Abstract
Gastric cancer remains a major unmet clinical problem with over 1 million new cases in 2018 worldwide. It is the fourth most commonly occurring cancer in men and the seventh most commonly occurring cancer in women. A major fraction of gastric cancer has been linked to variety of pathogenic infections including but not limited to Helicobacter pylori (H. pylori) or Epstein Barr virus (EBV). Strategies are being pursued to prevent gastric cancer development such as H. pylori eradication, which has helped to prevent significant proportion of gastric cancer. Today, treatments have helped to manage this disease and the 5-year survival for stage IA and IB tumors treated with surgery are between 60% and 80%. However, patients with stage III tumors undergoing surgery have a dismal 5-year survival rate between 18% and 50% depending on the dataset. These figures indicate the need for more effective molecularly driven treatment strategies. This review discusses the molecular profile of gastric tumors, the success and challenges with available therapeutic targets along with newer biomarkers and emerging targets.
Keywords: Gastric Cancer, Gastric Adenocarcinoma, Non Coding RNAs, lncRNA, piwi RNA, Autophagy, Novel Targets
Background
Gastric cancer is a deadly disease with poor overall survival statistics throughout the world. The majority of new diagnoses per year of gastric cancer occur mainly in Asian and South American countries (1). Within the United States, there are a projected 27,000 new cases to be diagnosed in 2020 (2). It is only recently that researchers started to understand how heterogenous gastric cancer actually is. There are three major subtypes of gastric cancer, according to the Lauren Classification system, and four subtypes according to the WHO classification system (3–4). The Lauren classification system was developed in the 1960’s and utilizes structural cellular components of the disease to separate patients into three subtypes: well differentiated (non-cardia/intestinal), poorly differentiated (cardia/diffuse) and mixed disease (3). The Lauren classification has been classified further to include the addition of a new subtype, solid gastric cancer, as well as including the addition of molecular markers in diagnosis criteria such as HER2 status (5–6). The other major classification system used is the World Health Organization (WHO) system, which elaborates further on the Lauren criteria (4). The WHO subtypes include the papillary, tubular, signet ring and mucinous (4).
Throughout the world, including the United States, the Lauren classification is commonly used as a diagnostic tool for gastric cancer due to its relative simplicity and long-term establishment within the medical community. The overall outcomes of disease have been well studied and generally differ based on the subtype of disease. We will focus on the Lauren classification system within this article for these aforementioned reasons. Well-differentiated (intestinal) gastric cancer is predominately found in individuals of an older age, >70 years, who are mostly male and patients present with larger tumor sizes (7). This subtype has overall better prognoses than the poorly differentiated (diffuse) subtype (8). The poorly differentiated subtype has poor survival statistics and is commonly found in younger women (8). The mixed subtype is present within a much smaller subset of patients, usually male, and it is known to be highly invasive and metastatic (9–10). The overall survival for cancer of the stomach remains at 31% within the United States and 25% worldwide (9). There are many reasons for the low survival rates. Most cases are diagnosed during late stage disease leading to overall poor outcomes which present with metastases, high intratumor heterogeneity and chemotherapeutic resistance (9, 11). Although the diagnosis of gastric cancer based on subtype can be rather ambiguous due to the various classification systems and differing terminologies for this disease used throughout the world, it is clear that gastric cancer remains a deadly disease that is not being controlled in a meaningful way with current treatment options or earlier detection strategies.
Gastric Cancer Development is Attributed to a Variety of Causes
A large majority of new gastric cancer cases can be attributed to a variety of pathogenic infections including but not limited to Helicobacter pylori (H. pylori) or Epstein Barr virus (EBV) (12–13). These two major pathogens influence disease progression through various intracellular mechanisms.
Helicobacter Pylori
Infection with H. pylori is the most commonly thought of pathogenic infection that leads to gastric cancer development. This bacterium is predicted to occur in over half of the world population, and it has recently been classified as a class I carcinogen. This infection leads to a predictable step-wise pattern of disease progression in a small percentage of the infected population (2%) that, if caught early enough, can be reversed (14–15). One way in which infection with H. pylori can induce a carcinogenic effect is through cag pathogenicity islands (known as the CagA protein) (16–17). This pathogenic protein influences the development of peptic ulcers and 100% of Asian patients infected with an H. pylori strain and 70% of United States patients infected by an H. pylori strain express the CagA protein (18). CagA is first integrated into the cell through the Type IV secretion system and becomes pathogenetic by activating a signaling cascade, either SHP2, Abl or Src kinases, within the gastric cancer cell depending on its post-translational modifications (17). The phosphorylation site within the CagA protein is referred to as the EPIYA motif (glutamate-proline-isoleucine-tyrosine-alanine residues) and can vary depending on the strain of H. pylori. This polymorphism of the CagA protein influences the development of gastric cancer, especially with the EPIYA-ABD genotype found in the East Asian demographic and this may be a significant reason why the Korean population has one of the highest incidence rates of gastric cancer throughout the world (19–20). H. pylori can also secrete peptidoglycan into the host’s cell, which causes upregulation of various pro inflammatory cytokines such as IL-8 and COX leading to chronic inflammation and cancer development (18). H. pylori has also been shown to secrete VacA toxin, a compound which can suppress T-cell responses, allowing lesions to form with little push back from the immune system (18).
H. pylori eradication in gastric ulcer patients may reduce the risk of developing gastric cancer. Lee et al performed a meta-analysis of 22 published studies covering 715 incident gastric cancers among a total of 48,064 individuals/340,255 person-years. Their goal was to assess the effects, as well as their modification by baseline gastric cancer incidence and study design (randomized trial vs observational study) to understand the benefit of H. pylori eradication on gastric cancer outcomes (21). From this study they concluded that individuals with eradication of H. pylori infection had a lower incidence of gastric cancer than those who did not receive eradication therapy (pooled incidence rate ratio = 0.53; 95% confidence interval: 0.44–0.64). We further describe how H. pylori eradication is being performed below (See Section entitled Gut Microbiome).
Epstein Barr Virus
Epstein Barr virus (EBV) has also been shown to influence gastric cancer progression in a subset of cases (10%) (22). The mechanism of viral introduction and subsequent infection into a human cell is somewhat elusive but there are a few hypotheses that exist. The main observation is that EBV is resistant to infecting cells expressing low cluster of differentiation 21 marker (CD21) while high CD21 cells are vulnerable to EBV infection (23). It has also been found that external EBV virons coated with IgA can bind to the IgA human cellular receptor allowing the virus to be internalized and integrated into the cell via endocytosis (22). Another means of EBV integration is through the gH/gL (glycoprotein) ligands excreted by this virus. The gH/gL and gp42 ligands found within the virus can also bind to HLA class II surfaces of B-lymphocytes (24). This system has differing effects whereas the gp42 protein when bound to the surfaces of B-lymphocytes inhibits epithelial cell infection with EBV (24). The gH, gL and gB complexes can also form and fuse to the epithelial membrane causing infection (24). It has also been hypothesized that interactions between the EBV BMFR2 protein and host β2 integrin protein leads to viral fusion and infection (25).
Non-Pathogenic Influences
Other factors can contribute to gastric cancer including familial or hereditary changes and environmental exposures. Familial causes of gastric cancer represent a small proportion of all cases, 10% total cases, while 3% of that population has gastric cancer caused by germline mutations in the E-cadherin (CDH1) gene (26). This type of gastric cancer is referred to as hereditary diffuse gastric cancer (HDGC). Within families, the absence of functioning CDH1 contributes to incidence rates as high as 50%. Normally there are no preventative measures besides prophylactic total gastrectomy before disease occurs within these cases (27). Lifestyle and environmental factors influence a smaller proportion of gastric cancer cases. These include poor diet, smoking and salt intake. A high intake of red and processed meats has been linked to statistically significant increase in gastric cancer development (28). Various factors can also influence gastric cancer progression including eating a midnight snack, swallowing hot food and having perturbations in circadian rhythm genes, i.e. not having a regular sleep schedule (29–30). Although the majority of people who do these activities do not develop gastric cancer, a small proportion may benefit from changing some aspects of their lifestyle (28–29). Smoking also increases risk for developing gastric cancer, particularly the diffuse or cardia subtype, which increases with the time and duration of cigarette smoking (31).
Although there are a variety of ways in which gastric cancer can develop, it is clear that more work is needed to understand how to not only treat this deadly disease but also to overcome resistance to chemotherapeutics, which is commonly seen in the majority of cases. In this article, we will focus on and discuss current ways in which researchers are approaching these critical issues.
Gastric Cancer Treatment Based on Disease Stage
To date there is no gold standard therapy used in gastroesophageal junction (GEJ cancer)/gastric cancer. Treatment options are mainly selected based on the stage of disease, the presence of biomarkers and the physicians preferred regimen. The American Joint Committee on Cancer (AJCC) cancer staging system (cTNM) has substantially enhanced decisions on treatment of gastric cancer, the latest 8th edition was introduced in 2017 (32). This staging system describes the invasion of the tumor through the stomach wall layers (T), lymph node involvement (N), and whether there are distant metastases present (M).
Surgical Intervention
For early stage disease, there is greater emphasis placed on the tumor resection rather than systemic chemotherapy (33). Patients with early stage disease (Stage II or less) undergo resection procedures to remove the malignancy. The type of surgery depends on the location of the tumor and the depth of the invasion and it could also vary between institutions, but in general, it includes endoscopic mucosal resection, distal esophagectomy, subtotal or total gastrectomy. Due to the high prevalence of elderly gastric cancer patients within the United States, it is critical for physicians and surgeons to optimize surgical intervention to extend patient quality of life while considering comorbidities that may present (34). A recently published study showed elderly gastric cancer patients who have undergone a laparoscopic assisted gastrectomy had lower risk of complications, such as respiratory compromise (35).
Cytotoxic Therapies
Despite the fact that surgery is the only curative approach in the treatment of gastric cancer, however, the addition of chemotherapy either pre (neoadjuvant), post (adjuvant) or peri-operatively has added a survival benefit. A large-scale study evaluated 206 gastric cancer patients and found that Stage II and III patients had higher survival rates with adjuvant therapy compared to surgery alone (36). Within the United States, there are a variety of options for treating metastatic gastric cancer, including cytotoxic monotherapy first line agents (antimetabolites, microtubule inhibitors, pyrimidine analogs) or combinations in doublet or triplet, which will be explained below.
For locally advanced disease (clinically T2–4 or positive lymph node), National Comprehensive Cancer Network (NCCN) guidelines recommend giving preoperative chemoradiotherapy or perioperative chemotherapy (37). Since 2005, there has been an increase in the administration of preoperative chemotherapy. Based on the results from the MAGIC trial (perioperative chemotherapy with the ECF regimen; epirubicin, cisplatin, and continuous infusion 5-fluorouracil) and SWOG INT-0116 trial (MacDonald’s regimen: postoperative chemoradiotherapy using 5FU as radiosensitizer), it was found that multimodality approach to locally advanced gastric cancer improved overall survival compared to surgery alone (38). Since the 1970s, gastric cancer therapy has steadily improved with chemotherapeutic regimens administered in either doublet or triplet combinations. A timeline of recent treatment strategies below will detail the progress we have made towards treating gastric cancer in recent years. An early treatment regimen was FAMTX, used in the 1990’s, and consists of fluorouracil, doxorubicin and methotrexate (39). FAMTX was replaced by the ECF regimen (epirubicin, cisplatin, and fluorouracil) after MAGIC trial showed improved 5-year survival of 36% with perioperative ECF compared with 23% in patients treated with surgery alone (hazard ratio for death, 0.75; 95% confidence interval, 0.60–0.93; P = 0.009) with also improved progression-free survival using perioperative chemotherapy (hazard ratio for progression, 0.66; 95% confidence interval, 0.53–0.81; P < .001) (40). The ECF regimen was utilized until head-to-head trial with the EOX regimen (epirubicin, oxaliplatin and capecitabine) showed capecitabine and oxaliplatin were non-inferior to 5FU and Cisplatin (ECF vs EOX) with less grade 3 or higher adverse events seen in EOX compared to ECF (41). Most recently, the preferred perioperative regimen became the FLOT (docetaxel, 5FU, leucovorin and oxaliplatin) after the positive results from the phase III FLOT4-AIO trial. In this study, the FLOT triplet was given over 8 weeks (2-week cycle) before and after surgery and compared to ECF or ECX control arm in a randomized fashion. After enrolling 716 patients with resectable gastric and Gastroesophageal junction adenocarcinoma, with median follow-up of 43 months, the study met its primary endpoint achieving a significant improvement in median overall survival (50 vs. 35 months, HR 0.77, 95% CI 0.63–0.94) in favor of FLOT (42). In the clinical practice, FLOT has become the standard modality perioperatively in resectable gastric and gastroesophageal cancer patients (T2 or higher and/or lymph node positive) who have good performance status and don’t have competing comorbidities. For those with less that ideal performance status or have medical conditions that could limit their ability to tolerate FLOT, we tend to utilize FOLFOX or CAPOX perioperatively (43–44). Efforts are ongoing to improve on FLOT. For example, a phase II RAMSES study which added Ramucirumab to FLOT and showed a higher (97% vs 83%) microscopic negative margin (R0), however, no benefit seen in the pathological complete response (27% vs 30%). The phase III trial will confirm if adding Ramucirumab will add any survival advantage (45). In HER2 positive tumors, there is also an extensive work to improve outcomes by adding HER2 directed therapy. PETRARCA trial, showed that the addition of both Trastuzumab and Pertuzumab to FLOT in perioperatively significantly improved the compete pathological response rate (35% vs 12%) with improvement in node-negative outcome (68% vs. 39%). However, due to increased toxicities when adding both HER2 agents including grade3/4 diarrhea (41% vs 5%), leukopenia (23% vs. 13%), this study is not being moved to the phase III (46).
In the metastatic setting, first line chemotherapy consists of a platinum-based agent, usually oxaliplatin, and a cytotoxic compound such as 5FU, mainly the FOLFOX or CAPOX regimens with or without Trastuzumab (if HER2 is overexpressed) (47). HER2 overexpression is found in 4–54% of all gastric cancer disease and testing is recommended by NCCN guidelines in all metastatic gastric cancer patients either through immunohistochemistry (IHC), in situ hybridization assays (ISH) or fluorescent in situ hybridization (FISH) (48). Once disease progresses, Ramucirumab (VEGFR-2 monoclonal antibody) plus Paclitaxel is the preferred regimen (49). Pembrolizumab (PD-L1 monoclonal antibody) started to have a major role in the treatment paradigm of gastric cancer since May 2017 when the FDA announced its approval for Pembrolizumab in all solid tumors with micro-instability high (MSI-H) or mismatch repair deficiency (dMMR) that progressed on previous lines of therapies and don’t have other alternative satisfactory treatment options (50). Later in September 2017, FDA approved Pembrolizumab for gastric cancer that is microsatellite stable (MSS)/ mismatch repair proficient (pMMR) as a 3rd line treatment (after failure of at least 2 previous lines of therapy) if the tumor is expressing PD-L1 with combined positive score (CPS; the number of PD-L1 staining cells including tumor cells, lymphocytes, macrophages divided by the total number of viable tumor cells, multiplied by 100) of 1% or above based on KEYNOTE 059 Trial (51). Subsequently, FDA announced their latest approval for Pembrolizumab in June 2020, for all solid tumors with high tumor mutational burden (TMB-H), adding another opportunity for gastric cancer patients to receive immunotherapy. Irinotecan (Topoisomerase I inhibitor) with or without 5FU and TAS-102 can also be used as third line or beyond therapies depending on the previous treatments (52–54), however, it is always preferred to enroll those patients in clinical trials if available.
Advancements in chemotherapeutic regimens have steadily improved the 5-year survival rate of gastric cancer within the United States to 31%. This is a large increase from the 15% survival rate previously observed in the 1970s (55). Worldwide the 5-year survival rate remains at 25%. Although there are a variety of treatment options available for gastric cancer patients, the majority of patients succumb to their disease quick due to (1) the high inter and intra tumor heterogeneity and (2) the majority of diagnoses occur during late stage disease and are met with high rates of chemotherapeutic resistance. It is clear that there is a lot of work that needs to be done in order to increase survival rates for this deadly disease.
