Abstract
Background
In early 2020, the coronavirus disease 2019 (COVID-19) pandemic outbreak has posed the risk of critical care resources overload in every affected country. Collective interhospital transport of critically ill COVID-19 patients as a way to mitigate the localised pressure from overloaded intensive care units at a national or international level has not been reported yet. The aim of this study was to provide descriptive data about the first six collective aeromedical evacuation (MEDEVAC) of COVID-19 patients performed within Europe.
Methods
This retrospective study included all adult patients transported by the first six collective MEDEVAC missions for COVID-19 patients performed within Europe on the 18th, 21st, 24th, 27th, 31st of March and the 3rd of April 2020.
Results
Thirty-six patients with acute respiratory distress syndrome (ARDS) were transported aboard six MEDEVAC missions. The median duration of mechanical ventilation in ICU before transportation was 4 days (3−5.25). The median PaO2/FiO2 ratio obtained before, during the flight and at day 1 after the transport was 180 mmHg (156–202,5), 143 mmHg (118,75–184,75) and 174 mmHg (129,5–205,5), respectively, with no significant difference. The median norepinephrine infusion rate observed before, during the flight and at day 1 after the transport was 0,08 µg/kg-1. min-1 (0,00-0,20), 0,08 (0,00-0,25), and 0,07 (0,03-0,18), respectively, with no significant difference. No life-threatening event was reported.
Conclusion
Collective aero-MEDEVAC of COVID-19 critically ill patients could provide a reliable solution to help control the burden of the disease at a national or international level.
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ICU, intensive care unit; MEDEVAC, medical evacuation; MoRPHEE, Module de Réanimation pour Patient à Haute Elongation d’Evacuation; PEEP, positive end-expiratory pressure; PBW, predicted body weight; SARS-CoV-2, severe acute respiratory syndrome coronavirus; SOFA, sequential organ failure assessment
Keywords: Acute respiratory distress syndrome, Aeromedical evacuation, Interhospital transport, COVID-19, Mechanical ventilation
Introduction
Caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus disease 2019 (COVID-19) outbreak became established in France, as in much of mainland Europe, in early March 2020 [1]. As in other countries, the distribution of the disease among the territories was heterogeneous and most cases were initially located in the Grand Est French region where a sudden rise of intensive care unit admissions was observed [1]. A specific risk during the pandemic was the risk of saturation of some local facilities, especially regarding critical care facilities.
Hence, the French Government made the decision to transport ICU patients with SARS-CoV-2 from the Grand Est region to other areas with ICU availability. The French Air Force and the French Military Health Service were engaged to organise and deploy their collective airborne medical evacuation (MEDEVAC) service, an Airbus A330 Multi-Role Tanker Transport plane equipped with the MoRPHEE (Module de Réanimation pour Patient à Haute Elongation d’Evacuation) system, which transforms the plane into an ICU for the long-distance transport of critically ill patients [2]. Aboard six flights, the French Ministry of the Armed Forces has carried out the medical transportation of 36 critically ill patients from hospitals in the north east of France. During the same period, more than 600 patients have been transported by road, train or helicopter in France.
Medical transportation of critically ill has been considered in case of pandemics or disaster but to date, no pandemic had imposed to resort to this mean [3]. Moreover, interhospital transport of critically ill patients is a known high-risk period [4], [5]. The transport of mechanically ventilated patients suffering from acute respiratory distress syndrome (ARDS) has been regularly reported, however always on an individual basis [6], [7], [8]. Little is known about the specific challenges and risks of the transport of multiple victims with ARDS, especially in a pandemic context.
The aim of this study was to provide descriptive data about the first six MEDEVAC missions of SARS-CoV-2 patients that were performed within Europe.
Methods
Study design
This retrospective cohort study included all of the adult patients transported by the first six airborne MoRPHEE MEDEVAC missions for SARS-CoV-2 patients performed within French national territory on the 18th, 21st, 24th, 27th, 31st of March and the 3rd of April 2020.
The study was approved by the Ethics Committee of the French Society of Anaesthesia and Intensive Care and was registered with the French Data Protection Authority (Commission Nationale d’Informatique et Libertés, CNIL) under the number MR 0509270320.
