Highlights
-
•
COVID-19 is a global pandemic caused due to SARS-CoV-2.
-
•
Etiopathogenesis of SARS-CoV-2 in severe and critical COVID-19 cases showed exaggerated immune response.
-
•
Increased secretion of pro-inflammatory cytokines and chemokines observed in COVID-19.
-
•
Vitamin D deficiency: a potential link between respiratory infection and immune evasion.
-
•
Vitamin D: a pleiotropic factor known to modulate immune response against pathogen.
Keywords: COVID-19, Coronavirus, SARS-CoV-2, Acute respiratory distress syndrome (ARDS), Immune response, Cytokine storm, Treatment, Vitamin D, Dexamethasone
Abstract
The first incidence of COVID-19 was reported in the Wuhan city of Hubei province in China in late December 2019. Because of failure in timely closing of borders of the affected region, COVID-19 spread across like a wildfire through air travel initiating a pandemic. It is a serious lower respiratory track viral infection caused by highly contagious, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Coronavirus including COVID-19 causing SARS-CoV-2 causes zoonotic diseases and thought to be originated from bats. Since its first incidence, the virus has spread all across the world, causing serious human casualties, economic losses, and disrupting global supply chains. As with SARS-CoV, COVID-19 causing SARS-CoV-2 follows a similar path of airborne infection, but is less lethal and more infectious than SARS and MERS. This review focusses on the pathogenesis of SARS-CoV-2, especially on the dysfunctional immune responses following a cytokine storm in severely affected persons. The mode of entry of SARS-CoV-2 is via the angiotensin converting enzyme 2 (ACE-2) receptors present on the epithelial lining of lungs, gastrointestinal tract, and mucus membranes. Older persons with weaker immune system and associated co-morbidities are more vulnerable to have dysfunctional immune responses, as most of them concomitantly have severe hypovitaminosis D. Consequently, causing severe damage to key organs of the body including lungs and the cardiovascular system. Since, vast majority of persons enters to the intensive care units and died, had severe vitamin D deficiency, thus, this area must be investigated seriously. In addition, this article assesses the role of vitamin D in reducing the risk of COVID-19. Vitamin D is a key regulator of the renin-angiotensin system that is exploited by SARS-CoV-2 for entry into the host cells. Further, vitamin D modulates multiple mechanisms of the immune system to contain the virus that includes dampening the entry and replication of SARS-CoV-2, reduces concentration of pro-inflammatory cytokines and increases levels of anti-inflammatory cytokines, enhances the production of natural antimicrobial peptide and activates defensive cells such as macrophages that could destroy SARS-CoV-2. Thus, this article provides the urgency of needed evidences through large population based randomized controlled trials and ecological studies to evaluate the potential role of vitamin D in COVID-19.
1. Introduction
We are amidst a socioeconomic and health catastrophic global pandemic, which brought the global economic collapse, that worsens by prolonged lockdowns and curfews. The latter two has little effects on controlling the disease but has major impact on destructing the likelihoods of millions of people and the economies of all affected countries.
In December 2019, reports came about a cluster of patients suffering from severe acute respiratory syndrome linked to seafood and wet animal market in the Wuhan city, Hubei province of China (Wang et al., 2020a), but later found that the said location was incorrect. World Health Organization (WHO) declared COVID-19 as a pandemic on March 11, 2020 and the disease causing virus was classified as severe acute respiratory syndrome–coronavirus-2 (SARS-CoV-2) on same day by International Committee on Taxonomy of Viruses (ICTV) (Jarvis, 2020). Previously, two major epidemics of coronavirus, the severe acute respiratory syndrome (SARS)-CoV (Zhong et al., 2003) and middle east respiratory syndrome (MERS)-CoV (Assiri et al., 2013), both originated from wild animals and were transmitted to humans. Major cause of death was reported as severe atypical pneumonia (Song et al., 2019; Yin and Wunderink, 2018). SARS-CoV-2 enters the human body by attaching to its cognate angiotensin converting enzyme-2 (ACE-2) receptor; located on the epithelium lining of lungs and other organs via its spike protein (Wang et al., 2020a). Once internalized, viral RNA strands replicate uninterruptedly using the host’s polymerase system and further invades other host cells causing severe pathological changes, including cell death. The virus also evade the host’s immune system through multiple mechanisms including excessive stimulation of renin-angiotensin system (RAS) (Kuba et al., 2005). SARS-CoV-2 seems to hijack and modulate the RAS system, not only initiating an excess generation of angiotensin-II fuelling the cytokine storm but also down regulating host’s immune system thus favouring the propagation of the virus. In some COVID-19 cases, manifestation of dysfunctional immune responses includes, triggering a cytokine storm involving increased secretion of pro-inflammatory cytokines in circulation which leads to acute respiratory distress syndrome (ARDS) and ultimately death.
Vitamin D regulates the immune modulatory mechanisms by decreasing pro-inflammatory environment in-vivo, and increases secretion of anti-inflammatory cytokines. It has been reported that 25(OH)D deficiency, a physiologically quantifiable form of vitamin D, is strongly associated with unfavourable clinical outcome. Lastly, no adverse effects of using high doses of vitamin D in COVID-19 and other circumstances has been reported.
In addition to above, immune suppressor role of dexamethasone, a potent corticosteroid, has also been discussed in preventing the need of ventilator support in severely and critically ill COVID-19 patients. Thus, this review addresses pathogenesis of SARS-CoV-2 and mechanisms opted for immune evasion along with plausible role of vitamin D in regulating these immune evasion mechanisms for the successful eradication of pathogen from the host.
2. COVID-19: epidemiology, pathogenesis, and treatment
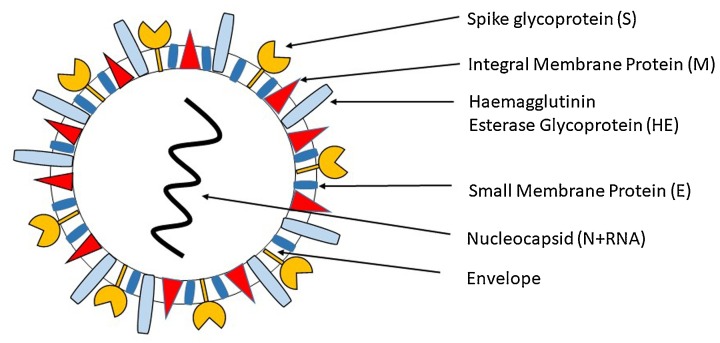
Coronavirus (CoVs) are enveloped, positive single-stranded RNA virus having ability to infect both humans and animals. Under electron microscope, they look like spherical entities along with core shell and have glycoprotein projections on their envelop, which gives them appearance like crown, hence named as coronavirus (Fig. 1 ) (Tyrrell and Bynoe, 1965). In 1966, Tyrell and Bynoe for the first-time identified coronavirus (CoVs) in patients suffering from acute upper respiratory illnesses (Tyrrell and Bynoe, 1965). Most coronavirus (CoVs) cause infections limiting to upper respiratory tract, while some CoVs such as beta-CoVs subgroup, including SARS-CoV, SARS-CoV-2 (COVID-19), and MERS-CoV cause lower respiratory tract infections resulting in severe complications and higher mortality. By 14th November 2020, the total confirmed COVID-19 cases were 53,164,803 and 1,300,576 deaths with a case fatality rate of 2.4 % globally. In India, till 14th November, the total confirmed COVID-19 cases were 8,774,479 with a total of confirmed 129,188 deaths (World Health Organization, 2020).
Fig. 1.
Diagrammatic representation of SARS-CoV-2 coronavirus virion.
