Abstract
A significant number of SARS-CoV-2 (COVID-19) pandemic patients have developed chronic symptoms lasting weeks or months which are very similar to those described for myalgic encephalomyelitis/chronic fatigue syndrome. This study reviews the current literature and understanding of the role that mitochondria, oxidative stress and antioxidants may play in the understanding of the pathophysiology and treatment of chronic fatigue. It describes what is known about the dysfunctional pathways which can develop in mitochondria and their relationship to chronic fatigue. It also reviews what is known about oxidative stress and how this can be related to the pathophysiology of fatigue, as well as examining the potential for specific therapy directed at mitochondria for the treatment of chronic fatigue in the form of antioxidants. This study identifies areas which require urgent, further research in order to fully elucidate the clinical and therapeutic potential of these approaches.
Keywords: Chronic fatigue syndrome, Mitochondria, Oxidative stress, Antioxidants, SARS-CoV-2
Introduction
A significant proportion of patients recovering from the current SARS-CoV-2 pandemic, termed ‘longhaulers’ or ‘Long COVID’, are reporting a broad range of persistent and debilitating symptoms centred around fatigue but also including symptoms such as ‘brain fog’, pain, breathlessness, and dysrhythmias, that have extended several months into the post infection period.1,2 These symptoms are a characteristic of an already well-documented, potentially devastating and largely unexplained post-viral illness, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). This disease is already known to be associated with other viral infections such as Epstein Barr or glandular fever.3 ME/CFS is a disease of unknown pathophysiology affecting 0.2%–2.0% of the world's population.4,5 It is characterised by severe post-exertional fatigue that is not relieved by rest, and as well as pain, severe cognitive dysfunction, insomnia and/or lack of restful sleep, and sensitivity to light and sound, lasts for longer than six months.6 There is now growing belief that, with millions of SARS-CoV-2 infections worldwide, there will be a spike in ME/CFS, with some people's post viral fatigue developing into the a long term illness that is symptomatically the same as ME/CFS —the unknown question is how many will be affected?
Knowing how many ME/CFS patients there are is difficult: withdrawal from society is common,7 especially as one in four individuals can be bed- or house-bound lifelong, and many more whilst in the acute phase.4,5 Demographic data collection is also hampered because there is no simple diagnostic test for this disease, (twenty published, differing clinical definitions exist).4 It is approximately two to six times more common in females than males,8 and affects all ages, socio-economic groups, ethnic groups and geographical locations.4 It is unknown, as yet, whether the ‘long-haulers’ from SARS-CoV-2 infections with ME/CFS like symptoms will share the same gender bias and other characteristics. Similar conditions to ME/CFS have been known for the last two centuries but not recognised as such.9 In 2019, the Senate of the United States passed a resolution acknowledging that ME/CFS was a serious disease that affected many people and in great need of more research.10 Spurred on by the SARS-CoV2 pandemic, research into ME/CFS may see such a resurgence. This would be a cruel irony for long-term ME/CFS sufferers if their condition, which has taken so long to be acknowledged as a serious debilitating disease, now gets ready acceptance through COVID-19.
The lack of a known aetiology or pathophysiology, the variability in the symptoms and the lack of outward physical signs of the illness have all contributed to the societal stigma of ME/CFS.11 It has often been referred to as an ‘invisible disease’,12, 13, 14, 15 as people may appear outwardly healthy and well, but be severely ill.11,15 Previous classification of ME/CFS as a psychiatric disorder has hindered the care of sufferers of the disease and their interactions with their physicians and the public for many years.15,16 It is hoped the SARS-CoV-2 ‘long-haulers’ will not have to undergo these experiences before their illness is properly acknowledged. A 2016 survey found that 60% of affected individuals were not diagnosed for at least one year and this delay can worsen the psychosocial impacts of the disease.4 These diagnostic difficulties make it hard to know whether the syndrome is a single disease entity with a range of symptoms, rather like multiple sclerosis (MS), or rather several different diseases with very similar and overlapping symptomatology. For example, Booth et al17 argues for two or more distinct pathophysiologies that are grouped together as ME/CFS. ME/CFS can have a number of initiating triggers, the most common being a viral infection.
The focus of this review is the links between some of the dysfunctional pathways found in ME/CFS – specifically mitochondrial dysfunction and oxidative stress – and the possible therapeutic effects of antioxidants levels upon these pathways. Simple remedies like antioxidants that are readily available and that can control oxidative stress might prove helpful to improve the lives of ME/CFS patients, as well as those suffering from post-viral illness as a result of SARS-CoV2 infections before other therapies become available. Mitochondria are organelles that are present in every cell of the body and produce 90%–95% of the body's total energy. Mitochondrial respiration produces the energy-carrier adenosine triphosphate (ATP) which drives all the necessary chemical reactions in the body. Cells only contain a small steady state concentration of ATP molecules in cells which needs to be constantly re-generated because of energy demands. This is done by oxidative phosphorylation (OXPHOS) or by glycolysis.18 Maximal mitochondrial respiration increases with stress, and there can be up to a 100-fold increase in ATP consumption during vigorous activity compared with sleep. Mitochondria are also involved in a wide range of other important cellular actions including redox signalling and cell danger responses.19,20 Dysfunction in any of these functions may possibly account for clinical symptoms in ME/CFS,20 however, mitochondrial bioenergetics is the main area of focus of this review, because dysfunction in mitochondrial respiration and metabolic changes have been found in ME/CFS,21, 22, 23 and these give a biologically plausible link to some of the symptoms seen in ME/CFS.24 A recent review25 has looked at mitochondrial changes in ME/CFS patients compared to healthy controls across 19 studies. They concluded that it was difficult to establish the role of mitochondria in the pathology of ME/CFS due to inconsistencies across the published studies. They argued for future studies to be well-designed, with patients diagnosed with the same ME/CFS clinical case definition and with analyses using the same methods. Despite this review, we argue that there is evidence of mitochondrial disturbances in ME/CFS and our review focuses on the potential of exploiting these for therapeutic approaches to alleviating both this complex disease and for SARS-CoV-2 chronically ill patients.
The search for peer-reviewed articles was conducted using Medline and Google Scholar up to March 2020. The following search terms were used: myalgic encephalomyelitis/chronic fatigue syndrome, coenzyme Q10 (CoQ10), MitoQ, bioenergetic health index (BHI), oxidative stress, mitochondrial dysfunction. Variations of these terms were used to ensure all possible papers were found. Inclusion criteria included the English language.
Mitochondrial energy pathways and dysfunction
A growing body of literature has investigated mitochondrial bioenergetics in ME/CFS patients, and is reporting evidence of mitochondrial dysfunction21,26,27 but with varying results.25,28 Since mitochondria produce most of the body's energy, they could contribute to ME/CFS symptoms of muscle pain, weakness and cognitive decline.29 Mitochondrial dysfunction found with classical mitochondrial disease caused by genetic mutations, share symptoms of fatigue, muscle weakness, cognitive decline and a waxing and waning pattern such as seen in ME/CFS.29
A range of different markers has been used to measure mitochondrial dysfunction in ME/CFS including the levels of certain mitochondrial proteins,30 production of ATP17,27,31 and more recently, oxygen consumption of live plated cells using a new technology with the Seahorse Analyzer.21,22,32 The earliest evidence of mitochondrial dysfunction in ME/CFS was structural changes seen in the skeletal muscle cell mitochondria.33 This study observed branching and fusion of cristae within the mitochondria, and variable shape and size in these patients. Lawson et al34 also found condensation of cristae, but no difference in the shape and size of the mitochondria in peripheral blood mononuclear cells (PBMCs). Both studies concluded that the increased energy demand in ME/CFS caused this excess branching of cristae in mitochondria.
