Introduction
The pathophysiology of Crohn’s disease (CD) remains obscure. From early descriptions, a defect in lymphatic transport was described1, but this issue remains unresolved.
A tractable approach for investigating transport of lymphatic cargo is to quantify postprandial chylomicron appearance in plasma. Chylomicrons, large lipoproteins synthesized by enterocytes during dietary absorption, pass through intestinal and mesenteric lymphatics before arriving in plasma2. Although chylomicron absorption typically occurs in the duodenum and jejunum, variables like enterocyte membrane status can shift absorption to distal parts of the intestine where CD most commonly involves3.
Beyond concerns of lymphatic dysfunction, other observations raise the possibility that postprandial chylomicron responses might be abnormal in CD. First, ileal inflammation may cause bile acid malabsorption and thereby impair fat emulsification during absorption4. Second, downregulation of genes encoding chylomicron-associated apoproteins apoB, apoA1, and apoC3 has been observed in ileal CD5. Third, enteroendocrine hormones like glucagon-like peptide 2 (GLP-2) released by specialized enterocytes located in the ileum regulate chylomicron output, increasing mesenteric lymph flow rates in rats2.
Here, we quantified postprandial chylomicron transport after feeding a mixed meal containing 13C-triolein to healthy participants compared with those having active CD of the ileum.
Materials and Methods
This study, including 28 participants with ileal CD and 19 healthy controls, was conducted as approved by the Human Research Protection Office at Washington University (Protocol #201712103). Inclusion criteria for CD participants required presence of active small bowel inflammatory disease. Patients underwent assessment for active small bowel CD through a combination of ileocolonoscopy and/or computed tomography and/or magnetic resonance enterography. All ileocolonoscopy was scored for disease activity by a single gastroenterologist with fellowship training in IBD. All enterography scans were conducted at the Mallinckrodt Institute of Radiology, Washington University School of Medicine and read with clinical reports generated by board certified and fellowship trained abdominal radiologists. Time between clinical evaluation and study visit averaged 40 ± 20 (SD) days. Exclusion criteria were previous relevant abdominal resections (e.g., bowel resection, gall bladder removal); use of steroids in the past 7 days; a diagnosis of or treatment for underlying diabetes or other metabolic diseases (eg., gout, thyroid disease). Biologic therapy for CD was not excluded and is reported in Supplemental Table 1 along with other participant characteristics. Control participants, recruited from the community, received a complete metabolic panel screen of fasting plasma to verify healthy status, applying above exclusion criteria. Other methods used in this study can be found in Supplemental Methods.
Results
We quantified accumulation of 13C-triolein and associated meal triglycerides within plasma chylomicron fractions over a 6 h-postprandial window. Tracer to tracee ratio enrichment of test meal oleate was 2.1% ± 0.5% (SD). Chylomicron-triglyceride (TG) oleate derived from the test meal was similar between healthy controls and CD participants (Fig. 1A). The proportion of test meal tracer expired in breath was also similar between groups (Fig. 1B), suggesting no obvious malabsorption. Fractional clearance rate of chylomicron-triglyceride from plasma was also not different between groups (Fig. 1C).