Targeted Therapies
Even with the added benefit to OS upon using the current targeted agents in gastric cancer (Trastuzumab, Ramucirumab and pembrolizumab), there is a consensus that further improvement is needed. Within this section, we will provide an overview of new-targeted therapies and small molecule inhibitors that are under pre-clinical or clinical investigation.
Tyrosine Kinase Inhibitors (TKIs)
The broadest category of targeted therapies currently being investigated are tyrosine kinase inhibitors (TKIs), such as Imatinib (Gleevec/STI571), Vandetanib and Sunitinib. Tyrosine kinase inhibitors work to inhibit either the phosphorylation or dephosphorylation of the tyrosine kinase protein cascade. Currently, over 90 tyrosine kinases are encoded within the human genome (56). The most common tyrosine kinase inhibitors target broad phosphorylation cascades controlling large cellular survival processes such as HER2, EGFR, VEGF and MET.
Although a variety of TKIs are being investigated clinically, the results are less than encouraging for gastric cancer. For example, Imatinib, a BCR/ABL, c-KIT and PDGFR potent inhibitor effective in treating chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors (GISTs), in a phase II clinical study of metastatic gastric cancer showed no clinical efficacy or response (57). Sunitinib, a VEGF inhibitor, used as single agent therapy showed no significant clinical benefit with an overall survival of 6.8 months, comparative to standard of care (58). It is unclear as to why Sunitinib, a multi kinase inhibitor, is ineffective in reducing gastric cancer disease burden. KIT mutations, lysosomal sequestration and drug efflux pathways have been identified in rendering Sunitinib ineffective in gastrointestinal stromal tumors (GISTs), which may be the case in gastric cancer (59–60). Ramucirumab, a VEGFR2 antibody, has shown clinical benefit by improving gastric cancer outcomes and is being used as standard of care for this disease, which we have mentioned previously (49). Vandetanib, a multi-kinase VEGFR inhibitor, was tested in a Phase I clinical trials in combination with Docetaxel but due to limited patient accrual, the study was terminated early (61). A separate Phase I trial showed Vandetanib in combination with Paclitaxel, carboplatin and 5-fluoruracil exhibited efficacy and resulted in disease free progression of 3.7 years (62) in early stages of disease compared to standard of care. This drug has a way to go through the clinical trial process to fully understand its impact on gastric cancer. Besides anti-angiogenic agents, various preclinical TKIs are also under investigation in gastric cancer. AZD4547, a compound that inhibits FGFR2 activity, was found to elicit anti-tumor response in gastric cancer cell lines, cell line xenograph and PGDGX models (63). Tucatinib, an oral HER2 inhibitor, was found to sensitize gastric cancer cells that were resistant to HER2 inhibition via trastuzumab and has improved patient survival with advanced gastric cancer (64).
It is clear that more investigatory work is needed to identify subsets of gastric cancer patients that would benefit from TKI therapy or to identify novel actionable targets that would be of greater clinical benefit to the patient population as a whole. TKIs are clearly not a one-size fits all type of therapy and because of the modest effectiveness of TKIs within gastric cancer research into the TKI sensitive gastric cancer populations are needed to maximize the effectiveness.
Cell Structure Remodeling Therapies
Another category of targeted therapies being currently investigated are inhibitors of cytoskeleton components. It was found in the poorly differentiated (diffuse) gastric cancers that inhibition of cytoskeleton components could induce apoptosis and enhance cellular death (65). This concept is not novel as inhibiting cytoskeleton components such as microtubules with taxol compounds is somewhat effective in managing this disease. Cytoskeletal components such as tubulins, myosin, kinesins and dynamins are some of the targets being explored. T900607 is a tubulin inhibitor, which binds irreversibly to colchicine binding sites, currently being investigated in Phase I clinical Trials for gastroesophageal junction (GEJ) cancer (65). This treatment showed no dose limiting toxicities but there were some cardiac toxicities (65). It is unclear whether this regime will be more effective than the taxol compounds such as paclitaxel or docetaxel but further investigation is needed. Microtubule associated proteins (MAPs) have also been shown to be effective anti-cancer strategy in pre-clinical investigations. For example, FAM83 is a MAP and was found to be upregulated in gastric cancer and knockdown of this protein lead to tumor regression (66). Eg5, a protein involved in spindle formation, can be inhibited with compounds such as monastrol and this treatment strategy is potent in eliciting a response even in taxol resistant cells due to upregulated drug efflux or other tubulin mutations (67). This phenomenon was also seen with MAP4, MAP2 and tau inhibition (65). Finding other ways to target cell division away from the naturally derived taxol compounds that are prone to cellular resistance mechanisms is a new focus of research and may be a promising avenue to pursue in gastric cancer.
Targeting of DNA Damage Repair Proteins
DNA damage repair proteins are mutated in a subset of gastric cancer patients. For example, ATM, BRCA1, BRCA2 and ATR mutations are found in ~14–20% of the gastric cancer population, according to the TCGA publicly available database. Mutations in these DNA repair proteins allow a normal cell to repair damaged portions of DNA in an ineffective manner which causes the cell to acquire further mutations which ultimately leads to a cancerous phenotype (68). Alkylating agents, such as nitrogen mustards, are administered with the hopes of targeting DNA repair proteins but there is little efficacy noted and various resistance mechanisms that emerge have been identified (69).
Poly (ADP-ribose) polymerase (PARP) inhibitors are a novel way in which we can target the DNA repair machinery and this class of compound is under clinical investigation in gastric cancer. PARP is an essential protein that is involved in a number of cellular processes including chromatin binding and recruitment of various DNA damage response proteins (such as XRCC1/ATM/MRE11) to the sites of DNA damage for the cell to effectively execute repair processes (70). Inhibiting PARP has been a therapeutic strategy in a variety of cancers including ovarian, breast and prostate. PARP inhibitors work by blocking single stranded DNA damage response via the inhibition of PARP1. Cancers with mutations in BRCA proteins and double-stranded DNA repair proteins, are sensitive to PARP inhibition via synthetic lethality (71). The PARP inhibitors Olaparib, rucaparib and niraparib are being tested in gastric cancer within pre-clinical and clinical settings. Recently, Olaparib was tested in an Asian gastric cancer cohort to identify whether patients with ATM deficiencies were sensitive to PARP inhibition. Unfortunately, the primary endpoint was not reached, i.e. the overall survival was not statistically significant (72). These results correlate with another clinical trial that determined Olaparib and paclitaxel versus paclitaxel alone was not beneficial to patient overall survival but there was a statistical trend for benefit in patients with low ATM activity (73). As single agent therapy, PARP inhibitors seem to have subpar overall benefit but there is a current phase II trial testing the standard of care therapy Ramucirumab with Olaparib. There is clear scientific rationale to justify this combination as it was found that hypoxic mimetic agents can sensitize the cells to PARP inhibitors due to their suppressive effect on homologous recombination pathways in gastric cancer cells (74). It is clear that more work is needed to elucidate the mechanism of PARP inhibition either in single agent or combination therapy within gastric cancer as there has been minimal successes compared to breast or ovarian malignancies.
Other preclinical compounds involved in cellular repair are also being tested including MHY440 and APG-115. MHY440, a topoisomerase inhibitor, has shown efficacy inhibiting the DNA damage response pathway and increases apoptotic death in a ROS dependent manner (75). APG-115, a MDM2-p53 inhibitor, was tested in a panel of gastric cancer cell lines and was found to enhance radiosensitivity in p53 wild type gastric cells inducing apoptosis and decreasing proliferating cells via Ki-67 (76). BCI hydrochloride is another compound that allosterically inhibits Dusp6, a negative feedback pathway mediating ERK associated proteins, which prevents the normal dephosphorylation and subsequent activation of ERK2. Although Dusp6 is not directly related to DNA repair, it was shown that Dusp6 inhibition can overcome cisplatin resistance, a cytotoxic DNA repair damaging agent (77) which suggests this protein may have some alternative functions involved in DNA repair. Inhibition of non-homologous end joining (NHEJ) with the compound AZD7648 showed potent activity and sensitized lung cancer cell lines to radiation, chemotherapy and Olaparib treatment and may be a new avenue worth exploring in gastric cancer (78). Future preclinical and clinical investigation is necessary to determine if there is survival benefit with these compounds.
Immunotherapy
Immune modulation is also being explored in gastric cancer. An immune panel analysis was performed on a cohort of gastric cancer patients and PD-L1 expression correlated with worse overall survival in later stage (Stage II/III) gastric cancer patients (79). PD-L1 is a cancer cell surface marker that when overexpressed avoids T-cell related targeting and thus causes the gastric tumors to evade immune system detection. Testing individual gastric cancer patient populations led to the realization that a large percentage of Chilean patients may be candidates for immunotherapy according to the FORCE1 study (80). This may be related to the prevalence of Epstein-Barr virus related gastric cancer carcinogenesis leading to microsatellite instable disease within this region (81). Although the Epstein-Barr virus is found to be the cause of disease in a small percentage of gastric cancer patients worldwide, it was found this disease has better survival outcomes compared to Epstein-Barr negative patients and these patients may further benefit from immunotherapy and have even better 5-year survival statistics (82). Treatment of gastric cancer with the PD-L1 inhibitor pembrolizumab (Keytruda) has been approved for third line use in gastric cancer based on the results from the phase II KEYNOTE-059 trial where response rates were increased to 11.6% compared to 2.3% in control arm (83). Around 40% of the patients with high expressing PD-L1 gastric tumors have lower rates of metastatic disease according to a recent analysis but because these patients have worse overall survival outcomes, immunotherapy could be a better treatment option for this patient population. Further work needs to be done to identify more vulnerable subpopulations of gastric populations with high expressing PD-L1 tumors to PD-L1 inhibition due to the vast heterogeneity of gastric cancer we will explore further in subsequent sections.
Besides PD-L1 immunotherapy, there are other treatment options including PD1 inhibition. PD1 is a marker found on immune T-cells which scan for cancers, which binds to PD-L1 on other cells causing destruction when bound. PD-L1 expression, as we have discussed above, is found to be highly expressed in malignant cells and this immune surveillance protects the body from malignancies. PD1 inhibition with nivolumab is also being tested clinically and in a phase 3 study, it was found that there was increased survival with this immunotherapy over a 12-month period indicating this may be another immunotherapeutic option for gastric cancer patients (84). It was further found that PD1 inhibition enhanced the effect of Ramucirumab, a standard of care treatment used in gastric cancer, in a phase I/II clinical study (85). Besides targeting the PD1/PD-L1 axis, there is preclinical research attempting to target the immune system through other markers including Car T cell therapy, tumor-infiltrating lymphocyte (TIL) therapy, interferons, and interleukins.
Interferons, a class of glycoproteins which are excreted in response to viral or bacterial infection, such as interferon gamma (IFN-y) has been found to be upregulated within the gastric mucosa after H. pylori infection. IFN-y was found to propagate carcinogenesis via upregulating NF-kB signaling (86). Interleukins, or cytokines excreted mainly by the leukocyte cells, such as interleukin-6 (IL-6) is a pro-inflammatory secretory protein that feeds gastric cancer growth and progression through various paracrine signaling mechanisms. Inhibition of IL-6 with the monoclonal antibody tocilizumab was found to induce chemotherapy directed apoptosis in gastric cancer models (87). There is further work being done to understand the role interleukins play in gastric cancer such as IL-32, IL17A and IL-11 but further work needs to be done to not only understand but to identify targeted therapies that can be utilized in gastric cancer patients as this is a rationale and feasible strategy for a subset of patients such as EBV-associated and microsatellite instable (MSI) patients (88–92).
Utilizing and manipulating T-cell receptor therapy to recognize and attack gastric cancer is a new strategy being utilized. Chimeric antigen receptor (Car T) therapy, a novel type of therapy being explored in a variety of mainly liquid malignancies are now being explored in solid tumors, such as gastric cancer. In a recent clinical study, it was found that Car T cells which target claudin 18.2 or EpCAM, specific receptors in gastric cancer, were found to be potent in targeting gastric cancer as well as pancreatic ductal adenocarcinoma in pre-clinical and Phase I open label clinical investigations (93–94). Tumor infiltrating lymphocytes (TILs) are cells that are present in malignant gastric tumors and are mainly used as a prognostic tool in gastric cancer to predict responses to adjuvant chemotherapy (95) but can be used as a therapeutic option. It has been shown in solid malignancies, such as melanoma, that specific TILs which recognize malignant cells are extracted from the tumor, treated with the IL-2 cytokine, and those that are reactive to the tumor cells are expanded and reinfused into the body (96). This process is being investigated in Phase II clinical trials for a variety of solid malignancies, including gastric cancer, with the infusion of autologous TILs and concurrent treatment with fludarabine and cyclophosphamide (97).
Adapting immune therapeutic options to gastric cancer is an emerging branch of research. There is a lot of information suggesting there may be a select group of gastric cancer patients would benefit from this therapy. Due to the known heterogeneity of gastric cancer cases, further work needs to be done to explore gastric cancer subpopulations in order to find these vulnerable groups.
Tumor Microenvironment
Gastric cancer is a disease with known high intra and inter tumor heterogeneity. One aspect of this heterogeneity is within the tumor microenvironment which is highly infiltrated by cancer associated fibroblasts (CAFs), immune cells and stromal components. CAFs are spindle shaped cells that can control signaling to other cells, such as fibroblast activation proteins (FAP) (98). CAFs have also been shown to control signaling of gastric cancer cells specifically by inducing migration, invasion and proliferation of the cancer cells as well as regulating interactions within the stroma (99). We discuss some of the ways in which CAFs can influence gastric cancer and ways in which targeting this subset of cells is being achieved.
Cancer Associated Fibroblasts and Immune Modulation
CAFs can regulate gastric tumor cell behavior through the control of immune components. As found with single cell genomic characterization, gastric tumors have a high level of heterogeneity regarding immune cells (macrophages, dendritic cells and regulatory T cells) all of which are prominent within the gastric tumor microenvironment (100). Presence of certain cell populations in the immune microenvironment has been shown to play a beneficial role in improving survival in western patients (101). Uppal et al. demonstrated that high expression of immune cells, such as CD3+ or FOXP3, correlated with improved overall survival and this trend was not found in eastern patients (100). This discrepancy may be another reason why the survival rate within the United States is higher than in other countries. Not only does high infiltration of immune cells correlate with overall survival, but the presence of immune cell alterations within a tumor may also predict a patient’s sensitivity to chemotherapy. Jiang et al. found that the patients with stage II and III gastric cancer with high CD3+, CD8+, CD45RO+ and other immune markers could sensitize the tumor to adjuvant chemotherapy better than those with low expressing immune infiltration (102). Further, it was found that those gastric cancers induced by H. pylori need an immunosuppressive environment which the stepwise tumor process can propagate from pre-malignancy to a malignant tumor (103). This immunosuppressive environment is attributed to perturbations in the tumor microenvironment and immune specific cells and secretion of various proteins and immune signaling factors are present within the immune infiltrative subset of gastric cancer patients. Although this subset is not found within the WHO or Lauren classification, some gastric cancer patients have high immune cell infiltration within their tumors.
Thrombospondin-4, a cell-cell and cell-matrix interaction promoting protein, was found to be expressed within stromal cells absent of cytokeratin expression, which correlated with higher invasion, metastasis and worse overall outcome clinically (104). IL-6, a pro-inflammatory cytokine that is excreted in the CAFs via tumor stroma, progresses gastric cancer through JAK2/STAT3 signaling pathway, enhances epithelial-to-mesenchymal transition (EMT) and migration of gastric cancer cells (105). Lactic acid, a secondary metabolite found as a byproduct of the Warburg effect, has been shown to promote macrophage polarization through angiogenic signaling via HIF1a and is highly prominent within the gastric cancer microenvironment (106). High glucose serum levels were found to activate and increase the incidence of H. pylori induced gastric carcinogenesis in people as well as having the ability to stimulate neighboring CAFs towards participating in the Warburg effect (107–108). A reciprocal feedback mechanism can occur between gastric cancer tumor cells and CAFs which modulates the immune system. CAFs were shown to perpetuate gastric cancer through IL-33 signaling which activates the ERK1/2 pathway in ST2L dependent manner while gastric cancer cells can further induce IL-33 signaling via TNF-a secretion (109). A better understanding of this intricate balance between the immune system and the gastric microenvironment is needed in order to find better-suited targets that can attack gastric cancer growth and development.