Participants
The transported patients were selected the day before the flight in coordination with their ICU doctors and regional health agencies. Among the patients requiring invasive mechanical ventilation, several simple criteria were used to select those who would be most suitable for transport: confirmed SARS-CoV-2 pneumonia, body-weight < 120 kg, PaO2/FiO2 ratio > 120 mmHg, no ongoing prone position ventilation, and moderate infusion rates of catecholamine (norepinephrine infusion rate < 0.5 μg.kg−1. min−1)
Exclusion criteria were: age < 18 years, incapability, consent withdrawal after written information was provided to the patient or relatives.
MEDEVAC procedures
Collective, strategic MoRPHEE MEDEVACs were originally conceived by the French Air Force and the French Military Health Service to repatriate severely injured war casualties. The MoRPHEE system provides a “flying ICU” facility that also complies with international aviation security regulations [9]. The system is based on mission-tailored ‘plug-and-play’ modules that can be installed into non-dedicated aircraft, in this case, an Airbus A330, to allow the repatriation of up to six critically ill patients at a time over long distances. Onboard facilities included mechanical ventilation with the transport ventilator LTV 1200 (CareFusion, Yorba Linda, CA), continuous monitoring (Welch Allyn Propaq CS), drug infusion, echography (M-Turbo; Sonosite, Bothell, WA), and delocalised biology [2]. The intensive care modules were set as illustrated in Fig. 1 . The medical crew, specifically trained to perform MEDEVACs, was composed of three ICU physicians, two flight surgeons, three anaesthetist nurses, three flight nurses, and two critical care nurses [9]. Due to the high risk of contagion posed by the COVID-19 disease, the teams were reinforced by four experts in the management of biological risk from two specialised units of the French Military Health Service, and the French Air Force. The specific management of the biological risk associated with COVID-19 during flights will be discussed in another publication. Due to regulatory reasons, a three-day interval was imposed between the MEDEVACs.
Fig. 1.
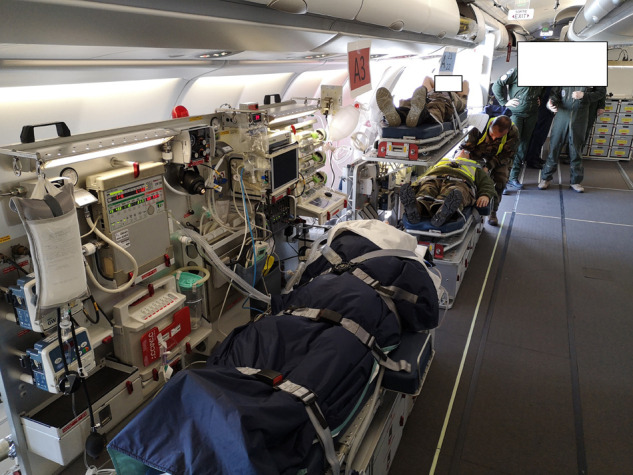
Intensive Care Module of the MoRPHEE system (Crédit photo: Ministère des Armées).
The patients’ current healthcare teams were encouraged to maintain any ongoing sedation, to induce muscle relaxation with long acting muscle relaxant, and to use closed-suction devices in order to limit the risk of tracheal tube disconnection and cabin contamination. A protective ventilation strategy using a low tidal volume and high positive end-expiratory pressure (PEEP) was established in order to prevent alveolar collapse, hypoxemia, and to minimise the risk of ventilator-induced lung injury.
Data collection
Data were extracted from the medical records using a standardised data collection form and from the missions’ detailed reportings. Demographics, chronic disease states, biologics and norepinephrine requirements were obtained during the doc-to-doc call the day before the transport. Onboard ventilator settings, acute physiologic data and arterial blood gas analysis were recorded after a stabilisation period of 10 min after the take-off. Follow-up was done in collaboration with receiving units.
The Charlson comorbidity index [10] and the Sequential Organ Failure Assessment (SOFA) score [11] were calculated for each patient before transportation. ARDS was defined and graded according to the Berlin definition [12]. Predicted body weight (PBW) was calculated according to the ARDS Network predicted body weight calculator [13]. The Richmond Agitation-Sedation scale was used to assess each patient [14].