Despite the public health processes and efforts from the frontline workers, the global incidence of COVID-19 keeps rising, so as the mortality. The preventive efforts were somewhat hampered due to lack of understanding of the biology and pathological processes of SARS-CoV-2 and suboptimal policies implemented by certain administrations. This issue warrants immediate actions in unveiling and understanding the host–pathogen biology of COVID-19 which will further help in gaining insights in management of disease and opening windows for cost-effective therapeutic interventions.
2.1. Description of coronavirus
Coronavirus are the largest group of virus belonging to the Nidovirales order that comprises three families i.e. arteriviridae, roniviridae and coronaviridae (Hui et al., 2020). Among various β-CoVs, SARS-CoV-2 shares 79 % genetic similarities to SARS-CoV (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020), whereas it demonstrates 98 % similarities with bat coronavirus RaTG13 (Zhou et al., 2020a), and also shares high similarities with pangolin (a scaly anteater) coronavirus sequences (Andersen et al., 2020). Like other respiratory coronavirus, SARS-CoV-2 virus spread majorly through respiratory droplets during sneezing and coughing. The first symptom arises at a median incubation period of 4–5 days while nearly 97.5 % patients develop symptoms within 11.5 days (Andersen et al., 2020; Guan et al., 2020). Symptomatically, COVID-19 patient displays fever, dry cough, muscle pain, difficulty in breathing, diarrhoea, nausea and vomiting (Guan et al., 2020; Huang et al., 2020; Chen et al., 2020a). After onset of symptoms, viral load achieve its maximal within next 5–6 days (Pan et al., 2020; Zou et al., 2020).
2.2. Pathophysiological processes of SARS-CoV-2
Patho-physiologically, SARS-CoV-2 resembles to SARS-CoV in exhibiting features of severe inflammatory response in damaging the airways (Wong et al., 2004a). Thus, the combination of anti-viral and host response leads to the severity of disease as also observed in SARS-CoV and MERS-CoV infected cases (Guan et al., 2020; Huang et al., 2020; Chen et al., 2020b; Tay et al., 2020). In COVID-19 patients, ARDS develops due to difficulty in breathing and low blood oxygen levels which ultimately leads to respiratory failure as observed in 70 % of severe COVID-19 cases (Zhang et al., 2020a). Further, a spurt of cytokines release in response to viral infection results in cytokine storm and sepsis leading to 28 % deaths in critical COVID-19 cases (Zhang et al., 2020a). Multiple organs failure are also observed in some patients due to uncontrolled inflammation caused by these pro-inflammatory cytokines (Chu et al., 2005; Wimalawansa, 2020).
Initially, SARS-CoV-2 attaches with ACE2 receptor which is present on majority of lung cells including epithelial cells, alveolar epithelial cells, vascular endothelial cells and macrophages and then enters via endocytosis (Jia et al., 2005; Hamming et al., 2004). The SARS-CoV-2 mode of entry is similar to SARS-CoV, thus it is likely that SARS-CoV-2 uses the similar cell subsets and related pathogenesis (Walls et al., 2020). SARS-CoV reduces the expression of ACE2 receptor which in turn is associated with acute lung injury and thus disease pathology. ACE2 regulates RAS, thus its downregulation will disrupt RAS homeostasis and ultimately affecting blood pressure, electrolyte balance, increasing inflammation and vascular permeability in the airways (Kuba et al., 2005). Coronavirus attaches to the receptor of host cell through spike glycoprotein (S), and once inside the cell this spike protein divides into S1 and S2 subunits. S1 regulates virus-host range and cellular tropism with the receptor binding domain (RBD) (Xiao et al., 2003; Wong et al., 2004b), whereas S2 helps in virus cell membrane fusion with the help of two tandem domains, heptad repeats 1 and 2 (HR1 & HR2) (Bosch et al., 2004; Liu et al., 2004). After the fusion, it releases viral RNA genome into cytoplasm thus initiating replication and transcription of virus in host cell cytoplasm. Positive RNA genome acts as a messenger RNA (mRNA) and is translated into pp1a and pp1ab, two large precursor polyproteins and these polyproteins in the presence of ORF 1a-encoded viral proteinases, 3C-like proteinases (3CLpro) and papain-like proteinase (PLpro) gets processed into 16 mature non-structural proteins (nsp1- nsp16) (Gorbalenya et al., 2000; Báez-Santos et al., 2015). During viral RNA replication and transcription these non-structural proteins (nsps) perform vital functions. Despite sharing of only 79 % genome sequence identity of SARS-CoV-2 with SARS-CoV, it displays high infectivity rate which could be due to the presence of furin-like cleavage site in S protein which is absent in SARS-CoV (Coutard et al., 2020).
Cytopathic virus including SARS-CoV-2 during virus replicative cycle causes tremendous destruction of infected cells and tissues thus triggering a local immune response due to virus linked pyroptosis and recruiting macrophages and monocytes (Park et al., 2020; Zhang et al., 2020b). Pyroptosis, a type of programmed cell death leads to extensive inflammation and secretion of IL-1β, a crucial inflammatory molecule (Huang et al., 2020; Fink and Cookson, 2005). Alveolar macrophage detects the pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs) present on the virus particle using pattern recognition receptors (PRRs) and generates local inflammation and releasing a spurt of cytokines and chemokines including IL-6, IFN-γ, MCP1 and IP-10 in the circulation of COVID-19 cases (Huang et al., 2020; Zhang et al., 2020a). Their secretion attracts various immune cells at affected organ including monocytes and T-lymphocytes thus conforming the lymphopenic situation along with increased neutrophil-lymphocyte ratio as observed in 80 % of the COVID-19 cases (Guan et al., 2020; Qin et al., 2020).
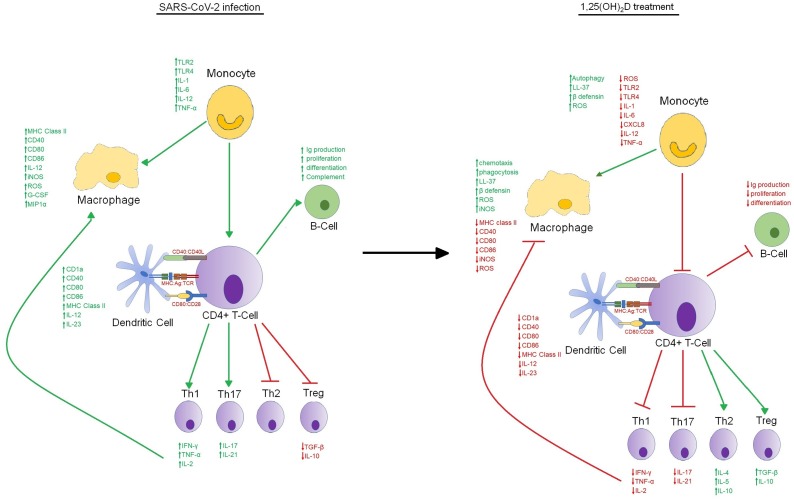
In majority of COVID-19 patients, these recruited cells eradicate the infection leading to patient’s recovery, but in some severe cases, a defective immune response arises, which activates a cytokine storm causing extensive lung inflammation. Severe COVID-19 patients have elevated levels of IL-2, IL-7, MCP1, G-CSF, IP-10, IL-10, MIP1α and TNF (Huang et al., 2020). Literature reported that IL-6 levels were considerably higher in non-survivors compared to survivors (Zhou et al., 2020b). Further, increased percentage of CD14+CD16+ inflammatory monocytes has also been observed in peripheral blood of severe COVID-19 patients than patients with mild infection, thus further triggering of cytokines release (Fig. 2 ) (Zhou et al., 2020c).