Dysfunctional energy producing pathways have been shown in ME/CFS.17,26,34 Booth et al17 and Lawson et al34 each suggested compensatory enhancement of other pathways such as anaerobic glycolysis occurred in ME/CFS, to compensate for dysfunctional oxidative phosphorylation. An increase in glycolysis causes damage and contributes to other dysfunctions.17 In contrast, Tomas et al21 found no change in glycolysis compared to controls. However they did find an overall decreased OXPHOS. Armstrong et al26 suggested glycolysis may be inhibited as lactate levels were low, when investigating metabolic pathways.
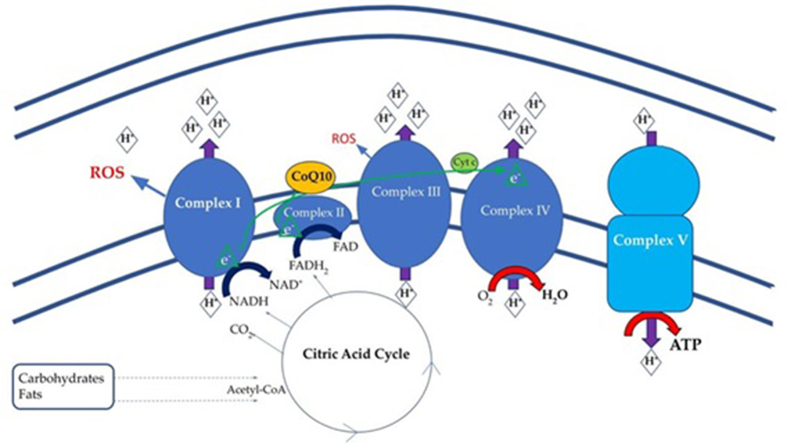
Tomas et al21 found that OXPHOS in PBMCs of ME/CFS patients was significantly depressed when compared to controls, and Booth et al17 found decreased ATP production by OXPHOS. Missailidis et al22 determined that ATP synthesis, as a percentage of basal respiration, was decreased in ME/CFS compared to controls which suggested a defect in ATP synthase (complex V) in ME/CFS. They inferred this was causing compensatory increases in the rest of the electron transport chain (ETC) (Fig. 1), as they found increased levels of other complexes: this suggested mitochondria were unable to increase energy from baseline when required. Two studies21,35 found mitochondria had a lower reserve capacity for energy production in ME/CFS compared to healthy controls, and also suggested mitochondria in ME/CFS are unable to meet energy demands during exercise or acute stress, giving a potential reason for the severe post-exertional fatigue seen in patients.
Fig. 1.
Electron transport chain on the inner mitochondrial membrane. Electrons move from carriers NADH and FADH2 to oxygen, the final acceptor. CoQ10 (yellow) moves from Complex I or Complex II carrying electrons to Complex III. The electrons then are carried from Complex III to Complex IV by cytochrome C (labelled Cyt C, green). NADH: Reduced nicotinamide adenine dinucleotide; FADH2: Reduced flavin adenosine dinucleotide; ROS: Reactive oxygen species; CoQ10: coenzyme Q10. (Image source: Sweetman et al,8 with permission).
Lawson et al34 and Tomas et al32 found no differences in the levels of specific complexes I-IV of the ETC between ME/CFS and healthy controls, despite finding changes in OXPHOS as a whole in their previous paper.21 They suggested that any dysfunction, therefore, was not due to these complexes but instead to an external stimuli or other cause such as oxidative damage. Oxidative damage may be a contributor to the dysfunction seen in mitochondrial pathways.36 Castro-Marrero37 reported higher lipid peroxidation as a cause of lowered ATP levels in ME/CFS compared to healthy controls. Morris and Maes36 suggested reactive oxygen species and inflammation might be a cause of mitochondrial damage and dysfunction. Missailidis et al,22 however, suggested that the cause of dysfunction was a defect in the control pathway of ATP synthase and Vermeulen et al35 suggested a decreased ability for ME/CFS to deliver oxygen to cells caused dysfunction and decreased ATP production. Lawson et al34 described changes in the mitochondria indicating ME/CFS patients have an increased and unmet energy demand instead of overt mitochondrial dysfunction.
Referring again to Fig. 1, an analysis of the proteomes of a cohorts of ME/CFS patients and healthy controls by Sweetman et al8 showed a significant proportion of proteins with changed expression in the ME/CFS cohort, were involved in mitochondrial function, oxidative phosphorylation, ETC complexes (shown in blue) and redox regulation or reactive oxygen species. These changes would contribute to oxidative stress.
Oxidative stress
Oxidative stress is a condition of increased amounts of reactive oxygen/nitrogen species. It is an important pathological feature of many acute and chronic diseases as well as the normal aging process. Oxidative stress results from an imbalance between free radicals and antioxidants which causes an increase in free radicals.38 Free radicals have an additional unpaired single electron, which is unstable and can cause damage to biological proteins and DNA.39 Free radicals are also produced in normal physiological cell action and signalling.40 Reactive oxygen species (ROS) are molecules which include free radicals and at least one oxygen atom, such as superoxide or hydrogen peroxide. Antioxidants are scavengers that remove free radicals and are necessary to prevent cellular damage.39 Mitochondria are a major producer of ROS in the cell, which makes them very susceptible to damage by oxidative stress.40 In the mitochondrial ETC, electrons en route to oxygen may be diverted in the conversion of oxygen into superoxide.41 In the mitochondria there are enzymes and coenzymes, such as vitamin E and CoQ10, that remove ROS to prevent damage. If there is damage or dysfunction in the ETC and/or in the ROS-removal system of mitochondria – for example with low levels of CoQ10 – ROS will increase causing further damage.40
Oxidative stress in ME/CFS
Increased oxidative stress is important in causing and perpetuating a range of pathologies.42 Jammes et al43 showed increased markers of oxidative stress in 38 ME/CFS participants and also showed these individuals had greater oxidative stress after exercise. Richards et al44 also found an association between increased levels of oxidative stress and ME/CFS symptoms in 33 patients. Castro-Marrero et al37 found increased signs of oxidative damage and decreased antioxidants (CoQ10) in mitochondria from patients with ME/CFS. Miwa and Fujita45 found levels of oxidative stress were increased in the relapse state of ME/CFS. Oxidative stress found in ME/CFS could be contributing to mitochondrial dysfunction, inflammatory pathways, and brain dysfunction.36
Suitable biomarkers of oxidative modifications of disease are oxidation products that are stable, can be measured, and which correlate with disease severity so that they can be used as diagnostic tools. Commonly used biomarkers of oxidative stress are: (a) a physiological ketoaldehyde produced from decomposition of unsaturated lipids that combines with free amino groups, mainly lysine of proteins; (b) isoprostanes, a family of prostaglandin F2a -like compounds that can be analysed in body fluids including urine and plasma; (c) glutathione disulphide/glutathione ratios; (d) protein carbonyls that are very stable in plasma samples stored at −80 °C degrees; and (e) 8-oxydeoxy guanosine from modified DNA. A number of specific analytical methods have been developed to analyse these oxidation products as an indicator of oxidative stress.46
Mitochondrial therapies for ME/CFS
As more knowledge of the mitochondrial effects has been learnt, potential treatments are being targeted to improve mitochondrial health.47, 48, 49, 50, 51, 52, 53, 54, 55 Teitelbaum et al50 has investigated the effect of d-ribose, the sugar moiety of ATP that stimulates energy production, on ME/CFS symptoms in an uncontrolled, open-label study. They found significant improvement in fatigue as measured by visual analogue scales. ME/CFS patients taking methylphenidate (Ritalin®) with vitamins and minerals which support mitochondrial function, reported some improvement in fatigue scores, although this was non-significant when compared to placebo.51 In an uncontrolled, open-label study, 45% of participants had reduced fatigue when treated with sodium dichloroacetate, a pyruvate dehydrogenase/glycolysis inhibitor.52 These studies may be relevant to SARS-CoV-2 recovering patients.
