Abstract
Palmatine is a naturally occurring isoquinoline alkaloid with various pharmacological properties. Given its antioxidant and anti-inflammatory properties, palmatine may be able to impede the effects of metabolic syndrome (MetS) and its related diseases triggered by inflammation and oxidative stress. This review summarises the existing literature about the effects of palmatine supplementation on MetS and its complications. The evidence shows that palmatine could protect against MetS, and cardiovascular diseases, osteoporosis and osteoarthritis, which might be associated with MetS. These protective effects are mediated by the antioxidant and anti-inflammatory properties of palmatine. Although preclinical experiments have demonstrated the efficacy of palmatine against MetS and its related diseases, no human clinical trials have been performed to validate these effects. This research gap should be bridged to validate the efficacy and safety of palmatine supplementation in protecting humans against MetS and its related diseases.
Keywords: anti-inflammation, antioxidant, myocardial reperfusion injury, obesity, osteoporosis, osteoarthritis
Introduction
Metabolic syndrome (MetS) is a strong risk factor for type 2 diabetes, cardiovascular diseases and stroke, which carry high morbidity and threaten global health.1 Although the definition of MetS has evolved with time, it is usually identified by the presence of 3 out of 5 metabolic anomalies, which include obesity, hyperglycaemia, dyslipidaemia [high triglycerides (TG) and low high-density lipoprotein cholesterol (HDL-c) and hypertension].2,3 Around 20–25% of the adult populations worldwide suffer from MetS.4 Data from National Health and Nutrition Examination Survey between 2003 and 2012 revealed that the prevalence of MetS in the United States was 36.5% for women and 30.3% for men.5 A systematic review reported that the incidence of MetS in the Asia-Pacific regions was between 11.9% and 37.1%.6
Oxidative stress, characterised by an imbalance between oxidants and antioxidants in the body, plays a major role in the development of MetS.7 Increased biomarkers of oxidative stress and diminished antioxidant defences were detected in the blood of patients with MetS, suggesting overproduction of oxidising agents in vivo.8 This imbalance involved enzymatic [superoxide dismutase (SOD) activity] and non-enzymatic antioxidant defences (circulating vitamin C and E), as well as increased oxidation products of proteins and lipids.8,9 Increased adipose/fat tissue observed in obesity can trigger oxidative stress.10 Tissue hypoxia due to the inadequate blood supply is a complication in obesity, which triggers cellular necrosis, and migration and phagocytosis of cellular debris by white blood cells.11 The process will elevate oxidative stress due to the release of free radicals, such as nitric oxide and hydrogen peroxide, which may worsen the existing metabolic condition of the patients.12–14 For example, blood pressure was positively associated with oxidative stress biomarkers and negatively associated with the antioxidant status of patients.15 On the other hand, chronic inflammation instigated by a hyperactive immune response is another contributor to MetS.16 Increased circulating pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), and C-reactive protein (CRP), were reported in patients with MetS.17 These cytokines, produced by adipocytes and white blood cells, bind to the respective receptors on target cells and trigger a cascade of signalling events leading to adverse metabolic changes.18
Natural compounds with anti-inflammatory and antioxidant properties, such as vitamin C, E and resveratrol, are hypothesised to prevent or reverse MetS complications.19–21 Palmatine is one of the candidate agents being investigated for its anti-MetS properties. Palmatine is a naturally-occurring quaternary protoberberine in the class of isoquinoline alkaloids.22,23 Structurally, it is an ammonium salt with four methoxyl moieties attached to the phenyl rings at C2 and C3 position.24 The preventive and curative actions of palmatine on cancer, cardiac hypertrophy, diabetes and its complications, osteoporosis, osteoarthritis, Alzheimer’s disease and atopic dermatitis have been reported.24–26 The biological effects of palmatine could be attributed to its anti-inflammatory27–29 and antioxidant properties.30–32 Of note, palmatine is shown to suppress Toll-like receptor (TLR) signalling by downregulating the expression of TLR4, Toll/IL-1 receptor-domain-containing adaptor protein inducing interferon-β and nuclear factor-kappa B in goat endometrial epithelial cells.29 This event leads to increased synthesis of prostaglandins E2 and IL-10 but reduced synthesis of TNF-α, IL-1β, IL-6, nitric oxide, matrix metalloproteinase (MMP)-9 and MMP-2.29 Therefore, the current review aims to summarise the effects of palmatine on MetS and its associated diseases.
The Effects of Palmatine on Components of MetS
Central obesity is a key feature of MetS. The excess nutrients are stored in the adipose tissue as lipids, leading to hypertrophy of adipocytes and increased fat mass.33 Weight reduction through lifestyle, dietary, pharmacological and surgical interventions are often prescribed to patients with MetS.34,35 The antiadipogenic activities of palmatine have been demonstrated in in vitro and in vivo models. Choi et al reported that 3T3-L1 pre-adipocytes treated with palmatine (12.5, 25 and 50 µM) for 24 hrs showed reduced lipid accumulation compared to the untreated cells during differentiation.36 Expression of proliferator-activated receptor-γ (PPAR-γ) and CCAAT/enhancer-binding protein-α (C/EBP-α) was also reduced with palmatine treatment, which signifies a reduction in adipogenesis. These positive findings justify further investigation into the anti-obesity effects of palmatine.
In an animal study by Ning et al, 4-week old Syrian golden hamsters with hyperlipidemia induced by with high-fat diet (HFD) showed reduced body weight, serum total cholesterol (TC), TG, and low-density lipoprotein cholesterol (LDL-c) after being treated with palmatine (23.35, 46.70 and 70.05 mg/kg/day) for four weeks.37 Palmatine increased the expression of low-density lipoprotein receptor (LDLR) and cholesterol 7α-hydroxylase (CYP7A1), which mediate the endocytosis of cholesterol and the conversion of cholesterol to bile acid, respectively.38–41 It also decreased apical sodium-dependent bile acid transporter (ASBT) mRNA and protein expression in these hamsters, which subsequently reduces the bile acid reabsorption in the intestine and increases the excretion of total bile acids in the faeces.42,43 Besides, CYP7A1 also will be upregulated to compensate for the low bile acid level by converting it to cholesterol.44
Similarly, in another study by He et al, 5-week old Syrian golden hamsters with hyperlipidemia induced by high-fat high-cholesterol diet were treated with palmatine (46.7 mg/kg/day for 140 days).45 The treated hamsters showed reduced body weight and epididymal adipose weight, improved lipid profile and increased LDLR and CYP7A1 mRNA and protein expression. Additionally, palmatine supplementation also reduced 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMGCR) mRNA and protein expression and increased uncoupling protein (UCP)-2 mRNA expressions.45 HMGCR is the rate-limiting enzyme in the mevalonate pathway responsible for cholesterol synthesis.46 A decrease in HMGCR expression suggests a reduction in cholesterol production.47,48 UCP-2 is a mitochondrial protein involved in thermogenesis and energy expenditure.49 An increase in UCP-2 expression is associated with a reduction in body weight due to improved energy metabolism.50
In another study by Ma et al, female KK-Ay mice with hyperlipidemia and hyperglycemia induced by HFD showed a decrease in serum TC, TG and LDL-c but an increase in HDL-c after being treated with palmatine (25, 50, 100 mg/kg/day) for 40 days.51 11-hydroxypalmatine (100 mg/kg/day) derived from tubers of Stephania glabra also reduced blood glucose level in alloxan-induced diabetic Swiss albino mice.52 Palmatine (10 mg/kg for 6 weeks) also reduced blood glucose and increased insulin in streptozocin-induced diabetic rats.32 These changes were attributed to reduced oxidative stress, marked by the lowering of malondialdehyde (MDA) and nitrite levels and the increasing of SOD activity.32 However, 24 hrs of palmatine treatment (up to 14.6 µM) inhibited the glucose uptake in HepG2 cells via a non-concentration-dependent manner51 which suggest the hypoglycaemic properties of palmatine may not be related to glucose transportation or insulin sensitivity.
