Abstract
Purpose:
To investigate the association between imaging biomarkers of radiation-induced white matter (WM) injury within perisylvian regions and longitudinal language decline in brain tumor patients.
Methods and Materials:
Primary brain tumor patients (n=44) on a prospective trial underwent brain MRI, diffusion-weighted imaging, and language assessments of naming [Boston Naming Test (BNT)] and fluency [DKEFS-Category Fluency (DKEFS-CF)] at baseline, 3, 6, and 12-months post-fractionated radiation therapy (RT). Reliable change indices of language function (0-6 months), accounting for practice effects (RCI-PE), evaluated decline. Bilateral perisylvian WM regions (superficial WM subadjacent to Broca’s Area and superior temporal gyrus (STG); inferior longitudinal fasciculus (ILF), inferior fronto-occipital fasciculus (IFOF), and arcuate fasciculus) were autosegmented. We quantified volume and diffusion measures of WM microstructure: fractional anisotropy (FA; lower values indicate disruption) and mean diffusivity (MD; higher values indicate injury). Linear mixed-effects models assessed mean dose as predictor of imaging biomarker change, and imaging biomarkers as longitudinal predictors of language scores.
Results:
DKEFS-CF scores declined at 6 months post-RT (RCI-PE −0.483, p=0.01), whereas BNT scores improved (RCI-PE 0.262, p=0.04). Higher mean dose to left and right regions was predictive of decreased volume (Left-STG, p=0.02; Right-ILF and IFOF, p=0.03), decreased FA (Left-WM tracts, all p<0.01; Right-STG and IFOF, p<0.02), and increased MD of left-WM tracts (all p<0.03). Volume loss within Left-Broca’s area (p=0.01), Left-ILF (p=0.01), Left-IFOF (p=0.01), and Left-arcuate fasciculus (p=0.04) were associated with lower BNT scores. Lower FA correlated with poorer DKEFS-CF and BNT scores within Left-ILF (p=0.02, NS), Left-IFOF (p=0.02, 0.04), and Left-arcuate fasciculus (p=0.01, 0.01), respectively. Poorer DKEFS-CF scores correlated with increased MD values within the Left-arcuate fasciculus (p=0.03). Right-sided biomarkers did not correlate with language scores.
Conclusions:
Primary brain tumor patients experience language fluency decline post-RT. Poorer fluency and naming function may be explained by microstructural injury to left-sided perisylvian white matter, representing potential dose-avoidance targets for language preservation.
INTRODUCTION
Radiotherapy (RT) plays a vital role in the treatment of primary brain tumors, but can also contribute to long-term neurocognitive decline1. Neurocognitive functioning has thus become a critical endpoint in brain tumor clinical trials2–6. Language functioning is known to be affected post-treatment1, which can have a significant impact on patients’ quality of life, including the ability to communicate effectively and interpret complex information. Despite the clinical importance of language functioning, this domain is largely understudied in radiation oncology and ongoing clinical trials.
Radiation injury to normal brain tissue is characterized by vascular damage, neuroinflammation, and microenvironmental changes that lead to loss of supportive glial cells and ultimately demyelination and axonal injury7. Diffusion tensor imaging (DTI) biomarkers, including fractional anisotropy (FA) and mean diffusivity (MD), provide quantification of this white matter disruption and help elucidate the pathophysiology of RT induced injury in-vivo with non-invasive means8–10. FA measures the directionality of water diffusion, with a decrease representing disruption in white matter integrity. MD is an average of three eigenvalues that represent the relative motility of water, with an increase indicating white matter disruption. Volumetric analysis allows detection of longitudinal atrophy within specific white matter regions of interest (ROIs). Previous studies have demonstrated that these biomarkers of regional white matter injury are associated with radiation-induced neurocognitive decline in various domains, including memory11–13 and executive functioning14. To our knowledge, a similar investigation into the critical aspects of language functioning has not been performed in primary brain tumor patients.
Language functioning encompasses many different component skills, including naming, fluency, and comprehension. Among these aspects of language, naming and fluency are often very sensitive to decline, as both require intact semantic processing, as well as lexical search and retrieval15. In this prospective study, we target both important aspects of language with two distinct yet complimentary measures. The Delis–Kaplan Executive Function System Category Fluency (DKEFS-CF) measures semantic fluency by assessing one’s ability to rapidly generate words according to specific semantic categories. The Boston Naming Test (BNT) measures naming performance by assessing one’s ability to spontaneously name objects presented visually, either with or without semantic or phonemic cues.
Despite the complexity of language functions, there is an extensive literature associating damage to left perisylvian regions to naming and fluency16–19. While this lateralization can be influenced by handedness, the vast majority of the population are left-hemisphere dominant for language20. Broca’s area is most commonly associated with expressive language, while the superior temporal gyrus (STG), which includes Wernicke’s area, is critical for receptive language. The main white matter tract that forms the connection between these areas is the arcuate fasciculus. In addition, two other white matter association tracts implicated in language functioning include the inferior longitudinal fasciculus (ILF) and the inferior fronto-occipital fasciculus (IFOF)21.
The ILF connects anterior temporal to occipitotemporal cortex and the IFOF connects lateral prefrontal to occipitotemporal cortex. Together, these three tracts form a rich network of fibers implicated in multiple aspects of language processing and when damaged, lead to different types of language impairment22,23.
Here, within a cohort of primary brain tumor patients receiving fractionated RT, we investigate biomarkers of white matter injury within language-associated perisylvian ROIs (including the superficial white matter subadjacent to Broca’s area and the STG, and white matter tracts including the arcuate fasciculus, ILF, and IFOF) as they relate to ROI-specific dose and language functioning. We aim to identify regions that represent potential targets for dose-avoidance in order to preserve language function. We hypothesized that longitudinal measurements of ROI volume and DTI imaging biomarkers would predict poorer language function over time.
MATERIALS AND METHODS
Study Participants
Overall, 59 subjects were consented for this IRB-approved, prospective clinical trial. This analysis includes 44 adult patients with primary brain tumors treated with fractionated brain RT, with available cognitive and imaging data (Supplemental eFigure 1). All subjects were native English language speakers and provided written informed consent. Inclusion criteria included: ability to complete neurocognitive assessments in English, age ≥18 years, Karnofsky performance status (KPS) ≥70, and estimated life expectancy ≥1 year. Patients who received prior RT were excluded from the study.