CoQ10 and mitochondria
CoQ10 (also called ubiquinone) is a potential treatment under investigation. It is an important antioxidant in the mitochondria. CoQ10 is the only lipid-soluble antioxidant that is endogenously synthesised in humans.56 It consists of a quinone head which can complete reduction–oxidation reactions and a long polyisoprenoid lipid tail making it extremely hydrophobic; this allows it to move freely within the phospholipid bilayer.56 Ubiquinol (reduced CoQ10) acts as an antioxidant by reducing ROS, or by regenerating other antioxidants such as vitamin E.57 After neutralising an oxygen radical, enzymes in the ETC can recycle and reduce CoQ10 back to ubiquinol.
A significant proportion of CoQ10 (50%) is found in the mitochondrial membranes,58 but it is also present in all membranes of the body and in the plasma where it is carried in the lipoproteins low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL).59 CoQ10 is found in the highest concentrations in highly metabolic tissues such as the heart, liver and muscle.58 Detection of CoQ10 in muscle cells is considered the gold standard for detecting defects in the synthesis of CoQ10.57
CoQ10 levels vary amongst individuals. Tissue levels are controlled by endogenous synthesis,60 while plasma levels can be influenced by a range of other factors, including diet,61 gender,62,63 hormones64 and exercise.65,66 Vitetta et al67 also suggested tissue breakdown, associated with aging, may increase plasma CoQ10 levels due to cell CoQ10 leaking into plasma. Niklowitz et al,62 however, measuring serum CoQ10 levels in 840 individuals, showed an inverse U-shaped curve with age, with middle-aged adults having the highest levels. Different studies have found widely varying ranges in healthy populations.68
When a person is well, quantities of CoQ10 in tissues are thought to be sufficient due to the endogenous production.69 Beal70 suggested that the level of CoQ10 in membranes reaches a saturation level in young animals, so that supplementation with CoQ10 does not further increase brain tissue levels. CoQ10 levels did increase in the brain tissue of older individuals and it was suggested that supplements may be beneficial in humans with decreased synthesis such as the elderly or for those with primary CoQ10 deficiency or other diseases.71 López-Lluch et al72 discussed that cells may only take up CoQ10 from plasma, if they are unable to produce it sufficiently. Therefore, CoQ10 supplementation may only produce a clinical effect if the disease is associated with low CoQ10 levels.
CoQ10's role is vital in the mitochondria. Genetic disorders which result in little to no CoQ10 have severe debilitating symptoms.56,73 A genetically-based failure of the synthesis of CoQ10 causes some forms of mitochondrial disease.74 There is no reported genetic disorder caused by an excess CoQ10. Lowered levels of plasma CoQ10 have been reported in a range of diseases including Alzheimer's disease, diabetes, and ‘low-energy syndromes’, such as Prader–Willi syndrome, Friedrich's ataxia, cancers, as well as hereditary mitochondrial disorders.52
Primary CoQ10 deficiency is defined as a deficiency due to mutations in the genes encoding CoQ10 synthesis. Primary CoQ10 deficient patients can have a range of phenotypes that affect highly metabolic tissues such as the brain, skeletal muscle and kidneys, resulting in nephrotic syndrome, cerebellar ataxia, myopathy and/or encephalopathy.57 Supplemental CoQ10 can treat primary CoQ10 deficiency.74
Secondary CoQ10 deficiency is caused by mutations in genes in other pathways such as those causing OXPHOS defects, which in turn produce low CoQ10 levels, or it can be associated with disease or drugs such as statins.56,74 Rodríguez-Aguilera et al74 discusses that secondary CoQ10 deficiency could be caused by compensating for a dysfunctional OXPHOS, which can occur in ME/CMS.
CoQ10 and ME/CFS
There is reported evidence that CoQ10 levels are decreased in ME/CFS.37,75,76 There is not strong enough evidence, however, to call ME/CFS a secondary CoQ10 deficiency syndrome, as measurement of fibroblast CoQ10 levels is necessary to define secondary CoQ10 deficiency.55,77 Maes et al76 studied 58 ME/CFS participants and 22 healthy controls, and measured CoQ10 levels in plasma and severity of symptoms using a ‘fibrofatigue’ (FF) scale. They found many ME/CFS participants had CoQ10 concentrations lower than the lowest healthy control value (490 μg/L), and that the CoQ10 concentration had significant negative correlation with the FF scores. They concluded that CoQ10 had a role in the pathophysiology causing ME/CFS symptoms. Castro-Marrero et al37 found significantly lower levels of CoQ10 in peripheral blood mononuclear cells (PBMCs) in 23 ME/CFS participants compared to 15 control participants. They also found significantly decreased ATP levels and increased lipid peroxidation and argued these indicated mitochondrial dysfunction in ME/CFS. Kurup and Kurup75 found statistically significant lower levels of CoQ10 in plasma of 15 ME/CFS patients compared to 15 control participants. They suggested magnesium deficiency, low CoQ10 levels and dysfunction of ion transporter Na+/K + ATPase all contribute to mitochondrial dysfunction in ME/CFS. By contrast, Wood,78 found no statistical differences between an ME/CFS cohort and healthy controls for CoQ10 in PBMCs or plasma.
CoQ10 has been shown to restore respiration and act as an antioxidant.79 Oral supplementation of CoQ10 has been researched in low-CoQ10 conditions including mitochondrial disease,80 neurodegenerative diseases81 and ME/CFS.47,48,54 Primary CoQ10 deficiency can be treated by supplementation with CoQ10, however there is a mixed response to supplementation for those affected by secondary CoQ10 deficiency.74 Fukuda et al54 found that supplementation with CoQ10 significantly increased ME/CFS patients plasma levels of CoQ10. There was a significant correlation between increasing CoQ10 concentration and decreasing oxidative stress and fatigue.
Castro-Marrero et al47,48 assessed clinical outcomes after supplementation with reduced nicotinamide adenine dinucleotide (NADH) and CoQ10 in ME/CFS. Castro-Marrero et al47 also claimed a direct improvement in mitochondrial function by finding a statistically significant increase in ATP levels and NAD+/NADH ratio in the intervention group. It is not clear if positive effects are due to CoQ10 alone or due to the combination of additional NADH, especially as treatment with NADH or CoQ10 alone had shown no changes in fatigue scores in previous studies.53,54
A systematic review82 on sixteen studies of the effects of CoQ10 supplementation on fatigue for both healthy and cohorts with disorders, concluded that CoQ10 was better at decreasing fatigue in some disorders such as fibromyalgia and statin-related fatigue rather than ME/CFS. Mizuno et al83 explored anti-fatigue effects of CoQ10 in healthy individuals and found that there was a non-significant trend for decreased perception of fatigue following exercise when CoQ10 was taken. They did find significant improvement in physical testing in CoQ10-supplemented groups compared to control groups, and suggested it was due to its antioxidant or OXPHOS roles. However, Orlando et al65 found taking exogenous CoQ10 did not improve physical performance or markers of muscular damage despite CoQ10 being depleted post-exercise in healthy individuals. These results of these studies are confusing and need clarification.
