Abstract
The outbreak of the coronavirus disease of 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 that has created huge trepidation worldwide, has a mortality rate of 0.5% to 1% and is growing incessantly. There are currently no therapies and/or vaccines that may help abate this viral disease, but the use of masks and social distancing can limit the spread. Boosting immunity has been a simple way to resist viral infection and limit fatalities. In this context, the use of nutraceuticals appears to be a potential panacea. The ability of algae-based nutraceuticals, mainly Spirulina, to boost immunity against viral diseases has already been reported clinically. Spirulina-based nutraceuticals boost the adaptive and innate immunity, and bioactive compounds, such as angiotensin-converting enzyme (ACE) inhibitor peptides, phycobiliproteins, sulfated polysaccharides, and calcium-Spirulan, can serve as antiviral agents. The presence of these molecules indicates its potential role in resisting infection and COVID-19 disease progression. This review focuses on the potential role of algal nutraceuticals as immune boosters to combat the human coronavirus and other viral diseases. The potential use of Spirulina-based nutraceuticals for combating COVID-19, its mechanism, and future directions have also been discussed.
Keywords: Algae, Nutraceuticals, Immune-booster, Antiviral, Coronavirus, COVID-19
Introduction
Since the beginning of the 21st century, humankind has suffered from beta-coronavirus diseases, such as severe acute respiratory syndrome (SARS) coronavirus (CoV), Middle East respiratory syndrome, and SARS-CoV2 [1]. The recent outbreak of the coronavirus disease of 2019 (COVID-19) caused by SARS-CoV2 has created panic around the world because of its higher rate of infection and comorbidity combined with the unavailability of standard therapies and/or vaccines [2]. CoVs are single-stranded, positive-sense encapsulated RNA viruses that have membrane augmented with glycoprotein spikes. These viruses attack the lower respiratory system of the host and affect the lungs, leading to acute respiratory distress causing pneumonia and later leading to the failure of multiple organs, such as the heart, kidneys, liver, and central nervous system [2], [3], [4].
SARS-CoV2 uses ACE2 cellular receptor for entry into the host cell through binding of its spike (S) protein, followed by S protein priming using the serine transmembrane protease 2 [5]. SARS-CoV 2 contains four structural proteins (spike [S], nucleocapsid [N], membrane [M], and envelop [E]) that may act as antigens. These antigens may induce neutralizing antibodies in the human body and increase cluster of differentiation (CD) 4+/8+ T-cell responses. These mechanisms of action serve as the basis for the treatment regimens used for this disease. At present, no specific or clinically proved therapies/vaccines are available; therefore, the outbreak requires an urgent response from the scientific community for new developments in this area. According to the World Health Organization, the development of a vaccine for COVID-19 might take >18 mo, because of the multiple steps required to ensure its effectiveness and safety. A study conducted by Gordon et al. [6] identified 67 druggable host proteins targeted by 69 existing U.S. Food and Drug Administration-approved drugs [6]. However, at present, only a few potential therapies (favipiravir, remdesivir, lopinavir, and hydroxychloroquine or chloroquine) are reported to be at the final stage of human testing [7].
The elderly and people with underlying medical conditions, such as chronic lung disease, diabetes, kidney and liver diseases, obesity, immunocompromised people (cancer, immune deficiencies), and smokers, are at a high risk [8]. The use of immune-boosting nutraceuticals has the potential to help to combat and control coronavirus infections through the activation of an immune response and alleviating oxidative stress [9], [10], [11], [12]. To date, many types of nutraceuticals, which are derived from natural resources such as animals, plants, marine organisms, and microorganisms, have been reported and are in use. Algae-comprising prokaryotic cyanobacteria and other eukaryotic forms are a rich bioresource of bioactive compounds of nutraceutical and therapeutic importance [13], [14], [15], [16], [17]. The use of cyanobacterium Spirulina-based nutraceuticals is well explored in in vitro and clinical studies and commercially available. Spirulina-based nutraceuticals have been reported to boost innate and adaptive immunity [18], [19], [20], and possess antiviral properties against different enveloped viral infections, such as human immunodeficiency virus (HIV) and herpes simplex virus (HSV) [21], [22], [23], [24]. This review discusses the potential therapeutic role of algae-based Spirulina nutraceuticals in addressing SARS-CoV and related viral infections.