Cancer Associated Fibroblasts and Stromal Interactions
Not only are CAFs involved with immune signaling but they can also control stromal changes. Stroma engulfing and/or surrounding malignant gastric cells has been identified as important for determining the prognostic outcome of gastric cancer. It was found, via meta-analysis, the tumor-stroma ratio (TSR) was a strong prognostic factor in gastric cancer and patients with high stroma to tumor ratio had better 5-year survival rates (110).
The stroma contributes to gastric cancer progression in a non-immune signaling manner. For example, SALM3 expression, a synaptic adhesion molecule, was found to be overexpressed in the tumor cells and fibroblasts in patient tissues and publicly available databases and was found to correlate to poorer patient survival (111). ADAMTS-2, a procollagen and N-proteinase, is present in both the tumor and the stroma and is an independent prognostic marker of survival (112). CAFs have also been found to signal gastric cancer cells through hepatocyte growth factor (HGF) which promotes MET-unamplified gastric cancers via enhancement of proliferation, invasion and metastasis (113). KLF-5, a zinc-finger protein, was found to be expressed within the tumor stroma, which regulates CAFs by promoting CCL5 secretion contributing to poorer overall survival correlating with clinicopathologic features (114). Lumican, an extracellular matrix protein, was also found to be secreted by gastric CAFs and correlates with poor prognostic factors via signaling through integrin-B1/FAK pathways (115). High TEM1 expression found within the gastric CAFs also can contribute to progression, metastasis, and contributing to poor overall survival (116). These are not the only examples of the signaling axis of CAFs/GC cells but it illustrates the functionality of the tumor microenvironment on enhancing and perpetuating gastric cancer progression in an immune-independent manner.
Cancer Associated Fibroblasts and Angiogenesis
Angiogenesis, or blood vessel formation, is another important part of the gastric tumor microenvironment. CAFs can stimulate either angiogenic or hypoxic conditions through various signaling mechanisms. Blood vessel formation in cancers are pathologically abnormal resulting in hypoxic conditions that the tumors rely on for growth. Hypoxia, a state of anoxic cellular conditions, leads to the proliferation and perpetuation of gastric cancer through HIF-a, VEGF, FGF and PDGF signaling. CAFs can stimulate angiogenesis through VEGF/IL-6 signaling axis but also can secrete other signaling factors for angiogenesis including but not limited to CCL-5, CXCL-12, EGF, MMP-3 and MAPK (117). Sun et al. found dysregulation of various bone morphogenic proteins (BMPs), within TCGA gastric cancer and GEO database cohorts, which these factors can signal for angiogenic markers (118). Not only are BMPs upregulated, but also a recent study has found that Gremlin1 (GREM1), a stimulator of BMPs, was upregulated in gastric cancers correlating with poorer survival (119). Common ways which angiogenesis can be targeted is through VEGF inhibitors (such as Ramucirumab). Not only are VEGF inhibitors directly responsible for downregulation of angiogenesis but the literature has suggested other compounds not specific to VEGF inhibition can downregulate angiogenesis. Bae et al. suggested metformin can inhibit the indirect feedback loop between CXCL8 and PTPRD (Protein tyrosine phosphatase receptor delta) (120). PTPRD downregulation is seen in gastric cancer leading to poorer overall survivals, while this axis has been shown to promote angiogenesis (120). A new compound, 5,6,7-Trimethoxy flavonoid salicylate derivatives, was found to potently inhibit not only VEGF but also HIFa, and may be effective in altering the angiogenic and hypoxic conditions of the tumor microenvironment (121). Arsenic trioxide, a commonly used remedy within the Chinese culture, has been found to inhibit gastric cancer angiogenesis through regulation of FOXO3a expression, VEGF and MMP9 downregulation (122). GX1, a novel peptide, was found to inhibit angiogenesis via binding to TGM2 in the endothelial gastric cancer cells (123). A recent study has shown targeting stromal cells via inhibition of PDGF-B with the tyrosine kinase inhibitor Imatinib caused a decrease in stromal cells and sensitized the cancer cells to chemotherapy (124).
Although new avenues are being explored for inhibition of angiogenesis within gastric cancer away from the known VEGF/HIF1a inhibitors, more work needs to be done to understand the safety and efficacy of these while also enhancing our knowledge on other stromal factors specific to gastric cancer.
Gut Microbiome
Not only are classical signaling mechanisms responsible for gastric cancer growth and progression but other environmental factors beyond poor diet, smoking and alcohol intake can also influence this disease. The microbiome consists of a variety of bacteria, predominately gram negative (Bacteroidetes) and gram positive (Firmicutes), which reside within the digestive tract (125). Interestingly, bacterial cells outnumber human cells within the body by a factor of 10 (126). Normal gut microbiome functions include innate immune protection of the host from invasive pathogens by secreting defensing molecules or cytokines (127). Various metabolic functions occur within the microbiome including the production of vitamins, synthesis of all amino acids and metabolism of carbohydrates, many of which cannot be digested normally (128). These metabolic functions ultimately lead to a supply of pyruvate, an essential component of cellular energy via the TCA cycle, as well as lipid catabolism. Dysfunctional microbiota has been linked to an increase in obesity (129). Finally, the gut microbiome can communicate with the brain, via signaling molecules, allowing for the brain to secrete hormonal and neurotransmitters such as gamma-amino butyrate (GABA), serotonin, norepinephrine and dopamine (130). The literature suggests alterations in any of the normal functions of the microbiota can contribute to pathogenic diseases including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), metabolic diseases such as type II diabetes, and neurocognitive disorders such as Parkinson’s disease (PD) (131–133). Interestingly, the use of probiotics, fecal microbiota transplantation (FMT), dietary intervention and antibiotics are being studied within PD patients (134). Due to the abundant role the gut microbiome plays into the health of an individual, it is not incorrect to assume that gastric cancer development may be impacted by the microbiota composition.
The Gut Microbiome and Gastric Cancer
The gut microbiome differs between individuals and it was found that gastric cancer patients exhibit even greater differences in microbiome composition compared to healthy individuals. Many of the studies conducted have been performed in Asian populations, where gastric cancer is predominant. Within a 276 patient Asian cohort, specific bacteria were found to be deregulated, many of these bacteria are not commonly studied such as Bacteroides uniformis and Sphingobium yanoikuyae which was found using shotgun sequencing on Chinese patient samples, (135–136). Bacteroides uniformis degrades the isoflavone compound genistein in human feces (137). A recent meta-analysis showed that ingestion of genistein decreased cancer incidences especially with breast and prostate (137) and the high consumption of soy in Asian countries correlates with the downregulation of these malignancies. Sphingobium yanoikuyae is a gram-negative bacterium found to be downregulated within the gut of gastric cancer patients. Its main function is to degrade pollutants such as biphenyl, naphthalene, phenanthrene, toluene and anthracene which are pollutants that can be found in the environment such as cigarettes (138–139).
This 276 cohort study also found an enrichment of other bacterium such as Streptococcus anginosus, Peptostreptococcus stomatis, Parvimonas micra, Slackia exigua and Dialister pneumosintes (135). Streptococcus anginosus is a gram-positive bacterium part of the normal gut microbiome has been found to cause intra-abdominal masses and primary peritonitis (oral infections). S. anginosus plays a role in the metabolism of glucose and lactose which gastric cancer cells rely on for energy (140). Peptostreptococcus stomatis and Slackia exigua are gram-positive bacterium found in the human oral cavity and produces a variety of end products of fermentation including acetic, butyric, isobutyric, isovaleric and isocaproic acids (148–149). These end products cause cellular injury and induce gastric cancer development and were found to be prominent bacterium in infections (141–142). Parvimonas micra is a gram-positive bacterium found in abundance within periodontal infections and has been associated with several diseases including septic arthritis and colon carcinoma (143–144). Found to distinguish gastric cancer from earlier dysplastic disease states, this bacterium may be an important bacterial target to study (145). Finally, Dialister pneumosintes is a gram negative bacterium is found in brain abscesses and has been found to be a endodontic pathogen (146–147). Comprehensive list of microbes involved in gastric cancer development can be found in Table 1.
Table 1. Bacterium found in the microbiome involved with the development of gastric cancer.
These abundant bacterium are involved in gastric cancer development and perpetuation of this disease. These bacterium have been found to be relevant to gastric cancer in numerous studies and are found in the oral cavity as well as the GI tract. Many of these bacteria are influenced by the presence of H. pylori, a well-known pathogen involved in the carcinogenesis and development of gastric cancer.
| BACTERIUM | PHYLUM | EXPRESSION | LOCATION | GASTRIC CANCER RISK | H. PYLORI INVOLVEMENT IN BACTERIAL ABUNDANCE |
|---|---|---|---|---|---|
| Neisseria | Proteobacteria | Absent | GI Tract | Preventative 219,220 | Increases 221 |
| Aggregatibacter | Proteobacteria | Abundant | Oral Cavity | Causative 222,223 | Increases 221 |
| Sphingobium yanoikuyae | Proteobacteria | Absent | Oral Cavity | Causative223 | Increases 221 |
| Haemophilias | Proteobacteria | Abundant | GI Tract | Causative 219 | Increases 221 |
| Escherichia coli | Proteobacteria | Abundant | GI Tract | Causative 219 | Increases 221 |
| Burkholderia flingorum | Proteobacteria | Abundant | GI Tract | Causative219 | Increases 221 |
| Streptococcus anginosus | Firmicutes | Abundant | Oral Cavity, GI Tract | Preventative/Causative 219 | Decreases 221 |
| Clostridium | Firmicutes | Absent | GI Tract | Causative 224 | Decreases 221 |
| Lactobacillus | Firmicutes | Absent | GI Tract | Preventative/Causative 220,225 | Decreases 221 |
| Veillonella | Firmicutes | Abundant | Oral Cavity | Causative 223 | Decreases 221 |
| Staphylococcus | Firmicutes | Abundant | GI Tract | Causative 226,227 | Decreases 221 |
| Lachnospiraceae | Firmicutes | Abundant | GI Tract | Causative 220,226 | Decreases 221 |
| Parvinomas micra | Firmicutes | Abundant | Oral Cavity | Causative 220,226 | Decreases 221 |
| Dialister pneumosintes | Firmicutes | Abundant | Oral Cavity | Causative 220,226,228 | Decreases 221 |
| Peptostreptococcus | Firmicutes | Abundant | Oral Cavity | Causative 220,226 | Decreases 221 |
| Phorhyromonas | Bacteroidetes | Abundant | Oral Cavity | Causative 220,226 | Decreases 221 |
| Alloprevotella | Bacteroidetes | Abundant | Oral Cavity | Causative 220,226 | Decreases 221 |
| Nitrospirales | Nitrospirae | Abundant | GI Tract | Causative 220,226 | Undetermined |
H. pylori and Gastric Cancer
We have discussed above the mechanisms by which H. pylori impacts gastric cancer development. It was further found that H. pylori was downregulated within the 276 patient cohort highlighted in Liu et al (135). Although it is counterintuitive that H. pylori would be in less abundance within the gut due to its carcinogenic role in gastric cancer development, its pro-inflammatory signaling is necessary and prominent mainly during the early stages of carcinogenesis (148). It is incorrect to think H. pylori is the only pathogen known to contribute to gastric cancer but microbiome studies correlating to gastric cancer within Western countries are lacking.
Attempts to Target the Gut Microbiome
Due to the global alterations of the microbiome found within gastric cancer patients, it is necessary to find ways to target or reduce these perturbations to identify if therapeutic benefit exists. H. pylori is a well-known bacterial pathogen that has been identified as a direct cause of gastric cancer through the development of ulcers. This bacterial presence increases abundance of developing non-cardia (well-differentiated) gastric cancers 5.9-fold (149). Due to this significant finding, eradication of H. pylori at the dysplastic stage of disease is being attempted in order to minimize the risk of gastric cancer development. Various drugs can be prescribed for H. pylori eradication including proton-pump inhibitors (PPIs), Bismuth, Metronidazole and a variety of antibiotics (Clarithromycin, Amoxicillin and Tetracycline) (150). Antibiotic eradication of H. pylori is met with concerns about antibiotic resistance developing so research is being done to explore other options of targeting the gut microbiome away from these antibiotic options (151). Testing the consumption of probiotics, live bacteria and yeasts is being investigated for therapeutic efficacy. Studies in colon cancer have found that probiotics, such as the ingestion of fermented milk, containing lactobacillus can work to mitigate the H. pylori infection as well as prophylactically preventing gastric cancer development (152). Not only are probiotics being investigated as a protective measure against gastric cancer. A recent study has found that consumption of Lactobacillus spp. and Bifidobacterium spp. may decrease toxicities seen with standard of care compounds, such as 5-FU, in colorectal cancer (153). It was also found that probiotics may increase antioxidant levels and prevented epithelial cellular injury in intestinal mucositis (154). Although Lactobacillus is a popular probiotic under investigation, there are other bacterial species, which have anti-cancerous effects in gastric cancer including Propionibacterium freudenreichii. This bacterium was found to inhibit cellular proliferation of the HGT-1 Gastric cancer cell line (155)
Not only are bacterial species the only organisms under investigation, but research into the therapeutic benefit of yeast, i.e. Saccharomyces, to prevent gastric ulcers is under investigation. Many of the studies are aimed to eliminate the H. pylori bacterium within the gut but a one-pronged approach is likely not the answer as the gut microbiome is globally perturbed and those perturbations may influence a host of factors contributing to disease development. Although using probiotics medicinally is a novel avenue of investigation, further investigation is required.
Biomarkers of Gastric Cancer: Small Non-coding RNA focused
Due to the deadly nature of gastric cancer, there are various attempts to uncover biomarkers for earlier detection either blood based or other means of detection besides the classic biomarkers, CEA or CA 19–9, which we will explore below. The carcinoembryonic antigen (CEA) is a biological marker, glycoprotein, found within the blood and is part of the CD66 family. CEA detection is used clinically in diagnostics of gastric cancers (156–157). CA 19–9 is another commonly used tumor antigen in various gastrointestinal malignancies. This biomarker is found within the serum and is used to monitor malignancy growth or regression and is being investigated for earlier detection of malignancies in gastrointestinal cancers (158). Small noncoding RNAs, such as microRNAs, long noncoding RNAs, and piwi RNAs are currently being investigated within gastric cancer as new avenues for biomarker detection.
MicroRNA based Biomarkers: A major focus of biomarker studies
MicroRNAs are small noncoding RNAs between 18 and 25 nucleotides which can modulate gene transcription either pre or post transcriptionally. MicroRNA-21 was found to be upregulated in a small gastric cancer cohort (50 normal/50 gastric cancer patients) within the serum and the mononuclear peripheral blood cells suggestive of its biomarker potential (159). Serum evaluations have found that there are distinct differences in the microRNA profiles of Asian and Western patients. Due to the prevalence of different gastric cancer subtypes within these regions, the differences are expected. Urine specific microRNAs are also under investigation and were found to be dysregulated in gastric cancer patients compared to normal control subjects. It was found that miR-6807–5p and miR-6856–5p were independent biomarkers of H. pylori status while distinguishing between healthy controls and stage I patients and could be normalized back to almost zero after removal of tumor (160)
One focus within our laboratory is to study and identify how to target gastric cancer related microRNAs. We have found that XPO1 inhibition in gastric cancer with the drug Selinexor (XPOVIO), which has recently been FDA approved for Multiple Myeloma, has the potential to differentially express a subset of gastric related microRNAs and piwi-RNAs (piRNAs) (161). We have found in the SNU-1 cell line that miR-1246 and miR-1275 are significantly upregulated (2.24 fold, 2.18 fold) after treatment with Selinexor indicating their probable roles as tumor suppressor microRNA (data unpublished). This correlates with the findings of Shi et al., which found miR-1246 is highly expressed in exosomal compartments via ELAV1 in gastric cancer patients and has tumor suppressive activity that could be used as an early diagnostic biomarker for gastric cancer (162). Mei et al found that miR-1275 has a tumor suppressive role in gastric cancer and its upregulation is indicative of decreased metastasis through vimentin/E-cadherin downregulation (163). Our group is currently pursuing this further but preclinical studies are needed to understand how to use microRNAs not only as an early biomarker of detection but to use the circulating microRNAs to monitor patient responses to treatment and enhance therapeutic treatment options for common chemotherapeutics such as platinum based agents and 5-flourouracil.
