Abstract
Translation of cardioprotective interventions aimed at reducing myocardial injury during ischaemia–reperfusion from experimental studies to clinical practice is an important yet unmet need in cardiovascular medicine. One particular challenge facing translation is the existence of demographic and clinical factors that influence the pathophysiology of ischaemia–reperfusion injury of the heart and the effects of treatments aimed at preventing it. Among these factors, age and sex are prominent and have a recognised role in the susceptibility and outcome of ischaemic heart disease. Remarkably, some of the most powerful cardioprotective strategies proven to be effective in young animals become ineffective during ageing. This article reviews the mechanisms and implications of the modulatory effects of ageing and sex on myocardial ischaemia–reperfusion injury and their potential effects on cardioprotective interventions.
LINKED ARTICLES
This article is part of a themed issue on Risk factors, comorbidities, and comedications in cardioprotection. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.23/issuetoc
Abbreviations
- AMI
acute myocardial infarction
- CAD
coronary artery disease
- CK
creatine kinase
- ECM
extracellular matrix
- GSK3β
glycogen synthase kinase 3β
- IHD
ischaemic heart disease
- IPC
ischaemic preconditioning
- IPostC
ischaemic postconditioning
- IR
ischaemia–reperfusion
- IS
infarct size
- LV
left ventricle
- MI
myocardial infarction
- MOM
mitochondrial outer membrane
- mPTP
mitochondrial permeability transition pore
- MtCK
mitochondrial creatine kinase
- OXPHOS
oxidative phosphorylation
- PCr
phosphocreatine
- RCS
respiratory chain supercomplexes
- RIPC
remote ischaemic preconditioning
- RISK
reperfusion injury salvage kinase
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- STEMI
ST‐segment elevation myocardial infarction
- VDAC
voltage‐dependent anion channel
1. INTRODUCTION
Key consequences of ischaemic heart disease (IHD) are contractile dysfunction, irreversible myocardial injury, heart failure, arrhythmia, and death. Their magnitude depends mainly on the extension, severity, and duration of the ischaemic insult. Although myocardial ischaemia may occur under different clinical conditions, including elevated oxygen demand, cardiac arrest, or cardiac therapeutic interventions such as surgery, the most important clinical condition is acute coronary occlusion leading to severe transmural ischaemia and development of myocardial infarction (MI). In this case, the mainstay of treatment is limitation of the duration of ischaemia through emergency re‐opening of the occluded vessel, generally through percutaneous coronary intervention, with pharmacological treatments to prevent re‐occlusion. Even after a successful re‐canalisation of the affected artery, restoration of blood flow is accompanied by a paradoxical worsening of the functional changes associated with ischaemia (“reversible reperfusion injury”), particularly arrhythmias and stunning, and in more severe cases by reperfusion‐induced cell death (“lethal reperfusion injury”). This comes in addition to potential microvascular dysfunction or injury when establishing reflow. The contribution of lethal reperfusion injury to the final extent of MI has been irrefutably demonstrated in the experimental setting (Piper, Garcia‐Dorado, & Ovize, 1998) and in patients with segment elevation myocardial infarction (STEMI), although here the efficacy of the therapeutic interventions has been less consistent (Hausenloy et al., 2017). The time window to effectively prevent or limit myocardial injury in patients with STEMI is very short (only a few hours from the onset of chest pain), and the development of ways to reduce the myocardial consequences of ischaemia of a given severity and duration by interfering with the molecular mechanisms of ischaemic injury is a priority (Ibanez et al., 2017).
Preclinical studies have identified a variety of molecular pathways potentially involved in cell death during ischaemia–reperfusion (IR) that are targetable by a therapeutic intervention (Hausenloy et al., 2017). Nevertheless, and despite promising outcomes in the experimental setting, the translation of such cardioprotective interventions to the clinical practice for the benefit of patients with IHD has repeatedly failed (Hausenloy, Garcia‐Dorado, et al., 2017; Heusch, 2017). The causes for this failure are multiple and have been reviewed elsewhere (Botker et al., 2018; Davidson et al., 2019; Heusch, 2017; Heusch, 2018; Kleinbongard, Botker, Ovize, Hausenloy, & Heusch, 2019). The inadequate simulation of the clinical conditions of patients (lack of confounders present in “real life”) has been the object of intense debate, as the patients with IHD recruited into cardioprotection clinical trials normally have an advanced age and several confounders, including one or more co‐morbidities (hyperlipidaemia, hypertension, and metabolic disorders) and co‐medications, conditions that have not been properly reproduced in the young and healthy animals used for research purposes. Among confounders, ageing and sex and their interplay (loss of hormonal influence is part of normal ageing) have been increasingly recognised as major determinants of the outcomes of IR injury (Davidson et al., 2019). Neglecting their role is proposed as a fundamental contributor of the translational gap and may explain the suboptimal and sometimes inconsistent effects of cardioprotective strategies (Ferdinandy, Hausenloy, Heusch, Baxter, & Schulz, 2014). Here, we review the current knowledge about the role of ageing and sex as (patho)physiological modulators of IR injury development, cardioprotection signalling, and efficacy of the therapeutic interventions.
2. AGE AND TOLERANCE TO ISCHAEMIA–REPERFUSION INJURY
Ageing of the human population is a global demographic phenomenon with profound effects on the epidemiology, severity, and mortality rates of IHD (Moran et al., 2014). About two thirds of myocardial infarctions occur in patients older than 65 years and one third occurs in patients older than 75 years (Engberding & Wenger, 2017). Despite this, randomised clinical trials preferentially include patients younger than 75 years, consistently underestimating the real number of elderly patients that cardiologists encounter in clinical practice (Lee, Alexander, Hammill, Pasquali, & Peterson, 2001). Therefore, most treatments applied to elderly patients have been extrapolated from the results obtained in studies performed with younger patients and are not based on specific evidence that supports their efficacy and safety in this age range.
Advanced age not only increases the incidence of IHD due to progressive arterial disease among other factors, but also exacerbates its clinical manifestations and impairs the prognosis and survival of the patients (Shih, Lee, Lee, & Boyle, 2011). Elderly patients are more likely to die after MI due to an increased rate of mechanical complications (papillary muscle rupture, left ventricular free wall rupture, and ventricular septal defects), in‐hospital mortality, and long‐term mortality after discharge (Mehta et al., 2001; Shih et al., 2011) According to the GISSI‐1 study, each year of age is associated with a 6% increase in the relative risk of mortality secondary to an acute coronary syndrome (Maggioni et al., 1993). This is in part due to the fact that age is a main determinant of the extension of necrosis within the myocardial segment exposed to ischaemia (Shih et al., 2011), which, in turn, is critically associated with the future occurrence of arrhythmias and development of heart failure. Indeed, being old significantly increases the likelihood of developing adverse cardiac remodelling and heart failure secondary to MI (Ezekowitz et al., 2009).
The effects of ageing on IS result from many factors including compromised functional reserve of the myocardium in elderly patients and increased burden of co‐morbidities (Barnett et al., 2012; Ekerstad et al., 2011). The heart develops some age‐dependent structural and physiological adaptations, such as changes in cardiomyocyte number, size, and function (Bernhard & Laufer, 2008) and altered composition of the extracellular matrix (ECM) (Horn & Trafford, 2016) that may influence the severity of myocardial dysfunction after an ischaemic episode. Remarkably, the increased vulnerability of the aged heart to develop cardiomyocyte dysfunction during IR has been reproduced in a myriad of experimental models, including isolated cardiomyocytes, in which the contribution of cell‐to‐cell interaction, extracellular components, vascular factors, and co‐morbidities is absent (Fernandez‐Sanz et al., 2015; Willems, Zatta, Holmgren, Ashton, & Headrick, 2005). This evidence supports the concept that beyond other factors, aged cardiomyocytes develop endogenous changes that reduce their ability to tolerate and survive an IR insult (Strait & Lakatta, 2012; Willems et al., 2005).
Efficient therapeutic strategies specifically addressed to treat IHD in elderly patients are challenged by the fact that some well‐characterised endogenous cardioprotective pathways and targets are attenuated in aged cardiomyocytes (see Section 4.1), rendering them more resistant to otherwise powerful therapeutic interventions, such as ischaemic preconditioning and postconditioning. To what extent this feature affects other drug‐induced cardioprotective strategies is a matter of research. Alterations in the inflammatory response induced by ischaemia, together with a gradual deterioration of the reparative fibrosis by mechanisms that involve the up‐regulation of β‐galactosidase and other key senescent markers, such as p53 (Zhu et al., 2013), have been shown to contribute to the higher prevalence of adverse remodelling and heart failure after MI in the elderly (Bujak et al., 2008; Westman et al., 2016).
3. MECHANISMS OF REDUCED TOLERANCE TO ISCHAEMIA–REPERFUSION INJURY
3.1. Intracellular changes involved in IR susceptibility during ageing
Aged cardiomyocytes develop a multiplicity of abnormalities in mitochondrial function, calcium handling, metabolism, and signalling pathways that can affect their susceptibility to IR (Jahangir et al., 2007; Figure 1). Some of the most prevalent pathophysiological traits of aged cardiomyocytes, that is, energy demand/supply mismatch and excessive oxidative damage, resemble those of failing cardiomyocytes (Morita & Komuro, 2018). Regular arrangement of mitochondria and adequate mitochondria‐sarcoplasmic reticulum (SR) communication are essential for metabolic plasticity, playing an important role in cell death or cardioprotection (Anmann et al., 2014; Ruiz‐Meana, Fernandez‐Sanz, & Garcia‐Dorado, 2010). Calcium transferred from SR to mitochondria adjusts ATP production with cell energy demand and maintains the pool of the antioxidant GSH through the regeneration of Krebs cycle‐coupled NADPH (Kohlhaas, Nickel, & Maack, 2017). Importantly, efficient inter‐organelle calcium exchange strongly depends on the tight alignment (50–100 nm distance) between ryanodine receptors (RyR) at the SR and the voltage‐dependent anion channel (VDAC) at the mitochondria (Garcia‐Perez, Hajnoczky, & Csordas, 2008). Previous studies indicate that the anatomical proximity between SR and mitochondria is partially disrupted in aged hearts, altering calcium exchange and favouring oxidative stress at the interface between both organelles (Fernandez‐Sanz et al., 2014). Recently, the RyR has been demonstrated to be a target of glycative damage during ageing in human and murine myocardium (Ruiz‐Meana et al., 2019). Therefore, glycation of RyRs could be responsible for the disruption of the RyR–VDAC bridges, with detrimental functional consequences. Indeed, RyR glycation induces a SR‐dependent calcium leak. In intact cells, chronic exposure of mitochondria to dysfunctional SR favours the concomitant increase of calcium precipitation in their matrix (Ruiz‐Meana et al., 2019). Excess of ROS and mitochondrial calcium are important triggers of mitochondrial permeability transition pore (mPTP) opening (Ong, Samangouei, Kalkhoran, & Hausenloy, 2015). Therefore, mitochondria from aged cardiomyocytes meet the conditions necessary to be more susceptible to undergo mitochondrial permeabilisation during IR. As predicted, the contribution of mPTP in cell death has been consistently demonstrated to be more relevant in the aged cardiomyocytes exposed to IR than in young cells (Escobales et al., 2014; Fernandez‐Sanz et al., 2015). Moreover, the endogenous increase in mitochondrial calcium prevents mitochondria from aged cardiomyocytes to actively buffer cytosolic calcium during IR, a feature that leads to a more severe cytosolic calcium overload, hypercontracture, and cell death by mechanisms unrelated to mPTP (Fernandez‐Sanz et al., 2015; Ruiz‐Meana et al., 2011).
FIGURE 1.