Study Design
Patients completed comprehensive neurocognitive testing, high-resolution 3D volumetric brain MRI, and DTI at baseline (pre-RT), 3 months, 6 months, and 12 months post-RT. Post-RT assessments within two weeks of the time point were accepted per study protocol. We investigated brain white matter regions strongly linked to language function (Figure 1, Supplemental eTable 1), including the superficial white matter subadjacent to Broca’s area and the STG, and white matter tracts including the arcuate fasciculus, ILF, and IFOF.
Figure 1.
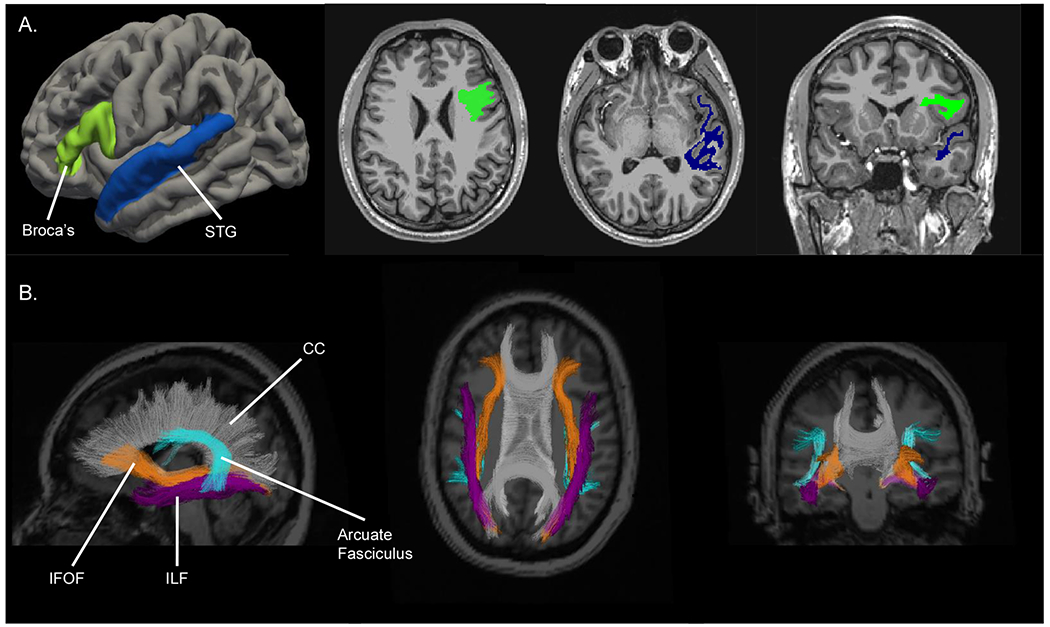
Representative segmentation of ROIs overlaid on an MRI image. A) Left Broca’s Area (bright green) and left STG (navy blue) superficial WM ROIs. B) WM tracts of IFOF (orange), ILF (purple), and Arcuate fasciculus (turquoise) ROIs. The CC is segmented in gray and labeled for reference only.
Abbreviations: WM, white matter; ROIs, regions of interest; STG, superior temporal gyrus; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; CC, corpus callosum.
Neurocognitive Assessment
Patients completed a comprehensive neurocognitive evaluation at each time point, administered by an expert neuropsychologist team with clinical and research experience with cancer patients. This comprehensive testing took 1-2 hours depending on the functioning of the study subject (i.e. response time, need for breaks). Study subjects were monitored for fatigue and given breaks as needed. Language functioning was measured via the DKEFS-CF24 and the BNT25. DKEFS-CF is a measure of semantic fluency and requires self-generated, rapid lexical search and retrieval. For the DKEFS-CF, participants are instructed to generate exemplars of first animals and then boy names within two 60 second periods. These categories were used at the baseline and 6-month sessions. For the 3- and 12-month time points, alternative forms were used and the categories were clothing items and girls’ names. Alternate forms of the test help minimize the role of practice effects. Participants were also administered the 60-item BNT, a confrontational naming test in which participants are presented with line drawings of objects with increasing difficulty; credit is given if the participant correctly names the object within 20 seconds or after a semantic cue is provided. No alternative forms exist for the BNT assessment.
Imaging Acquisition and Processing
The imaging acquisition of high-resolution volumetric and diffusion-weighted MRIs for this study have been described in detail elsewhere26. Briefly, imaging for all patients at each time point were acquired on a 3.0T 750 GE system (GE Healthcare, Milwaukee, Wisconsin) equipped with an 8-channel head coil. Sequences selected for the protocol included a 3D volumetric T1-weighted inversion recovery spoiled gradient echo sequence (echo time [TE]/repetition time [TR]=2.8/6.5 ms; inversion time [TI]=450 ms; flip angle=8 degrees; field of view [FOV]=24 cm), a 3D FLAIR sequence (TE/TR=125/6000 ms, TI=1868 ms, FOV=24cm, matrix=256x256, slice thickness=1 mm), and a diffusion weighted imaging (DWI) sequence using a single-shot pulsed-field gradient spin EPI sequence (TE/TR=96 ms/17 s; FOV=24 cm, matrix=128x128x48; inplane resolution 1.87x1.875; slice thickness=2.5 mm; 48 slices) with b=0, 500, 1500, and 4000 s/mm2, with 1, 6, 6, and 15 unique gradient directions for each b-value respectively and one average for each non-zero b-value. Two additional b=0 volumes were acquired with either forward or reverse phase-encode polarity for use in nonlinear B0 distortion correction.
Preprocessing of imaging data was completed using in-house algorithms derived in MATLAB. We corrected for anatomical imaging distortions due to gradient nonlinearities27, as well as diffusion scan spatial distortions caused by susceptibility and eddy currents28,29. Patient ROIs were segmented using the automated FreeSurfer processing pipeline (version 5.3; available at http://surfer.nmr.harvard.edu), available on the Neuroscience Gateway Portal30. FA and MD maps were derived by fitting the DWI data from b-values of 0, 500, and 1500 s/mm2 to a tensor. Within each voxel, an ellipsoid defined by three perpendicular axes (eigenvectors) approximated the diffusion process. FA is a unitless expression of the degree of directional bias of diffusion that range from 0-1 and decreases with white matter (WM) injury. MD is an average of the three eigenvalues expressed in μm2/millisecond that represents the average mobility of water molecules. Volume is reported here as cm3.
