Abstract
Chlorine (Cl2) and bromine (Br2) are produced in large quantities throughout the world and used in the industry and the sanitation of water. They pose a significant threat to public health when released into the atmosphere during transportation and industrial accidents as well as acts of terrorism. In this review, we discuss evidence showing that Cl2, Br2, and products formed by their interaction with biomolecules fragment high-molecular-weight hyaluronan (HMW-HA), a key component of the interstitial space and also present in epithelial cells, to form proinflammatory, low-molecular-weight hyaluronan (LMW-HA) fragments, increasing intracellular calcium (Ca2+) and activating Ras homolog family member A (RhoA) in airway smooth muscle, epithelial, and microvascular cells. These changes result in airway hyperresponsiveness (AHR) to methacholine and increased epithelial and microvascular permeability. The increase of intracellular Ca2+ is the result of the activation of the calcium-sensing receptor (CaSR) by Cl2, Br2, and their byproducts. Post-halogen administration of a commercially available form of HMW-HA to mice and airway cells reverses the increase of Ca2+ and the activation of RhoA and restores AHR to near-normal levels of airway function. These data establish the potential of HMW-HA as a countermeasure against Cl2 and Br2 toxicity.
Keywords: chlorine, bromine, chlorinated lipids, airway reactivity, membrane potential
Graphical abstract:
In this review, we discuss evidence showing that Cl2, Br2, and products formed by their interaction with biomolecules fragment high-molecular-weight hyaluronan, a key component of the interstitial space and also present in epithelial cells, to form proinflammatory, low-molecular-weight hyaluronan fragments, increasing intracellular calcium and activating Ras homolog family member A in airway smooth muscle, epithelial, and microvascular cells
Introduction and purpose
The purpose of our review is to discuss the mechanisms by which exposure of animals and humans to chlorine (Cl2) and bromine (Br2) results in acute airway injury, manifesting in airway hyperresponsiveness (AHR) to methacholine. This is a test used clinically to diagnose whether an individual has or is likely to develop asthma (1). These halogens, because of their high reactivity with biomolecules, interact with components of the airway lining fluid and apical membranes of the airway and alveolar epithelial cells (ECs). The mechanisms by which these halogens damage airway smooth muscle cells (ASMCs) and result in airway vasoconstriction before or after a subsequent challenge with methacholine or allergens have not been elucidated. Herein, we review our published work, as well as the work of others, showing that the halogens Cl2 and Br2, and their hydration products, hypochlorous (HClO) and hypobromous (HBrO) acids, react with plasmalogens (a class of membrane glycerophospholipids containing alcohol with vinyl-ether bond at the sn-1 position, and enriched in polyunsaturated fatty acids at the sn-2 position of the glycerol backbone), which are key components of pulmonary surfactant and membrane lipids. These reactions form reactive intermediates, halogenated lipids, with a much longer half-life, detected in alveolar spaces, lung tissues, and plasma. These intermediates act as biomarkers and mediators of halogen injury. Halogens and halogenated lipids fragment hyaluronan or hyaluronic acid (HA), a key component of the interstitial space, and also present in ECs, to form the proinflammatory, low-molecular-weight (LMW)-HA fragments, capable of increasing intracellular calcium (Ca2+) and activating Ras homolog family member A (RhoA),(2) increasing AHR, and alveolar permeability to proteins (2;3;4). The increase in Ca2+ is brought about by the activation of the calcium-sensing receptor (CaSR) of ASMCs by Cl2, Br2, and halogenated lipids. Finally, we will present evidence showing that post halogen administration of a commercially available form of high-molecular-weight (HMW)-HA to mice, reverses the toxic effects of halogens and restores AHR and alveolar permeability to normal levels. We will concentrate mainly on the pathophysiology of Cl2-induced lung injury and provide some data showing that similar results were obtained following exposure to Br2.
Exposure to toxic gases presents a significant threat to public health
The halogens Cl2 and Br2 are produced in large quantities throughout the world and used extensively in various manufacturing processes and the sanitation of water. They are very reactive gases and pose a significant threat to public health when released into the atmosphere in large quantities during transportation and industrial accidents, as well as acts of terrorism (5-7).
Worldwide production of Cl2 and Br2 exceeds millions of tons per year; the list of the producing countries is maintained by the World Chlorine Counsel and sustainable development (www.worldchlorine.com). Current industrial uses for Cl2 and Br2 include pulp bleaching, waste sanitation, organic compound and pharmaceutical manufacturing, drinking water treatment, and maintenance of pathogen-free swimming pools. Exposure to Cl2 or Br2 may lead to skin disorders (8), bronchospasm, acute lung injury (ALI), peribronchiolar abscess, delay in healing (9), and death from cardiorespiratory failure (10-14).
Cl2 and Br2, are produced in central facilities and transported to manufacturing plants by rail or trucks, raising the possibility that major accidents may occur. Indeed, industrial and transportation accidents have resulted in the release of these toxic gases into the atmosphere with disastrous consequences. For example, a Norfolk Southern train was diverted into a side rail by accident where it crashed into a parked tanker car filled with Cl2. The tanker ruptured spilling 11,500 gallons of Cl2, which engulfed the city of Graniteville, South Carolina. Eight persons died before reaching medical care. Of the 71 persons hospitalized for acute health effects because of Cl2 exposure, one died in the hospital and 25 (35%) were admitted to intensive care with a median length of stay of 3 days (15). The number of hospital admissions for pulmonary and nonpulmonary causes increased significantly, following the release of Cl2 in South Carolina (16). Eight to ten months after the Graniteville incident, cough and shortness of breath were still persistent in those victims (17). Preliminary evidence demonstrates that mill workers residing in Graniteville at the time of the chlorine spill experienced accelerated FEV1 annual decline in 18 months after the chlorine incident (5). A long-term follow-up of the known victims is ongoing. Patients who survive ALI from any source often develop chronic lung disease with airflow obstruction, fibrosis (18), AHR, and impaired gas exchange (14;19-21). These patients often require hospitalization (6;16) and are predisposed to bacterial infections(22). AHR, also termed reactive airways dysfunction syndrome (RADS), has also been reported in individuals exposed to Cl2, and asthma-like symptoms can persist for long periods (15;23).
Other notable Cl2 exposure incidents resulting in serious casualties include an industrial accident at a hazardous waste facility in Apex, NC, on October 5, 2006 and the malfunction of a Cl2 delivery system in a Sacramento, CA waterpark on August 15, 2011, both of which led to numerous hospitalizations for severe respiratory complaints. On May 25, 2019, the release of Cl2 at Birmingham, AL, water treatment plant resulted in 55 workers being transported to local emergency rooms. Exposures could be also encountered in swimming pools and through the mixing of domestic cleaning products (24). An estimated 9,000 calls occur annually to U.S. poison control centers for Cl2 related exposures. (25).