Algae nutraceuticals: Potential therapeutic role in coronavirus and other viral diseases
Compounds that induce the innate and adaptive immune responses are generally used to prevent and fight against various pathogenic infections. Algae derived bioactive compounds are well reported for their antimicrobial, antiinflammatory, immunostimulatory, and immunomodulatory properties that can be potentially used as immune boosters and/or therapeutic agents to control pathogen attacks and disease prevention in humans [15,19,25]. The cyanobacterium Spirulina is commercially produced for human consumption and typically used as a health food supplement due to its high protein content, lipids, vitamins, essential amino acids, minerals, photosynthetic pigments, and biologically active substances (phycocyanin, chlorophyll, and β-carotene).
Spirulina as an immune-booster and immune system potentiator
The use of Spirulina spp. has been found to improve immune function and disease resistance in animals and humans [20,26]. A study on human subjects by Selmi et al. [26] demonstrated that the use of Spirulina supplements ameliorated anemia and immunosenescence in senior citizens diagnosed with anemia (˂13 g dL−1 and ˂12 g dL−1 of hemoglobin in men and women, respectively, for the previous 12 mo) [26]. Both men and women (approximately 40%–60%) manifested an increase in corpuscular hemoglobin, complete cell count, and indoleamine 2,3-dioxygenase enzyme activity on Spirulina supplement consumption for a 6- to 12-wk period. However, randomized clinical trials would be helpful to ensure the validity of this health supplement. Rao et al. demonstrated the use, bioavailability, and antioxidant activity of different types of algal biomass (Spirulina platensis, Haematococcus pluvialis, and Botryococcus braunii) as a source of bioactive compounds (β-carotene, astaxanthin, and lutein) in plasma, liver, and eye tissues in healthy animal models [27]. The tested algae Spirulina platensis and Botryococcus braunii were isolates from India, and the microalga Haematococcus pluvialis was an isolate from Germany. Algal biomass grown under controlled conditions in standard growth media was suspended in olive oil for 15 d and administered to the rats with equal carotenoid concentration [27]. The administration of microalgal biomass helped restore enzyme activity and prevent lipid peroxidation through scavenging free and hydroxy radicals in living cells in the rat model. A significant (P ≤ .05) increase in the activity of antioxidant enzymes (catalase, superoxide dismutase, and peroxidase) in plasma and the liver was reported after repeated doses of algae biomass in rats, indicating their potential role in food, pharmaceutical, and nutraceutical applications [27].
Spirulina supplements and/or extracts are believed to potentiate the immune system, which may help fight and suppress different viral infections [20]. Soluble extracts of Spirulina have been found to enhance natural killer (NK) cell function, macrophage phagocytic activity, and red blood cell antibody response in in vitro studies and trials on different animals and humans [20]. Interferon gamma (IFN-γ) cytokine plays an important role in innate and adaptive immunity in humans, and is a primary activator of macrophages as well as a stimulator of NK cells and neutrophils [28]. The administration of Spirulina could enhance nonspecific preventive measures, such as the activation of CD4+ cells, which further enhance the production of IFN-γ in humans, for the prevention of viral infections [29].
Hirahashi et al. [20] evaluated the potential of condensed soluble extracts of S. platensis grown under outdoor open tanks in alkaline conditions as an immune potentiator in human subjects. Hot water extracts of S. platensis were prepared by autoclaving (1 h, 120°C) biomass, and pH was adjusted to 4.0 using citric acid. The water-soluble fraction was separated via centrifugation, and condensed soluble extracts were orally administered to healthy male volunteers, age 40 to 65 y [20]. The study demonstrated the immune potentiating ability of S. platensis and its mechanism through the analysis of blood cells with the pre- and post-oral consumption of hot water extracts in selected human subjects [20]. The administration of S. platensis extracts increased the production of IFN-γ (representative of NK function) in >50% of tested individuals in an interleukin (IL) 12- and 18- dependent manner. The oral administration of Spirulina in humans could aid signaling responses via toll-like receptors (TLR) and NK-mediated IFN-γ production. Bacille Calmette-Guerin cell wall skeleton is a strong immune adjuvant for various immune therapies and acts as a ligand for TLR 2 and 4 to raise the maturation stage of monocytes/macrophages [30]. In vitro study on the addition of Bacille Calmette-Guerin cell wall skeleton to fresh, human, peripheral blood, mononuclear cells or monocytes (expressing TLR2/4) augmented the potent production of IL-12 p40 in immune-competent cells taken after the administration of S. platensis extracts compared with before the administration [20]. Therefore, oral uptake of S. platensis could be involved in the signaling responses through TLR in blood cells in humans and improve immunity [20].