The DWI-derived maps and high-resolution volumetric MRI from each time point were co-registered and atlas-based tractography was used to segment the DWI into WM tracts31. FA and MD values were averaged across each ROI. Selected perisylvian ROIs were all WM based, including superficial WM of Broca’s Area and STG, and WM tracts, including the ILF, IFOF, and arcuate fasciculus (eTable 1; Figure 1). Superficial WM is defined as the volume of WM included up to 5 mm below the WM surface within the cortical ROIs described above and defined by FreeSurfer’s Desikan-Killiany atlas30. This superficial white matter is autosegmented using FreeSurfer. Herein, the ROIs referring to superficial WM subadjacent to Broca’s Area and STG will be referred to as “Broca’s” and “STG”. A censoring mask was manually drawn slice-by-slice on each image in order to exclude tissue affected by tumor, surgical cavity, surgical scars, or edema. The voxels within the censoring mask were excluded from the final ROI to avoid confounding by tumor and edema-related effects32. In order to control for longitudinal censoring trends in volume measurements, percent volume censored was controlled for in all models using volume as a predictor or outcome. Because FA and MD are average values, controlling for percent volume censored in those models was not necessary. Planning CT and radiation dose maps were co-registered to the baseline T1 and DWI volumes and used to estimate the average dose to each ROI.
Reliable Change Indices
In order to detect subacute changes in language, we assessed score differences from baseline to 6 months. This allows for the resolution of any acute treatment-related symptoms and instead quantifies what is understood to be a more irreversible cognitive decline emerging at the 6 month time point7,33. We calculated reliable change indices (RCI-PE) between the assessments, while controlling for practice effects and the measurement error of the test. This method predicts a given individual’s change score by adding the mean practice effect of a reference group (i.e., test-retest normative sample) to the individual’s baseline test score34. A RCI-PE score of 0 would reflect no change from baseline, while negative RCI-PE scores would reflect decline from baseline and positive scores would reflect improvement.
Statistical Analysis
A one-sample t-test was used to evaluate RCI-PEs of each assessment for change from baseline to 6 months post-RT (H0=0). Associations between RCI-PEs and patient characteristics were evaluated by independent samples t-test, one-way ANOVA, and Pearson correlations. Covariates investigated included patient sex, age, highest education level, ethnicity, baseline KPS, tumor location, radiation treatment modality, tumor histology, surgery, history of seizures, chemotherapy, and anxiety or depression symptoms.
We then investigated the longitudinal relationship between dose and imaging parameters (volume, FA, and MD) within ROIs. Linear mixed effects models, coded using the lme4 R package35, with subject-specific random intercepts investigated mean dose received to a region as a predictor of the volume, FA, and MD of that region over time. Time was included as a main effect; models predicting volume based on mean dose controlled for percentage censored as a main effect in addition to time.
We then evaluated these imaging biomarkers as predictors of language test scores over the entire study period. Linear mixed effects models with subject-specific random intercepts assessed the main effects of imaging parameters as predictors of raw language score, again controlling for time as a main effect. The BNT raw score was transformed using a logit function in order to minimize the ceiling effect of patients reaching the maximum score. Percentage of region censored was controlled for in all models that included volume as a predictor. We controlled for multiple comparisons using the false discovery rate36. Statistics were performed in R37.
RESULTS
Patient characteristics
Demographic and clinical characteristics are shown in Table 1. The 44 patients included in this analysis completed both neurocognitive assessments and DTI imaging on at least two time points within the study period. The median age was 47 years, with a slight majority of males (57%). Overall this cohort was comprised mostly of white, well-educated adults. Most patients had gliomas and were treated with intensity-modulated RT or volumetric modulated arc photon therapy (IMRT/VMAT). Half of the cohort received chemotherapy during the study period, including 19 patients who received concurrent and adjuvant temozolomide.
Table 1.
Demographic and clinical characteristics of patient cohort (n=44)
| Characteristic | No. of Participants (%) |
|---|---|
| Median age, years (range) | 47 (20-75) |
| Sex | |
| Male | 25 (57%) |
| Female | 19 (43%) |
| Ethnicity | |
| Non-Hispanic: | |
| Asian/Pacific Islander | 2 (5%) |
| Black | 1 (2%) |
| Middle Eastern | 2 (5%) |
| White | 34 (77%) |
| Hispanic | 5 (11%) |
| Highest Education Achieved, median (range) | 15.5 (10-20) |
| High school | 8 (18%) |
| College | 22 (50%) |
| Graduate School | 14 (32%) |
| Tumor Diagnosis | |
| Glioma: | |
| Low grade | 9 (20%) |
| High grade | 17 (39%) |
| Meningioma | 9 (20%) |
| Pituitary Adenoma | 4 (9%) |
| Schwannoma | 2 (5%) |
| Craniopharyngioma | 2 (5%) |
| Chondrosarcoma | 1 (2%) |
| Tumor Side | |
| Left | 21 (48%) |
| Right | 19 (43%) |
| Central | 4 (9%) |
| Tumor Region | |
| Frontal | 14 (32%) |
| Temporal | 10 (23%) |
| Suprasellar | 8 (18%) |
| Parietal | 3 (7%) |
| Base of skull | 3 (7%) |
| Cerebellar | 3 (7%) |
| Cavernous sinus | 2 (5%) |
| Sphenoid wing | 1 (2%) |
| RT type | |
| IMRT/VMAT | 31 (70%) |
| Proton | 13 (30%) |
| Dose Schedule | |
| 54 Gy / 30 Fx | 15 (34%) |
| 59.4 Gy / 33 Fx | 15 (34%) |
| 50.4 Gy / 28 Fx | 7 (16%) |
| 60 Gy / 30 Fx | 6 (14%) |
| 70 Gy / 35 Fx* | 1 (2%) |
| PTV Volume, cc | |
| Median (IQR) | 155.8 cc (48.3-228.2) |
| Baseline KPS | |
| 80 | 3 (7%) |
| 90 | 27 (61%) |
| 100 | 14 (32%) |
| Surgery | |
| GTR | 9 (20%) |
| STR | 27 (61%) |
| Biopsy | 3 (7%) |
| None | 5 (12%) |
| Chemotherapy | 22 (50%) |
| Concurrent + Adjuvant TMZ | 14 |
| Concurrent + Adjuvant TMZ + Other† | 5 |
| Adjuvant PCV only | 3 |
| Seizures ‡ | 23 (52%) |
| AED§ | 28 (64%) |
| Steroids§ | 23 (52%) |
| Previously treated: | |
| Anxiety | 9 (20%) |
| Depression | 8 (18%) |
Abbreviations: IMRT, intensity-modulated radiotherapy; VMAT, volumetric-modulated arc therapy; Fx, fractions; IQE, interquartile range; KPS, Karnofsky performance status; GTR, gross total resection; STR, sub-total resection; AED, anti-epileptic drug; TMZ, Temozolomide; PCV, Procarbazine, Lomustine, and Vincristine
= Prescription dose to low grade chondrosarcoma
= includes additional adjuvant therapy or clinical trials: Vaccine clinical trial (n=3), Parp-inhibitor clinical trial (n=1), adjuvant CCNU (n=1)
= history of seizures or active seizures during study period
= received during study period
Language Function over Time: Subacute Effects
Of the 44 patients included in the overall analysis, 36 completed neurocognitive assessments at the baseline and 6-month time points. There was a significant decline from baseline to 6 months post-RT in DKEFS-CF scores (mean RCI-PE −0.483, p=0.013). For BNT, the RCI-PE showed a slight improvement in scores compared to a standard population of older adults (mean RCI-PE 0.262, p=0.038).