Chlorine was used as a chemical warfare agent during the First World War when German forces waited for favorable meteorological conditions before opening cylinders of Cl2 to surprise unsuspecting forces caught downwind of the suffocating greenish-yellow cloud. More recently, during the Iraqi conflict, Cl2 cylinders have been bundled with traditional explosives, raising significant concerns regarding the possible reemergence of this agent as a chemical weapon against both combatants and civilians (26). Indeed, barrel bombs filled with Cl2 gas were used recently in Syria against civilians with disastrous consequences (https://www.bbc.com/news/world-middle-east-42944033/). Cl2 facilities are also implicated as a potential terrorist target. The U.S. Department of Homeland Security considers Cl2 production and storage facilities as potential high-risk targets that, according to the projections, could result in thousands of fatalities and up to 100,000 hospitalizations (the Homeland Security Council. Planning Scenarios: Executive Summaries. 004; 8–1).
Treatments following halogen gas exposure are mainly supportive, focusing on alleviating developed symptoms, such as bronchospasm, pulmonary edema, and upper and lower airway obstruction, including humidified oxygen administration, β2-agonists for bronchospasms and antibiotics for potential infections (27). In severe cases of acute respiratory distress syndrome (ARDS), supported ventilation with or without intubation and positive pressure ventilation may be necessary. Corticosteroids and sodium bicarbonate have been shown to alleviate ARDS following Cl2 exposure in animal studies, but their efficacy in improving respiratory function following human exposures has not been established (3;28). Therefore, animal models of halogen gas inhalation induced acute lung injury and ARDS provide us a unique opportunity to elucidate its basic mechanisms and to test novel therapeutic agents to reduce morbidity and mortality.
Animal models of Cl2-induced lung injury
Animal models in multiple species, including mice, rats, rabbits, pigs, sheep, and dogs (12,13;16;23;29-35), have been developed to study mechanisms of Cl2 toxicity and test the efficacy of a variety of therapeutic agents, which when administered postexposure either resolve or mitigate the injury. Rodents, rabbits, and other small animals are exposed to Cl2 in either environmental chambers or in the nose-only fashion. The advantages and disadvantages of each method have been described (36). Large animals, such as sheep, pigs, and dogs, are anesthetized and intubated at least during the exposure. Studies on large animals complement rodent studies and provide valuable information. However, the effects of anesthetics complicate the interpretation of the findings. Levels and duration of exposures can be based on studies (37) modeling the 2005 Graniteville Cl2 accident using the hazard prediction and assessment capability (HPAC) plume modeling system. Persons within 0.5 km from the epicenter were exposed to 1000 ppm Cl2 for 30 min and developed lethal cardiopulmonary injuries. Those within 2 km were exposed to 400–600 ppm for 30 min and developed severe but recoverable injuries. In agreement with these data, our data also show that exposure of adult male and female mice to 600 ppm for 45 min results in 90% mortality within 48 h (38;39) while mice exposed to 400–600 ppm for 30 min developed significant but recoverable injuries (19;20;29;40-43)
Mechanisms of Cl2-induced toxicity
When inhaled, Cl2 and Br2 and their hydration products, HOCl and HOBr, react with lung plasmalogens to form halogenated aldehydes (44;45). Major molecular species of the reactions of Cl2 with plasmalogens include 2-chloropalmitaldehyde (2-ClPALD) and 2-chlorostearaldehyde (2-ClSALD), which are either reduced to alcohol or oxidized to 2-chloropalmitic acid (2-ClPA) and 2-chlorostearic acid (2-ClSA), respectively. The fatty acids exist either in the esterified or free forms and may exert significant injury (45). We have shown the presence of significant levels of 2-ClPA and 2-ClSA in the lungs and plasma of mice even at 72 h post Cl2 exposure (45). These species were also detected in the plasma of persons exposed to Cl2 during the recent release of Cl2 in Birmingham, AL (46), in concentrations at least 100-fold higher than what has been reported in patients with sepsis (due to the endogenous production of HOCl by activated neutrophils) (47). The corresponding brominated species were also identified in the lungs and plasma of mice post Br2 exposure (44;48). Thus, these species along with additional ones formed by the reactions of HOCl with protein side chains, DNA, and lipids of the cells that line the airway epithelium; for instance, chloramines, byproducts of Cl2(49), could activate inflammatory cascades through stimulation of mitogen-activated protein kinase (MAPK) (40) and activation of nuclear factor-κB (NF-κB) via reversible IκBα (inhibitor of NF-κB) oxidation. The halogen injury will be amplified by the inflammatory response occurring as early as 12–24 h postexposure. Chlorinated and brominated species also increase the fragility of red blood cells resulting in the release of hemoglobin, which is oxidized to heme (46). Free heme stimulates the production of reactive species in the cytoplasm and mitochondria and causes extensive injury to all components of the blood–gas barrier (50).
Mechanisms of Cl2-induced AHR
Increased airway resistance and AHR are important pathological events of oxidative lung injury in general and Cl2 toxicity in particular, and result in persistent asthma-like symptoms, exacerbation of allergic airway inflammation, (19;20;23) which may progress to lung fibrosis (18;31). The transient receptor potential A1 (TRPA1) family of channels, present in sensory airway neurons, play an important role in the development of AHR in response to low (<50 ppm) concentrations of Cl2 (51). However, the mechanisms responsible for Cl2-induced AHR (when inhaled at concentrations, likely to be encountered near industrial accidents) have not been investigated adequately. For a thorough discussion of the various therapeutic agents that show promise in reversing chlorine-induced lung injury when administered postexposure, please see reviews in Refs. 3,16, and 52.
Below we discuss our studies showing low and HMW-HA play an important role in the development (low-molecular-weight) and treatment (high-molecular-weight) of chlorine-induced injury to airways. We start with a general discussion of the biochemistry and metabolism of HA, the evidence that it plays a role in the development and progression of acute and chronic lung injury, and then we discuss the role of these two forms of HA in the development and treatment of chlorine lung injury.
