Abstract
In this paper we have attempted to identify missed opportunities to change the trajectory of smoking and smoking caused diseases in America over the past 100 years. Many of the missed opportunities identified are due to the actions of cigarette manufacturers who misled the public about the dangers of cigarette smoking, the addictiveness of nicotine, and the feasibility of providing lower risk alternative nicotine delivery products to addicted smokers. An important lesson learned from the past is that treating all tobacco/nicotine products as equivalently harmful is counterproductive to public health as it only serves to protect the most lethal nicotine product - cigarettes. Since 2000, the evolving marketplace of lower risk nicotine products combined with regulatory authority over tobacco products represents a new opportunity to dramatically transform the cigarette business in ways that were never imagined when the war on tobacco was raging decades ago. However, this requires embracing risk-proportionate regulation, taxation policies, and providing consumers with accurate public messaging on product relative risks. A regulatory framework based on sound science that encourages and rewards new or existing manufacturers to invest in consumer acceptable lower risk products to replace cigarettes needs to be encouraged. The past is indeed not the future in smoking control, but it may be difficult to escape the past unless a realignment of market forces and policies can be achieved.
Keywords: Cigarette smoking prevalence, smoking initiation, smoking cessation, quit ratios, nicotine addiction, tobacco control, harm reduction, electronic cigarettes, product regulation
Introduction
The epidemic of smoking-caused disease in the United States (U.S.) during the twentieth century ranks among the greatest public health catastrophes in our history. More than 10 times as many U.S. citizens have died prematurely from cigarette smoking than have died in all the wars fought by the U.S. during its history [1]. Efforts to reduce cigarette smoking over the past 60 years have also been said to be one of public health’s great successes [2]. Between 1965 and 2019, cigarette smoking prevalence in the U.S. declined from 43% to 14% [3,4].
However, cigarette smoking prevalence can be a deceiving indicator of public health success since there is underreporting of socially disapproved behaviors and many of those with the greatest likelihood of cigarette smoking (the homeless, those with significant mental health conditions) are also least likely to be included in surveys [5]. In addition, population increases have offset declining smoking prevalence. As shown in figure 1, total cigarette consumption did not peak in the U.S. until the early 1980s [1].
Figure 1.
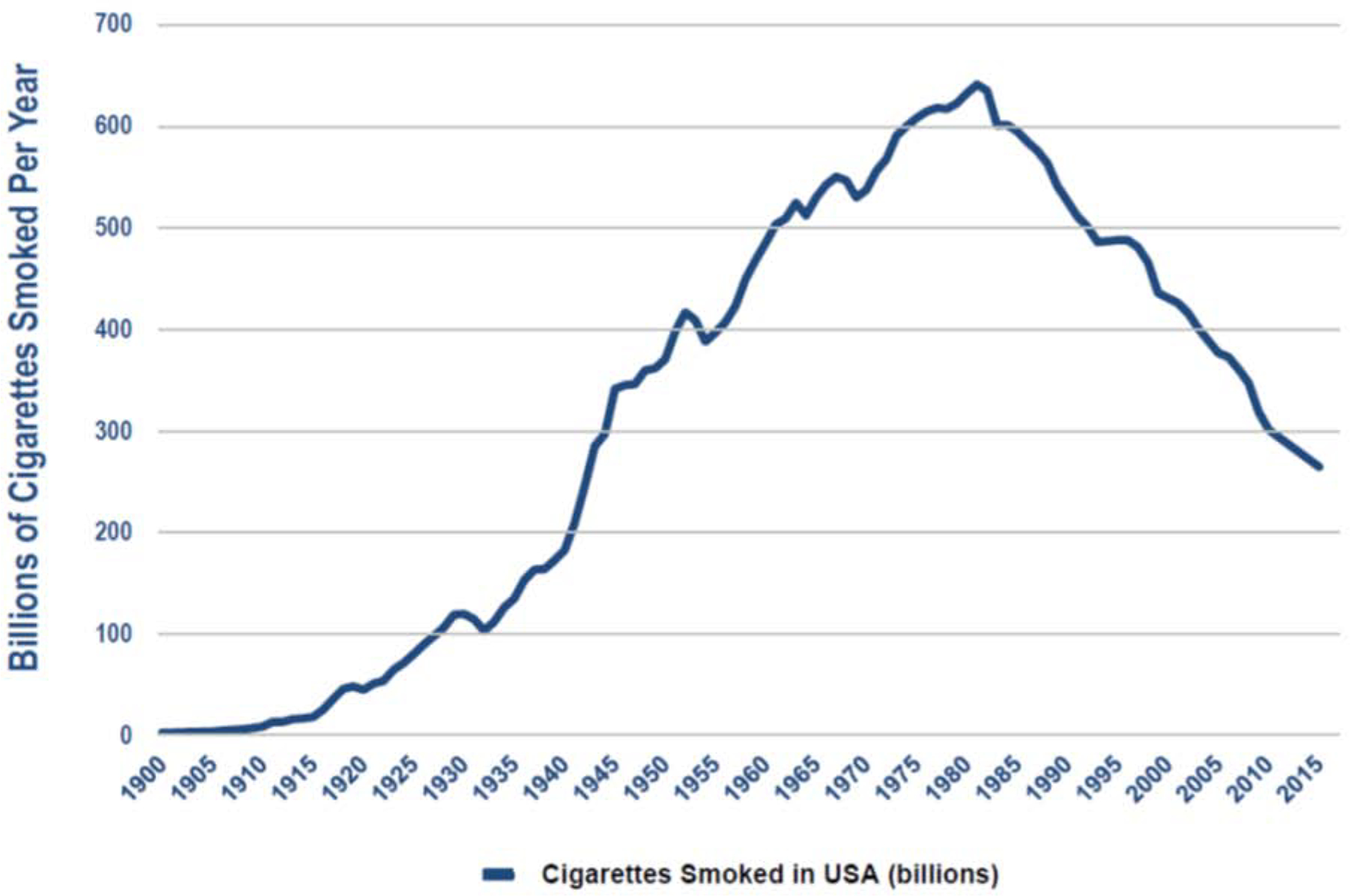
Cigarette consumption in the United States 1900–2016
Smoking prevalence in any given year is a function of two dynamic factors: 1) the number of people in the population who take up smoking (entry rates), and 2) the number of people who discontinue smoking (exit rates). Entry rates have been largely determined by the number of teens and young adults who initiate smoking since studies show that, historically, less than 1% of smokers in the population report starting smoking after the age of 26 years [6]. The number of smokers who either stop smoking or die in a given year determines exit rates. While many smokers attempt to stop smoking, the number who succeed is low, with less than 5% of daily smokers succeeding in staying off cigarettes on any given quit attempt, although quit success rates do gradually accumulate overtime which is why it is important to continue to encourage smokers to keep trying [7–9].
Since the mid-1990s, the entry rates for smoking have fallen dramatically; past 30-day smoking rates among grade 8, 10 and 12 students combined dropped from 28.3% in 1997 to 3.7% in 2019 [10]. By contrast, a survey of high school students, commissioned by a cigarette company in the late 1950s, found that 56% of high students and 75% of college students were regular smokers [11]. Trends in quit ratios suggest that progress in helping adult smokers to stop smoking has been much slower [7]. The quit ratio reflects the ratio of former smokers to ever smokers in a given year. Quit ratios should be expected to improve over time even if quitting rates do not, simply because more of those who do not quit will have died prematurely and thus be lost to the population. A flat quit ratio calls into question the effectiveness of existing interventions designed to promote smoking cessation. As shown in figure 2, between 1990 and 2010, population quit ratios were flat, but have slightly increased since 2010, due mostly to increasing rates of quitting among younger adults [7].
Figure 2.