No clinical covariates showed significant associations with RCI-PE score for the DKEFS-CF. Age was the only covariate with a significant relationship to BNT RCI-PE (r=−0.552, p <0.001) and indicated that less improvement on the BNT was associated with older age.
Mean Dose as a Predictor of Injury Biomarkers
Descriptive statistics of mean dose to each segmented ROI (after censoring) are shown in Table 2. There was no significant difference between mean doses to right and left ROIs. Results of linear mixed effects modeling investigating mean dose to ROIs as a predictor of longitudinal volume, FA, and MD are shown in Table 3.
Table 2.
Per patient dose (Gy) to each ROI
| Brain Region | Left-sided | Right-sided | p-value | ||
|---|---|---|---|---|---|
| Mean dose (Gy) | Standard deviation | Mean dose (Gy) | Standard deviation | ||
| Broca’s area superficial WM | 18.79 | 20.18 | 17.08 | 18.27 | 0.465 |
| STG superficial WM | 16.52 | 18.13 | 16.61 | 18.95 | 0.966 |
| WM tracts: | |||||
| Arcuate | 15.46 | 17.03 | 15.81 | 20.85 | 0.881 |
| ILF | 15.70 | 17.96 | 16.61 | 19.35 | 0.686 |
| IFOF | 17.23 | 17.22 | 17.28 | 18.22 | 0.983 |
P-values represent t-test of left and right sided ROI dose. Abbreviations: ROI, region of interest; WM, white matter; STG, superior temporal gyrus; ILF, inferior longitudinal fasciculus; IFOF, inferior fronto-occipital fasciculus
Table 3.
Mean dose to ROI as a predictor of imaging biomarkers
| Outcome | Brain Region# | β coefficient (SE) | p-value | |
|---|---|---|---|---|
| Volume* | Left | Broca’s area | −0.0115 (0.0074) | 0.122 |
| STG | −0.0204 (0.0082) | 0.015 | ||
| WM tracts: | ||||
| Arcuate | 0.00119 (0.0035) | 0.730 | ||
| ILF | −0.0139 (0.0086) | 0.110 | ||
| IFOF | −0.00443 (0.0046) | 0.332 | ||
| Right | Broca’s area | −0.00471 (0.0073) | 0.520 | |
| STG | −0.00384 (0.0069) | 0.577 | ||
| WM tracts: | ||||
| Arcuate | −0.00644 (0.0034) | 0.060 | ||
| ILF | −0.0155 (0.0071) | 0.031 | ||
| IFOF | −0.0120 (0.0054) | 0.030 | ||
| FA | Left | Broca’s area | −0.00726 (0.0169) | 0.668 |
| STG | −0.00967 (0.0237) | 0.684 | ||
| WM tracts: | ||||
| Arcuate | −0.0773 (0.0292) | 0.011 | ||
| ILF | −0.128 (0.0274) | <0.001 | ||
| IFOF | −0.152 (0.0296) | <0.001 | ||
| Right | Broca’s area | −0.0371 (0.0225) | 0.104 | |
| STG | 0.0408 (0.0166) | 0.018 | ||
| WM tracts: | ||||
| Arcuate | −0.0427 (0.0255) | 0.100 | ||
| ILF | −0.0406 (0.0215) | 0.065 | ||
| IFOF | −0.0712 (0.0249) | 0.006 | ||
| MD | Left | Broca’s area | 0.000115 (0.0003) | 0.727 |
| STG | 0.000213 (0.0003) | 0.529 | ||
| WM tracts: | ||||
| Arcuate | 0.00106 (0.0004) | 0.020 | ||
| ILF | 0.00103 (0.0005) | 0.030 | ||
| IFOF | 0.00116 (0.0005) | 0.015 | ||
| Right | Broca’s area | 0.0000592 (0.0004) | 0.869 | |
| STG | 0.0000762 (0.0003) | 0.820 | ||
| WM tracts: | ||||
| Arcuate | 0.000286 (0.0004) | 0.425 | ||
| ILF | 0.000615 (0.0004) | 0.123 | ||
| IFOF | 0.000571 (0.0004) | 0.202 | ||
Significant results (p<0.05) are shown in bold
Units for the β coefficient are as follows: volume, Gy/(month*cm3); FA, Gy/month; MD, Gy/(month*μm2/ms). Abbreviations: ROI, region of interest; FA, fractional anisotropy; MD, mean diffusivity; STG, superior temporal gyrus; ILF, inferior longitudinal fasciculus; IFOF, inferior fronto-occipital fasciculus
= Models control for percent volume censored
= Broca’s area and STG refer to superficial WM subadjacent to these regions
Volume
After controlling for time and percentage of volume censored, higher mean dose was significantly associated with a decrease in longitudinal volume for left STG superficial WM (β=−0.0204 Gy/cm3, p=0.015), right ILF (β=−0.0155 Gy/cm3, p=0.031), and right IFOF (β=−0.0120 Gy/mm3, p=0.030).
FA
Increased mean dose to the left arcuate fasciculus (β=−0.0773 Gy/month, p=0.011), ILF (β=−0.128 Gy/month, p<0.001), and IFOF (β=−0.152 Gy/month, p<0.001), as well as right-sided IFOF (β=−0.0712 Gy/month, p=0.006) were significantly associated with decreased FA within these regions. Higher dose to the right STG was significantly correlated with increased FA (β=0.0408 Gy/month, p=0.018).
MD
For all of the left-sided white matter tracts, higher mean dose was associated with increased MD: arcuate fasciculus (β=0.00106 Gy/[month*μm2/ms], p=0.020), ILF (β=0.00103 Gy/[month*μm2/ms], p=0.030), IFOF (β=0.00116 Gy/[month*μm2/ms], p=0.015).
Biomarkers of Injury as Longitudinal Language Predictors
Longitudinal volume change, FA, and MD within left and right-sided ROIs were investigated as predictors of raw DKEFS-CF and BNT logit score over time. Results are shown in Table 4.
