Abstract
Emm55 is a bacterial gene derived from Streptococcus pyogenes (S. pyogenes) that was cloned into a plasmid DNA vaccine (pAc/emm55). In this study, we investigated the anti-tumor efficacy of pAc/emm55 in a B16 murine melanoma model. Intralesional (IL) injections of pAc/emm55 significantly delayed tumor growth compared to the pAc/Empty group. There was a significant increase in the CD8+ T cells infiltrating into the tumors after pAc/emm55 treatment compared to the control group. In addition, we observed that IL injection of pAc/emm55 increased antigen-specific T cell infiltration into tumors. Depletion of CD4+ or CD8+ T cells abrogated the anti-tumor effect of pAc/emm55. Combination treatment of IL injection of pAc/emm55 with anti-PD-1 antibody significantly delayed tumor growth compared to either monotherapy. pAc/emm55 treatment combined with PD-1 blockade enhanced anti-tumor immune response and improved systemic anti-tumor immunity. Together, these strategies may lead to improvements in the treatment of patients with melanoma.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02634-4) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, emm55, Streptococcus pyogenes, Bacterial protein, Intralesional injection
Introduction
Intralesional (IL) therapies are a therapeutic strategy to increase anti-tumor immunity while avoiding off-target effects and decreasing toxicity of traditional therapies such as radiation and chemotherapy. Injection of intralesional therapies, such as cytokines, oncolytic viruses, and TLR agonists, can lead to local tumor disruption and act as adjuvants to stimulate the immune system [1]. Direct injection of oncolytic viruses, such as Talimogene laherparepvec (T-VEC), can cause tumor regression by selectively replicating within tumor cells. This results in cell lysis, release of more viral particles, and the continued infection and lysis of tumor cells. Stimulation of the innate immune system in response to the viral infection and antigen release from dying tumor cells can cause adaptive and systemic anti-tumor immunity resulting in regression of metastatic lesions. IL injection of TVEC has led to improved durable response rates in patients with advanced melanoma [2]. Additionally, IL Rose Bengal leads to the direct ablation of tumor cells and the induction of systemic tumor-specific immune responses in multiple tumor models as well as patients with metastatic melanoma [3-5].
The use of bacterial products to reduce tumor burden has been investigated in cancer therapy since 1891 beginning with Coley’s toxin [6, 7]. Heat inactivated streptococcal organisms and Serratia marcescens were used intravenously, intramuscularly, or intratumorally to stimulate the immune system [6]. More recent, local delivery of Bacillus Calmette–Guerin (BCG), an attenuated mycobacteria used to treat tuberculosis, is successfully used to prevent recurrence and progression in non-muscle invasive bladder cancer [8, 9]. Although the mechanism of action of BCG is not fully understood, it is known that response requires infection of urothelial cells and internalization of BCG and results in the stimulation of immune response through upregulation of antigen presenting molecules and cytokine release [8, 10]. Bacterial components such as DNA and synthetic oligodeoxynucleotides (ODN) containing unmethylated CpG motifs have been shown to act as “adjuvants” in inducing anti- tumor immune responses [11–14]. Lastly, activating TLRs on dendritic cells, for example, using microbial extracts containing TLR ligands, can lead to increased antigen presentation to T cells and enhanced anti-tumor immunity [15–19].
A major limitation to these therapies is introducing an active infection or virus into patients. Emm55 is a serotyping protein normally expressed on the surface of the bacterium S. pyogenes and is highly antigenic but not rheumatogenic [20]. The use of Emm55 as a priming antigen for the induction of a tumor-specific immune response has been shown in a clinical study in dogs in which the DNA plasmid containing the emm55 gene was transfected into canine lymphoma cells and used as a vaccine [21]. This plasmid was also successfully used to treat naturally occurring equine melanoma [22].
In this study, we evaluated the anti-tumor efficacy of IL injection of pAc/emm55 in a B16 melanoma model as a single agent and in combination with anti-PD-1. Our findings demonstrate that IL injection of pAc/emm55 enhances anti-tumor T cell responses, leading to delayed tumor growth in melanoma-bearing mice.
Materials and methods
Animals
Female C57BL/6 mice (6–8 weeks old) were purchased from Charles River Laboratories for these studies. OT-I transgenic mice (C57BL/6-Tg (TcraTcrb) 1100Mjb/J) were purchased from The Jackson Laboratory. Mice were bred and housed at the Animal Research Facility of the H. Lee Moffitt Cancer Center and Research Institute (Tampa, FL). Mice were observed daily and humanely euthanized if a solitary subcutaneous tumor exceeded 1.5 cm in diameter or mice demonstrated signs referable to metastatic cancer. Mice were humanely euthanized by CO2 inhalation according to the American Veterinary Medical Association Guidelines. All efforts were made to minimize suffering.
Tumor cell lines
B16 tumor cells were purchased from ATCC and were cultured in complete RPMI medium (CM) as described previously [23, 24]. For tumor experiments, mice were subcutaneously (s.c.) injected with 1 × 105 B16 tumor cells or 3 × 105 M05 tumor cells s.c. M05 is an OVA-expressing B16 melanoma cell line maintained in selection medium containing 0.8 mg/mL of the antibiotic G418. M05 tumor cells were obtained from Dr. Kenneth Rock (Dana-Farber Cancer Institute). Transfection of tumor cells with pAc/empty or pAc/emm55 plasmid was performed by plating tumor cells in a 6-well plate. Cells were 70–90% confluent at the time of transfection. Lipofectamine 2000 Reagent (9 µl/well, Invitrogen) was diluted in Opti-MEM Reduced Serum Medium (150 µl/well, Gibco). pAc/emm55 or pAc/Empty DNA plasmids (2.5 µg/well) were diluted in Opti-MEM Reduced Serum Medium (150 µl/well). Diluted DNA and diluted Lipofectamine 2000 Reagent were mixed 1:1 and incubated for 5 min at room temperature. The DNA–lipid mixture (300 µl) was added to cells for 3 days.
