Abstract
Background
The emergence of SARS-CoV-2 pandemic has upset health systems around the world and caused immeasurable losses and costs. Until a vaccine will become available, the recommended prevention measures remain physical distancing and enhanced hygiene.
Methods and findings
The proteic structure external to the virus is the main target that may eventually lead to reduce or block its replication in the upper airways. We developed a protocol based of repeated steam inhalation cycles aimed at reducing the risk of progression to full blown infection if performed soon after contagion. The protocol has been used in a single-center open label trial on ten infected asymptomatic or pauci-symptomatic health care professionals.
Conclusions
The promising results we obtained with this easily accessible, non-invasive and inexpensive procedure should prompt controlled trials.
Keywords: COVID-19, Steam inhalation, Treatment, Prevention, Healthcare professionals
1. Introduction
The new coronavirus (SARS-CoV-2) causing COVID-19 is an RNA virus coated with a capsid and a peri-capsid crossed by glycoprotein structures. The external proteic structure, which attacks human cells, is a potential target to therapeutic interventions against virus replication in airways.
Since high temperature can cause irreversible denaturation of proteins and loss of SARS CoV and SARS CoV-2 infectivity was obtained after heating at 56 °C for 15 and 30 min in liquid environments respectively [[1], [2], [3]], we designed a simple protocol aimed at damaging SARS-CoV-2 capsid through steam inhalation cycles. Although the ominous consequences of COVID 19 infections has directed medical attention toward solidly established medical approaches, the European Pharmacopoeia VI edition also quotes steam inhalations as a procedure to treat of respiratory diseases [4]. Based on these suggestions we established a single-centered open label interventional study with the limited objective of enrolling up to 10 asymptomatic or pauci-symptomatic healthcare professionals in whom RN-swab revealed a SARS-CoV-2 infection. The study protocol consisted of exposure of airway mucosae to humidified steam through steam inhalation for at least 20 min (5 cycles of 4 min) within 1 h for at least 4 consecutive days. The ensuing reduction of viral shedding was measured by real time PCR on rhino-pharyngeal (RN) swab self-sampling [5] 24 h after each cycle of treatment.
2. Patients and methods
This single-centered open label interventional study had the limited objective of enrolling up to 10 adults in whom RN-swab (eSwab, Copan, Italy) revealed a SARS-CoV-2 infection during a systematic screening of healthcare professionals (physicians, MD; pediatric nurses, PN) actively working at Meyer Children's University Hospital, Florence, consecutively screened between March–April 2020.
The protocol was proposed to the first 10 asymptomatic or pauci-symptomatic (up to 2–3 mild to moderate symptoms) healthcare professionals (7 PN and 3 MD) with a positive RN-swab (inclusion criteria) providing them with informed consent, protocol instructions plus a video tutorial and a form to record symptoms and protocol adherence. We set as exclusion criteria for enrolment a history of pre-existing allergies and/or asthma, ongoing drug therapies against SARS-CoV-2, and individuals with multiple symptoms consistent with COVID infection.
The study protocol consisted of exposure of airway mucosae to humidified steam through inhalation for at least 20 min (4 cycles of 5 min or 5 cycles of 4 min) within 1 h, with a temperature maintained between 55 and 65 °C in the first 4/5 min after initiation of water boiling (experimental measurements in triplicate). The patient was asked to drape the towel over the back of his/her head lowering toward the hot steam down to about 25 to 30 cm from the water.
The primary outcome was a reduction of viral shedding after 4 days (at least 6 Cycle Threshold Values measured with RT-PCR) and the secondary outcome complete virus elimination after the 4-day protocol.
Seven out of 10 subjects met the inclusion criteria (nos. 1–7). Subject 8 (PN) declared to be allergic; subject 9 (MD), after following the protocol for three days, reported to have also started hydroxychlorochine and azytromicine treatment since day 1; subject 10 (PN) manifested more than three symptoms, self-reported to be from moderate to severe, which likely indicated active exponential viral growth. While not satisfying inclusion criteria, these three individuals asked to join the protocol all the same, based on its simplicity and potential benefits. Their results will be dealt with separately.
