Abstract
The association between chronic inflammatory diseases (CIDs) and increased cardiovascular (CV) risk is well documented and can be a most threatening complication in these patients. However, the pathogenetic mechanisms underlying increased CV risk remain elusive, especially in their cellular and biochemical pathways. Using animal models to understand mechanisms underlying cardiac involvement are limited. Additionally, treatments may influence cardiovascular events through different outcomes. Some drugs used to treat CIDs can negatively affect cardiac function by a direct toxicity, whereas others may protect the myocardium. In the present article, we focus on the cardiac manifestations and risk factors, the pathogenetic mechanisms, and the effect of treatments on myocardial function and cardioprotection for five common worldwide CIDs (rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, psoriasis and inflammatory bowel disease). We also give recommendations in order to evaluate common targets between CID and CV disease (CVD) and to design therapies to alleviate CID‐related CVD.
LINKED ARTICLES
This article is part of a themed issue on Risk factors, comorbidities, and comedications in cardioprotection. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.23/issuetoc
Abbreviations
- CAD
coronary artery disease
- CCTA
coronary CT angiography
- CD
Crohn's disease
- CIDs
chronic inflammatory diseases
- CV
cardiovascular
- CVD
cardiovascular disease
- CXCR
chemokine C‐X‐C motif receptor
- DMARD
disease‐modifying antirheumatic drugs
- ECs
endothelial cells
- IBD
inflammatory bowel disease
- IHD
ischaemic heart disease
- MVD
microvascular dysfunction
- PTP‐PEST
tyrosine‐protein phosphatase non‐receptor type 12
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- SSc
systemic sclerosis
- TC
total cholesterol
- Th1
T helper cell type 1
- Th17
T helper cell type 17
- UC
ulcerative colitis
1. INTRODUCTION
Chronic inflammatory systemic diseases like rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and systemic multiple sclerosis are a heterogeneous group of disorders characterized by an excessive immune response due to the interaction between predisposing genetic factors, dysregulation of the immune system and environmental factors. The association of increased cardiovascular (CV) risk with chronic inflammatory conditions is well recognized and can be one of the most threatening complications in these patients (Mason & Libby, 2015). The manifestations vary by disease and all structures in the heart can be affected resulting in significant morbidity and mortality (Hollan et al., 2013; Roifman, Beck, Anderson, Eisenberg, & Genest, 2011). Traditional CV risk factors such as hypertension, dyslipidaemia, aging and smoking do not fully account for the increased CV risk in these patients and the disease is likely an independent risk factor.
Although the role of systemic inflammation in the development of premature atherosclerosis, ischaemic heart disease (IHD) and heart failure in these patients has been demonstrated, and immune mechanisms shared by CV and systemic inflammatory diseases have been identified, the pathogenetic mechanisms underlying increased CV risk in patients with chronic inflammatory disorders still remain elusive, especially in their cellular and biochemical pathways. The heart can be damaged either directly by a localized autoimmune process involving deposition of immune complexes and activation of the complement system leading to functional impairment of various cardiac structures or indirectly by the general chronic inflammation induced by other injured organs. In this respect, chronic inflammation has been suggested as an important contributing factor to the development of atherosclerotic disease, ultimately leading to myocardial infarction in patients with chronic inflammatory diseases (CIDs; Mason & Libby, 2015). In addition, disease treatments may precipitate CV events, although this is rather complex. Some drugs used to treat chronic inflammatory diseases can, sometimes, negatively affect cardiac function by a direct toxicity (such as antimalarials, glucocorticoids and non‐steroidal anti‐inflammatory drugs) or by worsening a pre‐existing cardiac disease. On the other hand, other drugs (such as methotrexate) may represent paradigms of potential treatments for CV disease (CVD) co‐morbidities (Mangoni, Zinellu, Sotgia, Carru, & Erre, 2017).
In this review, we aim to describe the cardiac manifestations and the pathophysiology of CV involvement in the most frequent chronic inflammatory diseases, such as rheumatoid arthritis (RA), psoriasis, systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and inflammatory bowel disease (IBD). In addition, we discuss the contribution of inflammatory disorders as potential CV risk factors and the role of treatment in chronic inflammatory disease patients.
Published articles for inclusion in this narrative review were sourced from the authors' record of papers on atherosclerosis and chronic inflammatory diseases, collected from January 2000 to August 2019, as well as from PubMed searches with the terms “rheumatoid arthritis,” “systemic lupus erythematosus,” “systemic sclerosis,” “psoriasis,” “inflammatory bowel disease,” “myocardial,” cardiovascular,” and “cardioprotection” in particular those papers presenting comprehensive reviews or meta‐analyses, plus in vivo studies and clinical trials on the aforementioned chronic inflammatory diseases that associate with CVD and cardioprotection.
2. RHEUMATOID ARTHRITIS
2.1. Cardiac manifestations and risk factors
Rheumatoid arthritis is a common autoimmune disease characterized by synovial inflammation, autoantibody production (rheumatoid factor and anti‐citrullinated protein antibody), joint deformity, and systemic features and affects 1% of the general population (McInnes & Schett, 2011). Patients with rheumatoid arthritis have increased risk of developing heart disease from both ischaemic and non‐ischaemic causes in comparison with the general population (Meune, Touze, Trinquart, & Allanore, 2009). Common CV manifestations include pericarditis (Voskuyl, 2006), myocardial dysfunction (Hurd, 1979), valvular heart disease (Roldan, DeLong, Qualls, & Crawford, 2007), atherosclerosis (Aubry et al., 2007; Chung et al., 2005; Ikonomidis, Makavos, et al., 2019; Maradit‐Kremers et al., 2005) and increased aortic stiffness and dysfunction of the coronary microcirculation (Roldan, 2008; Table 1).
TABLE 1.
Main manifestations of cardiac involvement in chronic inflammatory diseases
| Cardiovascular manifestations | Chronic inflammatory disease | ||||
|---|---|---|---|---|---|
| Rheumatoid arthritis | Systemic lupus erythematosus | Systemic sclerosis | Psoriasis | Inflammatory bowel disease | |
| Acute myocardial infarction | ✓ | ✓ | ✓ | ||
| Aortic root dilation/regurgitation | ✓ | ||||
| Aortic stiffness | ✓ | ✓ | |||
| Arterial stiffness | ✓ | ||||
| Atherosclerosis | ✓ | ✓ | ✓ | ✓ | ✓ |
| Coronary artery disease | ✓ | ✓ | |||
| Coronary microcirculation dysfunction | ✓ | ✓ | |||
| Fibrosis | ✓ | ||||
| Heart failure | ✓ | ✓ | |||
| Ischaemic heart disease | ✓ | ||||
| LV hypertrophy | ✓ | ||||
| Microvascular dysfunction | ✓ | ||||
| Myocarditis | ✓ | ||||
| Pericarditis | ✓ | ✓ | |||
| Peripheral arterial disease | ✓ | ||||
| Pulmonary arterial hypertension | ✓ | ||||
| Stroke | ✓ | ✓ | |||
| Systolic/diastolic dysfunction | ✓ | ✓ | ✓ | ||
| Valvular heart disease | ✓ | ||||
Although co‐morbidities affecting CV and respiratory system occur twice as often in rheumatoid arthritis patients (Batko et al., 2019), these patients display an increased risk of heart failure independent of traditional CV risk factors (del Rincon, Williams, Stern, Freeman, & Escalante, 2001; Maradit‐Kremers et al., 2005). Systemic inflammation plays a pivotal role in the atherosclerotic process in rheumatoid arthritis (Chung et al., 2005) and the amount of systemic inflammation and immune dysregulation are major risk factors at least partly responsible for CVD burden in rheumatoid arthritis patients.
There is a “paradoxical” relation between serum lipid levels and CV risk in rheumatoid arthritis. Patients with high disease activity have lower total cholesterol (TC) and LDL values compared with the general population, while the CV risk is increased (Myasoedova et al., 2011). Moreover, HDL levels are also reduced, whereas triglycerides levels are elevated compared to healthy controls. Therefore, TC/HDL ratio is a better CV risk predictor in rheumatoid arthritis than individual lipid levels (Toms et al., 2011).
2.2. Pathogenetic mechanisms
The pathophysiology of rheumatoid arthritis involves inflammatory mediators, including cytokines such as TNF‐α and IL‐1, IL‐6, IL‐12, IL‐15, IL‐17, IL‐18 and IL‐23. These are released by type 1 helper (Th1) and type 17 helper (Th17) T cells and dendritic cells (McInnes & Schett, 2011) and may induce endothelial dysfunction, accelerated atherosclerosis and atheromatous plaque instability or even have a negative inotropic action (Choy, 2012; Ikonomidis et al., 2008; Liang et al., 2009; Sattar & McInnes, 2005). Furthermore, cytokines such as IL‐1 may cause myocardial dysfunction through impairment of mitochondrial function, generation of nitro‐oxidative stress with increased production of intracellular ROS, inducible NO synthase, peroxynitrite and induction of cytokines with negative inotropic action such as IL‐6 (Ikonomidis et al., 2008, 2014). Apoptosis has a crucial role in the development of myocardial dysfunction in the setting of high inflammatory activity in rheumatoid arthritis.
In an animal model of inflammatory arthritis, designated as SKG mice, changes in the number and phenotype of cardiac immune cells were observed both before and after myocardial infarction which likely influences cardiac repair and ventricular remodelling post‐myocardial infarction, contributing to detrimental cardiac outcomes with inflammatory arthritis (Hsieh et al., 2017). Furthermore, an acquired form of cardiomyopathy was shown in collagen antibody‐induced arthritis mouse model, which mimics human rheumatoid arthritis. The heart displayed molecular and functional maladaptation including hypertrophy and increased fibrotic deposition, impaired cardiomyocyte Ca2+ signalling, mildly reduced cardiac contractility and oxidation‐dependent modification of important Ca2+ handling proteins, which resulted in long‐lasting cardiac remodelling and impaired contractile function (Pironti et al., 2018).
The main molecular pathogenetic mechanisms involved in cardiac manifestations in rheumatoid arthritis are shown in Figure 1.
FIGURE 1.
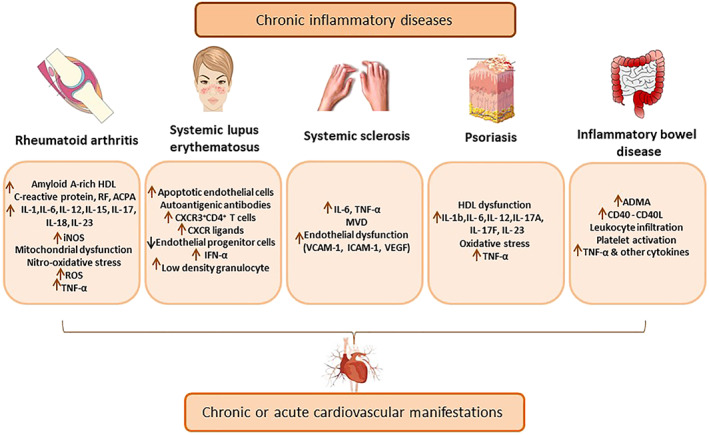
Main pathogenetic mechanisms of cardiovascular involvement by different chronic inflammatory diseases. ACPA, anti‐citrullinated protein antibody; ADMA, asymmetric dimethylarginine; ICAM‐1, intercellular adhesion molecule 1; MVD, microvascular dysfunction; RF, rheumatic factor; VCAM‐1, vascular cell adhesion molecule
2.3. Effect of rheumatoid arthritis treatment on myocardial function and cardioprotection
Treatment of rheumatoid arthritis aims towards the suppression of disease activity and reduction of CV risk by decrease of systemic inflammation. Mainstays of rheumatoid arthritis treatment are corticosteroids, disease‐modifying antirheumatic drugs (DMARDs; e.g. methotrexate) and cytokine inhibitors (e.g. anti‐TNF‐α, anti‐IL‐1 and anti‐IL‐6 agents). The effects of these agents on cardiac disease observed in rheumatoid arthritis are summarized in Table 2. Both methotrexate and TNF‐α antagonists have been associated with a cardioprotective effect due to suppression of systemic inflammation (Barnabe et al., 2011; Lee et al., 2018; Micha et al., 2011; Roubile et al., 2015). These studies have shown prospectively that biological agents reduce the number of adverse cardiac events while this response was not observed in those patients that ceased biological agents during follow‐up (Lee et al., 2018). A beneficial effect on myocardial and vascular function has been also indicated after treatment with cytokine inhibitors (Ikonomidis et al., 2008, 2014, 2009). Anti‐IL‐1 regimens seem to have a more favourable profile on myocardial function when compared to anti‐IL‐6 regimens, whereas anti‐IL‐6 regimens mostly benefit vascular function (Ikonomidis, Pavlidis, et al., 2019). In addition, two recent meta‐analyses indicated that tocilizumab (an anti‐IL‐6 agent) is safe in terms of CV outcomes compared to disease‐modifying antirheumatic drugs (Castagné et al., 2019) and may also be associated with decreased risk of major adverse cardiovascular events in comparison to TNF‐α inhibitors (Singh et al., 2019).