Bioavailability may account for this confusion (at least in part). CoQ10 has low bioavailability, due in part to its low water solubility and high molecular mass (M = 863 g/mol), which may explain why supplement studies have not always been consistent in producing clinical effects. It is also better absorbed if taken with a fatty meal, like other lipid-soluble supplements such as vitamin E.58 Langsjoen and Langsjoen84 found that ubiquinol had a greater bioavailability than ubiquinone in healthy subjects. Vitetta et al67 also showed a non-significant trend for greater plasma CoQ10 levels at 2 hours after ubiquinol capsule supplementation compared to ubiquinone capsules in healthy individuals. Therefore, when assessing whether CoQ10 supplements might be beneficial for ME/CFS, the oxidative state of CoQ10 is important as well as the mode of administration.
Another important aspect to consider is the uptake of CoQ10 by tissues from plasma supplementation. In many clinical trials the extent to which CoQ10 enters cells is unknown. It has been found in rodent models that CoQ10 supplementation can increase tissue levels in the liver and spleen, but much higher doses are needed to increase levels in brain and muscle tissue.58 Niklowitz et al85 found that supplementing with CoQ10 increased levels in white blood cells and platelets but not in red blood cells. This low uptake in highly metabolic tissues may be a limitation of CoQ10 supplementation, as these are the tissues most influenced by CoQ10 deficiency.56 Knowing how the molecule distributes across body tissues may be critical for CoQ10 to be used as an effective treatment for ME/CFS.
Mitoquinol mesylate (MitoQ ®)
A CoQ10 analogue, mitoquinol mesylate (MitoQ ®86), developed originally as a tool to target molecules to mitochondria,87 is a powerful antioxidant and may be helpful in ME/CFS.78,87 It is now sold commercially as a supplement to improve health, energy levels and promote healthy aging. MitoQ is known ‘to manipulate mitochondrial ubiquinone content in vitro.87 It has better bioavailability than CoQ10 due to its higher watersolubility. MitoQ consists of a CoQ10 molecule covalently attached to a lipophilic triphenylphosphonium (TPP+) cation. The positive charge allows MitoQ to accumulate in the negatively charged mitochondria88 at a concentration 50–1000 times higher than CoQ10.87 This higher accumulation in the mitochondria and higher bioavailability accounts for its much lower recommended daily supplemental dosage of 10–20 mg compared to 200 mg for CoQ10. Thus, MitoQ may be a better candidate for mitochondrial therapy compared to CoQ10, as it may overcome the bioavailability and pharmacodynamic challenges of CoQ10 described above.
In the mitochondria, MitoQ is reduced by complex II of the ETC to form its reduced or ubiquinol state, becoming an antioxidant.79,87,88 Unlike CoQ10, it cannot be easily reduced by complex I or oxidised by complex III because the TPP + tail stops effective binding to these complexes. MitoQ does not restore respiration in the electron transport chain/OXPHOS as CoQ10 does, and is therefore not consumed by this process, allowing higher concentrations of its reduced state to persist within the mitochondria.89
Kelso et al87 found that MitoQ performed better than non-targeted (non-complex II-specific) antioxidants in the mitochondria, and prevented apoptotic cell death caused by hydrogen peroxide. MitoQ stopped mitochondrial dysfunction caused by oxidative stress. Park et al90 found reduced reactive oxygen species in MitoQ-treated endothelial cells, possibly associated with decreased breakdown of the critical mitochondrial antioxidant enzyme, manganese-catalysed superoxide dismutase (SOD). Animal and human studies have been performed with MitoQ as a supplement.91 Individuals with chronic Hepatitis C virus showed positive benefits on liver function by decreasing alanine transaminase.92 A clinical trial found that MitoQ improved vascular function of healthy older adults, by showing increased brachial artery dilation, suggesting it may reduce the risk of cardiovascular disease.93 Research investigating the effects on Parkinson's disease was, in contrast, inconclusive.94
Johnson and Grant55 performed a randomised controlled trial of MitoQ versus placebo in participants with ME/CFS conducted online via a website. Fifty one participants with ME/CFS were sent either MitoQ or placebo in the mail. No statistically significant improvement occurred. There were several limitations and potential biases to this study including a lack of ability to confirm the diagnosis, the usage of self-reported data and an inability to confirm compliance with the study. By contrast, another group of participants who took MitoQ without a matching placebo group, by purchasing the supplement themselves, found significant results and a reduction in clinical symptoms. So far, this online study is the only published study investigating MitoQ for ME/CFS.
Bioenergetic health index
The Bioenergetic Health Index (BHI) is an emerging measure in biochemistry that has been referred to as the ‘new BMI’ by the American Physiological Society.95 It is a representation of mitochondrial health derived from calculating the ‘spare respiratory capacity’ multiplied by the ‘ATP production of mitochondria under stress’, divided by the ‘measured proton leak’ multiplied by the ‘non-mitochondrial respiration’ (glycolysis). Chacko et al96 discussed the use of BHI as a marker of disease progression in particular for areas such as cancer, cardio-metabolic syndromes and neurodegenerative diseases, which are now being found to be affected by mitochondrial dysfunction.97,98 BHI is sensitive to oxidative stress and therefore may reflect changes in oxidative stress in these diseases.96 BHI may help predict who is more susceptible to these diseases and who may benefit from emerging therapies targeting mitochondrial health. For example, post-cardiac surgery patients with the larger decreases in BHI values measured in leucocytes collected from their pericardial fluid were more likely to have cardiac complications (atrial fibrillation), than those with less significant changes in BHI values.99, 100, 101, 102, 103, 104 As mitochondrial dysfunction and oxidative damage have been implicated in ME/CFS,28,29 BHI could be a potential biomarker for revealing how well the mitochondria are functioning over time and whether mitochondrial therapies such as MitoQ could be useful in ME/CFS. Only three analyses of BHI with ME/CFS patients have been completed with contradictory results.21,22,32
Key papers regarding mitochondrial functions, oxidative stress and supplementation trials with ME/CFS patients are summarised in Table 1.
Table 1.