An overview of the effects of palmatine on components of MetS is presented in Table 1. Overall, palmatine supplementation attenuated the adverse effects associated with MetS. The cellular studies showed that palmatine could suppress the formation of adipocytes. Palmatine could also reduce cholesterol production and reabsorption, as well as enhancing energy metabolism, thereby improving lipid profile in animals. A general limitation for the in vivo studies is the absence of a positive control group, ie, pharmacological treatment for MetS. Therefore, the effects of palmatine and the standard treatment cannot be compared. Besides, clinical trial validating the effect of palmatine on MetS is absent in the literature. These research gaps should be considered in future studies.
Table 1.
Protective Effect of Palmatine on MetS Components
| Researcher | Study Design | Findings |
|---|---|---|
| Cell Culture Studies | ||
| Choi et al 201436 | Cell line: 3T3-L1 mouse pre-adipocytes Mode of disease/model induction: Adipocyte differentiation Treatment: 12.5, 25 and 50 µM palmatine for 24 hrs NC: Untreated cells DC: Cells with adipocyte differentiation media PC: n.a. |
↓ lipid accumulation, PPAR-γ and C/EBP-α compared to DC |
| Ma et al 201651 | Cell line: Human liver cancer HepG2 cells Mode of disease/model induction: none Treatment: 0.584, 2.92, 14.6 µM of palmatine for 24 hrs NC: Untreated cells PC: Metformin (0.2–5 µg/mL) |
Inhibit glucose uptake in non-concentration dependent manner |
| Animal Studies | ||
| Ning et al 201537 | Animals: 60 male Syrian golden hamsters (4 weeks old, 100 ± 5 g) Mode of disease induction: HFD-induced hyperlipidaemia Treatment: HFD with 23.35, 46.70 and 70.05 mg/kg/day of palmatine for 4 weeks NC: Distilled water DC: HFD group PC: 1.2 mg/kg/day of simvastatin for 4 weeks |
↓ HFD-induced body weight gain and upregulation of serum TC, TG & LDL-c in a dose-dependent manner NS for HDL-c level compared to DC Further ↑ faecal excretion of TC and TBA compared to DC Restore HFD-induced downregulation of LDLR and CYP7A1 mRNA and protein expression ↓ HFD-induced upregulation of ASBT NS for HMGCR mRNA and protein expression on DC or palmatine groups compared to NC |
| He et al 201645 | Animals: 54 male Syrian golden hamsters (5 weeks old, 100 ± 10 g) Mode of disease induction: HFHC-induced hyperlipidemia Treatment: 46.70 mg/kg/day of palmatine for 140 days NC: Distilled water DC: HFHC group PC: 1.2 mg/kg/day of orlistat for 140 days |
↓ body weight and epididymal adipose weight gain compared to DC ↓ HFHC-induced upregulation of serum TC and LDL-c Further ↑ serum HDL-c and faecal excretion of TC & TBA compared to DC ↓ HMGCR and ↑ LDLR, CYP7A1 and UCP-2 mRNAs and proteins expression compared to DC |
| Ma et al 201651 | Animals: Female KK-Ay mice (8 weeks old, 40 ± 5 g) Mode of disease induction: HFD-induced diabetes Treatment: 25, 50, 100 mg/kg/day of palmatine for 40 days NC: n.a. DC: 0.9% saline PC: 0.32 mL of 225 mg/kg/day of metformin for 40 days |
↓ serum TC, TG and liver/body weight ratio and ↑ HDL-c compared to DC ↓ water intake but NS for body weight gain, food consumption, urine output, fasting blood glucose, postprandial blood glucose and LDL-c levels compared to DC |
| Semwal et al 201052 | Animals: Swiss albino mice of either sex (35–50 g) Mode of disease induction: Alloxan-induced diabetes Treatment: 25, 50, and 100 mg/kg 11-hydroxypalmatine NC: 0.9% saline without alloxan DC: 60 mg/kg alloxan intravenous injection PC: 5 mg/kg/day of glibenclamide for 24 hours on diabetic mice |
↓ blood glucose level compared to NC |
| Pakseresht et al 201632 | Animals: 32 male Wistar rats (35–50 g) Mode of disease induction: STZ-induced diabetes Treatment: 10 mg/kg/day of palmatine for 6 weeks NC: no treatment DC: Intraperitoneal STZ injection (55 mg/kg) PC: n.a. |
↓ STZ-induced hyperglycaemia and ↑ STZ-induced hypoinsulinemia Restore STZ-induced upregulation of nitric oxide activity and downregulation of SOD level |
Abbreviations: ↓, decrease or downregulate; ↑, increase or upregulate; ASBT, apical sodium-dependent bile acid transporter; C/EBP-α, CCAAT/enhancer-binding protein-α; CYP7A1, cholesterol 7α-hydroxylase; DC, disease control/model; HDL-c, high-density lipoprotein cholesterol; HFD, high-fat diet; HFHC, high-fat high-carbohydrate diet; HMGCR, 3-hydroxy-3-methyl glutaryl coenzyme A reductase; LDL-c, low-density lipoprotein cholesterol; LDLR, low-density lipoprotein receptor; MDA, malondialdehyde; n.a., not available; NC, negative control; NS, not significant; PC, positive control; PPAR-γ, proliferator-activated receptor-γ; SOD, superoxide dismutase; STZ, streptozotocin; TBA, total bile acids; TC, total cholesterol; TG, triglyceride; UCP-2, uncoupling protein-2.
The Effects of Palmatine on Cardiovascular Diseases Associated with MetS
MetS is a significant risk factor for myocardial infarction, a common obstructive heart disease and one of the leading causes of death in developed countries.53 Apart from the pro-atherogenic environment, the pro-inflammatory and pro-oxidative conditions of MetS could damage the blood vessel wall, triggering coronary microvascular dysfunction.54,55 Insufficient supply of blood and oxygen to the heart is the initial cause of cardiac damage, thus an immediate blood supply restoration is crucial in minimising myocardial injury. However, sudden reperfusion causes tissue damage despite the restored blood flow, thereby worsening the initial ischemic injury.56
The cardioprotective effects of palmatine have been reported in several studies. In a study by Kim et al, lipopolysaccharides (LPS)-stimulated RAW 264.7 cells treated with palmatine (1, 5, 10 µM for 16 hours) showed decreased high mobility group box 1 (HMGB1) release compared to the untreated LPS-stimulated cells.31 HMGB1 regulates the activation of neutrophils during inflammation.57 The reduction in HMGB1 by palmatine suggests that it could reduce inflammation during MI by lowering the influx of neutrophils into the dying tissues.31 Human aortic endothelial cells (HAEC) treated with palmatine (1, 2, 5 and 10 µM for 8 hours) also showed increased heme oxygenase-1 (HO-1) with antioxidant and anti-inflammatory properties.31 Together, these observations imply the potential effects of palmatine in reducing inflammatory damage to the myocardium during myocardial infarction.