TABLE 2.
Effects of currently used medication for chronic inflammatory diseases on cardiac manifestations
| Inflammatory disease | Drug | Effect on CV outcome | |
|---|---|---|---|
| Clinical studies | Experimental studies and underlying mechanisms | ||
| Rheumatoid arthritis (RA) | Anti‐IL‐1 (Anakinra) |
Improvement of vascular and left ventricular function in RA patients (Ikonomidis et al., 2008) Improvement of myocardial deformation in RA patients (Ikonomidis et al., 2009) Improvement of vascular function, myocardial deformation, and twisting in CAD patients with RA (Ikonomidis et al., 2014) |
|
| Anti‐IL‐6 (Tocilizumab) |
Improvement of vascular function in RA patients (Ikonomidis, Pavlidis, et al., 2019) Decreased risk of major adverse CV events (non‐fatal MI, need for coronary revascularization, cardiovascular death with or without angina, CHF, peripheral artery disease, or abdominal aortic aneurysm) in RA patients (Singh et al., 2019) |
||
| Anti‐TNF‐α |
Reduced risk of CV events including MI in RA patients (Barnabe, Martin, & Ghali, 2011) Reduced risk of major CV events (defined as angina, MI, coronary artery bypass graft, PCI, other heart disease, stroke/transient ischaemic attack, or death from cardiovascular causes) in patients with inflammatory arthritis including RA (Lee et al., 2018) |
||
| Auranofin | Reduction of infarct size and improved cardiac function after I/R likely via PTP‐PEST‐ErbB‐2 signalling axis in an in vivo model of AMI in mice (Yang et al., 2019) | ||
| Methotrexate | Reduction of CV events in RA patients (Micha et al., 2011; Roubile et al., 2015) | ||
| Systemic Lupus Erythematosus (SLE) | Antimalarials (chloroquine and hydroxychloroquine) | Antimalarial‐induced cardiomyopathy in SLE patients (Tselios et al., 2016; Tselios et al., 2019) |
Conferred atheroprotection through a p53‐dependent mechanism in atherosclerosis‐prone ApoE‐null mice (Razani, Feng, & Semenkovich, 2010) Improved NO‐mediated vasorelaxation of mesenteric arteries in a murine model of SLE (Virdis et al., 2015) Reduced heart rate and LV pressure in Langendorff perfused rat hearts and exerted cardiotoxic effects in isolated cardiomyocytes (Blignaut, Espach, van Vuuren, Dhanabalan, & Huisamen, 2019) Reduce pressure overload‐induced cardiac hypertrophy but impaired cardiac relaxation and contractility in rats (Chaanine et al., 2015) |
| Glucocorticoids | Deleterious effects in heart and vessels in patients with SLE (Wu et al., 2016) | ||
| Immunosuppressants (mycophenolate mofetil) | Attenuation of atherosclerosis in lupus‐prone mice (van Leuven et al., 2012) | ||
| Methotrexate | Reduction of cardiac vasculopathy via activation of AMPK‐CREB in murine model of inflammatory vasculopathy (Thornton et al., 2016) | ||
| Systemic sclerosis (SS; lacks a specific therapy) | Nintedanib (inhibitor of GF‐RTKs) | Antifibrotic effects and amelioration of histological features of pulmonary arterial hypertension, microangiopathy and pulmonary fibrosis in Fra2 mouse model of SS (Huang et al., 2017) | |
| Psoriasis | Anti‐IL‐12/23 (ustekinumab) |
Improvement of coronary microcirculatoy function, arterial stiffness, and myocardial function in psoriasis patients—greater improvement than Anti‐TNF‐α or cyclosporine treatment (Ikonomidis et al., 2017) Reduction of aortic vascular inflammation with reduction in inflammatory cytokines associated with CV disease in psoriasis patients (Gelfand et al., 2019) |
|
| Anti‐IL‐17A (secukinumab) |
Improvement of vascular and myocardial function in psoriasis patients—greater improvement than methotrexate or cyclosporine treatment (Makavos et al., 2019) Improvement of arterial flow‐mediated dilation in psoriasis patients suggesting beneficial effects on CV risk by improving endothelial function (von Stebut et al., 2019) |
||
| Anti‐TNF‐α (etanercept), | Improvement of cardiac function in psoriasis patients (Ikonomidis et al., 2017) | ||
| Anti‐TNF‐α | Reduced risk of major CV events (angina, MI, coronary artery bypass graft, PCI, other heart disease, stroke/transient ischaemic attack, or death from cardiovascular causes) in patients with inflammatory arthritis including psoriasis (Lee et al., 2018) | ||
| Cyclosporine | Improvement of cardiac function in psoriasis patients (Ikonomidis et al., 2017) | ||
| Diverse biological therapies (anti‐TNF‐α, anti‐IL‐12/23, or anti‐IL‐17) |
Reduction of coronary inflammation (indicated by reduction of perivascular fat attenuation index) in psoriasis patients (Elnabawi, Oikonomou, et al., 2019) Reduction of non‐calcified plaque burden in psoriasis patients suggesting beneficial modulation of coronary plaque composition (Elnabawi, Dey, et al., 2019) |
||
| Methotrexate | Improvement of vascular and myocardial function in psoriasis patients (Makavos et al., 2019) | ||
| NF‐κB inhibitor (dimethyl fumarate) | Reduction of myocardial infarct size after I/R in rats in vivo likely through inhibition of NF‐κB in cardiac endothelial cells (Meili‐Butz et al., 2008) | ||
| Inflammatory bowel disease (IBD) | 5‐Aminosalicylic acids | Decreased risk of IHD in IBD patients (Rungoe et al., 2013) | |
| 6‐Mercaptopurine | Induction of calcium deposition in in vitro cultured vascular smooth muscle cells and in ex vivo media layer of rat aorta rings which might accelerate the evolution of arteriosclerosis and CVD (Prüfer et al., 2014) | ||
| Anti‐TNF‐α | Non‐significant decrease of IHD in IBD patients (Rungoe et al., 2013) | ||
| Azathioprine + aminosalicylates | Lower risk of CVD (calculated by Framingham Risk Score, a gender‐specific algorithm for estimating the 10‐year CV risk) in comparison to aminosalicilates only, and increased anti‐inflammatory cytokines in women with ulcerative colitis (UC; dos Santos et al., 2015) | ||
Abbreviations: AMI, acute myocardial infarction; AMPK, AMP‐activated protein kinase; ApoE, apolipoprotein E; CREB, cAMP response element‐binding protein; CHF, congestive heart failure; CV, cardiovascular; Fra2, fos‐related antigen‐2; GF, growth factor; IHD, ischaemic heart disease; PTP‐PEST, tyrosine‐protein phosphatase non‐receptor type 12; RTK, receptor tyrosine‐kinases.
As far as the lipid control is concerned, management of disease activity (mainly by methotrexate or anti‐TNF‐α agents) results in an overall increase of serum lipids, mainly of HDL, with a neutral effect (Daïen et al., 2012) or even improvement of the TC/HDLc ratio (Navarro‐Millán et al., 2013). Early aggressive treatment of inflammation in rheumatoid arthritis with methotrexate monotherapy or combination therapies including anti‐TNF‐α improves the atheroprotective composition of HDL (Charles‐Schoeman et al., 2017). Statins have been proved effective in the reduction of cholesterol, atherosclerotic burden, CV events and cumulative mortality in rheumatoid arthritis patients (Schoenfeld et al., 2016), whereas combination of their anti‐inflammatory properties when co‐administered with non‐steroidal anti‐inflammatory drugs and disease‐modifying antirheumatic drugs may improve inflammation and serum lipid profile (McCarey et al., 2004) and may result in an incremental CV risk reduction (Lv et al., 2015).
Data on the effect of treatment for rheumatoid arthritis on cardioprotection are scarce. Very recently, it has been demonstrated that Auranofin, a drug being used in clinical practice for treating rheumatoid arthritis reduces infarct size and results in better cardiac function when it is administered in mice before reperfusion. The potential underlying mechanism may involve the protein tyrosine phosphatases (PTP)‐PEST‐ErbB‐2 signalling axis (Yang et al., 2019). In a mouse model of adjuvant‐induced arthritis, which mimics RA in humans, ischaemic preconditioning did not result in a significant reduction of myocardial necrosis area induced by 30 min of ischaemia and 60 min of reperfusion (Wojciechowska et al., 2013). This effect might be associated with the presence of chronic systemic inflammation in these animals. The reduced benefits of ischaemic preconditioning may be one reason for the increased CV risk in patients with rheumatoid arthritis. However, to the best of our knowledge, there are no other studies that have investigated the effects of rheumatoid arthritisin conditioning cardioprotective mechanisms of the myocardium.
In summary, there is a clear correlation between rheumatoid arthritis and myocardial function mainly through increased inflammation, oxidative stress and apoptosis. However, since the majority of anti‐inflammatory cardioprotective strategies have failed in the clinical setting mainly because these drugs are directed to a particular cytokine (reviewed in detail in Andreadou et al., 2019), studies in clinically relevant animal rheumatoid arthritis models need to be performed, in order to investigate if rheumatoid arthritis interferes with the conditioning cardioprotective mechanisms.
3. SYSTEMIC LUPUS ERYTHEMATOSUS
3.1. Cardiac manifestations and risk factors
Systemic lupus erythematosus is a chronic autoimmune disease characterized by high levels of antinuclear antibodies in the serum and the most specific clinical features are skin rash and glomerulonephritis (Larosa, Iaccarino, Gatto, Punzi, & Doria, 2016). According to a recent review of epidemiological data, systemic lupus erythematosus prevalence displays an increasing trend over the last decades (Rees, Doherty, Grainge, Lanyon, & Zhang, 2017) with the female population being affected much more frequently compared with males. However, regardless of the sex, there is a strong correlation between systemic lupus erythematosus and CVD development, which represents one of the main reasons of morbidity and mortality among systemic lupus erythematosus patients (O'Sullivan, Bruce, & Symmons, 2016). Coronary heart disease, acute myocardial infarction, ischaemic stroke and peripheral arterial disease (Arkema, Svenungsson, Von Euler, Sjöwall, & Simard, 2017; Chuang et al., 2015; Hak, Karlson, Feskanich, Stampfer, & Costenbader, 2009) are among the main CV complications in systemic lupus erythematosus (Table 1).
Traditional along with disease‐specific risk factors might be considered for understanding the increased CVD risk in SLE. Hypertension, dyslipidaemia and diabetes are more common in systemic lupus erythematosus patients than controls (Bruce, Urowitz, Gladman, Ibañez, & Steiner, 2003). Related to this, metabolic syndrome and an exacerbated inflammatory state, measured as C‐reactive protein plasma levels, are also more prevalent in systemic lupus erythematosus compared with the general population (Chung et al., 2007). But traditional factors alone do not explain the higher CVD incidence. All these factors converge into atherosclerosis, which appears to be more frequent among systemic lupus erythematosus patients, similar to other autoimmune disorders, but with a more accelerated progression (Tektonidou et al., 2017).