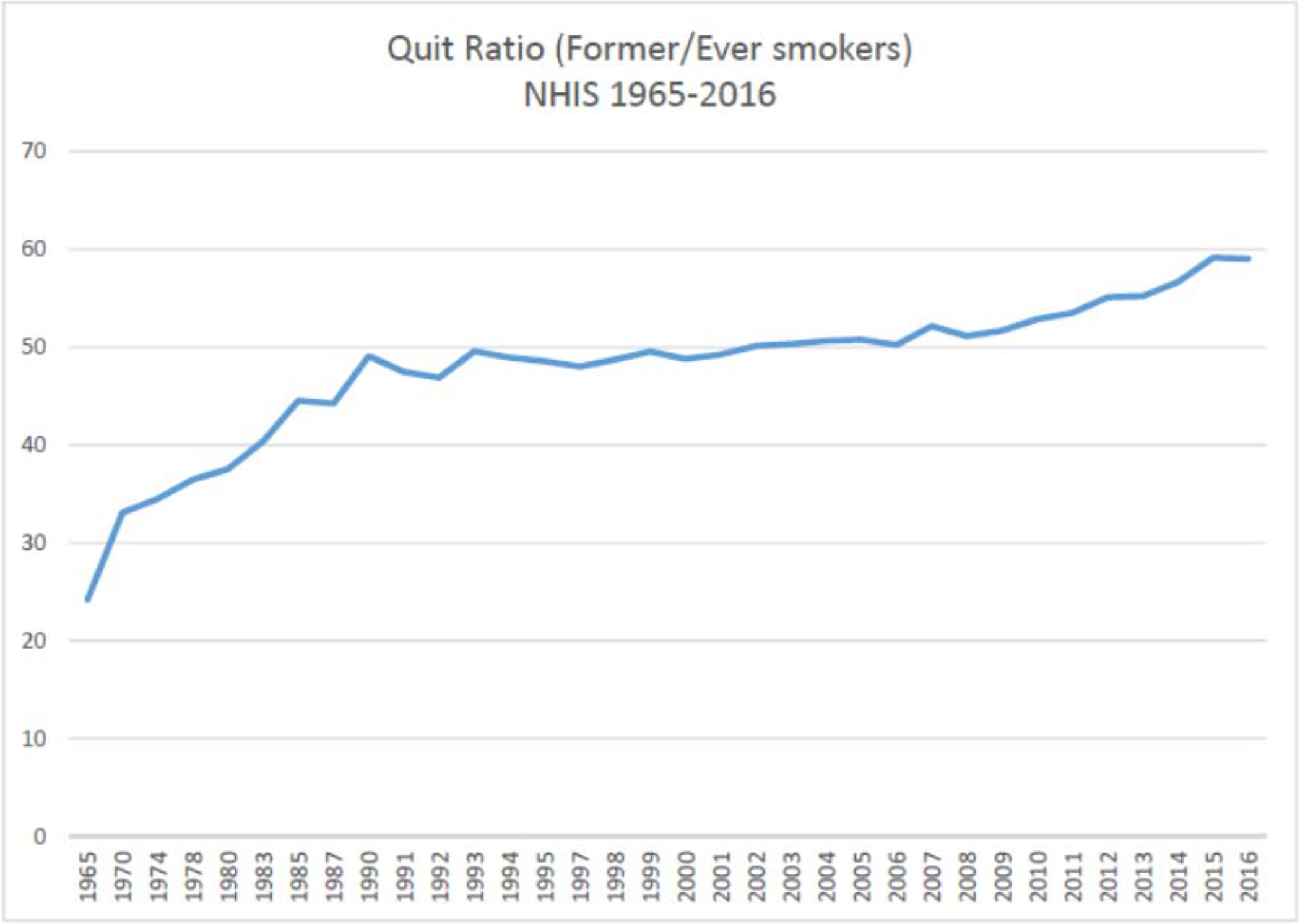
Trends in quit ratios in the United States 1965–2016
Collectively, these trends suggest that declining smoking rates over the past 30 years have been driven primarily by declining entry rates into the cigarette market. In other words, fewer teens and young adults are taking up smoking, which reflects positively on public health interventions to prevent smoking, but poorly upon efforts to increase the population rate of smoking cessation.
In this paper, we discuss factors that have contributed to the rise and fall of cigarette consumption over the past century, noting missed opportunities to change the trajectory of smoking, but also opportunities available today to markedly accelerate what continues to be slow progress in reducing adult smoking. Our analysis is divided into four chronological periods intended to roughly describe factors influencing the trajectory of cigarette consumption in America over the past 100 years. These four periods include: 1) the invention and mass marketing of the modern cigarette; 2) the discovery that smoking is harmful to health; 3) the era of tobacco control; and 4) the opportunity to accelerate a reduction in cigarette consumption.
Methods
The data for this paper come from five primary sources: 1) published scientific articles and government reports (i.e., Surgeon General Reports); 2) books chronicling the history of cigarette industry and the smoking and health problem in the United States; 3) tobacco industry documents accessed from the Truth Tobacco Industry Documents website; 4) product patents describing alternative nicotine product designs; and 5) the Family Smoking Prevention and Tobacco Control Act (hereafter referred to as the Tobacco Control Act)[12].
Results
The invention and mass marketing of the modern cigarette
Cigarette smoking grew rapidly in America in the early part of the twentieth century, largely displacing other forms of tobacco that had previously been popular such as cigars, pipe and chewing tobacco [13–15]. There are three factors that contributed to the rapid growth: 1) the wide scale use of automated cigarette rolling machines which lowered the cost of producing cigarettes; 2) the use of fine cut tobaccos and blends which were milder to inhale, delivering nicotine into the large surface area of the lungs making cigarettes highly addictive; and 3) anti-trust litigation that created different cigarette companies spurring competition in marketing on an unprecedented scale. Between 1900 and the end of Prohibition in the early 1930s, cigarette use grew despite opposition from temperance advocates and religious leaders who were concerned that smoking would lead to the abuse of alcohol and narcotic drugs, especially among youth [13–15]. However, with the entrance of the United States into World War I in 1917, cigarette use increased dramatically among United States military personnel with many previously anti-cigarette organizations supporting efforts to distribute cigarettes to troops [14].
However, neither the public nor most physicians appreciated the significant health threat from smoking [13–15]. The rapid rise in lung cancer deaths from a few hundred cases per year to several thousand per year by the early 1930s stimulated scientific theories about the possible causes for the increase in lung cancer deaths, but cigarette smoking was only one of many possible causes implicated [13–15]. With the end of Prohibition and the decline of the temperance movement, advertising in the 1930s and 1940s was defined by campaigns which often included explicit health claims, such as “They don’t get your wind” (Camel, 1935), “gentle on my throat” (Lucky Strike, 1937), “play safe with your throat” (Phillip Morris, 1941), and “Fresh as mountain air” (Old Gold, 1946). With World War II, cigarette companies continued to foster this culture of smoking by sending free cigarettes to troops and supporting the inclusion of cigarettes into the soldiers’ rations [13–15]. Advertisements for cigarettes in the 1940s often featured military themes and some encouraged citizens back home to support the troops by sending cigarettes. Except for a brief period around the Great Depression, per capita cigarette consumption increased steadily until 1953, by which time 47% of American adults were smoking cigarettes (58% of males and 36% of females), including half of all physicians [16–18].
The discovery that smoking is harmful to health
Research linking smoking to the rise in lung cancer deaths began to mount during the 1950s, with several landmark publications in leading medical journals [19–26]. Cigarette sales declined in 1953 as evidence implicating smoking as a cause of cancer began to be covered in the popular press [13–15, 27]. By 1957 the evidence establishing smoking as a causative factor in lung cancer had been established to a high degree of scientific certainty, leading to the first official statement from the US Public Health Service implicating smoking as a cause of lung cancer [28–30].
Once-secret internal business records of the cigarette companies revealed that senior scientists and executives suspected the potential cancer risk of smoking as early as the 1940s, and most accepted the fact that smoking caused cancer by the late-1950s [31–37]. However, the cigarette companies rejected the opportunity to publicly acknowledge what they knew to be true. Instead they collectively pooled their resources to finance a nearly 50-year disinformation campaign to deliberately mislead the public about the dangers of smoking, the addictiveness of nicotine, and the feasibility of providing lower risk alternative nicotine products to addicted smokers [38–40].
Patents dating back to the 1920s and 30s discuss the feasibility of removing nicotine from tobacco leaves and describe inventions to separate the delivery of nicotine from smoke (see figures 3 & 4) [41–43]. Paradoxically, these are the very same product design strategies that are central to the U.S. Food and Drug Administration’s (FDAs) science-based comprehensive nicotine focused regulatory framework for tobacco products today [44]. However, despite recognizing and even studying these alternative design strategies in the 1950s and 60s, manufacturers rushed to market filter tipped cigarettes to allay consumer health concerns [13–15, 45, 46]. The emergence of filtered cigarettes was a direct response to the publicity given to evidence linking smoking and cancer, and consumers reacted by shifting to filter tipped cigarettes. In 1952, filtered cigarettes accounted for less than 2% of sales; by 1957 this had grown to 40% and would surpass 60% by 1966 [46]. The switch to filter tipped cigarettes demonstrated that cigarette smokers were willing to change products in pursuit of reduced health risks. Consumers switched to filtered cigarettes largely based on manufacturers’ explicit or implied marketing claims, perceiving filters to be lower risk compared to using unfiltered cigarettes [47, 48] Epidemiologic studies comparing the cancer risks of those smoking filtered and unfiltered cigarettes suggested there might be a benefit from switching to a filtered cigarette [49–51]. However, these studies failed to consider the essential fact that filtered tipped cigarettes burned less tobacco compared to unfiltered cigarettes; the filter itself made no difference [51, 52]. In fact, the advertised benefits of filters were illusory [53].