In an animal study by Kim et al, male Sprague Dawley rats with ischemic reperfusion (I/R)-mediated acute myocardial infarction were treated with palmatine (25 and 50 mg/kg) 1 hr before ischemia.31 Reduced MDA and increased SOD and catalase (CAT) activities suggestive of reduced oxidative stress were observed in I/R rats with palmatine treatment.31 Palmatine treated I/R rats also showed reduced lactate dehydrogenase (LDH) and creatine phosphokinase (CK), which are intracellular metabolic enzymes of cardiomyocytes elevated after infarction.31 Palmatine also increased the first derivative (±dp/dt) of left ventricular pressure and reduced infarct size of the heart.31 In the myocardial tissues of I/R rats, cyclooxygenase-2 (COX-2) and inducible NO synthase (iNOS) expression were reduced with palmatine supplementation, suggestive of decreased inflammation in I/R-mediated myocardial infarction.31
Taken together, palmatine is effective in reducing myocardial injury by suppressing inflammatory and oxidative damage associated with reperfusion in animal models. It could be used as an adjuvant to the standard therapy in the management of myocardial infarction. However, its putative use should be validated in a human clinical trial.
The Effects of Palmatine on Musculoskeletal Disorder Associated with MetS
Osteoporosis is a degenerative bone disease that can be triggered by low-grade inflammation and oxidative stress. Pro-inflammatory cytokines, such as TNF-α and IL-1, increase the production of receptor activator of nuclear factor-kB ligand (RANKL), which promote osteoclast formation and activation.58,59 They also suppress apoptosis and prolong the lifespan of osteoclasts.60 Reactive oxygen species (ROS) trigger phosphorylation and degradation of nuclear factor-kappa B inhibitor, leading to the activation of nuclear factor-kappa B, thereby promoting osteoclast formation and activity.61 ROS also induce apoptosis of osteoblasts by activating mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinases, c-Jun-N terminal kinase and p38.62,63 Altogether, these changes lead to altered bone remodelling favouring bone loss.
The simultaneous presence of inflammation and oxidative stress in MetS could potentially result in osteoporosis. The increase in adipocyte differentiation in bone marrow prevents osteoblast differentiation because they are derived from a common progenitor, the multipotential mesenchymal stem cells.64 Adipose tissue is a significant source of pro-inflammatory cytokines which triggers bone resorption via mechanisms mentioned above.65,66 Products of lipid oxidation in dyslipidaemia induce oxidative stress on osteoblast and prevent their differentiation but activate PPAR-γ signalling and promote adipocyte differentiation.16 In hypertension, competition between sodium and calcium ions reduces calcium reabsorption in the renal proximal tubule and contributes to increased urinary excretion of calcium.16 In hyperglycaemia, the accumulation of advanced glycosylation end products (AGEs) in collagen could suppress bone remodelling and reduce bone quality, leading to increased fracture risk.16,67,68
In a study by Ishikawa et al, palmatine (1, 5, 10, 40 and 100 μM for five days) decreased the number of osteoclasts formed from RAW 264.7 macrophages and bone resorption activity indicated by the reduction of total pit formation and fluorescent intensity of conditioned media.69 Ishikawa et al also studied the effects of palmatine (1, 5, 10, 40, 100 and 200 μM for 3 days) on LPS-stimulated MC3T3-E1 pre-osteoblasts.70 Palmatine decreased RANKL and osteoprotegerin (OPG) levels in culture supernatants of LPS-stimulated MC3T3-E1 pre-osteoblasts.70 Similarly, the RANKL and OPG mRNAs expression of palmatine-treated pre-osteoblasts also reduced compared to untreated LPS-stimulated pre-osteoblasts.70 In another study, RAW264.7 cells, mouse clonal stromal cells from bone marrow (ST2 cells), bone marrow cells (BMCs) and bone marrow macrophages (BMMs) were induced with RANKL or 1α,25-dihydroxyvitamin D3 to form osteoclasts followed by palmatine treatment (1, 10, 20 and 40µM) for 1 hr.27 Palmatine lowered pit formation and actin ring formation in 1α,25-dihydroxyvitamin D3-treated BMC and ST2 co-culture.27 RANKL (but not OPG and macrophage colony-stimulating factor) mRNA expression was also reduced upon palmatine treatment in 1α,25-dihydroxyvitamin D3-stimulated ST2 cells.27,71 Palmatine, however, did not significantly reduce the osteoclast formation in RANKL-treated BMMs.27 Ishikawa et al validated the in vitro findings using ICR female mice with bilateral ovariectomy (OVX)-induced osteoporosis.70 They were treated with 1 and 10 mg/kg/day of palmatine for 13 weeks.70 The results showed that palmatine treatment reduced osteoclast number, serum RANKL, OPG and RANKL/OPG ratio in OVX-treated mice.70 These results suggest that palmatine may be able to prevent osteoporosis by reducing bone turnover.70 However, the effects of palmatine on bone in animal models of MetS have not been established. Further studies should consider validating the antiosteoporotic effects of palmatine using animal models induced by high-fat high-carbohydrate diet, which has been shown to induce deterioration of bone mass, structure and strength.18,20
Osteoarthritis is a degenerative joint disease marked by deterioration in articular cartilage, subchondral bone sclerosis and formation of osteophyte with significant clinical signs, such as chronic pain, joint instability, stiffness, and radiographic joint space narrowing.72 In 2015, the World Health Organization estimated that 9.6% of people ≥60 years suffered from symptomatic osteoarthritis.73 MetS is one of the primary risk factors of osteoarthritis, apart from age and joint injury.74 Apart from increasing mechanical loading to the joint, obesity is associated with increased leptin production, which promotes inflammation and MMP synthesis.75,76 Cartilage degradation at the joint leads to localised inflammation and promote the further breakdown of cartilage through MMPs, forming a vicious cycle.77
According to Zhou et al, IL-1β-activated proline chondrocytes treated with palmatine (10, 25, 50, and 100 mg/L for 6 hrs) showed decreased MMP-1, MMP-3, and MMP-13, and increased expression of tissue inhibitor of MMP (TIMP)-1, collagenase II, aggrecan and Dickkopf-related protein 1 (DKK-1).78 These changes were attributable to decreased expression of β-catenin, glycogen synthase kinase-3β (GSK-3β), Indian hedgehog (Ihh), sonic hedgehog (Shh) and Gli-2 caused by palmatine treatment.78 These effects of palmatine were found similar when chondrocytes co-treated with IL-1β and DKK-1 (the Wnt/β-catenin signalling inhibitor).78 The co-incubation of cyclopamine (a Hedgehog signalling inhibitor) further enhanced the palmatine-mediated downregulation of GSK-3β, Ihh, Shh and Gli-2, suggestive the possibly inhibition of both Wnt/β-catenin and Hedgehog signalling pathways upon palmatine treatment.78 These changes were validated by Zhou et al in an in vivo model, whereby decreased expressions of MMP-3, β-catenin, Ihh, Shh, Gli-2 and TNF-α with concomitant increased of TIMP-1 expression were observed in New Zealand rabbits with bilateral anterior cruciate ligament transections (ACLTs)-induced osteoarthritis and treated with palmatine (0.3 mL articular injection of 100 mg/L/week for five weeks).78 Histologically, palmatine decreased erosion, osteophyte formation and cartilage degradation in the joints of the treated osteoarthritic rabbits.78 These results suggest that palmatine may possess antiarthritic effects through reduction/inhibition of inflammatory mediators with the inhibition of Wnt/β-catenin and Hedgehog signalling pathways.78 The effectiveness of palmatine on MetS-induced osteoarthritis model remains hypothetical due to the absence of animal models mimicking this process.