Key papers for ME/CFS patient studies.
| Activity | Reference | Finding | Therapeutic Investigated | Cell Type/Tissue Investigated |
|---|---|---|---|---|
| Mitochondrial metabolic pathways | Fluge et al 2016100 | Impaired pyruvate dehydrogenase, consistent with inadequate ATP production, excessive lactate on exertion | NA | PBMCs |
| Metabolic response to exercise | Brown et al 2015101 | Impaired AMP kinase activation, glucose uptake | NA | Skeletal muscle cells |
| Cellular metabolism | Naviaux et al 201623 | Reduced metabolites in 20 biochemical pathways consistent with hypometabolic syndrome | NA | Plasma |
| Mitochondrial metabolism | Comhaire et al 201852 | Reduction in fatigue severity consistent with existing mitochondrial hypometabolism and impaired pyruvate dehydrogenase | Sodium Dichloroacetate (Oral administration) | NA |
| Mitochondrial metabolism | Forsyth et al 199953 | 31% of patients had a positive reaction to NADH | NADH (Oral administration ENADA) | NA |
| Cellular energy synthesis | Teitelbaum et al 200650 | Improvement in energy, sleep, mental clarity, pain intensity and well-being | d-ribose (Oral administration) | NA |
| Cellular stress, Inflammation | Sweetman et al 2019102 | Transcriptome -changes in stress, inflammation pathways, metabolic regulation, mitochondrial function, and circadian clock. | NA | PBMCs |
| Metabolic, Immune and Neurological | Helliwell et al 2020103 | Changes in DNA methylation indicate abnormal immune, neurological and metabolic functions | NA | PBMCs |
| Oxidative stress | Jammes et al 2005104 | Incremental exercise resulted in oxidative stress with alterations in muscle membrane | NA | Blood samples |
| Mitochondrial stress | Tomas et al 201721 | Lower maximal respiration indicating inability to compensate for stress | NA | PBMCs |
| Mitochondrial ATP production | Lawson et al 201634 | Increased cristae density in patients. Increased ATP from non-mitochondrial sources | NA | PBMCs |
| Mitochondrial ATP production | Missailidis et al 202022 | Complex V inefficiency in ATP production | NA | Immortalised lymphocytes |
| Mitochondrial complex activity | Tomas et al 201932 | No observed differences in mitochondrial complex activity | NA | PBMCs, skeletal muscle cells |
| Mitochondrial Complexes, ATP production and oxidative stress | Sweetman et al 20208 | Disturbances in proteins of (a) mitochondrial complexes I & V, (b) regulating reactive oxygen species, and in mitochondrial pathways | NA | PBMCs |
| CoQ10 | Maes et al 200976 | Deficiency in CoQ10 in patient group correlating with symptom severity | CoQ10 | Plasma |
| CoQ10 | Castro-Marrero et al 201548 | Improvement in fatigue levels and biochemical parameters (NAD+, CoQ10, ATP, citrate synthase and lipoperoxides) | CoQ10 (Oral supplementation) | |
| CoQ10/MitoQ | Wood 202078 | An association of CoQ10 levels with mitochondrial function, enhanced by supplementation of cells with exogenous C0Q10. Supplementation of MitoQ orally improved bioenergetic profiles of a subject. | CoQ10 MitoQ (oral supplementation) |
PBMCs, |
| ∗MitoQ | Kelso et al 200187 | MitoQ protects mitochondria from hydrogen peroxide-induced apoptosis | MitoQ | Human osteosarcoma cells |
| ∗MitoQ | James et al 200579 | MitoQ10 was an effective antioxidant against lipid peroxidation, peroxynitrite and superoxide. | MitoQ | Rat heart mitochondria |
Non-patient studies showing MitoQ is a powerful antioxidant in mitochondria. PBMC: Peripheral blood mononuclear cell; NA: Not applicable; NADH: Reduced nicotinamide adenine dinucleotide; CoQ10: Coenzyme Q10.
Can antioxidant therapy help the ‘long haulers’ with SARS–CoV-2 infection?
The relatively high proportion of people chronically infected with SARS-CoV-2 (‘the long haulers’), who do not make a straight-forward recovery in the post viral period of their illness, almost certainly reflects damage done by the host response to the initial infection. A severe body response such as a cytokine storm can give rise to oxidative and inflammatory damage and generalised oxidative stress, and this suggests that the antioxidant therapies discussed in this review might be beneficial. Antioxidant therapy is known to improve the levels of the abundant natural antioxidant, glutathione (which is important for redox balance), and to strengthen the immune response.105 Physiological changes in SARS-CoV-2 that enhance the production of reactive oxygen species could be ameliorated by free radical scavengers.106 Oxidative stress and ongoing pathogenesis in SARS-CoV-2 are almost certainly linked.107 Indeed Wu108 believes the cytokine storm and free radical storm should be regarded as the actual pathogens.
These observations suggest antioxidant therapies should be trialled in patients struggling to recover after SARS-CoV-2. N-acetylcysteine is one therapeutic agent that has been suggested as an effective method of improving redox status, especially when under oxidative stress. Earlier clinical trials have used this compound to replenish glutathione stores and increase the proliferative response of immune T cells,109 and indeed a new clinical trial to investigate vitamin C infusion for the treatment of severe SARS-CoV-2 infected pneumonia has already begun in Wuhan, China.110 Ouyang and Gong111 hypothesize that MitoQ could act as a potential treatment for SARS-CoV-2 in the acute phase. Recent evidence indicates that mitochondrial dysfunction participates in the development of the illness and may be responsible for the ongoing abnormal immune response. On the basis of the research done with ME/CFS patients, we propose that MitoQ may alleviate the effects of the cytokine storm and restore the function of exhausted T cells through improving mitochondrial function, in ‘long haul’ or chronically ill SARS-CoV-2 patients as well.
Conclusion
Research into mitochondrial function of ME/CFS has been increasing in recent years and appears to hold potential for better understanding this enigmatic disease that may be increasing its prevalence as a result of the SARS-CoV-2 pandemic. So far, however, the results have proved somewhat contradictory. There are several reasons for this. An accurate diagnosis for ME/CFS is difficult in the absence a molecular biomarker diagnostic test, individuals in the ME/CFS group are often heterogeneous and have differing disease severity. There are multiple clinical case definitions that are being used for diagnosis. A vicious circle exists: without a biochemical diagnostic test it is difficult to be certain which patients have ME/CFS and not another fatigue illness with overlapping symptoms, but determining a valid test requires identifying an homogenous group of test subjects. If ME/CFS is indeed a state of CoQ10 deficiency – particularly in high energy-demand organs like the brain – then an effective way of assessing CoQ10 is needed. This could then be a cellular biomarker that is monitored after oral supplementation in treatment of ME/CFS. Changes in mitochondrial function and markers of oxidative stress may be the biological component most impacted upon by CoQ10 supplementation, due to CoQ10's vital role in the electron transport chain. There is also clear need to establish whether MitoQ does indeed improve mitochondrial function and oxidative stress and lessens symptoms in both ME/CFS and chronic symptoms from SARS-CoV-19. There is a biologically plausible mechanism for expected improvement with MitoQ's superior bioavailability to cells and to mitochondria within them, and by how it can restore oxidative balance and therefore improve mitochondrial function. A respiratory index, like the BHI, also has the potential to predict and monitor pathophysiology in both ME/CFS and ‘long-haulers’ from SARS-CoV-2. Further research into ME/CFS and SARS-CoV-2, two perplexing and seriously debilitating diseases, is urgently required.
Funding
This study was support by a grant from Otago Medical School Scholarship for Ms Wood.
Conflicts of interest
None.