Table 4.
Imaging biomarkers as predictors of language score
| Brain Region# | BNT Logit Score | Raw DKEFS CF Score | |||||
|---|---|---|---|---|---|---|---|
| Biomarker | β coefficient (SE) | p-value | Biomarker | β coefficient (SE) | p-value | ||
| Left sided: | Broca’s area | Volume† | 0.238 (0.081) | 0.006* | Volume† | 1.71 (0.971) | 0.091 |
| FA | 0.0300 (0.031) | 0.337 | FA | 0.757 (0.383) | 0.052 | ||
| MD | −0.176 (1.13) | 0.876 | MD | −18.8 (14.3) | 0.191 | ||
| STG | Volume† | 0.146 (0.076) | 0.061 | Volume† | 0.803 (0.905) | 0.377 | |
| FA | 0.0109 (0.022) | 0.629 | FA | 0.270 (0.278) | 0.335 | ||
| MD | 0.423 (1.08) | 0.700 | MD | −13.1 (13.7) | 0.342 | ||
| WM tracts: | |||||||
| Arcuate | Volume† | 0.466 (0.212) | 0.038* | Volume† | 0.0306 (2.63) | 0.991 | |
| FA | 0.0607 (0.022) | 0.008* | FA | 0.701 (0.243) | 0.007* | ||
| MD | −1.51 (0.918) | 0.139 | MD | −24.5 (10.6) | 0.027* | ||
| Volume† | 0.245 (0.096) | 0.012* | Volume† | −1.03 (1.15) | 0.373 | ||
| ILF | FA | 0.0378 (0.025) | 0.137 | FA | 0.686 (0.274) | 0.018* | |
| MD | 0.800 (0.960) | 0.413 | MD | −19.8 (12.3) | 0.116 | ||
| IFOF | Volume† | 0.430 (0.163) | 0.010* | Volume† | 0.266 (1.98) | 0.894 | |
| FA | 0.0456 (0.022) | 0.039* | FA | 0.595 (0.244) | 0.021* | ||
| MD | 0.552 (0.921) | 0.552 | MD | −13.7 (11.8) | 0.250 | ||
| Right-sided: | Broca’s area | Volume† | 0.0760 (0.126) | 0.550 | Volume† | −0.244 (1.38) | 0.860 |
| FA | 0.0301 (0.025) | 0.232 | FA | 0.124 (0.304) | 0.684 | ||
| MD | −0.153 (1.12) | 0.892 | MD | −16.6 (14.2) | 0.243 | ||
| STG | Volume† | 0.0801 (0.115) | 0.490 | Volume† | −0.996 (1.32) | 0.456 | |
| FA | 0.0184 (0.023) | 0.421 | FA | −0.123 (0.291) | 0.673 | ||
| MD | 0.306 (1.06) | 0.774 | MD | −14.4 (13.5) | 0.288 | ||
| WM tracts: | |||||||
| Arcuate | Volume† | 0.331 (0.216) | 0.129 | Volume† | −1.87 (2.53) | 0.467 | |
| FA | −0.00960 (0.024) | 0.697 | FA | 0.396 (0.282) | 0.163 | ||
| MD | 0.891 (1.07) | 0.406 | MD | −22.0 (13.5) | 0.106 | ||
| ILF | Volume† | 0.124 (0.103) | 0.231 | Volume† | −1.41 (1.19) | 0.240 | |
| FA | −0.0322 (0.035) | 0.357 | FA | 0.409 (0.389) | 0.297 | ||
| MD | 1.04 (1.11) | 0.352 | MD | −20.7 (13.9) | 0.141 | ||
| IFOF | Volume† | 0.161 (0.148) | 0.278 | Volume† | −1.73 (1.72) | 0.314 | |
| FA | −0.0379 (0.030) | 0.208 | FA | 0.255 (0.341) | 0.460 | ||
| MD | 0.996 (1.02) | 0.332 | MD | −17.7 (12.9) | 0.170 | ||
Significant results (p<0.05) are shown in bold, with asterisks (*) next to results that remain significant after corrections for multiple comparisons.
Units for the β coefficient are as follows: volume, points/(month*cm3); FA, points/month; MD, points/(month*μm2/ms). Abbreviations: ROI, region of interest; FA, fractional anisotropy; MD, mean diffusivity; STG, superior temporal gyrus; ILF, inferior longitudinal fasciculus; IFOF, inferior fronto-occipital fasciculus
Models control for percent volume censored
= Broca’s area and STG refer to superficial WM subadjacent to these regions
Broca’s Area
Smaller left Broca’s superficial WM volume was a significant predictor of poorer BNT score after correction for multiple comparisons (β=0.238 points/cm3, p=0.006). The association between decreased left Broca’s FA (β=0.757 points/month, p=0.052) and worse DKEFS-CF scores approached significance. No right-sided associations were significant.
Superior Temporal Gyrus
An association between smaller left STG superficial WM volume and worse BNT score approached significance (β=0.146 points/cm3, p=0.061). No right-sided associations were significant.
White Matter Tracts
Smaller volume of the left arcuate fasciculus (β=0.466 points/cm3, p=0.038), ILF (β=0.245 points/cm3, p=0.012), and IFOF (β=0.430 points/cm3, p=0.010) was associated with worse BNT score. Decreased FA in left arcuate fasciculus (β=0.0607 points/month, p=0.008) and IFOF (β=0.0456 points/month, p=0.039) was significantly associated with poorer scores in BNT. All three white matter tracts investigated showed a significant correlation between decreased FA and decreased DKEFS-CF scores: arcuate fasciculus (β=0.701 points/month, p=0.007), ILF (β=0.686 points/month, p=0.017), and IFOF (β=0.595 points/month, p=0.021). Increase in MD within the arcuate fasciculus was associated with poorer DKEFS-CF scores (β= −24.5 points/[month*μm2/ms], p=0.027). All of these relationships remained significant after correction for multiple comparisons. No right-sided associations were significant.
DISCUSSION
In this prospective, longitudinal investigation of primary brain tumor patients receiving RT, we found that radiation-mediated injury to left perisylvian white matter is significantly correlated with poorer language scores over time. To our knowledge, this is the first study to investigate DTI-quantified axonal disruption within perisylvian regions to longitudinal language functioning in brain tumor patients. Overall, we also found that this cohort experienced a significant subacute decline in language fluency from baseline to 6 months post-RT. This finding demonstrates the critical role RT may play in core aspects of language functioning. Interestingly, fluency but not naming showed decline when analyzed by RCI-PE. This dissociation is not surprising given that language is not a monolithic function and different regions of the brain and neuropsychological measures are differentially sensitive to distinct, but complementary aspects of language.