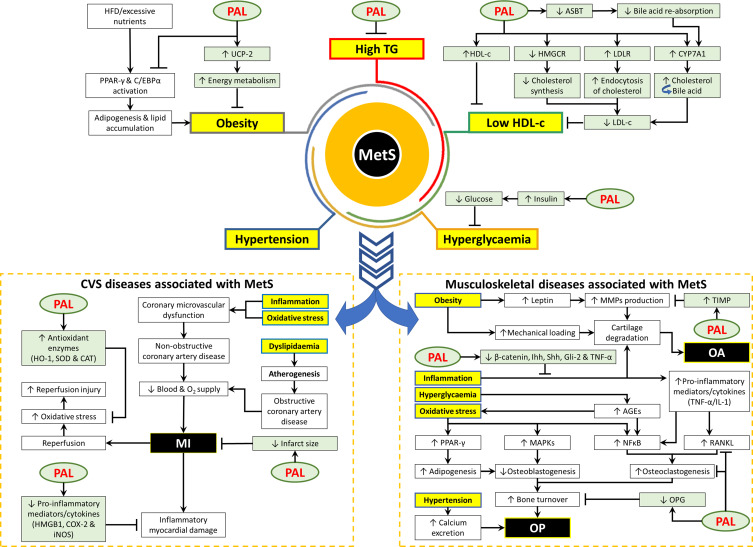
The detailed health benefits of palmatine have been summarised in Table 2. Figure 1 summarises the protective effects of palmatine on MetS and its associated diseases.
Table 2.
Health Benefits of Palmatine Related to the Cardiovascular and Musculoskeletal System
| Researcher | Study Design | Findings |
|---|---|---|
| Cardioprotective activity | ||
| Kim et al 200931 | Cell lines: Human aortic endothelial HAEC and murine macrophage RAW 264.7 cells Mode of disease induction: LPS-induced inflammation (RAW 264.7 cells) Treatment: 1, 2, 5, 10 µM of palmatine for 8 hrs (HAEC) or 16 hrs (RAW 264.7 cells) NC: Untreated cells PC: 1 µg/mL LPS-induced cells (RAW 264.7 cells) Negative: no treatment PC: n.a. |
↑ HO-1 expression in HAEC cells via a concentration-dependent manner ↓ LPS-induced HMGB1 release in RAW264.7 cells via a concentration-dependent manner and restore to baseline level at 10 µM |
| Kim et al 200931 | Animals: Male Sprague–Dawley rats (200–250 g) Mode of disease induction: I/R-mediated acute myocardial injury Treatment: 25 and 50mg/kg of palmatine 1 hr before ischemia NC: Sham with 0.25 mL DMSO 1 hr before ischemia DC: 30 mins ischemia followed by either 6h reperfusion (biochemical analysis) or 24 hrs reperfusion (infarct size and heart function) PC: n.a. |
Restore I/R-increased LVEDP to baseline ↑ I/R-reduced ±dp/dt of left ventricular pressure ↓ infarct size compared to DC ↓ I/R-upregulated serum MDA, LDH, CK and ↑ I/R-reduced SOD and CAT activities ↓ I/R-upregulated COX-2 and iNOS protein expressions in myocardial tissues |
| Antiarthritic activity | ||
| Zhou et al 201678 | Cell line: Primary rabbit chondrocytes Mode of disease induction: IL-1β-induced OA Treatment: 29.20, 73.01, 146.02, and 292.05 µM palmatine for 6 hrs pre-treatment NC: Untreated cells DC: 24 hrs of 10 ng/mL IL-1β PC: n.a. |
↑ IL-1β-downregulated TIMP, collagenase II, aggrecan and DKK-1 mRNAs and ↓ IL-1β-upregulated MMP-1, MMP-3, MMP-13, β-catenin, GSK-3β, Ihh, Shh and Gli-2 mRNAs in a concentration-dependent manner ↓ β-catenin, Ihh & Shh mRNAs compared to NC ↑ IL-1β-downregulated TIMP protein expression and ↓ IL-1β-upregulated MMP-3, β-catenin, Ihh, Shh and Gli-2 protein levels in a concentration-dependent manner |
| Zhou et al 201678 | Animals: 21 New Zealand rabbits weighing (4-week-old, 2.0–2.5 kg) Mode of disease induction: bilateral ACLTs-induced OA Treatment: 0.3 mL articular injection of 100 mg/L/week of palmatine for 5 weeks NC: 0.3 mL of D-Hanks Balanced Salt Solutions DC: OA by ACLTs on knee joints PC: n.a. |
↑ TIMP-1 mRNA compared to DC and NC groups ↓ OA-upregulated MMP-3, β-catenin, Ihh, Shh, Gli-2, and TNF-α mRNAs ↓ OA-upregulated MMP-3, β-catenin, Ihh, Shh, Gli-2, and TNF-α mRNAs ↓ OA-upregulated β-catenin, Shh and Gli-2 protein expression in articular cartilage via immunohistochemical staining ↓ erosion and osteophyte formation compared to DC ↓ cartilage degradation compared to DC |
| Antiosteoporotic activity | ||
| Lee et al 201027 | Cell lines: RAW264.7, BMCs, ST2 cells and BMMs | NS for RANKL-induced osteoclast formation in mouse BMMs |
| Lee et al 201071 | Mode of disease induction: 1α,25-dihydroxyvitamin D3-induced osteoclastogenesis Treatment: 1, 10, 20 and 40 µM of palmatine for 1 hr NC: Untreated cells DC: RANKL or 1α,25-dihydroxyvitamin D3-treated cells PC: n.a. |
↓ RANKL (NS for OPG & M-CSF) mRNA expression in ST2 cells compared to DC Inhibit pit formation and actin ring formation by BMCs and ST2 co-culture compared to DC |
| Ishikawa et al 201570 | Cell line: MC3T3-E1 cells Mode of disease induction: LPS induction Treatment: 1, 5, 10, 40, 100, and 200 μM of palmatine for 3 days Negative: no treatment NC: Untreated cells DC: 1 µg/mL LPS for 24 hrs PC: n.a. |
↓ LPS-upregulated RANKL and OPG levels in culture supernatants and their mRNAs expression |
| Ishikawa et al 201669 | Cell lines: RAW 264.7 and MC3T3-E1 cells Treatment: 1, 5, 10, 40 and 100 μM of palmatine for 5 days NC: Untreated co-culture DC: no PC: n.a. |
↓ osteoclast number compared to NC ↓ bone resorption activity with the reduced total pit formation and fluorescent intensity of conditioned media compared to NC |
| Ishikawa et al 201570 | Animals: Pathogen-free ICR female mice (7-week-old) Mode of disease induction: OVX-induced OP model Treatment: Daily gavage of 1 and 10 mg/kg/day of palmatine for 13 weeks NC: Sham operation mice DC: Bilaterally ovariectomised mice PC: n.a. |
↓ OVX-mediated upregulation of osteoclast number, serum RANKL, OPG and RANKL/OPG ratio in a dose-dependent manner |
Abbreviations: ↓, decrease or downregulate; ↑, increase or upregulate; ±dp/dt, first derivative; ACLTs, anterior cruciate ligament transections; BMMs, bone marrow macrophages; CAT, catalase; CK, creatine phosphokinase; COX-2, cyclooxygenase-2; DC, disease control/model; DKK-1, Dickkopf-related protein 1; DMSO, dimethyl sulfoxide; GSK-3β, glycogen synthase kinase-3β; HEAC, human aortic endothelial cells; HMGB1, high mobility group box 1; HO-1, heme oxygenase-1; Ihh, Indian hedgehog, IL-1β, interleukin-1β; iNOS, inducible NO synthase; I/R, ischemia/reperfusion; LDH, lactate dehydrogenase; LPS, lipopolysaccharride; MDA, malonaldehyde; MMP-3, matrix metalloproteinases-3; n.a., not available; NC, negative control; NS, not significant; OA, osteoarthritis; OP, osteoporosis; OPG, osteoprotegerin; OVX, ovariectomy; PC, positive control; RANKL, receptor activator of nuclear factor-kB ligand; Shh, Sonic hedgehog; SOD, superoxide dismutase; ST2, mouse stromal cells from bone marrow; TIMP-1, tissue inhibitors of metalloproteinases; TNF-α, tumor necrosis factor-α.