Fig. 2.
Comparative presentation of the immunomodulatory actions of 1,25(OH)2D. Image on the left represent the possible mechanism adopted by the SARS-CoV-2 in immune evasion during pathogenesis. Image on the right described the mechanism used by 1,25(OH)2D in modulating both innate and adaptive immune responses to mitigate the viral infection.
2.3. Mechanism of action of SARS- CoV-2 in harming host cells
Though disruptive mechanisms used by SARS-CoV-2 in inhibiting the body’s innate antiviral cytokine response are yet to be unveiled, the initial evidences on SARS-CoV revealed that viral structural and non-structural proteins might inhibit the interferon pathway activation through multiple mechanism including prevention of PRRs recognition, inhibition of signalling pathways, host mRNA degradation, and hindering host protein degradation. This antagonism of interferon response promotes viral replication and leads to aberrant inflammatory responses (Siu et al., 2014; Versteeg et al., 2007).
This unrestrained infiltration of inflammatory cells causes extensive destruction of lung cells and tissue via protease and reactive oxygen species secretion leading to massive pathological changes in the lung including desquamation of alveolar cells, hyaline membrane formation, pulmonary oedema (Xu et al., 2020). These pathological changes decrease efficient gaseous exchanges and low blood oxygen levels. Apart from local damage, the spurt of cytokine levels including TNF-α causes septic shock and leads to multi organ failure (Ruan et al., 2020). Patients with more than 60 years and those associated with co-morbidities are more susceptible to display dysfunctional immune response. In contrast, children despite having high viral titres do not develop severe disease (Kam et al., 2020).
The first autopsy of COVID-19 patient showed mononuclear cells accumulation (probably monocytes and T cells) in lungs, and deficit of hyperactive T cells in peripheral blood (Xu et al., 2020). One week after onset of COVID-19 symptoms, both T and B cells against SARS-CoV-2 have been detected in the peripheral blood. CD8+ T cells directly attacks and kills the virus whereas CD4+ T cells primed the CD8+ T cells and B cells. Further, CD4+ T cells are involved in immune cells recruitment through cytokines production. SARS-CoV-specific CD4+ T cells displays IFN-γ, TNF, IL-2 chemokines, thereby suggesting Th1 immune response and cell mediated immunity, a reason for extensive immunopathogenesis (Janice Oh et al., 2012).
Although several vaccine formulations against SARS-CoV showed signs of immunopathology, still this T cells vaccine holds promising avenues in eliminating the virus, but it must be ascertained whether they alone are capable of preventing infection in human setting or need to be complemented with other conjugates is a matter of further investigation (Deming et al., 2006; Yasui et al., 2008). Similarly, in COVID-19, B cells response is reported to occur after 1-week post onset of symptoms. In SARS-CoV infection, initially response is against nucleocapsid (N) protein, 4–8 days later post symptoms, antibody response against spike (S) protein can also be seen and reaches to its peak by the end of third week. In contrast, viral titre peaks earlier for SARS-CoV-2 than SARS, thus antibody response might also arise earlier (Pan et al., 2020; Zou et al., 2020).
Antibody response holds great promise as convalescent plasma therapy have demonstrated good clinical results in COVID-19 cases (Xinhua, 2020). In China, initial efforts in treating COVID-19 patients with polyclonal antibodies hold great promise in reducing viral titre and mortality (Li et al., 2020; CNA, 2020). Further, various monoclonal antibodies raised against RBD of S protein working efficiently against SARS-CoV also neutralized SARS-CoV-2 virus, but majority do not, thus underline the importance of dissimilar protein sequence in the RBDs of SARS-CoV-2 and SARS-CoV.
2.4. Management of persons with COVID-19
Current management of COVID-19 is supportive, and respiratory failure from ARDS is the leading cause of mortality. Numerous drugs avenues are being explored for the treatment and management of COVID-19. Remdesivir was initially developed for the treatment of Ebola, MERS-CoV, and SARS-CoV (Sheahan et al., 2017). It is a potential nucleoside inhibitor that causes premature termination of viral RNA replication. Atleast eight clinical trials are currently underway in China (NCT04252664, NCT04257656), France (NCT04314817, NCT04315948) and the USA (NCT04315948, NCT04292730, NCT04280705, NCT04302766).
Lopinavir and ritonavir are two protease inhibitors initially designed for anti-retroviral therapy (ART) and showed promising results in SARS (Chu et al., 2004). Hydroxychloroquine is an anti-malarial drug and it works on the principle of inhibition of viral replication (Savarino et al., 2003). Further, it also acts as immunosuppressant by reducing IL-6 and TNF-α levels. The drug interferes with the ACE2 receptor glycosylation and ultimately blocks the viral entry into the host cells. Secondarily, this drug has evolved as a major pre-exposure and post-exposure prophylactic agent. Various clinical trials are undergoing in multiple countries to test the potential of hydroxychloroquine. Further, ACE inhibitors, antibodies, convalescent plasma therapy, and vaccines are also being explored for the management of COVID-19. Recently, studies are focussing on curbing the complement system pathway, a component of innate immune system, in reducing hyperinflammatory and hypercoagulation stage in severe COVID-19 patients (Satyam and Tsokos, 2020). Furthermore, Ibrahim et al. (2020) reported that intravenous injection of N-acetyl cysteine in small cases of COVID-19, demonstrated significantly decreased inflammation and clinical improvement along with markedly reduced CRP levels in all patients and ferritin in 9/10 patients (Ibrahim et al., 2020). Lastly, other options such as Imatinib a tyrosine kinase inhibitor and colchicine, an anti-inflammatory drug have also been explored in few COVID-19 cases (Morales-Ortega et al., 2020; Della-Torre et al., 2020).
Final approval of these drugs will require time to develop and commercialize and poses equally large hurdles. In contrast, studies done in the last few decades to unveil the potential roles of vitamin D has given significant hopes for the current management of COVID-19. Further, widespread deficiency, correlation with clinical outcomes, immunomodulatory potential, regulation of RAS and less toxicity at very high doses proposed this drug as a likely candidate to study as a therapeutic modality alone or in combination with standard drug regimen in the COVID-19 patients.
3. Vitamin D physiology and metabolism
Vitamin D is a secosteroid hormone, initially discovered during England’s industrial revolution when workers were suffering from rickets problem (DeLuca, 2014; Gallo et al., 2018). Since then, multiple functions including skeletal and extra skeletal have been attributed to the vitamin D. Vitamin D specifically is of two types: vitamin D2 (ergocalciferol) derived mainly from plant sources and vitamin D3 (cholecalciferol), which is present in higher animals constitutes nearly 80–90 % (Holick, 2014). Initially, 7-dehydrocholesterol is converted to cholecalciferol by exposure to ultraviolet B radiation (UV B) (Holick, 2014). Both endogenous and exogenous forms are inactive and requires two successive hydroxylation steps by cytochrome P450 (CYP) enzymes to form fully active vitamin D. Circulatory vitamin D is initially transported to liver by vitamin D binding protein (DBP). First hydroxylation takes place in liver at 25-carbon position by cytochrome P450 vitamin D hydroxylases (CYP2R1, CYP2D11 and CYP2D25) leading to formation of calcidiol (25(OH)D) (Christakos et al., 2010). Due to its longer shelf life in humans, it is used as a universal biomarker of vitamin D status (Pludowski et al., 2018). For second hydroxylation step, calcidiol leaves the liver and is internalized into kidney where C-1 hydroxylation takes place by renal 1α-hydroxylase (CYP27B1) strictly regulated by parathyroid hormone (PTH). Renal hydroxylation forms the fully functional, active form of vitamin D i.e. calcitriol (1α, 25-(OH)2 vitamin D) (Christakos et al., 2016).