3.2. Pathogenetic mechanisms
Although the pathogenic mechanisms of accelerated atherosclerosis remain unclear, possible explanations have been proposed. Such putative mechanisms have been recently reviewed elsewhere (Liu & Kaplan, 2019). Certainly, high BP, dyslipidaemia and other traditional risk factors contribute to the primary lesion of the intima layer, endothelium activation and plaque formation. However, specific disease factors seem to be responsible for the remarkable endothelial damage‐repair imbalance manifested in systemic lupus erythematosus. Reflecting this profound imbalance, circulating endothelial progenitor cells able to repair endothelial damage are decreased in systemic lupus erythematosus patients (Moonen et al., 2007). In contrast, circulating apoptotic endothelial cells (ECs) are increased, probably linked to the impairment of NO vasorelaxation pathway (Rajagopalan et al., 2004). Evidence suggests that IFN‐α, secreted by plasmacytoid dendritic cells located in plaques, plays a central role in this disequilibrium, contributing to endothelium and platelet activation, diminished EC differentiation (Thacker et al., 2012), impaired endothelium‐dependent vasorelaxation (Buie, Renaud, Muise‐Helmericks, & Oates, 2017), foam cell maturation, and plaque destabilization (Li et al., 2011). Likewise, a systemic lupus erythematosus special kind of neutrophil encountered in plaques, the low‐density granulocyte, could provoke EC death through enhanced metalloproteinase externalization (Carmona‐Rivera, Zhao, Yalavarthi, & Kaplan, 2015). In addition, chemokine receptor (CXCR) 3 expressed in CD4+T cells as well as CXCR ligands are increased in both SLE patients and animal models, and such levels correlate with the thickness of the internal carotid wall and plaque formation (Clement et al., 2015). Autoantigenic antibodies, which are the hallmark of systemic lupus erythematosus, are implied in the first term in plasmacytoid dendritic cell activation leading to an unspecific amplification of the humoral immune response, thus establishing a feedforward loop (Döring et al., 2012). Together, endothelial dysfunction and chronic inflammation may ultimately lead to the observed accelerated progression of atherosclerosis in systemic lupus erythematosus. Finally, an example of the synergistic effect of traditional and disease‐specific CVD risk factors is the case of systemic lupus erythematosus patients with metabolic syndrome. These patients have reduced circulating endothelial progenitor cells, higher plasmatic pro‐inflammatory cytokines levels and more pronounced arterial stiffness, when compared to systemic lupus erythematosus patients without metabolic syndrome (Castejon et al., 2016). The main pathogenetic molecular mechanisms that are involved in CVD manifestations in systemic lupus erythematosus are illustrated in Figure 1.
Various mouse models have been developed to dissect the cellular and genetic mechanisms of systemic lupus erythematosus, to identify therapeutic targets and to screen treatments (Li, Titov, & Morel, 2017). However, apart from an early study demonstrating multiple small infarcts in mice with systemic lupus erythematosus and small coronary artery disease (CAD; Yoshida, Fujiwara, Fujiwara, Ikehara, & Hamashima, 1987), there are no studies focusing on cardiac manifestations in these animal models.
3.3. Effect of systemic lupus erythematosus treatment on myocardial function and cardioprotection
Medication in systemic lupus erythematosus may contribute to the observed CVD in these patients (Table 2). Current treatment for systemic lupus erythematosus encompasses antimalarials, glucocorticoids, immunosuppressive and biological agents (Fanouriakis et al., 2019). Widely used antimalarials exhibit contradictory effects regarding CVD. In mice, chloroquine confers atheroprotection through a p53‐dependent process (Razani et al., 2010), while in humans it does not reduce the carotid intima‐media layer thickness (McGill et al., 2019). More specifically, in a murine model of systemic lupus erythematosus, hydroxychloroquine safeguards endothelial integrity, thus restoring vasorelaxation mediated by NO in mesenteric arteries (Virdis et al., 2015). Furthermore, although hydroxychloroquine‐induced cardiomyopathy is considered to be extremely rare (Yogasundaram et al., 2014), there are recent claims that antimalarial cardiotoxicity may be underdiagnosed and suggest a causal link between antimalarials and cardiomyopathy in systemic lupus erythematosus (Tselios et al., 2016; Tselios et al., 2019). Even a single high dose of chloroquine significantly reduces heart rate and left ventricular pressure in an ex vivo rat model, in the absence of mitochondrial damage (Blignaut et al., 2019). Hydroxychloroquine and chloroquine toxicity is understood in terms of lysosomal accumulation, hydrolase inhibition, enhanced lysosomal storage and impaired autophagy that culminates in degenerated mitochondria, left ventricle hypertrophy and fibrosis (Chaanine et al., 2015).
Concerning glucocorticoids in systemic lupus erythematosus, evidence is more straightforward showing deleterious side effects in heart and vessels (Wu et al., 2016) and clinicians agree in minimizing or withdrawing glucocorticoid dosage whenever it is possible (Fanouriakis et al., 2019). Cumulative doses of steroids, consumed as systemic lupus erythematosus treatment, strongly correlate with carotid plaque formation and carotid intima‐media thickness increase (Wu et al., 2016). Other immunosuppressive agents such as mycophenolate mofetil and methotrexate have been shown to reduce atheromatous plaque formation (van Leuven et al., 2012) and cardiac vasculopathy (Thornton et al., 2016) in systemic lupus erythematosus‐prone and inflammatory vasculopathy mice models respectively.
In summary, CVD is a major cause of morbidity and mortality in systemic lupus erythematosus. Together, traditional and disease‐related factors synergistically act to produce arterial intima layer damage, inflammation, atherosclerosis and subsequent CVD. Pro‐inflammatory factors play a key role in accelerated atherosclerosis progression of systemic lupus erythematosus, although the precise mechanisms are not fully understood. Current treatment of systemic lupus erythematosus could either exacerbate or counteract CVD. Further research is necessary in order to identify common targets of systemic lupus erythematosus and CVD that may lead to novel therapeutic strategies.
4. SYSTEMIC SCLEROSIS
4.1. Cardiac manifestations and risk factors
Systemic sclerosis is a multi‐systemic immune‐mediated disorder characterized by excessive fibrosis of the skin and internal organs (Spethman et al., 2014). As shown in Table 1, patients with systemic sclerosis present various types of CV complications, ranging from subclinical atherosclerosis, LV hypertrophy and diastolic dysfunction to acute coronary syndromes even in the absence of significant coronary artery stenosis (Au et al., 2011; Chu et al., 2013; Derk & Jimenez, 2007; Spethman et al., 2014; Ungprasert et al., 2014). In parallel, they exhibit significant microvascular dysfunction (MVD) which is responsible for multiple manifestations of this disease, such as Raynaud phenomenon, pulmonary artery hypertension or renal sclerosis (Faccini, Kaski, & Camici, 2016).
Contribution of traditional CV risk factors to CAD is small in systemic sclerosis, whereas there is independent involvement of chronic inflammatory disease contributing to endothelial dysfunction and atherosclerosis (Kakuta et al., 2015).
4.2. Pathogenetic mechanisms
Chronic systemic inflammation, a critical component of atherosclerosis, is present in systemic sclerosis and has been demonstrated to be associated with a significant degree of endothelial dysfunction (Ungprasert et al., 2014). Therefore, it is very likely that systemic sclerosis patients, who exhibit increased inflammation and endothelial dysfunction, are predisposed to development of atherosclerotic lesions. High concentrations of TNF‐α or IL‐6 contribute to the development of accelerated atherosclerosis and promote endothelial dysfunction, which is proved by elevated serum levels of endothelial injury biomarkers, such as vascular cell adhesion molecule 1, intercellular adhesion molecule 1 or VEGF (Faccini et al., 2016). At the same time, patients with systemic sclerosis exhibit an underlying coronary microvascular dysfunction, which may precede coronary atherosclerosis after many years, being correlated with a systemic inflammatory response (Faccini et al., 2016). Inflammation‐related microvascular dysfunction at the level of coronary arteries may be responsible for myocardial ischaemia in the absence of obstructive CAD, a frequent observation in systemic sclerosis patients. At the level of coronary circulation, microvascular dysfunction can lead to an increased rate of major CV events via complex mechanisms that involve endothelial dysfunction and increased platelet aggregation.
Contraction band myocardial necrosis resulting from ischaemia and myocardial fibrosis are the most common pathological findings at necropsy in patients with systemic sclerosis who suffered a cardiac death. Myocardial inflammation was also documented in systemic sclerosis patients by histological and MRI studies, who found a significantly higher amount of scar in systemic sclerosis patients as compared to controls (P < .0001), which followed a diffuse distribution pattern (Mavrogeni et al., 2015).
In summary, all these observations prove that chronic systemic inflammation and fibrosis play a pivotal role in initiation and progression of cardiac manifestations in systemic sclerosis (Figure 1).
4.3. Effect of systemic sclerosis treatment on myocardial function and cardioprotection
As systemic sclerosis is a clinically heterogeneous disease affecting multiple organ systems, the optimal treatment of this disease remains a challenge. In present, there is no recommendation for any specific drug that would stop progression of the disease. However, consensus recommendations clearly address the major clinical conditions associated with systemic sclerosis. The European League Against Rheumatism Scleroderma Trials and Research group released an updated consensus recommendation for the treatment of systemic sclerosis, which is structured in six distinct recommendations, one for each organ specific involvement: dihydropyridine‐type calcium antagonists and i.v. prostanoids for severe systemic sclerosis‐related digital vasculopathy, PDE type 5 inhibitors for systemic sclerosis‐related Raynaud phenomenon or digital ulcers, endothelin receptor antagonists, prostacyclin analogues and PDE type 5 inhibitors for systemic sclerosis‐related pulmonary arterial hypertension, methotrexate for systemic sclerosis‐related skin involvement, cyclophosphamide for interstitial lung disease, angiotensin‐converting enzyme inhibitors for scleroderma renal crisis and proton pump inhibitors for gastrointestinal manifestations (Kowal‐Bielecka et al., 2017). While the consensus recommendations clearly address these clinical conditions associated with systemic sclerosis, there is no recommendation for any specific drug that would stop progression of this systemic disease.
There are very few studies using animal models to investigate the potential of some new drugs to treat systemic sclerosis‐related cardiac fibrosis (Table 2). For instance, Fra‐2 transgenic mice were demonstrated to represent a suitable preclinical model for the study of systemic sclerosis‐related cardiomyopathy, resembling the clinical manifestations of systemic sclerosis and developing systemic fibrotic disease, systemic sclerosis‐like microvascular disease and pulmonary arterial hypertension (Huang et al., 2017; Venalis et al., 2015). Using this animal model, it has been demonstrated that Nintedanib, an inhibitor targeting growth factor receptor TK, exerts antifibrotic effects and ameliorates histological features of pulmonary arterial hypertension, microangiopathy and pulmonary fibrosis (Huang et al., 2017). However, human studies with Nintedanib have been reported only for lung diseases, while CV effects of this drug have not been tested yet in clinical applications.
In conclusion, systemic sclerosis is a highly heterogenous inflammatory disease which lacks a specific therapy that would stop its progression. The treatment of systemic sclerosis is currently focused on organ specific complication. Preclinical studies using murine models show promising results of several recent therapies on systemic sclerosis‐related cardiomyopathy, mainly based on their anti‐fibrotic effect and should be further tested in clinical trials.
5. PSORIASIS
5.1. Cardiac manifestations and risk factors
Psoriasis is an immune‐mediated disease affecting up to 3% of the adult general population characterized by skin lesions, arthritis in 10% of patients, and systemic manifestations (Gelfand et al., 2005). Main CV complications are summarized in Table 1 and include increased prevalence of atherosclerosis, increased aortic stiffness, coronary microcirculatory dysfunction and impaired myocardial function (Ikonomidis et al., 2015; Kimball et al., 2008; Ogdie et al., 2015).
Increased rates of risk factors such as hypertension, diabetes, dyslipidaemia and obesity in patients with psoriasis have been reported (Tam et al., 2008). Patients with psoriatic arthritis are at higher CV risk compared to plaque‐type psoriasis alone; thus, CVD risk assessment is recommended for all patients with psoriatic arthritis at least once every 5 years and should be reassessed following major changes in antirheumatic therapy (Agca et al., 2017).
5.2. Pathogenetic mechanisms
Psoriasis and CVD share common pathophysiological mechanisms, including inflammation, oxidative stress and common genetic susceptibility (Ogdie et al., 2015). Figure 1 summarizes the main pathophysiological molecules that link psoriasis with CVD.
Chronic inflammation is important in the development of both psoriasis and atherosclerosis with the involvement of inflammatory mediators such as TNF‐α, IL‐1β, IL‐6, IL‐12, IL‐17, IL‐22 and IL‐23, released primarily by activated dendritic cells and Th1 and Th17 lymphocytes (Prinz, 2010; Reich, 2012; Figure 2). Activated lymphocytes and high levels of circulating cytokines observed in psoriasis are responsible for endothelial inflammation and atheromatous plaque formation and rupture (Pryshchep, Sato, Goronzy, & Weyand, 2006). Several of these cytokines have been associated with myocardial and vascular dysfunction including arterial stiffness (Pryshchep et al., 2006; Yong et al., 2013), coronary atherosclerosis (Zhang et al., 2006), left ventricular hypertrophy and cardiac remodelling (Madhur et al., 2010; Sheu et al., 2013) and myocardial ischaemia (Ikonomidis et al., 2005, 2017). IL‐17A, one of the main inflammatory mediators in psoriasis (Lockshin, Balagula, & Merola, 2018), has pro‐atherogenic action (Karbach et al., 2014) and has been detected in patients with acute coronary syndromes (Cheng et al., 2008). IL‐17 induces the expression of pro‐inflammatory cytokines (TNF‐α, IL‐1, and IL‐6) in keratinocytes and fibroblasts (Eid et al., 2009) and consequently promotes the differentiation of T helper 17 (Th17) cells responsible for IL‐17A release (Figure 2).
