Abstract
Purpose of review
To review recent clinical evidence surrounding the use of cannabinoids and cannabis in gastrointestinal diseases, particularly inflammatory bowel disease (IBD) and functional gut disorders. A second aim is to evaluate the current status of gastrointestinal related adverse effects which have been linked to cannabis use, specifically cannabis hyperemesis syndrome (CHS) and acute pancreatitis.
Recent findings
Observational and prospective studies suggest that cannabinoids improve IBD symptoms. Small prospective clinical trials have not shown any effects on objective inflammatory findings, other than one recent paper in ulcerative colitis, in abstract form only, which suggests endoscopic improvement. Short duration mechanistic studies in functional gut disorders suggest cannabinoids may attenuate gastric emptying and slow colonic motility but appear to have less effect on sensory thresholds in the gut.
Summary
In general, while mostly uncontrolled data suggests cannabis may improve symptoms in IBD (and to a lesser degree functional gut disorders), this is not likely due to any substantial anti-inflammatory effect. Much remains unknown about CHS etiology and complete abstinence from cannabinoids remains the generally accepted treatment strategy. Population-based studies do not suggest that cannabis use is related to acute pancreatitis. Further research is certainly warranted.
Keywords: Cannabinoid, Inflammation, Irritable bowel syndrome, Abdominal pain, Cannabinoid hyperemesis syndrome, Pancreatitis
Introduction
Cannabis and cannabinoids have been used since time immemorial for their medical and recreational properties. In recent years, medical research on cannabis has increased substantially in conjunction with removal or lowering of legal restrictions on its use. However, while a biological rationale and animal data have existed for some time suggesting beneficial effects of cannabis on gastrointestinal related pain and slowing overactive motility [1], research confirming these effects clinically has lagged behind.
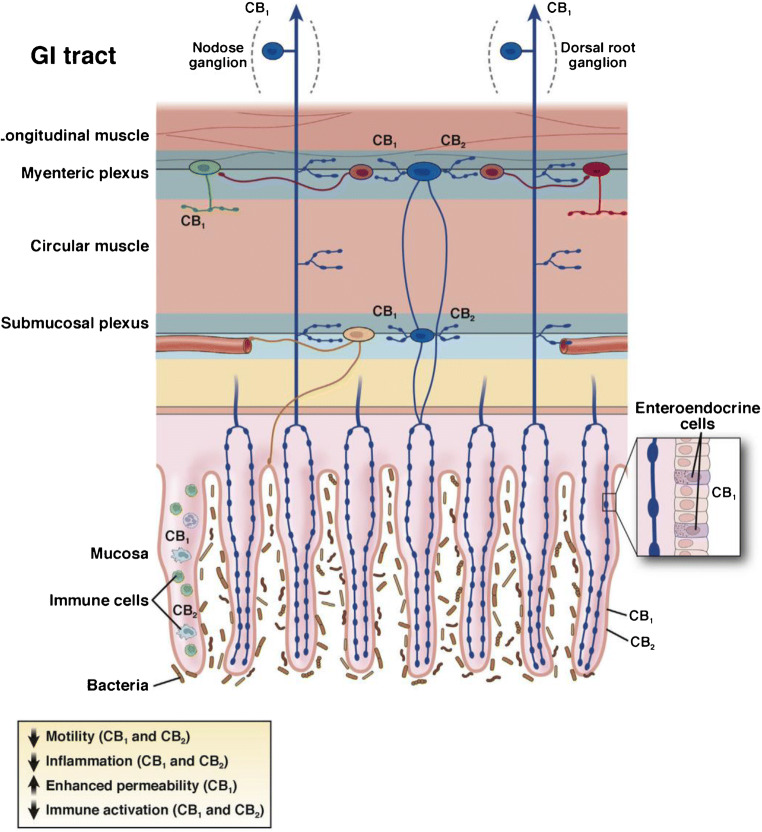
The body makes its own endogenous cannabinoids (anandamide and 2-arachidonoylglycerol) which exert their effects predominantly through CB1 and CB2 receptors that are widely distributed throughout the gastrointestinal tract and the nervous system (reviewed in [2•]). This “endocannabinoid” system is an important physiological regulator of gastrointestinal motility and is also involved in modulation of intestinal inflammation and visceral sensitivity. CB1 is expressed on motor, secretory, and sensory afferent neurons throughout the wall of the gut and peripheral nervous system, and acute stimulation of CB1 receptors causes a reduction of motility and secretion mediated by those neurons. CB2 receptors are predominantly located on immune cells and other neurons in the epithelium and gut wall, and are upregulated in inflammatory states. Stimulation of CB2 reduces inflammation and immune activation in vitro and in animal studies (see Fig. 1).
Fig. 1.
Effects of cannabinoids in the gastrointestinal (GI) tract are mediated by CB1 and CB2. Cannabinoid receptors are widely distributed in the GI tract. CB1 is expressed by all classes of cholinergic enteric neurons in the myenteric and submucosal plexuses (primary afferent neurons [blue], interneurons [purple], secretomotor [yellow], and excitatory motor neurons [green], but not on inhibitory motor neurons [red]). CB1 is also found on some enteroendocrine cells and in the epithelium. Extrinsic vagal and spinal primary afferent neurons express CB1, which is regulated by feeding (vagal) and stress (spinal), respectively. CB2 is expressed by enteric neurons and immune cells in the GI tract. Under conditions of inflammation and injury, CB2 is upregulated in the epithelium and its function on enteric neurons increases. The effects of activating cannabinoid receptors in the GI tract in animal studies include a reduction in motility, reduced inflammation, and reduced immune activation. Adapted from Sharkey et al. [2•].