Other Small RNAs: A Newer Avenue of Biomarker Detection
Long-noncoding (lncRNAs) RNAs are small 1000–10,000 nucleotide RNA molecules which are involved in controlling cellular processes including their involvement in chromatin remodeling and post-transcriptional regulation (164). This class of small noncoding RNAs can enhance or repress gene transcription based on cellular cues. Various lncRNAs have been found to be perturbed in gastric cancer including CASC15 (165). CASC15 has been found to be upregulated in gastric cancer and is correlated with the tumor stage of the patient and high expression was found to be a risk factor for gastric cancer through controlling genes such as TUG1 and TINCR (166). A bioinformatic analysis of long-noncoding RNAs found that LINC00982 was an inhibitor of gastric cancer while LL22NC03-N14H11.1 was a pro-proliferation marker in gastric cancer in an Asian cohort (167). The identification of these long-noncoding RNAs were found in gastric tumors and databases but future work is needed to develop assays for detection of these lncRNAs in the blood.
PiwiRNAs (piRNAs) are small non-coding RNAs (21–35 nucleotides) which are involved in regulating gene functions and are involved in responding to viral infections. A recent transcriptome-wide piRNA profiling found a variety of protein-coding genes were regulated by gastric cancer associated piRNAs in a large percentage of protein-coding genes (~65%) (168). This was confirmed in other studies which found an important role for piRNAs in gastric cancer (169–170). This new field is being currently studied for its potential biomarker applicability but more work is needed before a candidate piRNA is found.
Autophagy and New Age Drugs
Autophagy is a natural cellular process devoted to conserving cellular resources during times of stress. The major players in autophagy include Becn1, ATG type proteins, UVRAG, p62, parkin and lamp2. These proteins work together to induce autophagy in the presence of distinct cellular signals such as glucagon-induced autophagy or cellular starvation (either mTOR dependent or mTOR independent signaling) (See Figure 1).
Figure 1: Diverse Cellular Activation Processes of Autophagy.
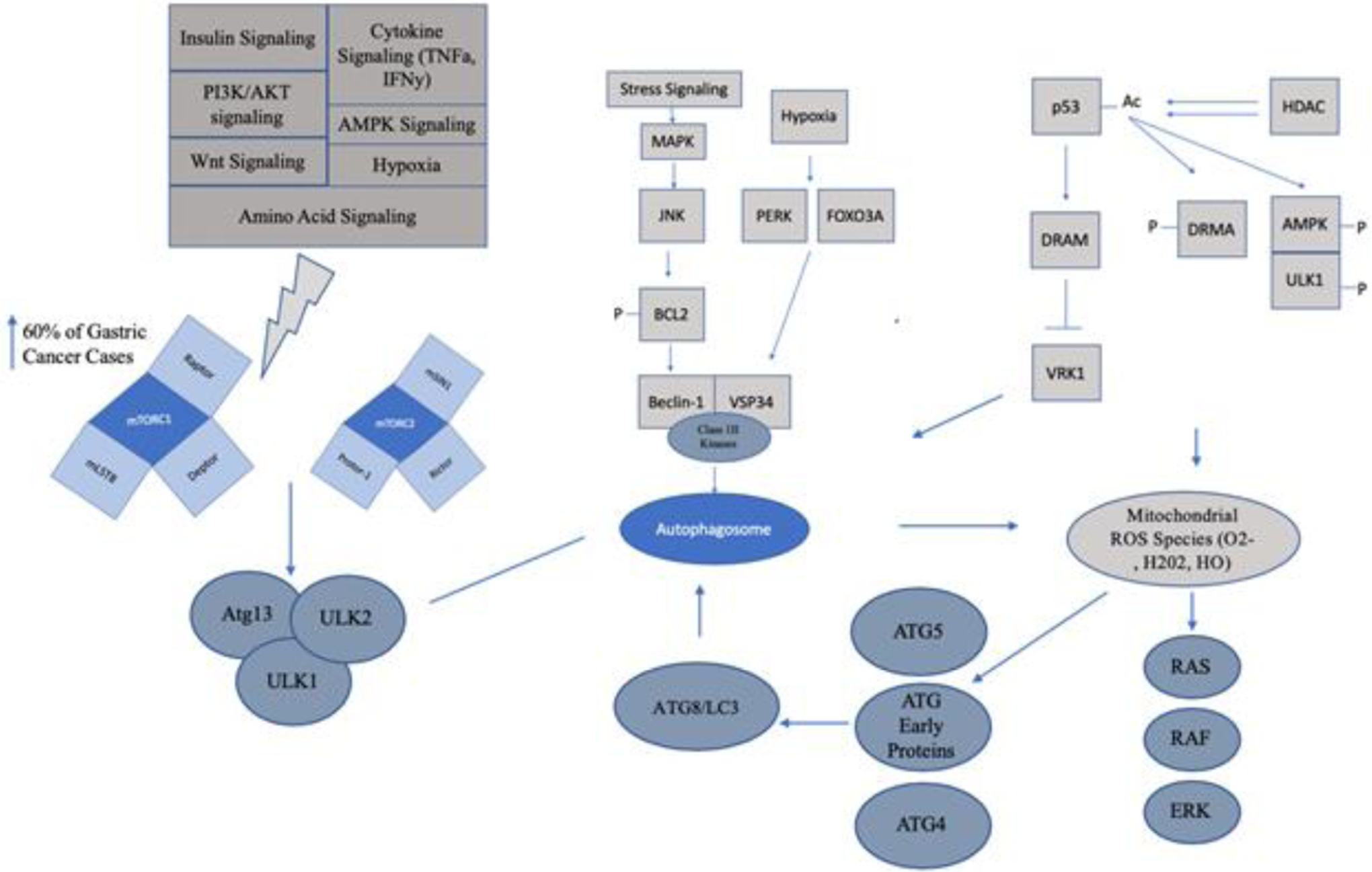
Autophagy occurs in mTOR dependent and mTOR independent manners as well as through activation of ROS species. Gastric cancer has been found to have autophagy activation in all of these manners. Targeting autophagy is being investigated both with mTOR inhibition, hypoxic gene inhibition or deacetylation inducing compounds (See Table 2).
Beclin
Beclin-1 is a Bcl-2 homologous protein (BH3 domain) and works as a key molecule in autophagy mediation. The structure of Beclin consists of three structural domains: BH3 domain (N-terminal located), coiled-coil domain and conserved domain (171). Due to the homologous structure to Bcl-2 like proteins, Beclin is involved in both autophagy and apoptosis. Through apoptosis, Beclin-1 signaling can inhibit apoptosis through interaction with either TRAIL or Bid. The high expression of this protein also has been shown to protect against chemotherapy, irradiation, immunotherapy and other small molecule inhibitors such as angiogenic agents (172). Through a signaling cascade, Beclin-1 is associated at the ER membrane through interaction and first associates with ATG14, VSP15 and VPS34 (PI3K-III complex) causing the autophagy cascade to begin via a phagophore, which begins to isolate the damaged cellular organelles or components with the cellular membrane, and fuses completely with the membrane to make an isolated body or autophagosome. After initiation of the autophagosome, the entity is activated by ULK1/2 phosphorylation and begins to fuse with the lysosome for degradation (173). Depending on the type of signal binding to the PI3K-III complex there can be inhibition of the lysosomal degradation, such as binding with Rubicon or UVRAG (173). Beclin-1 can also be initiated within the PI3K-III complex proteins through phosphorylation of other kinases including AMPK, SAPK and PGK1. Phosphorylation events depends on the individual stress signals such as cellular stress or glutamine depravation (173). Beclin-1 expression has been found to be high in early stage disease with later stage disease exhibiting lower expression of Beclin-1 and correlated with inflammatory cytokine infiltration of the tumor and is hypothesized to be mediated by IFN-y signaling (174). Beclin-1 was also found to be overexpressed in intestinal-type (well-differentiated) tumors and was correlated to poor prognosis (175). It is clear that the role of Beclin-1 remains elusive as its expression varies between stage of disease and subtype and further investigation is needed to fully elucidate its role within gastric cancer.
Inhibiting Beclin-1 has been shown with compounds such as Spautin-1, a PI3KCIII/VSP34 complex inhibitor, which targets Beclin-1 induced autophagy (Table 2) (176). Xestosponging B can also disrupt interactions between INS (1, 4, 5) P3 membrane cargo loading and Beclin-1 (176). These compounds have been tested preclinically in gastric cancer and have shown some therapeutic benefit in gastric cancer cell lines. According to the TCGA database, there are no Beclin-1 (BECN1) mutations found within their gastric cancer cohort suggesting that targeting Beclin-1 is not only feasible but this strategy may be met initially with less therapeutic resistance due to its wild type status. As we have previously stated, intestinal type gastric tumors have high expression of Beclin-1 compared to other subtypes and it would be interesting to see whether the highly expressing Beclin-1 gastric cancer population would receive any therapeutic benefit of these preclinical compounds.
Table 2: Autophagy can be targeted through a variety of small molecule inhibitors.
Literature search found a variety of small molecule compounds can target autophagy at various stages of the process. Autophagy can be targeted via PI3K/mTOR, Beclin, BCL2, lysosomal cellular components or p62. Targeting can either be beneficial (anti-cancerous) or harmful (precancerous) depending on the subtype of gastric cancer and the driver mutations within the tumor. Many compounds have been tested in clinical trials in various solid tumors but further work needs to be done to elucidate the effect specifically in gastric cancer.
| AUTOPHAGY TARGET GENE | COMPOUND | CLINICAL TRIAL VIA CLINICALTRIAL.GOV |
|---|---|---|
| Beclin-1/PI3KCIII/VSP34 | Spautin-1 | No- Preclinical176 |
| Beclin-1/INS (1, 4, 5) P3 | Xestosponging B | No- Preclinical 176 |
| Beclin-1 | Tat-beclin 1 (Tat-BECN1) | No |
| p62/FOXO3A | Selinexor | Planned |
| mTOR1/2 | Everolimus | Yes (22 Studies GC) 193 |
| mTOR1/2 | Temsirolimus | Yes (21 Studies GC) 193 |
| mTOR | CC-223 (Onatasertib) | |
| PI3K/mTOR | BMK120 | Yes (Breast Only) |
| PI3K/mTOR | PX-886 | No |
| PI3K/mTOR | XL 147 | Yes (Various Solid Tumors) |
| PI3K/mTOR | WX-037 | Yes (Various Solid Tumors) |
| PDK/mTOR | BYL719 | Yes (1 GC) |
| PDK/mTOR | GDC0032 | Yes (1 GC) |
| PDK/mTOR | INK1117 | Yes (Various Solid Tumors) |
| PI3K/mTOR | P7170 | Yes (Refractory Solid Tumors) |
| PI3K/mTOR | BEZ235 | Yes (Malignant Solid Tumors) |
| PI3K/mTOR | XL765 | Yes (Various Solid Tumors) |
| P13K/mTOR | GDC-0980 | Yes (Breast, NHL, Renal, Endometrial) |
| PI3K/mT0R | SF1126 | Yes (Advanced Metastatic Tumors) |
| PI3K/mT0R | PF-05212384 | Yes (Various Solid Tumors) |
| PI3K/mT0R | PF-4691502 | Yes (Breast Cancer) |
| PI3K/mT0R | VS-558 | No |
| P13K/mTOR | OSI-027 | Yes (Various Solid Tumor/Lymphoma) |
| P13K/mTOR | AZD2014 | Yes (2 Studies GC) |
| P13K/mTOR | AZD8055 | Yes (Various Solid Tumors) |
| P13K/mTOR | MK-2206 | Yes (2 Studies GC) |
| PI3K/mT0R | AZD5363 | Yes (2 Studies GC) |
| PI3K/mT0R | GSK690693 | Yes (2 Hematological Malignancies) |
| PI3K/mT0R | GDC0068 | Yes (2 Studies GC) |
| PI3K | Salidroside | No- Preclinical 204 |
| P13K/mTOR | B-elemene | No- Preclinical 205 |
| P13K/mTOR | B-elemene | No- Preclinical 205 |
| AMPK Phosphorylation | Chichoric acid | No- Preclinical 206 |
| AMPK phosphorylation | Perillaldehyde | No- Preclinical |
| ULK1 | LYN-1604 | No- Preclinical 229 |
| ULK1/ULK2 | SBI-0206965 | No- Preclinical 229 |
| ULK1 | MRT68921 HCL | No- Preclinical 229 |
| ULK1/ULK2 | ULK-101 | No- Preclinical 229 |
| ATG4B | NSC185058 | No- Preclinical 230 |
| ATG4B | Flubendazole | No- Preclinical 230 |
| Lysosome | DC661 | No- Preclinical 231 |
| Lysosome | Lys05 | No- Preclinical 231 |
| Lysosome/PI3K/mTOR | Hydroxychloroquine | No- Preclinical 232 |
| Autophagosome/Lysosome | CA-5f | No |
| Lysosome | Eliglustat | Yes (Gaucher Disease) |
| Autophagy/mitophagy | Liensinine | No- Preclinical 233 |
| PERK | GSK2606414 | No- Preclinical 234 |
| PERK | GSK2656157 | No- Preclinical 235 |
| PERK | ISRIB (Trans-isomer) | No |
| ROS | Acetylcysteine (N-Acetylcysteine) | No- Preclinical 236 |
| BCL2 | Venetoclax (ABT-199) | Yes (Solid Tumors/1 GC) |
| BCL-2 | TW-37 | No- Preclinical 237–238 |
| BCL-2 | BH3I-1 | No |
| BCL-2 | S63845 | No- Preclinical 239 |
| BCL-2 | HA14–1 | No- Preclinical 240 |
| BCL-2 | Navitoclax (ABT-263) | Yes- (Solid Tumors) |
| BCL-2 | ABT-737 | No- Preclinical 241–242 |
| BCL-2/p53 | Pifithrin-u | No- Preclinical 243 |
ATG Proteins
This family of proteins is highly conserved between humans and yeast and they bind with Beclin-1 to induce the autophagosome and can be subdivided into various groups, which has been extensively reviewed in Wesselborg et al (177). From these various subdivisions, it has been found that there are a subset of ATG genes involved in ubiquitination including Atg12, Atg7, Atg16, Atg8, Atg4, Atg2 and Atg5 in Saccharomyces cerevisiae, all of which form epistatic interrelationships during autophagy (178). During autophagy, these various ATG proteins form serine threonine kinase multicomplex subunits that conjugate within the membrane (either within the cytoplasm or on the membrane of the endoplasmic reticulum) to initiate the formation of autophagosomes for the enclosure of autophagosome substrates, such as damaged organelles or viral infections (179). Microtubule-associated protein 1 light chain 3 or LC3, which is part of the Atg8 protein family, is commonly used within research as an autophagic marker. LC3 mainly functions to conjugate phospholipids during autophagy induction. The LC3 protein is cleaved during this process, through cleavage of a C-terminal glycine, and is involved with the closure of the autophagosome (180). The cleavage is normally visualized via Western Blot through two distinct bands indicative of autophagy induction. The ULK complex proteins, such as ULK1 and ULK2, are serine threonine kinases, which is an early activator of autophagy, and are homologous to Atg1 proteins in Saccharomyces cerevisiae. It was previously thought that AMPK autophagy was linked through only mTOR phosphorylation cascade. It was found that there is an alternative pathway of autophagic induction through AMPK phosphorylation of ULK1/Atg13/FIP200 complex inhibiting the mTOR autophagy induction pathway (181). Class III phosphoinositide 3-kinases or PI3Ks are another member of the ATG family of proteins. The only known Class III PI3K is Vps34, which stands for vacuolar sorting protein. This protein is responsible for mTOR activation, which we will describe below, and is a main cellular sensor for nutrient availability (182). Various members of the Atg protein family have been found to be upregulated in gastric cancer. ULK1 has been found to be overexpressed in gastric cancer in both patients and cell line models, and has been shown to correlate with cancer relapse rates (183–184). This may be due to the autophagic response being a survival mechanism for gastric cancer cells. LC3 proteins were found to be overexpressed within the cytoplasm in a small gastric cohort and inhibition of the autophagic process lead to a sensitization to PD-L1 therapy (185).
p62
P62/SQSTM1 (Sequestosome-1) is a protein involved with both autophagy and the ubiquitin protease system (UPS). While p62 is mainly involved in the aggregation of ubiquitin tagged proteins, during autophagic events p62 is normally found to be downregulated. Although it can be used to measure autophagy within a cell, there are other factors that can cause p62 expression fluxuations including mTOR signaling or Nrf2 transcriptional activation (186). P62 has been found to be a prognostic marker in gastric cancer within the tubular and gastric adenocarcinomas found to be expressed in the nucleus or cytoplasm. Kim et al. has found that high cytoplasmic p62 and low nuclear p62 is correlated for poor prognosis and survival (187). Interestingly, p62 has a nuclear export sequence (NES) meaning its subcellular localization is controlled by an interaction with XPO1 (formally known as CRM1 or chromosome region maintenance protein-1). Nuclear retention of p62 may be a reason as to why XPO1 inhibitory therapy shows efficacy as a single agent therapy, work our lab has previously shown, and has the potential to synergize with various cytotoxic compounds that are normally ineffective in eliciting a therapeutic response due to autophagy (188). Sequestration of p62 into the nucleus may also cause sensitization to immunotherapeutic agents such as PD-L1 inhibitors (188).
mTOR Dependent Signaling Pathways
mTOR signaling is a cascade of serine/threonine kinases that are part of the phosphatidylinositol kinase related kinase (PIKK) family (189). Mainly, the signaling involved within this pathway influences protein synthesis, cell proliferation, lipid synthesis, immunologic responses and autophagy (190). The two main components of mTOR signaling are mTORC1 and mTORC2. These proteins are composed of multiprotein subunits, which include a catalytic subunit and other associated factors. For example, mTORC1 associates with proteins such as Raptor, Deptor and mLST8 where as mTORC2 associates with Rictor, mSIN1 and Protor-1 (190). Signaling pathways which mTOR activates or is activated by includes insulin signaling, via the IGF-1/AKT pathway, PI3K/AKT signaling, AMPK signaling, hypoxia signaling, amino acid signaling, Wnt signaling, and cytokine signaling through TNFa or IFNy (191). In normal cells, mTOR signaling is mainly present to sense dangerous cellular conditions such as nutrient starvation and regulating cellular functions, such as proliferation, as needed. Stimulation of mTOR signaling induces phosphorylation/inactivation of Atg13, ULK1 and ULK2 (192–193).