FIGURE 2.

Immune cells and cytokines implicated in the pathogenesis of psoriasis. Deregulation of innate immunity by genetic and/or environmental triggers results in the production of cytokines that activate myeloid dendritic cells. Activated dendritic cells present antigens and secrete mediators such as IL‐23 and IL‐12, leading to the differentiation of type 1 and type 17 helper T cells (Th17 and Th1). T cells, in turn, secrete mediators (e.g. TNF‐α, IL‐17 and IL‐22) that activate keratinocytes and induce the production of proinflammatory cytokines (TNF‐α, IL‐1 and IL‐6) which feed back into the proinflammatory disease cycle. At the same time, secreted proinflammatory cytokines and chemokines (TNF‐α and INF‐α) contribute to the formation of atheromatic plaque
Oxidative stress biomarkers such as malondialdehyde and protein carbonyls are increased in psoriasis and are associated with vascular and impaired coronary microcirculatory function (Ikonomidis et al., 2015). It has been indicated that the above‐mentioned biomarkers of oxidative stress are also implicated in atherosclerosis (Stocker & Keaney, 2004) and in autoimmune rheumatic diseases (Kaiser, Abdulla, Henningsen, Skov, & Hansen, 2019).
Additionally, compositional alterations of psoriatic HDL have been reported which lead to impairment of cholesterol efflux capacity of HDL. The functional impairment of HDL may contribute to the excess CV mortality of psoriatic patients and may provide a link between psoriasis and CVD (Holzer et al., 2012).
5.3. Effect of psoriasis treatment on myocardial function and cardioprotection
Mainstays of psoriasis treatment are corticosteroids, disease‐modifying antirheumatic drugs (e.g. methotrexate), cyclosporine and cytokine inhibitors (e.g. anti‐TNF‐α, anti‐IL‐12/23, and anti‐IL‐17 agents). Treatment with cytokine inhibitors seems to have a favourable profile on CV function in psoriasis patients (see Table 2 for details). Treatment with anti‐IL‐12/23 resulted in reduction of aortic vascular inflammation and a greater improvement of coronary microcirculatory function, arterial stiffness, and myocardial function compared to TNF‐α inhibition or cyclosporine treatment in psoriasis patients (Gelfand et al., 2019; Ikonomidis et al., 2017). Additionally, inhibition of IL‐17A resulted in a greater improvement of vascular and myocardial function compared with cyclosporine or methotrexate treatment, suggestive of a stronger beneficial effect on overall CV function in psoriasis (Makavos et al., 2019; von Stebut et al., 2019). Treatment with biological agents was associated with reduction of coronary inflammation indicated by significant reduction of perivascular fat attenuation index measured by coronary CT angiography (CCTA; Elnabawi, Oikonomou, et al., 2019). Moreover, in psoriasis patients, treatment with biological agents resulted in 6% reduction of non‐calcified plaque burden assessed by CCTA, suggestive of a beneficial modulation of coronary plaque composition (Elnabawi, Dey, et al., 2019). Thus, CCTA may be a useful tool to assess the burden of CAD and monitor response to anti‐inflammatory treatment.
Over the past decades, murine models of psoriasis have been developed as tools for understanding the pathogenesis of this disease. These models include transgenic mice, knockout mice, and reconstituted models of psoriasis (reviewed in Nakajima & Sano, 2018). As far as cardioprotection studies are concerned, dimethyl fumarate, a small molecule drug for psoriasis, has been shown to reduce myocardial infarct size in rats undergoing 45‐min ischaemia followed by 120 min of reperfusion probably through inhibition of nuclear factor κ‐light‐chain‐enhancer of activated B cells (NF‐κB) in cardiac endothelial cells (Meili‐Butz et al., 2008). Studies using the K5.Stat3C mouse, an animal model of psoriasis, demonstrated the clinical relevance of STAT3 targeting as a novel therapy for psoriasis (Nakajima & Sano, 2018). Taking into account that STAT3 activation is an important signalling cascade for cardioprotection (Andreadou et al., 2017), we may conclude that psoriasis may interfere with the cardioprotective conditioning mechanisms. However, to the best of our knowledge, cardioprotection studies in animal models of psoriasis have not been performed.
6. INFLAMMATORY BOWEL DISEASE
6.1. Cardiac manifestations and risk factors
Inflammatory bowel disease is a chronic inflammatory, immunological mediated disorder that comprises ulcerative colitis (UC) and Crohn's disease (CD). Crohn's disease is characterized by transmural inflammation of any part of the gastrointestinal tract, mainly at ileum and/or colon (Gajendran, Loganathan, Catinella, & Hashash, 2018), whereas in ulcerative colitis, mucosal inflammation occurs from rectum to colon (Gajendran et al., 2019). Aetiology is defined by a complex interplay of genetic and environmental factors, as well as gut microbiota and lifestyle (Ananthakrishnan, 2015).
Besides gastrointestinal symptoms, there has been recorded a wide plethora of extra‐intestinal manifestations of inflammatory bowel disease, including CVD (Harbord et al., 2016), which represents the main cause of death in both ulcerative colitis and Crohn's disease (Fumery et al., 2014). As shown in Table 1, inflammatory bowel disease patients present premature signs of subclinical atherosclerosis (Cappello et al., 2017) and increased risk of ischaemic heart disease (IHD), acute myocardial infarction and heart failure (Aniwan, Pardi, Tremaine, & Loftus, 2018; Feng et al., 2017; Fumery et al., 2014). Additional cardiac manifestations are arrhythmias and pericarditis and myocarditis (Mitchell, Harrison, Junga, & Singla, 2018).
Systemic chronic inflammation has been pointed out as responsible for the larger CVD incidence among inflammatory bowel disease patients. Related to this last statement, inflammatory bowel disease clinical activity correlates in a considerable manner with CVD and systemic inflammation, measured as plasmatic C‐reactive protein levels (Le Gall et al., 2018). Consequent to inflammation, a hypercoagulable state has been reported in inflammatory bowel disease, which associates to thrombosis and to a dramatically exacerbated mortality risk (Andrade et al., 2018).
6.2. Pathogenetic mechanisms
Presumed pathogenic mechanisms that lead to CVD in inflammatory bowel disease are here briefly reviewed. Damage to intestinal mucosa permits exposure of bacterial hyper‐acylated LPS to the circulation, thus enabling the system to mount a generalized inflammatory response (McDonnell et al., 2011). TNF‐α along with other released cytokines then targets endothelium, producing an impairment of vasorelaxation pathways and an increase in adhesion molecules that trigger leukocyte infiltration, both phenomena implicated in atherosclerosis initiation and progression (Steyers & Miller, 2014). Accordingly, the levels of the endothelium NO synthase inhibitor asymmetric dimethylarginine are higher in inflammatory bowel disease patients than in controls, and such increase correlates with platelet count and other inflammation parameters (Owczarek, Cibor, & Mach, 2010). Moreover, inflammatory bowel disease endothelium exhibits abnormally high levels of CD40 whereas soluble CD40 levels, produced by platelets, are also high in inflammatory bowel disease patients, reflecting an enhanced platelet activation state (Danese et al., 2003). This enhanced platelet activation state may conduce to the reported procoagulant state and thromboembolism (Andrade et al., 2018). Also, CD40–CD40L interaction produces the release of multiple inflammation mediators, some of them implied in the Th17 subset differentiation that characterizes Crohn's disease and ulcerative colitis inflammation (Iezzi et al., 2009; Ueno et al., 2018). Finally, as CD40 activation by its ligand promotes secretion of TNF‐α and that TNF‐α induces endothelial CD40 and adhesion molecules expression, a feedforward loop establishes with the aforementioned deleterious consequences to endothelial integrity seen in inflammatory bowel disease (Cibor et al., 2016; Danese et al., 2006). The major signalling molecules involved in CVD complications in inflammatory bowel disease are illustrated in Figure 1.
6.3. Effect of inflammatory bowel disease treatment on myocardial function and cardioprotection
First line therapy of ulcerative colitis patients consists in 5‐aminosalicylates for both maintaining remission and treating active disease. Corticosteroids are also effective inducing remission in ulcerative colitis mesalamine‐resistant patients as well as in Crohn's disease. Other inflammatory bowel disease therapies comprise immunosuppressive agents, namely, azathioprine, 6‐mercaptopurine, methotrexate, tacrolimus, cyclosporine A and more recently, antibodies, fusion proteins and small molecules (Su et al., 2019). 5‐Aminosalicylic acid improves lipid profile in mice fed with a high‐fat and high‐cholesterol diet, possibly due to PPARα and PPARγ up‐regulation (Wang & Bao, 2017). In addition, 5‐aminosalicylic acid also ameliorates the insulin resistance state of mice subjected to a high‐fat diet (Luck et al., 2015). In line with this, the risk of CVD is suggested to be lower among ulcerative colitis patients receiving azathioprine and salicylate‐based therapy compared with those treated only with salicylates (dos Santos et al., 2015). In contrast, 6‐mercaptopurine, a by‐product of azathioprine metabolism, induces calcium deposition in in vitro cultured vascular smooth muscle cells and in ex vivo media layer of rat aorta rings. These findings point out that 6‐mercaptopurine might accelerate the evolution of atherosclerosis and CVD (Prüfer et al., 2014). With respect to anti‐TNF‐α agents, a slight non‐significant decrease of ischaemic heart disease is observed in users when compared to non‐users in an inflammatory bowel disease patient's cohort study (Rungoe et al., 2013). More recently, anti‐TNF‐α agents, infliximab and adalimumab, were shown to lower plasma insulin levels and the homeostatic model assessment of insulin resistance index in inflammatory bowel disease patients. Hence, such drugs could reduce the risk of CVD (Paschou et al., 2018).
Different effects have been reported concerning the use of steroids as inflammatory bowel disease therapy. Corticosteroids have been associated with an increased risk of CV events in a Danish cohort of patients with inflammatory bowel disease (Rungoe et al., 2013). On the other hand, another study could not demonstrate differences in vascular parameters (carotid intima‐media thickness, carotid plaque, and aortic stiffness) among patients treated with steroids (Cappello et al., 2017). The association of steroid therapy and CVD may be related to the unfavourable long‐term side effects (diabetes, hypertension, and obesity) which represent well‐known CV risk factors (Welsh, Grassia, Botha, Sattar, & Maffia, 2017). The effects of drugs currently used for inflammatory bowel disease treatment on cardiac manifestations are summarized in Table 2.
In conclusion, CVD risk is significant in inflammatory bowel disease evolution, accounting as the main cause of death among ulcerative colitis and Crohn's disease patients. This fact cannot be explained by traditional risk factors for CVD, since inflammatory bowel disease patients display less frequency of such factors. In addition, most of the immunosuppressive agents currently used as inflammatory bowel disease treatment are associated with a reduced risk of CVD. However, systemic chronic inflammation in inflammatory bowel disease seems to lead to endothelial dysfunction, more inflammation, and a hypercoagulable state that might evolve into CVD, posing a life‐threatening situation to inflammatory bowel disease patients.