Main measures
In order to estimate potential adverse effects of the transport, we recorded and compared the PaO2/FiO2 ratio as well as norepinephrine requirement before, during and the day after the transport. Onboard events requiring medical intervention were identified from continuous monitoring and medical records. Life-threatening events (e.g., cardiac arrest, refractory hypoxemia or hypotension, accidental extubation) were also recorded.
Statistical analysis
Descriptive statistics were provided. Continuous and categorical variables were respectively presented as median (1st-3rd quartiles) and n (%). Given the small sample size, the PaO2/FiO2 ratio and norepinephrine infusion rates before, during and the day after the transport were compared with a non-parametric test (Friedman test) performed with GraphPad Prism 7 (GraphPad Software, USA).
Results
Thirty-six patients transported aboard six MEDEVAC missions performed on the 18th, 21st, 24th, 27th, 31st of March and the 3rd of April 2020 were included in the study. The patients were evacuated from four referring hospitals located in Mulhouse (n = 16), Metz (n = 10), Colmar (n = 6), Thionville (n = 2) in the Grand Est region into nine receiving hospitals located in Bordeaux (n = 12), Toulouse (n = 6), Brest (n = 4), Marseille (n = 3), Toulon (n = 3), Quimper (n = 2), France, and Kiel (n = 3) and Lubeck (n = 3), Germany. Median patient age was 64 years (58−72), and the age range was from 49 to 78 years. The male-to-female sex ratio was 2. The three most frequent comorbidities were hypertension (n = 18 (50%)), obesity (n = 17 (47%)) and diabetes (n = 13 (36%)) and the median Charlson comorbidity index was 1 (0-1,25).
All patients fulfilled the criteria of ARDS according to the Berlin definition and required mechanical ventilation. ARDS was moderate (PaO2/FiO2 ratio > 100 and ≤ 200) in 24 patients and mild (PaO2/FiO2 ratio > 200 and ≤ 300) in 12 patients. The median duration of mechanical ventilation in ICU before transportation was 4 days (3-5.25). One patient was weaned from veno-venous extracorporeal membrane oxygenation two days before her transportation. Baseline characteristics of all patients are shown in Table 1 as well as organ failures recorded before transportation and onboard ventilatory settings. All patients were placed in a semi-recumbent position and received a neuromuscular blockade and protective ventilation. Tidal volume was 6.5 mL.kg−1 (PBW) (6.2−7.0) and PEEP was 13 cmH20 (12−14).
Table 1.
Patients’ characteristics, organ failures recorded before transportation and onboard ventilatory settings.
| Baseline characteristics | n = 36 |
|---|---|
| Age, years, median (1st-3rd quartiles) | 64 (58−72) |
| Male gender, n (%) | 24 (67) |
| Comorbidities | |
| Diabetes, n (%) | 13 (36) |
| Hypertension, n (%) | 18 (50) |
| Obesity, n (%) | 17 (47) |
| Body Mass Index, kg.m−², median (1st - 3rd quartiles) | 29 (26−32,25) |
| Respiratory and associated organ failures, before transport | |
| ARDS severity | |
| Mild, n (%) | 12 (33) |
| Moderate, n (%) | 24 (67) |
| Severe, n (%) | 0 (0) |
| Previous prone positioning, n (%) | 5 (14) |
| Time from intubation to transportation, days, median (1st-3rd quartiles) | 4 (3−5,25) |
| Hemodynamic instability, n (%) | 21(58) |
| SOFA score, median (1st - 3rd quartiles) | 6 (3,5−7) |
| On-board ventilatory settings | |
| Volume control ventilation, n (%) | 35 (97) |
| Pressure control ventilation, n (%) | 1 (3) |
| Vt / PBW (mL. kg−1), median (1st-3rd quartiles) | 6,5 (6,2−7) |
| Respiratory Rate (. min−1), median (1st-3rd quartiles) | 25 (22−26) |
| FiO2, %, median (1st-3rd quartiles) | 60 (50−65,5) |
| PEEP, cmH20, median (1st-3rd quartiles) | 13 (12−14) |
ARDS severity was defined and graded according to the Berlin’s definition. Haemodynamic instability was defined as the need for vasopressor support. SOFA score was assessed from the closest available data before the transport. Onboard ventilator settings were recorded after a 10 min stabilisation period during the flight.