Many types of malignancies have either mutations or upregulation of mTORC1, which results in either the failure of the ULK1-Atg13-FIP200 complex formation or upregulation of mTOR activity (194). Upregulation of mTORC2 has also been observed, which directly inhibits mTORC1 and thus all downstream signaling cascades (195). Depending on the tumor type, autophagy plays a pro or anti carcinogenic role, which is normally dependent on the stage of disease; autophagy has an anti-pathogenic role within early stages and is normally protective during advanced disease (196–197). mTORC1 deregulation can also interfere with normal signaling of death-associated protein 1 (DAP1), WD repeat domain phosphoinositide-interacting protein 2 (WIPI2) and signaling to the Atg18 ortholog (198). In gastric cancer, it was found that mTOR is active in ~60% of patients and may be a poor prognostic factor (199). Interestingly, it was found that some poorly differentiated gastric cancer patients (diffuse subtype) are sensitive to mTOR inhibition using PDX-derived cell models (199). Within the intestinal subtype, normally initiated through H. pylori signaling, the exposure of normal gastric cells to the VacA protein causes induction of autophagy in early stages of malignancy (premalignant to primary tumor) whereas the prolonged exposure through the sequence of pathogenicity prevents normal induction of autophagy through the disruption of normal autolysosome formation (200). It is clear that autophagy plays a complex role within gastric cancer development and more work is needed to fully elucidate this mechanism.
A variety of mTOR inhibitors exists are being tested in gastric cancer. The most notable includes Everolimus and temsirolimus. According to the GRANITE-1 trial, Everolimus showed some clinical benefit compared to placebo (201) and has been FDA approved to treated advanced gastric cancer that has not responded to VEGF inhibitors (i.e. sunitinib). Although there has been approval, Everolimus is known to induce autophagy in a variety of tumors. Due to the elusive nature of the autophagic process within cancer, upregulation of autophagy can confer resistance to Everolimus in distinct mantle cell lymphoma (MCL) populations and thus downregulating autophagy can overcome this resistance (202). Based on this rationale, some groups have found ways to inhibit autophagy within gastric cancer using compounds such as hydroxychloroquine. Hydroxychloroquine inhibits autophagy via alterations in intracellular pH, which causes disruptions within the lysosome and does not allow for the fusion of the autophagosome to the lysosome. It was found that inhibition of hypoxic signaling, via VEGFR2, in combination with hydroxychloroquine can enhance the therapeutic effect and thus killing of gastric cancer cells (203). Salidroside, a natural ingredient derived from the Rhodiola rosea species decreased the phosphorylation of PI3K and thus induced autophagy (204). Induction of autophagy caused a protective effect towards the gastric cells and the addition of hydroxychloroquine caused cell induced apoptosis (204). In experimental models, the discrepancy between gastric subtypes and roles of autophagy has been observed. It was found that in two distinct diffuse gastric cancer cell lines, MGC803 (primary poorly differentiated mucoid adenocarcinoma) and SGC7901 (depressed subtype 3–4 according to the Bormann classification), that inhibition of autophagy caused cellular apoptosis after treatment with the natural compound B-Elemene (a PI3K/mTOR inhibitor) and either Beclin 1 knockdown or 3-methyladenine or chloroquine (205).
As has been extensively shown the protective effect autophagy has on gastric cancer cells, there has been equal publications showing autophagy induction causes apoptosis. Chichoric acid, which inhibits AMPK phosphorylation, induced expression of LC3II cleavage and caused significant ER stress leading to prevention of gastric cancer progression (206). Perillaldehyde, an oil found in Perilla frutescens, has also been shown to inhibit AMPK phosphorylation and induce autophagy and reduction of cellular growth in mouse gastric cancer cells (207). Although inhibition of AMPK has been shown to induce anti-cancerous induction of apoptosis, this is not always the case (208). Understanding the role of autophagy within gastric cancer and identification of distinct subsets of patients that would benefit from mTOR inhibition alone or with the addition of an autophagy inhibitor is needed to enhance therapeutic benefit and overall survival outcomes.
mTOR Independent Signaling Pathways
Autophagy within cancer can also be activated through mTOR independent mechanisms caused by stress induced signaling, such as starvation, DNA damage and hypoxia. The well-studied tumor suppressor p53 (tumor protein 53) has been shown to induce autophagy in a DRAM-dependent manner. DNA-damage regulated autophagy modulator 1 (DRAM) is a transcription factor that localizes to the lysosome and is located in a downstream pathway of p53 signaling. After induction of DNA damage, inhibition of the ubiquitin-proteasome system and upregulation of p53, by phosphorylation of MDM2, activation of the transcription factor DRAM ensues. DRAM inhibits VRK1 (vaccina-related serine/threonine kinase 1) causing cell cycle arrest and signaling for autophagy via the activation of autophagosome formation (209). This effect is enhanced by HDAC acetylation. Mrakovcic et al found that DNA acetylation induced p53-mediated autophagic cell death through activation of p53/DRMA and p53/AMPK/ULK1 phosphorylation whereas deacetylation reversed this phenotype (210). Starvation signals can activate MAPK signaling via Jnk (c-Jun N-Terminal Kinase) which can activate autophagy through phosphorylation of BCL2 and thus Beclin-1 association with Class III Kinases, such as Vsp34 and induction of the autophagosome (211). Hypoxia can induce PERK or FOXO3, which causes induction of the autophagosome (212). One common factor found within mTOR independent autophagy is the accumulation of reactive oxygen species (ROS) (213).
ROS results from the accumulation of different chemical species including superoxide anions (O2−), hydrogen peroxide (H202) and hydroxyl radicals (HO) within the mitochondria (214). ROS can induce autophagy in an mTOR independent manner through the introduction of cellular DNA damage. The DNA damage induces upregulation of p53 within the nucleus causing the upregulation of BAX, downregulation of BCL2 and freeing of Beclin1 do induce autophagy. ROS can also signal for the activation of ATG proteins, ATG4 and ATG5, which causes the upregulation of ATG8/LC3 and thus autophagy. Furthermore, ROS can activate the RAS/RAF signaling to induce autophagy through ERK/JNK signaling (215). In gastric cancer, it has been found that targeting ROS elicits an anti-tumor effect on gastric cancer cells in an ambiguous manner, as has been found for targeting autophagy as a whole. For example, the curcumin analog WZ26 has shown to have anti-proliferative effects by inducing ER stress and thus ROS activated autophagy through JNK mitochondrial pathway (216). Wogonin, an O-methylated flavone, has been shown to elicit synergy with paclitaxel and cisplatin in gastric cancer cell lines. Although the authors only looked at apoptosis as a means of cell death, a publication of this compound in pancreatic ductal adenocarcinoma (PDAC) showed wogonin is an inhibitor of ROS mediated autophagy, which lead to cellular death through apoptosis (217–218).
Conclusion
Gastric Cancer is a heterogenous disease that affects a large number of individuals per year and remains an unmet clinical problem. Gastric cancer far more acutely impacts populations in developing nations. Improvement in hygiene and eradication of H. Pylori has significantly improved that statistics of gastric cancer in developing nations. Nevertheless, there is a long way to go in terms of improving on the dismal survival statistics of advanced and metastatic gastric cancer. Although there are very few therapies that provide substantial benefit to survival, many different emerging therapies and targets are being evaluated in patients and are described in this review. As research moves forward, hopefully there will be more progress into uncovering of more effective therapeutic options to improve on the survival statistics of this deadly disease.
Funding:
Work in the lab of ASA is supported by NIH R37CA215427 and SKY Foundation Inc.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interests: The authors have no conflict to declare.
References
- 1.Prashanth R; Barsouk A Epidemiology of gastric cancer: global trends, risk factors and prevention. Przeglad Gastroenterologiczny. 2019; 14(1): 26–38. DOI: 10.5114/pg.2018.80001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Key Statistics About Stomach Cancer. Cancer.org Accessed 3/6/2020.
- 3.Chen YC; Fang WL; Wang RF; Liu CA; Yang MH; Lo SS; Wu CW; Li AF; Shyr YM; Huang KH Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol Oncol Res. 2016; 22(1): 197–202. DOI: 10.1007/s12253-015-9996-6. [DOI] [PubMed] [Google Scholar]
- 4.Hu B; Hajj N; Sittler S; Lammert N; Barnes R; Meloni-Ehrig A Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3(3):251–261. doi: 10.3978/j.issn.2078-6891.2012.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y; Xue XW; Luo YF; Wu HW; Chen J; Zhou WX Clinicopathologic features of gastric adenocarcinoma based on the revised Lauren’s classification. Zhonghua Bing Li Xue Za Zhi. 2018; 47(7): 486–91. DOI: 10.3760/cma.j.issn.0529-5807.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Miaozhen Q; Zhou Y; Zhang X; Wang Z; Wang F; Shao J; Lu J; Jin Y; Wei X; Zhang D; et al. Lauren classification combined with HER2 status is a better prognostic factor in Chinese gastric cancer patients. BMC Cancer. 2014; 14(823). DOI: 10.1186/1471-2407-14-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang YX; Deng JY; Guo HH et al. Characteristics and prognosis of gastric cancer in patients aged ≥ 70 years. World J Gastroenterol. 2013;19(39):6568–6578. doi: 10.3748/wjg.v19.i39.6568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrelli F; Berenato R; Turati L; Mennitto A; Steccanella F; Caporale M; Dallera P; de Braud F; Pezzica E; Di Bartolomeo M et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. Journal of Gastrointestinal Oncology. 2017; 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rugge M; Fassan M; Graham DY Epidemiology of Gastric Cancer. Gastric Cancer. 2015. 23–34.25209115 [Google Scholar]
- 10.Han JP; Hong SJ; Kim HK Long-term outcomes of early gastric cancer diagnosed as mixed adenocarcinoma after endoscopic submucosal dissection. JGH. 2014; 30(2): 316–320. DOI: 10.1111/jgh.12838. [DOI] [PubMed] [Google Scholar]
- 11.Stahl P; Seeschaaf C; Lebok P; et al. Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol. 2015;15:7 Published 2015 Feb 5. doi: 10.1186/s12876-015-0231-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae JM; Kim EH Epstein-Barr Virus and Gastric Cancer Risk: A Meta-analysis with Meta-regression of Case-control studies. J Prev Med Public Health. 2016; 49(2): 97–107. DOI: 10.3961/jpmph.15.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wroblewski LE; Peek RM; Wilson KT Helicobacter pylori and Gastric Cancer: Factors that Modulate Disease Risk. Clinical Microbiology Reviews. 2010; 23(4): 713–39. DOI: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cats A; Meuwissen SG; Forman D; Craanen ME; Kuipers EJ Helicobacter pylori: a true carcinogen? Eur J Gastroenterol Hepatol. 1998; 10(6): 447–50. DOI: 10.1097/00042737-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Conteduca V; Sansonno D; Lauletta G; Russi S; Ingravallo G; Dammacco FH pylori infection and gastric cancer: State of the art (Review). International Journal of Oncology. 2012. DOI: 10.3892/ijo.2012.1701. [DOI] [PubMed] [Google Scholar]
- 16.Rick JR; Goldman M; Semino-Mora C; Liu H; Olsen C; Rueda-Pedraza E; Sullivan C; Dubois A In situ expression of cagA and risk of gastroduodenal disease in Helicobacter pylori infected children. J Pediatr Gastroenterol Nut. 2010; 50(2): 167–72. DOI: 10.1097/MPG.0b013e3181bab326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatakeyama M Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004; 4(9): 688–94. DOI: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 18.Wroblewski LE; Peek RM Jr; Wilson KT Helicobacter pylori and Gastric Cancer: Factors that Modulate Disease Risk. Clinical Microbiology Reviews. 2010; 713–39. DOI: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones KR; Joo YM; Jang S; Yoo YJ; Lee HS; Chung IS; Olsen CH; Whitmire JM; Merrell S; Cha JH Polymorphism in the CagA EPIYA Motif Impacts Development of Gastric Cancer. Journal of Clinical Microbiology. 2009: 959–68. DOI: 10.1128/JCM.02330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KE Gastric Cancer in Korean Americans: Risks and reductions. Korean Korean Am Stud Bull. 2003; 13(1/2): 84–90. [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y Association between helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology (New York, N.Y. 1943). May/2016;150(5):1113–1124.e5. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Iizas H; Nanbo A; Nishikawa J; Yoshiyama H Epstein Barr virus (EBV)-associated gastric carcinoma. Viruses. 2012; 4(12): 3420–39. DOI: 10.3390/v4123420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YS; Paik SR; Kim HK; Yeom BW; Kim I; Lee D Epstein Barr Virus and CD21 Expression in Gastrointestinal Tumors. Pathology. 1998; 194(1): 705–711. DOI: 10.1016/S0344-0338(98)80130-1. [DOI] [PubMed] [Google Scholar]
- 24.Kirschner AN; Omerovic J; Popov B; Longnecker R; Jardetzky TS Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. J Virol. 2006;80(19):9444–9454. doi: 10.1128/JVI.00572-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao J; Palefsky JM; Herrera R; Berline J; Tugizov SM The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells. Virology. 2008;370(2):430–442. doi: 10.1016/j.virol.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogelaar IP; van der Post RS; Bisseling TM; van Krieken JHJ; Ligtenberg MJ; Hoogerbrugge N Familial gastric cancer: detection of a hereditary cause helps to understand its etiology. Hered Cancer Clin Pract. 2012;10(1):18. doi: 10.1186/1897-4287-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josefson D Prophylactic gastrectomy seems to extend life of patients with a family history of stomach cancer. BMJ. 2001; 322(7302): 1566. [Google Scholar]
- 28.Ferro A; Rosato V; Rota M; Costa AR; Morais S; Pelucchi C; Johnson KC; Hu J; Palli D; Ferraroni M et al. Meat Intake and risk of gastric cancer in the Stomach cancer Pooling (StoP) project. Int J. Cancer 2019. DOI: 10.1002/ijc.32707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islami F; Boffetta P; Ren JS; Pedoeim L; Khatib D; Kamangar F High-temperature beverages and Foods and Esophageal Cancer Risk- A systematic review. Int J Cancer. 2009; 125(3): 491–524. DOI: 10.1002/ijc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu ML; Yeh KT; Lin PM; Hsu CM; Hsiao HH; Liu YC; Lin HY; Lin SF; Yang MY Deregulated expression of circadian rhythm clock genes in gastric cancer. BMC Gastroenterol. 2014; 14:67 DOI: 10.1186/1471-230x-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Praud D; Rota M; Pelucchi C; Bertuccio P; Rosso T; Galeone C; Zhang ZF; Matsuo K, Ito H; Hu J; et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. European Journal of Cancer Prevention. 2018; 27(2): 124–33. DOI: 10.1097/CEJ.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 32.Amin MB; Greene FL; Edge SB; Compton CC; Gershenwald JE; Brookland RK; Meyer L; Gress DM; Byrd DR; Winchester DP The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017; 67(2): 93–99. DOI: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 33.Bollschweiler E; Berlth F; Baltin C; Mönig S; Hölscher AH Treatment of early gastric cancer in the Western World. World J Gastroenterol. 2014;20(19):5672–5678. doi: 10.3748/wjg.v20.i19.5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakahara T; Ueno N; Maeda T; Kanemitsu K; Yoshikawa T; Tsuchida S; Toyokawa A Impact of Gastric Cancer Surgery in Elderly Patients. Oncology. 2018; 94(2): 79–84. DOI: 10.1159/000481404. [DOI] [PubMed] [Google Scholar]