Inflammation plays an imperative role in innate immunity and, depending on the amount of inflammation caused, may lead to various chronic disorders. Betacoronavirus infection results in the activation of monocytes, macrophages, and dendritic cells, followed by the secretion of IL-6 and other inflammatory cytokines [1]. Acute respiratory distress syndrome in severe cases of coronavirus infections is generally a result of cytokine release syndrome (CRS) and secondary hemophagocytic lymphohistiocytosis [1,31]. CRS and cytokine IL-6 driven elevated serum C-reactive protein are common in patients infected with COVID-19, and has led to urgent clinical research on the use of therapies to suppress CRS [1,32].
The host immune system recognizes pathogen‐associated molecular patterns through pattern recognition receptors. The recognition of coronavirus-associated molecular patterns by the host immune system is mainly mediated by the use of various pattern recognition factors, such as TLR and NOD-like receptor (NLR) [33]. NLRP3 inflammasome activation plays an important role in the innate immune response to pathogenic infections in macrophages, including COVID-19 [34,35]. Spirulina extracts are found to prevent the activation of NLRP3 inflammasome through the inhibition of extracellular signal-regulated kinases (ERK) signaling [18]. Chei et al. recently reported on the antiinflammatory effect of Spirulina maxima extracts on mouse RAW264.7 macrophages and human THP-1 cells [18]. S. maxima biomass grown in volcano seawater in Jeju, Korea was subjected to ultrasound extraction in ethanol for Spirulina extract preparation. The resulting crude extract was dissolved in dimethyl sulfoxide, and used for cell culture studies.
The study showed that Spirulina extracts can suppress lipopolysaccharide-induced NLRP3 inflammasome activation and upregulation of the proinflammatory cytokines tumor necrosis factor-α, IL-12, IL-1β, and IL-18 by inhibiting the lipopolysaccharide-induced phosphorylation of ERK [18]. The phosphorylation of ERK results in the formation of reactive oxygen species, and the inhibition of phosphorylation by Spirulina extract attenuated the harmful effect of ROS. There was a significant reduction in superoxide dismutase and glutathione peroxidase (which removes toxic hydrogen peroxide) expression in RAW264.7 cells upon Spirulina extract treatment. This demonstrated the antiinflammatory potential of Spirulina extract in macrophages, human macrophages, and bone marrow-derived macrophages. Such Spirulina derived extracts could be further explored for the treatment of inflammasome-dependent disorders [18].
The posttreatment care of patients infected with COVID-19 is also highly essential due to the side effects related to drug toxicity. Spirulina and its extracts have been proven to play multiple roles as immune boosters, antiinflammatory, antioxidant, antiapoptotic, and immune stimulatory agents. In this scenario, the application of Spirulina biomass and/or its active ingredients may provide promising protection against drug-induced hepatotoxicity and immunosuppression. Khafaga and Sayed studied the effect of oral feeding of Spirulina platensis powder (DXN Company, Kedah, Malaysia) at 500 mg kg−1 bwt in adult male Wistar albino rats against the effect of the cytotoxic drug, methotrexate [36]. Methotrexate causes a reduction in leukocyte counts, hepatic antioxidant enzymes, reduced glutathione, glutathione peroxidase, catalase, superoxide dismutase; serum immunoglobulins, as well as immunoglobulin (Ig) A, IgM, and IgM levels. Spirulina intake helps ameliorate methotrexate toxicity through the restoration of liver enzymes and a significant reduction in proinflammatory cytokines and lipid peroxidation products. In summary, Spirulina has the potential to enhance immune components and reduce physiobiochemical stress, and therefore could be used as a supplement along with treatments or prevent COVID-19 infection and related symptoms.
Potential therapeutic compounds from Spirulina and mechanisms of action
In addition to immune-boosting agents, algae are potential resources for biologically active compounds with antiinflammatory and antiviral properties, and have been said to enhance the immune system and treat immune disorders related to coronavirus and other viral infections [15,16,19]. Much research has been done on the antiviral properties of algal extracts in vitro; however, studies on the practical implications are still underway and need to be explored at demonstration scale.