Treatment model
B16 tumor cells were injected sc. in C57BL/6 mice on day 0. B16-bearing mice with palpable tumors (approximately 25 mm2) received three doses of IL injection of 20 μg of pAc/emm55 or pAc/Empty vector at weekly intervals. Invivo-jetPEI transfection reagent (VWR) was mixed with pAc/emm55 or pAc/Empty vector (provided by Morphogenesis Inc.) to prepare a homogenous complex for intratumoral injection. Tumor growth was measured precisely 2–3 times per week. This experiment was repeated 5 times. For the antigen specificity experiments, mice received M05 tumor cells on day 0, followed by two doses of IL injection of pAc/emm55 or pAc/Empty vector on day 7 and day 14. Naïve OT-I T cells which contain a TCR specific for an ovalbumin peptide on CD8 T cells were adoptively transferred at 5 × 106/ 250 μl/ mouse on day 14 by tail-vein injection. After 48 h, mice were euthanatized and spleens and tumors were harvested. This experiment was repeated twice.
T cell depletion
CD4 and CD8 T cells were depleted as described previously [25]. Anti-CD4 (clone GK1.5, catalog# BE0003-1) and anti-CD8 (clone 2.43, catalog# BE0061) were given at 300 μg /200 μl /mouse/i.p starting day 3 before tumor injection and continued twice a week until the endpoint. Control mice received NrIgG isotype alone. All antibodies were purchased from BioXcell (West Lebanon, NH). B16 cells were injected s.c. followed by IL injection of pAc/emm55 or pAc/Empty vector as described above. Mice were monitored and measured for tumor growth every other day until endpoint. This experiment was repeated twice.
pAc/emm55 injection in combination with PD-1 blockade
B16 tumors were established in C57BL/6 mice as described above. Mice received IL injection of pAc/emm55 or pAc/Empty vector on days 7, 14, and 21. Starting on day 8, mice received intraperitoneal injection of isotype control antibody (clone HRPN, catalog# BP0088) or anti-PD-1 blocking antibody (250 μg /200 μl, clone RMP1-14, catalog# BE0146, BioXcell, West Lebanon, NH) twice per week in combination with IL injection of pAc/emm55 or pAc/Empty vector. Tumor measurements were recorded every other day. This experiment was repeated 3 times.
T cell co-cultures and measurement of IFN-gamma
To measure systemic T cell reactivity, T cells were isolated from the spleens of treated mice using a T cell column (R&D Systems, Minneapolis, MN) and co-cultured with CM or at a 10:1 ratio with irradiated B16 cells in a 96-well plate in technical triplicate. After 24 h, supernatants were collected. IFN-gamma was measured in supernatants using an IFN-gamma ELISA assay kit (BD Biosciences, San Jose, CA) and following the manufacturer’s instructions. In a second experiment, dendritic cells (DCs) were grown from mouse bone marrow as previously described [26]. Briefly, bone marrow was flushed from the femurs and tibiae of mice, and red blood cells were lysed with ACK lysis buffer. Single cells were plated in CM containing 20 ng/ml GM-CSF and 10 ng/ml IL-4 (PeproTech, Rocky Hill, NJ) for 5–7 days. DCs were isolated by Opti-Prep gradient (Alere Technologies AS, Oslo, Norway). M05 tumor cells were transfected with pAc/empty or pAc/emm55 in vitro. Cell lysate was generated by 3 cycles of rapid freeze–thawing as previously described [27]. Lysate was pulsed onto DC at a 3:1 ratio and cultured for 18–24 h. DCs were co-cultured with OT-I T cells at a 1:10 ratio for 24 h, and supernatants were collected. IFN-gamma was measured in supernatants using an IFN-gamma ELISA assay kit (BD Biosciences, San Jose, CA) and following the manufacturer’s instructions. This experiment was repeated in technical and experimental triplicate.
Flow staining and analysis
Single-cell suspensions from spleen or tumors were processed as described previously [23, 24]. For surface staining, cells were stained with relevant antibodies recognizing CD3 (catalog# 565,643), CD8 (catalog# 558,106), CD4 (catalog# 550,954), CD45.1 (catalog# 560,578, all from BD Biosciences, San Jose, CA), and OVA-tetramer (MBL International, Woburn, MA, catalog# TB5001-1), and incubated for 20 min, washed, fixed with 2% Paraformaldehyde and stored in PBS. Samples were analyzed using an LSRII (BD Biosciences, San Jose, CA), and the data were analyzed using FlowJo software (Tree Star).
Immunohistochemistry
Immunohistochemistry was performed as a blinded study by the microscopy core facility at Moffitt Cancer Center on both treated and untreated tumor tissues. Tissues were stained for anti-mouse CD3 (clone SP-7, catalog# ab16669, Abcam, Cambridge, MA), CD4 (clone 1, catalog# 50,134-M08H, Sino Biological, Wayne, PA) or CD8 (clone D4W2Z, catalog# 98,941, Cell Signaling Technology, Danvers, MA) antibody and were scanned using the Leica Aperio™ (Vista, CA) AT2 with a 200x/0.8NA objective lens at a rate of 3 min per slide via Basler tri-linear array detection. Each slide was then analyzed as previously described [28] using the default Positive Pixel Count v9.0 and Membrane v1 Macros available in the Spectrum Database. The Positive Pixel Count® v9.0 algorithm applies the following thresholds: [Hue Value = 0.1; Hue Width = 0.5; Color Saturation Threshold = 0.04; IWP(High) = 220; Iwp(Low) = Ip(High) = 175; Ip(low) = Isp(High) = 100 Isp(Low) = 0] to segment positive staining of various intensities. The algorithm was applied to the entire digital slide to determine the percentage of positive biomarker staining by applicable area. The membrane algorithm applies the following thresholds: [Background Intensity Threshold = 240; Weak Intensity Threshold = 200; Moderate Intensity Threshold = 170 and Strong Intensity Threshold = 105] to segment positive staining of various intensities in the membrane of each cell.
Quantitative PCR
Emm55 expression was analyzed in tumor tissues using quantitative PCR. RNA from tumor tissues of mice treated with pAc/emm55 or pAc/Empty vector was extracted using the Qiagen RNAeasy Plus Kit followed by cDNA synthesis using the Invitrogen SuperScript III cDNA Synthesis Kit. qPCR was run on the Applied Biosystems 7900HT machine for Emm55- N primer (5′- CGAAGCGTTTATGAGCCAGT-3′) and Emm55- C primer (5′-CGGCTCGCTTCTGAAATCTT-3′) for fold change expression (ΔCT value normalized to GAPDH). GAPDH-Forward (ACC ACA GTC CAT GCC ATC AC) and GAPDH-Reverse (TCC ACC ACC CTG TTG CTG TA) were used as a control.