7. CONCLUSION
CV manifestations present a considerable burden for patients with chronic inflammatory diseases. Although atherosclerosis is a common chronic inflammatory disease manifestation among the different diseases, ultimately leading to myocardial infarction in patients with chronic inflammatory diseases (Mason & Libby, 2015), different and specific CVD manifestations also exist among the different types of chronic inflammatory diseases (Table 1). Additionally, the different chronic inflammatory diseases share common but also different signalling molecules responsible for the chronic inflammatory disease complications that are apparent in these patients (Figure 1). Therefore, there is still considerable need to expand understanding of the underlying pathogenesis of cardiac complications in patients with chronic inflammatory diseases in order to identify potential novel targets to treat these patients. Appropriate animal models mimicking the different diseases should be indispensable tool in the search of pathogenetic mechanisms, although it is conceivable that no single animal model perfectly mimics a human disease. A range of inducible and spontaneous mouse models of systemic autoimmune diseases is available for mechanistic and therapeutic studies (reviewed in Sanghera et al., 2019; McNamee, Williams, & Seed, 2015). However, despite the significant clinical relevance of cardiac manifestations in chronic inflammatory diseases, experimental studies using these animal models to understand mechanisms underlying cardiac involvement are limited. Additionally, and importantly, it should be noted that despite the relevance and translatability of animal models of chronic inflammatory diseases (reviewed in McNamee et al., 2015), studies on myocardial infarction in these animals are scarce. Therefore, translational studies in animal models should be conducted in order to identify common molecular targets between chronic inflammatory diseases and myocardial infarction.
Treatment for chronic inflammatory diseases also results in divergent results in the myocardium. Hence, appropriate management strategies that consider the increased risk of CV complications in patients with chronic inflammatory diseases are important. Such strategies should focus on the prediction of CVD risk and its management through targeting the molecular pathogenetic mechanisms of each disease and traditional CVD risk factors.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This article is based upon work from COST Action EU‐CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). H.A.C.F. was supported by the Russian Government Program for competitive growth of Kazan Federal University, Kazan (Russian Federation), by the Singapore Heart Foundation (SHF/FG657P/2017), and by the Von Behring‐Röntgen‐Foundation (Marburg, Germany).
Lazou A, Ikonomidis I, Bartekova M, et al. Chronic inflammatory diseases, myocardial function and cardioprotection. Br J Pharmacol. 2020;177:5357–5374. 10.1111/bph.14975
Hector Cabrera Fuentes and Ioanna Andreadou have contributed equally to this work.
Contributor Information
Antigone Lazou, Email: lazou@bio.auth.gr.
Ioanna Andreadou, Email: jandread@pharm.uoa.gr.
REFERENCES
- Agca, R. , Heslinga, S. C. , Rollefstad, S. , Heslinga, M. , McInnes, I. B. , Peters, M. J. , … Nurmohamed, M. T. (2017). EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Annals of the Rheumatic Diseases, 76, 17–28. 10.1136/annrheumdis-2016-209775 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Keely, E. A. , Mathie, A. , Peter, J. A. , Veale, E. L. , Armstrong, J. H. , … GTP collaborators (2019). The concise guide to pharmacology 2019/2020: Introduction and other protein target. British Journal of Pharmacology, 176, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan, A. N. (2015). Epidemiology and risk factors for IBD. Nature Reviews Gastroenterology & Hepatology, 12(4), 205–217. 10.1038/nrgastro.2015.34 [DOI] [PubMed] [Google Scholar]
- Andrade, A. R. , Barros, L. L. , Azevedo, M. F. , Carlos, A. S. , Damião, A. O. , Sipahi, A. M. , & Leite, A. Z. (2018). Risk of thrombosis and mortality in inflammatory bowel disease. Clinical and Translational Gastroenterology, 9(4), 142 10.1038/s41424-018-0013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadou, I. , Cabrera‐Fuentes, H. A. , Devaux, Y. , Frangogiannis, N. G. , Frantz, S. , Guzik, T. , … Hausenloy, D. J. (2019). Immune cells as targets for cardioprotection: New players and novel therapeutic opportunities. Cardiovascular Research, 115, 1117–1130. 10.1093/cvr/cvz050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadou, I. , Efentakis, P. , Balafas, E. , Togliatto, G. , Davos, C. H. , Varela, A. , … Iliodromitis, E. K. (2017). Empagliflozin limits myocardial infarction in vivo and cell death in vitro: Role of STAT3, mitochondria, and redox aspects. Frontiers in Physiology, 19(8), 1077 10.3389/fphys.2017.01077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniwan, S. , Pardi, D. S. , Tremaine, W. J. , & Loftus, E. V. Jr. (2018). Increased risk of acute myocardial infarction and heart failure in patients with inflammatory bowel diseases. Clinical Gastroenterology and Hepatology, 16(10), 1607–1615. 10.1016/j.cgh.2018.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkema, E. V. , Svenungsson, E. , Von Euler, M. , Sjöwall, C. , & Simard, J. F. (2017). Stroke in systemic lupus erythematosus: A Swedish population‐based cohort study. Annals of the Rheumatic Diseases, 76(9), 1544–1549. 10.1136/annrheumdis-2016-210973 [DOI] [PubMed] [Google Scholar]
- Au, K. , Singh, M. , Bodukman, V. , Bae, S. , Maranian, P. , Ogawa, R. , … Khanna, D. (2011). Atherosclerosis in systemic sclerosis—A systematic review and meta‐analysis. Arthritis and Rheumatism, 63, 2078–2090. 10.1002/art.30380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry, M. C. , Maradit‐Kremers, H. , Reinalda, M. S. , Crowson, C. S. , Edwards, W. D. , & Gabriel, S. E. (2007). Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. The Journal of Rheumatology, 34, 937–942. http://www.jrheum.org/content/34/5/937 [PubMed] [Google Scholar]
- Barnabe, C. , Martin, B. , & Ghali, W. (2011). Systematic review and meta‐analysis: Anti‐tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Research (Hoboken), 63, 522–529. 10.1002/acr.20371 [DOI] [PubMed] [Google Scholar]
- Batko, B. , Urbański, K. , Świerkot, J. , Wiland, P. , Raciborski, F. , Jędrzejewski, M. , … Stajszczyk, M. (2019). Comorbidity burden and clinical characteristics of patients with difficult‐to‐control rheumatoid arthritis. Clinical Rheumatology, 38, 2473–2481. 10.1007/s10067-019-04579-1 [DOI] [PubMed] [Google Scholar]
- Blignaut, M. , Espach, Y. , van Vuuren, M. , Dhanabalan, K. , & Huisamen, B. (2019). Revisiting the cardiotoxic effect of chloroquine. Cardiovascular Drugs and Therapy, 33(1), 1–11. 10.1007/s10557-018-06847-9 [DOI] [PubMed] [Google Scholar]
- Bruce, I. N. , Urowitz, M. B. , Gladman, D. D. , Ibañez, D. , & Steiner, G. (2003). Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis and Rheumatism, 48(11), 3159–3167. 10.1002/art.11296 [DOI] [PubMed] [Google Scholar]
- Buie, J. J. , Renaud, L. L. , Muise‐Helmericks, R. , & Oates, J. C. (2017). IFN‐α negatively regulates the expression of endothelial nitric oxide synthase and nitric oxide production: implications for systemic lupus erythematosus. The Journal of Immunology, 199(6), 1979–1988. 10.4049/jimmunol.1600108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello, M. , Licata, A. , Calvaruso, V. , Bravatà, I. , Aiello, A. , Torres, D. , … Craxì, A. (2017). Increased expression of markers of early atherosclerosis in patients with inflammatory bowel disease. European Journal of Internal Medicine, 37, 83–89. 10.1016/j.ejim.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Carmona‐Rivera, C. , Zhao, W. , Yalavarthi, S. , & Kaplan, M. J. (2015). Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase‐2. Annals of the Rheumatic Diseases, 74(7), 1417–1424. 10.1136/annrheumdis-2013-204837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné, B. , Viprey, M. , Martin, J. , Schott, A. M. , Cucherat, M. , & Soubrier, M. (2019). Cardiovascular safety of tocilizumab: A systematic review and network meta‐analysis. PLoS ONE, 14(8), e0220178 10.1371/journal.pone.0220178 eCollection 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castejon, R. , Jimenez‐Ortiz, C. , Rosado, S. , Tutor‐Ureta, P. , Mellor‐Pita, S. , & Yebra‐Bango, M. (2016). Metabolic syndrome is associated with decreased circulating endothelial progenitor cells and increased arterial stiffness in systemic lupus erythematosus. Lupus, 25, 129–136. 10.1177/0961203315603138 [DOI] [PubMed] [Google Scholar]
- Chaanine, A. H. , Gordon, R. E. , Nonnenmacher, M. , Kohlbrenner, E. , Benard, L. , & Hajjar, R. J. (2015). High‐dose chloroquine is metabolically cardiotoxic by inducing lysosomes and mitochondria dysfunction in a rat model of pressure overload hypertrophy. Physiological Reports, 3(7), e12413 10.14814/phy2.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles‐Schoeman, C. , Yin Lee, Y. , Shahbazian, A. , Wang, X. , Elashoff, D. , Curtis, J. R. , … Reddy, S. T. (2017). Improvement of high‐density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis & Rheumatology, 69, 46–57. 10.1002/art.39833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X. , Yu, X. , Ding, Y. J. , Fu, Q. Q. , Xie, J. J. , Tang, T. T. , … Liao, Y. H. (2008). The Th17/Treg imbalance in patients with acute coronary syndrome. Clinical Immunology, 127, 89–97. 10.1016/j.clim.2008.01.009 [DOI] [PubMed] [Google Scholar]
- Choy, E. (2012). Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford), 51, v3–v11. 10.1093/rheumatology/kes113 [DOI] [PubMed] [Google Scholar]
- Chu, S. Y. , Chen, Y. J. , Liu, C. J. , Tseng, W. C. , Lin, M. W. , Hwang, C. Y. , & Liu, H. N. (2013). Increased risk of acute myocardial infarction in systemic sclerosis: A nationwide population‐based study. The American Journal of Medicine, 126, 982–988. 10.1016/j.amjmed.2013.06.025 [DOI] [PubMed] [Google Scholar]
- Chuang, Y. W. , Yu, M. C. , Lin, C. L. , Yu, T. M. , Shu, K. H. , & Kao, C. H. (2015). Risk of peripheral arterial occlusive disease in patients with systemic lupus erythematosus: A nationwide population‐based cohort study. Medicine, 94(46), e2121 10.1097/MD.0000000000002121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, C. P. , Avalos, I. , Oeser, A. , Gebretsadik, T. , Shintani, A. , Raggi, P. , & Stein, C. M. (2007). High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: Association with disease characteristics and cardiovascular risk factors. Annals of the Rheumatic Diseases, 66(2), 208–214. 10.1136/ard.2006.054973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, C. P. , Oeser, A. , Raggi, P. , Gebretsadik, T. , Shintani, A. K. , Sokka, T. , … Stein, C. M. (2005). Increased coronary artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis and Rheumatism, 52, 3045–3453. 10.1002/art.21288 [DOI] [PubMed] [Google Scholar]
- Cibor, D. , Domagala‐Rodacka, R. , Rodacki, T. , Jurczyszyn, A. , Mach, T. , & Owczarek, D. (2016). Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World Journal of Gastroenterology, 22(3), 1067–1077. 10.3748/wjg.v22.i3.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement, M. , Charles, N. , Escoubet, B. , Guedj, K. , Chauveheid, M. P. , Caligiuri, G. , … Sacre, K. (2015). CD4+ CXCR3+ T cells and plasmacytoid dendritic cells drive accelerated atherosclerosis associated with systemic lupus erythematosus. Journal of Autoimmunity, 63, 59–67. 10.1016/j.jaut.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Daïen, C. I. , Duny, Y. , Barnetche, T. , Daurès, J. P. , Combe, B. , & Morel, J. (2012). Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: A systematic review with meta‐analysis. Annals of the Rheumatic Diseases, 71, 862–868. 10.1136/annrheumdis-2011-201148 [DOI] [PubMed] [Google Scholar]