Basic biochemistry and metabolism of HA
HA is a linear polymer formed by a repeating disaccharide structure of glucuronic acid and N-acetyl glucosamine consisting of up to 25,000 disaccharide units (MW = 1–10 million Daltons). Mammalian HA is synthesized at the plasma membrane (53) by a family of membrane-bound glycosyltransferases, HA synthases (HAS)-1, -2, and -3. HAS enzymes are evolutionarily conserved, highly homologous (55–70% protein identity)(54), and catalyze the addition of UDP-D-glucuronic acid and UDP-N-acetyl-D-glucosamine monomers in an alternating assembly to form HA polymers (55). The HAS isoforms differ in half-life, stability, rate of HA synthesis, and affinity for HA substrates, which impacts their biological function (56) and, in particular, the molecular masses of their products. HAS1 and HAS2 synthesize larger polymers (2 × 105 to 2 × 106 Da), while HAS3 synthesizes shorter HA polymers (1 × 105 to 1 × 106 Da) (56). Because HA of different lengths also have different biological effects, differential expression of HAS enzymes may affect organ development, injury, and disease. HAS expression is regulated by a wide range of cytokines and growth factors (57). Dysregulation of HAS expression is relevant in disease and injury, consistent with the biological roles of HA in disease progression, wound healing, and tissue regeneration (57).
HA turnover is partly accomplished by hyaluronidases (HYALs). The HYAL family in mammals consists of hyaluronidases 1–4 (HYAL1–4), PH20, HYALP1, and possibly TMEM2 (58). HYALs are highly homologous endoglycosidases, which hydrolyze the β-1, for linkage of the HA molecule. HYALs functional range, however, is not identical; for example, HYAL1 to HYAL3 are primarily active at an acidic pH, while hyaluronidase PH20 has optimum activity at a neutral pH (58). HYAL1 and HYAL2 are the predominant isoforms functioning to catabolize HA in somatic tissues. HYAL1 and HYAL2 work together to catabolize HA into tetrasaccharides. HA is anchored to the cell surface through CD44 and HYAL2 and localized to lipid rafts in the cell membrane, where HA is first broken down to polymers of approximately 20 kDa (~50 disaccharide units). These fragments are internalized into lysosomes, where lysosomal HYAL1 further degrades the HA into tetrasaccharides (59)
Besides specific enzymatic degradation pathways, HA can also be fragmented by nonspecific pathways. This will be particularly relevant in the context of halogen-induced lung injury described below. Reactive oxygen species (ROS), including superoxide, hydrogen peroxide, nitric oxide, and peroxynitrite, and HClO and HBrO, degrade HA (2;4;60-62). Nonenzymatically produced, ambient ROS may be most relevant in environmental lung injury as described below; for example, inhaled ozone and Cl2 can fragment HMW-HA into LMW-HA in vitro, while neutralization of ROS through superoxide dismutase or its mimetics decreases HA degradation and resulting inflammation (63;64).
HMW-HA is a major structural component of the extracellular matrix; it promotes cell survival, has antiangiogenic properties and anti-inflammatory effects on immune cells, some of these mediated via binding to its receptors, CD44 Toll-like receptor (TLR)-2 and TLR4 (65-68). On the contrary, LMW-HA (L-HA ~300 kDa) fragments, act as endogenous innate immune ligands, and promote inflammatory responses, angiogenesis, and epithelial-to-mesenchymal transition (57;65;66;69). LMW-HA fragments stimulate cytokine production and activate the innate immune response via binding to CD44 and TLR signaling in a myeloid differentiation primary response 88 (MyD88)– and NF-κB–dependent fashion, whereas HMW-HA inhibits TLR2 signaling in vitro and in vivo (57). Binding of LMW-HA to CD44 is enhanced by inter-α-trypsin inhibitor (IαI), a serum protease inhibitor consisting of three polypeptides (70;71); the concentration of IαI in the bronchoalveolar lavage (BAL) increases considerably when blood gas permeability to plasma proteins increases (65;72). Increased concentrations of LMW-HA fragments have been found in a number of lung diseases, such as asthma, COPD, and pulmonary fibrosis. LMW-HA is both necessary and sufficient for the development of AHR after exposure to ozone (65;73), ischemia-reperfusion injury,(74) and various other forms of injury. LMW-HA increases vascular permeability by activating RhoA and ROCK (its downstream kinase), inducing cytoskeletal reorganization, and inhibiting cell – cell contacts (75). On the contrary, the administration of HMW-HA protects from the development of lung injury in ozone exposure (73), bleomycin administration (76), smoke inhalation, and sepsis (77;78). Others have also shown that HMW-HA inhibits the development of AHR in various models of lung injury (73;79-81) and the development of pulmonary fibrosis (82). A type of HMW-HA (Yabro®; ~1,000 kDa) has been shown to prevent exercise-induced bronchoconstriction in patients with asthma (83). No protective effects were seen following the administration of LMW-HA (84). Several studies indicate that the relevant factor for HMW-HA and Yabro protection is their size and not the source from which HMW-HA is derived (66;77;78).
Role of HA in halogen toxicity
Increased airway resistance and AHR are important pathological events following exposure to Cl2 and Br2, and results in persistent asthma-like symptoms and exacerbation of allergic airway inflammation (19;20;23), which may progress to lung fibrosis (14;18;31). The TRPA1 channels, present in sensory airway neurons, play an important role in the development of AHR in response to low (<50 ppm) concentrations of Cl2 (51). However, the mechanisms responsible for Cl2-induced AHR (when inhaled at concentrations, likely to be encountered near industrial accidents) have not been investigated adequately. We performed a number of studies to test the hypothesis that LMW-HA, generated by the action of Cl2, HOCl, and other secondary reactive intermediates on HMW-HA, was necessary and sufficient for the development of AHR in mice exposed to sublethal concentrations of Cl2. In a series of experiments, Lazrak et al. showed the presence of LMW-HA in the BAL (Fig. 1) and peribronchial spaces of C57BL/6 mice were exposed to Cl2 (400 ppm for 30 min) and returned to room air for up to 24 h (2;14). The increase in LMW-HA levels coincided with a significant increase in the expression of HA synthases, as well as hyaluronidases. There was also an increase in HA staining in the peribronchial space (Fig. 1). Concentrations of LMW-HA were also increased in the BAL fluid (BALF) of mice exposed to Br2 (600 ppm for 30 min) and returned to air (50). Instillation of HMW-HA in the external nares post Cl2 or Br2 exposure decreased the extent of AHR significantly (Fig. 2).(2;50) The Cl2-induced AHR was mitigated by instillation of an antibody against the IαI, which inhibits HA signaling (85). An additional, interesting finding was that mice infected with respiratory syncytial virus (RSV), the most common cause of lower respiratory tract infection in children (86), developed increased sensitivity to Cl2 gas, which was attributed to the presence of LMW-HA, a proinflammatory agent.
Figure 1.