Angiotensin-converting enzyme inhibitory peptides
The mechanism of infection by SARS-CoV2 includes the attachment of the viral glycosylated S protein to the ACE2 protein of host human cells, followed by the use of serine transmembrane protease 2 for S protein priming [2]. Severe coronavirus (i.e., SARS-CoV) infections generally lead to the downregulation of ACE2 and more severe lung injury [37,38]. ACE enhances the generation of angiotensin (Ang) II from AngI, which induces acute lung injury by stimulating the AngII type 1 receptor, whereas ACE2 and AngII type 2 receptor negatively regulate this pathway and are protective [37,39]. Upon SARS-CoV2 infection, once the defensive immune system is impaired, propagation of virus leads to tissue damage in organs with ACE2 receptors and induces innate inflammation mediated by proinflammatory macrophages and granulocytes. At this stage, a greater effort is required to suppress inflammation and manage life-threatening symptoms.
Generally, ACE inhibitors, which inhibit the renin–angiotensin –aldosterone system, are used as protective gear for the treatment of severe coronavirus infections [38,40,41]. ACE inhibitors help treat lung/organ injury by enhancing ACE2 activity. However, the use of ACE inhibitors for the treatment of COVID-19 is still under critical debate, especially in cases with medical conditions, such as hypertension, heart diseases, and diabetes [42]. In general, medication for the treatment of these conditions increases the expression of ACE2, and increased expression of ACE2 receptors is suspected to lead to an increased risk of SARS-CoV2 infection, because the virus uses ACE2 receptors for entry into the host. However, this concept is not yet clinically proven.
Moreover, the use of ACE inhibitors is clinically proven for the treatment of severe SARS-CoV infections. According to Kuster et al. [38], there are no current data available to show the relationship between ACE2 activity and mortality related to SARS-CoV2 [38]. The most common lethality in coronavirus infections and COVID-19 is associated with underlying lung injury due to the downregulation of ACE2 [38,43]. Therefore, the use of ACE inhibitors is continued for the treatment of multiple organ injury in severe cases of coronavirus infection [38,[44], [45], [46]].
Spirulina is a natural bioresource of ACE inhibitory peptides of therapeutic value that could be explored for their potential in the treatment of severe symptoms (lung injury) and inflammation related to beta-coronavirus infections, including COVID-19. Spirulina extracts have been reported to possess antiinflammatory properties and its potential use in humans, and its mechanism as an antiinflammatory agent has been demonstrated successfully [18]. Moreover, Spirulina nutraceuticals and derived ACE inhibitory peptides have been well demonstrated to boost immune response, reduction in cytokine-related inflammation, and enhancing ACE2 activity in in vitro, in vivo, and in silico studies on model animal organisms and humans in different diseases [18,[47], [48], [49], [50]]. Heo et al. [49] investigated the potential of ACE inhibitory peptide from hydrolyzed Spirulina sp. protein in inhibiting ACE activity in human endothelial cells. In their study, Spirulina sp. biomass was hydrolyzed using gastrointestinal enzymes to obtain hydrolysate. Different sizes of peptides were obtained by molecular weight fractionation of the hydrolysates. ACE inhibitory peptides were purified using ion-exchange chromatography, followed by reversed-phase high-performance liquid chromatography [49]. Molecular dynamics simulation revealed the formation of 5.76 ± 1.50 and 2.58 ± 0.83 pairs of H-bonds by purified peptides with ACE and AngII, respectively, indicating their potential to make dead-end complex through the formation of enzyme-inhibitor and enzyme-substrate-inhibitor complexes and inhibiting ACE catalytic activity [49]. The simulation also revealed that the application of ACE inhibitory peptide (250 μM concentration) decreased the AngII-induced production of nitric oxide and reactive oxygen species and downregulated the expression of inducible nitric oxide synthase and endothelin-1, and blocked the activation of mitogen-activated protein kinase (p38) in EA.hy926 endothelial cells. McCarty and DiNicolantonio [9] postulated that the possible use of nutraceuticals capable of inhibiting NADPH oxidase 2 production, promoting the clearance of hydrogen peroxide or aiding the restoration of the native structure of Cys98 in TLR7, might help boost the TLR7-mediated induction of type 1 IFN (innate immunity response) and antiviral antibodies. Thus, in this perspective, the use of algae-derived ACE inhibitory peptides seems to be a potential option that might play multiple roles as ACE inhibitors, immune boosters, and treating vascular dysfunction in human viral diseases, including COVID-19.