Apart from kidney, second hydroxylation also occurs in other cells and tissues types collectively called as extra renal hydroxylation and performs different functions depending on their locations. 1α, 25-dihydroxy vitamin D [1, 25(OH)2D], similar to other forms of steroid hormone combines with vitamin D receptor/retinoic X receptor (VDR/RXR) heterodimeric complex and transcriptionally regulates expression of plethora of genes by binding to the DNA responsive elements and mediate several biological responses (Haussler et al., 2011). After the function is over, 1α, 25-(OH)2 vitamin D is catabolized by 24-hydroxylase [24(OH)ase] to inactive metabolites which is then excreted through bile and urine (Pilz et al., 2019).
Vitamin D activation in skin is considerably affected by exposure of UV, age, 7-dehydrocholesterol reductase (DHCR7) polymorphism, and ethnicity. Other factors include CYP2R1 polymorphism, DBP, vitamin D receptor (VDR), body composition and diet (Holick, 2014; Bouillon et al., 2019). All above factors drastically regulate the vitamin D status in a gender related manner. Actually, a good relationship exist between status of vitamin D, sex and immunomodulation (Crescioli and Minisola, 2017). Current literature reported that the reference range of vitamin D in humans is between 30–100 ng/ml whereas the vitamin D insufficiency and deficiency correspond to levels < 30 ng/ml and <20 ng/ml respectively (Pilz et al., 2019; Bouillon et al., 2019; Cesareo et al., 2018). Vitamin D by binding to its receptor (VDR), regulates a plethora of genes. It has been reported that about 3 % of the human genome (> 200 genes) is directly or indirectly regulated by vitamin D (Christakos et al., 2016). This global role of vitamin D along with wide tissue distribution of VDR, indicated the extra renal regulation of vitamin D and was called as non-calcaemic effects of vitamin D.
Mentioned effects include anti-proliferative, pro-differentiating, induction of cancer cells apoptosis, increases insulin production, declines reactive oxygen species, regulates renin-angiotensin-aldosterone effects and numerous immune modulation effects including controlling immune activation on one side and enhancing anti‑infectious defence on the other (Christakos et al., 2016; Bouillon et al., 2019; Autier, 2016; Kumar et al., 2020; Himani et al., 2020a, 2020b). Importantly, these all effects were regulated by various cytokines and not by PTH as observed in calcaemic effects. Various pre-clinical and clinical trials have outlined the role of vitamin D in various health conditions with mixed results (Bouillon et al., 2019; Autier et al., 2017; Giustina et al., 2019).
4. Current evidences on the putative roles of vitamin D in the light of COVID-19 and other respiratory diseases
The original evidence of the role of vitamin D in immune defence was determined when Hansdottir et al. reported synthesis of vitamin D from human respiratory epithelial cells and that leads to induction of synthesis of cathelicidin, an antimicrobial peptide (Hansdottir et al., 2008). Similarly, Liu et al. and Heulens et al. in separate studies confirmed above finding in monocytes derived macrophages, thus consolidating the role of vitamin D in immune cells regulation (Liu et al., 2007; Heulens et al., 2016). Due to wide spread distribution of VDR and hydroxylating enzymes in variety of immune cells including dendritic cells (DCs), macrophages, natural killer cells and B-cells, plethora of research studies were done to unveil the role of vitamin D in immunity and diseases spanning both innate and adaptive immune system (Fig. 2).
Vitamin D deficiency is an important factor in the mitigation of the amplified and persistent inflammation, a cardinal feature of ARDS (Dancer et al., 2015; Parekh et al., 2013; Marik et al., 2020). In various diseases including influenza, respiratory syncytial virus infection (RSV), and tuberculosis, vitamin D deficiency has been a consistent factor (Berry et al., 2011; Cannell et al., 2006). A significant association has been observed between vitamin D deficiency and seasonal diseases like influenza during the winter periods (Ginde et al., 2009). Further Bergman et al. in a randomized controlled trial meta-analysis reported that prophylactic intake of vitamin D reduces the risk of respiratory tract infections (OR, 0.64; 95 %; CI, 0.49 to 0.84) (Bergman et al., 2013). Though, majority of the reports which have got positive outcome were either the association studies or they had been performed in in vitro environment. Till today, only single clinical trial in Japanese school children has been approved for the treatment of seasonal influenza A infection (Urashima et al., 2010).
In a retrospective study by Alipio in early 2020 involving data of 212 COVID-19 study subjects reported significant association between vitamin D deficiency and poor clinical outcome of these cases. He also reported that deficiency was highest in the most severe COVID-19 cases. Further, study demonstrated that for each increment in the standard deviation of 25(OH)D the probability of having mild clinical outcome rather than a severe outcome was 7.94 times while the odds of having mild clinical outcome than critical outcome was 19.61 times. Thus, it said that with increase in serum 25(OH)D levels, there was significant increase in clinical outcome of the body whereas decrease 25(OH)D levels could worsen the disease (Alipio, 2020). In another crucial study by Maghbooli et al. (2020) reported the association between serum 25(OH)D levels and clinical outcomes in 235 patients with COVID-19 infection. Study reported that after adjusting with confounding factor, a significant association was observed between 25(OH)D levels and disease severity, patients’ mortality, CRP levels and increased lymphocyte percentage. Further, vitamin D sufficiency was also associated with lower risk of unconsciousness and hypoxia. In addition to that, only 9.7 % patients succumbed to death who were vitamin D sufficient whereas mortality percentage was upto 20 % in those who had serum 25(OH)D levels <30 ng/ml. Thus, decreased levels of CRP and increased percentage lymphocyte in circulation indicates that vitamin D sufficiency might play important role in modulating the immune response of the infected patients (Maghbooli et al., 2020).
Vitamin D has been reported to mitigate the symptoms of common cold and influenza (Rondanelli et al., 2018). Further, it augments cell mediated immunity, regulates adaptive immunity, and increases expression of genes associated with antioxidants (Cantorna, 2010; Sharifi et al., 2019; Lei et al., 2017). It has been reported that nearly 40 % of the Europeans are deficient in the vitamin D, irrespective of their age, sex, skin textures and latitudes (Lips et al., 2019). Similarly, it has been seen that European countries (France; 27.3 %, Portugal; 21.2 % and Austria; 19.3 %) that were severely deficient in vitamin D exhibited significant cases of COVID-19 (Ilie et al., 2020; Garg et al., 2020; Brown and Sarkar, 2020). Further, countries (e.g. Norway, Finland, Sweden, Denmark and Netherlands; except for the United Kingdom; 23.7 %) lacking severely deficient vitamin D did not show COVID-19 cases, thus substantiating its potential in COVID-19 management (Kara et al., 2020).
Furthermore, few studies have highlighted that high doses of vitamin D may be given to COVID-19 patients especially with confirmed deficiency and also associated with comorbidities such as obese, elder, dark skin texture and those living in higher latitudes (Grant et al., 2020a). Additionally, due to its importance in protective effects in several diseases such as cardiovascular disease, diabetes, cancer, respiratory infections and hypertension, experts suggested that its supplementation and concomitant increased levels of 25(OH)D above 50 ng/ml might play important roles in mitigating the incidence and symptom of viral diseases including COVID-19 (Grant et al., 2020b). Therefore, several authors have emphasized the prophylactic use of vitamin D in the COVID-19 management (Wimalawansa, 2020; Grant et al., 2020c; Braiman, 2020). Further, in my knowledge, no clinical trial has been made till today to explore the potential of vitamin D in mitigating the effects of SARS-CoV-2 and ultimately COVID-19.