Detection of HA and IαI in the BALF of Cl2-exposed mice. (A) Mice were exposed to Cl2 (400 ppm for 30 min) and returned to air. Values are means ± 1 standard error of the mean (SEM); a number of mice (n) in each group = 5; *# P < 0.01 compared with air and the value to its left at the same time point, respectively. (B) Agar gel electrophoresis of Yabro. Lanes: 1, HA Mega-HA™ Ladder (Hyalose, L.L.C.); 2, Select-HA™ HiLadder; 3, Yabro; 4, Yabro exposed to Cl2 (400 ppm for 30 min) and stored at −4 °C for 24 h; 5, sonicated Yabro; and 6, Select-HA LoLadder. (C) Agar gel electrophoresis of concentrated BALF from air and Cl2 exposed mice. Lanes: 1, Select-HA HiLadder; 2, Select-HA LoLadder; 3, 95% air and 5% CO 2 (air); 4 and 5, immediately post-Cl2; 6, 6 h post-Cl2; 7, 24 h post-Cl2; and 8, as in 7, but the BALF was treated with hyaluronidase, which degrades HA. In all cases, proteins were visualized with Stains-All (Sigma, St. Louis, MO). (D–F.) Representative image of mouse airways in naïve state (D) or 6 h (E) and 24 h (F) after Cl2 exposure. Increased HA staining (green, arrows) at 24 h in the peribronchial area surrounding ASMCs (red). Magnification: 200× (reprinted from Ref. 2 with permission). Abbreviations: HA, total hyaluronan; IαI, inter-α-trypsin inhibitor.
Figure 2.

AHR in Cl2-exposed mice was reversed by instillation of Yabro or an antibody against IαI. (A-D.) Mice were exposed to Cl2 (400 ppm for 30 min) and returned to room air for 24 hours. Yabro (HMW-HA; 3 mg/mL; panels A and B) or a blocking antibody against IαI; panels C–D) were administered in the external at 0.5 and 23 h postexposure. Airway resistance (R; panels A and C) and Newtonian resistance (RN; panels B and D) measured by flexiVent. Values are means ± 1 SEM; N ≥ 6 (mice); * # P < 0.05 compared with the corresponding air and Cl2 values in the same group. (E) R, measured 1 h post-intranasal instillation of either Yabro or Cl2-exposed H-HA. Values are means ± 1 SEM; n = 4 (mice); *#P < 0.05 compared with the corresponding air or H-HA value in the same group (reprinted from Ref. 2 with permission).
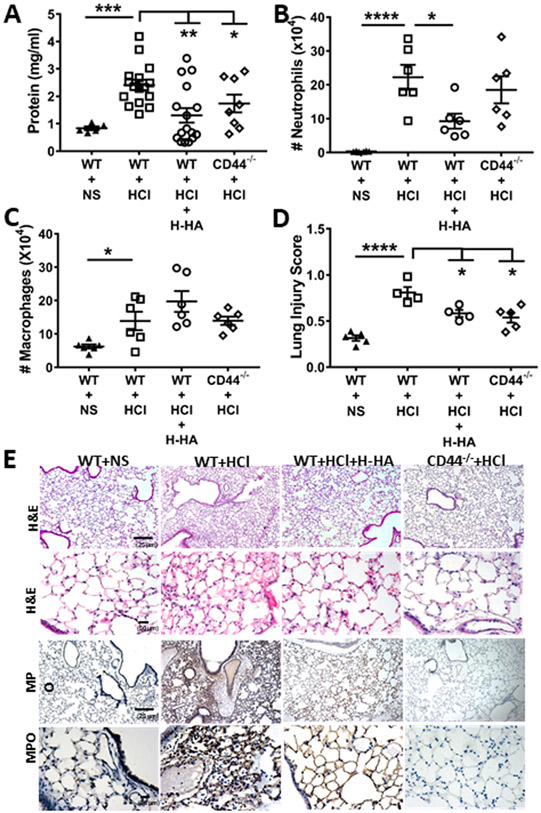
A major byproduct of the hydrolysis of Cl2, in addition to HOCl, is hydrochloric acid (HCl). HCl formed during Cl2 inhalation does not have a major contribution to injury caused by Cl2 and HOCl (87). At small concentrations, HCl is buffered by bicarbonate and unlike HOCl does not cause extensive lung injury. However, pulmonary aspiration of acid and/or gastric contents is considered to be a direct cause of ARDS (3),(88). There was a significant increase in LMW-HA mice IαI, as early as 1 h after HCl instillation, which persisted at least 24 h post HCl instillation (4). Intranasal instillation of HMW-HA post-HCl alleviated distal lung epithelial injury at 24 h postexposure, as shown by considerably lower levels of plasma protein concentration and a decreased number of inflammatory cells in their BAL, as well as near-normal lung histology (Fig. 3) and AHR. In addition, HMW-HA reversed HCl-induced lung injury to the pulmonary microvasculature, as shown by measurements of the filtration coefficients (Kf), a product of endothelial permeability and surface area, and of pulmonary edema, as shown by the reduction in the lung wet/dry weights (Fig. 4),(4) as well as the increase in transendothelial resistance across human lung bronchial ECs. Instillation of HCl in CD44−/− mice resulted in significantly less injury, strongly suggesting that LMW-HA was contributing, at least to a large extent, to this injury (Fig. 4). In subsequent in vitro studies, it was shown that the exposure of mouse or human lung airway cells to Cl2 or LMW-HA increased intracellular Ca2+ levels and RhoA activity in human airway smooth muscle (HASM) cells after exposure to Cl2. This resulted in activation of the RhoA downstream kinase ROCK2 and TMEM16, a Ca2+-activated Cl− channel present, resulting in membrane depolarization (50). Postexposure addition of HMW-HA reversed these effects (Fig. 5). Activation of RhoA contributes to the development of AHR, airway smooth muscle contractility, and increases microvascular permeability (89-91). Instillation of HCl in mice also activated lung RhoA and ROCK2, and postexposure instillation of HMW-HA returned these values to their control levels (4). Br2 and Cl2, as well as LMW-HA, increased the expression of the CaSR 24 h later (Fig. 6) (50). The effect of LMW-HA on the CaSR was reversed when cells were treated with HMW-HA, added after the incubation with LMW-HA. The importance of the CaSR in promoting AHR in mice is demonstrated by the fact that instillation of its inhibitor (calcilytic) NPS2143 at 1 and 23 h post Br2 exposure in the external nares resulted in normal AHR to methacholine (50).
Figure 3.