Molecular players involved in the mitochondrial cardioprotective response during IR that may be altered in aged cardiomyocytes. Supercomplex mitochondrial interactosome (MIt), consisting of mitochondrial ATP synthase, phosphate carrier (PiC), voltage‐dependent anion channel (VDAC), mitochondrial creatine kinase (MtCK), adenine nucleotide translocator (ANT), and respiratory chain complexes, is shown separately. AK, adenylate kinase; HK, hexokinase; IMS, intermembrane space; MIM, mitochondrial inner membrane; MOM, mitochondrial outer membranes; SR, sarcoplasmic reticulum
In addition to calcium handling, alterations in the VDACs (at the mitochondrial outer membrane [MOM]), the electron transport chain and the activity/expression of mitochondrial kinases have all been involved in ageing and cardioprotection. These components are part of the large protein supercomplex, that has been called the mitochondrial interactosome (Figure 1), located in the contact sites between inner and outer mitochondrial membranes, which is designed to deliver ATP more efficiently to the sites where energy demands are high (Timohhina et al., 2009). The restriction of ATP and ADP diffusion at the level of the MOM is an essential element of compartmentalised energy transfer. In adult cardiomyocytes, the MOM permeability to ADP is regulated by interaction of VDACs with cytoskeletal proteins, particularly with β tubulin II (Bagur et al., 2016). The permeability of the MOM increases in middle‐aged female rats, but not in male rats and this difference disappears during ageing and increased VDAC permeability becomes characteristic for both sexes. The IR itself can induce similar change in the VDAC permeability, thereby enhancing the contribution of hexokinase to the regulation of cellular energy fluxes (Halestrap, Pereira, & Pasdois, 2015).
Restriction of ATP/ADP diffusion through VDAC in cardiac cells is overcome by intracellular phosphoryl transfer through the phosphocreatine (PCr)/creatine kinase (CK) shuttle (Tepp et al., 2016). IR injury compromises mitochondrial oxidative phosphorylation (OXPHOS) and compartmentalised intracellular energy transfer via the PCr/CK network (Bagur et al., 2016). Similarly, for middle‐aged and old rats, OXPHOS activity is much lower than for young rats (Tepp et al., 2017). The efficiency of the CK shuttle, which is the main energy provider for ATPases in adult cardiomyocytes, decreases remarkably as a result of ageing, but reperfusion does not alter mitochondrial CK (MtCK) activity or expression or the ratio of dimeric : octameric isoforms of MtCK (Bagur et al., 2016). The decline in adenylate kinase system is very low with no sex‐related differences in its activity or in mitochondrial isoform contribution. The contribution of glycolysis to ATP production is increased during ageing while the hexokinase activity does not change during healthy ageing (Tepp et al., 2017). It remains unknown to what extent glycolysis replaces OXPHOS in the aged patients and whether that switch has cardioprotective effects.
The third possible players in ageing and cardioprotection are respiratory chain supercomplexes (RCS). In complexes I, III, and IV, the ubiquinone and cytochrome c binding sites are facing each other favouring substrate channelling together with association of ATP synthase, adenine nucleotide translocator, and phosphate carrier (Vonck & Schafer, 2009). Disruption of the RSCs and lack of complex IV may be important factors in the impairment of mitochondrial function in ageing (Ramirez‐Camacho, Flores‐Herrera, & Zazueta, 2019), although data obtained in aged rats show that changes in cardiac bioenergetics are not caused by the functional impairment of RCS (Tepp et al., 2017). Nevertheless, pre‐existing deficiencies in OXPHOS during ageing may compromise the plasticity of ETC activity (Agarwal, Dash, Stowe, Bosnjak, & Camara, 2014). Indeed, during preconditioning changes may occur in the RCS, as a consequence of alterations in the lipid composition of mitochondrial membranes, as suggested in aged animals (Kuznetsov et al., 2019).
3.2. Changes in extracellular matrix
ECM remodelling secondary to ageing may affect the response to IR injury in at least three different ways (Frangogiannis, 2019; Mendes et al., 2012; Meschiari, Ero, Pan, Finkel, & Lindsey, 2017): (a) It increases the distance for oxygen diffusion from the capillaries to the mitochondria of cardiomyocytes; (b) it disturbs integrin signalling and T‐tubule function in cardiomyocytes, hampering post‐ischaemic recovery of electromechanical function; and (c) it induces stiffening of the vessel wall, which in turn reduces the ability of the vessels to adequately adjust coronary blood flow to oxygen demand. Oxygen extraction is already high at rest in the heart. Limited dilatation of conductance vessels, arterioles, and precapillary sphincters results in low flow ischaemia.
The components of ECM are characterised by a slow turnover. In addition, they have a high degree of posttranslational modifications, both spontaneously and enzymically. The products of partial degradation, fragmentation, advanced glycation end‐products (AGE), and other modifications accumulate in the interstitial space of the heart, in the vessel wall, and in other organs with increasing age (Birch, 2018). As a consequence, extracellular deposits and matrix stiffness increase, and mechano‐transduction and normal integrin‐related cell signalling deteriorate.
The most abundant component of the ECM of the heart is collagen, mainly synthesised by fibroblasts. Collagen I, which controls stiffness, and collagen III, which adds elasticity, are the subtypes that dominate in the myocardium. Proteoglycans, consisting of glycosaminoglycan attached to a protein core, provide the tissue turgor. An ageing heart is characterised by ECM changes in parallel with loss of cardiomyocytes (and eventually slight hypertrophy of remaining cells). Microscopic measurements of collagen content revealed that collagen type I fibres were increased in number and thickness in hearts of old male individuals without structurally visible heart disease (Mendes et al., 2012). In healthy ageing, ECM changes are subtle, but as ECM matrix remodelling takes place both as a reparative and as a reactive process, reported changes in the ECM in the ageing heart are often due to interaction between age and subclinical or manifest age‐related diseases (Frangogiannis, 2019; Meschiari et al., 2017; Trial, Entman, & Cieslik, 2016), with hypertension in combination with the decline in gonadal hormone influence being one example (Ludvigsen et al., 2018).
The basement membrane of cardiomyocytes is part of the ECM and consists of a tiny layer of laminin A and non‐fibrillar‐forming collagen type IV, among other components. Examining the heart‐tube of Drosophila melanogaster at different ages, it was observed that age‐dependent thickening of the basement membrane led to restrictions in contractility and that contractility loss could be reversed by reducing laminin A expression (Sessions et al., 2017). Using super‐resolution microscopy, several collagen subtypes were detected in the T‐tubules potentially rendering T‐tubule structure and function sensitive to ageing, a hypothesis confirmed in male mice (Crossman et al., 2017; Kong et al., 2018).
ECM composition is dependent on the balance between synthesis and degradation of the ECM elements and network of macromolecular fibres. MMPs and tissue inhibitors of MMPs regulate degradation in a cascade of interrelated processes (Lindsey, 2018; Wang et al., 2012).
A role for MMP‐9 activation in ageing has evoked particular interest due to its multiple substrates. MMP‐9 promotes replacement of elastin with collagen thereby increasing stiffening of the vessel wall, since elastin has a particularly important role in the vessel wall in providing reversible passive extensibility. MMP9 expression increases with age, and MMP‐9 null male and female mice showed delayed development of age‐dependent diastolic dysfunction, reversal of age‐dependent increase in vascular permeability, and inflammation markers (Yabluchanskiy et al., 2014).
3.3. Change in function of endothelial cells, inflammatory cells, and platelets
Other mechanisms might contribute to reduced IR tolerance in ageing. In both sexes, ageing is associated with enhanced platelet activation, aggregation, and secretion, reduced bleeding time and alterations in coagulation factors that are stored, synthesised, and/or released by platelets. In addition to platelet abnormalities, monocyte phenotype and function are altered in older adults, resulting in enhanced platelet–monocyte interaction and activation of thrombo‐inflammatory pathways. Thus, platelets in older adults may be “primed” for exaggerated responses, enhancing susceptibility to adverse clinical outcomes in settings of IR (Mohebali, Kaplan, Carlisle, Supiano, & Rondina, 2014).
Ageing is also associated with chronic, low‐grade inflammation and characterised by increases in circulating pro‐inflammatory cytokines and ROS, which can both be produced within endothelial cells or by neighbouring immune cells (Chung et al., 2019). A vicious cycle activated by inflammation and oxidative stress, both of which exacerbate one another, impair NO bioavailability and endothelial function in aged arteries (Chung et al., 2019). Age‐associated dysfunction in cardiac microvascular endothelial cells and impaired induction of cardioprotective pathways may contribute to the increased severity of IR in older patients (Edelberg et al., 2002). Moreover, ageing hearts have impaired angiogenic function, and following acute arterial obstruction, a decline in collateral number, diameter, and remodelling can be observed in aged mice, associated with impaired angiogenesis and resulting in more severe ischaemic tissue injury (Faber et al., 2011). This age‐dependent impairment in ischaemia‐induced neovascularisation might be due, at least in part, to oxidative stress‐related dysfunction of endothelial progenitor cells (Lam, 2015).
4. EFFECT OF AGE ON CARDIOPROTECTION TREATMENTS
4.1. Ischaemic conditioning
The conditioning strategies by which IR‐induced damage of the myocardium is reduced include brief non‐lethal periods of IR, which are performed either (a) before the sustained phase of IR directly to the heart (ischaemic preconditioning, IPC) or to distant organs or tissues (remote ischaemic preconditioning, RIPC) or (b) at the onset of reperfusion (postconditioning, IPostC). Studies analysing myocardial conditioning in middle‐aged or old animals are reviewed here or elsewhere (Boengler, Schulz, & Heusch, 2009; Calabrese, 2016; Ferdinandy et al., 2014).
The effectiveness of IPC to protect against myocardial IR injury declines with age as shown in aged (18 months) mouse hearts in vitro (Peart et al., 2014) and in mice older than 13 months in vivo (Boengler et al., 2007). Also, the loss of IS reduction by IPC is demonstrated in 12–18 months (Adam, Sharp, Opie, & Lecour, 2013) or in 18 months old rat hearts in vitro (Schulman, Latchman, & Yellon, 2001). Whereas the majority of studies describes a loss of IPC‐induced cardioprotection with age, some recent investigations also indicate preserved myocardial protection by IPC in 17 months old rat hearts in vitro (Webster et al., 2017) or in 20 months old rat hearts in vivo (Dong, Guo, Yang, & Li, 2017).
Data on the effectiveness of the cardioprotection by IPostC are also controversial and seem to depend on the postconditioning algorithm as well as on the age of the animals. In middle‐aged mouse hearts in vivo (older 13 months), the effectiveness of IPostC depends on the strength of the postconditioning stimulus (Boengler et al., 2008), whereas the IS reduction by IPostC is lost in both middle‐aged (12 months) and aged (20 months) mice in vitro (Perez et al., 2016). The loss of cardioprotection by IPostC in mice seen with advancing age, is also evident in rats where IPostC is cardioprotective in aged (16–18 months) hearts in vivo (Yin et al., 2009), but not in old (24 months) hearts in vitro (Chen et al., 2016). The cardioprotection by RIPC is present in young rat hearts in vivo but is abolished in aged (22–24 months) animals (Behmenburg, Heinen, Bruch, Hollmann, & Huhn, 2017).