Figure 1.
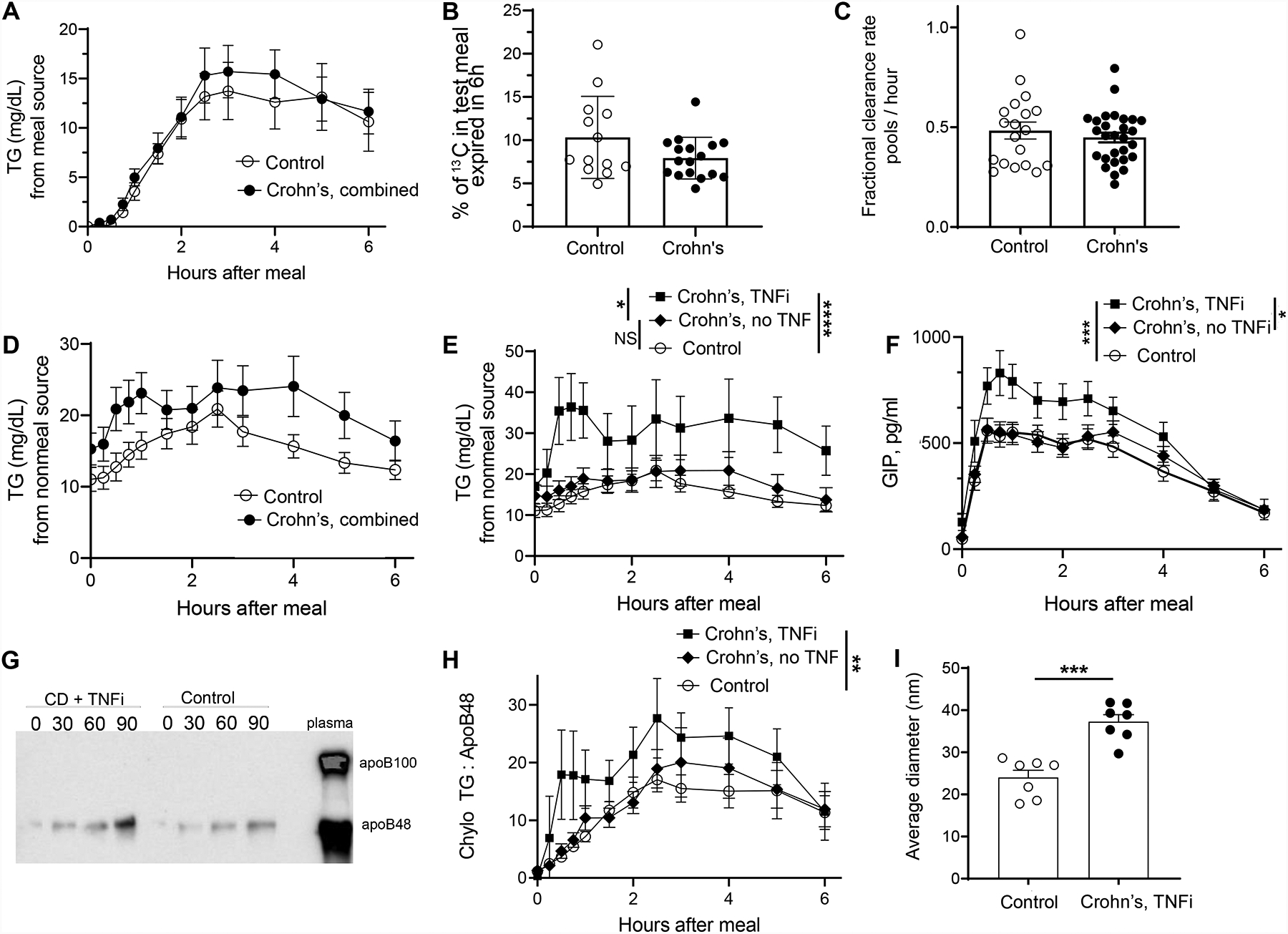
A) Chylomicron triglycerides (TG) derived from the test meal in all control versus all CD participants. B) % of test meal 13C-oleate tracer expired in breath over the 6 h study. Not all participants were analyzed due to equipment failure for a period of the study. C) Chylomicron-TG fractional clearance rate (pools/hr) derived from the monoexponential slope of chylomicron-TG glycerol after bolus injection of 2H5-glycerol. D) Chylomicron-TG derived from nonmeal sources in all control versus all CD participants. E) Data from panel D replotted to separately display the 8 CD participants taking TNFi adalimumab in the study (square symbols) versus all other CD participants not taking TNFi (diamond symbols) or control participants. F) Plasma GIP in the same groupings as shown in panel E. G) Immunoblot of the chylomicron fraction for apoB isoforms. H) A ratio of postprandial triglyceride (mg/l) to apoB48 (microgram/ml) concentration over the time course for each subject in the same experimental groupings as those in panels E and F, with baseline values subtracted to focus on the postprandial response. I) Analysis of mean chylomicron/chylomicron remnant size in the chylomicron fraction of plasma at the 90-minute time point, examining all CD + TNFi participants and 7 randomly selected control participants at the same time point. In all panels, statistical analyses were done using nonparametric tests; Mann U Whitney tests for panels B, C, I. Friedman tests for panels A, D, E, F, H. *, p <0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. The absence of a symbol for statistical significance indicates that statistically significant differences were not found.
There was a trend (not statistically significant) toward elevated postprandial chylomicron-triglyceride that did not arise from the test meal (Fig. 1D). One variable was able to account for this trend: the 8 CD participants taking adalimumab (all 8) or infliximab (1 subject transitioning from adalimumab) as TNF inhibitors (TNFi) displayed markedly elevated postprandial chylomicron-triglyceride derived from a nonmeal source, compared with controls or other CD participants (Fig. 1E). Furthermore, examination of enteroendocrine hormones revealed that CD participants taking TNFi uniquely displayed marked increases in postprandial glucose-dependent insulinotropic peptide (GIP, Fig. 1F, Supplemental Fig. 1A), the release of which occurs in response to secretion of chylomicrons6. Lesser to no changes in glucagon like peptide 1 (GLP-1) and GLP-2 were observed (Supplemental Fig. 1B–C). Immunoblots identified apoB48, not apoB100, protein in the fractions, confirming the traced lipids were from chylomicrons (Fig. 1G). Total apoB48 in the chylomicron fractions was similar after TNFi treatment (Supplemental Fig. 1D), such that the triglyceride:apo48 ratio was increased (Fig. 1H), suggesting enlarged chylomicrons in participants taking TNFi (Fig. 1I). Electron microscopy confirmed elevated size of plasma chylomicron remnants (Fig. 1I).
