 10.1172/JCI43068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poledne, R. , & Jurčíková‐Novotná, L. (2017). Experimental models of hyperlipoproteinemia and atherosclerosis. Physiological Research, 66, S69–S75. [DOI] [PubMed] [Google Scholar]
- Qu, S. , Perdomo, G. , Su, D. , D'Souza, F. M. , Shachter, N. S. , & Dong, H. H. (2007). Effects of apoA‐V on HDL and VLDL metabolism in APOC3 transgenic mice. Journal of Lipid Research, 48, 1476–1487. 10.1194/jlr.M600498-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja, B. , Saravanakumar, M. , & Sathya, G. (2012). Veratric acid ameliorates hyperlipidemia and oxidative stress in Wistar rats fed an atherogenic diet. Molecular and Cellular Biochemistry, 366, 21–30. 10.1007/s11010-012-1278-y [DOI] [PubMed] [Google Scholar]
- Reimer, R. A. , & Russell, J. C. (2008). Glucose tolerance, lipids, and GLP‐1 secretion in JCR: LA‐cp rats fed a high protein fiber diet. Obesity (Silver Spring), 16, 40–46. 10.1038/oby.2007.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, A. , & Thompson, J. S. (1976). Inbred mice and their hypbrids as an animal model for atherosclerosis research. Advances in Experimental Medicine and Biology, 67, 313–327. 10.1007/978-1-4614-4618-7_18 [DOI] [PubMed] [Google Scholar]
- Romain, C. , Piemontese, A. , Battista, S. , Bernini, F. , Ossoli, A. , Strazzella, A. , … Zanotti, I. (2018). Anti‐atherosclerotic effect of a polyphenol‐rich ingredient, Oleactiv®, in a hypercholesterolemia‐induced Golden Syrian hamster model. Nutrients, 10, 1–13, 1511. 10.3390/nu10101511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoni, G. , Gomaraschi, M. , Berti, F. , Sirtori, C. R. , Franceschini, G. , & Calabresi, L. (2004). Synthetic high‐density lipoproteins exert cardioprotective effects in myocardial ischemia/reperfusion injury. Journal of Pharmacology and Experimental Therapeutics, 308, 79–84. 10.1124/jpet.103.057141 [DOI] [PubMed] [Google Scholar]
- Sattler, K. , Gräler, M. , Keul, P. , Weske, S. , Reimann, C.‐M. , Jindrová, H. , … Levkau, B. (2015). Defects of high‐density lipoproteins in coronary artery disease caused by low sphingosine‐1‐phosphate content: Correction by sphingosine‐1‐phosphate‐loading. Journal of the American College of Cardiology, 66, 1470–1485. 10.1016/j.jacc.2015.07.057 [DOI] [PubMed] [Google Scholar]
- Sattler, K. , Lehmann, I. , Gräler, M. , Bröcker‐Preuss, M. , Erbel, R. , Heusch, G. , & Levkau, B. (2014). HDL‐bound sphingosine 1‐phosphate (S1P) predicts the severity of coronary artery atherosclerosis. Cellular Physiology and Biochemistry, 34, 172–184. 10.1159/000362993 [DOI] [PubMed] [Google Scholar]
- Sattler, K. J. E. , Herrmann, J. , Yün, S. , Lehmann, N. , Wang, Z. , Heusch, G. , … Levkau, B. (2009). High high‐density lipoprotein‐cholesterol reduces risk and extent of percutaneous coronary intervention‐related myocardial infarction and improves long‐term outcome in patients undergoing elective percutaneous coronary intervention. European Heart Journal, 30, 1894–1902. 10.1093/eurheartj/ehp183 [DOI] [PubMed] [Google Scholar]
- Scalia, R. , Gooszen, M. E. , Jones, S. P. , Hoffmeyer, M. , Rimmer, D. M. 3rd , Trocha, S. D. , … Lefer, D. J. (2001). Simvastatin exerts both anti‐inflammatory and cardioprotective effects in apolipoprotein E‐deficient mice. Circulation, 103, 2598–2603. 10.1161/01.cir.103.21.2598 [DOI] [PubMed] [Google Scholar]
- Shim, J. , Al‐Mashhadi, R. H. , Sørensen, C. B. , & Bentzon, J. F. (2016). Large animal models of atherosclerosis—New tools for persistent problems in cardiovascular medicine. The Journal of Pathology, 238, 257–266. 10.1002/path.4646 [DOI] [PubMed] [Google Scholar]
- Shiomi, M. , Koike, T. , & Ito, T. (2013). Contribution of the WHHL rabbit, an animal model of familial hypercholesterolemia, to elucidation of the anti‐atherosclerotic effects of statins. Atherosclerosis, 231, 39–47. 10.1016/j.atherosclerosis.2013.08.030 [DOI] [PubMed] [Google Scholar]