The loss of cardioprotection with ageing originates from reduced expression or phosphorylation and subcellular localisation of proteins involved in the protective signalling cascades, such as mitochondrial connexin43, components of the reperfusion injury salvage kinase (RISK) pathway (including the pro‐survival Akt and ERK1/2 cascades), PKC, and STAT3 (Boengler et al., 2009; Kang, Qin, Osei, & Hu, 2017; Whittington et al., 2013) as well as alterations of mitochondrial function (Figure 1). The activation of STAT3 is also attenuated in the presence of other cardiovascular risk factors (Pipicz, Demjan, Sarkozy, & Csont, 2018), conditions in which IPC and IPostC often are ineffective (Xia, Li, & Irwin, 2016). Therefore, for the cardioprotection of aged and diseased myocardium, new strategies to preserve signal transduction and mitochondrial function need to be developed.
4.2. Pharmacological treatments
Age is known to confound the cardioprotective efficacy of pharmacological treatments which protect the heart against acute IR injury by targeting protective signalling pathways (Boengler et al., 2009; see Table 1 for summary). Pharmacological preconditioning with volatile anaesthetic agents such as isoflurane and sevoflurane was attenuated in aged rat hearts (20–24 months), and this effect was attributed to a failure to activate the Akt pathway to generate signalling ROS and to inhibit mPTP opening (Nguyen et al., 2008; Sniecinski & Liu, 2004; Zhu et al., 2010). Furthermore, isoflurane preconditioning was also attenuated in human atrial cardiomyocytes harvested from aged (74 ± 6 years) compared to middle‐aged (54 ± 7 years) patients undergoing cardiac surgery, subjected to simulated IR injury (Mio et al., 2008).
TABLE 1.
Summary of major studies investigating the confounding effect of age on the cardioprotective efficacy of pharmacological conditioning agents on IR injury
| Pharmacological conditioning agent | Model of IR injury | Confounded by age | Mechanism | References |
|---|---|---|---|---|
| Preconditioning with adenosine A1 receptor agonist (CCPA1), PKC activator (DAG), and mitochondrial KATP channel activator (diazoxide) | Rat | Yes | Failure to activate known mediators of IPC–adenosine A1 receptor, PKC, and mitochondrial KATP channel | (Schulman et al., 2001) |
| Volatile anaesthetic preconditioning (isoflurane/sevoflurane) |
Rat Human atrial cardiomyocytes |
Yes | Failure to activate Akt, produce signalling ROS and inhibit MPTP opening | (Mio et al., 2008; Nguyen et al., 2008; Sniecinski & Liu, 2004; Zhu et al., 2010) |
| Helium preconditioning | Rat | Yes | Failure to activate Ca2+‐sensitive K+ channels and uncouple mitochondria | (Heinen et al., 2008) |
| Dexmedetomidine preconditioning | Rat | Yes | Failure to have antioxidant effect | (Dong, Guo, Yang, & Li, 2017) |
| High‐dose folic acid preconditioning | Rat | Yes | Failure to maintain dimerisation of eNOS | (Zuurbier et al., 2014) |
| Volatile anaesthetic postconditioning (isoflurane/sevoflurane) | Rat | Yes | Failure to activate Akt and ERK1/2 and inhibit MPTP opening | (Chang et al., 2012; Li et al., 2013) |
| SB‐216763 postconditioning (GSK‐3β inhibitor) | Mouse | Yes | Failure to inhibit MPTP opening | (Zhu et al., 2011) |
| Sphingosine | Rat | No | Persisting cardioprotection via PKG and PKA | (Vessey et al., 2008; Vessey et al., 2009) |
| Amobarbital postconditioning (reversible mitochondrial inhibitor) | Rat | No | (Chen, Ross, Hu, & Lesnefsky, 2012) |
Pharmacological postconditioning with the anaesthetic agents sevoflurane or isoflurane reduced IS in the myocardium of young (3–5 months age) but not aged (20–24 months age) rats, and this was attributed to the failure to activate the pro‐survival RISK signalling pathway, comprising Akt and ERK1/2, and lack of inhibition of the mPTP opening (Chang et al., 2012; Li et al., 2013). Furthermore, pharmacological inhibition of glycogen synthase kinase 3β (GSK3β), using SB‐216763, reduced IS and inhibited mPTP opening in young (3–5 months age) but not aged (20–24 months age) rats (Zhu, Rebecchi, Glass, Brink, & Liu, 2011). Interestingly, signalling through the PKG or PKA pathway by sphingosine was preserved in aged rat hearts suggesting that this signalling cascade may not be affected (Vessey, Kelley, Li, & Huang, 2009; Vessey, Li, Kelley, Zhang, & Karliner, 2008).
5. SEX AND GENDER
Several reports have shown that sex and gender play a major role in the development, perception, therapy, response to treatment, and outcome of various diseases, including cardiovascular diseases (Regitz‐Zagrosek & Kararigas, 2017). Sex and gender are often mistakenly used in the literature, and there is a generalised lack of prospective design to properly study their effect. Sex is a biological attribute based on sex‐chromosome assignment and defined by the resulting sex hormones, as well as genetic and epigenetic factors that lead to sex differences. Gender is a construct that includes socio‐cultural and behavioural aspects, which give rise to gender identity, norms, and relations. There are close interactions between sex and gender that affect human physiology and pathology, as sex hormones may influence behaviour and lifestyle, while gender may modify a patient's adherence to treatment, for example. However, for the purpose of the present review article, we consider here the biological aspects that affect disease and lead to pronounced sex differences. In particular, we discuss the hormonal, genetic, and epigenetic mechanisms that contribute to different types of IHD developing between men and women.
5.1. Sex differences in tolerance to IR injury in preclinical and clinical studies
It is well established that sex impacts on all aspects of cardiovascular health and disease. Sex‐specific differences exist in the epidemiology, clinical presentation, underlying pathophysiological mechanisms, treatment, and clinical outcomes in patients with acute MI (AMI; Mehta et al., 2016). However, experimental and clinical studies taking sex differences into account are scarce. Therefore, it is essential to include both sexes when undertaking experimental and clinical studies investigating AMI and consider the possible interactions between sex and co‐morbidities.
Women are often older than men when they present with their first AMI (mean age of 72 years compared to 65 years; Benjamin et al., 2019), and this has been attributed to the protective role of circulating oestrogens against endothelial dysfunction and lipid deposition in the vasculature (Chakrabarti, Morton, & Davidge, 2014). Consistent with this assumption, the incidence of AMI increases substantially in postmenopausal women (Benjamin et al., 2019). In terms of clinical presentation of AMI, women most often present with atypical chest pain and angina equivalent symptoms (such as dyspnoea, weakness, fatigue, and indigestion), when compared to men who present with typical chest pain (Rubini et al., 2014). This difference affects the management and mortality of AMI, as many female patients are misdiagnosed. A number of studies have shown that women with AMI are less likely to be managed using guideline‐recommended medical therapies (Jneid et al., 2008), to undergo cardiac catheterisation (Jneid et al., 2008), and to receive timely reperfusion (Mehilli et al., 2005). In terms of pathophysiology underlying AMI, there are important sex differences. Plaque rupture, the main cause of AMI, is less common in women, when compared to men (55% vs. 76%, respectively; Falk, Nakano, Bentzon, Finn, & Virmani, 2013), which is of major relevance given that AMI without obstructive coronary artery disease is more common among women (Chokshi et al., 2010).
In experimental animal studies of AMI, there are sex differences in terms of susceptibility to acute IR injury (see Blenck, Harvey, Reckelhoff, & Leinwand, 2016). In aged female animals, reduced tolerance to IR has been largely attributed to oestradiol deficiency. Preclinical studies have demonstrated the beneficial effects of chronic or acute exogenous oestradiol administration in adult male and female, gonad‐intact, and gonadectomised animals (Korzick & Lancaster, 2013). However, conflicting evidence exists regarding the efficacy of menopausal hormone therapy in cardioprotection of postmenopausal women (Korzick & Lancaster, 2013). Similarly, in older men, the decline in testosterone levels with advancing age is associated with a number of symptoms and adverse cardiovascular outcomes. Yet the advantages of testosterone therapy in older hypogonadal men have not been adequately resolved (Orwoll, 2017). Despite some inconsistencies observed using rodent models of AMI, in general, female mice and rats appear to be protected against acute myocardial IR injury compared to males (Dow, Bhandari, Hale, & Kloner, 2015), specifically after more prolonged ischaemic time (Dow et al., 2015; Penna et al., 2009). Neutrophil infiltration, post‐AMI adverse left ventricular (LV) remodelling, cardiac rupture, and mortality were lower in female, than in male mice, irrespective of IS (Cavasin, Tao, Menon, & Yang, 2004; Fang et al., 2007; Shioura, Geenen, & Goldspink, 2008). The mechanisms underlying sex‐dependent differences in the susceptibility to acute myocardial IR injury include the presence of oestrogen, lower calcium levels and enhanced NO signalling (Murphy & Steenbergen, 2007), less apoptosis (Wang, He, Sun, Dai, & Yang, 2010), more efficient ROS‐generated aldehydes detoxification (Lagranha, Deschamps, Aponte, Steenbergen, & Murphy, 2010), and more favourable interplay between ROS and the expression/activation of some kinase‐dependent survival pathways (Ciocci et al., 2018; Pagliaro & Penna, 2017; Wang et al., 2010) in females than in males.
5.2. Biological mechanisms
5.2.1. Effect of hormones on IR injury
All the steroid sex hormones, oestrogens, progesterone, and androgens modify IR injury, especially when given at pharmacological concentrations. These hormones exert a wide variety of responses via their nuclear receptors and have additional effects via non‐genomic cellular responses in the cardiovascular system (Lucas‐Herald, Alves‐Lopes, Montezano, Ahmed, & Touyz, 2017). The non‐genomic effects are particularly well described for oestradiol in the context of acute cardioprotection (Deschamps & Murphy, 2009; Sovershaev, Egorina, Andreasen, Jonassen, & Ytrehus, 2006). Non‐nuclear receptors consist of a G‐protein coupled oestrogen receptor and extra‐nuclear oestrogen receptors subtypes α and β (Deschamps, Murphy, & Sun, 2010). Both male and female hearts harbour oestrogen receptors. Oestradiol is a cardioprotective agent both in male and female hearts when given as acute pretreatment, although a slightly weaker effect was observed in male rat hearts (Sovershaev et al., 2006). The cardioprotection is due to the activation of the PI3K pathway converging on GSK3β and eventually leading to mitochondrial protection. Another cardioprotective mechanism stimulated by oestradiol is the up‐regulation of NO‐signalling and S‐nitrosylated proteins (Deschamps et al., 2010). Interestingly, antiblastic‐induced cardiotoxicity, which shares some mechanisms of cardiomyocyte damage with those of IR injury (excessive ROS production and mitochondrial dysfunction) has been shown to be ameliorated by oestrogens (Cadeddu et al., 2019). The effect of acute administration of progesterone is variable and clearly dependent on the model. Progesterone reduces calcium sensitivity, contractility, and action potential duration in female hearts but not in male hearts (Feridooni, MacDonald, Ghimire, Pyle, & Howlett, 2017). Acute administration of the main androgen subtype, testosterone, might also provide cardioprotection. The heart of both sexes contains aromatase, the enzyme responsible for converting androgens to oestrogens. Some of the reported effects of testosterone might therefore be oestrogen‐mediated. Interestingly, increasing aromatase in male mice leads to up‐regulation of Akt phosphorylation, reduction in ischaemic contracture but reduced recovery of systolic function at reperfusion in isolated hearts (Bell et al., 2014).