Statistical analysis
A Student’s t test was used to compare between two treatment groups. Statistical analysis of tumor growth curves was performed using a CGGC permutation test [29]. A log-rank test was used to compare survival curves (Fig. 1e). Bonferroni correction was utilized when multiple pairwise comparisons were investigated (Fig. 5d). All statistical evaluations of data were performed using GraphPad Prism version 8.4.1, GraphPad Software, San Diego, California USA or CGGC permutation test. Statistical significance was achieved at p < 0.05.
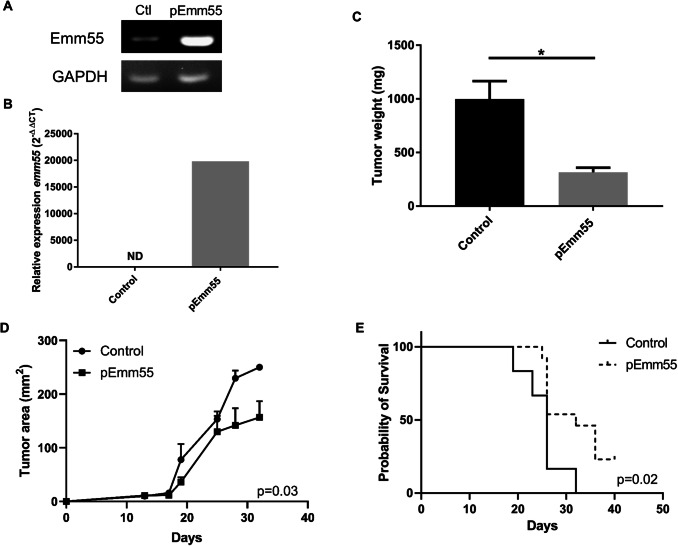
Fig. 1.
pAc/emm55 delays tumor growth in B16 melanoma-bearing mice. B16-bearing mice with palpable tumors (approximately 25 mm2) received three doses of IL injection of pAc/emm55 or pAc/Empty vector on a weekly interval. Invivo-jetPEI transfection reagent (VWR) was mixed with pAc/emm55 or pAc/Empty vector to get homogenous complex for IL injection. Tumor growth was measured and monitored 2–3 times a week. a End point PCR expression on tumor cells transfected with pAc/emm55 in vitro and b qRT-PCR on one representative tumor per group; c tumor weight *p < 0.05 Student’s t test. d Tumor growth curves, CGGC permutation test. e Survival curves, log-rank test. Data represent mean + SEM, N = 12 per group
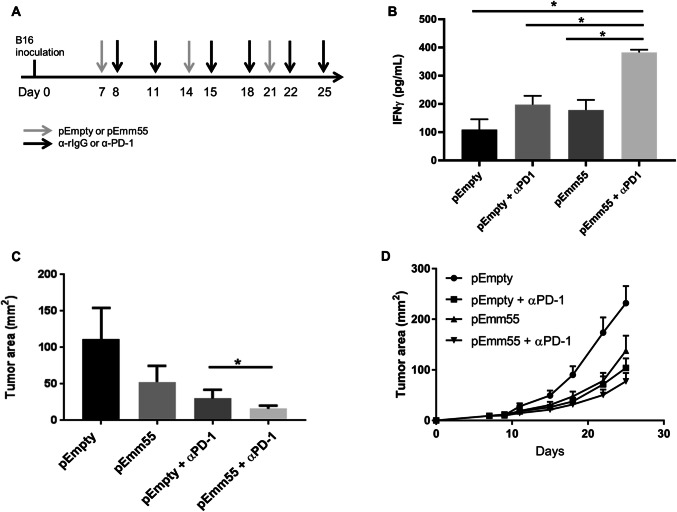
Fig. 5.
IFNγ production increases upon the addition of pAc/emm55 vector and anti-PD-1 therapy. Murine melanoma B16 cells were injected subcutaneously on day 0. pEmpty vector or pEmm55 vector was injected on days 7, 14, and 21. Starting on day 8, mice received intraperitoneal injection of either isotype control antibody or anti-PD-1 blocking antibody (250 μg/200μL/IP) twice weekly (a). b Splenocytes collected from mice treated with emm55 vector ± anti-PD-1 therapy were co-cultured with B16 melanoma cells, and culture supernatants were collected after 24 h. Levels of IFNγ were measured (p < 0.01 utilizing unpaired T test with Bonferroni correction). c Tumor sizes for these B16 tumors at day 25 were also measured and demonstrated an increased response with pEmm55/anti-PD-1 compared to anti-PD-1 therapy alone (p < 0.05; unpaired T test). d Tumor growth curves from each treatment group. (N = 12 for treatment groups, N = 9 for control pEmpty group). *p < 0.05
Results
IL injection of pAc/emm55 delays tumor growth in B16-bearing mice
Using the B16 melanoma model, the anti-tumor efficacy of pAc/emm55 was examined. Tumor-bearing mice were treated with three IL injections of pAc/emm55 or plasmid DNA controls on days 7, 14, and 21 post tumor cell injections. As shown in Fig. 1a, emm55 RNA was measured in tumor cells after in vitro transfection (Fig. 1a). A faint band can be seen in the control lane, as nonspecific amplification can be detected by endpoint PCR in pAc/empty transfected tumor cells according to the manufacturers. Successful transfection of emm55 after intratumoral injection of plasmid DNA was confirmed using qRT-PCR using tumor lysates harvested 7 days after the third IL injection. Emm55 expression was detected by qRT-PCR and normalized to GAPDH expression with a 20,000-fold increase in a pAc/emm55 treated tumor compared to a control treated tumor (Fig. 1b). B16 tumor-bearing mice that received weekly IL injection of emm55 plasmid DNA had a significant decrease in tumor burden (Fig. 1c p < 0.0076 and 1d p = 0.03 CGGC permutation test) and improved survival (Fig. 1e log rank p = 0.02) compared to control treated mice. These data confirm the expression of emm55 in tumors after transfection of pAc/emm55 plasmid and suggest that this treatment could be used to delay tumor growth and improve survival.