Exogenous cannabinoids may be derived from the Cannabis sativa plant or may be synthesized. The two exogenous cannabinoids which are best understood and are typically used to guide prescribing and recreational purchases are delta-9 tetrahydrocannabinol (THC) and cannabidiol (CBD). THC is associated with the psychoactive and intoxicating effects of cannabis, whereas CBD is not psychoactive on its own and appears to modulate effects of THC.
In the USA, synthetic oral THC (dronabinol) has been approved for loss of appetite and weight loss associated with acquired immune deficiency syndrome as well as nausea and vomiting caused by chemotherapy since the 1980s [3]. In the last decades, many jurisdictions in the USA and worldwide have also approved cannabis products (derived from the naturally occurring plant) for medical purposes, which commonly entail gastrointestinal disorders. However, the vast majority of these uses have not been formally tested for efficacy. A lack of standardization of cannabis products, which may contain hundreds of bioactive compounds, also hampers research in the field. Finally, chronic use of cannabinoids, particularly inhaled cannabis amongst youth and young adults, has clearly shown risks of causing or worsening mental health in addition to other negative effects [4]. Thus, patients need to “watch their head” as they step into the ambiguous treatment space of cannabinoids for their gut.
Given this uncertainty and potential risks of harm with chronic recreational use, a number of national medical bodies have suggested that cannabinoids should not be used first-line for gastrointestinal related indications [5, 6]. Patients may ask their gastroenterologist about the use of cannabinoids for their symptoms, or there may be concern that chronic cannabis use may be contributing to their symptoms. The most commonly encountered situations are discussed below.
Cannabinoids and inflammatory bowel disease
Cannabinoids act as endogenous inhibitors of intestinal inflammation, modulating immune cell function, promoting wound healing, and decreasing intestinal permeability [2, 7, 8]. Pre-clinical data from animal models of colitis have shown that both CB1 and CB2 receptor agonists, as well as THC and CBD, can limit intestinal inflammation and disease severity [9]. Accordingly, there has been great interest in investigating the therapeutic role of cannabinoids in inflammatory bowel disease (IBD).
The endocannabinoid system is altered in patients with active IBD, with studies reporting both an increase as well as a decrease in endocannabinoid tone [10]. Genetic polymorphisms in cannabinoid receptors have also been associated with IBD. A CB2 receptor variant (R63) was found to convey increased risk for developing IBD, along with increased disease severity in one case-control study [11]. Another study demonstrated that single nucleotide polymorphisms (SNPs) in the CB1 receptor modulated the susceptibility to ulcerative colitis and altered the phenotype of patients with Crohn’s disease [12]. SNPs in the endocannabinoid degradative enzyme fatty acid amide hydrolase (FAAH) were associated with earlier disease onset in ulcerative colitis and increased disease severity in Crohn’s with associated fistulizing disease and extraintestinal manifestations [13]. These data indicate that the endocannabinoid system plays a role in IBD pathogenesis.
Patients with IBD often turn to complementary and alternative therapies to treat their symptoms. Cannabis use in IBD is common, and a seminal paper which used the National Health and Nutrition Examination Survey reported a higher incidence of cannabis/hashish use in IBD patients (67.3%) when compared to controls (60%) [14]. Patients report cannabis as helpful for the control of abdominal pain and diarrhea as well as nausea and decreased appetite [14–19]. Cannabis is generally well-tolerated in IBD, although prolonged cannabis use (> 6 months) in Crohn’s disease may be associated with an increased risk of surgery [15]. However, it is difficult to interpret whether this association is causal or reflects cannabis use in patients with more aggressive disease [6, 15]. Taken together, cannabis use appears effective for symptom management in IBD, although smoked cannabis may be detrimental in Crohn’s disease.
Observational studies of cannabinoids in patients with IBD have generally shown positive outcomes regarding disease severity. An early retrospective observational study of 30 IBD patients who consumed cannabis revealed a significant decrease in the Harvey Bradshaw Index (HBI) after cannabis consumption [20]. Similarly, a retrospective case-control study of Crohn’s disease patients which analyzed data from the Healthcare Cost and Utilization Project-National Inpatient Sample concluded that cannabis users had lower rates of fistulizing disease, lower total parenteral nutrition requirements, and underwent fewer colonic surgical resections [21], in contrast with data from a previous patient survey [15]. Naftali et al. conducted a prospective observational study of 127 IBD patients applying for a medical cannabis license. The average HBI was significantly decreased over the follow-up period while steroid, immunomodulator, and thiopurine use were all significantly decreased following cannabis consumption. However, 14% of patients discontinued their medications without consulting the prescribing physician, which is extremely concerning [22]. It should be noted that these observational studies do not report objective indices of disease activity such as fecal calprotectin or endoscopic activity and thus should be interpreted with caution.
