BRAF-activating mutations have been detected in nearly all craniopharyngiomas of the papillary subtype,1 with interesting implications for patients with suboptimal tumor control. Here we report that combined treatment with BRAF and MEK inhibitors resulted in major clinical benefit in the case of a patient with a BRAF V600E-mutant papillary craniopharyngioma rapidly progressing after surgery.
A 55-year-old woman was diagnosed with a suprasellar neoplasm after a rapid increase in body weight and combined pituitary hormone deficiency. The visual field test was normal.
Partial surgical resection was performed via a transsphenoidal approach achieving approximately 15% of tumor debulking with excision limited by intraoperative bleeding.
Histological analysis showed a BRAF V600E-positive papillary craniopharyngioma, qPCR–high-resolution melting confirming the presence of the somatic BRAF c.1799T>A, p.Val600Glu mutation.
Postoperative follow-up MRI showed a dramatic increase in tumor volume (up to 67% compared to postoperative imaging) 3 months after surgery, with pressure on the optic chiasma.
Based on the large tumor volume, its worryingly rapid growth, and the potential detrimental effect on the visual and hormonal functions, the multidisciplinary tumor board of our institution proposed a front-line treatment with BRAFi and MEKi.
According to previous case reports,2,3 we inferred that our patient might benefit from medical tumor debulking with BRAFi followed by a second surgery and/or local irradiation. We opted for combined BRAF/MEK inhibition, which demonstrated superior oncological outcomes compared to BRAFi monotherapy in BRAF V600E-mutant melanoma.4
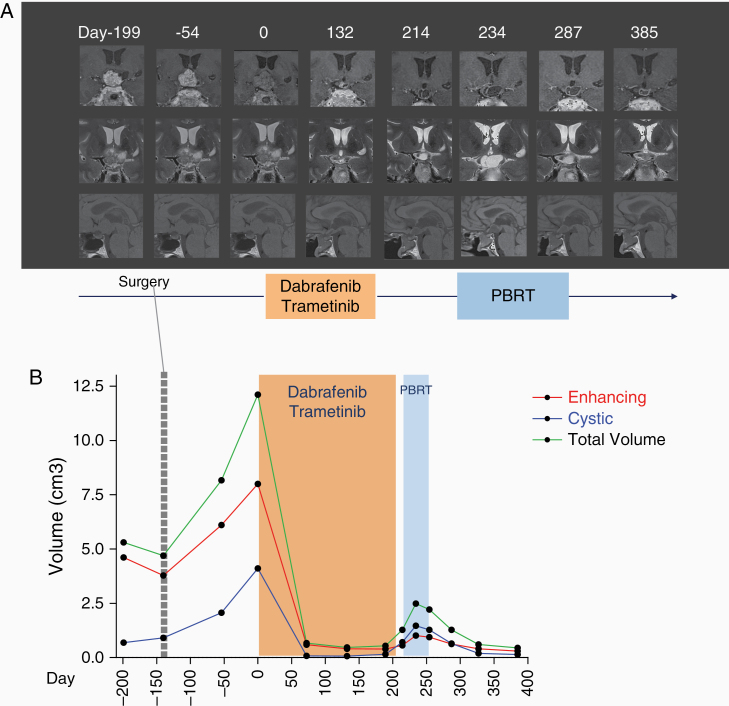
The patient started a combination of oral dabrafenib 150 mg BID and oral trametinib 2 mg QD 5 months after surgery. MRI performed the day treatment was started (day 0) showed a further increase in tumor volume reaching 12.1 cm3.
Control MRI performed 72 days after starting BRAFi/MEKi showed dramatic tumor shrinkage (−94.5% compared to day 0 imaging).
Combined treatment was well tolerated with grade 1 fatigue (CTCAE v4.0), coughing, and peripheral edema requiring temporary interruption of trametinib.
After 132 days of treatment, given the persistence of a residual tumor measuring 0.5 cm3 in an area hardly accessible to a second surgery, we proposed that the patient should undergo proton beam radiotherapy (PBRT). Dabrafenib and trametinib were discontinued 7 days (day 208 in Figure 1) before starting PBRT to avoid all adverse events related to radiosensitization.2,5
Figure 1.