IL injection of pAc/emm55 increases T cell infiltration within the tumor
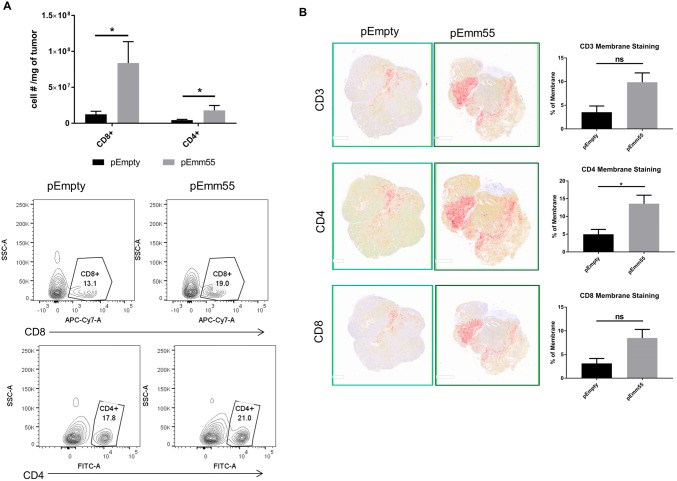
The immune-mediated anti-tumor response to IL injection of pAc/emm55 plasmid was investigated. C57BL/6 mice were injected with B16 cells and treated with pAc/emm55 or pAc/empty vector, and immune cell infiltration was examined. As shown in Fig. 2a, there was a significant increase in CD3+ CD8+ T cell and CD4+ T cell infiltration measured by flow cytometry within the tumors of pAc/emm55 treated tumor-bearing mice compared to pAc/empty vector treated mice (p < 0.05, gating strategy Supplemental Fig. 1). Immunohistochemistry analysis revealed an increased infiltration of CD3+, CD4+, and CD8+ T cells within the tumors injected with pAc/emm55 vector compared to the pAc/empty vector treatment group; however, this was only significant for CD4+ infiltration (Fig. 2b, p = 0.049). No significant differences were seen in other immune cell subtypes as determined by flow cytometry (Supplemental Fig. 2a, b).
Fig. 2.
IL injection of pAc/emm55 enhances T cell infiltration in B16 tumors. a B16 melanoma tumors were harvested, and the numbers of CD3 + CD8+ T cells and CD4+ T cells were measured within the tumor by flow cytometry. The cell number per mg of tumor was compared (p < 0.05, unpaired T test). b Representative images of Immunohistochemistry analysis for T cell infiltration within tumors. Bar graphs represent the positive staining of T cell intensities in the membrane of each cell. We observed trends in the numbers of infiltrating CD3+ and CD8+ cells with CD4+ cells reaching statistical significance p = 0.049; *p < 0.05
Anti-tumor efficacy of pAc/emm55 is T cell dependent
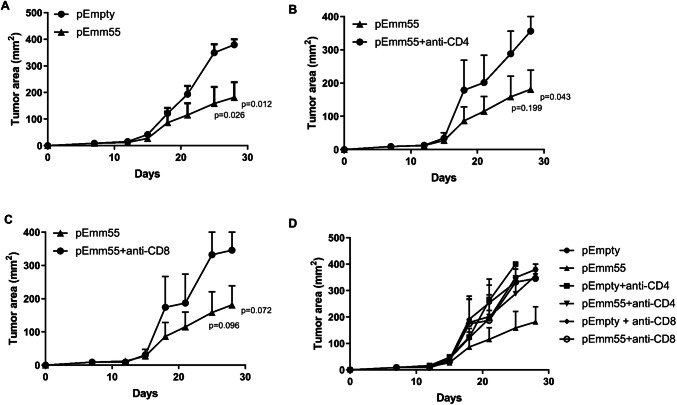
To investigate whether the anti-tumor immunity elicited by pAc/emm55 in B16 tumor-bearing mice was T cell dependent, C57BL/6 mice received three doses of IL injection of pAc/emm55 or pAc/empty vector with control, anti-CD4, or anti-CD8 depleting monoclonal antibodies. As shown in Fig. 3, treatment with anti-CD4 (Fig. 3b) or CD8 (Fig. 3c) T cell depleting antibodies abrogated the anti-tumor immune response induced by IL injection of pAc/emm55 vector. The tumor growth curve again demonstrated statistical significance between emm55 and empty vector with the CGGC permutation test (p = 0.02). While the tumor growth curve comparing emm55 to depletion of CD4 or CD8 positive cells demonstrated trends, the last two time points on the growth curve demonstrate that removing the CD4 or CD8 T cells abrogate the activity of the emm55 vector. These data suggest that both CD4+ and CD8+ T cells contributed to the anti-tumor efficacy of the pAc/emm55.
Fig. 3.