Very few randomized clinical trials of cannabinoids have been conducted in IBD. Two small clinical trials have been conducted in Crohn’s disease. Naftali et al. conducted a double-blinded randomized placebo-controlled clinical trial of 21 patients with moderate to severe refractory Crohn’s disease. Patients were randomized to smoke cigarettes containing 115 mg of THC or “placebo” cigarettes (without THC) twice daily for 8 weeks. Although there was no significant difference in the incidence of complete remission between groups, 10/11 patients in the THC group vs 4/10 patients in the placebo group had a significant decrease in the Crohn’s disease activity index (CDAI > 100). No significant side effects were reported and patients in the THC arm reported an improvement in sleep, pain, and quality of life. However, no endoscopic measure of disease activity was performed [23•]. The same group examined the effect of the non-psychoactive cannabinoid, CBD, in 20 patients with moderate to severe refractory Crohn’s disease. Patients were randomized to receive oral CBD 10 mg twice daily or placebo for 8 weeks. CBD had no effect on disease activity and no adverse side effects were reported [24]. A recent Cochrane review considered both these studies at high risk of bias [25]; further studies in larger cohorts are needed prior to drawing any conclusion on the role of cannabinoids in active Crohn’s.
CBD was also evaluated in a trial in ulcerative colitis. In this relatively larger study, 60 patients with mild to moderate ulcerative colitis were randomized to placebo or 10 weeks of oral CBD in a “botanic extract” containing up to 5% THC. The dose of the extract was escalated over the first 2 weeks of the trial to a maximum of 500 mg/day. No differences were seen between groups in the rate of clinical remission; a per-protocol analysis of the total and partial Mayo scores favored CBD while secondary outcomes such as quality of life showed a trend to improvement in patients given the CBD extract. However, 38% of patients in the CBD group did not complete the trial and 90% of patients reported side effects due to the poor tolerability of the extract [26]. Another study by Naftali et al. in ulcerative colitis is only reported in abstract format [27]. A total of 28 patients with moderate ulcerative colitis were randomized to smoke cigarettes containing 11.5 mg of THC or “placebo” cigarettes (without THC) twice daily for 8 weeks. A significant improvement in the disease activity index was seen in patients given THC. Importantly an endoscopic endpoint was included in this trial and the Mayo endoscopic score was significantly improved in those that received THC when compared to placebo [27]. A recent Cochrane review considered the data from this last study at high risk of bias [28]; examination of the published data is needed to fully judge the quality of the study.
In conclusion, although pre-clinical studies in rodent models have shown great promise, no recommendations can be made on the use of cannabinoids for the treatment of active IBD. Larger randomized placebo-controlled trials, with examination of objective/endoscopic outcomes are needed.
Cannabinoids and functional gut disorders
Cannabis has historically been used as an anti-diarrheal and appetite stimulant. A meta-analysis of randomized controlled trials of cannabinoid use for symptom control found moderate-quality evidence to support the use of cannabinoids for the treatment of chronic pain, and low-quality evidence suggesting that cannabinoids were associated with improvements in nausea and vomiting due to chemotherapy and weight gain in human immunodeficiency virus infection [29]. None of the chronic pain studies analyzed were for abdominal or gastrointestinal related pain. The vast majority of these trials used oral formulations of cannabinoids and adverse effects (typically mild) related to cannabis were common.
More recently, a double blind randomized placebo-controlled trial of oral THC vs placebo was evaluated in 65 patients with chronic abdominal pain due to chronic pancreatitis or post-surgical pain [30]. THC doses were increased over an introductory period up to 8 mg three times daily orally and the primary outcome was pain severity change at the end of the study compared to baseline. There were no significant differences between THC or placebo in pain scores or quality of life at 50 days.
A handful of mechanistic studies with cannabinoids in irritable bowel syndrome (IBS) have been published in humans (reviewed in [31]). A crossover study of 10 IBS and 12 healthy controls did not show any differences in rectal sensitivity to barostat after single-dose 5 mg or 10 mg of THC vs placebo [32]. Wong et al. [33] also studied 75 IBS patients (35 IBS-D, 90% female) with descending colon barostat, randomized to single dose of placebo, 2.5 mg, or 5.0 mg of dronabinol. In that study, dronabinol was found to decrease colonic motility index and increased colonic compliance. However, it did not alter sensation or tone. Effects were most pronounced in patients with diarrhea-predominant or alternating IBS. A follow-up study by the same group of 36 mostly female patients with IBS-D randomized to placebo, 2.5 mg, or 5 mg of dronabinol orally twice daily did not show any differences in gastric, small bowel, or colonic transit by radioscintigraphy [34].
CB2 expression is upregulated in inflammatory states [2•]. CB2 receptor mRNA and protein was recently shown to be increased in colonic mucosal biopsies of IBS patients compared to asymptomatic controls [35]. Olorinab (Arena Pharmaceuticals, Boston, MA), a peripherally restricted CB2 agonist, is in phase 2 clinical trials for IBS-related pain [36].
In terms of gastric function, Esfandyari and colleagues evaluated cannabinoid effects on upper gut function by randomizing 30 healthy volunteers to placebo or dronabinol 5 mg orally twice daily for three doses [37]. There was an overall slowing of gastric emptying with dronabinol compared to placebo. The effect was stronger in females compared to males. There were no significant treatment differences in gastric volumes, satiation testing, or scintigraphic small bowel or colonic transit. Despite apparent slowing of gastric emptying in healthy volunteers, observational [38] and uncontrolled open label data [39] suggest some symptomatic benefit of oral or inhaled cannabinoid in patients with gastroparesis.