The results of studies investigating the role of these hormones on IR injury in a chronic (more physiological) setting are variable. Some authors report female hearts to be less vulnerable; others do not find significant differences. These discrepancies may be due to differences in the endpoint‐selection and the experimental models. In addition to age, several pathophysiological changes might nullify the female advantage (Bell, Porrello, Huggins, Harrap, & Delbridge, 2008). Exposure to angiotensin II leads to marked up‐regulation of collagen and microscopic fibrosis and substantial impairment of post‐ischaemic function after IR compared to sham rats. In this setting, sex‐dependent differences in functional recovery were not significant despite differences in angiotensin II‐induced molecular remodelling. When IR injury was compared among rats of the same sex with and without intact gonads, loss of gonadal function was associated with increased IR injury in box sexes (Ross & Howlett, 2012). These results might differ somewhat depending on the timing of the gonadectomy (i.e., young or fully mature animals) and the duration of the study. However, experiments using specific oestrogen receptor knockout mice confirm the beneficial role of oestrogen in IR injury (Deschamps et al., 2010).
Other hormones with sex‐dependent concentration differences in a lifetime perspective are oxytocin and the insulin‐like peptide relaxin, both produced in increasing amounts during pregnancy. Under experimental conditions, both have been shown to be cardioprotective during IR (Gonzalez‐Reyes et al., 2015; Sarwar, Du, Dschietzig, & Summers, 2017).
5.2.2. Genetic and epigenetic mechanisms
Genetic mechanisms strongly affect susceptibility to IR insult. Surprisingly, the number of X chromosomes negatively affects cardiac IR injury in mice, suggesting that one X is better than two, irrespective of gonadal sex (Li et al., 2014). Higher susceptibility of XX mice is due to the number of X chromosomes rather than the absence of a Y chromosome. Interestingly, most X chromosome genes do not show large sex differences in their levels of expression, since one X chromosome is randomly transcriptionally silenced in XX female adult somatic cells. However, some genes “escape” inactivation and are expressed from each X chromosome, so that they have higher gene transcript levels in XX cells compared to XY cells (Arnold, Cassis, Eghbali, Reue, & Sandberg, 2017).
After completion of the human genome project, several candidate gene studies have been conducted to estimate the effects of specific gene variants on coronary artery disease (CAD) risk in both sexes (Khera et al., 2016). Several sex‐specific genetic CAD risk predictors have been identified, and while some variants conferred risk in both sexes, others showed significant effects only in males (Khera et al., 2016; Silander et al., 2008; Yamada et al., 2002). However, the majority of common CAD variants identified so far only cause modest increases in CAD risk by unknown mechanisms, and whether sex‐specific effects exist has been rarely tested. As mitochondria are mainly derived from the mother (Byars & Inouye, 2018), variations in mtDNA might also account, at least in part, for sex differences. In addition, gene variants on the Y chromosome might also contribute to cardiovascular phenotypes in men. The Y chromosome is routinely excluded from large‐scale genome‐wide association studies, and it is therefore the most underexplored portion of the human genome to date.
In addition to gene variants, a number of epigenetic modifications can alter gene expression and IHD risk in a sex‐specific manner (Hartman, Huisman, & den Ruijter, 2018). First, epigenetic mechanisms control sex‐specific gene expression during development, and such early epigenetic changes can alter the phenotype much later and even affect subsequent generations (Hartman et al., 2018). Prenatal hypoxia has been shown to modulate DNA methylation and repression of cardiac PKCε gene in a sex‐dependent manner, linking fetal hypoxia and pathophysiological consequences in the hearts of adult offspring (Patterson, Chen, Xue, Xiao, & Zhang, 2010). Consistent with these preclinical results, the risk of MI in women is associated with enhanced DNA methylation at specific loci sensitive to prenatal conditions (Talens et al., 2012). In post‐natal life, oestrogens have been shown to protect the myocardium against IR through epigenetic regulation of urocortin receptor type 2 levels in female rats (Cong et al., 2013). Unfortunately, the majority of studies investigating the role of epigenetics in cardioprotection during post‐natal life did not stratify data by sex (Hartman et al., 2018). Therefore, further studies will be needed to precisely determine the role of sex on epigenetic regulatory processes in IR injury.
5.2.3. Sex differences in cardiovascular ageing
The heart exhibits sex‐biased distinct structural and functional features at all ages that contribute to differences in the pattern of biological ageing between males and females. Morphometric analysis using autopsied human hearts has revealed that men experience a more pronounced age‐dependent decline in the number of ventricular cardiomyocytes (and compensatory increase in cardiomyocyte volume) than women (Olivetti et al., 1995). Consistent with this, it has been shown that cardiomyocytes from aged male rats develop a more significant reduction in peak calcium transients and systolic function than those from aged females (Howlett, 2010). Whereas some prevalent pathophysiological changes present in ageing, such as atrial fibrosis, do not clearly differ between the sexes, others may differ in their magnitude and prevalence, such as aortic valve calcification (men > women), reduced sensitivity to β‐adrenoceptor stimulation (men > women), increased LV wall thickness (women > men), concentric remodelling and diastolic dysfunction (women > men; Keller & Howlett, 2016). Particularly, the latter might be due to higher levels of ECM components in left ventricular samples of older women compared with those of younger women or younger and older men (Dworatzek, Baczko, & Kararigas, 2016). However, the molecular mechanisms are poorly understood. Collectively, the contribution of biological sex to cardiovascular ageing deserves further investigation.
5.3. Sex‐specific pharmacology in the heart
Basic anatomical and physiological features, such as body and organ weight, body composition and fat content and distribution, liver metabolism, and renal function, influence the effects of drugs. Given that these features differ between males and females, efficacy and safety of pharmacological treatments may also be sex‐specific. Consequently, the risk of experiencing adverse drug reactions and their severity is different between male and female patients. Sex‐specific drug therapeutic and deleterious effects are expected to stem from sex differences in pharmacokinetic and pharmacodynamic properties of drugs, which, however, are under‐investigated (Gaignebet & Kararigas, 2017). Nevertheless, it is well known that there are pronounced sex differences in the levels of the cytochrome P450 enzymes and their metabolism of drugs (Freire, Basit, Choudhary, Piong, & Merchant, 2011; Schwartz, 2007). Notably, protection from preconditioning and postconditioning by nitrite depends on ALDH2 genotype (Ormerod et al., 2017). A major contradiction, though, is the fact that these differences are usually not considered when defining drug dosing. Similarly, it is well established that women are under‐represented in the design and development of novel therapeutics and technologies. As a result, assessment of drug efficacy and safety is primarily performed in one sex, and it is taken for granted that they should be the same in the other sex, which, in many situations, is not the case. Collectively, this practice leads to non‐effectively treated patients contributing to further suffering and important socio‐economic implications.
6. SUMMARY AND RECOMMENDATIONS FOR FUTURE RESEARCH
Biological age is the strongest predictor of cardiovascular health. Due to the progressive ageing of the human population, heart‐related diseases have become the leading cause of death and disability worldwide and, among them, IHD accounts for the majority of this medical effect (Benjamin et al., 2019; Moran et al., 2014). Importantly, being old has been identified to be an independent determinant of the extension of MI and the outcome of cardioprotective strategies after an IR episode. During ageing, a plethora of molecular changes occur (Figure 2). Aged cardiomyocytes display excessive mitochondrial calcium accumulation and ROS production that play a fundamental role in the susceptibility to mPTP opening and cell death upon IR injury in many experimental models (Briston, Selwood, Szabadkai, & Duchen, 2019). Nevertheless, the exact molecular nature of the mPTP remains to be defined and attempts to translate experimental strategies aimed at preventing cell death, secondary to mPTP opening, to the clinical context have failed (Trankle, Thurber, Toldo, & Abbate, 2016). Moreover, ageing induces a variable degree of mismatch in the cell energy demand and supply, secondary to altered cytoarchitecture (deficient SR‐mitochondria calcium exchange and defective supramolecular assembly of the RSCs) but also to inadequate transfer of intracellular energy and progressive metabolic remodelling (i.e., partial replacement of OXPHOS by glycolysis; Fernandez‐Sanz et al., 2014; Ramirez‐Camacho et al., 2019; Ruiz‐Meana et al., 2019; Tepp et al., 2017). Whether these mechanisms affect the outcome of CAD in elderly patients remains unclear.
FIGURE 2.

Intracellular targets altered by ageing and sex that can be targeted by specific cardioprotective strategies
Advanced age may also aggravate adverse remodelling and the occurrence of arrhythmias secondary to MI due to changes in the composition of ECM and altered inflammatory response (Meschiari et al., 2017; Mohebali et al., 2014). Platelet abnormalities and enhanced platelet–monocyte interactions present in ageing modify the thrombo‐inflammatory profile of elderly patients and might require therapeutic adjustments and/or differential approaches, the efficacy of which has not been specifically addressed in clinical trials. Some canonical intracellular signalling pathways involved in the endogenous cardioprotection are disrupted in the aged heart, rendering conditioning strategies less or non‐effective (Boengler et al., 2009). This is partly explained not only by reduced expression or phosphorylation and altered trafficking and localisation of intracellular proteins that participate in protective signalling cascades, but also by mitochondrial dysfunction during ageing, increased production of circulating cytokines and ROS within endothelial cells, reduced NO bioavailability and less neovascularisation, all factors that increase the threshold necessary to trigger cardioprotection (Edelberg et al., 2002). Cardioprotection includes multitarget strategies (Figure 3). Further studies are needed to determine whether the confounding effect of age can be overcome by increasing the cardioprotective stimulus (such as combining agents) and to elucidate the mechanisms through which age negatively affects cardioprotective signalling pathways.
FIGURE 3.
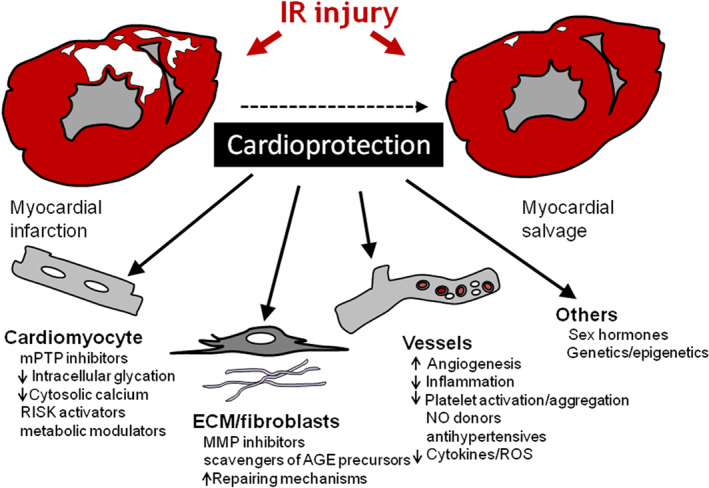
Multitarget strategies involved in cardioprotection. Multiple cardiac and extra‐cardiac factors modulate IR injury in the heart and are potentially targetable by cardioprotective interventions
The intersection between ageing and sex has pathophysiological consequences in terms of cardioprotection. Ageing leads to a decline in the oestrogen and testosterone levels. It is well established that the oestrogen status of females modulates the susceptibility of the heart to IR injury. Similarly, reduction in testosterone levels in older men worsens cardiovascular outcomes. Yet the benefit of menopausal hormone therapy (either oestradiol or testosterone) is controversial (Korzick & Lancaster, 2013; Orwoll, 2017). Sex also has specific genetic and epigenetic implications, the contribution of which to the susceptibility to IR injury has not been fully assessed. The efficacy and safety profiles of therapeutic interventions differ among the sexes. With some exceptions, the risk of experiencing adverse drug reactions is higher in women than in men. Nevertheless, these differences are not considered when defining the dose and timing of treatments, and women tend to be under‐represented in clinical trials. Therefore, a better understanding of the effects of sex in pathophysiology and pharmacology would lead to the identification of targets and the development of pharmacotherapies applied in a sex‐specific manner, thereby contributing towards a more appropriate and individualised medical care for both men and women (Kararigas, Seeland, Barcena de Arellano, Dworatzek, & Regitz‐Zagrosek, 2016). To this extent, artificial intelligence by means of in silico models may prove helpful in predicting sex‐specific drug responses, on a large scale, which could be translated into clinical practice (Cui et al., 2018; Huang et al., 2018).