Systemic immune responses elicited by the pAc/emm55 vector in B16-bearing mice are T cell dependent. C57BL/6 mice were injected with a 3 weekly doses of pAc/Empty or pAc/emm55 plasmid IL. CGGC permutation test p = 0.02. Mice were pretreated with b anti-CD4 or c anti-CD8 antibody (300 μg/200μL/intraperitoneal) starting on day 3 and continuing twice a week until the endpoint. Control mice received isotype control. CD4 or CD8 T cells abrogate the activity of the emm55 vector. The p values utilizing the Student’s t test are presented for the last two time points on the growth curve. d Combined graph with all controls represented. N = 6 per group
IL injection with pAc/emm55 increases antigen-specific T cell infiltration
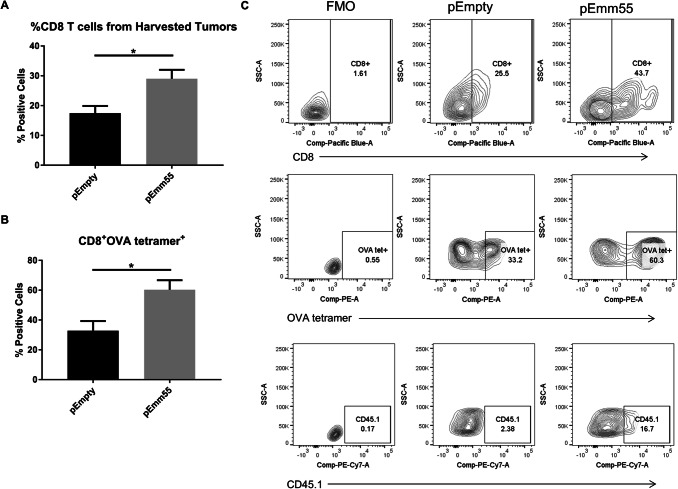
We next asked whether the T cells that infiltrate into tumors after injection of pAc/emm55 vector are tumor-specific. To answer this, pAc/empty or pAc/emm55 was injected into M05 tumors on days 7 and 14. In addition, mice received OT-I T cells as described in the methods section. The OVA model system used for these studies allowed us to track CD8+ T cells from mice with a TCR that recognizes the OVA-derived peptide SIINFEKL expressed by M05 tumor cells. OT-I T cells express the congenic marker CD45.1 which can be used to detect adoptively transferred cells. Two IL injections were performed in this experiment instead of three because adoptive transfer of OT-I T cells could reduce tumor burden. After 48 h, tumors were harvested and the percent of CD45.1 + OVA specific T cells within the tumors were measured by flow cytometry (gating strategy Supplemental Fig. 3). The overall percent of CD8+ T cells measured by flow cytometry within the tumor was much higher in mice that received pAc/emm55 vector compared to pAc/empty vector treated mice (Fig. 4a, c). There was a significant increase in OVA tetramer positive CD8+ T cells in mice that received pAc/emm55 vector compared to pAc/empty vector treated mice (Fig. 4b, c). Since the tumor expresses OVA, there is an endogenous T cell response against OVA that is enhanced in mice treated with emm55 plasmid therapy. These data suggest that IL injection of pAc/emm55 increases antigen-specific T cell infiltration within the tumor.
Fig. 4.
Systemic immune responses elicited by pAc/emm55 vector in B16-bearing mice are antigen specific. C57BL/6 were injected subcutaneously with M05 cells and mice received intralesional injection of pAc/empty or pAc/emm55 vector on days 7 and 14. In addition, the mice received 5 × 106 OT-1 T cells (CD45.1 +) systemically. After 48 h, tumors were harvested and tetramer-specific CD8+ T cells were measured by flow cytometry. The bar graphs represent the percent of a CD8+ and b CD8+ OVA tetramer+ positive cells (n = 5 in each group). c Representative flow plots comparing both treatment groups illustrating the increase in tetramer recognition with emm55 plasmid therapy. *p < 0.05
pAc/emm55 in combination with PD-1 blockade improve anti-tumor efficacy
Since we observed expression of co-inhibitory immune checkpoint receptors on TIL-infiltrating B16 tumors (Supplemental Fig. 4), it was investigated whether blocking these checkpoint receptors could improve the efficacy of IL injection of pAc/emm55 vector. We were particularly interested in blocking PD-1, as it has translational relevance in melanoma. B16 cells were injected into C57BL/6 mice. Mice were treated with three weekly doses (days 7, 14, and 21) of IL injection of pAc/emm55 or pAc/empty vector alone or in combination with anti-PD-1 blocking antibody given twice a week (Fig. 5a). Splenocytes were collected from treated mice, and IFN-γ production was measured in response to re-stimulation with tumor cells. As shown in Fig. 5b, splenocytes from pAc/emm55 vector or anti-PD-1 antibody treated mice alone had a significant increase in IFN-γ production in response to re-stimulation with B16 tumor cells. However, mice that received pAc/emm55 in combination with anti-PD-1 antibody demonstrated a significant increase in IFN-γ production compared to the tumor-bearing mice that received monotherapy or pAc/Empty vector. Mice that received pAc/emm55 vector or anti-PD-1 antibody alone demonstrated smaller tumor sizes compared to the pAc/empty vector (Fig. 5c). Combination therapy with anti-PD-1 antibody and pAc/emm55 enhanced the anti-tumor efficacy in B16 tumor-bearing mice (Fig. 5d). These data suggest that PD-1 blockade enhances the anti-tumor efficacy of pAc/emm55 in a B16 melanoma model and increases systemic anti-tumor immunity.
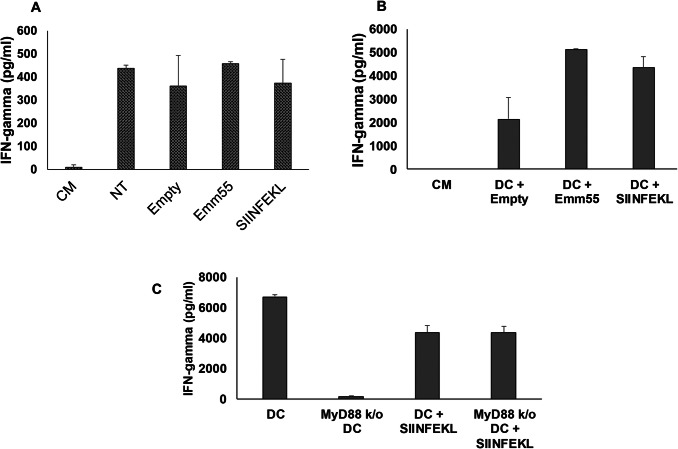
Role of emm55 expression on T cell activation
M05 cells were transfected in vitro with pAc/empty or pAc/emm55 vectors. No difference in tumor cell viability or growth kinetics was measured (data not shown). Non-transfected and transfected tumor cells were co-cultured with OT-I T cells, and IFN-γ was measured by ELISA. As shown in Fig. 6a, nonsignificant differences were measured and OT-I T cells responded equally to non-transfected (NT), pAc/empty, and pAc/emm55 transfected M05 cells. Next, tumor lysates from tumor cells transfected with pAc/empty or pAc/emm55 were pulsed onto bone marrow-derived dendritic cells (DCs) for 18 h. DCs were co-cultured with OT-I T cells for 24 h. Increased production of IFN-gamma was measured after co-culture of OT-I T cells with DC pulsed with M05-pAc/emm55 lysate (Fig. 6b). This increase was ablated if tumor lysate was pulsed onto DC lacking the innate immune signal transduction adaptor, MyD88 (Fig. 6c). Together these data support the role of DCs to enhance T cell responses through a TLR-dependent mechanism.
Fig.6.