Figure 3.
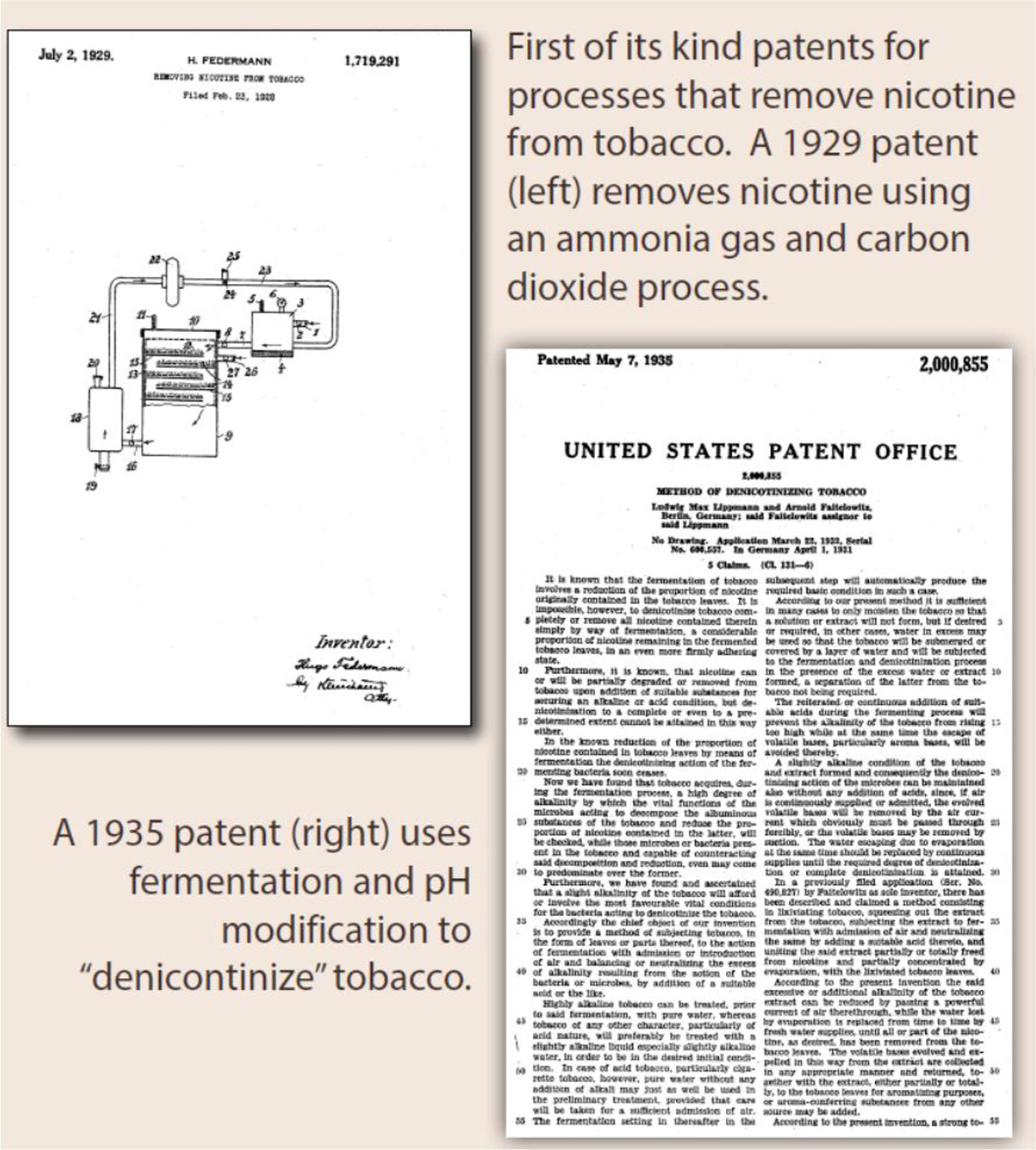
Early patents for removing nicotine from tobacco
Figure 4.
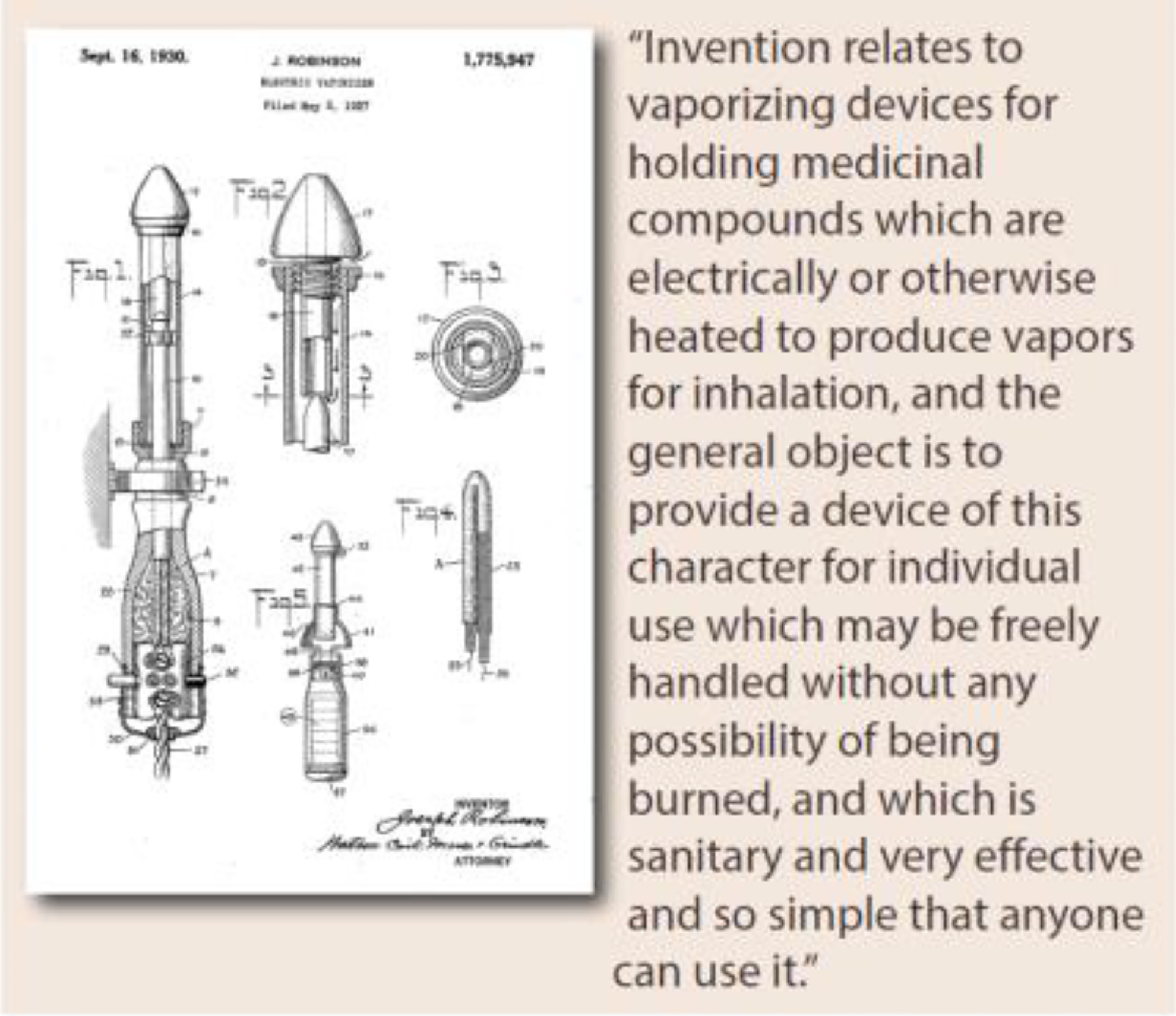
1930 patent for electronic vaporizer
Publicity surrounding the 1964 Surgeon General’s report provided yet another opportunity for cigarette companies to compete for smokers, more and more of whom were becoming concerned about the dangers of smoking [13–15, 27]. To do so, the companies engineered and marketed cigarette brands offering lower machine-measured tar and nicotine yields, even though they recognized that smokers would adjust their smoking in ways to compensate for nicotine delivery and would not necessarily get less tar and therefore suffer less disease [54–56]. Unfortunately, many smokers switched to these so-called light and lower yield cigarette brands believing them to be lower risk to their health [54–56]. The evidence today is that the introduction of filtered and low tar cigarettes can be even more dangerous—since smokers tend to smoke such cigarettes more intensively—drawing the smoke more deeply into the lungs, for example [52, 54–56].