Cannabis—gastrointestinal related adverse effects
Cannabinoid hyperemesis syndrome
A finding of severe vomiting episodes in some chronic cannabis users has been noted in recent decades. First described in 2004 by Allen et al., cannabinoid hyperemesis syndrome (CHS) is now defined by the Rome IV criteria as stereotypical episodic vomiting, occurring in patients who consume cannabis long-term, who experience symptom resolution with cannabis cessation, and may be associated with pathologic bathing behavior [40, 41]. Chronic cannabis use is the sine qua non of the diagnosis of CHS, and cannabis use of > 4 times/week for at least a year preceding the onset of cyclic vomiting is considered necessary for the development of CHS. Symptom relief with hot baths is also seen in cyclic vomiting syndrome (CVS); hence CHS may be a subset of CVS [42]. On a population level, the relatively recent emergence of this disorder appears to correlate with increased access to and THC concentration of potency of cannabis, although the mechanism by which this occurs is unclear.
Cannabis inhibits centrally and peripherally mediated emesis via interactions of THC with centrally-located CB1 receptors in the dorsal vagal complex [43]. Why cannabis use is pro-emetogenic in CHS is incompletely understood, but likely multifactorial. Downregulation of CB1 receptors in the central nervous system of chronic cannabis users may impair the ability of the hypothalamic-pituitary-adrenal axis to recover from stress, and cannabinoids have been shown to increase hypothalamic expression of corticotrophin-releasing hormone and stress hormones (e.g., adrenocorticotropic hormone and glucocorticoid); the combination of which may lead to dysregulation of the sympathetic nervous system [44–46].
As previously mentioned, the endocannabinoid system has inhibitory effects on gut motility. In humans, oral THC reduces gastric emptying and patients with slow transit constipation have increased expression of endogenous endocannabinoids and higher CB1 receptor expression [37, 47]. Transient receptor potential vanilloid 1 (TRPV1) receptors are co-expressed on neurons alongside CB1 receptors throughout the gastrointestinal tract, as well as the chemoreceptor trigger zone, and may exert anti-emetic effects through depletion of substance P. Prolonged exposure to cannabis can lead to dephosphorylation of TRPV1, receptor desensitization, and uncontrolled emesis [48]. TRPV1 is expressed in skin, and receptor activation with elevated temperature (e.g., hot showers) or agonists (e.g., topical capsaicin) may explain the apparent symptomatic improvement from these therapies during attacks [49, 50].
Currently, complete cannabis abstinence has been the only reported approach providing long-term symptom resolution [51]. It is unclear how long patients must be abstinent for their symptoms to start improving, which can vary based on the cyclical nature of attacks, as well as the propensity for THC to accumulate within adipose tissue leading to a prolonged elimination half-life. Traditional anti-emetics, such as anti-histamines (e.g., dimenhydrinate), serotonin 5-HT3 receptor antagonists (e.g., ondansetron), and dopamine antagonists (e.g., metoclopramide), have proven minimally effective [52]. There is a dearth of high-quality studies looking at the efficacy of specific pharmacologic agents as prophylaxis or abortive therapy in CHS [53•]. Benzodiazepines have been reported to be effective in case studies [53•]. Two prospective studies examined the role of tricyclic antidepressants for long-term therapy in CVS; in both these studies, patients who were reported to use cannabis had symptomatic improvement (74–93% at 3 months) [54, 55]. However, these patients did not necessarily meet defined criteria for CHS, and the concurrent cessation of cannabis use may be a potential confounder. Due to a lack of rigorous studies on prophylactic and abortive therapies in CHS, CVS therapies may be offered to patients with CHS; however, there are specific therapies that show some promise on CHS.
Topical capsaicin shows promise as abortive therapy and has been described to be effective in case reports and series, although the quality of data is low [56]. A retrospective cohort study of 43 patients reported no significant reduction in the length of stay in the emergency department with topical capsaicin, but the use of topical capsaicin resulted in a fewer additional medication doses (4 vs. 3 doses, p = 0.015) and a numerical reduction in opioid use (166.5 vs. 69 mg oral morphine equivalents) [57]. There may be a role for haloperidol in the acute and chronic management of CHS, possibly through interactions between CB1 and dopamine D2 receptors, although this has not yet been prospectively evaluated [53, 58].
Pancreatitis
The data for cannabis as a risk factor for acute pancreatitis is conflicting, with case reports and series suggesting a link, and population-based studies suggesting the opposite. Barkin et al reported 26 cases of cannabis-induced acute pancreatitis; in 69.2% of patients, development of acute pancreatitis correlated with increased cannabis use, and recurrent acute pancreatitis temporally related to cannabis use was seen in 57.7% of patients [59]. In a prospective study of 66 patients aged ≤ 35 years with acute pancreatitis, cannabis was the putative etiology in 13% of cases, which was significantly higher than the 1% of patients with acute pancreatitis in a comparative cohort of 243 patients aged > 35 years (p < 0.0001) [60]. Furthermore, in a retrospective study of 460 patients admitted with an initial episode of acute pancreatitis, cannabis use was documented in 10% patients diagnosed with idiopathic pancreatitis, with an overall prevalence of 2% in all patients with acute pancreatitis; however, cannabis use was not associated with mortality, length of stay, or recurrence [61].