- Danese, S. , Katz, J. A. , Saibeni, S. , Papa, A. , Gasbarrini, A. , Vecchi, M. , & Fiocchi, C. (2003). Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut, 52(10), 1435–1441. 10.1136/gut.52.10.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese, S. , Sans, M. , Scaldaferri, F. , Sgambato, A. , Rutella, S. , Cittadini, A. , … Fiocchi, C. (2006). TNF‐α blockade down‐regulates the CD40/CD40L pathway in the mucosal microcirculation: A novel anti‐inflammatory mechanism of infliximab in Crohn's disease. The Journal of Immunology, 176(4), 2617–2624. 10.4049/jimmunol.176.4.2617 [DOI] [PubMed] [Google Scholar]
- del Rincon, I. , Williams, K. , Stern, M. P. , Freeman, G. L. , & Escalante, A. (2001). High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis and Rheumatism, 44, 2737–2745. [DOI] [PubMed] [Google Scholar]
- Derk, C. T. , & Jimenez, S. A. (2007). Acute myocardial infarction in systemic sclerosis patients: A case series. Clinical Rheumatology, 26, 965–968. 10.1007/s10067-006-0211-8 [DOI] [PubMed] [Google Scholar]
- Döring, Y. , Manthey, H. D. , Drechsler, M. , Lievens, D. , Megens, R. T. , Soehnlein, O. , … Daemen, M. J. (2012). Auto‐antigenic protein‐DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation, 125(13), 1673–1683. 10.1161/CIRCULATIONAHA.111.046755 [DOI] [PubMed] [Google Scholar]
- dos Santos, L. C. , Costa, A. V. , Lopes, L. G. , Leonel, A. J. , Aguilar, E. C. , Noviello, M. D. L. , … Alvarez‐Leite, J. I. (2015). Combination of azathioprine and aminosalicylate treatment prevent risk of cardiovascular disease in women with ulcerative colitis by reducing inflammation. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 21, 2305–2315. 10.12659/MSM.893865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid, R. E. , Rao, D. A. , Zhou, J. , Lo, S. F. , Ranjbaran, H. , Gallo, A. , … Tellides, G. (2009). Interleukin‐17 and interferon‐gamma are produced concomitantly by human coronary artery‐infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation, 119, 1424–1432. 10.1161/CIRCULATIONAHA.108.827618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnabawi, Y. A. , Dey, A. K. , Goyal, A. , Groenendyk, J. W. , Chung, J. H. , Belur, A. D. , … Mehta, N. N. (2019). Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: Results from a prospective observational study. Cardiovascular Research, 115(4), 721–728. 10.1093/cvr/cvz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnabawi, Y. A. , Oikonomou, E. K. , Dey, A. K. , Mancio, J. , Rodante, J. A. , Aksentijevich, M. , … Mehta, N. N. (2019). Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. The Journal of The American Medical Association of Cardiology, 4(9), 885–891. 10.1001/jamacardio.2019.2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccini, A. , Kaski, J. C. , & Camici, P. G. (2016). Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. European Heart Journal, 37(23), 1799–1806. 10.1093/eurheartj/ehw018 [DOI] [PubMed] [Google Scholar]
- Fanouriakis, A. , Kostopoulou, M. , Alunno, A. , Aringer, M. , Bajema, I. , Boletis, J. N. , … Houssiau, F. (2019). 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Annals of the Rheumatic Diseases, 78(6), 736–745. 10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- Feng, W. , Chen, G. , Cai, D. , Zhao, S. , Cheng, J. , & Shen, H. (2017). Inflammatory bowel disease and risk of ischemic heart disease: An updated meta‐analysis of cohort studies. Journal of the American Heart Association, 6(8), e005892 10.1161/JAHA.117.005892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumery, M. , Xiaocang, C. , Dauchet, L. , Gower‐Rousseau, C. , Peyrin‐Biroulet, L. , & Colombel, J. F. (2014). Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: A meta‐analysis of observational studies. Journal of Crohn's and Colitis, 8(6), 469–479. 10.1016/j.crohns.2013.09.021 [DOI] [PubMed] [Google Scholar]
- Gajendran, M. , Loganathan, P. , Catinella, A. P. , & Hashash, J. G. (2018). A comprehensive review and update on Crohn's disease. Disease‐a‐Month, 64(2), 20–57. 10.1016/j.disamonth.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Gajendran, M. , Loganathan, P. , Jimenez, G. , Catinella, A. P. , Ng, N. , Umapathy, C. , … Hashash, J. G. (2019). A comprehensive review and update on ulcerative colitis. Disease‐a‐Monthpii S0011‐5029, (19), 30031–30038. 10.1016/j.disamonth.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Gelfand, J. M. , Shin, D. B. , Alavi, A. , Torigian, D. A. , Werner, T. , Papadopoulos, M. , … Mehta, N. N. (2019). A phase IV, randomized, double‐blind, placebo‐controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP‐U trial). Journal of Investigative Dermatologypii: S0022‐202X(19)32537–0, 140, 85–93.e2. 10.1016/j.jid.2019.07.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand, J. M. , Weinstein, R. , Porter, S. B. , Neimann, A. L. , Berlin, J. A. , & Margolis, D. J. (2005). Prevalence and treatment of psoriasis in the United Kingdom: A population‐based study. Archives of Dermatology, 141, 1537–1541. 10.1001/archderm.141.12.1537 [DOI] [PubMed] [Google Scholar]
- Hak, A. E. , Karlson, E. W. , Feskanich, D. , Stampfer, M. J. , & Costenbader, K. H. (2009). Systemic lupus erythematosus and the risk of cardiovascular disease: Results from the nurses' health study. Arthritis Care & Research, 61(10), 1396–1402. 10.1002/art.24537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbord, M. , Annese, V. , Vavricka, S. R. , Allez, M. , Barreiro‐de Acosta, M. , Boberg, K. M. , … Juillerat, P. (2016). The first European evidence‐based consensus on extra‐intestinal manifestations in inflammatory bowel disease. Journal of Crohn's and Colitis, 10(3), 239–254. 10.1093/ecco-jcc/jjv213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollan, I. , Meroni, P. L. , Ahearn, J. M. , Cohen Tervaert, J. W. , Curran, S. , Goodyear, C. S. , … Wasko, M. C. (2013). Cardiovascular disease in autoimmune rheumatic diseases. Autoimmunity Reviews, 12, 1004–1015. 10.1016/j.autrev.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Holzer, M. P. , Wolf, S. , Curcic, R. , Birner‐Gruenberger, W. , Weger, M. , Inzinger, D. , … Marsche, G. (2012). Psoriasis alters HDL composition and cholesterol efflux capacity. Journal of Lipid Research, 53, 1618–1624. 10.1194/jlr.M027367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, C. , Imhof, I. , Li, L. , Niemi, E. , Bell, M. , Karliner, J. , & Nakamura, M. (2017). Cardiac immune cells in SKG mice with inflammatory arthritis before and after myocardial infarction. Arthritis & Rheumatology, 69(suppl 10). https://acrabstracts.org/abstract/cardiac-immune-cells-in-skg-mice-with-inflammatory-arthritis-before-and-after-myocardial-infarction/ [Google Scholar]
- Huang, J. , Maier, C. , Zhang, Y. , Soare, A. , Dees, C. , Beyer, C. , … Distler, J. H. W. (2017). Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Annals of the Rheumatic Diseases, 76(11), 1941–1948. 10.1136/annrheumdis-2016-210823 [DOI] [PubMed] [Google Scholar]
- Hurd, E. R. (1979). Extra‐articular manifestations of rheumatoid arthritis. Seminars in Arthritis & Rheumatism, 8, 151–176. 10.1016/S0049-0172(79)80005-0 [DOI] [PubMed] [Google Scholar]
- Iezzi, G. , Sonderegger, I. , Ampenberger, F. , Schmitz, N. , Marsland, B. J. , & Kopf, M. (2009). CD40–CD40L cross‐talk integrates strong antigenic signals and microbial stimuli to induce development of IL‐17‐producing CD4+ T cells. Proceedings of the National Academy of Sciences, 106(3), 876–881. 10.1073/pnas.0810769106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidis, I. , Athanassopoulos, G. , Lekakis, J. , Venetsanou, K. , Marinou, M. , Stamatelopoulos, K. , … Nihoyannopoulos, P. (2005). Myocardial ischemia induces interleukin‐6 and tissue factor production in patients with coronary artery disease: A dobutamine stress echocardiography study. Circulation, 112, 3272–3279. 10.1161/CIRCULATIONAHA.104.532259 [DOI] [PubMed] [Google Scholar]
- Ikonomidis, I. , Lekakis, J. P. , Nikolaou, M. , Paraskevaidis, I. , Andreadou, I. , Kaplanoglou, T. , … Kremastinos, D. T. (2008). Inhibition of interleukin‐1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation, 117, 2662–2669. 10.1161/CIRCULATIONAHA.107.731877 [DOI] [PubMed] [Google Scholar]
- Ikonomidis, I. , Makavos, G. , Katsimbri, P. , Boumpas, D. , Parissis, J. , & Iliodromitis, E. (2019). Imaging risk in multisystem inflammatory diseases. Journal of the American College of Cardiology Cardiovascular Imaging, 12(12), 2517–2537. 10.1016/j.jcmg.2018.06.033 [DOI] [PubMed] [Google Scholar]
- Ikonomidis, I. , Makavos, G. , Papadavid, E. , Varoudi, M. , Andreadou, I. , Gravanis, K. , … Lekakis, J. (2015). Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: The role of oxidative stress and inflammation. Canadian Journal of Cardiology, 31, 287–295. 10.1016/j.cjca.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Ikonomidis, I. , Papadavid, E. , Makavos, G. , Andreadou, I. , Varoudi, M. , Gravanis, K. , … Rigopoulos, D. (2017). Lowering Interleukin‐12 activity improves myocardial and vascular function compared with tumor necrosis factor‐a antagonism or cyclosporine in psoriasis. Circulation Cardiovascular Imaging, 10(9), e006283 10.1161/CIRCIMAGING.117.006283 [DOI] [PubMed] [Google Scholar]
- Ikonomidis, I. , Pavlidis, G. , Katsimbri, P. , Andreadou, I. , Triantafyllidi, H. , Tsoumani, M. , … Iliodromitis, E. (2019). Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clinical Research in Cardiology, 108, 1093 –1101. 10.1007/s00392-019-01443-9 [DOI] [PubMed] [Google Scholar]
- Ikonomidis, I. , Tzortzis, S. , Andreadou, I. , Paraskevaidis, I. , Katseli, C. , Katsimbri, P. , … Lekakis, J. (2014). Increased benefit of interleukin‐1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circulation. Cardiovascular Imaging, 7, 619–628. 10.1161/CIRCIMAGING.113.001193 [DOI] [PubMed] [Google Scholar]
- Ikonomidis, I. , Tzortzis, S. , Lekakis, J. , Paraskevaidis, I. , Andreadou, I. , Nikolaou, M. , … Kremastinos, D. T. (2009). Lowering interleukin‐1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart, 95, 1502–1507. 10.1136/hrt.2009.168971 [DOI] [PubMed] [Google Scholar]
- Kaiser, H. , Abdulla, J. , Henningsen, K. M. A. , Skov, L. , & Hansen, P. R. (2019). Coronary artery disease assessed by computed tomography in patients with psoriasis: A systematic review and meta‐analysis. Dermatology, 3, 1–10. 10.1159/000502138 [DOI] [PubMed] [Google Scholar]
- Kakuta, K. , Dohi, K. , Sato, Y. , Yamanaka, T. , Kawamura, M. , Ogura, T. , … Ito, M. (2015). Chronic inflammatory disease is an independent risk factor for coronary flow velocity reserve impairment unrelated to the process of coronary artery calcium deposition. Journal of the American Society of Echocardiography, 29(2), 173–180. 10.1016/j.echo.2015.09.001 [DOI] [PubMed] [Google Scholar]
- Karbach, S. , Croxford, A. L. , Oelze, M. , Schüler, R. , Minwegen, D. , Wegner, J. , … Münzel, T. (2014). Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis‐like skin disease. Arteriosclerosis, Thrombosis and Vascular Biology, 34, 2658–2668. 10.1161/ATVBAHA.114.304108 [DOI] [PubMed] [Google Scholar]