In 1968 the National Cancer Institute launched a 10-year long research initiative to develop a less hazardous cigarette [12, 13, 57]. The cigarette industry was invited to participate in the research effort and responded by sending scientists to participate in the research program as advisors. However, internal company documents reveal that the industry’s intent in participating in the program was to steer the direction of the research away from any real solutions that would have reduced cigarette sales, representing yet another missed opportunity to change the trajectory of cigarette use in America [13–15, 57–59]. The industry’s focus on profits and fear of telling the truth about cigarettes due to liability and regulatory concerns prevented the companies from acting on real research solutions to the cigarette problem, which they recognized required at least one of the following two critical design modifications to cigarettes: 1) keeping smoke out of the lungs, and/or 2) keeping the nicotine levels low, to not reinforce the need to smoke [60].
Of course, what was missing during this period was any real regulatory oversight of the cigarette industry and its products. Indeed, cigarette companies told government officials that they could self-regulate themselves [13–15]. When industry whistleblowers finally came forward in the 1990s and told the world about the industry’s decades-long mass deception campaign, attitudes about the cigarette companies shifted further in favor of public health efforts to end the cigarette epidemic that had plagued American for nearly a century [13–15, 27, 38–40, 61]
The Era of Tobacco Control
The 1964 Surgeon General’s report marks the beginning of the era of tobacco control [1, 27]. Declining smoking rates in the U.S. corresponds to increased public awareness of the dangers of smoking, changing social norms about smoking and other tobacco products, and increased governmental actions to regulate the use, sale, and advertising of tobacco [1, 27, 62–64]. In 1966 the first cautionary label appeared on cigarette packs, stating that cigarette smoking “may be hazardous to your health.” The warnings were updated in 1970 and again in 1985, although their effectiveness has been the subject of much scientific debate [64]. In 1967, anti-smoking advertisements began to air on television as part of a Federal Communications Commission Fairness Doctrine ruling requiring broadcasters to run one anti-smoking advertisement for every three cigarette ads aired [62]. Cigarette ads were banned from television and radio in 1971, and soon after the ban was extended to include small cigars [62, 63].
Before the 1980s smokeless tobacco was a niche product that was not very popular. This changed in the 1980s with the introduction of newer styles of flavored smokeless tobacco products that appealed to younger males and were marketed using popular athletes [94]. Studies in the early 1980s also appeared suggesting that smokeless tobacco products contained carcinogens and could potentially cause oral cancer, although the health risks appeared to be markedly lower compared to cigarettes [94]. Nonetheless public health groups urged regulators to place health warnings on products and restrict their marketing much in the same way as cigarettes had been regulated. One of the required product warnings on smokeless tobacco products which persists to this day is a warning that states “This Product Is Not a Safe Alternative to Cigarettes” [95]. Public health groups at the time had argued the warning was justified to discourage the use of smokeless tobacco because of its potential addictive nature, never considering its potential as a lower risk substitute for cigarettes [96]. Today, most smokers perceive smokeless tobacco to be as dangerous or more dangerous compared to cigarettes, even though the scientific evidence does not justify this finding [97].
As evidence regarding the health consequences of secondhand smoke strengthened in the 1970s and ‘80s, policies limiting where people could use cigarettes also became more common [1, 27, 63]. The 1988 Surgeon General’s Report helped to further stigmatize tobacco use. The report examined why people persist in smoking despite recognition of its harms and concluded that smoking was not just a “habit” but was in fact addictive in ways like heroin, cocaine and other drugs of abuse [65].
When cigarette company executives appeared before a Congressional committee in 1994 and testified that they still did not accept that smoking was proven to be harmful to human health and stated that nicotine was not addictive, it was clear the industry had missed yet another historic opportunity to tell the truth [13–15]. Political support for the cigarette companies was diminishing in the 1990s, although the long ingrained political power of manufacturers persists to this day. In the mid-1990s, a lawsuit filed against cigarette companies by various state attorneys general intended to recoup public tax dollars spent on public insurance (Medicaid) for treating smoking caused diseases gained momentum, as did other lawsuits filed on behalf of injured smokers [66].
In 1998, the attorneys general of 46 states and cigarette makers reached an historic agreement to settle the various state lawsuits under what is known as the Master Settlement Agreement (MSA) [66]. Four other states reached individual state settlements with cigarette manufacturers prior to the MSA. The MSA required cigarette companies to pay billions of dollars in perpetuity to reimburse states for their Medicaid expenditures allocated to treat smoking caused diseases [66, 67]. The MSA also required companies to agree to marketing restrictions on cigarettes and disband their jointly funded public relations and research programs (i.e., the Tobacco Institute and Council for Tobacco Research) [27, 40, 66]. As part of the deal, states agreed not to pursue future efforts to recoup public health expenditures for treating tobacco caused diseases. Importantly, the MSA required the release of previously secret internal company records, revealing much of what companies had known about smoking and disease, the marketing of cigarette brands, and the engineering of cigarettes to make them hard to stop using. At the same time, though, the MSA agreement protected the major cigarette companies from competition; and since the MSA the companies have become even more profitable [68, 69].
Shortly after the release of their internal business records, the cigarette companies quietly adjusted their decades-long position that cigarettes were not harmful or addictive. For example, in October 2000, Philip Morris on its website stated: “an overwhelming medical and scientific consensus that cigarette smoking causes lung cancer, heart disease, emphysema and other serious disease in smokers” [70]. Around this time Philip Morris would be the first major cigarette company to break from the rest of the industry acknowledging that there needed to be FDA oversight of the industry.
In 1999, the US Department of Justice (DOJ) filed its own suit against the cigarette industry for violating the Racketeer Influenced and Corrupt Organizations (RICO) Act [66]. In August 2006, U.S. District Judge Gladys Kessler concluded that the cigarette companies “conspired to violate the substantive provisions of RICO” and in fact “violated those substantive provisions.” [39] Although monetary claims were not permitted in the government’s case, the judge ordered the companies to run “corrective statements” to educate the public about the companies’ past lies and deception about the health risks of smoking [64].
Following the MSA, state and local governments increasingly adopted comprehensive clean indoor air laws to protect nonsmokers from secondhand smoke, some resources were allocated to enforce laws preventing the sale of tobacco products to minors, most states set up dedicated toll-free quit lines for smokers to call to get help to stop smoking, and some states funded robust public education campaigns intended to discourage smoking [63]. Federal, state and local governments increasingly hiked cigarette taxes to discourage smoking, with some states even dedicating a percentage of the funding to support smoking control programming [63, 71]. However, less was accomplished than might have been expected. Few states put any significant proportion of MSA or tax collections into efforts to combat the smoking epidemic. Policy and public education efforts during this time period mostly lumped all tobacco products into the same risk basket inadvertently protecting cigarette sales, even though the science was beginning to show that lower risk nicotine-based product might offer an opportunity to transform the cigarette market [1, 98]. In this pre-FDA period, both R.J. Reynolds and Altria (i.e., Philip Morris) acquired smokeless tobacco manufacturers, perhaps to hedge their bets as to how the cigarette business might be transformed in the future.
In 2009, Congress passed the Family Smoking Prevention and Tobacco Control Act, which finally gave FDA regulatory authority over cigarettes and smokeless tobacco [12]. The Tobacco Control Act was written in part to rein in the cigarette industry’s decades of fraud, conspiracy and misrepresentation, which at the time was fully understandable. However, the statute, a long time in the making, was in many ways outdated on the day it was signed into law, particularly in terms of the way it dealt with new nicotine delivery products that could potentially offer addicted smokers a lower risk alternative [72]. The Tobacco Control Act was passed with the active participation and support of Altria, the parent company of Philip Morris, which at the time had half the cigarette market. Altria’s support for the Tobacco Control Act likely stemmed from the fact that the law provided protection for cigarettes that were on the market as of 2007, while making it extremely difficult to introduce new lower risk alternative nicotine products that had the potential to accelerate a decline in cigarette use, the leading preventable cause of death [72].
It is important to recognize that, like the MSA, the Tobacco Control Act was a political compromise involving stakeholders including cigarette manufacturers and a few select members of the public health community. Based on over a decade of the law protecting cigarettes from lower risk competition, in stark contrast to past FDA laws (i.e., the 1906 and 1938 FDA laws on food and drugs) that profoundly influenced the food and pharmaceutical industries to develop lower risk products, in retrospect it might be fair to say that the cigarette companies got the better part of the legislative deal [73]. It is important to recognize that today’s environment is very different than it was when the Tobacco Control Act was conceived. The internet and global product innovations have allowed for a growing spectrum of lower risk nicotine delivery products to reach consumers, threatening to replace cigarettes, much as sanitary food and science-based pharmaceuticals replaced their far more hazardous precursors [72, 73].