Targeted inhibition of BRAF/MEK pathway in a patient with a recurrent papillary craniopharyngioma. (A) Timeline from the first presentation is indicated in days. T1-weighted (sagittal), contrast-enhanced (coronal) and T2-weighted (coronal) images of MRI performed at diagnosis (day –199). Follow-up MRI performed 3 months after surgery showing progression (day –54). Medical therapy with dabrafenib and trametinib was started on day 0. Subsequent MRI during medical therapy showed major tumor response on days 72 and 132. Anti-BRAF and anti-MEK inhibitors were stopped on day 208 and RT was started on day 215. After the anti-BRAF and anti-MEK discontinuation and during RT we observed an initial increase of the tumor, mainly of the cystic component (on days 214 and 234) followed by stabilization and final reduction (day 327, corresponding to the last follow-up). Tumor volume measure at corresponding time points is shown in (B). Enhancing (red) and cystic (blue) components as well as the total volume (green) variations are indicated. Tumor volumes were calculated by semiautomatic segmentation using Myrian Expert, v2.7.1, Intrasense, France.software. Of note, in MRI on day 0 contrast-enhancement is reduced (ie, half gadolinium dose compared to other MRIs) due to incidental extravasation during the contrast-agent infusion. PBRT, proton beam radiation therapy.
Control MRI performed the day before starting PBRT (day 214 in Figure 1) showed an increase of the cystic component of the tumor (13%, 0.7 cm3), which further progressed to 1.5 cm3 (with a total volume of 2.5 cm3) during the third week of PBRT (day 234 in Figure 1A) and then stabilized at subsequent MRI.
The patient was able to continue PBRT without relevant toxicity, for a total dose of 52.2 Gy relative biological effectiveness (RBE) in 29 fractions.
At last follow-up performed 18 weeks after the end of PBRT (corresponding to day 385 in Figure 1A), the patient is free of new symptoms, with the cystic component as well as the total tumor volume decreased to 0.14 cm3 and 0.45 cm3, respectively. Despite sustained tumor control, pituitary function did not improve, but the visual field remained normal.
In summary, our case showed that combined treatment with BRAFi/MEKi can achieve rapid tumor control in patients with BRAF-mutant craniopharyngiomas rapidly progressing after surgery.
Combined short-course BRAFi/MEKi therapy produced an efficient tumor debulking and allowed the dramatic reduction of the volume treated with local radiotherapy.
Interestingly, close imaging follow-up showed an initial cystic recurrence 7 days after BRAFi/MEKi discontinuation, suggesting that some patients may present a BRAFi/MEKi dependent evolution requiring adequate treatment planning.
While treatment with BRAFi/MEKi should be considered a potential life-saving strategy in relapsing patients, neoadjuvant approaches should also be discussed in order to reduce the morbidity associated with local treatments and to contribute to functional sparing.
Funding
The authors received no specific funding for this work.
Conflict of interest statement. The authors declare no conflict of interest.
Authorship statement. A.L.D.S. and S.G.: study concept and design, acquisition and interpretation of data, drafting the manuscript, and responsible for research integrity. A.B., B.B., V.D.B., L.F., D.G., E.H., K.S., and C.V.: acquisition and interpretation of data, critical revision of the manuscript for intellectual content. G.B.: critical revision of the manuscript for intellectual content. All read and approved the manuscript.
References
- 1. Brastianos PK, Taylor-Weiner A, Manley PE, et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet. 2014;46(2):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brastianos PK, Shankar GM, Gill CM, et al. Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Inst. 2016;108(2):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rostami E, Witt Nyström P, Libard S, Wikström J, Casar-Borota O, Gudjonsson O. Recurrent papillary craniopharyngioma with BRAFV600E mutation treated with neoadjuvant-targeted therapy. Acta Neurochir (Wien). 2017;159(11):2217–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anker CJ, Grossmann KF, Atkins MB, Suneja G, Tarhini AA, Kirkwood JM. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95(2):632–646. [DOI] [PMC free article] [PubMed] [Google Scholar]