Discussion
We addressed whether the lymphatic-dependent transport of chylomicrons was decreased in individuals with active CD, given the possibility that lymphatic defects may occur in CD. However, chylomicron transport was not impaired. Rather, a detailed analysis of chylomciron-triglyceride appearance in the postprandial state indicated normal lipid absorption and delivery to plasma in CD. It remains possible and perhaps likely that since chylomicrons are mainly carried by lymphatics in the duodenum and jejunum3, upstream of where disease is focused, that ileal lymphatic transport defects exist and were not captured in this study. Methods to trace ileal lymphatic cargo remain to be defined but will be of interest for future studies.
This study also does not support the idea that fat malabsorption typifies CD, at least in the inflammatory phenotype of CD without fistulizing complications or prior resection of bowel segments or gall bladder.
Although we did not set out to study the impact of specific therapeutics on the postprandial response, we observed that TNFi promoted an early output of enlarged chylomicrons loaded with lipids not derived from the test meal. These lipids may arise from fats from prior meals retained in an epithelial storage pool2.The enhanced output of chylomicrons was strongly associated with increased secretion of the enteroendocrine hormone glucose-dependent insulinotropic peptide (GIP), secreted downstream of chylomicron release6. GIP has been linked to weight gain and increased bone mass7. Although GIP has scarcely been investigated in association with CD or CD therapy, it is known that treatment of CD with TNFi increases fat mass8 and bone mass. The mechanisms underlying how TNFi might affect lipid loading on chylomicrons and whether a rise in GIP might account for some effects linked with TNFi should be addressed in future prospective studies. It will also be important in future studies to determine if the effect of TNFi in the postprandial response is observed only in CD participants or in other patient populations taking TNFi.
Supplementary Material
Acknowledgements
This work was funded by NIH grant DP1 DK1109668 and the Rainin Foundation to GJR and American Heart Association Career Development Award (AHA: 18CDA34110273) to LH. Additional funding for resources accessed at Washington University core facilities included Digestive Diseases Research Core Center (P30 DK052574), Washington University Nutrition Obesity Research Center (P30 DK056341), the Institute of Clinical and Translational Science (UL1TR002345), and the Diabetes Research Center (NIH P30 DK020579). Washington University IBD Center research is supported by the Lawrence C. Pakula, MD IBD Innovation Fund and givinitallforguts.org (MAC). We are indebted to those who had important roles in advising on methods including statistics, providing technical assistance, recruiting patients, and/or providing overall advice and resources including Washington University colleagues Nicole K. H. Yiew, Shashi B. Kumar, George P. Christophi, Adewole L. Okunade, Ling Chen, Sewuese E. Akuse, Adam J. Bittel, W. Todd Cade, and Nicholas O. Davidson. We thank Shaji Chacko (Baylor University) and the Baylor Children’s Nutrition Research Center core facility for mass spectroscopy analysis of breath samples. We thank Ross Kossina and Gregory Strout for assistance with electron microscopy, performed at the Washington University Center for Cellular Imaging, which is supported in part by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (CDI-CORE-2015-505 and CDI-CORE-2019-813) and the Foundation for Barnes-Jewish Hospital (3770 and 4642). We extend additional thanks to the nursing and kitchen staff of the CRTU for their expert assistance. We are grateful for the advice and insight generously shared in conversations with Drs. Robert Hirten and Jean-Frederic Colombel (Mount Sinai), and Elizabeth Parks (University of Missouri).
Footnotes
No authors declare a financial conflict of interest with this work.
References
- 1.Van Kruiningen HJ, et al. Gut 2008; 57:1–4. [DOI] [PubMed] [Google Scholar]
- 2.Xiao C, et al. Cell Mol Gastroenterol Hepatol. 2019;7:487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang B, et al. Cell Metabolism 2016; 23:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchiyama K, et al. J. Immunol. Res 2018; 7270486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haberman Y, et al. J Clin Invest 2015; 124:3617–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gribble FM, et al. Nat Rev Endocrinol 2019;15:226–237. [DOI] [PubMed] [Google Scholar]
- 7.Møller CL, et al. J Clin Endocrinol Metab 2016;101:485–493. [DOI] [PubMed] [Google Scholar]
- 8.Parmentier-Decrucq E, et al. 2009; 15:1476–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