Figure 1.
The protective effects of palmatine on MetS and the associated diseases.
Abbreviations: ↓, decrease or downregulate; ↑, increase or upregulate; ┬, inhibit or suppress; AGEs, advanced glycosylation end products; ASBT, apical sodium-dependent bile acid transporter; CAT, catalase; COX, cyclooxygenase; C/EBP-α, CCAAT/enhancer-binding protein-α; HDL-c, high-density lipoprotein cholesterol; HFD, high fat diet; HMGB1, high mobility group box 1; HMGCR, 3-hydroxy-3-methyl glutaryl coenzyme A reductase; HO-1, heme oxygenase −1; iNOS, inducible NO synthase; Ihh, Indian hedgehog; LDL-c, low-density lipoprotein cholesterol; LDLR, low-density lipoprotein receptor; MAPKs, mitogen-activated protein kinases; MI, myocardial infarction; MMP, matrix metalloproteinases; NFκB, nuclear factor kappa B; O2, oxygen; OA, osteoarthritis; OP, osteoporosis; OPG, osteoprotegerin; PAL, palmatine; PPAR-γ, proliferator-activated receptor-γ; RANKL, receptor activator of nuclear factor-kB ligand; Shh, Sonic hedgehog; SOD, superoxide dismutase; TG, triglyceride; TIMP-1, tissue inhibitors of metalloproteinases 1; TNF-α, tumor necrosis factor-α; UCP-2, uncoupling protein-2.
Safety Evaluations of Palmatine
Similar to other isoquinoline alkaloids, palmatine can bind to DNA through groove-binding mechanism by forming hydrogen and van der Waals bonds.79 Other studies demonstrated that palmatine binds to poly (A) and tRNA,80,81 adenine-thymine homo and hetero polymers through the intercalation.82 It also binds preferentially to DNAs that have a long sequence of AT base pairs.83 Besides, it exerts phototoxic effects by generating singlet molecular oxygen after forming complexes with DNA in water solution after photosensitization. The singlet molecular oxygen causes guanine photooxidation, and eventually cleavage of DNA structure.84 These phenomena contribute to the anticancer activity of palmatine. As evidence, palmatine is reported to cause singlet oxygen generation and DNA fragmentation in human skin epithelial carcinoma cells in a dose- and time-depend manner.85 Similar to cisplatin, a DNA-intercalating agent,86 the mutagenic potential of palmatine can cause cellular damage in normal cells and induce secondary malignancy. However, this safety aspect of palmatine has not been explored using normal cells/animal models.
In a study by Yi et al, half-maximal inhibitory concentrations of palmatine on 3T3-L1 and HepG2 cells were 84.32 and 112.80 µg/mL, respectively.87 An acute toxicity study showed a median lethal dose (LD50) of 1533.68 mg/kg in mice.87 In the subchronic toxicity study, no irregularities in clinical signs, body and organ weight, haematological parameters, gross necropsy and histopathology were observed following oral administration of palmatine in rats (156 mg/kg; 5 female/5 male for 90 days).87 All pharmacological studies reviewed in previous sections did not report mortality following palmatine supplementation.87 Thus, palmatine is safe within the therapeutic range but this speculation needs to be validated in human studies. One of the highest doses used for MetS prevention in the reviewed studies is 100 mg/kg in mice.51,52 After translation using Reagan-Shaw formula,88 this dose is equivalent to 486.5 mg for a 60-kg human, which is feasible for supplementation in humans.
Berberine, an isoquinoline alkaloid belonging to the same group as palmatine, has been demonstrated to have the potential to treat MetS and osteoporosis.89–91 Comparison of the therapeutic potential between berberine and palmatine against MetS and its complications is limited, thus it is unsure which compound is more efficacious. In term of toxicity, the LD50 of berberine (oral administration) in mice is 329 mg/kg,92 and a dose as low as 10 mg/kg (intraperitoneal administration) suppressed cellular and humoral immune functions in BALB/c mice.93 Therefore, it could be more toxic than palmatine.
Palmatine is a substrate for P-glycoprotein (P-gp; an excretion transporter/efflux pump), but not for multidrug resistance-associated protein-2, which may justify its relative poor bioavailability.94 Consequently, combining palmatine with P-gp substrates at this particular concentration range did not affect its intestinal absorption.94,95 In diabetic rats, the level of P-gp protein decrease in the small intestine, which could significantly improve palmatine’s intestinal permeability.96,97 This effect can help patients with insulin resistance in absorbing palmatine. However, delivering this drug in sufficient concentration to exert its systemic effects remains a challenge.
Conclusion
The evidence reviewed suggests that palmatine possesses therapeutic effect against MetS and its related disorders like cardiovascular disease, osteoporosis and osteoarthritis. Despite the generally positive effects derived from preclinical studies, there is a lack of human clinical trial to validate these findings. For this reason, well-planned human clinical trials should be conducted to bridge this research gap.