The DKEFS-CF is a speeded, self-generating lexical retrieval test that is sensitive to deficits in semantic processing, processing speed, and word retrieval. Conversely, the BNT is a confrontational naming test that requires semantic processing and word retrieval, but does not tax retrieval speed or self-generated responses. However, both tasks depend on a complex interaction of perisylvian structures, including the underlying white matter of the arcuate fasciculus and related fiber tracts. In order to generate an RCI-PE for BNT, we were required to use a reference population with a mean age that was 30 years older than this cohort38. In covariate analysis, we found a strong negative correlation between age and BNT RCI-PE, which clarifies that the older adults within our cohort have significantly greater evidence of decline, as we would expect. Thus, within this study the BNT RCI-PE underestimated decline of our relatively young cohort. The finding of significant language decline in semantic fluency, measured by DKEFS-CF, is more representative of the primary brain tumor population and is consistent with existing literature39. This may imply that the influence of RT on language decline is more tied to the processing speed required for speeded lexical processing and retrieval versus a primary naming impairment.
We found significant correlations between higher RT dose and lower FA, higher MD, and decreased volume in perisylvian ROIs, regardless of hemisphere. This is consistent with other studies examining dose effects on DTI imaging biomarkers8,10,40 and volumetric changes after RT41,42. White matter damage after RT is mediated by axonal injury, demyelination, neuroinflammation, and changes in vascular permeability7. These processes contribute to variations in diffusion properties of water within white matter, represented by decreasing FA and increasing MD. Similarly, atrophy to defined brain regions has previously been shown to be radiation dose-dependent, and may be reflective of microenvironmental changes including neuroinflammation and glial cell death. DTI biomarkers have been increasingly used in identifying disruption of the microstructural integrity of the brain as it relates to cognitive decline in cancer patients12,14, mild cognitive impairment and Alzheimer’s Disease43, systemic lupus erythematosus44, small vessel disease45, and epilepsy46.
Quantitative imaging biomarkers of axonal injury and demyelination within left, but not right sided, perisylvian regions were significantly correlated with language performance after brain RT. In addition to white matter association tracts, we investigated the superficial white matter directly beneath Broca’s area and the STG, which is the superior temporal gyrus region that includes Wernicke’s area. This superficial white matter contains short range U-fibers that promote rapid communication across neighboring gyri and have been implicated in multiple aspects of cognition47. Decreased white matter volume within left Broca’s Area was significantly correlated with poorer semantic fluency, whereas decreased white matter volume of left STG and decreased FA of left Broca’s Area showed a trend toward an association with worse fluency and naming performance. The most consistent associations were seen within the white matter tracts, where decreased white matter volume, decreased FA, and increased MD were significantly correlated with poorer language functioning. Similar studies within cohorts of patients with left-sided stroke48 and temporal lobe epilepsy21, have shown lower FA within the arcuate fasciculus to be correlated to worse language performance. The current study takes a step further by investigating this relationship longitudinally and in reference to RT.
Our findings support the hypothesis that RT-mediated atrophy and axonal injury to left perisylvian white matter plays a critical role in core aspects of language decline in primary brain tumor patients. After correction for multiple comparisons, the detected relationships remained significant, demonstrating the strength of the results. In a previous study investigating patients with nasopharyngeal cancer, patients who received higher RT dose to temporal lobes showed significant short-term decline in language fluency49. Similarly, our results highlight the importance of multiple fronto-temporal and fronto-occipital white matter tracts in language fluency and may reflect a unique effect of RT on compromise to these tracts, primarily through impairing speeded lexical processing and retrieval. Significant associations between both naming and fluency with the integrity of multiple left-sided perisylvian tracts demonstrate the importance of complex neuronal networks in language functioning17, and highlight the detrimental effects of RT-mediated damage to these critical regions.
Despite the clinical relevance and prevalence of language difficulties among primary brain tumor patients, which are often misperceived as memory problems, there has been little investigation into better understanding the neural underpinnings of language impairment in this clinical population. Our study shows that longitudinal, RT-associated axonal injury within left perisylvian regions is associated with poorer language performance, suggesting that dose-avoidance to these regions may preserve long-term language function. Unlike hippocampal50 or optic nerve avoidance51, delineation of perisylvian structures is challenging and not standard of care. Automated tractography as used in this study is now more widely available52, and can assist with identifying these language-associated areas neuroanatomically. While definitive “mapping” of regional language centers at the individual patient level, as done in presurgical planning for eloquent tumor resections, involves more complex functional imaging53 or invasive Wada tests54, this study suggests that left-sided perisylvian WM regions represent dose avoidance targets. Future studies investigating a more personalized approach to language preservation via radiation techniques and planning are needed.
Our study has potential limitations. First, the current analysis provides strong evidence of associations between imaging biomarkers of white matter damage and language function, but cannot definitively determine causation. ROIs were defined by FreeSurfer and AtlasTract31, which are semi-automated programs shown to decrease inter-operator variability, but were developed on healthy controls and not patients with distorted anatomy. However, these programs are robust, well-validated, and have been used in prior brain tumor studies40,55 . Additionally, segmentation outputs of each patient and each time point were meticulously inspected slice-by-slice in order to manually and generously censor out areas of tumor, surgical cavities, and edema10,32,41. However, this may not have entirely controlled for the possibility of tumor cells beyond tumor/surgical margins in high grade glioma cases. While our neurocognitive battery is robust within our study design, when patients complete repeated assessments there is a concern for practice effects. RCI-PE calculations control for these practice effects, but we cannot completely rule out the influence of practice on our data and are limited by availability of an appropriate control reference group. In addition, although we tested two components of language that are sensitive to language decline, we did not test other aspects of language that may be influenced by RT-mediated perisylvian damage (i.e., comprehension, repetition). During neurocognitive testing on this clinical trial, subjects were carefully monitored for fatigue and given ample time for responses and breaks; however there may be aspects of fatigue that could affect cognitive performance, as in all prospective trials with neurocognitive endpoints. Finally, although we have a relatively small sample size, the cohort size is comparable to other similar prospective, longitudinal studies8,11,12 and allows us to collect and analyze a rich longitudinal data set with specialized, consistently acquired imaging and detailed neurocognitive data. A quarter of enrolled patients did drop out of the study, but this is not unusual in brain tumor clinical trials, and attrition was not related to tumor progression or clinical decline (eFigure 1). Thus, the likelihood of selection bias significantly altering the results of this study is low. The heterogeneity of primary brain tumor histology (benign/low grade and high grade) and location within our cohort may introduce additional confounding that we were not able to control for. However, this also allows for the generalization of these results to all primary brain tumor patients presenting for radiation therapy treatment.