- Sithu, S. D. , Malovichko, M. V. , Riggs, K. A. , Wickramasinghe, N. S. , Winner, M. G. , Agarwal, A. , … Srivastava, S. (2017). Atherogenesis and metabolic dysregulation in LDL receptor‐knockout rats. Journal of Clinical Investigation Insight, 2, 1–15, 9. 10.1172/jci.insight.86442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, A. A. S. , Carvalho, L. S. F. , Bonilha, I. , Virginio, V. W. , Nadruz Junior, W. , Coelho‐Filho, O. R. , … Brasilia Heart Study Group (2019). Adverse interaction between HDL and the mass of myocardial infarction. Atherosclerosis, 281, 9–16. 10.1016/j.atherosclerosis.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Szilvassy, Z. , Ferdinandy, P. , Szilvassy, J. , Nagy, I. , Karcsu, S. , Lonovics, J. , … Koltai, M. (1995). The loss of pacing‐induced preconditioning in atherosclerotic rabbits: Role of hypercholesterolaemia. Journal of Molecular and Cellular Cardiology, 27, 2559–2569. 10.1006/jmcc.1995.0043 [DOI] [PubMed] [Google Scholar]
- Temel, R. , & Rudel, L. (2007). Diet effects on atherosclerosis in mice. Current Drug Targets, 8, 1150–1160. 10.2174/138945007782403847 [DOI] [PubMed] [Google Scholar]
- Theilmeier, G. , Schmidt, C. , Herrmann, J. , Keul, P. , Schäfers, M. , Herrgott, I. , … Levkau, B. (2006). High‐density lipoproteins and their constituent, sphingosine‐1‐phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation, 114, 1403–1409. 10.1161/CIRCULATIONAHA.105.607135 [DOI] [PubMed] [Google Scholar]
- Thim, T. , Hagensen, M. , Drouet, L. , Bal Dit Sollier, C. , Bonneau, M. , Granada, J. , … Falk, E. (2010). Familial hypercholesterolaemic downsized pig with human‐like coronary atherosclerosis: A model for preclinical studies. EuroIntervention, 6, 261–268. 10.4244/EIJV6I2A42 [DOI] [PubMed] [Google Scholar]
- Timmis, A. , Townsend, N. , Gale, C. , Grobbee, R. , Maniadakis, N. , Flather, M. , … ESC Scientific Document Group (2018). European Society of Cardiology: Cardiovascular disease statistics 2017. European Heart Journal, 39, 508–579. 10.1093/eurheartj/ehx628 [DOI] [PubMed] [Google Scholar]
- Trask, A. J. , Katz, P. S. , Kelly, A. P. , Galantowicz, M. L. , Cismowski, M. J. , West, T. A. , … Luccessi, P. A. (2012). Dynamic micro‐ and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. Journal of Applied Physiology, 113, 1128–1140. 10.1152/japplphysiol.00604.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi, K. , Hagi, A. , & Inoue, Y. (2001). The relationship between plasma high density lipoprotein cholesterol levels and cholesteryl ester transfer protein activity in six species of healthy experimental animals. Biological and Pharmaceutical Bulletin, 24, 579–581. 10.1248/bpb.24.579 [DOI] [PubMed] [Google Scholar]
- Vaidya, M. , Jentsch, J. A. , Peters, S. , Keul, P. , Weske, S. , Gräler, M. H. , … Levkau, B. (2019). Regulation of ABCA1‐mediated cholesterol efflux by sphingosine‐1‐phosphate signaling in macrophages. Journal of Lipid Research, 60, 506–515. 10.1194/jlr.M088443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilahur, G. , Casani, L. , Juan‐Babot, O. , Guerra, J. M. , & Badimon, L. (2012). Infiltrated cardiac lipids impair myofibroblast‐induced healing of the myocardial scar post‐myocardial infarction. Atherosclerosis, 224, 368–376. 10.1016/j.atherosclerosis.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Vilahur, G. , Gutiérrez, M. , Casaní, L. , Cubedo, J. , Capdevila, A. , Pons‐Llado, G. , … Badimon, L. (2015). Hypercholesterolemia abolishes high‐density lipoprotein‐related cardioprotective effects in the setting of myocardial infarction. Journal of the American College of Cardiology, 66, 2469–2470. 10.1016/j.jacc.2015.08.901 [DOI] [PubMed] [Google Scholar]