Due to widespread expression of VDR and enzyme CYP27B1 on majority immune cells, vitamin D has got extensive effects on immune cells (Baeke et al., 2010; Provvedini et al., 1983). The most common is the LL-37, an antimicrobial peptide residing in monocytes, B-cells, NK cells, epithelial cells, and γδ T-cells (Agerberth et al., 2000; Currie et al., 2013) and is also released by respiratory epithelial cells to form a first line of defense against various invading pathogens (Bals et al., 1998; Greiller and Martineau, 2015). Vitamin D in combination with VDR/RXR, binds to the promoter region of cathelicidin, and enhances hCAP-18 production (Yim et al., 2007), an innate mechanism to respiratory viruses. Similarly, vitamin D and VDR complex enhances the production of β-defensins which could activate chemotaxis and is shown to prevent RSV infection (Kota et al., 2008). In the innate counterpart, vitamin D activates monocyte differentiation into macrophage, activates PI3K signalling pathways for generation of ROS and iNOS oxidative pathway by monocyte and macrophage, an important antiviral mechanism (Sly et al., 2001). It also triggers autophagy, which is known to prevent severe immunopathology generally associated with viral infections (Wu et al., 2011). Additionally, vitamin D modulates the expression of PRRs including TLR2 and TLR4 in monocytes. This reduction in expression and consequent reduction in PAMPs mediated signalling is required to dampen the excessive inflammation caused due to our innate immune system (Baeke et al., 2010; Sadeghi et al., 2006).
Vitamin D demonstrates a very potent impact on the antigen presentation function of DCs by inhibiting their differentiation, maturation and antigen presentation along with decreased expression of specific molecules like MHC class II, CD1a, co-stimulatory molecules including CD40, CD80, & CD86 and chemotactic molecules such as CCL4 & CCL19 (Gauzzi et al., 2005). Activation of T cells by DCs is also hampered by reducing expression of co-stimulatory and MHC class II molecules, resulting in a tolerogenic phenotype. Further, vitamin D treatment also results in decreased production of IL-12 and IL-23 by DCs cells. Additionally, vitamin D by inhibiting secretion of IL-12 switches the immune axis from Th1 to Th2 phenotype (Lemire et al., 1995). In addition to that, enhanced production of IL-10 is observed after treatment with vitamin D, leading to development of regulatory T cells, thus these roles of vitamin D suggest benefits in attenuating the development of immunopathogenesis caused by some viral infections.
Lastly, vitamin D also showed direct effects on the fate of T and B cells, irrespective of antigen presenting cells (APC). They can directly alter the proliferation and cytokine profile of T cells i.e. inhibiting IFN-γ, TNF-α, IL-2, IL-17, and IL-21 cytokines and upregulating secretion of IL-4, IL-5 and IL-10 cytokines (Boonstra et al., 2001). A study reported that 1,25(OH)2D treatment of primary human tracheobronchial epithelial (hTBE) cells increased the expression of the IκBα (an NF-κB inhibitor) (Brockman-Schneider et al., 2014). Another study reported that both tocilizumab and vitamin D might controls cytokine storm occurred in COVID-19 by modulating IL-6 levels (Silberstein, 2020). Additionally, this treatment also reduces interferon stimulated genes (ISGs) levels along with other important components (IFN-β, CXCL10, STAT1, MxA and ISG15), viral replication and viral load, thus dampening the inflammatory response to RSV infection whilst maintaining the antiviral state. Similarly, in alveolar A549 cells infected with RSV, 1,25(OH)2D treatment increased the expression of IκBα protein and decreased the expression of phosphorylated STAT-1 along with lower mRNA levels of IRF1, IRF7, IFN-β and CXCL8 (Stoppelenburg et al., 2014).
5. Combination of dexamethasone and vitamin D may improve critically ill Covid-19 patients
ARDS features usually develops due to viral replication and hyperactive innate immune response leading to cytokine storm. This spurt of cytokines leads to massive inflammation, disseminated coagulation, shock and hypotension ultimately leading to multi organ failure and death. Since long, dexamethasone, a corticosteroid, has been established as a potent immuno suppressor, that acts by impairing the innate immune response. Further, it has also been seen that patients on long term dexamethasone therapy did not show any features related to severe or critical pneumonia in the presence of COVID-19 (Isidori et al., 2020).
The major drawback associated with dexamethasone therapy is the continual or exacerbation of viral replication and its progression that might worsen the condition or delay the recovery. Recently, a Randomized Evaluation of COVID-19 thERapY (RECOVERY) Trial performed in the University of Oxford, UK highlighted the importance of dexamethasone in the treatment of critically ill COVID-19 patients. Results showed 35 % decreased mortality rate in ventilated and 20 % decreased rate in patients on supplemental oxygen therapy. Dexamethasone did not show any difference in the recovery of patients with milder disease (Horby et al., 2020). Though majority of COVID-19 patients undergo asymptomatic disease, around 14 % patients progress to severe pneumonia and 5 % of the patients develop critical pneumonia. Similarly, another study done by Selvaraj et al. in only 21 COVID-19 patients reported that early and low dose of dexamethasone significantly improved the mortality outcome in COVID-19 patients with a sharp reduction in C-reactive protein levels and no escalation in the care related to mechanical ventilation (Selvaraj et al., 2020).
Two observational studies showed better clinical outcome in SARS-CoV-2 positive patients (Fadel et al., 2020; Wu et al., 2020). Further Villar et al. in a clinical trial over 277 COVID-19 patients reported early removal of ventilation and reduced mortality in patients treated with dexamethasone (Villar et al., 2020). In contrary, few studies from China have reported routine usage of dexamethasone but did not show significant benefits to COVID-19 patients (Wang et al., 2020b; Yang et al., 2020). Additionally, one study reported that dexamethasone stimulated the action of vitamin D levels by upregulating the transcription of VDR gene (Hidalgo et al., 2011). Also, dexamethasone decreases the calcitriol induced β-cathelicidin levels in human monocytes and bronchial epithelial cell line (Kulkarni et al., 2016).
In addition to above, combination of calcitriol and dexamethasone significantly modulates Erk and Akt signaling pathways leading to cell cycle arrest and apoptosis in cancer cells (Bernardi et al., 2001). Thus, it has been demonstrated that dexamethasone holds great promise for severely or critically ill COVID-19 patients but more research or trials are required to set up specific protocols for drug dosage and duration along with combination with other drugs to prevent anti-viral replication and propagation.
In conclusion, this review provides a brief information about the COVID-19 disease and its immune pathogenesis. Initial evidences involved in the mechanism opted by SARS-CoV-2 in the immune evasion were also discussed with few studies. Further, unavailability of potential drugs and their targets warrants intensive research in the repurposing of drugs. Strong association between vitamin D deficiency and severe viral infections including COVID-19 provided evidences of a simple solution to a complex problem like COVID-19. Additionally, potential role of vitamin D in modulating the immune response in viral infection further substantiated its importance in the current pandemic situation. In addition to above, potential role of dexamethasone has been highlighted as an anti-inflammatory drug in severe or critically ill COVID-19 patients. Lastly, large scale population based randomized controlled clinical trials are required to unveil the potential of vitamin D alone or in combination with dexamethasone in mitigating the viral infection is required.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This review article did not receive grant funding from any funding agencies in the public, commercial, or not-for-profit sectors or professional writing assistance.