- Kimball, A. B. , Robinson, D. Jr. , Wu, Y. , Guzzo, C. , Yeilding, N. , Paramore, C. , … Bala, M. (2008). Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001‐2002. Dermatology, 217, 27–37. 10.1159/000121333 [DOI] [PubMed] [Google Scholar]
- Kowal‐Bielecka, O. , Fransen, J. , Avouac, J. , Becker, M. , Kulak, A. , Allanore, Y. , … Müller‐Ladner, U. (2017). Update of EULAR recommendations for the treatment of systemic sclerosis. Annals of the Rheumatic Diseases, 76(8), 1327–1339. 10.1136/annrheumdis-2016-209909 [DOI] [PubMed] [Google Scholar]
- Larosa, M. , Iaccarino, L. , Gatto, M. , Punzi, L. , & Doria, A. (2016). Advances in the diagnosis and classification of systemic lupus erythematosus. Expert Review of Clinical Immunology, 12(12), 1309–1320. 10.1080/1744666X.2016.1206470 [DOI] [PubMed] [Google Scholar]
- Le Gall, G. , Kirchgesner, J. , Bejaoui, M. , Landman, C. , Nion‐Larmurier, I. , Bourrier, A. , … Beaugerie, L. (2018). Clinical activity is an independent risk factor of ischemic heart and cerebrovascular arterial disease in patients with inflammatory bowel disease. PLoS ONE, 13(8), e0201991 10.1371/journal.pone.0201991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. L. , Sinnathurai, P. , Buchbinder, R. , Hill, C. , Lassere, M. , & March, L. (2018). Biologics and cardiovascular events in inflammatory arthritis: A prospective national cohort study. Arthritis Research and Therapy, 20(1), 171 10.1186/s13075-018-1669-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Fu, Q. , Cui, H. , Qu, B. , Pan, W. , Shen, N. , & Bao, C. (2011). Interferon‐α priming promotes lipid uptake and macrophage‐derived foam cell formation: A novel link between interferon‐α and atherosclerosis in lupus. Arthritis and Rheumatism, 63(2), 492–502. 10.1002/art.30165 [DOI] [PubMed] [Google Scholar]
- Li, W. , Titov, A. A. , & Morel, L. (2017). An update on lupus animal models. Current Opinion in Rheumatology, 29, 434–441. 10.1097/BOR.0000000000000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, K. , Kremers, H. , Crowson, C. , Snyder, M. , Therneau, T. , Roger, V. , & Gabriel, S. E. (2009). Autoantibodies and the risk of cardiovascular events. Journal of Rheumatology, 36, 2462–2469. 10.3899/jrheum.090188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , & Kaplan, M. J. (2019). Pathogenesis of accelerated atherosclerosis and vascular injury in systemic lupus erythematosus. Dubois' Lupus Erythematosus and Related Syndromes, 294–304. 10.1016/B978-0-323-47927-1.00021-9 [DOI] [Google Scholar]
- Lockshin, B. , Balagula, Y. , & Merola, J. F. (2018). Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. Journal of the American Academy of Dermatology, 79, 345–352. 10.1016/j.jaad.2018.02.040 [DOI] [PubMed] [Google Scholar]
- Luck, H. , Tsai, S. , Chung, J. , Clemente‐Casares, X. , Ghazarian, M. , Revelo, X. S. , … Copeland, J. K. (2015). Regulation of obesity‐related insulin resistance with gut anti‐inflammatory agents. Cell Metabolism, 21(4), 527–542. 10.1016/j.cmet.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Lv, S. , Liu, Y. , Zou, Z. , Li, F. , Zhao, S. , Shi, R. , … Tian, H. (2015). The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: A meta‐analysis. Clinical and Experimental Rheumatology, 33, 69–76. https://www.ncbi.nlm.nih.gov/pubmed/25327393 [PubMed] [Google Scholar]
- Madhur, M. S. , Lob, H. E. , McCann, L. A. , Iwakura, Y. , Blinder, Y. , Guzik, T. J. , & Harrison, D. G. (2010). Interleukin 17 promotes angiotensin II‐induced hypertension and vascular dysfunction. Hypertension, 55, 500–507. 10.1161/HYPERTENSIONAHA.109.145094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makavos, G. , Ikonomidis, I. , Andreadou, I. , Varoudi, M. , Kapniari, M. , Loukeri, M. , … Papadavid, E. (2019). Effects of interleukin 17A inhibition on myocardial deformation and vascular function in psoriasis. Canadian Journal of Cardiologyin press, 36, 100–111. 10.1016/j.cjca.2019.06.021 [DOI] [PubMed] [Google Scholar]
- Mangoni, A. A. , Zinellu, A. , Sotgia, S. , Carru, C. , & Erre, G. L. (2017). Methotrexate and cardiovascular protection: Current evidence and future directions. Clinical Medicine Insights: Therapeutics, 9, 1–12. 10.1177/1179559X17741289 [DOI] [Google Scholar]
- Maradit‐Kremers, H. , Crowson, C. S. , Nicola, P. J. , Ballman, K. V. , Roger, V. L. , Jacobsen, S. J. , & Gabriel, S. E. (2005). Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population‐based cohort study. Arthritis and Rheumatism, 52, 402–411. 10.1002/art.20853 [DOI] [PubMed] [Google Scholar]
- Mason, J. C. , & Libby, P. (2015). Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. European Heart Journal, 36, 482–489. 10.1093/eurheartj/ehu403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrogeni, S. , Bratis, K. , Karabela, G. , Spiliotis, G. , Wijk, K. V. , Hautemann, D. , … Kolovou, G. (2015). Cardiovascular magnetic resonance imaging clarifies cardiac pathophysiology in early, asymptomatic diffuse systemic sclerosis. Inflammation & Allergy ‐ Drug Targets, 14(1), 29–36. 10.2174/1871528114666150916112551 [DOI] [PubMed] [Google Scholar]
- McCarey, D. W. , McInnes, I. B. , Madhok, R. , Hampson, R. , Scherbakov, O. , Ford, I. , … Sattar, N. (2004). Trial of atorvastatin in rheumatoid arthritis (TARA): Double‐blind, randomized placebo controlled trial. Lancet, 363(9426), 2015–2021. 10.1016/S0140-6736(04)16449-0 [DOI] [PubMed] [Google Scholar]
- McDonnell, M. , Liang, Y. , Noronha, A. , Coukos, J. , Kasper, D. L. , Farraye, F. A. , & Ganley‐Leal, L. M. (2011). Systemic toll‐like receptor ligands modify B‐cell responses in human inflammatory bowel disease. Inflammatory Bowel Diseases, 17(1), 298–307. 10.1002/ibd.21424 [DOI] [PubMed] [Google Scholar]
- McGill, J. B. , Johnson, M. , Hurst, S. , Cade, W. T. , Yarasheski, K. E. , Ostlund, R. E. , … de las Fuentes, L. (2019). Low dose chloroquine decreases insulin resistance in human metabolic syndrome but does not reduce carotid intima‐media thickness. Diabetology & Metabolic Syndrome, 11(1), 61 10.1186/s13098-019-0456-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes, I. , & Schett, G. (2011). The pathogenesis of rheumatoid arthritis. New England Journal of Medicine, 365, 232205–232219. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- McNamee, K. , Williams, R. , & Seed, M. (2015). Animal models of rheumatoid arthritis: How informative are they? European Journal of Pharmacology, 759, 278–286. 10.1016/j.ejphar.2015.03.047 [DOI] [PubMed] [Google Scholar]
- Meili‐Butz, S. , Niermann, T. , Fasler‐Kan, E. , Barbosa, V. , Butz, N. , John, D. , … Zaugg, C. E. (2008). Dimethyl fumarate, a small molecule drug for psoriasis, inhibits nuclear factor‐κB and reduces myocardial infarct size in rats. European Journal of Pharmacology, 586, 251–258. 10.1016/j.ejphar.2008.02.038 [DOI] [PubMed] [Google Scholar]
- Meune, C. , Touze, E. , Trinquart, L. , & Allanore, Y. (2009). Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: A systematic review and meta‐analysis of cohort studies. Rheumatology (Oxford), 48, 1309–1313. 10.1093/rheumatology/kep252 [DOI] [PubMed] [Google Scholar]
- Micha, R. , Imamura, F. , Wyler Von Ballmoos, M. , Solomon, D. , Hernan, M. , Ridker, P. , & Mozaffarian, D. (2011). Systematic review and meta‐analysis of methotrexate use and risk of cardiovascular disease. American Journal of Cardiology, 108, 1362–1370. 10.1016/j.amjcard.2011.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, N. E. , Harrison, N. , Junga, Z. , & Singla, M. (2018). Heart under attack: cardiac manifestations of inflammatory bowel disease. Inflammatory Bowel Diseases, 24(11), 2322–2326. 10.1093/ibd/izy157 [DOI] [PubMed] [Google Scholar]
- Moonen, J. R. A. , de Leeuw, K. , van Seijen, X. J. G. Y. , Kallenberg, C. G. , van Luyn, M. J. , Bijl, M. , & Harmsen, M. C. (2007). Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Research & Therapy, 9(4), R84 10.1186/ar2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myasoedova, E. , Crowson, C. S. , Kremers, H. M. , Roger, V. L. , Fitz‐Gibbon, P. D. , Therneau, T. M. , & Gabriel, S. E. (2011). Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Annals of the Rheumatic Diseases, 70, 482–487. 10.1136/ard.2010.135871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, K. , & Sano, S. (2018). Mouse models of psoriasis and their relevance. Journal of Dermatology, 45, 252–263. 10.1111/1346-8138.14112 [DOI] [PubMed] [Google Scholar]
- Navarro‐Millán, I. , Charles‐Schoeman, C. , Yang, S. , Bathon, J. M. , Bridges, S. L. Jr. , Chen, L. , … Curtis, J. R. (2013). Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: Results from the treatment of early rheumatoid arthritis trial. Arthritis and Rheumatism, 65, 1430–1438. 10.1002/art.37916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogdie, A. , Yu, Y. , Haynes, K. , Love, T. J. , Maliha, S. , Jiang, Y. , … Gelfand, J. M. (2015). Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: A population‐based cohort study. Annals of the Rheumatic Diseases, 74, 326–332. 10.1136/annrheumdis-2014-205675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan, M. , Bruce, I. N. , & Symmons, D. P. (2016). Cardiovascular risk and its modification in patients with connective tissue diseases. Best Practice & Research Clinical Rheumatology, 30(1), 81–94. 10.1016/j.berh.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Owczarek, D. , Cibor, D. , & Mach, T. (2010). Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), arginine, and 8‐iso‐prostaglandin F2α (8‐iso‐PGF2α) level in patients with inflammatory bowel diseases. Inflammatory Bowel Diseases, 16(1), 52–57. 10.1002/ibd.20994 [DOI] [PubMed] [Google Scholar]
- Paschou, S. A. , Kothonas, F. , Lafkas, A. , Myroforidis, A. , Loi, V. , Terzi, T. , … Vryonidou, A. (2018). Favorable effect of anti‐TNF therapy oninsulin sensitivity in nonobese, nondiabetic patients with inflammatorybowel disease. International Journal of Endocrinology, 2018, 6712901 10.1155/2018/6712901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pironti, G. , Bersellini‐Farinotti, A. , Agalave, N. M. , Sandor, K. , Fernandez‐Zafra, T. , Jurczak, A. , … Andersson, D. C. (2018). Cardiomyopathy, oxidative stress and impaired contractility in a rheumatoid arthritis mouse model. Heart, 104, 2026–2034. 10.1136/heartjnl-2018-312979 [DOI] [PubMed] [Google Scholar]
- Prinz, J. C. (2010). From bench to bedside–translational research in psoriasis. Journal of the European Academy of Dermatology and Venereology, 24(suppl 6), 1–4. 10.1111/j.1468-3083.2010.03829.x [DOI] [PubMed] [Google Scholar]
- Prüfer, J. , Schuchardt, M. , Tölle, M. , Prüfer, N. , Höhne, M. , Zidek, W. , & van der Giet, M. (2014). Harmful effects of the azathioprine metabolite 6‐mercaptopurine in vascular cells: Induction of mineralization. PloSOne, 9(7), e101709 10.1371/journal.pone.0101709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryshchep, S. , Sato, K. , Goronzy, J. J. , & Weyand, C. M. (2006). T cell recognition and killing of vascular smooth muscle cells in acute coronary syndrome. Circulation Research, 98, 1168–1176. 10.1161/01.RES.0000220649.10013.5c [DOI] [PubMed] [Google Scholar]
- Rajagopalan, S. , Somers, E. C. , Brook, R. D. , Kehrer, C. , Pfenninger, D. , Lewis, E. , … Kaplan, M. J. (2004). Endothelial cell apoptosis in systemic lupus erythematosus: A common pathway for abnormal vascular function and thrombosis propensity. Blood, 103(10), 3677–3683. 10.1182/blood-2003-09-3198 [DOI] [PubMed] [Google Scholar]