The potential pathogenesis of cannabis-induced pancreatitis remains unknown, but ostensibly involves CB1 receptors which are present in pancreatic β cells. In rodent models, CB1 receptor activation was shown to increase pancreatic tissue damage (through activation of serum pancreatic enzymes and increased serum concentrations of pro-inflammatory interleukin-1β) [62], and induce β cell death (via activation of the pro-apoptotic protein, BCL2 associated agonist of cell death) [63]. Conversely, a nonselective cannabinoid agonist ameliorated pancreatitis in CB1 receptor-negative mice, ostensibly through activation of CB2, preventing activation of pro-apoptotic kinases via inhibition of pro-inflammatory cytokines (e.g., IL-6) [64].
Population-based studies have not suggested any significant risk of acute pancreatitis with cannabis. Analysis of self-reported data from the National Surveys on Drug Use and Health (2005–2007) involving 29,195 respondents aged 35–49 showed no association between duration of cannabis use and lifetime risk of pancreatitis, once controlled for confounders [65].
Cannabis use was actually shown to be protective in an analysis of the National Inpatient Sample (NIS) database (2003–2013), where acute pancreatitis patients with exposure to cannabis (defined by ICD-9 coding) had significantly lower inpatient mortality (OR: 0.17 [0.06–0.53]) and were less likely to develop systemic organ failure including acute kidney injury, acute respiratory distress syndrome, and shock [66]. Further analysis from the NIS database demonstrated that cannabis use was associated with a decreased incidence of acute pancreatitis and chronic pancreatitis (aOR: 0.50 [0.48 to 0.53] and 0.77 [0.71 to 0.84] respectively), in patients with a history of alcohol abuse [67].
Hence, while the link between cannabis use and pancreatitis is poorly defined and warrants further investigation, a thorough history querying cannabis use may shed light on patients with otherwise unexplained pancreatitis.
Summary and future directions
In general, while substantial observational data suggests cannabis improves symptoms in IBD, prospective experimental data (other than a preliminary observation in abstract form) does not support any material improvement in inflammation based on studies to date. This may not be surprising given the burden of inflammation seen in active IBD patients, many of whom have not responded to much stronger anti-inflammatory medications such as steroids in those trials.
Mechanistic studies in functional gut disorders suggest probable effects on slowing gastric emptying and reducing colonic contractile activity, but the magnitude of these effects appears modest in short-term dosing. Although sensory thresholds were not changed, it remains likely that cannabinoids, like other neuromodulator medications, may take some time to work; single or a few doses may not be enough to show changes in sensation, let alone achieve steady state kinetics. Despite the traditional use for cannabis as an anti-diarrheal, its known anti-cholinergic effects, and the positive symptomatic benefits in IBD, it remains surprising that it has not yet been prospectively studied in IBS.
In the vast majority of cases, gastroenterologists at present are more likely to ask patients to stop cannabis rather than prescribing it for them. Chronic cannabis use may cause CHS, in addition to other non-gastrointestinal related adverse effects [4]. The risk of pancreatitis, however, appears to be less of a concern. In any case, the prudent approach would be to suggest abstinence where cannabis use is felt to be linked to symptoms. Until then, clinicians can encourage lower-risk cannabis use advice for their patients (such as avoiding daily use and using lower THC formulations if abstinence cannot be achieved) [4].
For both IBD and functional disorder research, many aspects of cannabinoid use still need to be determined. Ideal dosing and duration, and balancing desired clinical effects against intoxicating psychoactive effects, in particular are major considerations. Cannabis is also in the unusual position of being simultaneously available for both medical and recreational use in many places, which further affects the ability to conduct research. Considerable headwinds to larger scale trials still exist, mostly based on legal and intellectual property considerations. Despite that being the case, promise still exists for the use of cannabinoids in gastrointestinal related gut function and more research is warranted.
Compliance with Ethical Standards
Conflict of Interest
CNA has been on medical advisory boards for Abbvie, Allergan, Pendopharm, Lupin, Gemelli Biotech, and Newstrike. He has received research funding from Allergan and Janssen. He is a board member of Callitas Health, and a principal of Visceral Therapeutics, Inc. YN reports speaker fees and grant support from Allergan. MW reports speaker fees from Proctor and Gamble.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Storr MA, Yüce B, Andrews CN, Sharkey KA. The role of the endocannabinoid system in the pathophysiology and treatment of irritable bowel syndrome. Neurogastroenterol Motil. 2008;20(8):857–868. doi: 10.1111/j.1365-2982.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 2.Sharkey KA, Wiley JW. The role of the endocannabinoid system in the brain-gut axis. Gastroenterology. 2016;151(2):252–266. doi: 10.1053/j.gastro.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinol Monograph. doi:https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf. Accessed 8 June 2020.
- 4.Fischer B, Russell C, Sabioni P, van den Brink W, Le Foll B, Hall W, et al. Lower-risk cannabis use guidelines: a comprehensive update of evidence and recommendations. Am J Public Health. 2017;107(8):e1–e12. doi: 10.2105/AJPH.2017.303818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overview | Cannabis-based medicinal products | Guidance | NICE. doi:https://www.nice.org.uk/guidance/ng144. Accessed 8 June 2020.