H-HA treatment or the lack of CD44 protein attenuates persistent lung injury and inflammation after HCl instillation. BALF protein (A), neutrophils (B), macrophages (C) and lung injury score (D) in WT or CD44−/− mice at 24 h post-HCl or NS; WT mice were also treated with H-HA (Yabro). (E) H&E staining of lung tissue at 24 h after HCl instillation shows the presence of hyaline membranes and protein exudates in alveolar spaces, as well as MPO staining (third row: 10×; and fourth row:40×) in WT mice. Reprinted from Ref. 4 with permission.
Figure 4.
Beneficial effects of H-HA following HCL injury in vivo. (A and B) Airway resistance prior by flexiVent® at 24 h in after NS, HCl instillation or H-HA treatment post HCl exposure. (means ± 1 SEM; ** P < 0.01 or less when comparing the WT + HCl group with the corresponding WT + NS control at the same dose of methacholine. (C and D) Pulmonary filtration coefficients (Kf) and wet-to-dry lung weights 24 h after HCl instillation in WT mice; (E) Electrical resistance values (RT) of confluent primary human bronchial epithelial cell monolayers seeded on ECSIS arrays before and L-HA or PBS followed H-HA or PBS. Reprinted from Ref. 4 with permission.
Figure 5.

Exposure of HASM cells to Cl2 or LMW-HA activates Rho and causes depolarization. (A) HASM cells were exposed to Cl2 and returned to 95% air/5% CO2. RhoA activity and protein, levels were measured by GLISA/ELISA. Values are means ± SEM; * P < 0.01; (B and C) HASM cells were exposed to different concentrations of LMW-HA with either HMW-HA (Yabro), IgG, or anti-IαI antibody; RhoA activation was measured as above. * P < 0.01. (D and E) Measurements of the membrane potential of HASM cells 1 h postexposure to Cl2 incubated with the reagents shown. *P < 0.05 compared with air (E). Reprinted from Ref. 2 with permission.
Figure 6.

(A) hpASMC (ASMCs harvested from normal human lungs, cultured for 2–4 passages), were exposed to air (lanes 1–3) or Br2 (100 ppm for 10 min; lanes 4–9). At 1 h post Br2 exposure, saline (lanes 4–6) or Yabro (150 μg/mL; lanes 7–9). Western blots showing immunostaining with an antibody against the CaSR 24 h later. (B) Quantification of the 250-kDa band for the conditions shown. Individual values for each lane and means ± 1 SEM; n = 5 for each condition. (C) Saline (lanes 1–3) or LMW-HA (150 μg/mL; lanes 4–6) were added into the medium of phASMC followed 1 h later by saline (lanes 4–6) or Yabro (150 μg/mL; lanes 7–9). They were immunostained with an antibody against the CaSR at 24 h postexposure. (D) Quantification of the 250-kDa band (the active form of the CaSR) for the conditions shown. (E) pmASMCs (mouse ASMCs in primary culture) treated with saline (lanes 1 and 2) or 150 μg/mL LMW-HA (lanes 3–6) for 24 h as described above. Western blots showing immunostaining with an antibody against the CaSR. (F and G) Quantitation of the 120- and 250-kDa bands of the CaSR. Data are individual values and means ± SE, n = 5 (reprinted from Ref. 50 with permission).
HMW-HA reverses LMW-HA–induced CaSR expression by either inhibiting downstream signaling cascades or by displacing LMW-HA from its receptors. It is also possible that activation of the CaSR may activate TPRC or other Ca2+ channels on the surface of ASMCs and HMW-HA prevents this from happening.
It is interesting to note that the exposure of C57BL/6 mice to Br2 (600 ppm for 30 min) also increases the concentration of LMW-HA in the BALF and AHR with similar mechanisms as for Cl2 (50). However, whereas the AHR in Cl2-exposed mice is caused by an increase in the Newtonian resistance (i.e., the resistance of the large airways), Br2-induced AHR is due to the tissue resistance (parameter G of the constant phase equation) that reflects tissue viscoelasticity and possibly the resistance of the small airways, which normally account for less than 10% of the total airway resistance.
Conclusions
Developing countermeasures, which when administered post Cl2 exposure, decrease acute and long-term injury, and mortality is a high priority of the CounterACT network. Yabro shows considerable promise in reversing acute lung injury to airways and distal lung regions when administered post Cl2 exposure by counteracting the deleterious effects of LMW-HA (Fig. 7). LMW-HA activates innate and adaptive immunity and increases permeability and airway resistance by activating RhoA and ROCK2, while HMW-HA has strong anti-inflammatory and prohomeostasis functions. The reason for this difference may be because of differences in receptor engagement, or cell uptake depending on size, but ultimately remains elusive. Recent work suggested that HMW-HA may create a transmembrane “picket fence” barrier, tethered on CD44 and the cellular cytoskeleton, which prevents ligands from reaching and activating their respective receptors, an effect that was abolished after HA degradation (92). HMW-HA also binds several extracellular proteins with strong anti-inflammatory potential, such as IαI, which is associated with decreased endothelial injury and organ dysfunction in sepsis (93). Furthermore, HMW-HA is a recently recognized crucial constituent of the endothelial glycocalyx, and HA homeostasis is central to the maintenance of a healthy endothelial barrier and the avoidance of tissue injury (94). Finally, HA has well-described antimicrobial properties, inhibiting bacterial adhesion and promoting phagocytosis (95). Thus, HMW-HA acts along with its binding partners to reduce inflammation and promote the antibacterial properties of the host. A major theoretical concern, whenever anti-inflammatory applications of HMW-HA are being discussed, is its potential degradation into smaller, proinflammatory fragments. It is interesting to note, however, that in our study, HMW-HA retained its large MW despite being in a presumably prodegradation environment for several hours. This agrees with the existing literature on the use of HMW-HA in lung inflammation and suggests that pharmacologically-dosed HMW-HA either overwhelms or somehow escapes the degrading activity of the inflammatory milieu and, therefore, is safe to use in this setting.
Figure 7.
The summary diagram depicting the damaging effects of LMW-HA and the protective effects of HMW-HA (Yabro) on the airway function.
Acknowledgments
Supported by the CounterACT Program, the National Institutes of Health Office of the Director (NIH OD), the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute of Environmental Health Sciences (NIEHS), Grant Numbers (5UO1 ES026458 03; 3UO1 ES026458 03S1; 5UO1 ES027697 02) to S.M., the National Heart, Lung and Blood Institute (NHLBI). S.G. is supported by the Division of Intramural Research, NIEHS (ZIA ES102605). Yabro was donated gratis by the IBSA Institut Biochimique, Lugano, Switzerland. We would like to thank Ms. Janet McDaniel and Ms. Emily Sher for their editorial assistance in the preparation of this manuscript.