ARDS: Acute Respiratory Distress Syndrome.
SOFA score: Sequential Organ Failure Assessment score.
Vt/PBW: Tidal Volume/Predicted Body Weight.
FiO2: Inspired fraction of oxygen.
PEEP: Positive End Expiratory Pressure.
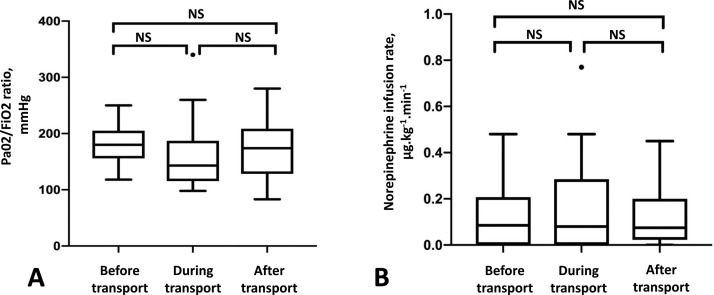
Arterial blood gas analysis data were obtained from arterial lines in 29 patients. The PaO2/FiO2 ratio was 180 mmHg (156-202,5) before transport, 143 mmHg (118,75-184,75) during the flight, and 174 mmHg (129,5-205,5) at day 1 after the transport; there was no significant difference between these values (p = 0.99), as reported in Fig. 2 , panel A. The median arterial to end-tidal CO2 tension difference during flight was 15,5 (11-19) mmHg. Twenty-three (64%) of the 36 patients had haemodynamic instability that required norepinephrine infusion during the flight; the median rates of norepinephrine infusion pre, per and post transport did not differ (p = 0.53), as reported in Fig. 2, panel B.
Fig. 2.
Pre, per and post transport evolution of PaO2/FiO2 ration and norepinephrine infusion rate.
Panel A shows box plots for PaO2/FiO2 ratio (mmHg) measured successively in the referring intensive care unit on the day before transport (Before transport), during the aeromedical transport after a stabilisation period of 10 min after the take-off (During transport), and at day 1 after transport in the receiving unit (After transport). Boxes show median and quartiles, whiskers show 5th and 95th percentiles, dots are outliers.
Panel B shows box plots for norepinephrine infusion rate (µg.kg-1.min-1) measured successively in the referring intensive care unit on the day before transport (Before transport), during the aeromedical transport after a stabilisation period of 10 min after the take-off (During transport), and at day 1 after transport in the receiving unit (After transport). Boxes show median and quartiles, whiskers show 5th and 95th percentiles, dots are outliers.
Thirty-seven events occurred during the flights that required medical intervention in 23 patients. All were promptly controlled by the onboard medical team. No cardiac arrests or accidental tracheal extubation were observed. The Table 2 summarises the events that required medical intervention during flight. Cardiovascular events often occurred during the early phase of the transportation, often before the take-off. They were typically related to the changeover of norepinephrine infusion pumps. When receiving the patients, we encountered an unexpected large variety of perfusion assemblies: various concentrations of catecholamine in the syringe, association of the catecholamine with other perfusions used as carriers to accelerate the flow in the distal part of the catheter, etc. The number of infusion pumps onboard was limited to three of them; hence, we often had to modify the set-up of the catecholamine infusion. Among four equipment-related events, three were due to a vascular line obstruction and one was related to the dysfunction of the end-tidal CO2 monitoring line.
Table 2.
Number of events requiring a medical intervention.
| Cardiovascular | |
| Mean arterial pressure < 55 mmHg | 7 |
| Mean arterial pressure < 55 mmHg over at least 5 min | 2 |
| Mean arterial pressure > 110 mmHg | 3 |
| Mean arterial pressure > 110 mmHg over at least 5 min | 1 |
| Bradycardia < 50 bpm | 1 |
| Respiratory | |
| Patient-ventilator asynchrony, including cough | 8 |
| Patient disconnexion | 5 |
| SpO2 < 90 % over at least 5 min | 2 |
| Other | |
| Equipment related | 4 |
| Richmond Agitation Sedation Scale > 0 | 4 |
Thirty-seven events occurred in 23 among 36 transported patients.