Nicotine vaping products (also referred to as electronic cigarettes, or e-cigarettes) began to be sold over the internet by Chinese manufacturers in the early part of the 21st century, although their popularity in the U.S. market does not to start to grow rapidly until after 2010 [74]. In 2017, NVPs accounted for approximately 4% of the nicotine product market in the U.S., with millions of customers, mostly current cigarette smokers, using them to quit or reduce their cigarette smoking [75]. Growing consumer demand for NVPs spurred competition, which in turn stimulated product innovation and kept prices low. Dedicated retail outlets known as vape shops began selling NVPs in communities across the U.S. As NVPs sales increased after 2010, so did quit ratios, which had been flat for nearly two decades before (see figure 2) [7]. The trend in increasing in quit ratios is most apparent in smokers age 18 to 44 years where NVP use is more prevalent and largely unchanged in smokers 45 years and older where NVP use is not very common [7]. In response to shifting consumer preferences, cigarette manufacturers began to develop and market their own NVP brands (e.g., Vuse, Mark X, IQOS) and in some cases acquired NVP brands from competitors (e.g., Blu, Logic, Juul).
The opportunity to accelerate a reduction in cigarette consumption
The most recent report of the Surgeon General on the topic of adult smoking cessation finds that, despite significant progress made in reducing smoking rates, there are still an estimated 34 million people smoking cigarettes in this country, most of whom are persistent daily smokers [7]. The report makes it clear why progress with smoking cessation has been painfully slow: 1) nicotine addiction makes it very hard to stop smoking; and 2) current treatments for nicotine addiction have limited effectiveness.
The reality is most adults who smoke want to stop but find it hard to stay smoke-free because of the way cigarettes are designed [7–9, 39, 44, 53, 54, 76–81]. In other words, the crux of the smoking cessation problem has to do with the way cigarettes are engineered to cause and sustain nicotine addiction. A 1982 internal memo from R.J. Reynolds discussing the dynamics of the cigarette market acknowledged that, “we cannot ever be comfortable selling a product which most of our customers would stop using if they could. That is to say, if the exit gate from our market should suddenly open, we could be out of business almost overnight” [77]. The memo goes on to say that Reynolds planned to remain in the conventional cigarettes business as long as possible, but recognized that at some future time, they would need to be prepared to shift away from conventional cigarettes to other products which met the same needs cigarettes met, but without the associated negatives [77].
In July 2017, the FDA announced an innovative new framework for regulating tobacco products [44, 78]. The science-based strategy recognized that there is a continuum of risk across different nicotine delivery products and suggested that public health could be markedly improved by reducing the addictiveness of combustible tobacco products while at the same time increasing smokers’ access to less harmful tobacco and nicotine products (i.e., both consumer and medicinal nicotine products). The guiding principle behind the strategy was finding ways to reduce the diseases and premature deaths caused by tobacco products, the vast majority of which are currently the result of addiction to conventional, combustible tobacco cigarettes [44, 78–82]. As the FDA’s Center for Tobacco Products’ press release noted:
“Envisioning a world where cigarettes would no longer create or sustain addiction and where adults who need or want nicotine could get it from less harmful alternative sources, needs to be the cornerstone of our efforts - and we believe it’s vital that we pursue common ground.”[78]
One study estimated that if all adult smokers in the U.S. who could or would not quit combustible cigarettes by other means were to switch to NVPs, more than 6.6 million premature deaths could be averted and 87 million years of life lost would then be avoided [83]. Setting regulations based on risk is an approach that has been used by the FDA in many other areas such as pharmaceuticals and food products.
However, not everyone is convinced that offering smokers less harmful nicotine delivery products would be beneficial [84, 85, 93]. One concern with the rapidly growing popularity of NVPs among youth is that those - including some non-smoking adults - who would otherwise never have taken up smoking might transition to smoking if they begin using an NVP [86–88]. However, a recent study suggests that government estimates of the number of teens who will eventually die prematurely because of smoking addiction have been exaggerated given current trends in smoking behavior [100]. Moreover, several studies have shown a positive association between NVP use and later trying smoking, although it is yet unclear if the association is causal or due to a common shared characteristic of adolescents who are more prone to take risks and experiment with smoking, nicotine vaping, and other drugs [88].
In response to the surge in NVP use by never smoking adolescents, several health organizations have called for a prohibition on the sale of some or all NVPs [85]. However, some experts are concerned that completely prohibiting lower risk alternative products for adult smokers who do not otherwise stop smoking conventional tobacco cigarettes, including by FDA-approved nicotine replacement therapies, may be counterproductive to public health [85, 89]. The recent outbreak of electronic cigarette vaping associated lung injury (EVALI) in the United States provides a cautionary lesson: EVALI is primarily attributable to vitamin E acetate in cannabis oils distributed through illicit channels [90]. There is concern that prohibiting the sale of nicotine containing e-cigarettes altogether may have the effect of driving consumers back to smoking and/or to acquisition of unregulated products through illicit channels [85, 89].
That said, the unfettered marketing of nicotine containing e-cigarettes is not a reasonable option either, as nonsmokers should not be enticed to use these products. Thus, the question for government regulators is how to strike the right balance between making accessible potentially lifesaving lower risk nicotine products intended for current addicted smokers, while discouraging use by nonsmokers, especially youth [89]. In fact, this is exactly why FDA was given authority to regulate tobacco products to begin with. The recent experiences with the rapid increase of nicotine vaping by youth involving JUUL and other similarly designed NVPs has raised important questions about the unintended consequences of allowing alternative nicotine delivery products to be sold. At the same time, it also demonstrates the value of robust but workable regulatory oversight [91]. Youth vaping is an unintended consequence of aggressive industry marketing in an unfettered marketplace born of poor regulatory oversight [72]. Ironically, it is also likely that regulatory restrictions preventing marketing of such products as a carefully regulated reduced risk option for adults who smoke cigarettes has inadvertently contributed to the youth vaping problem, since the simultaneous lack of regulatory action on other marketing has permitted widespread lifestyle advertising and no health-related messaging which would be appealing to current adult smokers [72, 89]. Post-market product surveillance supported by FDA was quick to pick up on the growing trend of youth vaping which in turn allowed FDA to use its regulatory authority to implement remedial interventions to address the problem [91]. However, at the same time FDA has been slow to require manufacturers to submit applications for review of presumably lower risk products that have been allowed onto the market under FDA’s regulatory discretion.
Unfortunately, controversy and politics have impeded efforts to identify and effectuate solutions that serve to balance the need to protect nonsmokers while also potentially providing access to lower risk products for tens of millions of addicted current smokers [72, 83, 85]. We advise skepticism of manufacturers whose motives are to maximize shareholder value which may not always align with the goals of public health. The recent Federal Trade Commission complaint regarding the Juul-Altria deal reinforces the need for extreme skepticism in accepting at face-value claims made by cigarette manufacturers to transform their core cigarette business [99]. However, we must also be careful not to miss genuine opportunities to support evidence-based innovations that offer the potential to advance public health by offering smokers lower risk alternative nicotine products. Effective regulatory oversight can compel business interests to align with public health goals, as has been done with other consumer products, food, airline and auto safety, air quality, unleaded paint and motor fuels and myriad other goods and services.
Discussion
In this paper we have looked back in time to understand the factors that influenced the rise and fall of cigarette use in America. We have attempted to identify missed opportunities to change the trajectory of smoking caused deaths. Many of the missed opportunities identified are due to the actions of cigarette manufacturers who deliberately misled the public about the dangers of cigarette smoking, the addictiveness of nicotine, and the feasibility of providing lower risk alternative nicotine delivery products to addicted smokers. An important lesson learned from the past is that treating all tobacco/nicotine products as equivalently harmful is counterproductive to public health goals as it only serves to protect the most lethal nicotine product - cigarettes. In 2009, Congress passed the Tobacco Control Act which finally gave FDA regulatory authority over cigarettes and smokeless tobacco and later was extended to include all tobacco products. The Tobacco Control Act was written in part to rein in the cigarette industry’s decades of misconduct. However, the statute was in many ways outdated on the day it was signed into law, particularly in terms of the way it dealt with new nicotine delivery products that could potentially offer addicted smokers a lower risk alternative.
The evolving marketplace of lower risk nicotine products represents a new opportunity to dramatically transform the cigarette business in ways that were never imagined when the war on tobacco was raging decades ago. Today public health groups are in a unique position to align market forces with public health goals to reduce the premature deaths caused by cigarettes. However, this requires embracing risk-proportionate regulation and taxation policies along with providing consumers with accurate public messaging on product relative risks.
Disruptive technology is a huge ongoing threat to the market for and profitability of cigarettes. In what has been and will continue to be a rapidly changing environment there are both challenges but more importantly opportunities that require action [72, 92–93]. The natural evolution of the marketplace requires FDA - within the dictates of the statute - to adapt so regulations can consider new ideas and options that can better address the devastating health consequences caused by cigarettes [72]. Below we describe four strategies that public health groups could be embracing today in order to better align market forces to accelerate a decline in cigarette use.