- Vilahur, G. , Padro, T. , & Badimon, L. (2011). Atherosclerosis and thrombosis: Insights from large animal models. Journal of Biomedicine and Biotechnology, 2011, 1–12. 10.1155/2011/907575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilahur, G. , Padró, T. , Casaní, L. , Mendieta, G. , López, J. A. , Streitenberger, S. , & Badimon, L. (2015). Polyphenol‐enriched diet prevents coronary endothelial dysfunction by activating the Akt/eNOS pathway. Revista Española de Cardiología (English Edition), 68, 216–225. 10.1016/j.rec.2014.04.021 [DOI] [PubMed] [Google Scholar]
- Vrána, A. , & Kazdová, L. (1990). The hereditary hypertriglyceridemic nonobese rat: An experimental model of human hypertriglyceridemia. Transplantation Proceedings, 22, 2579. [PubMed] [Google Scholar]
- Wei, J. , Ouyang, H. , Wang, Y. , Pang, D. , Cong, N. X. , Wang, T. , … Deng, X. (2012). Characterization of a hypertriglyceridemic transgenic miniature pig model expressing human apolipoprotein CIII. The FEBS Journal, 279, 91–99. 10.1111/j.1742-4658.2011.08401.x [DOI] [PubMed] [Google Scholar]
- Wei, S. , Zhang, Y. , Su, L. , He, K. , Wang, Q. , Zhang, Y. , … Ma, S. (2015). Apolipoprotein E‐deficient rats develop atherosclerotic plaques in partially ligated carotid arteries. Atherosclerosis, 243, 589–592. 10.1016/j.atherosclerosis.2015.10.093 [DOI] [PubMed] [Google Scholar]
- Wong, S. K. , Chin, K.‐Y. , Suhaimi, F. H. , Fairus, A. , & Ima‐Nirwana, S. (2016). Animal models of metabolic syndrome: A review. Nutrition & Metabolism, 13, 1–12, 65. 10.1186/s12986-016-0123-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudberg, N. J. , Pedretti, S. , Lecour, S. , Schulz, R. , Vuilleumier, N. , James, R. W. , & Frias, M. A. (2017). Pharmacological intervention to modulate HDL: What do we target? Frontiers in Pharmacology, 8, 1–16, 989. 10.3389/fphar.2017.00989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagyu, H. , Lutz, E. P. , Kako, Y. , Marks, S. , Hu, Y. , Choi, S. Y. , … Goldberg, I. J. (2002). Very low density lipoprotein (VLDL) receptor‐deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. The Journal of Biological Chemistry, 277, 10037–10043. 10.1074/jbc.M109966200 [DOI] [PubMed] [Google Scholar]
- Yin, W. , Carballo‐Jane, E. , McLaren, D. G. , Mendoza, V. H. , Gagen, K. , Geoghagen, N. S. , … Strack, A. M. (2012). Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. Journal of Lipid Research, 53, 51–65. 10.1194/jlr.M019927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadelaar, S. , Kleemann, R. , Verschuren, L. , de Vries‐Van der Weij, J. , van der Hoorn, J. , Princen, H. M. , & Kooistra, T. (2007). Mouse models for atherosclerosis and pharmaceutical modifiers. Arteriosclerosis, Thrombosis and Vascular Biology, 27, 1706–1721. 10.1161/ATVBAHA.107.142570 [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Jin, Y. , Liu, T. , Liu, F. , & Ito, T. (2009). Hypertriglyceridemia in Watanabe heritable hyperlipidemic rabbits was associated with increased production and reduced catabolism of very‐low‐density lipoproteins. Pathobiology, 76, 315–321. 10.1159/000245897 [DOI] [PubMed] [Google Scholar]
- Zhao, J.‐L. , Yang, Y.‐J. , You, S.‐J. , Cui, C.‐J. , & Gao, R.‐L. (2007). Different effects of postconditioning on myocardial no‐reflow in the normal and hypercholesterolemic mini‐swines. Microvascular Research, 73, 137–142. 10.1016/j.mvr.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Yang, Y. , Xing, R. , Cui, X. , Xiao, Y. , Xie, L. , … Liu, M. (2018). Hyperlipidemia induces typical atherosclerosis development in Ldlr and Apoe deficient rats. Atherosclerosis, 271, 26–35. 10.1016/j.atherosclerosis.2018.02.015 [DOI] [PubMed] [Google Scholar]
- Zulet, M. A. , Barber, A. , Garcin, H. , Higueret, P. , & Martínez, J. A. (1999). Alterations in carbohydrate and lipid metabolism induced by a diet rich in coconut oil and cholesterol in a rat model. Journal of the American College of Nutrition, 18, 36–42. 10.1080/07315724.1999.10718825 [DOI] [PubMed] [Google Scholar]