The median flight duration was 71,5 min (64-74,5) for a median distance between airports of 800 km (717-830). The total duration of onboard transport (i.e., from boarding to disembarking) was 185 min (145-198,5). Total oxygen consumption was 1650 L.patient-1 (1350-1950) per flight and 564 L.patient-1. h-1 (482-675).
Discussion
We have here reported the transport of 36 COVID-19 ARDS patients during 6 collective aero MEDEVAC operations. All the patients could be transported from overloaded ICUs in the Grand Est region to 9 other hospitals in France and Germany where the ICU availability was greater. To our knowledge, this is the first experience of collective aero-MEDEVAC of patients with ARDS. Our study has outlined the selection criteria that we used to select patients transport and their management on board. We do not report any life-threatening event or significant respiratory or haemodynamic aggravation during flight.
Comorbidities as assessed by Charlson comorbidities index, obesity, or diabetes were frequent in our population, which is consistent with other work on COVID-19 ARDS [15] or ICU [16] patients. Also in agreement with previous other published data in COVID-19 patients, half of our patients were aged over 64 years, and a majority of our study population was male [15], [16], [17], [18]. On the other hand, it is also important to note that the patients were carefully selected for MEDEVAC, based on pre-established criteria. As a consequence, all of them presented with ARDS and were under invasive mechanical ventilation when they boarded the flight, but no one suffered from severe ARDS or required high-dose of norepinephrine.
Transportations between medical facilities are well-known periods of risk [4], [5]. Of note, we did not identify an aggravation of the PaO2/FiO2 ratio or an increase of the norepinephrine requirements throughout the follow-up of patients. These data have been monitored by other authors during interhospital transport of ARDS critically ill patients [19]. They appear as a good approach to assess the potential adverse effects of interhospital transport, even if they cannot provide certain information on long-term outcomes of these patients.
Before these flights, the MEDEVAC crew had both received training about and had experience with strategies to minimise the occurrence of adverse events, such as checklists. Despite this, most patients still presented with an event that required medical intervention during the flight. All were promptly managed and none was life-threatening. The frequency of events was higher than previously reported [7], [19]. Some authors observed similar rate of events during intrahospital transport of critically ill patients [20]. Overall, the proportion of respiratory events was lower than expected, whereas we faced many cardiovascular events, mainly related to the changeover of norepinephrine infusion pumps. A standardisation of the infusion set-up with no more than three infusion pumps might have helped limiting these events, but in the very complicated situation that our colleagues were facing in the Grand Est region, we chose not to surimpose external constraints. The relative ease of patient management and the stability of clinical symptoms during the MEDEVAC, however, strongly support the feasibility and practical implementation of such evacuations, both in France and elsewhere. In summary, although no intervention of this scale can be without risk, we do believe that the in-flight events that our patients encountered did not outweigh the benefits of the evacuation, which should be assessed both at an individual and collective point of view.
Our study has some limitations. First, it was a retrospective study and thus vulnerable to the inherent limitations of such study design, especially the risk of bias due to the small patient population and small dataset. Secondly, patients were selected before the transportation and our results may not be reproducible with other selection criteria. Third, we chose to design the study in order to describe the patient management during the flight and we limited the follow-up of subsequent outcomes at day 1 after the transport. The data collection of subsequent aspects is still in progress, and the report will be of great interest, but we chose to rapidly share our experience with colleagues. Fourth, these results are not directly replicable and require a specific training program of the MEDEVAC team. Finally, the heterogeneous distribution of both the disease and the strain on ICU facilities was patent in France but this may not be reproducible in other countries or continents. However, the strain of the disease on critical care resources reveals to be critical all over the world.
Conclusion
This is the first report about collective MEDEVAC of patients with ARDS in an epidemic context. We hope that this information will be useful to those colleagues around the world who need to organise similar evacuations as the COVID-19 pandemic progresses, especially as we encountered neither life-threatening events nor significant respiratory or haemodynamic exacerbations during the flight. In a pandemic context, collective aero-MEDEVAC of ARDS patients could provide a possible, reliable solution to help mitigate the localised pressure from overloaded ICUs at a national or international level.