Expression of emm55 enhances the ability of DC to stimulate T cells. M05 tumor cells were not transfected (NT) or transfected with pAc/empty or pAc/emm55 plasmid. a Tumor cells or b DCs pulsed with tumor cell lysates were co-cultured alone or with OT-I T cells for 24 h, and supernatants were collected. Controls included OT-I T cells alone (CM) or co-cultured with OVA-SIINFEKL peptide (SIINFEKL). IFN-gamma was measured by ELISA. c Lysates of tumor cells transfected with pEmpty or pEmm55 were pulsed onto wild-type or MyD88 k/o DC and co-cultured with OT-I T cells. Controls included OT-I T cells alone or co-cultured with DC pulsed with OVA-SIINFEKL peptide. IFN-gamma was measured in supernatants after a 24-h co-culture
Discussion
Intralesional delivery of immunotherapeutic agents has been used to elicit anti-tumor immune responses in various cutaneous malignancies [1, 2]. In a phase 3 clinical trial, T-VEC has shown an improved overall clinical response in melanoma patients [3]. Previous studies in our laboratory have shown that IL Rose Bengal induced T cell-mediated tumor-specific immune responses in MT901 breast cancer and in B16 melanoma [4, 5]. A phase I clinical trial of IL therapy with Rose Bengal induced 48% objective response in treated lesions and 27% in untreated lesion in melanoma patients. Granulocyte macrophage colony-stimulating factor (GM-CSF) for intralesional therapy has been used for the treatment of metastatic melanoma [30]. Here, we report novel IL immunotherapy using a plasmid DNA vaccine, pAc/emm55, expressing the emm55 gene derived from S. pyogenes.
IL injection of pAc/emm55 DNA plasmid led to a delay in tumor growth in the B16 melanoma model. We demonstrate that reduction in tumor burden after IL pAc/emm55 was dependent on both CD4+ and CD8+ T cells. In addition, an increase in CD8+ T cell infiltration was found in tumors of mice treated with IL pAc/emm55. This datum is consistent with a previous study that has shown IL injection of live S. pyogenes resulted in complete tumor regression in Panc02 tumor model [31] and resulted in increased infiltration of immune cells and increased IFN-γ production within tumor microenvironment. These data support our findings that S. pyogenes or its related protein is highly antigenic and may act as an adjuvant for priming or activating the tumor-specific T cells. In addition, the data suggest that delivery of plasmid DNA vaccine, pAc/emm55 derived from S. pyogenes, directly to the tumor site could be a promising therapeutic approach for the treatment of local lesions in melanoma patients.
Pulsing DC with pAc/Emm55 transfected tumor cell lysate improved T cell stimulation and was dependent on MyD88 expression. This finding is supported in the literature by a number of groups studying the immune response to a variety of bacterial proteins [32–35]. These studies demonstrate that DCs exposed to bacterial proteins were found to have improved survival, maturation, and potent cross-presentation to CD8 + T cells [32–35]. We hypothesize that pAc/Emm55 may function by promoting Th1 T cell immunity through DC stimulation; however, further studies would need to be performed to confirm this.
Co-inhibitory checkpoint molecules have been shown to inhibit T cell activation and function. Blockade of checkpoint receptor/ligand interaction restored T cell function and anti-tumor immune responses in various cancers [23, 36]. The current response rate with anti-PD-1 therapy in melanoma patients is approximately 30% [37–39]. Having observed high levels of PD-1 expression on TIL from pAc/emm55 treated mice, we next investigated the efficacy of IL injection of pAc/emm55 vector in combination with PD-1 blockade. PD-1 blockade significantly improved the anti-tumor efficacy of pAc/emm55 with increased IFN-γ secretion compared to mice that received treatment with pAc/emm55 or anti-PD-1 antibody alone. We observed delayed tumor growth in mice that received IL injection of pAc/emm55 in combination PD-1 blockade. This strategy of combining checkpoint inhibitors and bacterial factors is being investigated in a variety of cancer types. A Listeria vaccine secreting HPV-derived protein has been previously shown to have anti-tumor efficacy [40]. A Phase I/II clinical trial using a combination approach with HPV-16-E7 fusion protein and anti-PD-L1 in cervical cancer patients is ongoing. In addition, a live-attenuated Listeria strain encoding for prostate-specific antigen level in combination with anti-PD-1 (pembrolizumab) is being tested in patients with prostate cancer. These studies support the use of immunogenic bacterial proteins as a combination therapeutic approach for the treatment of melanoma. A first in man Phase I clinical trial is currently underway to test the safety of the single agent plasmid therapy at Moffitt Cancer Center [41].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Charles James for technical assistance. This work has been supported in part by the Flow Cytometry and the Analytic Microscopy Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292).
Abbreviations
- BCG
Bacillus Calmette–Guerin
- DC
Dendritic cell
- IFN-γ
Interferon-gamma
- IL
Intralesional
- OVA
Ovalbumin
- PD-1
Programmed death receptor-1
- s.c.
Subcutaneous
- TIL
Tumor-infiltrating lymphocytes
- TLR
Toll-like receptor
- T-VEC
Talimogene laherparepvec
Author contributions
Shari Pilon-Thomas and Joseph Markowitz conceived and planned the experiments; Brittany Bunch, Krithika Kodumudi, Ellen Scott, Jennifer Morse, and Amy Weber carried out the experiments; Brittany Bunch, Krithika Kodumudi, Anders E Berglund Shari Pilon-Thomas, and Joseph Markowitz analyzed and interpreted the data; Brittany Bunch and Krithika Kodumudi wrote the manuscript with support from Shari Pilon-Thomas and Joseph Markowitz; Shari Pilon-Thomas and Joseph Markowitz supervised the project.
Funding
This work was supported by a sponsored research agreement from Morphogenesis, Inc. to the H Lee Moffitt Cancer Center and Research Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We wish to acknowledge the Donald A Adam Comprehensive Melanoma Research Center at Moffitt Cancer Center. KK was supported by a Phi Beta Psi sorority award.