References
- Agerberth B., Charo J., Werr J., Olsson B., Idali F., Lindbom L., Kiessling R., Jörnvall H., Wigzell H., Gudmundsson G.H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- Alipio M. Social Science Research Network; Rochester, NY: 2020. Vitamin D Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected with Coronavirus-2019 (COVID-19) [DOI] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Torre E., Della-Torre F., Marija Kusanovic P.C.P., Scotti R., Alvise-Ramirez G., Dagna L., Tresoldi M. Treating COVID-19 with colchicine in community healthcare setting. Clin. Immunol . 2020;217:108490. doi: 10.1016/j.clim.2020.108490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T., Alabdullatif Z.N., Assad M., Almulhim A., Makhdoom H., Madani H., Alhakeem R., Al-Tawfiq J.A., Cotten M., Watson S.J., Kellam P., Zumla A.I., Memish Z.A. Hospital outbreak of middle east respiratory syndrome coronavirus. N. Engl. J. Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier P. Vitamin D status as a synthetic biomarker of health status. Endocrine. 2016;51:201–202. doi: 10.1007/s12020-015-0837-x. [DOI] [PubMed] [Google Scholar]
- Autier P., Mullie P., Macacu A., Dragomir M., Boniol M., Coppens K., Pizot C., Boniol M. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986–1004. doi: 10.1016/S2213-8587(17)30357-1. [DOI] [PubMed] [Google Scholar]
- Baeke F., Takiishi T., Korf H., Gysemans C., Mathieu C. Vitamin D: modulator of the immune system. Curr. Opin. Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Báez-Santos Y.M., John S.E.St., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antivir. Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R., Wang X., Zasloff M., Wilson J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman P., Lindh Å.U., Björkhem-Bergman L., Lindh J.D. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8:e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi R.J., Trump D.L., Yu W.D., McGuire T.F., Hershberger P.A., Johnson C.S. Combination of 1alpha,25-dihydroxyvitamin D(3) with dexamethasone enhances cell cycle arrest and apoptosis: role of nuclear receptor cross-talk and Erk/Akt signaling. Clin. Cancer Res. 2001;7:4164–4173. [PubMed] [Google Scholar]
- Berry D.J., Hesketh K., Power C., Hyppönen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011;106:1433–1440. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- Boonstra A., Barrat F.J., Crain C., Heath V.L., Savelkoul H.F.J., O’Garra A. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4 +T cells to enhance the development of Th2 cells. J. Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- Bosch B.J., Martina B.E.E., van der Zee R., Lepault J., Haijema B.J., Versluis C., Heck A.J.R., de Groot R., Osterhaus A.D.M.E., Rottier P.J.M. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8455–8460. doi: 10.1073/pnas.0400576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R., Marcocci C., Carmeliet G., Bikle D., White J.H., Dawson-Hughes B., Lips P., Munns C.F., Lazaretti-Castro M., Giustina A., Bilezikian J. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr. Rev. 2019;40:1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. Latitude dependence of the COVID-19 mortality rate—a possible relationship to vitamin D deficiency? SSRN Electron. J. 2020 doi: 10.2139/ssrn.3561958. [DOI] [Google Scholar]
- Brockman-Schneider R.A., Pickles R.J., Gern J.E. Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication. PLoS One. 2014;9:e86755. doi: 10.1371/journal.pone.0086755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R., Sarkar A. Vitamin D deficiency: a factor in COVID-19, progression, severity and mortality?–An urgent call for research. MitoFit Preprint Arch. 2020 doi: 10.26124/mitofit:200001. [DOI] [Google Scholar]
- Cannell J.J., Vieth R., Umhau J.C., Holick M.F., Grant W.B., Madronich S., Garland C.F., Giovannucci E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M.T. Mechanisms underlying the effect of vitamin D on the immune system. Proc. Nutr. Soc. 2010;69:286–289. doi: 10.1017/S0029665110001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesareo R., Attanasio R., Caputo M., Castello R., Chiodini I., Falchetti A., Guglielmi R., Papini E., Santonati A., Scillitani A., Toscano V., Triggiani V., Vescini F., Zini M., on behalf of AME and Italian AACE Chapter Italian Association of Clinical Endocrinologists (AME) and Italian chapter of the American Association of Clinical Endocrinologists (AACE) position statement: clinical management of vitamin D deficiency in adults. Nutrients. 2018;10:546. doi: 10.3390/nu10050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S., Ajibade D.V., Dhawan P., Fechner A.J., Mady L.J. Vitamin D: metabolism. Endocrinol. Metab. Clin. N. Am. 2010;39:243–253. doi: 10.1016/j.ecl.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Cheng V., Hung I., Wong M., Chan K., Chan K., Kao R., Poon L., Wong C., Guan Y., Peiris J., Yuen K. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F., Fung K.S., Tang H.L., Yan W.W., Chan H.W.H., Lai T.S.T., Tong K.L., Lai K.N. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese doctors “using plasma therapy” on COVID-19 patients, CNA. (n.d.). https://www.channelnewsasia.com/news/asia/chinese-doctors-using-plasma-therapy-on-covid-19-patients-12444244 (accessed June 8, 2020).
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescioli C., Minisola S. Vitamin D: autoimmunity and Gender. Curr. Med. Chem. 2017;24 doi: 10.2174/0929867323666161220105821. [DOI] [PubMed] [Google Scholar]
- Currie S.M., Findlay E.G., McHugh B.J., Mackellar A., Man T., Macmillan D., Wang H., Fitch P.M., Schwarze J., Davidson D.J. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS One. 2013;8:e73659. doi: 10.1371/journal.pone.0073659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer R.C.A., Parekh D., Lax S., D’Souza V., Zheng S., Bassford C.R., Park D., Bartis D.G., Mahida R., Turner A.M., Sapey E., Wei W., Naidu B., Stewart P.M., Fraser W.D., Christopher K.B., Cooper M.S., Gao F., Sansom D.M., Martineau A.R., Perkins G.D., Thickett D.R. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70:617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca H.F. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014;3 doi: 10.1038/bonekey.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming D., Sheahan T., Heise M., Yount B., Davis N., Sims A., Suthar M., Harkema J., Whitmore A., Pickles R., West A., Donaldson E., Curtis K., Johnston R., Baric R. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3:e525. doi: 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel R., Morrison A.R., Vahia A., Smith Z.R., Chaudhry Z., Bhargava P., Miller J., Kenney R.M., Alangaden G., Ramesh M.S., Ford Henry. COVID-19 management task force, early short course corticosteroids in hospitalized patients with COVID-19. Clin. Infect. Dis. 2020:ciaa601. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink S.L., Cookson B.T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo D., Piantanida E., Badino P., Mortara L. The story of a vitamin for bone health that upgraded to hormone for systemic good health. Med. Hist. 2018;2:152–160. [Google Scholar]
- Garg M., Al-Ani A., Mitchell H., Hendy P., Christensen B. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North-supports vitamin D as a factor determining severity. Authors’ reply. Aliment. Pharmacol. Ther. 2020;51:1438–1439. doi: 10.1111/apt.15796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauzzi M.C., Purificato C., Donato K., Jin Y., Wang L., Daniel K.C., Maghazachi A.A., Belardelli F., Adorini L., Gessani S. Suppressive effect of 1α,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. J. Immunol. 2005;174:270–276. doi: 10.4049/jimmunol.174.1.270. [DOI] [PubMed] [Google Scholar]