- 35.Cha JH; Jang JS correlation between healing type of lesion and recurrence in gastric neoplastic lesions after endoscopic submucosal dissection. Turk J Gastroenterol. 2020. 31(1): 36–41. DOI: 10.5152/tjg.2020.18764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasako M; Sakuramoto S; Katai H; Kinoshita T; Furukawa H; Yamaguchi T; Nashimoto A; Fujii M; Nakajima T; Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. Journal of clinical oncology. 2011;29(33):4387–4393. DOI: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 37.Gastric Cancer. National Comprehensive Cancer Network (NCCN) Guidelines. Verison 1.2020. March 19, 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- 38.Jayanathan M; Erwin RP; Molacek N; Fluck M; Hunsinger M; Wild J; Arora TK; Shabahang MM; Franko J; Blansfield JA MAGIC versus MacDonald treatment regimes for gastric cancer: Trends and predictors of multimodal therapy for gastric cancer using the National Cancer Database. American Journal of Surgery. 2019. DOI: 10.1016/j.amjsurg.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz GK; Christman K; Saltz L; Casper E; Quan V; Bertino J; Martin DS; Colofiore J; Kelsen D A phase I Trial of modified, dose intensive FAMTX regimen (High dose 5-fluorouracil + doxorubicin + high dose methotrexate + leucovorin) with oral uridine rescue. ACS Journals. 1996. DOI: . [DOI] [PubMed] [Google Scholar]
- 40.Webb A; Cunningham D; Scarffe JH; Harper P; Norman A; Joffe JK; Hughes M; Mansi J; Findlay M; et al. Randomized trial comparing epirubicin, cisplatin and fluorouracil versus fluorouracil, doxorubicin and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997; 15(1): 261–7. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham D; Starling N; Rao S; Iveson T; Nicolson M; Coxon F; Middleton G; Daniel F; Oates J; Norman AR Capecitabine and Oxaliplatin for Advanced Esophagogastric cancer. N Engl J Med. 2008; 358: 36–46. [DOI] [PubMed] [Google Scholar]
- 42.Al-Batran SE; Homann N; Pauligk C; Goetze TO; Meiler J; Kasper S; Koop HG; Mayer F; Haag GM; Luley K; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, rescectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomized, phase 2/3 trial. Lancet. 2019; 393(10184): 1948–57. DOI: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 43.Masuishi T; Kadowaki S; Kondo M; Komori A; Sugiyama K; Mitani S; Honda K; Narita Y; Taniguchi H; Ura T et al. FOLFOX as First-line Therapy for Gastric Cancer with Severe Peritoneal Metastasis. Anticancer Res. 2017; 37(12): 7037–42. DOI: 10.21873/anticanres.12174. [DOI] [PubMed] [Google Scholar]
- 44.Ahn HS; Jeong SH; Son YG; Lee HJ; Im SA; Bang YJ; Kim HH; Yang HK Effect of neoadjuvant chemotherapy on postoperative morbidity and mortality in patients with locally advanced gastric cancer. Br J Surg. 2014; 101(12): 1560–5. DOI: 10.1002/bjs.9632. [DOI] [PubMed] [Google Scholar]
- 45.Al-Batran S-E, Hofheinz RD, Schmalenberg H; Strumberg D; Goekkurt E; Angermeier S; Zander T; Potenberg J; Koop HG; Pink D; et al. Perioperative ramucirumab in combination with FLOT versus FLOT alone for resectable esophagogastric adenocarcinoma (RAMSES/FLOT7): Results of the phase II-portion—A multicenter, randomized phase II/III trial of the German AIO and Italian GOIM. J Clin Oncol. 2020; 38(15). [Google Scholar]
- 46.Hofheinz RD; Haag GM; Ettrich TJ; Borchert K; Kretzschmar A; Teschendorf C; Siegler GM; Ebert MP; Goekkurt E; Welslau M; et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2 positive resectable esophagogastric adenocarcinoma: Final results of the PETRARCA multicenter randomized phase II trail of the AIO. J Clin Oncol. 2020; 35(18). [Google Scholar]
- 47.Bang YJ; Kim YW; Yang HK; Chung HC; Park YK; Lee KH; Kim YH; Noh SI; Cho JY; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomized controlled trial. The Lancet (British edition). 2012;379(9813):315–321. DOI: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 48.Bang YJ; Van Cutsem E; Feyereislova A; Chung HC; Shen L; Sawaki A; Lordick F; Ohtsu A; Omuro Y; Satoh T et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. The Lancet. 2010; 376(9742): 687–97. DOI: 10.1016/S0140-6736(10)6121-X [DOI] [PubMed] [Google Scholar]
- 49.Abrahao-Machado LF; Scapulatempo-Neto C HER2 testing in gastric cancer: An update. World J Gastroenterol. 2016; 22(19): 4619–4625. DOI: 10.3748/wjg.v22.i19.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.An Cutsem E; Muro K; Cunningham D; Bodoky G; Sobrero A; Cascinu S; Ajani J; Al-Batran SE; Wainberg ZA; Wijayawardana SR et al. Biomarker analyses of second-line Ramucirumab in patients with advanced gastric cancer from RAINBOW, a global, randomized, double-blind, phase 3 study. Eur J Cancer. 2020; 127: 150–57. DOI: 10.1016/j.ejca.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 51.FDA grands accelerated approval to pembrolizumab for first tissue/site agnostic indication. FDA. Accessed July 28, 2020G [Google Scholar]
- 52.Fuchs CS; Doi T; Jang RW; Muro K; Satoh T; Machado M; Sun W; Jalal SI; Shah MA; Metges JP; et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4:e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.FDA approves pembrolizumab for adults and children with TMB-H solid tumors. FDA. Accessed July 25, 2020. [Google Scholar]
- 54.Makiyama A; Arimizu K; Hirano G; Makiyama C; Matsushita Y; Shirakawa T; Ohmura H; Komoda M; Uchino K; Inadomia K; et al. Irinotecan monotherapy as third-line or later treatment in advanced gastric cancer. Gastric Cancer. 2018; 21(3): 464–72. DOI: 10.1007/s10120-017-0759-9. [DOI] [PubMed] [Google Scholar]
- 55.Bando H; Doi T; Muro K; Yasui H; Nishina T; Yamaquchi K; Takahashi S; Nomura S; Kumo H; Shitara K; et al. A multicenter phase II study of TAS-102 monotherapy in patients with pretreated advanced gastric cancer (EPOC1201). DOI: 10.1016/j.ejca.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Hansson LE; Sparén P; Nyrén O Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Ann Surg. 1999;230(2):162–169. doi: 10.1097/00000658-199908000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson FM; Gray NS Kinase inhibitors: the road ahead. Nature Reviews Drug Discovery. 2018; 17:353–77. [DOI] [PubMed] [Google Scholar]
- 58.Clinicaltrials.gov. A Phase II Trial of STI571 in the Treatment of Metastatic Gastric Cancer. Identifier: NCT00068380
- 59.Bang YJ; Kang YK; Kang WK; Boku N; Chung HC; Chen JS; Doi T; Sun Y; Shen L; Qin S; et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011; 29(6): 1449–58. DOI: 10.1007/s10637-010-9438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gotink KJ; Broxterman HJ; Labots M; de Haas RR; Dekker H; Honeywell RJ; Rudek MA; Beerepoot LV; Musters RJ; Jansen G; et al. Lysosomal Sequestration of Sunitinib: A Novel Mechanism of Drug Resistance. Clin Cancer Res. 2011; 17(23):7337–46. DOI: 10.1158/1078-0432.CCR-11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo T; Hajdu M; Agaram NP; Shinoda H; Veach D; Clarkson BD; Maki RG; Singer S; DeMatteo RP; Besmer P; et al. Mechanisms of sunitinib resistance in gastrointestinal stromal tumors harboring KITAY502–3ins mutation: an in vitro mutagenesis screen for drug-resistance. Clin Cancer Res. 2009; 15(22): 6862–70. DOI: 10.1158/1078-0432.CCR-09-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clinicaltrials.gov. Docetaxel With or Without Vandetanib in Treating Patients With Metastatic Stomach Cancer. Identifier: NCT00683787
- 63.Boland PM; Meyer JE; Berger AC; Cohen SJ; Neuman T; Cooper HS; Olszanski AJ; Davey M; Cheng JD; Lebenthal A; et al. Induction therapy for Locally Advanced, Resectable Esophagogastric Cancer: A Phase I trial of Vandetanib (ZD6474), Paclitaxel, Carboplatin, 5-Fluorouracil and Radiotherapy Followed by Resection. Am J Clin Oncol. 2018; 40(4): 393–98. DOI: 10.1097/COC.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie L; Su X; Zhang L; Yin X; Tang L; Zhang X; Xu Y; Gao Z; Liu K; Zhou M; et al. FGFR2 gene amplification in gastric cancer predicts sensitivity to the FGFR inhibitor AZD4547. Clin Cancer Res. 2013; 19(9): 2572–83. DOI: 10.1158/1078-0432.CCR-12-3898. [DOI] [PubMed] [Google Scholar]
- 65.Mitani S; Kawakami H Emerging Targeted Therapies for HER2 Positive Gastric Cancer That Can Overcome Trastuzumab Resistance. Cancers (Basel). 2020; 12(2): 400 DOI: 10.3390/cancers12020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhat KMR; Setaluri V Microtubule-Associated Proteins as Targets in Cancer Chemotherapy. Clin Cancer Res. 2007; 13(10). DOI: 10.1158/1078-043.CCR-06-3040. [DOI] [PubMed] [Google Scholar]
- 67.Huang M; Ma X; Shi H; Hu L; Fan Z; Pang L; Zhu F; Yang X; Xu W; Liu B; et al. FAM83D, a microtubule-associated protein, promotes tumor growth and progression of human gastric cancer. Oncotarget. 2017; 8(43): 74479–93. DOI: 10.18632/oncotarget.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cochran JC; Gatial III JE; Kapoor TM; Gilbert SP Monastrol Inhibition of the Mitotic Kinesin Eg5. JBC. 2005; 280(13): 12658–667. DOI: 10.1074/jbc.M413140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torgovnick A; Schumacher B DNA repair mechanisms in cancer development and therapy. Front Genet. 2015;6: 157 DOI: 10.3389/fgene.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marin JJ; Al-Abdulla R; Lozano E; Briz O; Bujanda L; Banales JM; Macias RI Mechanisms of Resistance to Chemotherapy in Gastric Cancer. Anticancer Agents Med Chem. 2016; 16(3): 318–34. [DOI] [PubMed] [Google Scholar]
- 71.Meehan RS; Chen AP New treatment option for ovarian cancer: PARP inhibitors. Gynecologic Oncology Research and Practice. 2016; 7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nijman SMB Synthetic lethality: General principles, utility and detection using genetic screens in human cells. FEBS Lett. 2011; 585(1): 1–6. DOI: 10.1016/j.febslet.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mo M; Yang J; Zhu X; Zhu J Use of olaparib in patients with advanced gastric cancer. Oncology. 2018; 19(2): 75 DOIL 10.1016/S1470-2045(18)30023-8. [DOI] [PubMed] [Google Scholar]
- 74.Bang YJ; Xu RH; Chin K; Lee KW; Park SH; Rha SY; Shen L; Qin S; Xu N; Im SA; et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomized, placebo-controlled, phase 3 trial. Oncology. 2017; 18(12): 1637–51. DOI: 10.1016/S1470-2045(17)30682-4. [DOI] [PubMed] [Google Scholar]
- 75.Cechini M; LoRusso P; Shyr Y; Ivy P; Sklar J; Dooley K; Lacy J NCI 10066: A phase I-II study of Olaparib in combination with ramucirumab in metastatic gastric and gastroesophageal junction (GEJ) adenocarcinoma. Journal of Clinical Oncology. 2018; 36(15). [Google Scholar]
- 76.Jang JY; Kang YJ; Sung B; Kim MJ; Park C; Kang D; Moon HR; Chung HY; Kim ND MHY440, a Novel Topoisomerase I Inhibitor, Induces Cell Cycle Arrest and Apoptosis via a ROS-Dependent DNA Damage Signaling Pathway in AGS Human Gastric Cancer Cells. Molecules. 2018; 24(1): E96 DOI: 10.3390/molecules24010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yi H; Yan X; Luo Q; Yuan L; Li B; Pan W; Zhang L; Chen H; Wang J; Zhang Y; et al. A novel small molecule inhibitor of MDM2-p53 (APG-115) enhances Radiosensitivity of gastric adenocarcinoma. J Exp Clin Cancer Res. 2018; 37(1): 97 DOI: 10.1186/s13046-018-0765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu QN; Liao YF; Lu YX; Wang Y; Lu JH; Zeng ZL; Huang QT; Sheng H; Yun JP; Xie D; et al. Pharmacological inhibition of DUSP6 suppresses gastric cancer growth and metastasis and overcomes cisplatin resistance. Cancer Lett. 2018; 412: 243–255. DOI: 10.1016/j.canlet.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 79.Fok JH; Ramos-Montoya A; Vazquez-Chantada M; Wijnhoven PWG; Follia V; James N; Farrington PM; Karmokar A; Willis SE; Cairns J; et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nature Communications. 2019; 10(5065). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tamura T; Ohira M; Tanaka H; Muquruma K; Toyokawa T; Kubo N; Sakurai K; Amano R; Kimura K; Shibutani M; et al. Programmed Death-1 ligand-1 (PD-L1) Expression is Associated with the Prognosis of Patients with Stage II/III Gastric Cancer. Anticancer Res. 2015; 35(10): 5369–76. [PubMed] [Google Scholar]
- 81.B; Mondaca S; Sanchez C; Galindo H; Nervi B; Ibanez C; et al. High Proportion of Potential Candidates for Immunotherapy in a Chilean Cohort of Gastric Cancer Patients: Results of the FORCE1 Study. Cancers (Basel). 2019; 11(9): E1275 DOI: 10.3390/cancers11091275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee HS; Chang MS; Yang HK; Lee BL; Kim WH Epstein-Barr Virus-Positive Gastric Carcinoma Has a Distinct Protein Expression Profile in Comparison with Epstein-Barr Virus-Negative Carcinoma. Clin Cancer Res. 2004; 10: 1698–1705. [DOI] [PubMed] [Google Scholar]
- 83.Fuchs CS; Doi T; Jang RW; Muro K; Satoh T; Machado M; Sun W; Jalal SI; Shah MA; Metges JP; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical Keynote-059 Trial. JAMA Oncol. 2018; 4(5):e180013 DOI: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xing X; Guo J; Ding G; Li B; Dong B; Feng Q; Li S; Zhang J; Ying X; Cheng X; et al. Analysis of PD1, PD-L1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. 2017; 7(3): e1356114 DOI: 1080/2162402X.2017.1356144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang YK; Boku N; Satoh T; Ryu MH; Chao Y; Kato K; Chung HC; Chen JS; Muro K; Kang WK; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): a randomized, double-blind, placebo controlled, phase 3 trial. The Lancet. 2017; 390(10111); 2461–71. DOI: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 86.Hara H; Shoji H; Takahari D; Esaki T; Machida N; Nagashima K; Aoki K; Honda K; Miyamoto T; Boku N; et al. Phase I/II study of ramucirumab plus nivolumab in patients in second-line treatment for advanced gastric adenocarcinoma (NivoRam study). Journal of Clinical Oncology. 2019; 37(4). [Google Scholar]
- 87.Xu YH; Li ZL; Qiu SF IFN-y Induces Gastric Cancer Cell Proliferation and Metastasis Through Upregulation of Integrin B3-Mediated NF-kB Signaling. Transl Oncol. 2018; 11(1): 182–92. DOI: 10.1016/j.tranon.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ham IH; Oh HJ; Jin H; Bae CA; Jeon SM; Choi KS; Son SY; Han SU; Brekken RA; Lee D; Hur H Targeting interleukin-6 as a stragety to overcome stroma-induced resistance to chemotherapy in gastric cancer. Molecular Cancer. 2019; 18(68). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Z; Zhang J; Miao L; Liu K; Yang S; Pan C; Jiao B Interleukin-11 Promotes the Progress of Gastric Carcinoma via Abnormally Expressed Versican. Int J Bio Sci. 2012; 8(3): 383–93. DOI: 10.7150/ijbs.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y; Wu H; Wu; Bian Z; Gao Q Interleukin 17A Promotes Gastric Cancer Invasiveness via NF-kB Mediated Matrix Metalloproteinases 2 and 9 Expression. PLoS One. 2014. DOI: 10.1371/journal.pone.0096678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khawar MB; Abbasi MH; Sheikh N IL-32: A Novel Pluripotent Inflammatory Interleukin, towards Gastric Inflammation, Gastric Cancer, and Chronic Rhino Sinusitis. Mediators of Inflammation. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ham IH; Oh HJ; Jin H; Bae CA; Jeon, Sm M; Choi KS; Son SY; Han SU; Brekken RA; Lee D; Hur H Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol Cancer. 2019; 18(1): 68 DOI: 10.1186/s12943-019-0972-8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lazar DC; Avram MF; Romosan I; Cornianu M; Taban S; Goldis A Prognostic significance of tumor immune microenvironment and immunotherapy: Novel insights and future perspectives in gastric cancer. World J Gastroenterol. 2018; 24(32): 3583–3616. DOI: 10.3748/wjg.v24.i32.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhan X; Wang B; Li L; Li J; Wang H; Chen L; Jiang H, Wu M; Xiao J; Peng X; et al. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. Journal of Clinical Oncology. 2019; 37(15). [Google Scholar]
- 95.Clinicaltrials.gov. Intraperitoneal Infusion of EpCAM CAR-T Cell in Advanced Gastric Cancer with Peritoneal Metastasis. Identifier: NCT03563326.