Authors’ contributions
JT, MBo contributed to the conception and design of the study, data analysis, drafting the manuscript. MBo obtained authorisations. All authors contributed to the data collection, revision of the manuscript, approval of the manuscript in its final form.
Funding
This study had no external funding source.
Ethical statement
The study was approved by the Ethics Committee of the French Society of Anaesthesia and Intensive Care and was registered with the French Data Protection Authority (Commission Nationale d’Informatique et Libertés, CNIL) under the number MR 0509270320.
Conflicts of interest
All the authors state that there are no conflicts of interest related to this study.
Acknowledgments
The authors acknowledge the patients, all the health professionals who participated to the management of the patients before, during and after the aeromedical evacuations, and all the colleagues from French Air Force and French Military Health Service without whom these collective MEDEVACs could not be performed.
References
- 1.Government of the French Republic. Map and data. Available from https://www.gouvernement.fr/info-coronavirus/carte-et-donnees. Accessed 28 March 2020.
- 2.Borne M., Tourtier J.P., Ramsang S., Grasser L., Pats B. Collective air medical evacuation: the French tool. Air Med J. 2012;31:124–128. doi: 10.1016/j.amj.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 3.King M.A., Niven A.S., Beninati W., Fang R., Einav S., Rubinson L., et al. Evacuation of the ICU: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146:e44S–60S. doi: 10.1378/chest.14-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strauch U., Bergmans D.C., Winkens B., Roekaerts P.M. Short-term outcomes and mortality after interhospital intensive care transportation: an observational prospective cohort study of 368 consecutive transports with a mobile intensive care unit. BMJ Open. 2015;28 doi: 10.1136/bmjopen-2014-006801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur K.R., Kelz R.R., Mills A.M., Reinke C.E., Robertson M.P., Sims C.A., et al. Interhospital transfer: an independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013;79:909–913. doi: 10.1177/000313481307900929. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann M., Bein T., Philipp A., Ittner K., Foltan M., Drescher J., et al. Interhospital transportation of patients with severe lung failure on pumpless extracorporeal lung assist. Br J Anaesth. 2006;96:63–66. doi: 10.1093/bja/aei274. [DOI] [PubMed] [Google Scholar]
- 7.Blecha S., Dodoo-Schittko F., Brandstetter S., Brandl M., Dittmar M., Graf B.M., et al. Quality of inter-hospital transportation in 431 transport survivor patients suffering from acute respiratory distress syndrome referred to specialist centers. Ann Intensive Care. 2018;15(8):5. doi: 10.1186/s13613-018-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt J., Boutonnet M., Goutorbe P., Raynaud L., Carfantan C., Luft A., et al. Acute Respiratory Distress Syndrome in the forward environment. Retrospective analysis of ARDS cases among French Army war casualties. J Trauma Acute Care Surg. 2020 doi: 10.1097/TA.0000000000002633. [DOI] [PubMed] [Google Scholar]
- 9.Boutonnet M., Pasquier P., Raynaud L., Vitiello L., Bancarel J., Coste S., et al. Ten years of en route critical care training. Air Med J. 2017;36(2):62–66. doi: 10.1016/j.amj.2016.12.004. Epub 2017 Feb 24. [DOI] [PubMed] [Google Scholar]
- 10.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A, Bruining H., et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 12.ARDS Definition Task Force, Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 13.ARDS Network Tools. Available from http://www.ardsnet.org/tools.shtml Accessed 28 March 2020.
- 14.Ely E.W., Truman B., Shintani A., Thomason J.W., Wheeler A.P., Gordon S., et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 15.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020:e200994. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauch U., Bergmans D.C., Winkens B., Roekaerts P.M. Short-term outcomes and mortality after interhospital intensive care transportation: an observational prospective cohort study of 368 consecutive transports with a mobile intensive care unit. BMJ Open. 2015;5(Apr 28(4)):e006801. doi: 10.1136/bmjopen-2014-006801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmentier-Decrucq E., Poissy J., Favory R., Nseir S., Onimus T., Guerry M.J., et al. Adverse events during intrahospital transport of critically ill patients: incidence and risk factors. Ann Intensive Care. 2013;3:10. doi: 10.1186/2110-5820-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]