1. Embrace the Concept of Regulating Tobacco Products Based on the Continuum of Risk
Regulating based on the continuum of risk was a major component of the FDA/CTP July 2017 announcement and has conceptually been supported by many in the public health and scientific community, consumers, and even many in the manufacturing sector [44, 78]. As a start, public health organizations should to be unified in supporting FDA’s logical 2018 plan to establish a very low nicotine standard for combustible tobacco rendering cigarettes non-addictive [44,78, 82]. If this plan were implemented, one analysis suggests that approximately 5 million additional adult smokers could quit smoking within one year of implementation and, over time, more than 33 million people - mostly youth and young adults - would avoid becoming regular smokers, thus avoiding many millions of tobacco-related deaths [82]. Simultaneously public health groups should recognize and support a more flexible and adaptable regulatory framework that that will allow science-based lower risk products into the marketplace more expeditiously, while working to ensure that such products are not available, targeted or used by any children or adolescents.
2. Update the Tobacco Control Act
It has been over 10 years since the passage of the Tobacco Control Act. The statute needs to be critically reviewed and updated to reflect the changing marketplace of nicotine delivery products. Such review and updating of FDA statutes is routine in other areas of regulation such as foods, drugs, and medical devices. As a follow-up to the Institute of Medicine’s 2000 report Clearing the Smoke, public health groups and others could ask the FDA/CTP to request that the Health and Medicine Division of the National Academies of Science to do a thorough assessment on how the Tobacco Control Act might be updated to adapt to a rapidly evolving tobacco and nicotine market place [98]. Areas of review might focus on and include: a) defining common terminologies and definitions that can allow for greater public understanding, and provide consistency in statutory, regulatory, and legal relevance; b) creating product standards for the various categories of products that includes combustible products, non-combustible tobacco, nicotine products, and other possible alternatives; c) developing comprehensive labeling, marketing and educational campaigns that would reflect the risks and relative risks of the products both in terms of product categories as well as individual products so that the public, users of products, the medical profession, and others would better understand the risks and relative risks of using one type of product over another; and d) restructuring oversight of products so that all tobacco and nicotine products are under the same regulatory authorization within the CTP.
3. Support Civil Dialogue on Issues of Smoking Harm Reduction
The current climate in smoking harm reduction has become toxic and emotional, non-scientific, and counterproductive to achieving the public health goal of reducing premature deaths caused by using smoked tobacco (i.e., mainly cigarettes). We are not suggesting that we dismiss the past bad actions of the cigarette manufacturers, nor accept the claims of manufacturers of alternative nicotine products. Rather, we need to heed the lessons of the past so as not to make the same mistakes going forward. The Tobacco Control Act created a framework that should incentivize manufacturers to move away from profiting from the sale of cigarettes that causes so much harm to consumers. Promoting dialogue summits would allow for participants to engage in a civil manner, educate one another about challenges and opportunities and agree to specific measurable goals and objectives. Bringing stakeholders together will not resolve all differences but it will allow serious and responsible stakeholders the opportunity to bring ideas forward and find areas of common ground that can more rapidly advance population health. As an example, issues and concerns related to adolescent use of tobacco and nicotine products should be a major topic of concern, not only by the public health and tobacco control communities but by federal, state, and local policy makers and regulators, parents and teachers, responsible retailers and distributers, and many of those associated with the manufacturing businesses. While many stakeholders share common ground in this area, the polarizing and media driven approach that has been taken over the last several years has, in our view, caused what has become a war of rhetoric, with a lot of finger pointing and a failure to bring interested parties together to discuss how to collectively deal with the issue and find workable solutions to protect youth while allowing smokers to have access to cleaner alternative nicotine products.
4. Encourage Collaborative Scientific Research and Product Innovation
It is often said that it should be good science that drives the implementation of sound policies. The FDA/CTP could be doing much more to encourage academic scientists to partner with new or existing manufacturers to advance science in ways that would accelerate the introduction of lower risk products into the market place. All parties and stakeholders should be held accountable to meeting and following the strictest standards for peer review. There should be greater collaboration and data sharing, and a shared commitment to open science. Science should not be cherry picked for public relations purposes. The FDA/CTP can play an important role in further facilitating such discussions, helping set research priorities which would have a positive impact on the regulatory decision-making. For example, the FDA/CTP could in theory invite manufacturers to voluntarily utilize their peer review system to vet proposals designed to assist manufacturers prepare their PMTA and MRTP applications thereby opening this process, making it more competitive, transparent, and less secretive. Product manufacturers also ought to be incentivized to share their internal research and market data more widely with public health scientists so that there is greater confidence in product claims. The FDA/CTP and other groups can and should do more to hold scientific workshops that allow scientists and researchers to meet in a safe-haven environment and where opportunities would be allowed for seemingly opposing interests to find common ground in areas of science, research and innovation. Innovators of products should not be shut out because some regard them as industry.
In summary, embracing risk-proportionate tobacco product regulation and taxation policies along with providing consumers with accurate public messaging on product relative risks offers the prospect of aligning market forces with public health goals to reduce deaths caused by cigarettes in ways that were never imagined decades ago. A regulatory framework based on sound science that encourages and rewards new or existing manufacturers to invest in consumer acceptable lower risk products to replace cigarettes needs to be encouraged. The past is indeed not the future in smoking control, but it may be difficult to escape the past unless a realignment of market forces and policies can be achieved.
Highlights.
Treating all tobacco/nicotine products as equivalently harmful is counterproductive to public health goals.
Public health needs to embrace the concept of regulating tobacco products based on the continuum of risk.
The Tobacco Control Act needs to be updated to adapt to the evolving marketplace of lower risk nicotine product.
We should encourage product innovation and support civil dialogue on issues related to smoking harm reduction.
Funding support:
Dr. Cummings receives funding support through a grant from the National Cancer Institute, P01 grant (P01CA200512). The funder of the study had no role in data analysis, data interpretation, or writing of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
K. Michael Cummings, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC.
Scott Ballin, Health Policy Consultant, Advisor to the University of Virginia Morven Dialogues
David Sweanor, Adjunct Professor of Law, University of Ottawa
References
- 1.The Surgeon General in The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General: Atlanta, GA2014. Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs—2014. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 2.Holford TR, Meza R, Warner KE, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964– 2012. JAMA, 2014;311(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovino GA, Schooley MW, Zhu BP, Chrismon JH, Tomar SL, Peddicord JP, et al. Surveillance for Selected Tobacco-Use Behaviors - United States, 1900–1994. Centers for Disease Control and Prevention. CDC Surveillance Summaries, 1994. MMWR, 1994; 43(No. SS-3):1–50. [PubMed] [Google Scholar]
- 4.Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, Jamal A, Neff L. Tobacco Product Use and Cessation Indicators Among Adults — United States, 2018. MMWR, 2019;68;1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gfroerer J, Dube SR, King BA, Garrett BE, Babb S, McAfee T. Vital signs: Current cigarette smoking among adults aged > 18 years with mental illness - United States, 2009–2011. MWWR, 2013;62:81–87. [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2012. [Google Scholar]
- 7.U.S. Department of Health and Human Services. Smoking Cessation A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020. [Google Scholar]
- 8.Hyland A, Li Q, Bauer J, Giovino G, Steger C, Cummings KM. Predictors of cessation in a cohort of current and former smokers followed over 13 years. Nicotine Tob Res, 2004;6 Suppl 3:S363–369. [DOI] [PubMed] [Google Scholar]
- 9.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting Smoking Among Adults — United States, 2000–2015. MMWR, 2017;65:1458–1464. [DOI] [PubMed] [Google Scholar]
- 10.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME Monitoring the Future national survey results on drug use 1975–2019: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan, 2020. https://www.drugabuse.gov/related-topics/trends-statistics/monitoring-future [Google Scholar]
- 11.William, Esty Company Inc. The Youth Research Institute Study Regarding Cigarette Smoking Among 7,521 High School and College Students in 80 Cities Throughout the United States, October – November, 1959: Summary of Findings. 1959. December Marketing to Youth MSA Collection. Unknown. https://www.industrydocuments.ucsf.edu/docs/qrgj0045