Footnotes
Competing interests:
The authors declare no competing interests.
References
- 1.Brannan JD, and Lougheed MD 2012. Airway hyperresponsiveness in asthma: mechanisms, clinical significance, and treatment. Front Physiol 3:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr., Stober VP, Trempus CS, Garantziotis S et al. 2015. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am. J. Physiol Lung Cell Mol. Physiol 308:L891–L903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou T, Song WF, Shang Y, Yao SL, and Matalon S 2018. Halogen Inhalation-Induced Lung Injury and Acute Respiratory Distress Syndrome. Chin Med. J. (Engl. ) 131:1214–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou T, Yu Z, Jian MY, Ahmad I, Trempus C, Wagener BM, Pittet JF, Aggarwal S, Garantziotis S, Song W et al. 2018. Instillation of hyaluronan reverses acid instillation injury to the mammalian blood gas barrier. Am. J. Physiol Lung Cell Mol. Physiol 314:L808–L821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark KA, Karmaus WJ, Mohr LC, Cai B, Balte P, Gibson JJ, Ownby D, Lawson AB, Vena JE, and Svendsen ER 2016. Lung Function before and after a Large Chlorine Gas Release in Graniteville, South Carolina. Ann. Am. Thorac. Soc 13:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackie E, Svendsen E, Grant S, Michels JE, and Richardson WH 2014. Management of Chlorine Gas-Related Injuries From the Graniteville, South Carolina, Train Derailment. Disaster. Med. Public Health Prep 1–6. [DOI] [PubMed] [Google Scholar]
- 7.Abara W, Wilson S, Vena J, Sanders L, Bevington T, Culley JM, Annang L, Dalemarre L, and Svendsen E 2014. Engaging a chemical disaster community: lessons from Graniteville. Int. J. Environ. Res. Public Health 11:5684–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers JV, Price JA, Wendling MQ, Perry MR, Reid FM, Kiser RC, and Graham JS 2011. An assessment of transcriptional changes in porcine skin exposed to bromine vapor. J Biochem. Mol. Toxicol 25:252–262. [DOI] [PubMed] [Google Scholar]
- 9.Schlagbauer M, and Henschler D 1967. [Toxicity of chlorine and bromine in single and repeated inhalation]. Int. Arch. Arbeitsmed 23:91–98. [PubMed] [Google Scholar]
- 10.Lam A, Vetal N, Matalon S, and Aggarwal S 2016. Role of heme in bromine-induced lung injury. Ann. N. Y. Acad. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, and Matalon S 2016. Heme Attenuation Ameliorates Irritant Gas Inhalation-Induced Acute Lung Injury. Antioxid. Redox. Signal 24:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaky A, Ahmad A, Dell'Italia LJ, Jahromi L, Reisenberg LA, Matalon S, and Ahmad S 2015. Inhaled matters of the heart. Cardiovasc. Regen. Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honavar J, Doran S, Ricart K, Matalon S, and Patel RP 2017. Nitrite therapy prevents chlorine gas toxicity in rabbits. Toxicol. Lett 271:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal S, Ahmad I, Lam A, Carlisle MA, Li C, Wells JM, Raju SV, Athar M, Rowe SM, Dransfield MT et al. 2018. Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema. JCI. Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ et al. 2009. Acute health effects after exposure to chlorine gas released after a train derailment. Am. J. Emerg. Med 27:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summerhill EM, Hoyle GW, Jordt SE, Jugg BJ, Martin JG, Matalon S, Patterson SE, Prezant DJ, Sciuto AM, Svendsen ER et al. 2017. An Official American Thoracic Society Workshop Report: Chemical Inhalational Disasters. Biology of Lung Injury, Development of Novel Therapeutics, and Medical Preparedness. Ann. Am. Thorac. Soc 14:1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark KA, Chanda D, Balte P, Karmaus WJ, Cai B, Vena J, Lawson AB, Mohr LC, Gibson JJ, and Svendsen ER 2013. Respiratory symptoms and lung function 8-10 months after community exposure to chlorine gas: a public health intervention and cross-sectional analysis. BMC. Public Health 13:945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mo Y, Chen J, Schlueter CF, and Hoyle GW 2013. Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis. Am. J Physiol Lung Cell Mol. Physiol 304:L92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E et al. 2014. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J Physiol Lung Cell Mol. Physiol 307:L158–L172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, and Matalon S 2011. Postexposure Administration of a {beta}2-Agonist Decreases Chlorine-Induced Airway Hyperreactivity in Mice. Am. J. Respir. Cell Mol. Biol 45:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes WW, Keyser BM, Paradiso DC, Ray R, Andres DK, Benton BJ, Rothwell CC, Hoard-Fruchey HM, Dillman JF, Sciuto AM et al. 2016. Conceptual approaches for treatment of phosgene inhalation-induced lung injury. Toxicol. Lett 244:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cucinell SA 1974. Review of the toxicity of long-term phosgene exposure. Arch. Environ. Health 28:272–275. [DOI] [PubMed] [Google Scholar]
- 23.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, and Maghni K 2003. Chlorine-induced injury to the airways in mice. Am. J. Respir. Crit Care Med 168:568–574. [DOI] [PubMed] [Google Scholar]
- 24.White CW, and Martin JG 2010. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc. Am. Thorac. Soc 7:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker M, and Forrester M 2008. Pattern of chlorine gas exposures reported to Texas poison control centers, 2000 through 2005. Tex. Med 104:52–7, 51. [PubMed] [Google Scholar]
- 26.Bell DG 2008. Management of Acute Respiratory Distress Syndrome (ARDS) Following Chlorine Exposure. Am. J. Respir. Crit. Care Med 176:A314 (Abstr.) [Google Scholar]
- 27.Sexton JD, and Pronchik DJ 1998. Chlorine inhalation: the big picture. J. Toxicol. Clin. Toxicol 36:87–93. [DOI] [PubMed] [Google Scholar]
- 28.Pascuzzi TA, and Storrow AB 1998. Mass casualties from acute inhalation of chloramine gas. Mil. Med 163:102–104. [PubMed] [Google Scholar]
- 29.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, and Matalon S 2008. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am. J. Physiol Lung Cell Mol. Physiol 295:L733–L743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyle GW, and Svendsen ER 2016. Persistent effects of chlorine inhalation on respiratory health. Ann. N. Y. Acad. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mo Y, Chen J, Humphrey DM Jr., Fodah RA, Warawa JM, and Hoyle GW 2014. Abnormal Epithelial Structure and Chronic Lung Inflammation after Repair of Chlorine-Induced Airway Injury. Am. J Physiol Lung Cell Mol. Physiolajplung [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demnati R, Fraser R, Ghezzo H, Martin JG, Plaa G, and Malo JL 1998. Time-course of functional and pathological changes after a single high acute inhalation of chlorine in rats. Eur. Respir. J 11:922–928. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Winskog C, Edston E, and Walther SM 2005. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta Anaesthesiol. Scand 49:183–190. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Oldner A, Winskog C, Edston E, and Walther SM 2006. Effects of endothelin receptor antagonism on acute lung injury induced by chlorine gas. Crit Care Med. 34:1731–1737. [DOI] [PubMed] [Google Scholar]