Current evidence argues for more research conducted in appropriate experimental models, where advanced age and biological sex are taken into consideration, as well as cardioprotective clinical trials carefully designed to integrate the basic scientific knowledge on these confounders to the patients.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Cidlowski et al., 2019; Alexander, Fabbro et al., 2019; Alexander, Kelly et al., 2019; Alexander, Mathie et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This article is based upon work from COST Action EU‐CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). M.R.M. and D.G.D. are funded by ISCIII (PI19‐01196), CIBER‐CV, Fundaciό MTV3‐122/C/2015, SEC‐2016, and the European Regional Development Fundings (ERDF‐FEDER); T.K. is funded by IUT23‐1 of the Estonian Ministry of Education and Research; D.J.H. is supported by the British Heart Foundation (CS/14/3/31002), the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Duke‐National University Singapore Medical School, Singapore Ministry of Health's National Medical Research Council under its Clinician Scientist‐Senior Investigator scheme (NMRC/CSA‐SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016‐T2‐2‐021); R.S. is funded by the German Research Foundation (Project 268555672‐SFI3‐B05); K.B. is funded by the German Research Foundation (BO 2955/4‐1); C.P. is funded by Ministero dell’Istruzione, Universitá e Ricerca Scientifica Grant (2015583WMX) and Programma STAR grant, financially supported by Federico II University and Compagnia di San Paolo. This work is dedicated to the memory of our colleague and friend Dr. David García‐Dorado, who sadly passed away during the final stage of the preparation of this manuscript.
Ruiz‐Meana M, Boengler K, Garcia‐Dorado D, et al. Ageing, sex, and cardioprotection. Br J Pharmacol. 2020;177:5270–5286. 10.1111/bph.14951
REFERENCES
- Adam, T. , Sharp, S. , Opie, L. H. , & Lecour, S. (2013). Loss of cardioprotection with ischemic preconditioning in aging hearts: Role of sirtuin 1? Journal of Cardiovascular Pharmacology and Therapeutics, 18, 46–53. [DOI] [PubMed] [Google Scholar]
- Agarwal, B. , Dash, R. K. , Stowe, D. F. , Bosnjak, Z. J. , & Camara, A. K. (2014). Isoflurane modulates cardiac mitochondrial bioenergetics by selectively attenuating respiratory complexes. Biochimica et Biophysica Acta, 1837, 354–365. 10.1016/j.bbabio.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Nuclear hormone receptors. British Journal of Pharmacology, 176, S229–S246. 10.1111/bph.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176, S397–S493. 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anmann, T. , Varikmaa, M. , Timohhina, N. , Tepp, K. , Shevchuk, I. , Chekulayev, V. , … Kaambre, T. (2014). Formation of highly organized intracellular structure and energy metabolism in cardiac muscle cells during postnatal development of rat heart. Biochimica et Biophysica Acta, 1837, 1350–1361. 10.1016/j.bbabio.2014.03.015 [DOI] [PubMed] [Google Scholar]
- Arnold, A. P. , Cassis, L. A. , Eghbali, M. , Reue, K. , & Sandberg, K. (2017). Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arteriosclerosis, Thrombosis, and Vascular Biology, 37, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagur, R. , Tanguy, S. , Foriel, S. , Grichine, A. , Sanchez, C. , Pernet‐Gallay, K. , … Guzun, R. (2016). The impact of cardiac ischemia/reperfusion on the mitochondria‐cytoskeleton interactions. Biochimica et Biophysica Acta, 1862, 1159–1171. 10.1016/j.bbadis.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Barnett, K. , Mercer, S. W. , Norbury, M. , Watt, G. , Wyke, S. , & Guthrie, B. (2012). Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross‐sectional study. Lancet, 380, 37–43. [DOI] [PubMed] [Google Scholar]
- Behmenburg, F. , Heinen, A. , Bruch, L. V. , Hollmann, M. W. , & Huhn, R. (2017). Cardioprotection by remote ischemic preconditioning is blocked in the aged rat heart in vivo. Journal of Cardiothoracic and Vascular Anesthesia, 31, 1223–1226. 10.1053/j.jvca.2016.07.005 [DOI] [PubMed] [Google Scholar]
- Bell, J. R. , Bernasochi, G. B. , Varma, U. , Boon, W. C. , Ellem, S. J. , Risbridger, G. P. , & Delbridge, L. M. (2014). Aromatase transgenic upregulation modulates basal cardiac performance and the response to ischemic stress in male mice. American Journal of Physiology. Heart and Circulatory Physiology, 306, H1265–H1274. 10.1152/ajpheart.00012.2014 [DOI] [PubMed] [Google Scholar]
- Bell, J. R. , Porrello, E. R. , Huggins, C. E. , Harrap, S. B. , & Delbridge, L. M. (2008). The Intrinsic resistance of female hearts to an ischemic insult is abrogated in primary cardiac hypertrophy. American Journal of Physiology. Heart and Circulatory Physiology, 294, H1514–H1522. 10.1152/ajpheart.01283.2007 [DOI] [PubMed] [Google Scholar]
- Benjamin, E. J. , Muntner, P. , Alonso, A. , Bittencourt, M. S. , Callaway, C. W. , Carson, A. P. , … Virani, S. S. (2019). Heart disease and stroke statistics‐2019 update: A report from the American Heart Association. Circulation, 139, e56–e528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Bernhard, D. , & Laufer, G. (2008). The aging cardiomyocyte: A mini‐review. Gerontology, 54, 24–31. [DOI] [PubMed] [Google Scholar]
- Birch, H. L. (2018). Extracellular matrix and ageing. Sub‐Cellular Biochemistry, 90, 169–190. 10.1007/978-981-13-2835-0_7 [DOI] [PubMed] [Google Scholar]
- Blenck, C. L. , Harvey, P. A. , Reckelhoff, J. F. , & Leinwand, L. A. (2016). The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circulation Research, 118, 1294–1312. 10.1161/CIRCRESAHA.116.307509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler, K. , Buechert, A. , Heinen, Y. , Roeskes, C. , Hilfiker‐Kleiner, D. , Heusch, G. , & Schulz, R. (Jan 4 2008). Cardioprotection by ischemic postconditioning is lost in aged and STAT3‐deficient mice. Circulation Research, 102(1), 131–135. [DOI] [PubMed] [Google Scholar]
- Boengler, K. , Konietzka, I. , Buechert, A. , Heinen, Y. , Garcia‐Dorado, D. , Heusch, G. , & Schulz, R. (2007). Loss of ischemic preconditioning's cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. American Journal of Physiology. Heart and Circulatory Physiology, 292, H1764–H1769. 10.1152/ajpheart.01071.2006 [DOI] [PubMed] [Google Scholar]
- Boengler, K. , Schulz, R. , & Heusch, G. (2009). Loss of cardioprotection with ageing. Cardiovascular Research, 83, 247–261. 10.1093/cvr/cvp033 [DOI] [PubMed] [Google Scholar]
- Botker, H. E. , Hausenloy, D. , Andreadou, I. , Antonucci, S. , Boengler, K. , Davidson, S. M. , … Heusch, G. (2018). Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Research in Cardiology, 113, 39–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briston, T. , Selwood, D. L. , Szabadkai, G. , & Duchen, M. R. (2019). Mitochondrial permeability transition: A molecular lesion with multiple drug targets. Trends in Pharmacological Sciences, 40, 50–70. 10.1016/j.tips.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Bujak, M. , Kweon, H. J. , Chatila, K. , Li, N. , Taffet, G. , & Frangogiannis, N. G. (2008). Aging‐related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. Journal of the American College of Cardiology, 51, 1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byars, S. G. , & Inouye, M. (2018). Genome‐wide association studies and risk scores for coronary artery disease: Sex biases. Advances in Experimental Medicine and Biology, 1065, 627–642. [DOI] [PubMed] [Google Scholar]
- Cadeddu, D. C. , Pepe, A. , Penna, C. , Gimelli, A. , Madonna, R. , Mele, D. , … Mercuro, G. (2019). Sex Differences in anthracycline‐induced cardiotoxicity: The benefits of estrogens. Heart Failure Reviews, 24(6), 915–925. [DOI] [PubMed] [Google Scholar]
- Calabrese, E. J. (2016). Pre‐ and post‐conditioning hormesis in elderly mice, rats, and humans: Its loss and restoration. Biogerontology, 17, 681–702. 10.1007/s10522-016-9646-8 [DOI] [PubMed] [Google Scholar]
- Cavasin, M. A. , Tao, Z. , Menon, S. , & Yang, X. P. (2004). Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sciences, 75, 2181–2192. [DOI] [PubMed] [Google Scholar]
- Chakrabarti, S. , Morton, J. S. , & Davidge, S. T. (2014). Mechanisms of estrogen effects on the endothelium: An overview. The Canadian Journal of Cardiology, 30, 705–712. [DOI] [PubMed] [Google Scholar]
- Chang, D. J. , Chang, C. H. , Kim, J. S. , Hong, Y. W. , Lee, W. K. , & Shim, Y. H. (2012). Isoflurane‐induced post‐conditioning in senescent hearts is attenuated by failure to activate reperfusion injury salvage kinase pathway. Acta Anaesthesiologica Scandinavica, 56, 896–903. 10.1111/j.1399-6576.2012.02702.x [DOI] [PubMed] [Google Scholar]
- Chen, J. , Gao, J. , Sun, W. , Li, L. , Wang, Y. , Bai, S. , … Xu, C. (2016). Involvement of exogenous H2S in recovery of cardioprotection from ischemic post‐conditioning via increase of autophagy in the aged hearts. International Journal of Cardiology, 220, 681–692. [DOI] [PubMed] [Google Scholar]
- Chen, Q. , Ross, T. , Hu, Y. , & Lesnefsky, E. J. (2012). Blockade of electron transport at the onset of reperfusion decreases cardiac injury in aged hearts by protecting the inner mitochondrial membrane. Journal of Aging Research, 2012, 753949–753957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokshi, N. P. , Iqbal, S. N. , Berger, R. L. , Hochman, J. S. , Feit, F. , Slater, J. N. , … Reynolds, H. R. (2010). Sex and race are associated with the absence of epicardial coronary artery obstructive disease at angiography in patients with acute coronary syndromes. Clinical Cardiology, 33, 495–501. 10.1002/clc.20794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, H. Y. , Kim, D. H. , Lee, E. K. , Chung, K. W. , Chung, S. , Lee, B. , … Yu, B. P. (2019). Redefining chronic inflammation in aging and age‐related diseases: Proposal of the senoinflammation concept. Aging and Disease, 10, 367–382. 10.14336/AD.2018.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocci, P. A. , Scuri, S. , Gonzalez Arbelaez, L. F. , Caldiz, C. , Fantinelli, J. , & Mosca, S. M. (2018). Survival kinase‐dependent pathways contribute to gender difference in the response to myocardial ischemia‐reperfusion and ischemic post‐conditioning. Cardiovascular Pathology, 33, 19–26. [DOI] [PubMed] [Google Scholar]
- Cong, B. , Zhu, X. , Cao, B. , Xiao, J. , Wang, Z. , & Ni, X. (2013). Estrogens Protect myocardium against ischemia/reperfusion insult by up‐regulation of CRH receptor type 2 in female rats. International Journal of Cardiology, 168, 4755–4760. [DOI] [PubMed] [Google Scholar]
- Crossman, D. J. , Shen, X. , Jullig, M. , Munro, M. , Hou, Y. , Middleditch, M. , … Soeller, C. (2017). Increased collagen within the transverse tubules in human heart failure. Cardiovascular Research, 113, 879–891. 10.1093/cvr/cvx055 [DOI] [PubMed] [Google Scholar]