Acknowledgments
We thank Universiti Kebangsaan Malaysia for funding the researchers through grant GUP-2020-021. Sophia Ogechi Ekeuku is a postdoctoral researcher funded by Faculty of Medicine, Universiti Kebangsaan Malaysia through FPR-1.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chan KL, Cathomas F, Russo SJ. Central and peripheral inflammation link metabolic syndrome and major depressive disorder. Physiology (Bethesda). 2019;34(2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang HH, Lee DK, Liu M, Portincasa P, Wang DQ. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr Gastroenterol Hepatol Nutr. 2020;23(3):189–230. doi: 10.5223/pghn.2020.23.3.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin K-Y, Wong SK, Ekeuku SO, Pang K-L. Relationship between metabolic syndrome and bone health – an evaluation of epidemiological studies and mechanisms involved. Diabetes Metab Syndr Obes. 2020;13:3667–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 5.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–1974. doi: 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]
- 6.Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: a systematic review. BMC Public Health. 2017;17(1):101. doi: 10.1186/s12889-017-4041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84(21–22):705–712. doi: 10.1016/j.lfs.2009.02.026 [DOI] [PubMed] [Google Scholar]
- 8.Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev. 2019;2019:8267234. doi: 10.1155/2019/8267234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spahis S, Borys JM, Levy E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid Redox Signal. 2017;26(9):445–461. doi: 10.1089/ars.2016.6756 [DOI] [PubMed] [Google Scholar]
- 10.Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13(10):423–444. doi: 10.1089/met.2015.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes. 2009;33(1):54–66. doi: 10.1038/ijo.2008.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya I, Dominguez AP, Dragert K, Humar R, Haas E, Battegay EJ. Hypoxia potentiates tumor necrosis factor-alpha induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in white and brown adipocytes. Biochem Biophys Res Commun. 2015;461(2):287–292. doi: 10.1016/j.bbrc.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 13.Kosacka J, Kern M, Kloting N, et al. Autophagy in adipose tissue of patients with obesity and type 2 diabetes. Mol Cell Endocrinol. 2015;409:21–32. doi: 10.1016/j.mce.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 14.Netzer N, Gatterer H, Faulhaber M, Burtscher M, Pramsohler S, Pesta D. Hypoxia, oxidative stress and fat. Biomolecules. 2015;5(2):1143–1150. doi: 10.3390/biom5021143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol. 2015;31(5):631–641. doi: 10.1016/j.cjca.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 16.Wong SK, Chin K-Y, Suhaimi FH, Ahmad F, Jamil NA, Ima-Nirwana S. Osteoporosis is associated with metabolic syndrome induced by high-carbohydrate high-fat diet in a rat model. Biomed Pharmacother. 2018;98:191–200. doi: 10.1016/j.biopha.2017.12.042 [DOI] [PubMed] [Google Scholar]
- 17.Bennett JM, Reeves G, Billman GE, Sturmberg JP. Inflammation-nature’s way to efficiently respond to all types of challenges: implications for understanding and managing “the epidemic” of chronic diseases. Front Med (Lausanne). 2018;5:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong SK, Chin KY, Suhaimi FH, Ahmad F, Ima-Nirwana S. Effects of metabolic syndrome on bone mineral density, histomorphometry and remodelling markers in male rats. PLoS One. 2018;13(2):e0192416. doi: 10.1371/journal.pone.0192416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Iglesia R, Loria-Kohen V, Zulet MA, Martinez JA, Reglero G, Ramirez de Molina A. Dietary strategies implicated in the prevention and treatment of metabolic syndrome. Int J Mol Sci. 2016;17(11):1877. doi: 10.3390/ijms17111877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SK, Chin KY, Suhaimi FH, Ahmad F, Ima-Nirwana S. The effects of Vitamin E from Elaeis guineensis (Oil Palm) in a rat model of bone loss due to metabolic syndrome. Int J Environ Res Public Health. 2018;15(9):1828. doi: 10.3390/ijerph15091828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang KL, Chin KY. The role of tocotrienol in protecting against metabolic diseases. Molecules. 2019;24(5):923. doi: 10.3390/molecules24050923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long J, Song J, Zhong L, Liao Y, Liu L, Li X. Palmatine: a review of its pharmacology, toxicity and pharmacokinetics. Biochimie. 2019;162:176–184. doi: 10.1016/j.biochi.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 23.Vrba J, Papouskova B, Pyszkova M, et al. Metabolism of palmatine by human hepatocytes and recombinant cytochromes P450. J Pharm Biomed Anal. 2015;102:193–198. doi: 10.1016/j.jpba.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 24.Tarabasz D, Kukula-Koch W. Palmatine: a review of pharmacological properties and pharmacokinetics. Phytother Res. 2020;34(1):33–50. doi: 10.1002/ptr.6504 [DOI] [PubMed] [Google Scholar]
- 25.Meng FC, Wu ZF, Yin ZQ, Lin LG, Wang R, Zhang QW. Coptidis rhizoma and its main bioactive components: recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin Med. 2018;13:13. doi: 10.1186/s13020-018-0171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rios JL, Francini F, Schinella GR. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015;81(12–13):975–994. doi: 10.1055/s-0035-1546131 [DOI] [PubMed] [Google Scholar]
- 27.Lee J-W, Mase N, Yonezawa T, et al. Palmatine attenuates osteoclast differentiation and function through inhibition of receptor activator of nuclear factor-kB ligand expression in osteoblast cells. Biol Pharm Bull. 2010;33(10):1733–1739. doi: 10.1248/bpb.33.1733 [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Wang X, Zhang SL, et al. Gastroprotective effect of palmatine against acetic acid-induced gastric ulcers in rats. J Nat Med. 2017;71(1):257–264. doi: 10.1007/s11418-016-1057-2 [DOI] [PubMed] [Google Scholar]
- 29.Yan B, Wang D, Dong S, et al. Palmatine inhibits TRIF-dependent NF-kappaB pathway against inflammation induced by LPS in goat endometrial epithelial cells. Int Immunopharmacol. 2017;45:194–200. doi: 10.1016/j.intimp.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 30.Khaksari M, Esmaili S, Abedloo R, Khastar H. Palmatine ameliorates nephrotoxicity and hepatotoxicity induced by gentamicin in rats. Arch Physiol Biochem. 2019:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Kim YM, Ha YM, Jin YC, et al. Palmatine from Coptidis rhizoma reduces ischemia-reperfusion-mediated acute myocardial injury in the rat. Food Chem Toxicol. 2009;47(8):2097–2102. doi: 10.1016/j.fct.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 32.Pakseresht Z, Norouzi P, Hojati V, Moghaddam KH. Effect of palmatine hydrochloride on oxidative stress in streptozotocin -induced diabetic rats. J Adv Med Biomed Res. 2016;24(107):119–129. [Google Scholar]
- 33.Ramli NZ, Chin KY, Zarkasi KA, Ahmad F. The beneficial effects of stingless bee honey from Heterotrigona itama against metabolic changes in rats fed with high-carbohydrate and high-fat diet. Int J Environ Res Public Health. 2019;16(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Lee S, Langleite T, et al. Subsarcolemmal lipid droplet responses to a combined endurance and strength exercise intervention. Physiol Rep. 2014;2(11):e12187. doi: 10.14814/phy2.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misra A, Gupta A, Tank N, Kaklotar D, Singh S, Sharma P Pharmacotherapy in metabolic syndrome; 2017:3.