He et al. found that ACE inhibitory peptides (Ile-Gln-Pro [IQP] and Val-Glu-Pro [VEP]) derived from Spirulina platensis (synthesized by SP Biomart Ltd., Beijing, China) could be absorbed intact via Caco-2 cell monolayers and is expected to have high bioavailability [51]. The transport of both peptides was energy-dependent and involved an apical-to-basolateral flux mediated by P-glycoprotein for the transport of VEP. Zheng et al. [50] also reported that the oral administration (10 mg−1kg−1d−1 for 6 wk) of Spirulina platensis derived bioactive peptides IQP, VEP, as well as biomass hydrolysates, exhibited ACE inhibiting activity, and could improve blood pressure in hypertensive rats [50]. The researchers found that there was a significant reduction (P ˂ .05) in ACE mRNA levels by 76.8%, 68.6%, and 87.7% in IQP, VEP, and biomass hydrolysates supplemented groups, respectively, compared with the control group. This was achieved by modulating the expression of local kidney renin angiotensin system components by downregulating the ACE, AngII, and Ang type 1 receptor while upregulating ACE2, Ang (1–7), Mas, and Ang type 2.
Additionally, peptic and tryptic protein hydrolysates of Spirulina platensis were also reported to inhibit the activity of peptidyl-peptidase IV (half maximal inhibitory concentration [IC50–] 3.4 and 0.1 mg mL−1) and ACE (IC50–3.0 and 0.28 mg mL−1) in an in vitro study and cellular assays in Caco-2 cells, respectively [47]. Aiello et al. found that S. platensis peptic and tryptic hydrolysates decreased the ACE activity in vitro (measuring the formation of hippuric acid [HA] from hippuryl-histidyl-leucine [HHL], a mimic substrate for AngI) in a dose-dependent manner with IC50 values of 0.1 ± 0.04 mg mL−1 and 0.28 ± 0.03 mg mL−1, respectively. Similarly, both hydrolysates inhibited ACE activity in cellular assays in Caco-2 cells with IC50 values of 2.7 ± 0.3 mg mL−1 and 2.8 ± 0.9 mg mL−1, respectively. Anekthanakul et al. [48] developed a SpirPep platform to assist in silico-based bioactive peptides discovery of bioactive compounds from Spirulina, and showed that peptides derived from Spirulina were mainly involved in ACE inhibitory activity. The researchers reported two new ACE substrate binding sites (R124 and S219) along with binding site residues (D121, E123, S516, and S517) from natural ACE inhibitory peptides (AngII and bradykinin-potentiating peptides) through which SpirPep1 indirectly bound to ACE. Natural ACE inhibitory peptides from Spirulina have great potential in ACE inhibition and enhancing ACE2 activity [48].
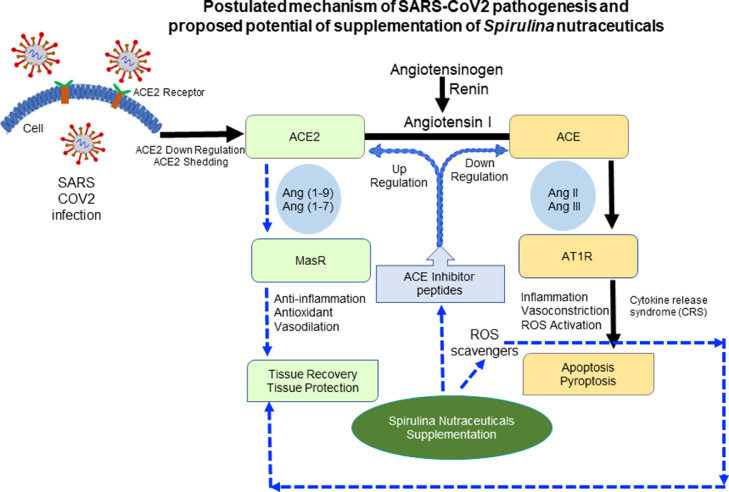
ACE inhibitors are generally used to enhance ACE2 activity for the treatment of tissue injury in various organs in severe cases of coronavirus infection. Different studies have shown that potential applications of Spirulina derived ACE inhibitory peptides as an antiinflammatory and antioxidative agent via enhancing ACE2 activity and reducing cytokine related inflammation [18,[47], [48], [49], [50]]. Therefore, based on these studies, supplementation of Spirulina-derived ACE inhibitory peptides may play a potential therapeutic or subsidiary role in the alleviation and treatment of oxidative stress, cytokine release syndrome, and tissue injury in SARS-CoV2 and other coronavirus infections through the regulation of ACE2 activity (Fig. 1 ). However, assessing the potentiality of these Spirulina-derived compounds in SARS-CoV2 infection is too early and needs to be reconnoitered with basic and clinical research.
Fig. 1.