Edited by Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Witvliet M.G. The Conversation; Australia: 2020 Aug 11. I'm a COVID-19 Long-Hauler and an Epidemiologist – Here's How it Feels when Symptoms Last for Months.https://theconversation.com/im-a-covid-19-long-hauler-and-an-epidemiologist-heres-how-it-feels-when-symptoms-last-for-months-143676 [Internet] [cited 2020 Aug 14]. Available from. [Google Scholar]
- 2.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020 Sep 14;585(7825) doi: 10.1038/d41586-020-02598-6. https://www.nature.com/articles/d41586-020-02598-6 [Internet] [cited 2020 Nov 3]. Available from. [DOI] [PubMed] [Google Scholar]
- 3.Katz B.Z., Shiraishi Y., Mears C.J., Binns H.J., Taylor R. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics. 2009;124:189–193. doi: 10.1542/peds.2008-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shephard C., Chaudhuri A. 9th ed. Key Cross Media; England: 2017. ME/CFS/PVFS an Exploration of the Key Clinical Issues.http://omegaoxon.org/media/booklets/shepherd.pdf [Internet] [cited 2020 Aug 13]. 26p. Available from. [Google Scholar]
- 5.Chu L., Valencia I.J., Garvert D.W., Montoya J.G. Onset patterns and course of myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatri. 2019;7:1–22. doi: 10.3389/fped.2019.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallings R. Calico Publishing; Auckland (New Zealand): 2012. Chronic Fatigue syndrome/ME: Symptoms, Diagnosis, Treatment; p. 352. [Google Scholar]
- 7.Drachler M.D.L., Carlos J., Leite D.C. The expressed needs of people with chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review. BMC Publ Health. 2009;9:458. doi: 10.1186/1471-2458-9-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweetman E., Kleffmann T., Edgar C., de Lange M., Mackay A., Vallings R. A SWATH-MS Analysis of Myalgic Encephalomyelitis/chronic Fatigue Syndrome Peripheral Blood Mononuclear Cell Proteomes Reveals Mitochondrial Dysfunction. J Trans Med. 2020;18:365. doi: 10.1186/s12967-020-02533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine . National Academies Press; Washington (DC): 2015. Board on the Health of Select Populations, Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Beyond Myalgic Encephalomyelitis/chronic Fatigue Syndrome: Redefining an Illness. [DOI] [PubMed] [Google Scholar]
- 10.United States Senate . 2019. A resolution supporting the goals of international myalgic encephalomyelitis/chronic fatigue syndrome awareness day S. Res. 225. 116th Congress.https://www.congress.gov/bill/116th-congress/senate-resolution/225 [cited 2020 Aug 13]. Accessed from. [Google Scholar]
- 11.Baken D.M., Harvey S.T., Bimler D.L., Ross K.J. Stigma in myalgic encephalomyelitis and its association with functioning. Fatigue. 2018;6:30–40. doi: 10.1080/21641846.2018.1419553. [DOI] [Google Scholar]
- 12.Gibson H. 2018 May 12. The Illness People Can't See: Living with Chronic Fatigue Syndrome [Internet]. Auckland (New Zealand): The Spinoff.https://thespinoff.co.nz/science/12-05-2018/the-illness-people-cant-see-living-with-chronic-fatigue-syndrome/ [cited 2020 August 12]. Available from. [Google Scholar]
- 13.Fabian R. What is invisible illness? (+ how to explain it to others) Talkspace. 2018 Mar 5 https://www.talkspace.com/blog/what-is-invisible-illness-how-to-explain-it-to-others/ [Internet] [cited 2020 August 8]. Available from. [Google Scholar]
- 14.Parker L. Scope; Stanford (CA): 2017 Jan 24. But You Look So Good: Living with an Invisible Illness. [Internet] [cited 2020 Aug 18] [Google Scholar]
- 15.Addison C. University of Auckland; 2013. Chronic Fatigue Syndrome/myalgic Encephalopathy: A Knowledge-Based Approach to an Indeterminate Illness. Master of Arts [thesis]. Auckland (New Zealand)https://researchspace.auckland.ac.nz/bitstream/handle/2292/22441/whole.pdf?sequence=6 Available from. [Google Scholar]
- 16.Wojcik W., Armstrong D., Kanaan R. Chronic fatigue syndrome: labels, meanings and consequences. J Psychosom Res. 2011;70:500–504. doi: 10.1016/j.jpsychores.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Booth N.E., Myhill S., McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Int J Clin Exp Med. 2012;5:208–220. [PMC free article] [PubMed] [Google Scholar]
- 18.Voet D., Voet J.G., Pratt C.W. 4th ed. John Wiley & Sons; Hoboken (NJ): 2003. Fundamentals of Biochemistry; p. 1208. [Google Scholar]
- 19.Schulz E., Wenzel P., Münzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naviaux R.K. Metabolic features and regulation of the healing cycle – a new model for chronic disease pathogenesis and treatment. Mitochondrion. 2019;46:278–297. doi: 10.1016/j.mito.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Tomas C., Brown A., Strassheim V., Elson J., Newton J., Manning P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PloS One. 2017;12:1–16. doi: 10.1371/journal.pone.0186802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Missailidis D., Annesley S.J., Allan C.Y. An isolated Complex V inefficiency and dysregulated mitochondrial function in immortalized lymphocytes from ME/CFS patients. Int J Mol Sci. 2020;21:1074. doi: 10.3390/ijms21031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naviaux R.K., Naviaux J.C., Li K., Bright T., Alaynick W.A., Wang L. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA. 2016;113:E5472–E5480. doi: 10.1073/pnas.1607571113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomas C., Newton J. Metabolic abnormalities in chronic fatigue syndrome/myalgic encephalomyelitis: a mini-review. Biochem Soc Trans. 2018;46:547–553. doi: 10.1042/BST20170503. [DOI] [PubMed] [Google Scholar]
- 25.Holden S., Maksoud R., Eaton-Fitch N., Cabanas H., Staines D., Marshall-Gradisnik S. A systematic review of mitochondrial abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med. 2020;18:290. doi: 10.1186/s12967-020-02452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong C.W., McGregor N.R., Lewis D.P., Butt H.L., Gooley P.R. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics. 2015;11:1626–1639. doi: 10.1007/s11306-015-0816-5. [DOI] [Google Scholar]
- 27.Myhill S., Booth N.E., McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med. 2009;2:1–16. PMCID: PMC2680051. [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson C. ME/CFS seahorse energy production study shows surprises. Incline Vill (NV): Simmaron Res. 2019 Aug 28 http://simmaronresearch.com/2019/08/me-cfs-seahorse-energy-production-study-shows-surprises/ [Internet] [cited 2020 Mar 23]. Available from. [Google Scholar]
- 29.Morris G., Maes M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis. 2014;29:19–36. doi: 10.1007/s11011-013-9435-x. [DOI] [PubMed] [Google Scholar]
- 30.Filler K., Lyon D., Bennett J. Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin. 2014;1:12–23. doi: 10.1016/j.bbacli.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smits B., van den Heuvel L., Knoop H. Mitochondrial enzymes discriminate between mitochondrial disorders and chronic fatigue syndrome. Mitochondrion. 2011;11:735–738. doi: 10.1016/j.mito.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Tomas C., Brown A.E., Newton J.L., Elson J.L. PeerJ; 2019. Mitochondrial Complex Activity in Permeabilised Cells of Chronic Fatigue Syndrome Patients Using Two Cell Types [Internet] [cited 2020 Aug 13];7:e6500. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behan W.M.H., More I.A.R., Behan P.O. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol. 1991;83:61–65. doi: 10.1007/BF00294431. [DOI] [PubMed] [Google Scholar]
- 34.Lawson N., Hsieh C.-H., March D., Wang X. Elevated energy production in chronic fatigue syndrome patients. J Nat Sci. 2016;2 http://www.jnsci.org/index.php?journal=nsci&page=article&op=view&path%5B%5D=221 Available from. [PMC free article] [PubMed] [Google Scholar]