Compliance with ethical standards
Conflict of interest
JM is the PI of an institutional grant from Morphogenesis for clinical trial activities. JM receives support from the Donald A. Adam Comprehensive Melanoma Research Center at Moffitt Cancer Center. Unrelated to this paper, JM was a member of an Array Biopharma Advisory Board in 2018 and is an advisory board member for Newlink Genetics. JM was also the recipient of a career enhancement program award under Melanoma Skin Cancer SPORE P50 CA158536 and the Institutional Research Grant number 17–173-22 from the American Cancer Society. JM received funding from Navigate BP and is currently funded by Jackson Laboratories for work unrelated to this paper. Moffitt Cancer Center has licensed Intellectual Property related to the proliferation and expansion of tumor-infiltrating lymphocytes (TILs) to Iovance Biotherapeutics. SPT is an inventor on such Intellectual Property. SPT participates in sponsored research agreements with Provectus Biopharmaceuticals, Iovance Biotherapeutics, Intellia Therapeutics, and Myst Therapeutics that are not related to this research. Dr. Pilon-Thomas has received research support that is not related to this research from the following entities: American Cancer Society -Leo and Anne Albert Charitable Foundation Research Scholar Grant (RSG-16–117-01-LIB), State of Florida Bankhead-Coley Cancer Research Program (7BC08), NIH-NCI (U01 CA244100-01 and R01 CA239219-01A1), V Foundation, and Swim Across America. Additionally, Dr. Pilon-Thomas is a co-Investigator on NIH-NCI (U54 CA193489-01A1 and R01 CA241559) research support, which is not related to this research. The other authors declare that they have no conflict of interest
Ethical approval
All experiments with mice were performed in compliance with the principles, and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals and protocols were approved after review by the Institutional Animal Care and Use Committee at the University of South Florida (Tampa, FL). The University of South Florida Comparative Medicine is fully accredited by AAALAC International as program #000434, is managed in accordance with the Guide for the Care and Use of Laboratory Animals, the Animal Welfare Regulations, the PHS Policy, the FDA Good Laboratory Practices, and the IACUC Principles and Procedures of Animal Care and Use, has an assurance #D16-00589 (A4100-01) on file with OLAW/PHS, and maintains registration #58-R-0015 with USDA/APHIS/AC.
Human and animal rights
C57BL/6 mice were obtained from Charles River (Frederick, MD). OT-I and MyD88 knock-out mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Cell line authentication
B16 melanoma cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). M05 melanoma cells were generously provided by Dr. Kenneth Rock (Dana-Farber Cancer Institute). B16 and M05 cells were verified for the lack of microbial contamination including mycoplasma by IDEXX BioAnalytics. Cell lines were expanded and cryopreserved according to the culture and cryopreserving conditions recommended by American Type Culture Collection.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Brittany Bunch and Krithika N Kodumudi have contributed equally to this work.
Shari Pilon-Thomas and Joseph Markowitz are shared senior authors.
Contributor Information
Shari Pilon-Thomas, Email: Shari.Pilon-Thomas@moffitt.org.
Joseph Markowitz, Email: joseph.markowitz@moffitt.org.
References
- 1.Agarwala SS. The role of intralesional therapies in melanoma. Oncology (Williston Park) 2016;30(5):436–441. [PubMed] [Google Scholar]
- 2.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 3.Lippey J, Bousounis R, Behrenbruch C, McKay B, Spillane J, Henderson MA, et al. Intralesional PV-10 for in-transit melanoma—a single-center experience. J Surg Oncol. 2016;114(3):380–384. doi: 10.1002/jso.24311. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Innamarato PP, Kodumudi K, Weber A, Nemoto S, Robinson JL, et al. Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1. Oncotarget. 2016;7(25):37893–37905. doi: 10.18632/oncotarget.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toomey P, Kodumudi K, Weber A, Kuhn L, Moore E, Sarnaik AA, et al. Intralesional injection of rose bengal induces a systemic tumor-specific immune response in murine models of melanoma and breast cancer. PLoS ONE. 2013;8(7):e68561. doi: 10.1371/journal.pone.0068561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64(3):529–564. doi: 10.1016/0163-7258(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 7.St Jean AT, Zhang M, Forbes NS. Bacterial therapies: completing the cancer treatment toolbox. Curr Opin Biotechnol. 2008;19(5):511–517. doi: 10.1016/j.copbio.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohle A, Schuller J, Knipper A, Hofstetter A. Bacillus Calmette–Guerin treatment and vesicorenal reflux. Eur Urol. 1990;17(2):125–128. doi: 10.1159/000464019. [DOI] [PubMed] [Google Scholar]
- 9.Rosevear HM, Lightfoot AJ, O'Donnell MA, Griffith TS. The role of neutrophils and TNF-related apoptosis-inducing ligand (TRAIL) in bacillus Calmette–Guerin (BCG) immunotherapy for urothelial carcinoma of the bladder. Cancer Metastasis Rev. 2009;28(3–4):345–353. doi: 10.1007/s10555-009-9195-6. [DOI] [PubMed] [Google Scholar]
- 10.Kresowik TP, Griffith TS. Bacillus Calmette–Guerin immunotherapy for urothelial carcinoma of the bladder. Immunotherapy. 2009;1(2):281–288. doi: 10.2217/1750743X.1.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong SD, Basha G, Wilson KD, Kazem M, Cullis P, Jefferies W, et al. The immunostimulatory activity of unmethylated and methylated CpG oligodeoxynucleotide is dependent on their ability to colocalize with TLR9 in late endosomes. J Immunol. 2010;184(11):6092–6102. doi: 10.4049/jimmunol.0802442. [DOI] [PubMed] [Google Scholar]
- 12.Chikh G, de Jong SD, Sekirov L, Raney SG, Kazem M, Wilson KD, et al. Synthetic methylated CpG ODNs are potent in vivo adjuvants when delivered in liposomal nanoparticles. Int Immunol. 2009;21(7):757–767. doi: 10.1093/intimm/dxp044. [DOI] [PubMed] [Google Scholar]