- Ginde A.A., Mansbach J.M., Camargo C.A. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch. Intern. Med. 2009;169:384. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustina A., Adler R.A., Binkley N., Bouillon R., Ebeling P.R., Lazaretti-Castro M., Marcocci C., Rizzoli R., Sempos C.T., Bilezikian J.P. Controversies in vitamin D: summary statement from an international conference. J. Clin. Endocrinol. Metab. 2019;104:234–240. doi: 10.1210/jc.2018-01414. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E., Snijder E.J., Ziebuhr J. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- Grant W.B., Al Anouti F., Moukayed M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D3 supplementation can have important patient and public health benefits. Eur. J. Clin. Nutr. 2020;74:366–376. doi: 10.1038/s41430-020-0564-0. [DOI] [PubMed] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Vitamin D supplementation could prevent and treat influenza, coronavirus, and pneumonia infections. Med. Pharmacol. 2020 doi: 10.20944/preprints202003.0235.v1. [DOI] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiller C., Martineau A. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J., Liang Z., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S., Monick M.M., Hinde S.L., Lovan N., Look D.C., Hunninghake G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M.R., Jurutka P.W., Mizwicki M., Norman A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Heulens N., Korf H., Mathyssen C., Everaerts S., De Smidt E., Dooms C., Yserbyt J., Gysemans C., Gayan-Ramirez G., Mathieu C., Janssens W. 1,25-Dihydroxyvitamin D modulates antibacterial and inflammatory response in human cigarette smoke-exposed macrophages. PLoS One. 2016;11:e0160482. doi: 10.1371/journal.pone.0160482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A.A., Deeb K.K., Pike J.W., Johnson C.S., Trump D.L. Dexamethasone enhances 1α,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription. J. Biol. Chem. 2011;286:36228–36237. doi: 10.1074/jbc.M111.244061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himani, Kumar R., Ansari J.A., Mahdi A.A., Sharma D., Karunanand B., Datta S.K. Blood lead levels in occupationally exposed workers involved in battery factories of Delhi-NCR region: effect on vitamin D and calcium metabolism. Indian J. Clin. Biochem. 2020;35:80–87. doi: 10.1007/s12291-018-0797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himani, Kumar R., Karunanand B., Datta S.K. Association of vitamin D receptor (VDR) gene polymorphism with blood lead levels in occupationally lead exposed male battery workers in Delhi - NCR region. Indian J. of Biochem. Bio. 2020;57(2):236–244. doi: 10.1007/s12291-018-0797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F. Cancer, sunlight and vitamin D. J. Clin. Transl. Endocrinol. 2014;1:179–186. doi: 10.1016/j.jcte.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J., RECOVERY Collaborative Group Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. Infect. Dis. (except HIV/AIDS) 2020 doi: 10.1101/2020.06.22.20137273. [DOI] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim H., Perl A., Smith D., Lewis T., Kon Z., Goldenberg R., Yarta K., Staniloae C., Williams M. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin. Immunol. 2020;219:108544. doi: 10.1016/j.clim.2020.108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020 doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori A.M., Arnaldi G., Boscaro M., Falorni A., Giordano C., Giordano R., Pivonello R., Pofi R., Hasenmajer V., Venneri M.A., Sbardella E., Simeoli C., Scaroni C., Lenzi A. COVID-19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J. Endocrinol. Invest. 2020:1–7. doi: 10.1007/s40618-020-01266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janice Oh H.-L., Ken-En Gan S., Bertoletti A., Tan Y.-J. Understanding the T cell immune response in SARS coronavirus infection. Emerg. Microbes Infect. 2012;1:1–6. doi: 10.1038/emi.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis L.M. Drug firms mobilize to combat coronavirus outbreak. C EN. 2020;98:5. [Google Scholar]
- Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K., Yung C.F., Cui L., Tzer Pin Lin R., Mak T.M., Maiwald M., Li J., Chong C.Y., Nadua K., Tan N.W.H., Thoon K.C. A well infant with coronavirus disease 2019 with high viral load. Clin. Infect. Dis. 2020:ciaa201. doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara M., Ekiz T., Ricci V., Kara Ö., Chang K.-V., Özçakar L. ‘Scientific strabismus’ or two related pandemics: COVID-19 & vitamin D deficiency. Br. J. Nutr. 2020:1–20. doi: 10.1017/S0007114520001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota S., Sabbah A., Chang T.H., Harnack R., Xiang Y., Meng X., Bose S. Role of human β-defensin-2 during tumor necrosis factor-α/NF-κB-mediated innate antiviral response against human respiratory syncytial virus. J. Biol. Chem. 2008;283:22417–22429. doi: 10.1074/jbc.M710415200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni N.N., Gunnarsson H.I., Yi Z., Gudmundsdottir S., Sigurjonsson O.E., Agerberth B., Gudmundsson G.H. Glucocorticoid dexamethasone down-regulates basal and vitamin D3 induced cathelicidin expression in human monocytes and bronchial epithelial cell line. Immunobiology. 2016;221:245–252. doi: 10.1016/j.imbio.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Kumar R., Himani, Gupta N., Singh V., Kumar V., Haq A., Mirza A.A., Sharma A. Unveiling molecular associations of polymorphic variants of VDR gene (FokI, BsmI and ApaI) in multiple myeloma patients of Indian population. J. Steroid Biochem. Mol. Biol. 2020;199:105588. doi: 10.1016/j.jsbmb.2020.105588. [DOI] [PubMed] [Google Scholar]
- Lei G.-S., Zhang C., Cheng B.-H., Lee C.-H. Mechanisms of action of vitamin D as supplemental therapy for pneumocystis pneumonia. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.01226-17. e01226-17, e01226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J.M., Archer D.C., Beck L., Spiegelberg H.L. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J. Nutr. 1995;125:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- Li X.Y., Du B., Wang Y.S., Kang H.Y.J., Wang F., Sun B., Qiu H.B., Tong Z.H. [The keypoints in treatment of the critical coronavirus disease 2019 patient(2)], Zhonghua Jie He He Hu Xi Za Zhi Zhonghua Jiehe He Huxi Zazhi Chin. J. Tuberc. Respir. Dis. 2020;43:277–281. doi: 10.3760/cma.j.cn112147-20200224-00159. [DOI] [PubMed] [Google Scholar]
- Lips P., Cashman K.D., Lamberg-Allardt C., Bischoff-Ferrari H.A., Obermayer-Pietsch B., Bianchi M.L., Stepan J., El-Hajj Fuleihan G., Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019:P23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- Liu S., Xiao G., Chen Y., He Y., Niu J., Escalante C.R., Xiong H., Farmar J., Debnath A.K., Tien P., Jiang S. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.T., Stenger S., Tang D.H., Modlin R.L. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosisIs dependent on the induction of cathelicidin. J. Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Maghbooli Z., Sahraian M.A., Ebrahimi M., Pazoki M., Kafan S., Tabriz H.M., Hadadi A., Montazeri M., Nasiri M., Shirvani A., Holick M.F. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One. 2020;15:e0239799. doi: 10.1371/journal.pone.0239799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Marik P.E., Kory P., Varon J. Does vitamin D status impact mortality from SARS-CoV-2 infection? Med. Drug Discov. 2020;6:100041. doi: 10.1016/j.medidd.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ortega A., Bernal-Bello D., Llarena-Barroso C., Frutos-Pérez B., Duarte-Millán Á M., de Viedma-García V.G., Farfán-Sedano A.I., Canalejo-Castrillero E., Ruiz-Giardín J.M., Ruiz-Ruiz J., San Martín-López J.V. Imatinib for COVID-19: a case report. Clin. Immunol. 2020;218:108518. doi: 10.1016/j.clim.2020.108518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh D., Thickett D., Turner A. Vitamin D deficiency and acute lung injury. Inflamm. Allergy-Drug Targets. 2013;12:253–261. doi: 10.2174/18715281113129990049. [DOI] [PubMed] [Google Scholar]