In conclusion, this study with robust imaging and language measures demonstrates important longitudinal associations between disruption of left-sided perisylvian white matter and language decline in patients with brain tumors undergoing fractionated partial RT. These findings suggest that damage to left perisylvian white matter via RT may impact early and potentially long-term language function for brain tumor patients. We encourage further work toward language-sparing techniques in order to support cognitive preservation efforts that will maximize the quality of life of brain tumor patients.
Supplementary Material
eFigure 1. CONSORT diagram of patient inclusion
Abbreviations: NCA, Neurocognitive assessment
Acknowledgments
Funding:
This work was supported by the National Institutes of Health (1TL1TR001444 to KT, 1TL1TR001443 to MDT, MS, and AY, and 1KL2TR001444, UL1TR000100, R01 CA238783-01 to JAH-G); National Cancer Institute and UC San Diego Moores Cancer Center (P30 CA02310029 to JAH-G); and American Cancer Society (ACS-IRG 70-002 to CRM). The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies, who had no direct role in designing, conducting, or reporting the study.
Conflict of Interest:
JAH-G reports grant funding from Varian Medical Systems, unrelated to the present study. CRM has research funding from GE Healthcare, unrelated to the current study. There are no financial or other relationships that might lead to a perceived conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing:
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
REFERENCES
- 1.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006. doi: 10.1200/JCO.2005.04.6086 [DOI] [PubMed] [Google Scholar]
- 2.Lin NU, Wefel JS, Lee EQ, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: Neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013;14(10):407–416. doi: 10.1016/S1470-2045(13)70308-5 [DOI] [PubMed] [Google Scholar]
- 3.Grosshans DR, Gondi V, Shih HA, Mahajan A, Tsien CI, Tseng YD. Proton Beam or Intensity-Modulated Radiation Therapy in Preserving Brain Function in Patients With IDH Mutant Grade II or III Glioma (NRG-BN005). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/study/NCT03180502 Published 2017.
- 4.Hassler MR, Elandt K, Preusser M, et al. Neurocognitive training in patients with high-grade glioma: A pilot study. J Neurooncol. 2010;97(1):109–115. doi: 10.1007/s11060-009-0006-2 [DOI] [PubMed] [Google Scholar]
- 5.Alexander BM, Brown PD, Ahluwalia MS, et al. Clinical trial design for local therapies for brain metastases: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018. doi: 10.1016/S1470-2045(17)30692-7 [DOI] [PubMed] [Google Scholar]
- 6.Meyers CA, Rock EP, Fine HA. Refining endpoints in brain tumor clinical trials. J Neurooncol. 2012;108(2):227–230. doi: 10.1007/s11060-012-0813-8 [DOI] [PubMed] [Google Scholar]
- 7.Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 2016. doi: 10.1038/nrneurol.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagesh V, Tsien CI, Chenevert TL, et al. Radiation-Induced Changes in Normal Appearing White Matter in Patients with Cerebral Tumors: A Diffusion Tensor Imaging Study. Int J Radiat Oncol Biol Phys. 2009;70(4):1002–1010. doi: 10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Wu EX, Qiu D, Leung LHT, Lau HF, Khong PL. Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Res. 2009;69(3):1190–1198. doi: 10.1158/0008-5472.CAN-08-2661 [DOI] [PubMed] [Google Scholar]
- 10.Connor M, Karunamuni R, McDonald C, et al. Dose-Dependent White Matter Damage After Brain Radiotherapy. Radiother Oncol. 2016;51(3):195–212. doi: 10.3109/10409238.2016.1172552.Mechanism [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman CH, Nagesh V, Sundgren PC, et al. Diffusion Tensor Imaging of Normal-Appearing White Matter as Biomarker for Radiation-Induced Late Delayed Cognitive Decline. Int J Radiat Oncol. 2012;82(5):2033–2040. doi: 10.1016/j.ijrobp.2011.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman CH, Zhu T, Nazem-Zadeh M, et al. Diffusion tensor imaging predicts cognitive function change following partial brain radiotherapy for low-grade and benign tumors. Radiother Oncol. 2016;120(2):234–240. doi: 10.1016/j.radonc.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tringale KR, Nguyen TT, Karunamuni R, et al. Quantitative Imaging Biomarkers of Damage to Critical Memory Regions Are Associated With Post–Radiation Therapy Memory Performance in Brain Tumor Patients. Int J Radiat Oncol Biol Phys. 2019;105(4):773–783. doi: 10.1016/j.ijrobp.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tringale KR, Nguyen T, Barhrami N, et al. Identifying early diffusion imaging biomarkers of regional white matter injury as indicators of executive function decline following brain radiotherapy: A prospective clinical trial in primary brain tumor patients. Radiother Oncol. 2019;132:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maseda A, Lodeiro-Fernández L, Lorenzo-López L, Núñez-Naveira L, Balo A, Millán-Calenti JC. Verbal fluency, naming and verbal comprehension: Three aspects of language as predictors of cognitive impairment. Aging Ment Heal. 2014; 18(8):1037–1045. doi: 10.1080/13607863.2014.908457 [DOI] [PubMed] [Google Scholar]
- 16.Skeide MA, Friederici AD. The ontogeny of the cortical language network. Nat Rev Neurosci. 2016. doi: 10.1038/nrn.2016.23 [DOI] [PubMed] [Google Scholar]
- 17.Dick AS, Bernal B, Tremblay P. The language connectome: New pathways, new concepts. Neuroscientist. 2014. doi: 10.1177/1073858413513502 [DOI] [PubMed] [Google Scholar]
- 18.Whatmough C, Chertkow H, Murtha S, Hanratty K. Dissociable brain regions process object meaning and object structure during picture naming. Neuropsychologia. 2002;40(2):174–186. doi: 10.1016/S0028-3932(01)00083-5 [DOI] [PubMed] [Google Scholar]
- 19.Costafreda SG, Fu CHY, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27(10):799–810. doi: 10.1002/hbm.20221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knecht S, Drager B, Deppe M, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(12):2512–2518. [DOI] [PubMed] [Google Scholar]
- 21.Mcdonald CR, Ahmadi ME, Tecoma ES, Dale AM, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaestner E, Reyes A, Macari AC, et al. Identifying the neural basis of a language-impaired phenotype of temporal lobe epilepsy. Epilepsia. 2019;60(8):1627–1638. doi: 10.1111/epi.16283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- 24.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- 25.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 26.Tringale KR, Nguyen TT, Karunamuni R, et al. Quantitative imaging biomarkers of damage to critical memory regions are associated with post-radiation therapy memory performance in brain tumor patients. Int J Radiat Oncol Biol Phys 2019;105:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- 28.Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. Neuroimage. 2010;50(1):175–183. doi: 10.1016/j.neuroimage.2009.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang J, Hrabe J, Kangarlu A, et al. Correction of eddy-current distortions in 29 diffusion tensor images using the known directions and strengths of diffusion gradients. J Magn Reson Imaging. 2006;24(5):1188–1193. doi: 10.1002/jmri.20727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivagnanam S, Majumdar A, Yoshimoto K, et al. Introducing the neuroscience gateway. 2013. [Google Scholar]
- 31.Hagler DJ, Ahmadi ME, Kuperman J, et al. Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Hum Brain Mapp. 2009;30(5):1535–1547. doi: 10.1002/hbm.20619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connor M, Karunamuni R, McDonald C, et al. Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother Oncol. 2017;123(2):209–217. doi: 10.1016/j.radonc.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown PD, Ballman KV., Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC-3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. doi: 10.1016/S1470-2045(17)30441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chelune GJ, Naugle RI, Luders H, Sedlak J, Awad IA. Individual change after epilepsy surgery: Practice effects and base-rate information. Neuropsychology. 1993;7(1):41–52. 10.1037/0894-4105.7.1.41. [DOI] [Google Scholar]
- 35.Bates DM, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. 2012. [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 37.Team RC. R: A Language and Environment for Statistical Computing. 2019. https://www.r-project.org/.