- 36.Choi JS, Kim JH, Ali MY, Min BS, Kim GD, Jung HA. Coptis chinensis alkaloids exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBP-alpha and PPAR-gamma. Fitoterapia. 2014;98:199–208. doi: 10.1016/j.fitote.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 37.Ning N, He K, Wang Y, et al. Hypolipidemic effect and mechanism of palmatine from coptis chinensis in hamsters fed high-fat diet. Phytother Res. 2015;29(5):668–673. doi: 10.1002/ptr.5295 [DOI] [PubMed] [Google Scholar]
- 38.Chen HY, Ye XL, Cui XL, et al. Cytotoxicity and antihyperglycemic effect of minor constituents from Rhizoma Coptis in HepG2 cells. Fitoterapia. 2012;83(1):67–73. doi: 10.1016/j.fitote.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 39.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50(10):1955–1966. doi: 10.1194/jlr.R900010-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han H, Xin P, Zhao L, et al. Excess iodine and high-fat diet combination modulates lipid profile, thyroid hormone, and hepatic LDLr expression values in mice. Biol Trace Elem Res. 2012;147(1–3):233–239. doi: 10.1007/s12011-011-9300-x [DOI] [PubMed] [Google Scholar]
- 41.Park WH, Pak YK. Insulin-dependent suppression of cholesterol 7alpha-hydroxylase is a possible link between glucose and cholesterol metabolisms. Exp Mol Med. 2011;43(10):571–579. doi: 10.3858/emm.2011.43.10.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lan T, Rao A, Haywood J, Kock ND, Dawson PA. Mouse organic solute transporter alpha deficiency alters FGF15 expression and bile acid metabolism. J Hepatol. 2012;57(2):359–365. doi: 10.1016/j.jhep.2012.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7(11):1189–1194. doi: 10.1016/j.cgh.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 44.Chiang JYL, Ferrell JM. Bile acid biology, pathophysiology, and therapeutics. Clin Liver Dis (Hoboken). 2020;15(3):91–94. doi: 10.1002/cld.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He K, Kou S, Zou Z, et al. Hypolipidemic effects of alkaloids from rhizoma coptidis in diet-induced hyperlipidemic hamsters. Planta Med. 2016;82(8):690–697. doi: 10.1055/s-0035-1568261 [DOI] [PubMed] [Google Scholar]
- 46.Wan Hasan WN, Chin KY, Abd Ghafar N, Soelaiman IN. Annatto-derived tocotrienol promotes mineralization of MC3T3-E1 cells by enhancing BMP-2 protein expression via inhibiting RhoA activation and HMG-CoA reductase gene expression. Drug Des Devel Ther. 2020;14:969–976. doi: 10.2147/DDDT.S224941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haerer W, Delbaere K, Bartlett H, Lord SR, Rowland J. Relationships between HMG-CoA reductase inhibitors (statin) use and strength, balance and falls in older people. Intern Med J. 2012;42(12):1329–1334. doi: 10.1111/j.1445-5994.2011.02622.x [DOI] [PubMed] [Google Scholar]
- 48.Notarnicola M, Messa C, Refolo MG, Tutino V, Miccolis A, Caruso MG. Synergic effect of eicosapentaenoic acid and lovastatin on gene expression of HMGCoA reductase and LDL receptor in cultured HepG2 cells. Lipids Health Dis. 2010;9:135. doi: 10.1186/1476-511X-9-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brondani LA, Assmann TS, de Souza BM, Boucas AP, Canani LH, Crispim D. Meta-analysis reveals the association of common variants in the uncoupling protein (UCP) 1-3 genes with body mass index variability. PLoS One. 2014;9(5):e96411. doi: 10.1371/journal.pone.0096411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh KS, Kim M, Lee J, et al. Liver PPARalpha and UCP2 are involved in the regulation of obesity and lipid metabolism by swim training in genetically obese db/db mice. Biochem Biophys Res Commun. 2006;345(3):1232–1239. doi: 10.1016/j.bbrc.2006.04.182 [DOI] [PubMed] [Google Scholar]
- 51.Ma H, Hu Y, Zou Z, Feng M, Ye X, Li X. Antihyperglycemia and antihyperlipidemia effect of protoberberine alkaloids from rhizoma coptidis in HepG2 cell and diabetic KK-Ay mice. Drug Dev Res. 2016;77(4):163–170. doi: 10.1002/ddr.21302 [DOI] [PubMed] [Google Scholar]
- 52.Semwal DK, Rawat U, Semwal R, Singh R, Singh GJ. Anti-hyperglycemic effect of 11-hydroxypalmatine, a palmatine derivative from Stephania glabra tubers. J Asian Nat Prod Res. 2010;12(2):99–105. doi: 10.1080/10286020903117325 [DOI] [PubMed] [Google Scholar]
- 53.Khademvatan K, Alinejad V, Eghtedar S, Rahbar N, Agakhani N. Survey of the relationship between metabolic syndrome and myocardial infarction in hospitals of Urmia University of medical sciences. Glob J Health Sci. 2014;6(7 Spec No):58–65. doi: 10.5539/gjhs.v6n7p58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maeda K, Ruel M. Prevention of ischemia-reperfusion injury in cardiac surgery: therapeutic strategies targeting signaling pathways. J Thorac Cardiovasc Surg. 2015;149(3):910–911. doi: 10.1016/j.jtcvs.2014.11.067 [DOI] [PubMed] [Google Scholar]
- 55.Monserrat-Mesquida M, Quetglas-Llabres M, Capo X, et al. Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants (Basel). 2020;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schanze N, Bode C, Duerschmied D. Platelet contributions to myocardial ischemia/reperfusion injury. Front Immunol. 2019;10:1260. doi: 10.3389/fimmu.2019.01260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park JS, Arcaroli J, Yum HK, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284(4):C870–C879. doi: 10.1152/ajpcell.00322.2002 [DOI] [PubMed] [Google Scholar]
- 58.Amarasekara DS, Yun H, Kim S, Lee N, Kim H, Rho J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018;18(1):e8. doi: 10.4110/in.2018.18.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo G, Li F, Li X, Wang ZG, Zhang B. TNFalpha and RANKL promote osteoclastogenesis by upregulating RANK via the NFkappaB pathway. Mol Med Rep. 2018;17(5):6605–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soysa NS, Alles N. Positive and negative regulators of osteoclast apoptosis. Bone Rep. 2019;11:100225. doi: 10.1016/j.bonr.2019.100225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33(4):359–370. doi: 10.1007/s00774-015-0656-4 [DOI] [PubMed] [Google Scholar]
- 62.Fontani F, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: involvement of JNK and ERK1/2 signalling. Calcif Tissue Int. 2015;96(4):335–346. doi: 10.1007/s00223-015-9961-0 [DOI] [PubMed] [Google Scholar]
- 63.Marathe N, Rangaswami H, Zhuang S, Boss GR, Pilz RB. Pro-survival effects of 17beta-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J Biol Chem. 2012;287(2):978–988. doi: 10.1074/jbc.M111.294959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21(12):1162–1169. doi: 10.1016/j.jnutbio.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(Suppl 3):S5–S78. doi: 10.1017/S0007114511005460 [DOI] [PubMed] [Google Scholar]
- 66.Campos RM, de Piano A, da Silva PL, et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: effects of long-term interdisciplinary therapy. Endocrine. 2012;42(1):146–156. doi: 10.1007/s12020-012-9613-3 [DOI] [PubMed] [Google Scholar]
- 67.Chin KY, Ima-Nirwana S, Mohamed IN, et al. Insulin-like growth factor-1 is a mediator of age-related decline of bone health status in men. Aging Male. 2014;17(2):102–106. doi: 10.3109/13685538.2014.896895 [DOI] [PubMed] [Google Scholar]
- 68.Jackuliak P, Payer J. Osteoporosis, fractures, and diabetes. Int J Endocrinol. 2014;2014:820615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishikawa S, Tamaki M, Ogawa Y, et al. Inductive effect of palmatine on apoptosis in RAW 264.7 cells. Evid Based Complement Alternat Med. 2016;2016:7262054. doi: 10.1155/2016/7262054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ishikawa S, Ogawa Y, Tamaki M, et al. Influence of palmatine on bone metabolism in ovariectomized mice and cytokine secretion of osteoblasts. In Vivo. 2015;29(6):671–677. [PubMed] [Google Scholar]
- 71.Lee WC, Kim JK, Kang JW, et al. Palmatine attenuates D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Food Chem Toxicol. 2010;48(1):222–228. doi: 10.1016/j.fct.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 72.Wang M, Shen J, Jin H, Im HJ, Sandy J, Chen D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61–69. doi: 10.1111/j.1749-6632.2011.06258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization. Chronic rheumatic conditions; 2020. Available from: https://www.who.int/chp/topics/rheumatic/en/. Accessed August20, 2020.