- Park W.B., Kwon N.-J., Choi S.-J., Kang C.K., Choe P.G., Kim J.Y., Yun J., Lee G.-W., Seong M.-W., Kim N.J., Seo J.-S., Oh M. Virus isolation from the first patient with SARS-CoV-2 in Korea. J. Korean Med. Sci. 2020;35:e84. doi: 10.3346/jkms.2020.35.e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S., Zittermann A., Trummer C., Theiler-Schwetz V., Lerchbaum E., Keppel M.H., Grübler M.R., März W., Pandis M. Vitamin D testing and treatment: a narrative review of current evidence. Endocr. Connect. 2019:R27–R43. doi: 10.1530/EC-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pludowski P., Holick M.F., Grant W.B., Konstantynowicz J., Mascarenhas M.R., Haq A., Povoroznyuk V., Balatska N., Barbosa A.P., Karonova T., Rudenka E., Misiorowski W., Zakharova I., Rudenka A., Łukaszkiewicz J., Marcinowska-Suchowierska E., Łaszcz N., Abramowicz P., Bhattoa H.P., Wimalawansa S.J. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018;175:125–135. doi: 10.1016/j.jsbmb.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Provvedini D., Tsoukas C., Deftos L., Manolagas S. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.-S. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020:ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanelli M., Miccono A., Lamburghini S., Avanzato I., Riva A., Allegrini P., Faliva M.A., Peroni G., Nichetti M., Perna S. Self-care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and Echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds—practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid. Based Complement. Altern. Med. 2018;2018:1–36. doi: 10.1155/2018/5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi K., Wessner B., Laggner U., Ploder M., Tamandl D., Friedl J., Zügel U., Steinmeyer A., Pollak A., Roth E., Boltz-Nitulescu G., Spittler A. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- Satyam A., Tsokos G.C. Curb complement to cure COVID-19. Clin. Immunol. 2020;221:108603. doi: 10.1016/j.clim.2020.108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V., Dapaah-Afriyie K., Finn A., Flanigan T.P. Short-term dexamethasone in Sars-CoV-2 patients. R. I. Med. J. 2020;103:39–43. 2013. [PubMed] [Google Scholar]
- Sharifi A., Vahedi H., Nedjat S., Rafiei H., Hosseinzadeh‐Attar M.J. Effect of single‐dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo‐controlled trial. APMIS. 2019;127:681–687. doi: 10.1111/apm.12982. [DOI] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein M. Vitamin D: a simpler alternative to tocilizumab for trial in COVID-19? Med. Hypotheses. 2020;140:109767. doi: 10.1016/j.mehy.2020.109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu K.-L., Chan C.-P., Kok K.-H., Chiu-Yat Woo P., Jin D.-Y. Suppression of innate antiviral response by severe acute respiratory syndrome coronavirus M protein is mediated through the first transmembrane domain. Cell. Mol. Immunol. 2014;11:141–149. doi: 10.1038/cmi.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly L.M., Lopez M., Nauseef W.M., Reiner N.E. 1α,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J. Biol. Chem. 2001;276:35482–35493. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Zhu H., Zhao W., Han Y., Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelenburg A.J., von Hegedus J.H., Huis in’t Veld R., Bont L., Boes M. Defective control of vitamin D receptor-mediated epithelial STAT1 signalling predisposes to severe respiratory syncytial virus bronchiolitis: vitamin D receptor and RSV infection. J. Pathol. 2014;232:57–64. doi: 10.1002/path.4267. [DOI] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D.A.J., Bynoe M.L. Cultivation of a novel type of common-cold virus in organ cultures. BMJ. 1965;1:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urashima M., Segawa T., Okazaki M., Kurihara M., Wada Y., Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- Versteeg G.A., Bredenbeek P.J., van den Worm S.H.E., Spaan W.J.M. Group 2 coronaviruses prevent immediate early interferon induction by protection of viral RNA from host cell recognition. Virology. 2007;361:18–26. doi: 10.1016/j.virol.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., Aguilar G., Alba F., González-Higueras E., Conesa L.A., Martín-Rodríguez C., Díaz-Domínguez F.J., Serna-Grande P., Rivas R., Ferreres J., Belda J., Capilla L., Tallet A., Añón J.M., Fernández R.L., González-Martín J.M., Aguilar G., Alba F., Álvarez J., Ambrós A., Añón J.M., Asensio M.J., Belda J., Blanco J., Blasco M., Cachafeiro L., del Campo R., Capilla L., Carbonell J.A., Carbonell N., Cariñena A., Carriedo D., Chico M., Conesa L.A., Corpas R., Cuervo J., Díaz-Domínguez F.J., Domínguez-Antelo C., Fernández L., Fernández R.L., Ferrando C., Ferreres J., Gamboa E., González-Higueras E., González-Luengo R.I., González-Martín J.M., Martínez D., Martín-Rodríguez C., Muñoz T., Ortiz Díaz-Miguel R., Pérez-González R., Prieto A.M., Prieto I., Rivas R., Rojas-Viguera L., Romera M.A., Sánchez-Ballesteros J., Segura J.M., Serna-Grande P., Serrano A., Solano R., Soler J.A., Soro M., Tallet A., Villar J. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalawansa S.J. Global epidemic of coronavirus—Covid-19: what can we do to minimize risks. European J. Biomedical. 2020;7(3):432–438. [Google Scholar]
- Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Lit L.C.W., Hui D.S.C., Chan M.H.M., Chung S.S.C., Sung J.J.Y. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Coronavirus Disease (COVID-19) Situation Reports 114.https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125 [Google Scholar]
- Wu S., Sun J., Vitamin D., Vitamin D. Receptor, and macroautophagy in inflammation and infection. Discov. Med. 2011;11:325–335. [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: expression and functional characterization. Biochem. Biophys. Res. Commun. 2003;312:1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinhua, 2020. China puts 245 COVID-19 patients on convalescent plasma therapy.
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. S2213260020300795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui F., Kai C., Kitabatake M., Inoue S., Yoneda M., Yokochi S., Kase R., Sekiguchi S., Morita K., Hishima T., Suzuki H., Karamatsu K., Yasutomi Y., Shida H., Kidokoro M., Mizuno K., Matsushima K., Kohara M. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J. Immunol. 2008;181:6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- Yim S., Dhawan P., Ragunath C., Christakos S., Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D3. J. Cyst. Fibros. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia: MERS, SARS and coronaviruses. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhou X., Qiu Y., Feng F., Feng J., Jia Y., Zhu H., Hu K., Liu J., Liu Z., Wang S., Gong Y., Zhou C., Zhu T., Cheng Y., Liu Z., Deng H., Tao F., Ren Y., Cheng B., Gao L., Wu X., Yu L., Huang Z., Mao Z., Song Q., Zhu B., Wang J. Clinical characteristics of 82 death cases with COVID-19. Infect. Dis. (except HIV/AIDS) 2020 doi: 10.1101/2020.02.26.20028191. [DOI] [Google Scholar]
- Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M., Zhang S., Cao T., Yang C., Li M., Guo G., Chen X., Chen Y., Lei M., Liu H., Zhao J., Peng P., Wang C.-Y., Du R. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N., Zheng B., Li Y., Poon L., Xie Z., Chan K., Li P., Tan S., Chang Q., Xie J., Liu X., Xu J., Li D., Yuen K., Peiris J., Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Sci. Rev. 2020:nwaa041. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]