- Cui, C. , Huang, C. , Liu, K. , Xu, G. , Yang, J. , Zhou, Y. , … Cui, Q. (2018). Large‐scale in silico identification of drugs exerting sex‐specific effects in the heart. Journal of Translational Medicine, 16, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, S. M. , Ferdinandy, P. , Andreadou, I. , Botker, H. E. , Heusch, G. , Ibanez, B. , … Garcia‐Dorado, D. (2019). Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. Journal of the American College of Cardiology, 73, 89–99. [DOI] [PubMed] [Google Scholar]
- Deschamps, A. M. , & Murphy, E. (2009). Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. American Journal of Physiology. Heart and Circulatory Physiology, 297, H1806–H1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps, A. M. , Murphy, E. , & Sun, J. (2010). Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends in Cardiovascular Medicine, 20, 73–78. 10.1016/j.tcm.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J. , Guo, X. , Yang, S. , & Li, L. (2017). The effects of dexmedetomidine preconditioning on aged rat heart of ischaemia reperfusion injury. Research in Veterinary Science, 114, 489–492. 10.1016/j.rvsc.2017.09.028 [DOI] [PubMed] [Google Scholar]
- Dow, J. S. , Bhandari, A. , Hale, S. L. , & Kloner, R. A. (2015). Does sex influence the incidence or severity of reperfusion‐induced cardiac arrhythmias? Springerplus, 4, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworatzek, E. , Baczko, I. , & Kararigas, G. (2016). Effects of aging on cardiac extracellular matrix in men and women. Proteomics. Clinical Applications, 10, 84–91. 10.1002/prca.201500031 [DOI] [PubMed] [Google Scholar]
- Edelberg, J. M. , Lee, S. H. , Kaur, M. , Tang, L. , Feirt, N. M. , McCabe, S. , … Hong, M. K. (2002). Platelet‐derived growth factor‐AB limits the extent of myocardial infarction in a rat model: Feasibility of restoring impaired angiogenic capacity in the aging heart. Circulation, 105, 608–613. [DOI] [PubMed] [Google Scholar]
- Ekerstad, N. , Swahn, E. , Janzon, M. , Alfredsson, J. , Lofmark, R. , Lindenberger, M. , & Carlsson, P. (2011). Frailty is independently associated with short‐term outcomes for elderly patients with non‐ST‐segment elevation myocardial infarction. Circulation, 124, 2397–2404. [DOI] [PubMed] [Google Scholar]
- Engberding, N. , & Wenger, N. K. (2017). Acute coronary syndromes in the elderly. F1000Res, 6, 1791–1797. 10.12688/f1000research.11064.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobales, N. , Nunez, R. E. , Jang, S. , Parodi‐Rullan, R. , Ayala‐Pena, S. , Sacher, J. R. , … Javadov, S. (2014). Mitochondria‐targeted ROS scavenger improves post‐ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats. Journal of Molecular and Cellular Cardiology, 77, 136–146. 10.1016/j.yjmcc.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz, J. A. , Kaul, P. , Bakal, J. A. , Armstrong, P. W. , Welsh, R. C. , & McAlister, F. A. (2009). Declining in‐hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. Journal of the American College of Cardiology, 53, 13–20. 10.1016/j.jacc.2008.08.067 [DOI] [PubMed] [Google Scholar]
- Faber, J. E. , Zhang, H. , Lassance‐Soares, R. M. , Prabhakar, P. , Najafi, A. H. , Burnett, M. S. , & Epstein, S. E. (2011). Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arteriosclerosis, Thrombosis, and Vascular Biology, 31, 1748–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk, E. , Nakano, M. , Bentzon, J. F. , Finn, A. V. , & Virmani, R. (2013). Update on Acute Coronary syndromes: The pathologists' view. European Heart Journal, 34, 719–728. 10.1093/eurheartj/ehs411 [DOI] [PubMed] [Google Scholar]
- Fang, L. , Gao, X. M. , Moore, X. L. , Kiriazis, H. , Su, Y. , Ming, Z. , … Du, X. J. (2007). Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. Journal of Molecular and Cellular Cardiology, 43, 535–544. [DOI] [PubMed] [Google Scholar]
- Ferdinandy, P. , Hausenloy, D. J. , Heusch, G. , Baxter, G. F. , & Schulz, R. (2014). Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacological Reviews, 66, 1142–1174. 10.1124/pr.113.008300 [DOI] [PubMed] [Google Scholar]
- Feridooni, H. A. , MacDonald, J. K. , Ghimire, A. , Pyle, W. G. , & Howlett, S. E. (2017). Acute exposure to progesterone attenuates cardiac contraction by modifying myofilament calcium sensitivity in the female mouse heart. American Journal of Physiology. Heart and Circulatory Physiology, 312, H46–H59. 10.1152/ajpheart.00073.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Sanz, C. , Ruiz‐Meana, M. , Castellano, J. , Miro‐Casas, E. , Nunez, E. , Inserte, J. , … Garcia‐Dorado, D. (2015). Altered FoF1 ATP synthase and susceptibility to mitochondrial permeability transition pore during ischaemia and reperfusion in aging cardiomyocytes. Thrombosis and Haemostasis, 113, 441–451. 10.1160/TH14-10-0901 [DOI] [PubMed] [Google Scholar]
- Fernandez‐Sanz, C. , Ruiz‐Meana, M. , Miro‐Casas, E. , Nunez, E. , Castellano, J. , Loureiro, M. , … Garcia‐Dorado, D. (2014). Defective sarcoplasmic reticulum‐mitochondria calcium exchange in aged mouse myocardium. Cell Death & Disease, 5, 1573–1587, e1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis, N. G. (2019). Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Molecular Aspects of Medicine, 65, 70–99. 10.1016/j.mam.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Freire, A. C. , Basit, A. W. , Choudhary, R. , Piong, C. W. , & Merchant, H. A. (2011). Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. International Journal of Pharmaceutics, 415, 15–28. [DOI] [PubMed] [Google Scholar]
- Gaignebet, L. , & Kararigas, G. (2017). En route to precision medicine through the integration of biological sex into pharmacogenomics. Clinical Science (London, England), 131, 329–342. [DOI] [PubMed] [Google Scholar]
- Garcia‐Perez, C. , Hajnoczky, G. , & Csordas, G. (2008). Physical coupling supports the local Ca2+ transfer between sarcoplasmic reticulum subdomains and the mitochondria in heart muscle. The Journal of Biological Chemistry, 283, 32771–32780. 10.1074/jbc.M803385200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Reyes, A. , Menaouar, A. , Yip, D. , Danalache, B. , Plante, E. , Noiseux, N. , … Jankowski, M. (2015). Molecular mechanisms underlying oxytocin‐induced cardiomyocyte protection from simulated ischemia‐reperfusion. Molecular and Cellular Endocrinology, 412, 170–181. 10.1016/j.mce.2015.04.028 [DOI] [PubMed] [Google Scholar]
- Halestrap, A. P. , Pereira, G. C. , & Pasdois, P. (2015). The role of hexokinase in cardioprotection—Mechanism and potential for translation. British Journal of Pharmacology, 172, 2085–2100. 10.1111/bph.12899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … Davies, J. A. (2018). The IUPHAR/BPS Guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman, R. J. G. , Huisman, S. E. , & den Ruijter, H. M. (2018). Sex differences in cardiovascular epigenetics—A systematic review. Biology of Sex Differences, 9, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy, D. J. , Botker, H. E. , Engstrom, T. , Erlinge, D. , Heusch, G. , Ibanez, B. , … Garcia‐Dorado, D. (2017). Targeting reperfusion injury in patients with ST‐segment elevation myocardial infarction: Trials and tribulations. European Heart Journal, 38, 935–941. 10.1093/eurheartj/ehw145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy, D. J. , Garcia‐Dorado, D. , Botker, H. E. , Davidson, S. M. , Downey, J. , Engel, F. B. , … Ferdinandy, P. (2017). Novel targets and future strategies for acute cardioprotection: Position paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovascular Research, 113, 564–585. 10.1093/cvr/cvx049 [DOI] [PubMed] [Google Scholar]
- Heinen, A. , Huhn, R. , Smeele, K. M. , Zuurbier, C. J. , Schlack, W. , Preckel, B. , … Hollmann, M. W. (2008). Helium‐induced preconditioning in young and old rat heart: Impact of mitochondrial Ca2+‐sensitive potassium channel activation. Anesthesiology, 109, 830–836. [DOI] [PubMed] [Google Scholar]
- Heusch, G. (2017). Critical issues for the translation of cardioprotection. Circulation Research, 120, 1477–1486. 10.1161/CIRCRESAHA.117.310820 [DOI] [PubMed] [Google Scholar]
- Heusch, G. (2018). Cardioprotection research must leave its comfort zone. European Heart Journal, 39, 3393–3395. 10.1093/eurheartj/ehy253 [DOI] [PubMed] [Google Scholar]
- Horn, M. A. , & Trafford, A. W. (2016). Aging and the cardiac collagen matrix: Novel mediators of fibrotic remodelling. Journal of Molecular and Cellular Cardiology, 93, 175–185. 10.1016/j.yjmcc.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett, S. E. (2010). Age‐associated changes in excitation‐contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. American Journal of Physiology. Heart and Circulatory Physiology, 298, H659–H670. 10.1152/ajpheart.00214.2009 [DOI] [PubMed] [Google Scholar]
- Huang, C. , Yang, W. , Wang, J. , Zhou, Y. , Geng, B. , Kararigas, G. , … Cui, Q. (2018). The DrugPattern tool for drug set enrichment analysis and its prediction for beneficial effects of OxLDL on type 2 diabetes. Journal of Genetics and Genomics, 45, 389–397. 10.1016/j.jgg.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Ibanez, B. , James, S. , Agewall, S. , Antunes, M. J. , Bucciarelli‐Ducci, C. , Bueno, H. , … Widimsky, P. (2017). 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Revista Española de Cardiología (Engl Ed), 70, 1082–1142. [DOI] [PubMed] [Google Scholar]
- Jahangir, A. , Sagar, S. , & Terzic, A. (2007). Aging and cardioprotection. Journal of Applied Physiology, 103, 2120–2128. [DOI] [PubMed] [Google Scholar]
- Jneid, H. , Fonarow, G. C. , Cannon, C. P. , Hernandez, A. F. , Palacios, I. F. , Maree, A. O. , … Wexler, L. (2008). Sex differences in medical care and early death after acute myocardial infarction. Circulation, 118, 2803–2810. [DOI] [PubMed] [Google Scholar]
- Kang, C. , Qin, J. , Osei, W. , & Hu, K. (2017). Regulation of protein kinase C‐epsilon and its age‐dependence. Biochemical and Biophysical Research Communications, 482, 1201–1206. [DOI] [PubMed] [Google Scholar]
- Kararigas, G. , Seeland, U. , Barcena de Arellano, M. L. , Dworatzek, E. , & Regitz‐Zagrosek, V. (2016). Why the study of the effects of biological sex is important. Commentary. Annali dell'Istituto Superiore di Sanità, 52, 149–150. 10.4415/ANN_16_02_03 [DOI] [PubMed] [Google Scholar]
- Keller, K. M. , & Howlett, S. E. (2016). Sex differences in the biology and pathology of the aging heart. The Canadian Journal of Cardiology, 32, 1065–1073. [DOI] [PubMed] [Google Scholar]