- Razani, B. , Feng, C. , & Semenkovich, C. F. (2010). p53 is required for chloroquine‐induced atheroprotection but not insulin sensitization. Journal of Lipid Research, 51(7), 1738–1746. 10.1194/jlr.M003681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, F. , Doherty, M. , Grainge, M. J. , Lanyon, P. , & Zhang, W. (2017). The worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology, 56(11), 1945–1961. 10.1093/rheumatology/kex260 [DOI] [PubMed] [Google Scholar]
- Reich, K. (2012). The concept of psoriasis as a systemic inflammation: Implications for disease management. Journal of the European Academy of Dermatology and Venereology, 26(Suppl 2), 3–11. 10.1111/j.1468-3083.2011.04410.x [DOI] [PubMed] [Google Scholar]
- Roifman, I. , Beck, P. L. , Anderson, T. J. , Eisenberg, M. J. , & Genest, J. (2011). Chronic inflammatory diseases and cardiovascular risk: A systematic review. Canadian Journal of Cardiology, 27, 174–182. 10.1016/j.cjca.2010.12.040 [DOI] [PubMed] [Google Scholar]
- Roldan, C. (2008). Valvular and coronary heart disease in systemic inflammatory diseases. Heart, 94, 1089–1101. 10.1136/hrt.2007.132787 [DOI] [PubMed] [Google Scholar]
- Roldan, C. A. , DeLong, C. , Qualls, C. , & Crawford, M. H. (2007). Characterization of valvular heart disease in rheumatoid arthritis by transesophageal echocardiography and clinical correlates. American Journal of Cardiology, 100, 496–502. 10.1016/j.amjcard.2007.03.048 [DOI] [PubMed] [Google Scholar]
- Roubile, C. , Richer, V. , Starnino, T. , McCourt, C. , McFarlane, A. , Fleming, P. , … Haraoui, B. (2015). The effects of tumour necrosis factor inhibitors, methotrexate, non‐steroidal anti‐inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta‐analysis. Annals of the Rheumatic Diseases, 74, 480–489. 10.1136/annrheumdis-2014-206624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungoe, C. , Basit, S. , Ranthe, M. F. , Wohlfahrt, J. , Langholz, E. , & Jess, T. (2013). Risk of ischaemic heart disease in patients with inflammatory bowel disease: A nationwide Danish cohort study. Gut, 62(5), 689–694. 10.1136/gutjnl-2012-303285 [DOI] [PubMed] [Google Scholar]
- Sanghera, C. , Wong, L. M. , Panahi, M. , Sintou, A. , Hasham, M. , & Sattler, S. (2019). Cardiac phenotype in mouse models of systemic autoimmunity. Disease Models & Mechanisms, 12(3), dmm036947 10.1242/dmm.036947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar, N. , & McInnes, I. B. (2005). Vascular comorbidity in rheumatoid arthritis: Potential mechanisms and solutions. Current Opinion in Rheumatology, 17, 286–292. 10.1097/01.bor.0000158150.57154.f9 [DOI] [PubMed] [Google Scholar]
- Schoenfeld, S. R. , Lu, L. , Rai, S. K. , Seeger, J. D. , Zhang, Y. , & Choi, H. K. (2016). Statin use and mortality in rheumatoid arthritis: A general population‐based cohort study. Annals of the Rheumatic Diseases, 75, 1315–1320. 10.1136/annrheumdis-2015-207714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu, J. J. , Sung, P. H. , Leu, S. , Chai, H. T. , Zhen, Y. Y. , Chen, Y. C. , … Yip, H. K. (2013). Innate immune response after acute myocardial infarction and pharmacomodulatory action of tacrolimus in reducing infarct size and preserving myocardial integrity. Journal of Biomedical Science, 20, 82–14. 10.1186/1423-0127-20-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S. , Fumery, M. , Singh, A. G. , Singh, N. , Prokop, L. J. , Dulai, P. S. , … Curtis, J. R. (2019). Comparative risk of cardiovascular events with biologic and synthetic disease‐modifying anti‐rheumatic drugs in patients with rheumatoid arthritis: A systematic review and meta‐analysis. Arthritis Care & Research. 10.1002/acr.23875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spethman, S. , Rieper, K. , Riemekasten, G. , Borges, A. C. , Schattke, S. , Burmester, G. R. , … Knebel, F. (2014). Echocardiographic follow‐up of patients with systemic sclerosis by 2D speckle tracking echocardiography of the left ventricle. Cardiovascular Ultrasound, 12, 13 10.1186/1476-7120-12-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyers, C. , & Miller, F. (2014). Endothelial dysfunction in chronic inflammatory diseases. International Journal of Molecular Sciences, 15(7), 11324–11349. 10.3390/ijms150711324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, R. , & Keaney, J. F. Jr. (2004). Role of oxidative modifications in atherosclerosis. Physiological Reviews, 84, 1381–1478. 10.1152/physrev.00047.2003 [DOI] [PubMed] [Google Scholar]
- Su, H. J. , Chiu, Y. T. , Chiu, C. T. , Lin, Y. C. , Wang, C. Y. , Hsieh, J. Y. , & Wei, S. C. (2019). Inflammatory bowel disease and its treatment in 2018: Global and Taiwanese status updates. Journal of the Formosan Medical Association, 118(7), 1083–1092. 10.1016/j.jfma.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Tam, L. S. , Tomlinson, B. , Chu, T. T. , Li, M. , Leung, Y. Y. , Kwok, L. W. , … Li, E. K. (2008). Cardiovascular risk profile of patients with psoriatic arthritis compared to controls—The role of inflammation. Rheumatology, 47, 718–723. 10.1093/rheumatology/ken090 [DOI] [PubMed] [Google Scholar]
- Tektonidou, M. G. , Kravvariti, E. , Konstantonis, G. , Tentolouris, N. , Sfikakis, P. P. , & Protogerou, A. (2017). Subclinical atherosclerosis in systemic lupus erythematosus: Comparable risk with diabetes mellitus and rheumatoid arthritis. Autoimmunity Reviews, 16(3), 308–312. 10.1016/j.autrev.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Thacker, S. G. , Zhao, W. , Smith, C. K. , Luo, W. , Wang, H. , Vivekanandan‐Giri, A. , & Eitzman, D. T. (2012). Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis and Rheumatism, 64(9), 2975–2985. 10.1002/art.34504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton, C. C. , Al‐Rashed, F. , Calay, D. , Birdsey, G. M. , Bauer, A. , Mylroie, H. , … Mason, J. C. (2016). Methotrexate‐mediated activation of an AMPK‐CREB‐dependent pathway: A novel mechanism for vascular protection in chronic systemic inflammation. Annals of the Rheumatic Diseases, 75(2), 439–448. 10.1136/annrheumdis-2014-206305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms, T. E. , Panoulas, V. F. , Douglas, K. M. J. , Nightingale, P. , Smith, J. P. , Griffiths, H. , … Kitas, G. D. (2011). Are lipid ratios less susceptible to change with systemic inflammation than individual lipid components in patients with rheumatoid arthritis? Angiology, 62, 167–175. 10.1177/0003319710373749 [DOI] [PubMed] [Google Scholar]
- Tselios, K. , Deeb, M. , Gladman, D. D. , Harvey, P. , Akhtari, S. , Mak, S. , … Urowitz, M. B. (2019). Antimalarial‐induced cardiomyopathy in systemic lupus erythematosus: As rare as considered? The Journal of Rheumatology, 46(4), 391–396. 10.3899/jrheum.180124 [DOI] [PubMed] [Google Scholar]
- Tselios, K. , Gladman, D. D. , Harvey, P. , Mak, S. , Chantal, M. , Butany, J. , & Urowitz, M. B. (2016). Hydroxychloroquine‐induced cardiomyopathy in systemic lupus erythematosus. Journal of Clinical Rheumatology, 22(5), 287–288. 10.1097/RHU.0000000000000400 [DOI] [PubMed] [Google Scholar]
- Ueno, A. , Jeffery, L. , Kobayashi, T. , Hibi, T. , Ghosh, S. , & Jijon, H. (2018). Th17 plasticity and its relevance to inflammatory bowel disease. Journal of Autoimmunity, 87, 38–49. 10.1016/j.jaut.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Ungprasert, P. , Charoenpong, P. , Ratanasrimeta, P. , Thongprayoon, C. , Cheungpasitporn, W. , & Suksaranjit, P. (2014). Risk of coronary artery disease in patients with systemic sclerosis: A systematic review and meta‐analysis. Clinical Rheumatology, 33, 1099–1104. 10.1007/s10067-014-2681-4 [DOI] [PubMed] [Google Scholar]
- van Leuven, S. I. , Mendez‐Fernandez, Y. V. , Wilhelm, A. J. , Wade, N. S. , Gabriel, C. L. , Kastelein, J. J. , … Major, A. S. (2012). Mycophenolate mofetil but not atorvastatin attenuates atherosclerosis in lupus‐prone LDLr−/− mice. Annals of the Rheumatic Diseases, 71(3), 408–414. 10.1136/annrheumdis-2011-200071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venalis, P. , Kumánovics, G. , Schulze‐Koops, H. , Distler, A. , Dees, C. , Zerr, P. , … Distler, J. H. (2015). Cardiomyopathy in murine models of systemic sclerosis. Arthritis& Rheumatology, 67(2), 508–516. 10.1002/art.38942 [DOI] [PubMed] [Google Scholar]
- Virdis, A. , Tani, C. , Duranti, E. , Vagnani, S. , Carli, L. , Kühl, A. A. , … Taddei, S. (2015). Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Arthritis Research & Therapy, 17(1), 277 10.1186/s13075-015-0790-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stebut, E. , Reich, K. , Thaçi, D. , Koenig, W. , Pinter, A. , Körber, A. , … Frueh, J. (2019). Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. Journal of Investigative Dermatology, 139(5), 1054–1062. 10.1016/j.jid.2018.10.042 [DOI] [PubMed] [Google Scholar]
- Voskuyl, A. E. (2006). The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology (Oxford), 45(Suppl4), iv4–iv7. 10.1093/rheumatology/kel313 [DOI] [PubMed] [Google Scholar]
- Wang, Z. , & Bao, Z. (2017). Effect of anti‐gut inflammatory agent on insulin resistance and lipid profile of mice fed different diets. Tropical Journal of Pharmaceutical Research, 16(11), 2651–2658. 10.4314/tjpr.v16i11.12 [DOI] [Google Scholar]
- Welsh, P. , Grassia, G. , Botha, S. , Sattar, N. , & Maffia, P. (2017). Targeting inflammation to reduce cardiovascular disease risk: A realistic clinical prospect? British Journal of Pharmacology, 174(22), 3898–3913. 10.1111/bph.13818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowska, M. , Wątroba, M. , Ciużyńska, G. , Bral, M. , Szukiewicz, D. , & Maśliński, S. (2013). Ischaemic heart preconditioning in rats with adjuvant‐induced arthritis. Kardiologia Polska, 8, 839–844. 10.5603/KP.2013.0196 [DOI] [PubMed] [Google Scholar]
- Wu, G. C. , Liu, H. R. , Leng, R. X. , Li, X. P. , Li, X. M. , Pan, H. F. , & Ye, D. Q. (2016). Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systemic review and meta‐analysis. Autoimmunity Reviews, 15(1), 22–37. 10.1016/j.autrev.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Yang, C. F. , Chen, Y. Y. , Singh, J. P. , Hsu, S. F. , Liu, Y. W. , Yang, C. Y. , … Meng, T. C. (2019). Targeting protein tyrosine phosphatase PTP‐PEST for therapeutic intervention in acute myocardial infarction. Cardiovascular Research, pii: cvz165. 10.1093/cvr/cvz165 [DOI] [PubMed] [Google Scholar]
- Yogasundaram, H. , Putko, B. N. , Tien, J. , Paterson, D. I. , Cujec, B. , Ringrose, J. , & Oudit, G. Y. (2014). Hydroxychloroquine‐induced cardiomyopathy: Case report, pathophysiology, diagnosis, and treatment. Canadian Journal of Cardiology, 30(12), 1706–1715. 10.1016/j.cjca.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Yong, K. , Dogra, G. , Boudville, N. , Chan, D. , Adams, L. , Ching, H. , … Lim, W. H. (2013). Interleukin‐12 is associated with arterial stiffness in healthy individuals. American Journal of Hypertension, 26, 159–162. 10.1093/ajh/hps032 [DOI] [PubMed] [Google Scholar]
- Yoshida, H. , Fujiwara, H. , Fujiwara, T. , Ikehara, S. , & Hamashima, Y. (1987). Quantitative analysis of myocardial infarction in (NZW x BXSB)F1 hybrid mice with systemic lupus erythematosus and small coronary artery disease. The American Journal of Pathology, 129, 477–485. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1899816 [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Niessner, A. , Nakajima, T. , Ma‐Krupa, W. , Kopecky, S. L. , Frye, R. L. , … Weyand, C. M. (2006). Interleukin 12 induces T‐cell recruitment in to the atherosclerotic plaque. Circulation Research, 98, 524–531. 10.1161/01.RES.0000204452.46568.57 [DOI] [PubMed] [Google Scholar]