Postulated mechanism of severe acute respiratory syndrome coronavirus (SARS-CoV) 2 pathogenesis and proposed potential of supplementation of Spirulina nutraceuticals in alleviating oxidative stress and tissue injury. Similar to SARS-CoV, SARS-CoV2 is believed to infect host cells via binding of the virus spike protein with angiotensin converting enzyme (ACE) 2 receptors of the host cell and lead to the downregulation of the ACE2. ACE2 is a suppressor of the renin-angiotensin system, where ACE catalyzes the conversion of angiotensin (Ang) I to Ang II, which further binds to angiotensin II type 1 receptors and induces acute tissue injury. On the other hand, ACE2 hydrolyzes Ang II to Ang 1 to 7 peptide that acts on the Mas receptor and protect from tissue injury. Supplementation of Spirulina nutraceuticals in SARS-CoV2 infection may help upregulate ACE2 activity and downregulate ACE activity that may further assist to overcome cytokine release syndrome and aid in tissue protection and repair.
Antiviral compounds
Novel sulfated polysaccharides from different algal resources are reported to exhibit antiviral properties and are of therapeutic use against different viral infections [23]. Novel sulfated polysaccharide derived from different species of Spirulina designated as calcium-spirulan was found to possess distinct antiviral activity against different enveloped viruses, including Herpes simplex virus type 1, human cytomegalovirus, measles virus, mumps virus, influenza A virus, and HIV-1 in different human cell lines [21,22,52]. Hayashi et al. [21] revealed that the main mechanism of antiviral activity of calcium-spirulan derived from Spirulina platensis is involved in the inhibition of viral replication via selective inhibition of penetration of the virus into the host cell. The main components of calcium-spirulan were calcium, sulfate, glucuronic acid, galacturonic acid, glucose, xylose, galactose, fructose, mannose, ribose, and rhamnose. The researchers suggested that the main antiviral activity of the calcium-spirulan might be exhibited due to the molecular conformation through the chelation of calcium-ion with sulfate groups.
In another study, Hayashi et al. [52] evaluated the antiviral potential of calcium-spirulan derived from Spirulina platensis against HIV-1 and HSV-1 compared with standard dextran sulfate. Mice treated intravenously with calcium-spirulan isolated from S. platensis showed increased serum concentration of 1000 µg mL−1 after 30 min of administration, which gradually decreased. Serum samples from calcium-spirulan administrated mice showed long-lasting antiviral activity against HIV-1 and HSV-1 even after 24 h of administration. This was significantly higher compared with the representative sulfated polysaccharide desxtran sulfate [52].
Phycobiliproteins are a group of water-soluble proteins that possess antioxidant, antiinflammatory, and antimicrobial properties [53]. Water-soluble extracts of Spirulina are generally rich in phycobiliproteins, which also exhibit antiviral properties. A study conducted by Chen et al. [29] showed that cold water extract of Spirulina (Arthrospira platensis, Far East Bio-Tec Co., Taipei, Taiwan) exhibited antiviral effect in influenza A/WSN/33 (H1N1) virus-infected mice. BALB/c female mice were given Spirulina extracts by oral gavage at concentrations of 5, 12.5, and 25 mg kg−1 4 h before intranasal inoculation of the H1N1 virus in mice (2.0 × 104 PFU per mouse). At the early stages of infection, Spirulina extracts reduced the virus yield in cells and improved the survival rate by 0%, 20%, 40%, and 60% on the oral gavage at 5, 12.5, and 25 mg kg−1 Spirulina extracts, respectively, in the infected mice. Hemagglutination was identified as one of the mechanisms involved in the inhibition of viral replication. The tested Spirulina extracts was composed of 39.33% of protein, 11.79% of polysaccharides, 19.29% of nucleic acids, 5% of water, 1.2% of ash, and approximately 23.39% of unknown components. C-phycocyanin and allophycocyanin constituted 50% and 10% of the protein fraction, respectively, which indicated that antiviral properties of Spirulina extracts are mainly contributed by phycobiliproteins. Antiinflammatory, antioxidant and immunomodulatory activities of Spirulina derived phycobiliproteins have also been extensively reported [25,53,54]; however, future clinical investigations on multiple roles of these bioactive compounds are still warranted.