- Khera, A. V. , Emdin, C. A. , Drake, I. , Natarajan, P. , Bick, A. G. , Cook, N. R. , … Kathiresan, S. (2016). Genetic risk, adherence to a healthy lifestyle, and coronary disease. The New England Journal of Medicine, 375, 2349–2358. 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbongard, P. , Botker, H. E. , Ovize, M. , Hausenloy, D. J. , & Heusch, G. (2019). Co‐morbidities and co‐medications as confounders of cardioprotection—Does it matter in the clinical setting? British Journal of Pharmacology, 2019(1‐18), 14839–14856. 10.1111/bph.14839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaas, M. , Nickel, A. G. , & Maack, C. (2017). Mitochondrial energetics and calcium coupling in the heart. The Journal of Physiology, 595, 3753–3763. 10.1113/JP273609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, C. H. T. , Bryant, S. M. , Watson, J. J. , Gadeberg, H. C. , Roth, D. M. , Patel, H. H. , … James, A. F. (2018). The effects of aging on the regulation of T‐tubular ICa by caveolin in mouse ventricular myocytes. The Journals of Gerontology. Series a, Biological Sciences and Medical Sciences, 73, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzick, D. H. , & Lancaster, T. S. (2013). Age‐related differences in cardiac ischemia‐reperfusion injury: effects of estrogen deficiency. Pflügers Archiv, 465, 669–685. 10.1007/s00424-013-1255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov, A. V. , Javadov, S. , Margreiter, R. , Grimm, M. , Hagenbuchner, J. , & Ausserlechner, M. J. (2019). The role of mitochondria in the mechanisms of cardiac ischemia‐reperfusion injury. Antioxidants (Basel), 8(10), 454–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagranha, C. J. , Deschamps, A. , Aponte, A. , Steenbergen, C. , & Murphy, E. (2010). Sex Differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circulation Research, 106, 1681–1691. 10.1161/CIRCRESAHA.109.213645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, Y. T. (2015). Critical roles of reactive oxygen species in age‐related impairment in ischemia‐induced neovascularization by regulating stem and progenitor cell function. Oxidative Medicine and Cellular Longevity, 2015, 1–14. 7095901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, P. Y. , Alexander, K. P. , Hammill, B. G. , Pasquali, S. K. , & Peterson, E. D. (2001). Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA, 286, 708–713. [DOI] [PubMed] [Google Scholar]
- Li, H. , Zhou, C. , Chen, D. , Fang, N. , Yao, Y. , & Li, L. (2013). Failure to protect against myocardial ischemia‐reperfusion injury with sevoflurane postconditioning in old rats in vivo. Acta Anaesthesiologica Scandinavica, 57, 1024–1031. 10.1111/aas.12156 [DOI] [PubMed] [Google Scholar]
- Li, J. , Chen, X. , McClusky, R. , Ruiz‐Sundstrom, M. , Itoh, Y. , Umar, S. , … Eghbali, M. (2014). The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: One X is better than two. Cardiovascular Research, 102, 375–384. 10.1093/cvr/cvu064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey, M. L. (2018). Assigning matrix metalloproteinase roles in ischaemic cardiac remodelling. Nature Reviews. Cardiology, 15, 471–479. 10.1038/s41569-018-0022-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas‐Herald, A. K. , Alves‐Lopes, R. , Montezano, A. C. , Ahmed, S. F. , & Touyz, R. M. (2017). Genomic and non‐genomic effects of androgens in the cardiovascular system: Clinical implications. Clinical Science (London, England), 131, 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsen, S. , Mancusi, C. , Kildal, S. , De Simone, G. , Gerdts, E. , & Ytrehus, K. (2018). Cardiac adaptation to hypertension in adult female Dahl salt‐sensitive rats is dependent on ovarian function, but loss of ovarian function does not predict early maladaptation. Physiological Reports, 6, 13593–13606, e13593 10.14814/phy2.13593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggioni, A. P. , Maseri, A. , Fresco, C. , Franzosi, M. G. , Mauri, F. , Santoro, E. , & Tognoni, G. (1993). Age‐related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. The investigators of the Gruppo Italiano Per Lo Studio Della Sopravvivenza Nell'Infarto Miocardico (GISSI‐2). The New England Journal of Medicine, 329, 1442–1448. 10.1056/NEJM199311113292002 [DOI] [PubMed] [Google Scholar]
- Mehilli, J. , Ndrepepa, G. , Kastrati, A. , Nekolla, S. G. , Markwardt, C. , Bollwein, H. , … Schomig, A. (2005). Gender and myocardial salvage after reperfusion treatment in acute myocardial infarction. Journal of the American College of Cardiology, 45, 828–831. 10.1016/j.jacc.2004.11.054 [DOI] [PubMed] [Google Scholar]
- Mehta, L. S. , Beckie, T. M. , DeVon, H. A. , Grines, C. L. , Krumholz, H. M. , Johnson, M. N. , … Wenger, N. K. (2016). Acute myocardial infarction in women: A scientific statement from the American Heart Association. Circulation, 133, 916–947. 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- Mehta, R. H. , Rathore, S. S. , Radford, M. J. , Wang, Y. , Wang, Y. , & Krumholz, H. M. (2001). Acute myocardial infarction in the elderly: Differences by age. Journal of the American College of Cardiology, 38, 736–741. 10.1016/s0735-1097(01)01432-2 [DOI] [PubMed] [Google Scholar]
- Mendes, A. B. , Ferro, M. , Rodrigues, B. , Souza, M. R. , Araujo, R. C. , & Souza, R. R. (2012). Quantification of left ventricular myocardial collagen system in children, young adults, and the elderly. Medicina (B Aires), 72, 216–220. [PubMed] [Google Scholar]
- Meschiari, C. A. , Ero, O. K. , Pan, H. , Finkel, T. , & Lindsey, M. L. (2017). The impact of aging on cardiac extracellular matrix. Geroscience, 39, 7–18. 10.1007/s11357-017-9959-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio, Y. , Bienengraeber, M. W. , Marinovic, J. , Gutterman, D. D. , Rakic, M. , Bosnjak, Z. J. , & Stadnicka, A. (2008). Age‐related attenuation of isoflurane preconditioning in human atrial cardiomyocytes: Roles for mitochondrial respiration and sarcolemmal adenosine triphosphate‐sensitive potassium channel activity. Anesthesiology, 108, 612–620. 10.1097/ALN.0b013e318167af2d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebali, D. , Kaplan, D. , Carlisle, M. , Supiano, M. A. , & Rondina, M. T. (2014). Alterations in platelet function during aging: Clinical correlations with thromboinflammatory disease in older adults. Journal of the American Geriatrics Society, 62, 529–535. 10.1111/jgs.12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, A. E. , Forouzanfar, M. H. , Roth, G. A. , Mensah, G. A. , Ezzati, M. , Flaxman, A. , … Naghavi, M. (2014). The global burden of ischemic heart disease in 1990 and 2010: The global burden of disease 2010 study. Circulation, 129, 1493–1501. 10.1161/CIRCULATIONAHA.113.004046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, H. , & Komuro, I. (2018). Heart failure as an aging‐related phenotype. International Heart Journal, 59, 6–13. 10.1536/ihj.17-519 [DOI] [PubMed] [Google Scholar]
- Murphy, E. , & Steenbergen, C. (2007). Cardioprotection in females: A role for nitric oxide and altered gene expression. Heart Failure Reviews, 12, 293–300. 10.1007/s10741-007-9035-0 [DOI] [PubMed] [Google Scholar]
- Nguyen, L. T. , Rebecchi, M. J. , Moore, L. C. , Glass, P. S. , Brink, P. R. , & Liu, L. (2008). Attenuation of isoflurane‐induced preconditioning and reactive oxygen species production in the senescent rat heart. Anesthesia and Analgesia, 107, 776–782. 10.1213/ane.0b013e318180419d [DOI] [PubMed] [Google Scholar]
- Olivetti, G. , Giordano, G. , Corradi, D. , Melissari, M. , Lagrasta, C. , Gambert, S. R. , & Anversa, P. (1995). Gender differences and aging: Effects on the human heart. Journal of the American College of Cardiology, 26, 1068–1079. [DOI] [PubMed] [Google Scholar]
- Ong, S. B. , Samangouei, P. , Kalkhoran, S. B. , & Hausenloy, D. J. (2015). The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. Journal of Molecular and Cellular Cardiology, 78, 23–34. [DOI] [PubMed] [Google Scholar]
- Ormerod, J. O. , Evans, J. D. , Contractor, H. , Beretta, M. , Arif, S. , Fernandez, B. O. , … Ashrafian, H. (2017). Human second window pre‐conditioning and post‐conditioning by nitrite is influenced by a common polymorphism in mitochondrial aldehyde dehydrogenase. JACC Basic Transl Sci, 2, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwoll, E. (2017). Further elucidation of the potential benefits of testosterone therapy in older men. JAMA Internal Medicine, 177, 459–460. [DOI] [PubMed] [Google Scholar]
- Pagliaro, P. , & Penna, C. (2017). Hypertension, hypertrophy, and reperfusion injury. Journal of Cardiovascular Medicine (Hagerstown, Md.), 18, 131–135. [DOI] [PubMed] [Google Scholar]
- Patterson, A. J. , Chen, M. , Xue, Q. , Xiao, D. , & Zhang, L. (2010). Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circulation Research, 107, 365–373. 10.1161/CIRCRESAHA.110.221259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart, J. N. , Pepe, S. , Reichelt, M. E. , Beckett, N. , See, H. L. , Ozberk, V. , … Headrick, J. P. (2014). Dysfunctional survival‐signaling and stress‐intolerance in aged murine and human myocardium. Experimental Gerontology, 50, 72–81. 10.1016/j.exger.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna, C. , Tullio, F. , Merlino, A. , Moro, F. , Raimondo, S. , Rastaldo, R. , … Pagliaro, P. (2009). Postconditioning cardioprotection against infarct size and post‐ischemic systolic dysfunction is influenced by gender. Basic Research in Cardiology, 104, 390–402. [DOI] [PubMed] [Google Scholar]
- Perez, V. D. , Annunzio, V. , Mazo, T. , Marchini, T. , Caceres, L. , Evelson, P. , & Gelpi, R. J. (2016). Ischemic postconditioning confers cardioprotection and prevents reduction ofTrx‐1 in young mice, but not in middle‐aged and old mice. Molecular and Cellular Biochemistry, 415(1‐2), 67–76. 10.1007/s11010-016-2677-2 [DOI] [PubMed] [Google Scholar]
- Piper, H. M. , Garcia‐Dorado, D. , & Ovize, M. (1998). A fresh look at reperfusion injury. Cardiovascular Research, 38, 291–300. 10.1016/s0008-6363(98)00033-9 [DOI] [PubMed] [Google Scholar]
- Pipicz, M. , Demjan, V. , Sarkozy, M. , & Csont, T. (2018). Effects of cardiovascular risk factors on cardiac STAT3. International Journal of Molecular Sciences, 19, 3572–3600. 10.3390/ijms19113572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez‐Camacho, I. , Flores‐Herrera, O. , & Zazueta, C. (2019). The relevance of the supramolecular arrangements of the respiratory chain complexes in human diseases and aging. Mitochondrion, 47, 266–272. 10.1016/j.mito.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Regitz‐Zagrosek, V. , & Kararigas, G. (2017). Mechanistic pathways of sex differences in cardiovascular disease. Physiological Reviews, 97, 1–37. [DOI] [PubMed] [Google Scholar]