- 35.Zaky A, Bradley WE, Lazrak A, Zafar I, Doran S, Ahmad A, White CW, Dell'Italia LJ, Matalon S, and Ahmad S 2015. Chlorine inhalation-induced myocardial depression and failure. Physiol Rep. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng YS, Bowen L, Rando RJ, Postlethwait EM, Squadrito GL, and Matalon S 2010. Exposing animals to oxidant gases: nose only vs. whole body. Proc. Am. Thorac. Soc 7:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jani DD, Reed D, Feigley CE, and Svendsen ER 2016. Modeling an irritant gas plume for epidemiologic study. Int. J. Environ. Health Res 26:58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, and Matalon S 2011. Ascorbate and deferoxamine administration after chlorine exposure decrease mortality and lung injury in mice. Am. J. Respir. Cell Mol. Biol 45:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarogiannis SG, and Matalon S 2011. Constrictive bronchiolitis in soldiers. N. Engl. J. Med 365:1743–1745. [DOI] [PubMed] [Google Scholar]
- 40.Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, and Matalon S 2010. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J. Biol. Chem 285:9716–9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazrak A, Chen L, Jurkuvenaite A, Doran SF, Liu G, Li Q, Lancaster JR Jr., and Matalon S 2012. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice. Am. J. Respir. Cell Mol. Biol 46:342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, and Matalon S 2012. Postexposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am. J. Respir. Cell Mol. Biol 46:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarogiannis SG, Wagener BM, Basappa S, Doran S, Rodriguez CA, Jurkuvenaite A, Pittet JF, and Matalon S 2014. Postexposure aerosolized heparin reduces lung injury in chlorine-exposed mice. Am. J Physiol Lung Cell Mol. Physiol 307:L347–L354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duerr MA, Palladino END, Hartman CL, Lambert JA, Franke JD, Albert CJ, Matalon S, Patel RP, Slungaard A, and Ford DA 2018. Bromofatty aldehyde derived from bromine exposure and myeloperoxidase and eosinophil peroxidase modify GSH and protein. J. Lipid Res 59:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ford DA, Honavar J, Albert CJ, Duerr MA, Oh JY, Doran S, Matalon S, and Patel RP 2016. Formation of chlorinated lipids post-chlorine gas exposure. J. Lipid Res 57:1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aggarwal S, Lazrak A, Ahmad I, Yu Z, Bryant A, Mobley J, Ford DA, and Matalon S 2020. Heme Impairs Alveolar Epithelial Sodium Channels Post Toxic Gas Inhalation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer NJ, Reilly JP, Feng R, Christie JD, Hazen SL, Albert CJ, Franke JD, Hartman CL, McHowat J, and Ford DA 2017. Myeloperoxidase-derived 2-chlorofatty acids contribute to human sepsis mortality via acute respiratory distress syndrome. JCI. Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmad S, Masjoan Juncos JX, Ahmad A, Zaky A, Wei CC, Bradley WE, Zafar I, Powell P, Mariappan N, Vetal N et al. 2019. Bromine inhalation mimics ischemia-reperfusion cardiomyocyte injury and calpain activation in rats. Am. J. Physiol Heart Circ. Physiol 316:H212–H223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Squadrito GL, Postlethwait EM, and Matalon S 2010. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am. J. Physiol Lung Cell Mol. Physiol 299:L289–L300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lazrak A, Yu Z, Doran S, Jian MY, Creighton J, Laube M, Garantziotis S, Prakash YS, and Matalon S 2020. Upregulation of airway smooth muscle calcium-sensing receptor by low-molecular-weight hyaluronan. Am. J. Physiol Lung Cell Mol. Physiol 318:L459–L471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bessac BF, and Jordt SE 2008. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology. (Bethesda. ) 23:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlisle M, Lam A, Svendsen ER, Aggarwal S, and Matalon S 2016. Chlorine-induced cardiopulmonary injury. Ann. N. Y. Acad. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laurent TC, and Fraser JR 1992. Hyaluronan. FASEB J. 6:2397–2404. [PubMed] [Google Scholar]
- 54.Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, and Jia X 2014. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 10:1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prehm P 1989. Identification and regulation of the eukaryotic hyaluronate synthase. Ciba Found. Symp 143:21–30. [DOI] [PubMed] [Google Scholar]
- 56.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y et al. 1999. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem 274:25085–25092. [DOI] [PubMed] [Google Scholar]
- 57.Jiang D, Liang J, and Noble PW 2011. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 91:221–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stern R, and Jedrzejas MJ 2006. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem. Rev 106:818–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erickson M, and Stern R 2012. Chain gangs: new aspects of hyaluronan metabolism. Biochem. Res. Int 2012:893947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rees MD, McNiven TN, and Davies MJ 2007. Degradation of extracellular matrix and its components by hypobromous acid. Biochem. J 401:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stankovska M, Hrabarova E, Valachova K, Molnarova M, Gemeiner P, and Soltes L 2006. The degradative action of peroxynitrite on high-molecular-weight hyaluronan. Neuro. Endocrinol. Lett 27 Suppl 2:31–34. [PubMed] [Google Scholar]
- 62.Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, and Arnhold J 2006. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 7:659–668. [DOI] [PubMed] [Google Scholar]
- 63.Gao F, Koenitzer JR, Tobolewski JM, Jiang D, Liang J, Noble PW, and Oury TD 2008. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J. Biol. Chem 283:6058–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campo GM, Avenoso A, D'Ascola A, Scuruchi M, Nastasi G, Micali A, Puzzolo D, Pisani A, Calatroni A, and Campo S 2013. The SOD mimic MnTM-2-PyP(5+) reduces hyaluronan degradation-induced inflammation in mouse articular chondrocytes stimulated with Fe (II) plus ascorbate. Int. J. Biochem. Cell Biol 45:1610–1619. [DOI] [PubMed] [Google Scholar]