- 96.Lu J; Xu Y; Wu Y; Huang XY; Xie JW; Wang JB; Lin JX; Zheng CH; Huang AM; Huang CM Tumor-inflitrating CD8+ T cells combined with tumor-associated CD68+ macrophages predict postoperative prognosis and adjuvant chemotherapy benefit in resected gastric cancer. BMC Cancer. 2019; 19(920). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee S; Margolin K Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep. 2013; 14(5): 468–74. DOI: 10.1007/s11912-012-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clinicaltrials.gov. Adoptive Transfer of Tumor Infiltrating Lymphocytes for Advanced Solid Cancers. Identifier: NCT03935893.
- 99.Lin HJ Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers (Basel) 2015; 7(4): 2443–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Q; Peng C Cancer-associated fibroblasts regulate the biological behavior of cancer cells and stroma in gastric cancer. Oncol Lett. 2018; 15(1): 691–98. DOI: 10.3892/ol.2017.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sathe A; Grimes SM; Lau BT; Chen J; Suarez C; Huang RJ; Poultsides GA; Ji HP Single cell genomic characterization reveals the cellular reprogramming of the gastric tumor microenvironment. Clin Cancer Res. 2020. DOI: 10.1158/1078-0432.CCR-19-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uppal A; Dehal A; Chang SC; Barrak D; Naeini Y; Jalas JR; Bilchik AJ The Immune Microenvironment Impacts Survival in Western Patients with Gastric Adenocarcinoma. J Gastrointest Surg. 2020; 24(1): 28–38. DOI: 10.1007/s11605-019-04403-w. [DOI] [PubMed] [Google Scholar]
- 103.Jiang Y; Xie J; Huang W; Chen H; Xi S; Han Z; Huang L; Lin T; Zhao LY; Hu YF; et al. Tumor Immune Microenvironment and Chemosensitivity Signature for Predicting Response to Chemotherapy in Gastric Cancer. Cancer Immunol Res. 2019; 7(12): 2065–73. DOI: 10.1158/2326-6066.CIR-19-0311. [DOI] [PubMed] [Google Scholar]
- 104.Protti LF; Soto-Molinari R; Calderon-Osorno M; Mora J; Alpizar-Alpizar W Gastric Cancer in the Era of Immune Checkpoint Blockade. Journal of Oncology. 2019. DOI: 10.1155/2019/107910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuroda K; Yashiro M; Sera T; Yamamoto Y; Kushitani Y; Sugimoto A; Kushiyama S; Nishimura S; Togano S; Okuno T; et al. The clinicopathological significance of Thrombospondin-4 expression in the tumor microenvironment of gastric cancer. PLoS One. 2019; 14(11): e0224727 DOI: 10.1371/journal.pone.0223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu X; Tao P; Zhou Q; Li J; Zhenjia Y; Wang X; Li J; Li C; Yan M; Zhu Z; et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017; 8(13): 20741–50. DOI: 19.18632/oncotarget.15119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shiga K; Hara M; Nagasaki T; Sato T; Takahashi H; Takeyama H Cancer-Associated Fibroblasts: Their characteristics and Their Roles in Tumor Growth. Cancers (Basel). 2015; 7(4): 2443–58. DOI: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan LW; Yamashita H; Seto Y Glucose metabolism in gastric cancer: The cutting edge. World J Gastroenterol. 2016; 22(6): 2046–59. DOI: 10.3748/wjg.v22.i6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang L; Li S Lactic acid promotes macrophage polarization through MCT-HIFa signaling in gastric cancer. Exp Cell Res. 2020: 111846 DOI: 10.1016/j.yexcr.2020.111846. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Q; Wu X; Wang X; Yu Z; Pan T; Li Z; Chang X; Jin Z; Li J; Zhu Z; Liu B; Su L The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-a/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene. 2020; 39(7): 1414–1428. DOI: 10.1038/s41388-019-1078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kemi N; Eskuri M; Kauppila JH Tumour-stroma ratio and 5-year mortality in gastric adenocarcinoma: a systematic review and meta-analysis. Sci Rep. 2019; 9(1): 16018 DOI: 10.1038/s41598-019-52606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y; Chen X; Chen X; Yang X; Song Q; Wu H High SALM3 Expression in Tumor Cells and fibroblasts is Correlated with Poor Prognosis in Gastric Cancer Patients. Dis Markers 2019: 8282414 DOI: 10.1155/2019/8282414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang C; Zhou Y; Huang Y; Wang Y; Wang W; Kuai X Overexpression of ADAMTS-2 in tumor cells and stroma is predictive of poor clinical prognosis in gastric cancer. Hum Pathol. 2019; 84: 44–51. DOI: 10.1016/j.humpath.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 114.Ding X; Ji J; Jiang J; Cai Q; Wang C; Shi M; Yu Y; Zhu Z; Zhang J HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. 2018; 9(9): 867 DOI: 10.1038/s41419-018-0922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang T; Chen M; Yang X; Zhang X; Zhang Z; Sun Y; Xu B; Hua J; He Z; Song Z Down-regualtion of KLF-5 in cancer-associated fibroblasts inhibits gastric cancer cells progression by CCL5/CCR5 axis. Cancer Biol Ther. 2017; 18(10): 806–815. DOI: 10.1080/15384047.2017.1373219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X; Zhou Q; Yu Z; Wu X; Chen X; Li J; Li C; Yan M; Zhu Z, Liu B; Su L Cancer-associated fibroblast-derived Lumican promotes gastric cancer progression via the integrin B1-FAK signaling pathway. Int J Cancer. 2017; 141(5): 998–1010. DOI: 10.1002/ijc.30801. [DOI] [PubMed] [Google Scholar]
- 117.Fujii S; Fujihara A; Natori K; Abe A; Kuboki Y; Higuchi Y; Aizawa M; Kuwata T; Kinoshita T; Yasui W; et al. TEM1 expression in cancer-associated fibroblasts is correlated with a poor prognosis in patients with gastric cancer. Cancer Med. 2015; 4(11): 1667–78. DOI: 10.1002/cam4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muz B; de la Puente P; Azab F; Azab AK The role of hypoxia in cancer progression, angiogenesis, metastasis and resistance to therapy. Hypoxia (Auckl). 2015; 3:83–92. DOI: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun Z; Liu C; Jiang WG; Ye L Deregulated bone morphogenic proteins and their receptors are associated with disease progression of gastric cancer. Comput Struct Biotechnol. 2020; 18:177–88. DOI: 10.1016/j.csbj.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun Z; Cai S; Liu C; Cui Y; Ji J; Jiang WG; Ye L Increased Expression of Gremlin1 Promotes Proliferation and Epithelial Mesenchymal Transition in Gastric Cancer Cells and Correlates With Poor Prognosis of Patients with Gastric Cancer. Cancer Genomics Proteomics. 2020; 17(1): 49–60. DOI: 10.21873/cgp.20167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bae WJ; Ahn JM; Byeon HE; Kim S; Lee D PTPRD-inactivation-induced CXCL8 promotes angiogenesis and metastasis in gastric cancer and is inhibited by metformin. J Exp Clin Cancer Res. 2019; 38(1): 484 DOI: 10.1186/s13046-019-1469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu R; Deng X; Peng Y; Feng W; Xiong R; Zou Y; Lei X; Zheng X; Xie Z; Tang G Synthesis and biological evaluation of novel 5,6,7-trimethyloxy flavonoid salicylate derivates as potential anti-tumor agents. Bioorg Chem. 2020; 96: 103652 DOI: 10.1016/j.bioorg.2020.103652. [DOI] [PubMed] [Google Scholar]
- 123.Zhang L; Liu L; Zhan S; Chen L; Wang Y; Zhang Y; Du J; Wu Y; Gu L Arsenic Trioxide Suppressed Migration and Angiogenesis by Targeting FOXO3a in Gastric Cancer Cells. Int J Mol Sci. 2018; 19(12): E3739 DOI: 10.3390/ijms19123739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lei Z; Chai N; Tian M; Zhang Y; Wang G; Liu J; Tian Z; Yi X; Chen D; Li X; et al. Novel peptide GX1 inhibits angiogenesis by specifically binding to transflutaminase-2 in the tumorous endothelial cells of gastric cancer. Cell Death Dis. 2018; 9(6):579 DOI: 10.1038/s41419-018-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uhlik MT; Liu J; Falcon BL; Iyer S; Stewart J; Celikkaya H; O’Mahony M; Sevinsky C; Lowes C; Douglass L; et al. Stromal-Based Signatures for the Classification of Gastric Cancer. Cancer Research. 2016; 76(9). DOI: 10.1158/0008-5472.CAN-16-0022. [DOI] [PubMed] [Google Scholar]
- 126.Ismail NA; Ragab SH; ElBaky AA; Shoeib ARS; Alhosary Y; Fekry D Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci. 2011; 7(3): 501–507. DOI: 10.5114/aoms.2011/23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bull MJ; Plummer NT Part 1: The Human Gut Microbiome in Health and Diseases. Interg Med (Encinitas). 2014; 13(6): 17–22. [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng HY; Ning MX; Chen DK; Ma WT Interactions Between the Gut Microbiota and the Host Innate Immune Response against Pathogens. Front Immunol. 2019; 10:607 DOI: 10.3389/fimmu.2019.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oliphant K; Allen-Vercoe E Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019; 7(91). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abenavoli L; Scarpellini E; Colica C; Boccuto L; Salehi B; Sharifi-Rad J; Aiello V; Romano B; De Lorenzo A; Izzo AA; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients. 2019;11(11):2690 DOI: 10.3390/nu11112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galland L The Gut Microbiome and the Brain. J Med Food. 2014; 17(12): 1261–72. DOI: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shin A; Preidis GA; Shulman R; Kashyap P The Gut Microbiome in Adult and Pediatric Functional Gastrointestinal Disorders. Clin Gastroenterol Hepatol. 2018; 17(2): 256–74. DOI: 10.10165/j.cgh.2018.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harsch IA; Konturek PC The Role of Gut Microbiota in Obesity and Type 2 and Type 1 Diabeties Mellitus: New Insights into “Old” Diseases. Med Sci (Basel). 2018; 6(2):32 DOI: 10.3390/medsci6020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gazerani P Probiotics for Parkinson’s Disease. Int J Mol Sci. 2019; 20(17): 4121 DOI: 10.3390/ijms20174121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nagano T; Otoshi T; Hazama D; Kiriu T; Umezawa K; Katsurada N; Nishimura Y Novel cancer therapy targeting microbiome. Onco Targets Ther. 2019; 12: 3619–24. DOI: 10.2147/OTT.S207546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu X; Shao L; Liu X; Ji F; Mei Y; Cheng Y; Liu F; Yan C; Li L; Lin Z Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine. 2019; 40:336–48. DOI: 10.1016/j.ebiom.2018.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu YL; Pang W; Huang Y; Zhang Y; Zhang CJ The Gastric Microbiome is Perturbed in Advanced Gastric Adenocarcinoma Identified Through Shotgun Metagenomics. Front Cell Infect Microbiol. 2018; 8:433 DOI: 10.3389/fcimb.2018.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Spanguolo C; Russo GL; Orhan IE; Habtemariam S; Dagila M; Sureda A; Nabavi SF; Devi KP; Loizzo MR; Tundis R; et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv Nut. 2015; 6(4): 408–19. DOI: 10.3945/an.114.008052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang y; Liu H; Peng Y; Tong L; Feng L; Ma K Characterization of the diethyl phthalate-degrading bacterium Sphingobium yanoikuyae SHJ. Data Breif. 2018; 20:1758–63. DOI: 10.1016/j.dib.2018.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao Q; Hu H; Wang W; Peng H; Zhang X Genome Sequencing of Sphingobium yanoikuyae B1, a Polycyclic Aromatic Hydrocarbon-Degrading Strain. Genome Announc. 2015; 3(1):e01522–14. DOI: 10.1128/genomeA.01522-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Terzi HA; Demiray T; Koroglu M; Cakmak G; Ciftci IH; Ozbek A; Altindis M Intra-Abdominal Abscess and Primary Peritonitis Caused by Streptococcus anginosus. 2016; 9(6): e33863 DOI: 10.5812/jjm.33863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Downes J; Wade WG Peptostreptococcus stomatis sp. nov., isolated from the human oral cavity. Int J Syst Evol Microbiol. 2006; 56(Pt 4): 751–54. DOI: 10.1099/ijs.0.64041-0. [DOI] [PubMed] [Google Scholar]
- 143.Kim KS; Rowlinson MC; Bennion R; Liu C; Talan D; Summanen P; Finegold SM Characterization of Slackia exigua Isolated from Human Wound Infections, Including Abscesses of Intestinal Origin. J Clin Microbiol. 2010; 48(4): 1070–75. DOI: 10.1128/JCM.01576-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baghban A; Gupta S Parvimonas micra: A rare cause of native joint septic arthritis. Anaerobe. 2016; 39: 26–7. DOI: 10.1016/j.anaerobe.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 145.Khan MS; Ishaq M; Hinson M; Potugari B; Rehman AU Parvimonas micra bacteremia in a patient with colonic carcinoma. Caspian J Intern Med. 2019; 10(4): 472–75. DOI: 10.22088/cjim.10.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Coker OO; Dai Z; Nie Y; Zhao G; Cao L; Nakatsu G; Wu WKK; Wong SH; Chen Z; Sung JJY; Yu J Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rousse JM; Bermond D; Piemont Y; Tournoud C; Heller R; Kehrli P; Harlay ML; Monteil H; Jaulhac B Dialister pneumosintes Associated with Human Brain Abscesses. J Clin Microbiol. 2002; 40(10): 3871–73. DOI: 10.1128/JCM.40.10.3871-3873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Contreras A; Doan N; Chen C; Rusitanonta T; Flynn MJ; Slots J The Importance of Dialister pneumosintes in human periodontitis. Oral Microbiology and Immunology. 2001. DOI: 10.1034/j.1399-302x.2000.150410.x. [DOI] [PubMed] [Google Scholar]
- 149.Schulz C; Schutte K; Mayerle J; Malfertheiner P The role of the gastric bacterial microbiome in gastric cancer and beyond. Therapeutic Advances in Gastroenterology. 2019. DOI: 10.1177/1756284819894062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liou JM; Lee YC; El-Omar EM; Wu MS Efficacy and Long-Term Safety of H. pylori Eradication for Gastric Cancer Prevention. Cancers (Basel). 2019; 11(5): E593 DOI: 10.3390/cancers11050593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Romano M; Cuomo A Eradication of Helicobacter pylori: A Clinical Update. MedGenMed. 2004; 6(1): 19. [PMC free article] [PubMed] [Google Scholar]
- 152.Hamilton-Miller JM The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int J Antimicrob Agents. 2003; 22(4): 360–6. DOI: 10.1016/s0924-8579(03)00153-5. [DOI] [PubMed] [Google Scholar]