- Ross, J. L. , & Howlett, S. E. (2012). Age and ovariectomy abolish beneficial effects of female sex on rat ventricular myocytes exposed to simulated ischemia and reperfusion. PLoS ONE, 7, 38425–38436, e38425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubini, G. M. , Reiter, M. , Twerenbold, R. , Reichlin, T. , Wildi, K. , Haaf, P. , … Mueller, C. (2014). Sex‐specific chest pain characteristics in the early diagnosis of acute myocardial infarction. JAMA Internal Medicine, 174, 241–249. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Meana, M. , Fernandez‐Sanz, C. , & Garcia‐Dorado, D. (2010). The SR‐mitochondria interaction: A new player in cardiac pathophysiology. Cardiovascular Research, 88, 30–39. 10.1093/cvr/cvq225 [DOI] [PubMed] [Google Scholar]
- Ruiz‐Meana, M. , Inserte, J. , Fernandez‐Sanz, C. , Hernando, V. , Miro‐Casas, E. , Barba, I. , & Garcia‐Dorado, D. (2011). The role of mitochondrial permeability transition in reperfusion‐induced cardiomyocyte death depends on the duration of ischemia. Basic Research in Cardiology, 106, 1259–1268. 10.1007/s00395-011-0225-5 [DOI] [PubMed] [Google Scholar]
- Ruiz‐Meana, M. , Minguet, M. , Bou‐Teen, D. , Miro‐Casas, E. , Castans, C. , Castellano, J. , … Garcia‐Dorado, D. (2019). Ryanodine receptor glycation favors mitochondrial damage in the senescent heart. Circulation, 139, 949–964. 10.1161/CIRCULATIONAHA.118.035869 [DOI] [PubMed] [Google Scholar]
- Sarwar, M. , Du, X. J. , Dschietzig, T. B. , & Summers, R. J. (2017). The actions of relaxin on the human cardiovascular system. British Journal of Pharmacology, 174, 933–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman, D. , Latchman, D. S. , & Yellon, D. M. (2001). Effect of aging on the ability of preconditioning to protect rat hearts from ischemia‐reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology, 281, H1630–H1636. 10.1152/ajpheart.2001.281.4.H1630 [DOI] [PubMed] [Google Scholar]
- Schwartz, J. B. (2007). The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clinical Pharmacology and Therapeutics, 82, 87–96. [DOI] [PubMed] [Google Scholar]
- Sessions, A. O. , Kaushik, G. , Parker, S. , Raedschelders, K. , Bodmer, R. , Van Eyk, J. E. , & Engler, A. J. (2017). Extracellular matrix downregulation in the drosophila heart preserves contractile function and improves lifespan. Matrix Biology, 62, 15–27. 10.1016/j.matbio.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, H. , Lee, B. , Lee, R. J. , & Boyle, A. J. (2011). The aging heart and post‐infarction left ventricular remodeling. Journal of the American College of Cardiology, 57, 9–17. 10.1016/j.jacc.2010.08.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioura, K. M. , Geenen, D. L. , & Goldspink, P. H. (2008). Sex‐related changes in cardiac function following myocardial infarction in mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 295, R528–R534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silander, K. , Alanne, M. , Kristiansson, K. , Saarela, O. , Ripatti, S. , Auro, K. , … Peltonen, L. (2008). Gender differences in genetic risk profiles for cardiovascular disease. PLoS ONE, 3, 3615–3629, e3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniecinski, R. , & Liu, H. (2004). Reduced efficacy of volatile anesthetic preconditioning with advanced age in isolated rat myocardium. Anesthesiology, 100, 589–597. 10.1097/00000542-200403000-00019 [DOI] [PubMed] [Google Scholar]
- Sovershaev, M. A. , Egorina, E. M. , Andreasen, T. V. , Jonassen, A. K. , & Ytrehus, K. (2006). Preconditioning by 17β‐estradiol in isolated rat heart depends on PI3‐K/PKB pathway, PKC, and ROS. American Journal of Physiology. Heart and Circulatory Physiology, 291, H1554–H1562. [DOI] [PubMed] [Google Scholar]
- Strait, J. B. , & Lakatta, E. G. (2012). Aging‐associated cardiovascular changes and their relationship to heart failure. Heart Failure Clinics, 8, 143–164. 10.1016/j.hfc.2011.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talens, R. P. , Jukema, J. W. , Trompet, S. , Kremer, D. , Westendorp, R. G. , Lumey, L. H. , … Heijmans, B. T. (2012). Hypermethylation at loci sensitive to the prenatal environment is associated with increased incidence of myocardial infarction. International Journal of Epidemiology, 41, 106–115. 10.1093/ije/dyr153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepp, K. , Puurand, M. , Timohhina, N. , Adamson, J. , Klepinin, A. , Truu, L. , … Kaambre, T. (2017). Changes in the mitochondrial function and in the efficiency of energy transfer pathways during cardiomyocyte aging. Molecular and Cellular Biochemistry, 432, 141–158. 10.1007/s11010-017-3005-1 [DOI] [PubMed] [Google Scholar]
- Tepp, K. , Timohhina, N. , Puurand, M. , Klepinin, A. , Chekulayev, V. , Shevchuk, I. , & Kaambre, T. (2016). Bioenergetics of the aging heart and skeletal muscles: Modern concepts and controversies. Ageing Research Reviews, 28, 1–14. [DOI] [PubMed] [Google Scholar]
- Timohhina, N. , Guzun, R. , Tepp, K. , Monge, C. , Varikmaa, M. , Vija, H. , … Saks, V. (2009). Direct measurement of energy fluxes from mitochondria into cytoplasm in permeabilized cardiac cells in situ: Some evidence for mitochondrial interactosome. Journal of Bioenergetics and Biomembranes, 41, 259–275. [DOI] [PubMed] [Google Scholar]
- Trankle, C. , Thurber, C. J. , Toldo, S. , & Abbate, A. (2016). Mitochondrial membrane permeability inhibitors in acute myocardial infarction: Still awaiting translation. JACC Basic Transl Sci, 1, 524–535. 10.1016/j.jacbts.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trial, J. , Entman, M. L. , & Cieslik, K. A. (2016). Mesenchymal stem cell‐derived inflammatory fibroblasts mediate interstitial fibrosis in the aging heart. Journal of Molecular and Cellular Cardiology, 91, 28–34. 10.1016/j.yjmcc.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey, D. A. , Kelley, M. , Li, L. , & Huang, Y. (2009). Sphingosine protects aging hearts from ischemia/reperfusion injury: Superiority to sphingosine 1‐phosphate and ischemic pre‐ and post‐conditioning. Oxidative Medicine and Cellular Longevity, 2, 146–151. 10.4161/oxim.2.3.8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey, D. A. , Li, L. , Kelley, M. , Zhang, J. , & Karliner, J. S. (2008). Sphingosine can pre‐ and post‐condition heart and utilizes a different mechanism from sphingosine 1‐phosphate. Journal of Biochemical and Molecular Toxicology, 22, 113–118. 10.1002/jbt.20227 [DOI] [PubMed] [Google Scholar]
- Vonck, J. , & Schafer, E. (2009). Supramolecular organization of protein complexes in the mitochondrial inner membrane. Biochimica et Biophysica Acta, 1793, 117–124. 10.1016/j.bbamcr.2008.05.019 [DOI] [PubMed] [Google Scholar]
- Wang, F. , He, Q. , Sun, Y. , Dai, X. , & Yang, X. P. (2010). Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension, 55, 1172–1178. 10.1161/HYPERTENSIONAHA.110.150839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Zhang, J. , Telljohann, R. , Jiang, L. , Wu, J. , Monticone, R. E. , … Lakatta, E. G. (2012). Chronic matrix metalloproteinase inhibition retards age‐associated arterial proinflammation and increase in blood pressure. Hypertension, 60, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, I. , Salie, R. , Marais, E. , Fan, W. J. , Maarman, G. , Huisamen, B. , & Lochner, A. (2017). Myocardial susceptibility to ischaemia/reperfusion in obesity: A re‐evaluation of the effects of age. BMC Physiology, 17, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman, P. C. , Lipinski, M. J. , Luger, D. , Waksman, R. , Bonow, R. O. , Wu, E. , & Epstein, S. E. (2016). Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. Journal of the American College of Cardiology, 67, 2050–2060. 10.1016/j.jacc.2016.01.073 [DOI] [PubMed] [Google Scholar]
- Whittington, H. J. , Harding, I. , Stephenson, C. I. , Bell, R. , Hausenloy, D. J. , Mocanu, M. M. , & Yellon, D. M. (2013). Cardioprotection in the aging, diabetic heart: The loss of protective Akt signalling. Cardiovascular Research, 99, 694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems, L. , Zatta, A. , Holmgren, K. , Ashton, K. J. , & Headrick, J. P. (2005). Age‐related changes in ischemic tolerance in male and female mouse hearts. Journal of Molecular and Cellular Cardiology, 38, 245–256. [DOI] [PubMed] [Google Scholar]
- Xia, Z. , Li, H. , & Irwin, M. G. (2016). Myocardial ischaemia reperfusion injury: The challenge of translating ischaemic and anaesthetic protection from animal models to humans. British Journal of Anaesthesia, 117(Suppl 2), ii44–ii62. 10.1093/bja/aew267 [DOI] [PubMed] [Google Scholar]
- Yabluchanskiy, A. , Ma, Y. , Chiao, Y. A. , Lopez, E. F. , Voorhees, A. P. , Toba, H. , … Jin, Y. F. (2014). Cardiac aging is initiated by matrix metalloproteinase‐9‐mediated endothelial dysfunction. American Journal of Physiology. Heart and Circulatory Physiology, 306, H1398–H1407. 10.1152/ajpheart.00090.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, Y. , Izawa, H. , Ichihara, S. , Takatsu, F. , Ishihara, H. , Hirayama, H. , … Yokota, M. (2002). Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. The New England Journal of Medicine, 347, 1916–1923. 10.1056/NEJMoa021445 [DOI] [PubMed] [Google Scholar]
- Yin, Z. , Gao, H. , Wang, H. , Li, L. , Di, C. , Luan, R. , & Tao, L. (2009). Ischaemic post‐conditioning protects both adult and aged Sprague‐Dawley rat heart from ischaemia‐reperfusion injury through the phosphatidylinositol 3‐kinase‐AKT and glycogen synthasekinase‐3beta pathways. Clinical and Experimental Pharmacology and Physiology, 36(8), 756–763. 10.1111/j.1440-1681.2009.05148.x [DOI] [PubMed] [Google Scholar]
- Zhu, F. , Li, Y. , Zhang, J. , Piao, C. , Liu, T. , Li, H. H. , & Du, J. (2013). Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS ONE, 8, 74535–74546, e74535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J. , Rebecchi, M. J. , Glass, P. S. , Brink, P. R. , & Liu, L. (2011). Cardioprotection of the aged rat heart by GSK‐3β inhibitor is attenuated: Age‐related changes in mitochondrial permeability transition pore modulation. American Journal of Physiology. Heart and Circulatory Physiology, 300, H922–H930. 10.1152/ajpheart.00860.2010 [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Rebecchi, M. J. , Tan, M. , Glass, P. S. , Brink, P. R. , & Liu, L. (2010). Age‐associated differences in activation of Akt/GSK‐3β signaling pathways and inhibition of mitochondrial permeability transition pore opening in the rat heart. The Journals of Gerontology. Series a, Biological Sciences and Medical Sciences, 65, 611–619. [DOI] [PubMed] [Google Scholar]
- Zuurbier, C. J. , Heinen, A. , Koeman, A. , Stuifbergen, R. , Hakvoort, T. B. , Weber, N. C. , & Hollmann, M. W. (2014). Cardioprotective efficacy depends critically on pharmacological dose, duration of ischaemia, health status of animals and choice of anaesthetic regimen: A case study with folic acid. Journal of Translational Medicine, 12, 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]