- 12.U.S. Congress. Family Smoking Prevention and Tobacco Control Act In: Congress US, ed. R. H 1256 Vol Public Law 111–31. Washington, D.C. : U. S. Government Printing Office; 2009. [Google Scholar]
- 13.Kluger R Ashes to Ashes. America’s Hundred-Year Cigarette War, the Public Health, and the Unabashed Triumph of Philip Morris. New York, NY: Alfred A. Knopf, Inc., 1996. [Google Scholar]
- 14.Brandt A The Cigarette Century: The Rise, Fall, and Deadly Persistence of the Product That Defined America. New York: Basic books; 2007. [Google Scholar]
- 15.Proctor RN. Golden Holocaust: Origins of the Cigarette Catastrophe and the Case for Abolition. Berkeley, CA: University of California Press, 2011. [Google Scholar]
- 16.Burgard JW. Copy of Study of Cigarette Advertising, 1953. Retrieved February 1, 2002, from Legacy Tobacco Documents Library, UCSF: http://legacy.library.ucsf.edu/tid/kmn99d00
- 17.Blum A The “More doctors smoke camels” campaign: The unfiltered truth about smoking and health. The University of Alabama: Center for the Study of Tobacco and Society. https://csts.ua.edu/ama/more-doctors-smoke-camels/ [Google Scholar]
- 18.Gardner MN, Brandt AM. The Doctors’ Choice Is America’s Choice: The Physician in US Cigarette Advertisements, 1930–1953. Am J Public Health, 2006;96(2): 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert PN. Angel H Roffo: The forgotten father of experimental tobacco carcinogenesis Bulletin of the World Health Organization, 2006;84(6):494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrek R, Baker LA, Ballard GP, Dolgoff S. Tobacco smoking as an etiologic factor in disease. I. Cancer. Cancer Research, 1950;10(1):49–58. [PubMed] [Google Scholar]
- 21.Wynder EL, Graham EA, Tobacco smoking as a possible etiologic factor in bronchogenic carcinoma, JAMA, 1950;143(4):329–336. [DOI] [PubMed] [Google Scholar]
- 22.Levin ML, Goldstein H, Gerhardt PR, Cancer and tobacco smoking, JAMA, 1950; 143(4):336–338. [DOI] [PubMed] [Google Scholar]
- 23.Wynder EL, Graham EA, Croninger AB, Experimental product of carcinoma with cigarette tar, Cancer Research, 1953;13:855–864. [PubMed] [Google Scholar]
- 24.Hammond EC, Horn D. The relationship between human smoking habits and death rates: a follow-up study of 187,766 men. JAMA, 1954;155(15):1316–1328. [DOI] [PubMed] [Google Scholar]
- 25.Doll R, Hill BA. The mortality of doctors in relation to their smoking habits - a preliminary report. BMJ, 1954;I:1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auerbach et al. Changes in the bronchial epithelium in relation to smoking and cancer of the lung. A report of progress. N Engl J Med, 1957;256(3):97–104. [DOI] [PubMed] [Google Scholar]
- 27.Cummings KM, Proctor RN. The changing public image of smoking in the United States: 1964–2014. Cancer Epidemiology, Biomarkers & Prev 2014. 23(1):32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Department Of Health Education And Welfare. -- No Title -- 1957. July 12 American Tobacco Records; https://www.industrydocuments.ucsf.edu/docs/nkjv0015
- 29.Burney LE. “Policy over Politics: The First Statement on Smoking and Health by the Surgeon General of the United States Public Health Service.” New York State Journal of Medicine, 1983;83:12–13. [PubMed] [Google Scholar]
- 30.Garland J Editorials. N Engl J Med, 1959;262(8):417–418. [Google Scholar]
- 31.Hanmer HR. Letter. May 11, 1939. American Tobacco Records. https://www.industrydocuments.ucsf.edu/docs/rmmp0140
- 32.Parmele HB Letter. July 29, 1946. Lorillard Records. https://www.industrydocuments.ucsf.edu/docs/gpnm0104
- 33.Teague C Survey of cancer research. February 2, 1953. RJ Reynolds Records. https://www.industrydocuments.ucsf.edu/docs/lphb0086
- 34.Bentley, Felton HR, Reid DGI, W.W. Report on Visit to U.S.A. and Canada - 17th April – 12th May 1958. June 11, 1958. Ness Motley Law Firm Documents. https://www.industrydocuments.ucsf.edu/docs/ksfd0040
- 35.Arthur D Little Inc; L & M - a Perspective Review. 15 March, 1961. Tobacco Institute Records. https://www.industrydocuments.ucsf.edu/docs/ytbl0135
- 36.Wakeham H Tobacco and Health R&D Approach, Presentation to R&D Committee. November 15, 1961. Philip Morris Records. https://www.industrydocuments.ucsf.edu/docs/sjgx0106
- 37.Rodgman A; Chemical Research. The Smoking and Health Problem--A Critical and Objective Appraisal. September 12, 1962. RJ Reynolds Records; Minnesota Documents. https://www.industrydocuments.ucsf.edu/docs/rmpp0092
- 38.Glantz SA, Slade J, Bero LA, Hanauer P, Barnes DE. The Cigarette Papers. Berkeley, California: University of California Press, 1996. [Google Scholar]
- 39.US v Philip Morris et al. , Civil Action 99–2496, Final opinion, issued August 17, 2006, US District, Lexis; 61412. [Google Scholar]
- 40.Cummings KM, Brown A, O’Connor R. The cigarette controversy. Cancer Epidemiol Biomarkers Prev. 2007. 16(6):1070–1076. [DOI] [PubMed] [Google Scholar]
- 41.Federman H Removing nicotine from tobacco. United States Patient Office. Patent: 1,719,291. July 2, 1929. [Google Scholar]
- 42.Lippmann LM, Faitelowitz A. Method of denicotinizing tobacco. United States Patient Office. Patent: 2,000,855. May 7, 1935. [Google Scholar]
- 43.Robinson J Electronic vaporizer. United States Patient Office. Patent: 1,775,947. September 16, 1930. [Google Scholar]
- 44.Gottlieb S, Zeller M. A Nicotine-Focused Framework for Public Health. N Engl J Med. 2017. September 21; 377(12):1111–1114. [DOI] [PubMed] [Google Scholar]
- 45.Blatnik JA (Committee Chairperson). False and Misleading Advertising (filter-tip cigarettes). Twentieth Report by the Committee on Government Operations, Legal and Monetary Affairs Subcommittee February 20, 1958 Lorillard Records. https://www.industrydocuments.ucsf.edu/docs/lrbd0124 [Google Scholar]
- 46.Federal Trade Commission. Report to Congress Pursuant to the Federal Cigarette Labeling and Advertising Act. Washington, DC: United States Federal Trade Commission, 1967. June 30, 1967 Lorillard Records. https://www.industrydocuments.ucsf.edu/docs/hswn0222 [Google Scholar]
- 47.Wakeham H Trends of tar and nicotine deliveries over the last 5 years. March 24, 1961, Philip Morris Records. https://www.industrydocuments.ucsf.edu/docs/pqjp0124
- 48.Pepples E Industry Response to Cigarette/Health Controversy. February 4, 1976. Brown & Williamson Records. https://www.industrydocuments.ucsf.edu/docs/jnxd0024
- 49.Bross IDJ, Gibson R. Risks of lung cancer in smokers who switch to filter cigarettes. AJPH, 1967; 58(8):1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris JE, Thun MJ, Mondul AM, Calle EE. Cigarette tar yields in relation to mortality from lung cancer in the cancer prevention study II prospective cohort, 1982–8. BMJ, 2004;328(7431)72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanner NT, Thomas NA, Ward R, et al. Association of Cigarette Type With Lung Cancer Incidence and Mortality: Secondary Analysis of the National Lung Screening Trial. JAMA Intern Med. 2019;179(12):1710–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston ME Jr. Special report No. 248: Market potential of a health cigarette. Philip Morris Records. June 1966. https://www.industrydocuments.ucsf.edu/docs/jfvc0123 [Google Scholar]
- 54.U.S. Department of Health and Human Services. The Health Consequences of Smoking: The Changing Cigarette A Report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office on Smoking and Health, 1981. DHHS Publication No. (PHS) 81–50156. [Google Scholar]
- 55.Burns D, Benowitz N, Amacher R, eds. Risks Associated With Smoking Cigarettes With Low Machine-Measured Yields of Tar and Nicotine Monograph No. 13. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute, NIH Publication No. 02–5074; 2001. [Google Scholar]
- 56.Shiffman S, Pillitteri JL, Burton SL, Rohay JM, Gitchell JG. Smokers’ beliefs about “light” and “ultra-light” cigarettes. Tob Control. 2001;10(suppl 1):i17–i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parascandola M Lessons from the History of Tobacco Harm Reduction: The National Cancer Institute’s Smoking and Health Program and the “Less Hazardous Cigarette”, Nicotine & Tob. Res. 2005; 7(5):779–789. [DOI] [PubMed] [Google Scholar]
- 58.Meeting of Committee of Counsel of the Tobacco Institute with regards to Dr. Gio Gori and the Tobacco Working Group on March 14, 1973 in New York. 1973. March 15 Tobacco Products Liability Project Collection. Unknown. https://www.industrydocuments.ucsf.edu/docs/ghgn0050
- 59.Senkus M re: Less Hazardous Cigarette. March 27, 1968. Ness Motley Law Firm Documents. https://www.industrydocuments.ucsf.edu/docs/gqmb0040
- 60.BAT. R & D Conference - Montreal 1967. October 24 Ness Motley Law Firm Documents. Unknown. https://www.industrydocuments.ucsf.edu/docs/ntfy0042 [Google Scholar]
- 61.LeBow BS. Videotaped deposition. 1997. July 18 Liggett & Myers Records; RICO Privilege Downgrades Collection. Unknown. https://www.industrydocuments.ucsf.edu/docs/fjvn0016
- 62.Warner KE. Effects of the antismoking campaign: an update. American Journal of Public Health, 1989; 79:144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cummings KM. Programs and policies to discourage the use of tobacco products. Oncogene. 2002; 21:7349–64. [DOI] [PubMed] [Google Scholar]
- 64.Cummings KM, Gdanski J, Veatch N, Sebrie E. Assumption of Risk and the Role of Health Warnings Labels in the United States. Nicotine Tob Res. 2019. May 25 pii: ntz089. doi: 10.1093/ntr/ntz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.United States Department of Health and Human Services. The Health Consequences of Smoking: Nicotine Addiction. A Report of the Surgeon General, 1988. Rockville, Maryland: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Health Promotion and Education, Office on Smoking and Health, 1988. [Google Scholar]
- 66.Douglas CE, Davis RM, Beasley JK. Epidemiology of the third wave of tobacco litigation in the United States, 1994–2005. Tob. Control, 2006;15:iv9–iv16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keller TE, Ju TW, Ong M, Sung HY. The US national tobacco settlement: the effects of advertising and price changes on cigarette consumption. Applied Economics, 2004; 36;1623–9. [Google Scholar]
- 68.Herzog B Wall Street Tobacco Industry Update: NATO Education Seminar. February 11, 2019.