- 74.Buchele G, Gunther KP, Brenner H, et al. Osteoarthritis-patterns, cardio-metabolic risk factors and risk of all-cause mortality: 20 years follow-up in patients after hip or knee replacement. Sci Rep. 2018;8(1):5253. doi: 10.1038/s41598-018-23573-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oztas B, Sahin D, Kir H, et al. The effect of leptin, ghrelin, and neuropeptide-Y on serum Tnf-Alpha, Il-1beta, Il-6, Fgf-2, galanin levels and oxidative stress in an experimental generalized convulsive seizure model. Neuropeptides. 2017;61:31–37. doi: 10.1016/j.npep.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 76.Williams RC, Skelton AJ, Todryk SM, Rowan AD, Preshaw PM, Taylor JJ. Leptin and pro-inflammatory stimuli synergistically upregulate MMP-1 and MMP-3 secretion in human gingival fibroblasts. PLoS One. 2016;11(2):e0148024. doi: 10.1371/journal.pone.0148024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16–21. doi: 10.1016/j.joca.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 78.Zhou X, Lin X, Xiong Y, et al. Chondroprotective effects of palmatine on osteoarthritis in vivo and in vitro: a possible mechanism of inhibiting the Wnt/beta-catenin and Hedgehog signaling pathways. Int Immunopharmacol. 2016;34:129–138. doi: 10.1016/j.intimp.2016.02.029 [DOI] [PubMed] [Google Scholar]
- 79.Mi R, Tu B, Bai XT, Chen J, Ouyang Y, Hu YJ. Binding properties of palmatine to DNA: spectroscopic and molecular modeling investigations. Luminescence. 2015;30(8):1344–1351. doi: 10.1002/bio.2904 [DOI] [PubMed] [Google Scholar]
- 80.Islam MM, Pandya P, Chowdhury SR, Kumar S, Kumar GS. Binding of DNA-binding alkaloids berberine and palmatine to tRNA and comparison to ethidium: spectroscopic and molecular modeling studies. J Mol Struct. 2008;891(1):498–507. doi: 10.1016/j.molstruc.2008.04.043 [DOI] [Google Scholar]
- 81.Giri P, Hossain M, Kumar GS. Molecular aspects on the specific interaction of cytotoxic plant alkaloid palmatine to poly(A). Int J Biol Macromol. 2006;39(4):210–221. [DOI] [PubMed] [Google Scholar]
- 82.Bhadra K, Maiti M, Kumar GS. Molecular recognition of DNA by small molecules: AT base pair specific intercalative binding of cytotoxic plant alkaloid palmatine. Biochim Biophys Acta. 2007;1770(7):1071–1080. doi: 10.1016/j.bbagen.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 83.Bhadra K, Maiti M, Kumar GS. Interaction of isoquinoline alkaloid palmatine with deoxyribonucleic acids: binding heterogeneity, and conformational and thermodynamic aspects. Chem Biodivers. 2008;5(4):575–590. [DOI] [PubMed] [Google Scholar]
- 84.Hirakawa K, Kawanishi S, Hirano T. The mechanism of guanine specific photooxidation in the presence of berberine and palmatine: activation of photosensitized singlet oxygen generation through DNA-binding interaction. Chem Res Toxicol. 2005;18(10):1545–1552. doi: 10.1021/tx0501740 [DOI] [PubMed] [Google Scholar]
- 85.Ali D, Ali H. Assessment of DNA damage and cytotoxicity of palmatine on human skin epithelial carcinoma cells. Toxicol Environ Chem. 2014;96(6):941–950. doi: 10.1080/02772248.2014.987510 [DOI] [Google Scholar]
- 86.Fung C, Dinh P, Ardeshir-Rouhani-Fard S, Schaffer K, Fossa SD, Travis LB. Toxicities associated with cisplatin-based chemotherapy and radiotherapy in long-term testicular cancer survivors. Adv Urol. 2018;2018:8671832. doi: 10.1155/2018/8671832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yi J, Ye X, Wang D, et al. Safety evaluation of main alkaloids from Rhizoma Coptidis. J Ethnopharmacol. 2013;145(1):303–310. doi: 10.1016/j.jep.2012.10.062 [DOI] [PubMed] [Google Scholar]
- 88.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- 89.Wong SK, Chin K-Y, Ima-Nirwana S. Berberine and musculoskeletal disorders: the therapeutic potential and underlying molecular mechanisms. Phytomedicine. 2020;73:152892. [DOI] [PubMed] [Google Scholar]
- 90.Cao C, Su M. Effects of berberine on glucose-lipid metabolism, inflammatory factors and insulin resistance in patients with metabolic syndrome. Exp Ther Med. 2019;17(4):3009–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu X, Zhang Y, Xue Y, Zhang Z, Wang J. Berberine is a potential therapeutic agent for metabolic syndrome via brown adipose tissue activation and metabolism regulation. Am J Transl Res. 2018;10(11):3322–3329. [PMC free article] [PubMed] [Google Scholar]
- 92.Singh N, Sharma B. Toxicological effects of berberine and sanguinarine. Front Mol Biosci. 2018;5:21. doi: 10.3389/fmolb.2018.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mahmoudi M, Zamani Taghizadeh Rabe S, Balali-Mood M, et al. Immunotoxicity induced in mice by subacute exposure to berberine. J Immunotoxicol. 2016;13(2):255–262. doi: 10.3109/1547691X.2015.1058306 [DOI] [PubMed] [Google Scholar]
- 94.Zhang X, Qiu F, Jiang J, Gao C, Tan Y. Intestinal absorption mechanisms of berberine, palmatine, jateorhizine, and coptisine: involvement of P-glycoprotein. Xenobiotica. 2011;41(4):290–296. doi: 10.3109/00498254.2010.529180 [DOI] [PubMed] [Google Scholar]
- 95.Yu CP, Huang CY, Lin SP, Hou YC. Activation of P-glycoprotein and CYP 3A by coptidis rhizoma in vivo: using cyclosporine as a probe substrate in rats. J Food Drug Anal. 2018;26(2S):S125–S132. doi: 10.1016/j.jfda.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobori T, Harada S, Nakamoto K, Tokuyama S. Functional alterations of intestinal P-glycoprotein under diabetic conditions. Biol Pharm Bull. 2013;36(9):1381–1390. doi: 10.1248/bpb.b13-00369 [DOI] [PubMed] [Google Scholar]
- 97.Neearti P, Barla R, Bedada SK. Influence of diabetes mellitus on P-glycoprotein function in rat intestine. Pharmacologia. 2011;2:293–298. doi: 10.5567/pharmacologia.2011.293.298 [DOI] [Google Scholar]