- 38.Sachs BC, Lucas JA, Smith GE, et al. Reliable change on the Boston naming test. J Int Neuropsychol Soc. 2012;18(2):375–378. doi: 10.1017/S1355617711001810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zucchella C, Bartolo M, Di Lorenzo C, Villani V, Pace A. Cognitive impairment in primary brain tumors outpatients: A prospective cross-sectional survey. J Neurooncol. 2013;112(3):455–460. doi: 10.1007/s11060-013-1076-8 [DOI] [PubMed] [Google Scholar]
- 40.Seibert TM, Karunamuni R, Kaifi S, et al. Cerebral Cortex Regions Selectively Vulnerable to Radiation Dose-Dependent Atrophy. In: International Journal of Radiation Oncology Biology Physics. ; 2017. doi: 10.1016/j.ijrobp.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seibert TM, Karunamuni R, Bartsch H, et al. Radiation Dose–Dependent Hippocampal Atrophy Detected With Longitudinal Volumetric Magnetic Resonance Imaging. Int J Radiat Oncol. 2017;97(2):263–269. doi: 10.1016/j.ijrobp.2016.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huynh-Le M-P, Karunamuni R, Moiseenko V, et al. Dose-dependent atrophy of the amygdala after radiotherapy. Radiother Oncol. 2019;136:44–49. https://www.sciencedirect.com/science/article/pii/S016781401930146X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Curr Opin Neurol. 2008;21(1):83–92. [DOI] [PubMed] [Google Scholar]
- 44.Kozora E, Filley CM, Erkan D, et al. Longitudinal evaluation of diffusion tensor imaging and cognition in systemic lupus erythematosus. Lupus. 2018;27(11):1810–1818. doi: 10.1177/0961203318793215 [DOI] [PubMed] [Google Scholar]
- 45.Van Norden AGW, De Laat KF, Van Dijk EJ, et al. Diffusion tensor imaging and cognition in cerebral small vessel disease. The RUN DMC study. Biochim Biophys Acta - Mol Basis Dis. 2012;1822(3):401–407. doi: 10.1016/j.bbadis.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 46.Leyden KM, Kucukboyaci NE, Puckett OK, et al. What does diffusion tensor imaging (DTI) tell us about cognitive networks in temporal lobe epilepsy? Quant Imaging Med Surg. 2015;5(2):247–24763. doi: 10.3978/j.issn.2223-4292.2015.02.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazeri A, Chakravarty MM, Rajji TK, et al. Superficial white matter as a novel substrate of age-related cognitive decline. Neurobiol Aging. 2015;36(6):2094–2106. doi: 10.1016/j.neurobiolaging.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 48.Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. Am J Neuroradiol. 2008;29(3):483–487. doi: 10.3174/ajnr.A0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsiao KY, Yeh SA, Chang CC, Tsai PC, Wu JM, Gau JS. Cognitive Function Before and After Intensity-Modulated Radiation Therapy in Patients With Nasopharyngeal Carcinoma: A Prospective Study. Int J Radiat Oncol Biol Phys. 2010;77(3):722–726. doi: 10.1016/j.ijrobp.2009.06.080 [DOI] [PubMed] [Google Scholar]
- 50.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J Clin Oncol. 2014. doi: 10.1200/JCO.2014.57.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J. Radiation Dose-Volume Effects of Optic Nerves and Chiasm. Int J Radiat Oncol Biol Phys. 2010;76(3 SUPPL.):28–35. doi: 10.1016/j.ijrobp.2009.07.1753 [DOI] [PubMed] [Google Scholar]
- 52.Jr DJH, Ahmadi ME, Kuperman J, et al. diffusion tensor atlas: Application to temporal lobe epilepsy. 2009;30(5):1535–1547. doi: 10.1002/hbm.20619.Automated [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang J, Thakur SB, Huang W, Narayana A. Magnetic resonance spectroscopy imaging (MRSI) and brain functional magnetic resonance imaging (fMRI) for radiotherapy treatment planning of glioma. Technol Cancer Res Treat. 2008;7(5):349–362. http://europepmc.org/abstract/MED/18783284. [PubMed] [Google Scholar]
- 54.Ishikawa T, Muragaki Y, Maruyama T, Abe K, Kawamata T. Roles of the wada test and functional magnetic resonance imaging in identifying the language-dominant hemisphere among patients with gliomas located near speech areas. Neurol Med Chir (Tokyo). 2017;57(1):28–34. doi: 10.2176/nmc.oa.2016-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karunamuni R, Bartsch H, White NS, et al. Dose-Dependent Cortical Thinning after Partial Brain Irradiation in High-Grade Glioma. Int J Radiat Oncol Biol Phys. 2016. doi: 10.1016/j.ijrobp.2015.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. CONSORT diagram of patient inclusion
Abbreviations: NCA, Neurocognitive assessment