- 13.Mahfouz M, Hashimoto W, Das Gupta TK, Chakrabarty AM. Bacterial proteins and CpG-rich extrachromosomal DNA in potential cancer therapy. Plasmid. 2007;57(1):4–17. doi: 10.1016/j.plasmid.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Pilon-Thomas S, Li W, Briggs JJ, Djeu J, Mule JJ, Riker AI. Immunostimulatory effects of CpG-ODN upon dendritic cell-based immunotherapy in a murine melanoma model. J Immunother. 2006;29(4):381–387. doi: 10.1097/01.cji.0000199199.20717.67. [DOI] [PubMed] [Google Scholar]
- 15.Gantier MP, Tong S, Behlke MA, Irving AT, Lappas M, Nilsson UW, et al. Rational design of immunostimulatory siRNAs. Mol Ther. 2010;18(4):785–795. doi: 10.1038/mt.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li K, Qu S, Chen X, Wu Q, Shi M. Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. Int J Mol Sci. 2017 doi: 10.3390/ijms18020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi M, Chen X, Ye K, Yao Y, Li Y. Application potential of toll-like receptors in cancer immunotherapy: systematic review. Medicine (Baltimore) 2016;95(25):e3951. doi: 10.1097/MD.0000000000003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendelac A, Medzhitov R. Adjuvants of immunity: harnessing innate immunity to promote adaptive immunity. J Exp Med. 2002;195(5):F19–23. doi: 10.1084/jem.20020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9(7):831–835. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 20.Ramiya VK, Jerald MM, Lawman PD, Lawman MJ. Autologous tumor cells engineered to express bacterial antigens. Methods Mol Biol. 2014;1139:243–257. doi: 10.1007/978-1-4939-0345-0_21. [DOI] [PubMed] [Google Scholar]
- 21.Glikin GC, Finocchiaro LM. Clinical trials of immunogene therapy for spontaneous tumors in companion animals. Sci World J. 2014;2014:718520. doi: 10.1155/2014/718520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown EL, Ramiya VK, Wright CA, Jerald MM, Via AD, Kuppala VN, Hazell WS, Lawman PD, Lawman MJ. Treatment of metastatic equine melanoma with a plasmid DNA vaccine encoding Streptococcus Pyogenes EMM55 protein. J Equine Vet Sci. 2014;34:704–708. doi: 10.1016/j.jevs.2013.11.012. [DOI] [Google Scholar]
- 23.Kodumudi KN, Siegel J, Weber AM, Scott E, Sarnaik AA, Pilon-Thomas S. Immune checkpoint blockade to improve tumor infiltrating lymphocytes for adoptive cell therapy. PLoS ONE. 2016;11(4):e0153053. doi: 10.1371/journal.pone.0153053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kodumudi KN, Weber A, Sarnaik AA, Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol. 2012;189(11):5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vohra N, Verhaegen M, Martin L, Mackay A, Pilon-Thomas S. TNF-alpha-treated DC exacerbates disease in a murine tumor metastasis model. Cancer Immunol Immunother. 2010;59(5):729–736. doi: 10.1007/s00262-009-0793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilon-Thomas S, Nelson N, Vohra N, Jerald M, Pendleton L, Szekeres K, et al. Murine pancreatic adenocarcinoma dampens SHIP-1 expression and alters MDSC homeostasis and function. PLoS ONE. 2011;6(11):e27729. doi: 10.1371/journal.pone.0027729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotera Y, Shimizu K, Mule JJ. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001;61(22):8105–8109. [PubMed] [Google Scholar]
- 28.Ibrahim-Hashim A, Abrahams D, Enriquez-Navas PM, Luddy K, Gatenby RA, Gillies RJ. Tris-base buffer: a promising new inhibitor for cancer progression and metastasis. Cancer Med. 2017;6(7):1720–1729. doi: 10.1002/cam4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elso CM, Roberts LJ, Smyth GK, Thomson RJ, Baldwin TM, Foote SJ, Handman E. Leishmaniasis host response loci (lmr13) modify disease severity through a Th1/Th2-independent pathway. Genes Immun. 2004;5(2):93–100. doi: 10.1038/sj.gene.6364042. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17(3):718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 31.Maletzki C, Linnebacher M, Kreikemeyer B, Emmrich J. Pancreatic cancer regression by intratumoural injection of live Streptococcus pyogenes in a syngeneic mouse model. Gut. 2008;57(4):483–491. doi: 10.1136/gut.2007.125419. [DOI] [PubMed] [Google Scholar]
- 32.Schetters STT, Jong WSP, Horrevorts SK, Kruijssen LJW, Engels S, Stolk D, Daleke-Schermerhorn MH, Garcia-Vallejo J, Houben D, Unger WWJ, den Haan JMM, Luirink J, van Kooyk Y. Outer membrane vesicles engineered to express membrane-bound antigen program dendritic cells for cross-presentation to CD8+ T cells. Acta Biomater. 2019 doi: 10.1016/j.actbio.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Kim WS, Jung ID, Kim JS, Kim HM, Kwon KW, Park YM, Shin SJ. Mycobacterium tuberculosis GrpE, a heat-shock stress responsive chaperone, promotes Th1-biased T cell immune response via TLR4-mediated activation of dendritic cells. Front Cell Infect Microbiol. 2018;27(8):95. doi: 10.3389/fcimb.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda Y, Azuma M, Funami K, Shime H, Matsumoto M, Seya T. Type I interferon-independent dendritic cell priming and antitumor T cell activation induced by a mycoplasma fermentans lipopeptide. Front Immunol. 2018;14(9):496. doi: 10.3389/fimmu.2018.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedrich C, Mamareli P, Thiemann S, Kruse F, Wang Z, Holzmann B, Strowig T, Sparwasser T, Lochner M. MyD88 signaling in dendritic cells and the intestinal epithelium controls immunity against intestinal infection with C. rodentium. PLoS Pathog. 2017;13(5):e1006357. doi: 10.1371/journal.ppat.1006357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184(7):3442–3449. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozgun A, Sondak VK, Markowitz J. Resistance patterns to anti-PD-1 therapy in metastatic melanoma. Chin Clin Oncol. 2016;5(6):75. doi: 10.21037/cco.2016.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 40.Jia YY, Tan WJ, Duan FF, Pan ZM, Chen X, Yin YL, et al. A Genetically modified attenuated listeria vaccine expressing HPV16 E7 Kill tumor cells in direct and antigen-specific manner. Front Cell Infect Microbiol. 2017;7:279. doi: 10.3389/fcimb.2017.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markowitz J, Kodumudi K, De Aquino DB, Sondak VK, Pilon-Thomas S (2019) Trial in progress: First in human Phase I study using a plasmid DNA coding for Emm55 streptococcal antigen (IFx-Hu2.0) in patients with unresectable stage III or stage IV cutaneous melanoma [abstract]. Cancer Res 79(13 Suppl):Abstract nr CT119
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.