- 35.Vermeulen R.C.W., Kurk R.M., Visser F.C., Sluiter W., Scholte H.R. Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. J Transl Med. 2010;8:93. doi: 10.1186/1479-5876-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris G., Maes M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis(ME)/chronic fatigue syndrome(CFS) Curr Neuropharmacol. 2014;12:168–185. doi: 10.2174/1570159x11666131120224653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro-Marrero J., Cordero M.D., Sáez-Francas N. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal. 2013;19:1855–1860. doi: 10.1089/ars.2013.5346. [DOI] [PubMed] [Google Scholar]
- 38.Betteridge D.J. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/S0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 39.Young I.S., Woodside J.V. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. https://doi.org/10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starkov A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann NY Acad Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/bj20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liguori I., Russo G., Curcio F. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jammes Y., Steinberg J.G., Delliaux S. Chronic fatigue syndrome: acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins. J Intern Med. 2012;272:74–84. doi: 10.1111/j.1365-2796.2011.02488.x. [DOI] [PubMed] [Google Scholar]
- 44.Richards R.S., Roberts T.K., McGregor N.R., Dunstan R.H., Butt H.L. Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome. Redox Rep. 2000;5:35–41. doi: 10.1179/rer.2000.5.1.35. [DOI] [PubMed] [Google Scholar]
- 45.Miwa K., Fujita M. Fluctuation of serum vitamin e (α-tocopherol) concentrations during exacerbation and remission phases in patients with chronic fatigue syndrome. Heart Ves. 2010;25:319–323. doi: 10.1007/s00380-009-1206-6. [DOI] [PubMed] [Google Scholar]
- 46.Dalle-Donne I., Rossi R., Colombo R., Giustarini D., Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 47.Castro-Marrero J., Cordero M.D., Segundo M.J. Does oral coenzyme Q 10 plus NADH supplementation improve fatigue and biochemical parameters in chronic fatigue syndrome? Antioxid Redox Signal. 2015;22:679–685. doi: 10.1089/ars.2014.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castro-Marrero J., Sáez-Francàs N., Segundo M.J. Effect of coenzyme Q 10 plus nicotinamide adenine dinucleotide supplementation on maximum heart rate after exercise testing in chronic fatigue syndrome: a randomized, controlled, double-blind trial. Clin Nutr ESPEN. 2016;35:826–834. doi: 10.1016/j.clnu.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Myhill S., Booth N.E., McLaren-Howard J. Targeting mitochondrial dysfunction in the treatment of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) - a clinical audit. Int J Clin Exp Med. 2013. 2013;6:1–15. [PMC free article] [PubMed] [Google Scholar]
- 50.Teitelbaum J.E., Johnson C., St Cy J. The use of D-ribose in chronic fatigue syndrome and fibromyalgia: a pilot study. J Alternative Compl Med. 2006;12:857–862. doi: 10.1089/acm.2006.12.857. [DOI] [PubMed] [Google Scholar]
- 51.Montoya J.G., Anderson J.N., Adolphs D.L. KPAX002 as a treatment for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a prospective, randomized trial. Int J Clin Exp Med. 2018;11:2890–2900. http://www.ijcem.com/files/ijcem0065685.pdf [Internet] Available from. [Google Scholar]
- 52.Comhaire F. Treating patients suffering from myalgic encephalopathy/chronic fatigue syndrome (ME/CFS) with sodium dichloroacetate: an open-label, proof-of-principle pilot trial. Med Hypotheses. 2018;114:45–48. doi: 10.1016/j.mehy.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Forsyth L.M., Preuss H.G., MacDowell A.L., Chiazze L., Birkmayer G.D., Bellanti J.A. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann Allergy Asthma Immunol. 1999;82:185–191. doi: 10.1016/S1081-1206(10)62595-1. [DOI] [PubMed] [Google Scholar]
- 54.Fukuda S., Nojima J., Kajimoto O. Ubiquinol-10 supplementation improves autonomic nervous function and cognitive function in chronic fatigue syndrome. Biofactors. 2016;42:431–440. doi: 10.1002/biof.1293. [DOI] [PubMed] [Google Scholar]
- 55.Johnson C., Grant J. Mendes; Leipzig: 2016 Aug. The Influence of MitoQ on Symptoms and Cognition in Fibromyalgia, Myalgic Encephalomyelitis and Chronic Fatigue. [Internet] [cited 2020 Aug 18]. 10p. Available from. [DOI] [Google Scholar]
- 56.Stefely J.A., Pagliarini D.J. Biochemistry of mitochondrial coenzyme Q biosynthesis. Trends Biochem Sci. 2017;42:824–843. doi: 10.1016/j.tibs.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yubero D., Montero R., Armstrong J. Molecular diagnosis of coenzyme Q10 deficiency. Expert Rev Mol Diagn. 2015;15:1049–1059. doi: 10.1586/14737159.2015.1062727. [DOI] [PubMed] [Google Scholar]
- 58.Bhagavan H.N., Chopra R.K. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 59.Kalén A., Appelkvist E.L., Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1987;24:579–584. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- 60.Varela-López A., Giampieri F., Battino M., Quiles J.L. Coenzyme Q and its role in the dietary therapy against aging. Molecules. 2016;21:1–26. doi: 10.3390/molecules21030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber C., Bysted A., Hølmer G. Coenzyme Q10 in the diet-daily intake and relative bioavailability. Mol Aspect Med. 1997;18:251–254. doi: 10.1016/S0098-2997(97)00003-4. [DOI] [PubMed] [Google Scholar]
- 62.Niklowitz P., Onur S., Fischer A. Coenzyme Q10 serum concentration and redox status in European adults: influence of age, sex, and lipoprotein concentration. J Clin Biochem Nutr. 2016;58:240–245. doi: 10.3164/jcbn.15-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miles M.V., Horn P.S., Morrison J.A., Tang P.H., DeGrauw T., Pesce A.J. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin Chim Acta. 2003;332:123–132. doi: 10.1016/s0009-8981(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 64.Mancini A., Festa R., Raimondo S., Pontecorvi A., Littarru G.P. Hormonal influence on coenzyme Q 10 levels in blood plasma. Int J Mol Sci. 2011;12:9216–9225. doi: 10.3390/ijms12129216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orlando P., Silvestri S., Galeazzi R. Effect of ubiquinol supplementation on biochemical and oxidative stress indexes after intense exercise in young athletes. Redox Rep. 2018;23:136–145. doi: 10.1080/13510002.2018.1472924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Pozo-Cruz J., Rodríguez-Bies E., Ballesteros-Simarro M. Physical activity affects plasma coenzyme Q10 levels differently in young and old humans. Biogerontology. 2014;15:199–211. doi: 10.1007/s10522-013-9491-y. [DOI] [PubMed] [Google Scholar]
- 67.Vitetta L., Leong A., Zhou J., Dal Forno S., Hall S., Rutolo D. The plasma bioavailability of coenzyme Q10 absorbed from the gut and the oral mucosa. J Funct Biomater. 2018;9:73. doi: 10.3390/jfb9040073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma A., Fonarow G.C., Butler J., Ezekowitz J.A., Felker G.M. Coenzyme Q10 and heart failure. Circ-Heart Fail. 2016;9:1–8. doi: 10.1161/CIRCHEARTFAILURE.115.002639. [DOI] [PubMed] [Google Scholar]
- 69.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta Biomembr. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Beal M.F. Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases. Biofactors. 1999;9:261–266. doi: 10.1002/biof.5520090222. [DOI] [PubMed] [Google Scholar]
- 71.Bentinger M., Tekle M., Dallner G. Coenzyme Q - biosynthesis and functions. Biochem Biophys Res Commun. 2010;396:74–79. doi: 10.1016/j.bbrc.2010.02.147. [DOI] [PubMed] [Google Scholar]