- 6.Andrews CN, Devlin SM, Le Foll B, Fischer B, Tse F, Storr M, et al. Canadian Association of Gastroenterology Position Statement: use of cannabis in gastroenterological and hepatic disorders. J Can Assoc Gastroenterol. 2019;2(1):37–43. doi: 10.1093/jcag/gwy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129(2):437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Picardo S, Kaplan GG, Sharkey KA, Seow CH. Insights into the role of cannabis in the management of inflammatory bowel disease. Ther Adv Gastroenterol. 2019;12:1756284819870977. doi: 10.1177/1756284819870977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couch DG, Maudslay H, Doleman B, Lund JN, O’Sullivan SE. The use of cannabinoids in colitis: a systematic review and meta-analysis. Inflamm Bowel Dis. 2018;24(4):680–697. doi: 10.1093/ibd/izy014. [DOI] [PubMed] [Google Scholar]
- 10.Alhouayek M, Muccioli GG. The endocannabinoid system in inflammatory bowel diseases: from pathophysiology to therapeutic opportunity. Trends Mol Med. 2012;18(10):615–625. doi: 10.1016/j.molmed.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Strisciuglio C, Bellini G, Miele E, Martinelli M, Cenni S, Tortora C, Tolone C, Miraglia del Giudice E, Rossi F. Cannabinoid receptor 2 functional variant contributes to the risk for pediatric inflammatory bowel disease. J Clin Gastroenterol. 2018;52(5):e37–e43. doi: 10.1097/MCG.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 12.Storr M, Emmerdinger D, Diegelmann J, Pfennig S, Ochsenkühn T, Göke B, Lohse P, Brand S. The cannabinoid 1 receptor (CNR1) 1359 G/A polymorphism modulates susceptibility to ulcerative colitis and the phenotype in Crohn’s disease. PLoS One. 2010;5(2):e9453. doi: 10.1371/journal.pone.0009453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storr M, Emmerdinger D, Diegelmann J, Yüce B, Pfennig S, Ochsenkühn T, et al. The role of fatty acid hydrolase gene variants in inflammatory bowel disease. Aliment Pharmacol Ther. 2009;29(5):542–551. doi: 10.1111/j.1365-2036.2008.03910.x. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A, Friedenberg F. Patterns of cannabis use in patients with inflammatory bowel disease: a population based analysis. Drug Alcohol Depend. 2015;156:84–89. doi: 10.1016/j.drugalcdep.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014;20(3):472–480. doi: 10.1097/01.MIB.0000440982.79036.d6. [DOI] [PubMed] [Google Scholar]
- 16.Phatak UP, Rojas-Velasquez D, Porto A, Pashankar DS. Prevalence and patterns of marijuana use in young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;64(2):261–264. doi: 10.1097/MPG.0000000000001474. [DOI] [PubMed] [Google Scholar]
- 17.Hoffenberg EJ, McWilliams S, Mikulich-Gilbertson S, Murphy B, Hoffenberg A, Hopfer CJ. Cannabis oil use by adolescents and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2019;68(3):348–352. doi: 10.1097/MPG.0000000000002189. [DOI] [PubMed] [Google Scholar]
- 18.Ravikoff Allegretti J, Courtwright A, Lucci M, Korzenik JR, Levine J. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(13):2809–2814. doi: 10.1097/01.MIB.0000435851.94391.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lal S, Prasad N, Ryan M, Tangri S, Silverberg MS, Gordon A, Steinhart H. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23(10):891–896. doi: 10.1097/meg.0b013e328349bb4c. [DOI] [PubMed] [Google Scholar]
- 20.Naftali T, Lev LB, Yablecovitch D, Half E, Konikoff FM. Treatment of Crohn’s disease with cannabis: an observational study. Isr Med Assoc J. 2011;13(8):455–458. [PubMed] [Google Scholar]
- 21.Mbachi C, Attar B, Wang Y, Paintsil I, Mba B, Fugar S, Agrawal R, Simons-Linares RC, Jaiswal P, Trick W, Kotwal V. Association between cannabis use and complications related to Crohn’s disease: a retrospective cohort study. Dig Dis Sci. 2019;64(10):2939–2944. doi: 10.1007/s10620-019-05556-z. [DOI] [PubMed] [Google Scholar]
- 22.Naftali T, Bar-Lev Schleider L, Sklerovsky Benjaminov F, Lish I, Konikoff FM, Ringel Y. Medical cannabis for inflammatory bowel disease: real-life experience of mode of consumption and assessment of side-effects. Eur J Gastroenterol Hepatol. 2019;31(11):1376–1381. doi: 10.1097/MEG.0000000000001565. [DOI] [PubMed] [Google Scholar]
- 23.Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11(10):1276–80.e1. doi: 10.1016/j.cgh.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 24.Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, Laish I, Benjaminov F, Konikoff FM. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig Dis Sci. 2017;62(6):1615–1620. doi: 10.1007/s10620-017-4540-z. [DOI] [PubMed] [Google Scholar]
- 25.Kafil TS, Nguyen TM, MacDonald JK, Chande N. Cannabis for the treatment of Crohn’s disease. Cochrane Database Syst Rev. 2018;11:CD012853. doi: 10.1002/14651858.CD012853.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irving PM, Iqbal T, Nwokolo C, Subramanian S, Bloom S, Prasad N, Hart A, Murray C, Lindsay JO, Taylor A, Barron R, Wright S. A randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflamm Bowel Dis. 2018;24(4):714–724. doi: 10.1093/ibd/izy002. [DOI] [PubMed] [Google Scholar]