3. Results
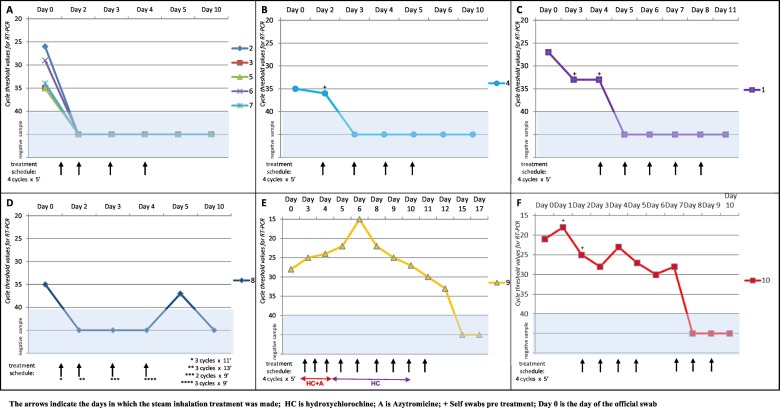
Of 1292 screened healthcare professionals, the first 10 with a SARS-CoV-2 positive RN-swab were enrolled in the study. The mean age of the 7 patients (6 women) completing the protocol, was 44.4 (SD 12.1) years. One patient (no. 5) was asymptomatic before and after the protocol, five exhibited one or two mild symptoms including dry cough (1/5), sore throat (1/5), headache (1/5), anosmia (2/5), ageusia (2/5), tiredness (2/5), soreness/muscle pain (1/5), and nasal congestion (1/5). One patient (no. 4) reported moderate headache and generalised muscle pain. All 6 patients with symptoms reported clinical improvement at the end of protocol: three reported to have been cleared of all symptoms; 2 declared persisting anosmia and ageusia; one reported mild muscle pain and nasal congestion. All seven patients achieved both outcomes, testing negative after the first day of steam inhalation (4 consecutive RN-swabs). All were asked to evaluate their negativity 3–5 days after protocol conclusion (Fig. 1A–C).
Fig. 1.
1A: Viral shedding diagram from patients 2, 3, 5, 6 and 7. All five patients performed the protocol (4 cycles/5 min each) on days 1, 2, 3 and 4 measuring the effect of treatment on days 2, 3, 4 and 5. A new swab on day 10 confirmed short term negativity.
1B: Viral shedding diagram from patient 4. The patient, who exhibited a low viral load (35 CT), was asked to perform a self-swabbing procedure before starting the protocol to test his/her ability to carry on autonomously the procedure on day 2. A new swab on day 10 confirmed short term negativity.
1C: Viral shedding diagram from patient 1. The patient with an official moderate viral load (27 CT) was asked to perform self-swabbing before starting the protocol, to test his/her ability to carry on autonomously the procedure on days 3 and 4. A new swab on day 11 confirmed short-term negativity.
1D: Viral shedding diagram from patient 8. This patient did not complete the cycle's protocol due to allergies and intolerance to a prolonged exposure to humidified steam: on day 1 this patient followed 11 min treatment divided in three cycle 5, 3 and 3 min respectively. On day 2 followed 13 min treatment divided in three cycles 5, 5 and 3 min respectively; on day 3, two cycles 5 and 4 min; on day 4, three cycles, 3 min each. On day 5 had a new low viral load positive swab and on day 10 she tested negative.
1E: Viral shedding diagram from patient 9. Subject performed the protocol for overall 11 days together with drug treatment based on hydroxychlorochine and azytromicine. She resulted in all positive swabs. She tested negative after 15 and 17 days.
1F: Viral shedding diagram in patient 10. This patient, who exhibited a high viral load (21 CT) was asked to perform a self-swabbing procedure (on day 2 and day 3) before starting the protocol to test his/her ability to carry on autonomously the procedure on day 3 and 4. This patient performed the protocol for overall 7 days testing negative since the 5th day of treatment.
Patient 8 (PN, 39 years old, allergic) experienced mild headache and nasal congestion, both of which resolved at the end of the study, after three steam inhalations on day 1 (5 + 3 + 3 min), three on day 2 (5 + 5 + 3 min), two on day 3 (5 + 4 min), three on day 4 (3 + 3+ 3 min). A first negative swab after the first session, was followed by a new weak positivity after 3 days. This patient stopped the protocol on day 5 and underwent an additional swab on day 10, which resulted negative (Fig. 1D).
Patient 9 (MD, 36 years old) reported moderate tiredness, headache and mild muscle pain. On the third day of the protocol this patient started hydroxychlorochine (HC) and azytromicine (AZ) (for 9 days and 3 days, respectively), which resulted in her being dropped from our study. This lady continued the steam inhalation protocol up to complete clearance on day 5, as she felt her symptoms had been eased. From day 6 she reported nasal congestion, muscle/bone pain, progressive anosmia and ageusia, which diminished till day 11. Steam inhalation was stopped on day 12. All swabs during the experimental protocol resulted positive (Fig. 1E), while further swabs on days 15 and 17 tested negative.
Patient 10 (PN, 38 years old) tested positive at first swab, 13 days after close contact with a known infected, symptomatic individual. When asked to participate to the study, this patient reported moderate muscle pain, tiredness, nasal congestion, sinusitis, anosmia, hypogeusia, and sore throat, with 4 days of fever (>38 °C) immediately prior to the official swab. All the swabs during the study protocol were positive (Fig. 1F). This lady continued the steam inhalation protocol, which she motivated with a beneficial effect on symptoms (complete clearance on day 10). Three additional swabs on days 8, 9 and 10 were negative (Fig. 1F).