- 65.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, and Hollingsworth JW 2010. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am. J. Respir. Crit Care Med 181:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 66.Jiang D, Liang J, and Noble PW 2010. Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat. Rec. (Hoboken. ) 293:982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, and Horton MR 2006. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol 177:1272–1281. [DOI] [PubMed] [Google Scholar]
- 68.Forteza R, Lieb T, Aoki T, Savani RC, Conner GE, and Salathe M 2001. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J. 15:2179–2186. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, and Noble PW 2011. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J. Exp. Med 208:1459–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bost F, Diarra-Mehrpour M, and Martin JP 1998. Inter-alpha-trypsin inhibitor proteoglycan family--a group of proteins binding and stabilizing the extracellular matrix. Eur. J. Biochem 252:339–346. [DOI] [PubMed] [Google Scholar]
- 71.Malki N, Balduyck M, Maes P, Capon C, Mizon C, Han KK, Tartar A, Fournet B, and Mizon J 1992. The heavy chains of human plasma inter-alpha-trypsin inhibitor: their isolation, their identification by electrophoresis and partial sequencing. Differential reactivity with concanavalin A. Biol. Chem. Hoppe Seyler 373:1009–1018. [DOI] [PubMed] [Google Scholar]
- 72.Lazrak A, Jurkuvenaite A, Ness EC, Zhang S, Woodworth BA, Muhlebach MS, Stober VP, Lim YP, Garantziotis S, and Matalon S 2014. Inter-alpha-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in DeltaF508 mice. Am. J Respir. Cell Mol. Biol 50:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA et al. 2009. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J. Biol. Chem 284:11309–11317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 74.Eldridge L, Moldobaeva A, and Wagner EM 2011. Increased hyaluronan fragmentation during pulmonary ischemia. Am. J. Physiol Lung Cell Mol. Physiol 301:L782–L788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lennon FE, and Singleton PA 2011. Hyaluronan regulation of vascular integrity. Am. J. Cardiovasc. Dis 1:200–213. [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA et al. 2005. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med 11:1173–1179. [DOI] [PubMed] [Google Scholar]
- 77.Huang PM, Syrkina O, Yu L, Dedaj R, Zhao H, Shiedlin A, Liu YY, Garg H, Quinn DA, and Hales CA 2010. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology. 15:1131–1139. [DOI] [PubMed] [Google Scholar]
- 78.Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H, Sheidlin A, Syrkina O, Huang PM, Garg HG, Hales CA et al. 2008. High-molecular-weight hyaluronan--a possible new treatment for sepsis-induced lung injury: a preclinical study in mechanically ventilated rats. Crit Care 12:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, and Garantziotis S 2011. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J. Biol. Chem 286:17435–17444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scuri M, and Abraham WM 2003. Hyaluronan blocks human neutrophil elastase (HNE)-induced airway responses in sheep. Pulm. Pharmacol. Ther 16:335–340. [DOI] [PubMed] [Google Scholar]
- 81.Scuri M, Forteza R, Lauredo I, Sabater JR, Botvinnikova Y, Allegra L, and Abraham WM 2000. Inhaled porcine pancreatic elastase causes bronchoconstriction via a bradykinin-mediated mechanism. J. Appl. Physiol 89:1397–1402. [DOI] [PubMed] [Google Scholar]
- 82.Garantziotis S, Zudaire E, Trempus CS, Hollingsworth JW, Jiang D, Lancaster LH, Richardson E, Zhuo L, Cuttitta F, Brown KK et al. 2008. Serum inter-alpha-trypsin inhibitor and matrix hyaluronan promote angiogenesis in fibrotic lung injury. Am. J. Respir. Crit Care Med 178:939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petrigni G, and Allegra L 2006. Aerosolised hyaluronic acid prevents exercise-induced bronchoconstriction, suggesting novel hypotheses on the correction of matrix defects in asthma. Pulm. Pharmacol. Ther 19:166–171. [DOI] [PubMed] [Google Scholar]
- 84.Telenga ED, and Kerstjens HA 2008. Effect of inhaled hyaluronic acid (HA) on exercised induced bronchoconstriction (EIB). Pulm. Pharmacol. Ther 21:430. [DOI] [PubMed] [Google Scholar]
- 85.Song W, Yu Z, Doran SF, Ambalavanan N, Steele C, Garantziotis S, and Matalon S 2015. Respiratory syncytial virus infection increases chlorine-induced airway hyperresponsiveness. Am. J. Physiol Lung Cell Mol. Physiol 309:L205–L210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smyth RL, and Openshaw PJ 2006. Bronchiolitis. Lancet 368:312–322. [DOI] [PubMed] [Google Scholar]
- 87.Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, and Matalon S 2010. Mechanisms and modification of chlorine-induced lung injury in animals. Proc. Am. Thorac. Soc 7:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ware LB, and Matthay MA 2000. The acute respiratory distress syndrome. N. Engl. J. Med 342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 89.Chiba Y, Matsusue K, and Misawa M 2010. RhoA, a possible target for treatment of airway hyperresponsiveness in bronchial asthma. J. Pharmacol. Sci 114:239–247. [DOI] [PubMed] [Google Scholar]
- 90.Goto K, Chiba Y, Matsusue K, Hattori Y, Maitani Y, Sakai H, Kimura S, and Misawa M 2010. The proximal STAT6 and NF-kappaB sites are responsible for IL-13- and TNF-alpha-induced RhoA transcriptions in human bronchial smooth muscle cells. Pharmacol. Res 61:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K et al. 2012. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med 18:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Freeman SA, Vega A, Riedl M, Collins RF, Ostrowski PP, Woods EC, Bertozzi CR, Tammi MI, Lidke DS, Johnson P et al. 2018. Transmembrane Pickets Connect Cyto- and Pericellular Skeletons Forming Barriers to Receptor Engagement. Cell 172:305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stober VP, Lim YP, Opal S, Zhuo L, Kimata K, and Garantziotis S 2019. Inter-alpha-inhibitor Ameliorates Endothelial Inflammation in Sepsis. Lung 197:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dogne S, Flamion B, and Caron N 2018. Endothelial Glycocalyx as a Shield Against Diabetic Vascular Complications: Involvement of Hyaluronan and Hyaluronidases. Arterioscler. Thromb. Vasc. Biol 38:1427–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garantziotis S, Brezina M, Castelnuovo P, and Drago L 2016. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am. J. Physiol Lung Cell Mol. Physiol 310:L785–L795. [DOI] [PMC free article] [PubMed] [Google Scholar]