- 27.Naftali T, Schlieder LBL, Benjaminov FS, Lish I, Konikoff FM. Sa1744-cannabis induces clinical and endoscopic improvement in moderately active ulcerative colitis. Gastroenterology. 2018;154(6):S-378. doi: 10.1016/s0016-5085(18)31568-3. [DOI] [Google Scholar]
- 28.Kafil TS, Nguyen TM, MacDonald JK, Chande N. Cannabis for the treatment of ulcerative colitis. Cochrane Database Syst Rev. 2018;11:CD012954. doi: 10.1002/14651858.CD012954.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 30.de Vries M, van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, van Goor H, Pain et al. Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. Clin Gastroenterol Hepatol. 2017;15(7):1079–86.e4. doi: 10.1016/j.cgh.2016.09.147. [DOI] [PubMed] [Google Scholar]
- 31.Camilleri M. Cannabinoids and gastrointestinal motility: pharmacology, clinical effects, and potential therapeutics in humans. Neurogastroenterol Motil. 2018;30(9):e13370. doi: 10.1111/nmo.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klooker TK, Leliefeld KEM, Van Den Wijngaard RM, Boeckxstaens GEE. The cannabinoid receptor agonist delta-9-tetrahydrocannabinol does not affect visceral sensitivity to rectal distension in healthy volunteers and IBS patients. Neurogastroenterol Motil. 2011;23(1):30–5, e2. 10.1111/j.1365-2982.2010.01587.x. [DOI] [PubMed]
- 33.Wong BS, Camilleri M, Busciglio I, Carlson P, Szarka LA, Burton D, et al. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology. 2011;141(5):1638–1647. doi: 10.1053/j.gastro.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong BS, Camilleri M, Eckert D, Carlson P, Ryks M, Burton D, Zinsmeister AR. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol Motil. 2012;24(4):358–e169. doi: 10.1111/j.1365-2982.2011.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dothel G, Chang L, Shih W, Barbaro MR, Cremon C, Stanghellini V, de Ponti F, Mayer EA, Barbara G, Sternini C. μ-opioid receptor, β-endorphin, and cannabinoid receptor-2 are increased in the colonic mucosa of irritable bowel syndrome patients. Neurogastroenterol Motil. 2019;31(11):e13688. doi: 10.1111/nmo.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olorinab (APD371) - Arena Pharmaceuticals.
- 37.Esfandyari T, Camilleri M, Ferber I, Burton D, Baxter K, Zinsmeister AR. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006;18(9):831–838. doi: 10.1111/j.1365-2982.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 38.Jehangir A, Parkman HP. Cannabinoid use in patients with gastroparesis and related disorders: prevalence and benefit. Am J Gastroenterol. 2019;114(6):945–953. doi: 10.14309/ajg.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 39.Barbash B, Mehta D, Siddiqui MT, Chawla L, Dworkin B. Impact of cannabinoids on symptoms of refractory gastroparesis: a single-center experience. Cureus. 2019;11(12):e6430. doi: 10.7759/cureus.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53(11):1566–1570. doi: 10.1136/gut.2003.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanghellini V, Chan FKL, Hasler WL, Malagelada JR, Suzuki H, Tack J, Talley NJ. Gastroduodenal disorders. Gastroenterology. 2016;150(6):1380–1392. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Venkatesan T, Levinthal DJ, Li BUK, Tarbell SE, Adams KA, Issenman RM, et al. Role of chronic cannabis use: cyclic vomiting syndrome vs cannabinoid hyperemesis syndrome. Neurogastroenterol Motil. 2019;31(Suppl 2):e13606. doi: 10.1111/nmo.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharkey KA, Darmani NA, Parker LA. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol. 2014;722:134–146. doi: 10.1016/j.ejphar.2013.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145(12):5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- 45.Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145(1):323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Richards JR. Cannabinoid hyperemesis syndrome: a disorder of the HPA axis and sympathetic nervous system? Med Hypotheses. 2017;103:90–95. doi: 10.1016/j.mehy.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S-C, Wang W-L, Su P-J, Jiang K-L, Yuan Z-W. Decreased enteric fatty acid amide hydrolase activity is associated with colonic inertia in slow transit constipation. J Gastroenterol Hepatol. 2014;29(2):276–283. doi: 10.1111/jgh.12346. [DOI] [PubMed] [Google Scholar]
- 48.Rudd JA, Nalivaiko E, Matsuki N, Wan C, Andrews PL. The involvement of TRPV1 in emesis and anti-emesis. Temperature (Austin) 2015;2(2):258–276. doi: 10.1080/23328940.2015.1043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards JR, Lapoint JM, Burillo-Putze G. Cannabinoid hyperemesis syndrome: potential mechanisms for the benefit of capsaicin and hot water hydrotherapy in treatment. Clin Toxicol. 2018;56(1):15–24. doi: 10.1080/15563650.2017.1349910. [DOI] [PubMed] [Google Scholar]