4. Discussion
Lowff's model, demonstrates that raising the mucosal temperature to 43 °C for three periods of 30 min each, at two-hour intervals, blocks the replication of rhinoviruses after virus inoculation [6,7]. Since SARS-COV-2 proved to be thermolabile in liquid environment [3], we hypothesized our procedure, if performed soon after contagion, would reduce the risk of progression to full blown infection. However, contagion occurs most often unknowingly, which does not allow a timely preventive procedure, unless carried on systematically in at risk individuals (for example healthcare professionals working in COVID units or personnel in contact with the public). Although applying the Lowff's model to benefit pre-symptomatic and untested individuals would be unrealistic in a pandemic context, we hypothesized it would still be effective in abating viral load in asymptomatic or pauci-symptomatic individuals who test positive to RN-swab and are both potentially infectious and at risk of symptoms worsening.
However, controlled trials of repeated steam inhalations at 43 °C for the treatment of airway viruses have generated controversial results [8,9]. On the other hand, the popular “grandma” remedy is variably applied and often implies a “one shot” steam inhalation procedure, exposing the upper airways mucosae for a short time at uncontrolled temperature. Neither approach may be effective, however, since repeated cycles at higher temperature have higher chances of weakening or halting virus spread. Our small study suggests that a protocol based on cycles of steam inhalation at temperature 55–65 °C might indeed be beneficial in halting SARS-CoV-2 virus infection in the upper airway mucosae during the initial stages of infection and possibly preventing further spread. It could also be helpful in pauci-symptomatic subjects during the last period of infection, when the immune system is already active against the virus but its presence is still detectable in the upper respiratory tract. Since steam cannot reach the bronchial tree, bronchi and lungs, it is unlikely it could be beneficial once the infection has reached the deep internal airway mucosa.
Our observation is only preliminary, it has obvious limitations and the beneficial effects we observed need confirmation in a controlled trial. In addition, self-swabbing procedures, although officially allowed in the interim guidelines for collecting, handling and testing clinical specimens from persons affected by COVID-19 [5], are a potential source of bias, which we tried to limit, by randomly asking 3/10 subjects (nos. 1, 4 and 10) to self-swab before starting the protocol procedures. A spontaneous remission from the virus 24 h after the treatment began is possible, but unlikely for all 7 enrolled patients.
Finally, the protocol cannot be intended to lead to an eradication of the virus from the body, as the steam inhalation procedure can only reach upper airways. However, the proposed steam inhalation cycles are safe (provided care is used against the possible burns, especially in children), inexpensive and easily self-managed. This simple approach, only tested in a small sample and in a biased population, might help easing the consequences of SARS-CoV-2 infection, if applied early in at risk individuals in whatever healthcare setting, especially in low income countries with limited access to equipped hospitals or intensive care units. Unequivocal proof of efficacy would require a controlled trial on a larger scale.
Should our preliminary observations be confirmed the protocol could be used against COVID19 or other viral infections using vapotherm masks, where temperature, time of exposure and size of steam particles can be set and monitored.
Ethics committee approval
The study has been approved by the Meyer Children's University Hospital Ethics Committee on 19/03/2020. The official number of protocol is 04/2020.
Role of funding source
We declare non funding source.
Declaration of competing interest
We declare non competing interests.
Acknowledgments
We appreciate the support and bravery of technicians of the Immunology Lab, Meyer Children's Hospital in providing swab analysis and Dr. Kathleen McGreevy for her suggestions.
References
- 1.First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network. https://www.who.int/csr/sars/survival_2003_05_04/en
- 2.Chan K.H., Peiris J.S., Lam S.Y., et al. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin A.W.H., Chu J.T.S., Perera M.R.A., et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30003-3. published online April 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Pharmacopoeia 6.0. http://www.uspbpep.com/ep60/preparations%20for%20inhalation%200671e.pdf
- 5.Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
- 6.Yerushalmi A., Karman S., Lwoff A. Treatment of perennial allergic rhinitis by local hyperthermia. PNAS. 1982;79:4766–4769. doi: 10.1073/pnas.79.15.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yerushalmi A., Lwoff A. Treatment of infectious coryza and persistent allergic rhinitis with thermotherapy. C R Seances Acad. Sci. D. 1980;291:957–959. [PubMed] [Google Scholar]
- 8.Tyrrell D., Barrow I., Arthur J. Local hyperthermia benefits natural and experimental common colds. BMJ. 1989;298:1280–1283. doi: 10.1136/bmj.298.6683.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendley J.O., Abbott R.D., Beasley P.P., Gwaltney J.M., Jr. Effect of inhalation of hot humidified air on experimental rhinovirus infection. JAMA. 1994;271:1112–1113. [PubMed] [Google Scholar]