Conclusions
The recent outbreak of COVID-19 caused by SARS-CoV2 has triggered multiple facets of new research to prevent infection and treat the disease. The role of nutraceuticals and similar compounds might have some answers for getting a handle on this pandemic. Spirulina-based nutraceuticals and bioactive compounds have been known for their antioxidant, antiviral, antiinflammatory, immunostimulatory, and immunomodulatory properties, and therefore could act as immune-boosting and therapeutic agents. The presence of natural ACE inhibitors (ACE inhibitory peptides), and antioxidants, antiviral compounds (calcium spirulan, phycocyanobilin) indicate beyond a doubt that Spirulina-based nutraceuticals can be integrated into the current research and clinical trials for immunity stimulation, disease prevention and treatment of disorders related to severe coronavirus infections, such as tissue repair in ACE2 dominated organs and antiinflammatory treatment. Despite the desired properties, their role in COVID-19 (SARS-CoV2 infections) remains limited and warrants urgent developments in this area. Although too early to design studies to assess the effectiveness of Spirulina against SARS-CoV2, the potential of nutraceutical supplements warrants basic and clinical research. Clinical studies in cell lines, animal model systems, and humans are required to understand and verify these speculations on the use of algal bioactive compounds as nutraceuticals for the treatment of COVID-19 and related symptoms.
Acknowledgments
The authors acknowledge the National Research Foundation (UID 84166), Republic of South Africa, and Durban University of Technology for providing financial assistance.
References
- 1.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. A rampage through the body. Science. 2020;368:356–360. doi: 10.1126/science.368.6489.356. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thanh Le T AZ, Kumar A, Gomez Roman R, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. People who are at higher risk for severe illness. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk. Accessed April 18, 2020.
- 9.McCarty MF, DiNicolantonio JJ. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog Cardiovasc Dis. 2020;63:383–385. doi: 10.1016/j.pcad.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muscogiuri G, Barrea L, Savastano S, Colao A. Nutritional recommendations for COVID-19 quarantine. Eur J Clin Nutr. 2020;74:850–851. doi: 10.1038/s41430-020-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caccialanza R, Laviano A, Lobascio F, Montagna E, Bruno R, Ludovisi S, et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laviano A, Koverech A, Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown EM, Allsopp PJ, Magee PJ, Gill CI, Nitecki S, Strain CR, et al. Seaweed and human health. Nutr Rev. 2014;72:205–216. doi: 10.1111/nure.12091. [DOI] [PubMed] [Google Scholar]
- 14.Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr Rev. 2017;75:731–767. doi: 10.1093/nutrit/nux047. [DOI] [PubMed] [Google Scholar]
- 15.Deng R, Chow TJ. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc Ther. 2010;28:e33–e45. doi: 10.1111/j.1755-5922.2010.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta V, Ratha SK, Sood A, Chaudhary V, Prasanna R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013;2:79–97. [Google Scholar]
- 17.Mogany T, Kumari S, Swalaha FM, Bux F. Extraction and characterisation of analytical grade C-phycocyanin from Euhalothece sp. J Appl Phycol. 2019;31:1661–1674. [Google Scholar]
- 18.Chei S, Oh HJ, Song JH, Seo YJ, Lee K, Kim KJ, et al. Spirulina maxima extract prevents activation of the NLRP3 inflammasome by inhibiting ERK signaling. Sci Rep. 2020;10:2075. doi: 10.1038/s41598-020-58896-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa S, Kawabe H, Ohori H, Mukai T, Matsumoto M. Preventive or therapeutic composition for viral infectious disease. Google Patents; 2008 United States patent: US7332475B2. [Google Scholar]
- 20.Hirahashi T, Matsumoto M, Hazeki K, Saeki Y, Ui M, Seya T. Activation of the human innate immune system by Spirulina: Augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int Immunopharmacol. 2002;2:423–434. doi: 10.1016/s1567-5769(01)00166-7. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, Hayashi K, Maeda M, Kojima I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J Nat Prod. 1996;59:83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Corona A, Nieves I, Meckes M, Chamorro G, Barron BL. Antiviral activity of Spirulina maxima against herpes simplex virus type 2. Antivir Res. 2002;56:279–285. doi: 10.1016/s0166-3542(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 23.Raposo MFDJ, de Morais RMSC, Bernardo de Morais AMM. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar Drugs. 2013;11:233–252. doi: 10.3390/md11010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaeffer DJ, Krylov VS. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Safe. 2000;45:208–227. doi: 10.1006/eesa.1999.1862. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Liu L, Miron A, Klímová B, Wan D, Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch Toxicol. 2016;90:1817–1840. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 26.Selmi C, Leung PSC, Fischer L, German B, Yang CY, Kenny TP, et al. The effects of Spirulina on anemia and immune function in senior citizens. Cell Mol Immunol. 2011;8:248–254. doi: 10.1038/cmi.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranga Rao A, Baskaran V, Sarada R, Ravishankar GA. In vivo bioavailability and antioxidant activity of carotenoids from microalgal biomass — A repeated dose study. Food Res Int. 2013;54:711–717. [Google Scholar]
- 28.Mah AY, Cooper MA. Metabolic regulation of natural killer cell IFN-γ production. Crit Rev Immunol. 2016;36:131–147. doi: 10.1615/CritRevImmunol.2016017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YH, Chang GK, Kuo SM, Huang SY, Hu IC, Lo YL, et al. Well-tolerated Spirulina extract inhibits influenza virus replication and reduces virus-induced mortality. Sci Rep. 2016;6:24253. doi: 10.1038/srep24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida S, Tsuboi A, Tanemura A, Ito T, Nakajima H, Shirakata T, et al. Immune adjuvant therapy using Bacillus Calmette-Guérin cell wall skeleton (BCG-CWS) in advanced malignancies: A phase 1 study of safety and immunogenicity assessments. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000016771. e16771–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khafaga AF, El-Sayed YS. Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation. Life Sci. 2018;196:9–17. doi: 10.1016/j.lfs.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, et al. SARS-CoV2: Should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41:1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adedeji AO, Severson W, Jonsson C, Singh K, Weiss SR, Sarafianos SG. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J Virol. 2013;87:8017. doi: 10.1128/JVI.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imai Y, Kuba K, Ohto-Nakanishi T, Penninger JM. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ J. 2010;74:405–410. doi: 10.1253/circj.cj-10-0045. [DOI] [PubMed] [Google Scholar]
- 42.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Resp Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowe D. Angiotensin and the coronavirus. Available at: https://blogs.sciencemag.org/pipeline/archives/2020/03/17/angiotensin-and-the-coronavirus. Accessed April 18, 2020.
- 45.Rico-Mesa JS, White A, Anderson AS. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. 2020;22:31. doi: 10.1007/s11886-020-01291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aiello G, Li Y, Boschin G, Bollati C, Arnoldi A, Lammi C. Chemical and biological characterization of spirulina protein hydrolysates: Focus on ACE and DPP-IV activities modulation. J Funct Food. 2019;63 [Google Scholar]
- 48.Anekthanakul K, Senachak J, Hongsthong A, Charoonratana T, Ruengjitchatchawalya M. Natural ACE inhibitory peptides discovery from Spirulina (Arthrospira platensis) strain C1. Peptides. 2019;118 doi: 10.1016/j.peptides.2019.170107. [DOI] [PubMed] [Google Scholar]
- 49.Heo SY, Ko SC, Kim CS, Oh GW, Ryu B, Qian ZJ, et al. A heptameric peptide purified from Spirulina sp. gastrointestinal hydrolysate inhibits angiotensin I-converting enzyme-and angiotensin II-induced vascular dysfunction in human endothelial cells. Int J Mol Med. 2017;39:1072–1082. doi: 10.3892/ijmm.2017.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng J, Wang J, Pan H, Wu H, Ren D, Lu J. Effects of IQP, VEP and Spirulina platensis hydrolysates on the local kidney renin angiotensin system in spontaneously hypertensive rats. Mol Med Rep. 2017;16:8485–8492. doi: 10.3892/mmr.2017.7602. [DOI] [PubMed] [Google Scholar]
- 51.He YY, Li TT, Chen JX, She XX, Ren DF, Lu J. Transport of ACE inhibitory peptides Ile-Gln-Pro and Val-Glu-Pro derived from Spirulina platensis across Caco-2 monolayers. J Funct Food. 2018;83:2586–2592. doi: 10.1111/1750-3841.14350. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi K, Hayashi T, Kojima I. A natural sulfated polysaccharide, calcium Spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res Hum Retrov. 1996;12:1463–1471. doi: 10.1089/aid.1996.12.1463. [DOI] [PubMed] [Google Scholar]
- 53.Pagels F, Guedes AC, Amaro HM, Kijjoa A, Vasconcelos V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol Adv. 2019;37:422–443. doi: 10.1016/j.biotechadv.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Qureshi MA, Ali RA. Spirulina platensis exposure enhances macrophage phagocytic function in cats. Immunopharmacol Immunotoxicol. 1996;18:457–463. doi: 10.3109/08923979609052747. [DOI] [PubMed] [Google Scholar]