- 50.Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ, et al. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci. 2007;25(9):2773–2782. doi: 10.1111/j.1460-9568.2007.05521.x. [DOI] [PubMed] [Google Scholar]
- 51.Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc. 2012;87(2):114–119. doi: 10.1016/j.mayocp.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lapoint J, Meyer S, Yu C, Koenig K, Lev R, Thihalolipavan S, Staats K, Kahn C. Cannabinoid hyperemesis syndrome: public health implications and a novel model treatment guideline. West J Emerg Med. 2018;19(2):380–386. doi: 10.5811/westjem.2017.11.36368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richards JR, Gordon BK, Danielson AR, Moulin AK. Pharmacologic Treatment of cannabinoid hyperemesis syndrome: a systematic review. Pharmacotherapy. 2017;37(6):725–734. doi: 10.1002/phar.1931. [DOI] [PubMed] [Google Scholar]
- 54.Hejazi RA, Reddymasu SC, Namin F, Lavenbarg T, Foran P, McCallum RW. Efficacy of tricyclic antidepressant therapy in adults with cyclic vomiting syndrome: a two-year follow-up study. J Clin Gastroenterol. 2010;44(1):18–21. doi: 10.1097/MCG.0b013e3181ac6489. [DOI] [PubMed] [Google Scholar]
- 55.Namin F, Patel J, Lin Z, Sarosiek I, Foran P, Esmaeili P, et al. Clinical, psychiatric and manometric profile of cyclic vomiting syndrome in adults and response to tricyclic therapy. Neurogastroenterol Motil. 2007;19(3):196–202. doi: 10.1111/j.1365-2982.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 56.McConachie SM, Caputo RA, Wilhelm SM, Kale-Pradhan PB. Efficacy of capsaicin for the treatment of cannabinoid hyperemesis syndrome: a systematic review. Ann Pharmacother. 2019;53(11):1145–1152. doi: 10.1177/1060028019852601. [DOI] [PubMed] [Google Scholar]
- 57.Wagner S, Hoppe J, Zuckerman M, Schwarz K, McLaughlin J. Efficacy and safety of topical capsaicin for cannabinoid hyperemesis syndrome in the emergency department. Clin Toxicol. 2020;58(6):471–475. doi: 10.1080/15563650.2019.1660783. [DOI] [PubMed] [Google Scholar]
- 58.Schulze DR, Carroll FI, McMahon LR. Interactions between dopamine transporter and cannabinoid receptor ligands in rhesus monkeys. Psychopharmacology. 2012;222(3):425–438. doi: 10.1007/s00213-012-2661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barkin JA, Nemeth Z, Saluja AK, Barkin JS. Cannabis-induced acute pancreatitis: a systematic review. Pancreas. 2017;46(8):1035–1038. doi: 10.1097/MPA.0000000000000873. [DOI] [PubMed] [Google Scholar]
- 60.Culetto A, Bournet B, Peron J-M, Alric L, Buscail L. Prospective evaluation of the aetiological profile of acute pancreatitis in young adult patients. Pancreatology. 2015;15(3):S66–SS7. doi: 10.1016/j.pan.2015.05.252. [DOI] [PubMed] [Google Scholar]
- 61.Simons-Linares CR, Barkin JA, Wang Y, Jaiswal P, Trick W, Bartel MJ, Barkin JS. Is there an effect of cannabis consumption on acute pancreatitis? Dig Dis Sci. 2018;63(10):2786–2791. doi: 10.1007/s10620-018-5169-2. [DOI] [PubMed] [Google Scholar]
- 62.Dembiński A, Warzecha Z, Ceranowicz P, Dembiński M, Cieszkowski J, Pawlik WW, et al. Cannabinoids in acute gastric damage and pancreatitis. J Physiol Pharmacol. 2006;57(Suppl 5):137–154. [PubMed] [Google Scholar]
- 63.Kim W, Lao Q, Shin Y-K, Carlson OD, Lee EK, Gorospe M, et al. Cannabinoids induce pancreatic β-cell death by directly inhibiting insulin receptor activation. Sci Signal. 2012;5(216):ra23. doi: 10.1126/scisignal.2002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michler T, Storr M, Kramer J, Ochs S, Malo A, Reu S, Göke B, Schäfer C. Activation of cannabinoid receptor 2 reduces inflammation in acute experimental pancreatitis via intra-acinar activation of p38 and MK2-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol. 2013;304(2):G181–G192. doi: 10.1152/ajpgi.00133.2012. [DOI] [PubMed] [Google Scholar]
- 65.Han B, Gfroerer JC, Colliver JD. Associations between duration of illicit drug use and health conditions: results from the 2005-2007 national surveys on drug use and health. Ann Epidemiol. 2010;20(4):289–297. doi: 10.1016/j.annepidem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Simons-Linares CR, Barkin JA, Jang S, Bhatt A, Lopez R, Stevens T, Vargo J, Barkin JS, Chahal P. The impact of cannabis consumption on mortality, morbidity, and cost in acute pancreatitis patients in the United States: a 10-year analysis of the National Inpatient Sample. Pancreas. 2019;48(6):850–855. doi: 10.1097/MPA.0000000000001343. [DOI] [PubMed] [Google Scholar]
- 67.Adejumo AC, Akanbi O, Adejumo KL, Bukong TN. Reduced risk of alcohol-induced pancreatitis with cannabis use. Alcohol Clin Exp Res. 2019;43(2):277–286. doi: 10.1111/acer.13929. [DOI] [PubMed] [Google Scholar]