- 69.Credit Suisse. Tobacco - US market data from Nielsen 4w to 2 Nov. November 12, 2019.
- 70.Szymanczyk ME. Philip Morris USA Website. October 11, 2000. Philip Morris Records. https://www.industrydocuments.ucsf.edu/docs/jnxf0061
- 71.Chaloupka FJ. Tobacco control lessons learned: The impact of state and local policies. Research Paper Series, No. 38 , January 2010. http://tobaccopolicycenter.org/wp-content/uploads/2017/11/153.pdf [Google Scholar]
- 72.Ballin S Is Civil Dialogue and Engagement Between Diverse Stakeholders with Respect to Tobacco Harm Reduction Feasible? A Review of the Past, Present and Future. 73rd Tobacco Science Research Conference July 26, 2019. [Google Scholar]
- 73.U.S. Food and Drug Administration. The History of FDA’s Fight for Consumer Protection and Public Health. June/29/2018. https://www.fda.gov/about-fda/history-fdas-fight-consumer-protection-and-public-health
- 74.Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, Cummings KM, McNeill Am, Thrasher JF, Hammond D, Fong GT. Electronic Nicotine Delivery Systems International Tobacco Control Four-Country Survey. Am J Prev Med. 2013. 44(3):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Global Trends in Nicotine. Retrieved October 19, 2018, from https://www.smokefreeworld.org/advancing-industry-transformation/global-trends-nicotine
- 76.Cummings KM, Brown A, Douglas CE. Consumer acceptable risk: how cigarette companies have responded to accusations that their products are defective. Tob Control. 2006, 15(Suppl 4):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teague CE. Nordine Study. December 1, 1982. R.J. Reynolds. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/jtjc0094
- 78.U.S. Food and Drug Administration, FDA announces comprehensive regulatory plan to shift trajectory of tobacco-related disease, death. July 27, 2017. https://www.fda.gov/news-events/pressannouncements/fda-announces-comprehensive-regulatory-plan-shift1trajectory-tobacco-related-disease-death
- 79.Food and Drug Administration. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. Federal Register (March 16, 2018), 83(52): 11818–11843. [Google Scholar]
- 80.Benowitz NL, Henningfield JE Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. [DOI] [PubMed] [Google Scholar]
- 81.Douglas CE, Henson R, Drope J, Wender RC. The American Cancer Society public health statement on eliminating combustible tobacco use in the United States. CA Cancer J Clin, 2018. July;68(4):240–245. [DOI] [PubMed] [Google Scholar]
- 82.Apelberg BJ, Feirman SP, Salazar E, et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med, 2018;378(18), 1725–1733. [DOI] [PubMed] [Google Scholar]
- 83.Levy DT, Borland R, Lindblom EN, et al. , 2018. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob. Control. 27 (1), 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abrams DB, Glasser AM, Pearson JL, Villanti AC, Collins LK, Niaura RS. Harm Minimization and Tobacco Control: Reframing Societal Views of Nicotine Use to Rapidly Save Lives. Annu. Rev. Public Health 2018. 39:193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fairchild A, Healton C, Curran J, Abrams D, Bayer R. Evidence, alarm, and the debate over e-cigarettes: Prohibitionist measures threaten public health. Science, 2019; 366(6471):1318–1320. [DOI] [PubMed] [Google Scholar]
- 86.Cullen KA, Gentzke AS, Sawdey MD, et al. e-Cigarette Use Among Youth in the United States, JAMA, 2019; 22(21):2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hammond D, Reid JL, Rynard VL, Fong GT, Cummings KM, McNeill A, Hitchman S, Thrasher JF, Goniewicz ML, Bansal-Travers M, O’Connor R, Levy D, Borland R, White CM. Prevalence of vaping and smoking among adolescents in Canada, England and the United States: repeat national cross-sectional surveys. BMJ, 2019;365:l2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levy DT, Warner KE, Cummings KM, et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob. Control, 2019;28:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cummings KM, Hammond D. E-cigarettes: striking the right balance. The Lancet Public Health, January 22, 2020. 10.1016/S2468-2667(20)30004-9 [DOI] [PubMed] [Google Scholar]
- 90.Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med, 2019:e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.U.S. Food and Drug Administration, FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint. January 2, 2020. https://www.fda.gov/news-events/pressannouncements/fda-finalizes-enforcement-policy-unauthorized-flavoredcartridge-based-e-cigarettes-appeal-children
- 92.US Food and Drug Administration, FDA permits sale of IQOS tobacco heating system through premarket tobacco product application pathway. April 30, 2019. https://www.fda.gov/news-events/pressannouncements/fda-permits-sale-iqos-tobacco-heating-system-throughpremarket-tobacco-product-application-pathway
- 93.Glantz SA. More evidence that IQOS and ecigs are as bad for human lungs as cigs. February 16, 2019. https://tobacco.ucsf.edu/more-evidence-iqos-and-ecigs-are-bad-human-lungs-cigs
- 94.United States Department of Health and Human Services. The health consequences of using smokeless tobacco: a report of the advisory committee to the Surgeon general, 1986. Bethesda, Maryland: U.S. Dept. of Health and Human Services, Public Health Service; 1986. [Google Scholar]
- 95.Kozlowski LT. Origins in the USA in the 1980s of the warning that smokeless tobacco is not a safe alternative to cigarettes: a historical, documents-based assessment with implications for comparative warnings on less harmful tobacco/nicotine products. Harm Reduct J 2018; 15, 21 10.1186/s12954-018-0228-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.United States Congress. Comprehensive smokeless tobacco health education act of 1986, 132 Congressional Record, H 245. Vol 132 no. 8 Pg H 245; 1986. [Google Scholar]
- 97.Fong GT, Elton-Marshall T, Driezen P, et al. U.S. adult perceptions of the harmfulness of tobacco products: Descriptive findings from the 2013–14 baseline wave 1 of the PATH study. Addict Behav. 2019. April;91:180–187. doi: 10.1016/j.addbeh.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Institute of Medicine. 2000. Clearing the Air: Asthma and Indoor Air Exposures. Washington, DC: The National Academies Press; 10.17226/9610. [DOI] [PubMed] [Google Scholar]
- 99.Federal Trade Commission BoC. In the Matter of Altria Group, Inc., a corporation; and JUUL Labs, Inc., a corporation. FTC Matter/File Number: 191 0075, Docket Number: 9393, Enforcement Type: Part III Administrative Complaint 2020. https://www.ftc.gov/system/files/documents/cases/d09393_administrative_part_iii_complaint-public_version.pdf
- 100.Warner K Will 5.6 million current American youth eventually die from smoking? The anatomy of a commonly accepted tobacco control measure. Tob Control 2020;0:1–3. doi: 10.1136/tobaccocontrol-2020-055672. [DOI] [PubMed] [Google Scholar]