- 72.López-Lluch G., Rodríguez-Aguilera J.C., Santos-Ocaña C., Navas P. Is coenzyme Q a key factor in aging? Mech Ageing Dev. 2010;131:225–235. doi: 10.1016/j.mad.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Emmanuele V., López L.C., Berardo A. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Arch Neurol. 2012;69:978–983. doi: 10.1001/archneurol.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodríguez-Aguilera J., Cortés A., Fernández-Ayala D., Navas P. Biochemical assessment of coenzyme Q10 deficiency. J Clin Med. 2017;6:1–9. doi: 10.3390/jcm6030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kurup R.K., Kurup P.A. Isoprenoid pathway dysfunction in chronic fatigue syndrome. Acta Neuropsychiatr. 2003;15:266–273. doi: 10.1034/j.1601-5215.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 76.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuroendocrinol Lett. 2009;30:470–476. [PubMed] [Google Scholar]
- 77.Yubero D., Montero R., Artuch R., Land J.M., Heales S.J.R., Hargreaves I.P. Biochemical diagnosis of Coenzyme Q10 deficiency. Mol Syndromol. 2014;5:147–155. doi: 10.1159/000362390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wood E. Bachelor of Medical Science with Honours Thesis. University of Otago; Dunedin (NZ): 2020. Effect of coenzyme Q10 and MitoQ on mitochondrial function in myalgic encephalomyelitis/chronic fatigue syndrome. [Google Scholar]
- 79.James A.M., Cochemé H.M., Smith R.A.J., Murphy M.P. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species: implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 80.Enns G.M. Treatment of mitochondrial disorders: antioxidants and beyond. J Child Neurol. 2014;29:1235–1240. doi: 10.1177/0883073814538509. [DOI] [PubMed] [Google Scholar]
- 81.Morris G., Anderson G., Berk M., Maes M. Coenzyme Q10 depletion in medical and neuropsychiatric disorders : potential repercussions and therapeutic implications. Mol Neurobiol. 2013;48:883–903. doi: 10.1007/s12035-013-8477-8. [DOI] [PubMed] [Google Scholar]
- 82.Mehrabani S., Askari G., Miraghajani M., Tavakoly R., Arab A. Effect of coenzyme Q10 supplementation on fatigue: a systematic review of interventional studies. Compl Ther Med. 2019;43:181–187. doi: 10.1016/j.ctim.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 83.Mizuno K., Tanaka M., Nozaki S. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition. 2008;24:293–299. doi: 10.1016/j.nut.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Langsjoen P.H., Langsjoen A.M. Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin Pharmacol Drug Dev. 2013;3:13–17. doi: 10.1002/cpdd.73. [DOI] [PubMed] [Google Scholar]
- 85.Niklowitz P., Sonnenschein A., Janetzky B., Andler W., Menke T. Enrichment of coenzyme Q10 in plasma and blood cells: defense against oxidative damage. Int J Biol Sci. 2007;3:257–262. doi: 10.7150/ijbs.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MitoQ . 2020. The Power Is Yours: World-First, Positively-Charged, CoQ10 to Help Power Energy, Health and Immunity [Internet]. Auckland (NZ); MitoQ.https://www.mitoq.com/ [cited 2020 August 13]. Available from. [Google Scholar]
- 87.Kelso G.F., Porteous C.M., Coulter C.V. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 88.Smith R.A.J., Porteous C.M., Gane A.M., Murphy M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.James A.M., Sharpley M.S., Manas A.-R.B. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem. 2007;282:14708–14714. doi: 10.1074/jbc.m611463200. [DOI] [PubMed] [Google Scholar]
- 90.Park S.-Y., Kwon O.S., Andtbacka R.H.I. Age-related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol. 2018;222:1–12. doi: 10.1111/apha.12893. [DOI] [PubMed] [Google Scholar]
- 91.Smith R.A.J., Murphy M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann NY Acad Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 92.Gane E.J., Weilert F., Orr D.W. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 93.Rossman M., Santos-Parker J.R., Steward C.A.C. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension. 2018;71:1056–1063. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Snow B.J., Rolfe F.L., Lockhart M.M. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 95.American Physiological Society . ScienceDaily; Rockville (MD): 2015 Sept 16. Could the Bioenergetic Health Index Become the Next BMI? [Internet]www.sciencedaily.com/releases/2015/09/150916165410.htm [cited 2020 July 17]. Available from. [Google Scholar]
- 96.Chacko B.K., Kramer P.A., Ravi S. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin Sci (Lond) 2014;127:367–373. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 98.Pieczenik S.R., Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 99.Kramer P.A., Chacko B.K., George D.J. Decreased Bioenergetic Health Index in monocytes isolated from the pericardial fluid and blood of post-operative cardiac surgery patients. Biosci Rep. 2015;35 doi: 10.1042/bsr20150161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fluge Ø., Mella O., Bruland O. Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight. 2016;1 doi: 10.1172/jci.insight.89376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brown A.E., Jones D.E., Walker M., Newton J.L. Abnormalities of AMPK activation and glucose uptake in cultured skeletal muscle cells from individuals with chronic fatigue syndrome. PloS One. 2015;10 doi: 10.1371/journal.pone.0122982102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sweetman E., Ryan M., Edgar C., Mackay A., Vallings R., Tate W. Changes in the transcriptome of circulating immune cells of a New Zealand cohort with Myalgic Encephalomyelitis/chronic fatigue syndrome. Int J Immunopathol Pharmacol. 2019;33:1–8. doi: 10.1177/2058738418820402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Helliwell A.M., Sweetman E.C., Stockwell P.A., Edgar C.D., Chatterjee A., Tate W.P. Changes in DNA methylation profiles of myalgic encephalomyelitis/chronic fatigue syndrome patients reflect systemic dysfunctions. Clin Epigenet. 2020;12:167. doi: 10.1186/s13148-020-00960-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jammes Y., Steinberg J.G., Mambrini O., Bregeon F., Delliaux S. Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med. 2005;257:299–310. doi: 10.1111/j.1365-2796.2005.01452.x. [DOI] [PubMed] [Google Scholar]
- 105.Soto M.E., Guarner-Lans V., Soria-Castro E., Pech L.M., Pérez-Torres I. Is antioxidant therapy a useful complementary measure for Covid-19 treatment? An algorithm for its application. Medicina. 2020;56:1–29. doi: 10.3390/medicina56080386. https://doi.org/10.3390%2Fmedicina56080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mironova G.D., Belosludtseva N.V., Ananyan M.A. Prospects for the use of regulators of oxidative stress in the comprehensive treatment of the novel Coronavirus Disease (COVID-19) and its complications. Eur Rev Med Pharmacol Sci. 2020;24:8585–8591. doi: 10.3390/medicina56080386. [DOI] [PubMed] [Google Scholar]
- 107.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide. 2020;102:39–41. doi: 10.1016/j.niox.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poe F.L., Corn J. N-acetylcysteine: a potential therapeutic agent for SARS-CoV-2. Med Hypotheses. 2020;143:109862. doi: 10.1016/j.mehy.2020.109862. https://doi.org/10.016/j.mehy.2020.10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carr A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24:133. doi: 10.1186/s13054-020-02851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ouyang L., Gong J. Mitochondrial-targeted ubiquinone: a potential treatment for COVID-19. Med Hypotheses. 2020;144:110161. doi: 10.1016/j.mehy.2020.110161. [DOI] [PMC free article] [PubMed] [Google Scholar]