- 153.Drago L Probiotics and Colon Cancer. Microorganisms. 2019; 7(3): 66 DOI: 10.3390/microorganisms7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Quaresma M; Damasceno S; Monteiro C; Lima F; Mendes T; Lima M; Justino P; Barbosa A; Souza M; Souza E; et al. Probiotic mixture containing Lactobacillus spp. And Bifidobacterium spp. Attenuates 5-fluorouracil-induced intestinal mucositis in mice. Nutr Cancer. 2019: 1–11. DOI: 10.1080/01635581.2019.167519. [DOI] [PubMed] [Google Scholar]
- 155.Prisciandaro LD; Geier MS; Butler RN; Cummins AG; Howarth GS Evidence Supporting the use of Probiotics for the Prevention and Treatment of Chemotherapy-Induced Intestinal Mucositis. Critical Reviews in Food Science and Nutrition. 2011; 51(3): 239–47. DOI: 10.1080/10408390903551747. [DOI] [PubMed] [Google Scholar]
- 156.Cousin FJ; Jouan-Lanhouet S; Dimanche-Boitrel MT; Corcos L; Jan G Milk Fermented by Propionibacterium freudenreichii Induces Apoptosis of HGT-1 Human Gastric Cancer Cells. PLoS One. 2012; 7(3):e31892 DOI: 10.1371/journal.pone.0031892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shimizu N; Wakatsuki T; Murakami A; Yoshioka H; Hamazoe R; Kanayama H; Maeta M; Koga S Carcinoembryonic Antigen in Gastric Cancer Patients. Oncology. 1987; 44:240–44. DOI: 10.1159/000226486. [DOI] [PubMed] [Google Scholar]
- 158.Tierman JP; Perry SL; Verghese ET; West NP; Yeluri S; Jayne DG; Hughes TA Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. J Cancer. 2013; 108(3): 662–7. DOI: 10.1038/bjc.2012.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pavai S; Yap SF The Clinical significance of elevated levels of serum CA 19–9. Med J Malaysia. 2003; 58(5): 667–72. [PubMed] [Google Scholar]
- 160.Wu J; Li G; Wang Z; Yao Y; Chen R; Pu X; Wang J Circulating MicroRNA-21 Is a Potential Diagnostic Biomarker in Gastric Cancer. Dis Markers. 2015:435656 DOI: 10.1155/2015/435656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Iwasaki H; Shimura T; Yamada T; Okuda Y; Natsume M; Kitagawa M; Horike S; Kataoka H A novel urinary microRNA biomarker panel for detecting gastric cancer. Journal of Gastroenterology. 2019; 54: 1061–69. [DOI] [PubMed] [Google Scholar]
- 162.Sexton R; Mahdi Z; Chaudhury R; Beydoun R; Aboukameel A; Khan HY; Baloglu E; Senapedis W; Landesman Y; Tesfaye A; Kim S; Philip PA; Azmi AS Targeting Nuclear Exporter Protein XPO1/CRM1 in Gastric Cancer. Int J Mol Sci. 2019; 20(19): E4826 DOI: 10.3390/ijms20194826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi Y; Wang Z; Zhu X; Chen L; Ma Y; Wang J; Yang X; Liu Z Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer. Int J Clin Oncol. 2020; 25(1): 89–99. DOI: 10.1007/s10147-019-01532-9. [DOI] [PubMed] [Google Scholar]
- 164.Mei JW; Yang ZY; Xiang HG; Bao R; Ye YY; Ren T; Wang XF; Shu YJ MicroRNA-1275 inhibits cell migration and invasion in gastric cancer by regulating vimentin and E-cadherin via JAZF1. BMC Cancer. 2019; 19(1): 740 DOI: 10.1186/s12885-019-5929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Novikova IV; Hennelly SP; Sanbonmatsu KY Sizing up long non-coding RNAs Do lncRNAs have secondary and tertiary structure? Bioarchitecture. 2012; 2(6): 189–99. DOI: 10.4161/bioa.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yao XM; Tang JH; Zhu H; Jing Y High expression of LncRNA CASC15 is a risk factor for gastric cancer prognosis and promote the proliferation of gastric cancer. European Review for Medical and Pharmacological Sciences. 2017; 21:5661–7. [DOI] [PubMed] [Google Scholar]
- 167.Qi D; Wang Q; Wu M; Zhang X Comprehensive bioinformatics analysis of lncRNAs in gastric cancer. Oncology Letters. 2018: 1279–91. DOI: 10.3892/ol.2018.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fei ZH; Yu XJ; Zhou M; Su HF; Zheng Z; Xie CY Upregulated expression of long-noncoding RNA LINC00982 regulates cell proliferation and its clinical relevance in patients with gastric cancer. Tumour Biol. 2016; 37(2): 1983–93. DOI: 10.1007/s13277-015-3979-9. [DOI] [PubMed] [Google Scholar]
- 169.Lin X; Xia Y; Hu D; Mao Q; Yu Z; Zhang H; Li C; Chen G; Liu F; Zhu W; et al. Transcriptome-wide piRNA profiling in human gastric cancer. Oncol Rep. 2019; 41(5): 3089–99. DOI: 10.3892/or.2019.7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martinez VD; Enfield KSS; Rowbotham DA; Lam WL An atlas of gastric PIWI-interacting RNA transcriptomes and their utility for identifying signatures of gastric cancer recurrence. Europe PMC. 2015; 19(2): 660–665. DOI: 10.1007/s10120-015-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cabral GF; Azevedo dos Santos Pinheiro J; Vidal AF; Santos S; Ribeiro-dos-Santos A piRNAs in Gastric Cancer: A New Approach Towards Translational Research. MDPI. 2020; 21(6): 2126 DOI: 10.3390/ijms21062126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kang R; Zeh HJ; Lotze MT; Tang D The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011; 18(4): 571–80. DOI: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Morris DH; Yip CK; Shi Y; Chait BT; Wang QJ Beclin-1VSP34 complex architecture: Understanding the Nuts and bolts of Therapeutic Targets. Front Biol (Beijing). 2015; 10(5): 398–426. DOI: 10.1007/s11515-015-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Galluzzi L; Baehrecke EH; Ballabio A; Boya P; Bravo-San Pedro JM; Cecconi F; Choi AM; Chu CT; Codogno P; Colombo MI et al. Molecular definitions of autophagy and related processes. EMBO J. 2017; 36(13): 1811–1836. DOI: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fei B; Ji F; Chen X; Liu Z; Li S; Mo Z; Fang X Expression and clinical significance of Beclin-1 in gastric cancer tissues of various clinical stages. Oncol Lett. 2016; 11(3): 2271–77. DOI: 10.3892/ol.2016.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Giatromanolaki A; Koukourakis MI; Georgiou I; Kouroupi M; Siviridis E LC3A, LC3B and Beclin-1 Expression in Gastric Cancer. Anticancer Res. 2018; 38(12): 6827–33. DOI: 10.21873/anticanres.13056. [DOI] [PubMed] [Google Scholar]
- 177.Levy JMM; Towers CG; Thorburn A Targeting autophagy in cancer. Nat Rev Cancer. 2017; 17(9): 528–42. DOI: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wesselborg S; Stork B Autophagy signal transduction by ATG proteins: from hierarchies to networks. Cell Mol Life Sci. 2015; 72: 4721–57. DOI: 10.1007/s00018-015-2034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Suzuki K; Noda T; Ohsumi Y Interrelationships among ATG proteins during autophagy in Saccharomyces cerevisiae. 2004. DOI: 10.1002/yea.1152. [DOI] [PubMed]
- 180.Yu L; Chen Y; Tooze SA Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 2018; 14(2): 207–215. DOI: 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cherra III SJ; Kulich SM; Uechi G; Balasubramani M; Mountzouris J; Day BW; Chu CT Regulation of the autophagy protein LC3 phosphorylation. J Cell Bio. 2010; 190(4): 553–39. DOI: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Roach PJ AMPKà ULK1à Autophagy. Mol Cell Biol. 2011; 31(15): 3082–84. DOI: 10.1128/MCB.05565-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Backer JM The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008; 410(1): 1–17. DOI: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 184.Chen MB; Ji XZ; Liu YY; Zeng P; Xu XY; Ma R; Guo ZD; Lu JW; Feng JF Ulk1 overexpression in human gastric cancer is correlated with patients’ T classification and cancer relapse. Oncotarget. 2017;; 8(20): 33704–12. DOI: 10.18632/Oncotarget.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen MB; Ji XZ; Liu YY; Zeng P; Xu XY; Ma R; Guo ZD; Lu JW; Feng JF Ulk1 overexpression in human gastric cancer is correlated with patients’ T classification and cancer relapse. Oncotarget. 2017; 8(20): 33704–12. DOI: 10.18632/oncotarget.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang X; Wu WKK; Gao J; Li Z; Dong B; Lin X; Li Y; Li Y; Gong J; Qi C; Peng Z; Yu J; Shen L Autophagy inhibition enhances PD-L1 expression in gastric cancer. J Exp Clin Cancer Res. 2019; 38:140 DOI: 10.1186/s13046-019-1148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Akin D; Wang SK; Habibzadegah-Tari P; Law B; Ostrov D; Li M; Yin XM; Kim JS; Horenstein N; Dunn WA A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy. 2014; 10(11): 2021–35. DOI: 10.4161/auto.32229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim JS; Bae GE; Kim KH; Lee SI; Chung C; Lee D; Lee TH; Kwon IS; Yeo MK Prognostic significance of LC3B and p62/SQSTM1 Expression in Gastric Adenocarcinoma. Anticancer Res. 2019; 39(12): 6711–6722. DOI: 10.21873/anticanres.13886. [DOI] [PubMed] [Google Scholar]
- 189.Sui X; Chen R; Wang Z; Huang Z; Kong N; Zhang M; Han W; Lou F; Yang J; Zhang Q; Wang X; He C; Pan H Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013; 4(10): 838 DOI: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jung CH; Ro SH; Cao J; Otto NM; Kim DH mTOR regulation of autophagy. FEBS Lett. 2010; 584(7): 1287–95. DOI: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Laplante M; Sabatini DM mTOR signaling at a glance. Journal of Cell Science. 2009; 122: 3589–94. DOI: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jung CH; Jun CB; Ro SH; Kim YM; Otto NM; Cao J; Kundu M; Kim DH ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009; 20:1992–2003. DOI: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ganley IG; Lam du H; Wang J; Ding X; Chen S; Jiang X ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009; 284:12297–305. DOI: 10.0174/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tian T; Li X; Zhang J mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int J Mol Sci. 2019; 20(3): 755 DOI: 10.3390/ijms20030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Galluzzi L; Pietrocola F; Bravo-San Pedro JM; Amaravadi RK; Baehrecke EH; Cecconi F; Codogno P; Debnath J; Gewirtz DA; Karantza V; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015; 34:856–880. DOI: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Amaravadi R; Kimmelman AC; White E Recent insights into the function of autophagy in cancer. Genes Dev. 2016; 30:1913–1930. DOI: 10.1101/gad.287524.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Riquelme I; Tapia O; Espinoza JA; Leal P; Buchegger K; Sandoval A; Bizama C; Araya JC; Peek RM; Roa JC The Gene Expression Status of the PI3K/AKT/mTOR Pathway in Gastric Cancer Tissues and Cell Lines. Pathol. Oncol. Res 2016; 22:797–805. DOI: 10.1007/s12253-016-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Byeon SJ; Han N; Choi J; Kim MA; Kim WH Prognostic implication of TSC1 and mTOR expression in gastric carcinoma. J. Surg. Oncol 2014;109:812–817. DOI: 10.1002/jso.23585 [DOI] [PubMed] [Google Scholar]
- 199.Tian T; Li X; Zhang J mTOR signaling in cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int J Mol Sci. 2019; 20(3): 755 DOI: 10.3390/ijms20030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fukamachi H; Kim SK; Koh J; Lee HS; Sasaki Y; Yamashita K; Nishikawaji T; Shimada S; Akiyama Y; Byeon SJ; et al. A subset of diffuse-type gastric cancer is susceptible to mTOR inhibitors and checkpoint inhibitors. Journal of Experimental & Clinical Cancer Research. 2019; 38(127). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Greenfield LK; Jones NL Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends in Microbiology. 2013; 21(11): 602–612. DOI: 10.1016/j.tim.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 202.Ohtsu A; Ajani JA; Bai YX; Bang YJ; Chung HC; Pan HM; Sahmoud T; Shen L; Yeh KH; Chin K; et al. Everolimus for Previously Treated Advanced Gastric Cancer: Results of the Randomized, Double Blind, Phase III GRANITE-1 Study. Gastrointestinal Cancer. 2013; 31: 3935–43. DOIL 10.1200/JCO.2012.48.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rosich L; Xargay-Torrent S; Lopez-Guerra M; Campo E; Colomer D; Roue G Counteracting Autophagy Overcomes Resistance to Everolimus in Mantle Cell Lymphoma. Cancer Therapy: Preclinical. 2012; 18(19): 10.1158/1078-0432.CCR-12-0351. [DOI] [PubMed] [Google Scholar]
- 204.Wang W; Lin L; Zhou Y; Ye Q; Yang X; Jiang J; Ye Z; Gao F; Tan X; Zhang G; et al. Hydroxychloroquine enhances the antitumor effects of BC001 in gastric cancer. International Journal of Oncology. 2019; 55(2): 405–414. DOI: 10.3892/ijo.2019.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rong L; Li Z; Leng X; Li H; Ma Y; Chen Y; Song F Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomedicine & Pharmacotherapy. 2020; 122: 109726. [DOI] [PubMed] [Google Scholar]
- 206.Liu J; Zhang Y; Qu J; Xu L; Hou K; Zhang J; Qu X; Liu Y B-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer. 2011; 11(183). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sun X; Zhang X; Zhai H; Zhang D; Ma S Chicoric acid (CA) induces autophagy in gastric cancer through promoting endoplasmic reticulum (ER) stress regulated by AMPK. Biomedicine & Pharmacotherapy. 2019; 118: 109144 DOI: 10.1016/j.biopha.2019.109144. [DOI] [PubMed] [Google Scholar]
- 208.Zhang Y; Liu S; Feng Q; Huang X; Wang X; Peng Y; Zhao Z; Liu Z Perialdehyde activates AMP-activated protein kinase to suppress the growth of gastric cancer via induction of autophagy. Journal of Cellular biochemistry. 2018. DOI: 10.1002/jcb.27491. [DOI] [PubMed] [Google Scholar]
- 209.Zhi X; Li B; Li Z; Zhang J; Yu J; Zhang L; Xu Z Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int J oncol. 2019; 54(5): 1625–38. DOI: 10.3892/ijo.2019.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ji CH; Kwon YT Crosstalk and Interplay between the Ubiquitin-Proteasome System and Autophagy. Biology, Medicine. 2017. DOI: 10.14348/molcells.2017.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mrakovcic M; Kleinheinz J; Frohlich LF Histone Deacetylase Inhibitor-Induced Autophagy in Tumor Cells: Implications for p53. Int J Mol Sci. 2017; 18(9): 1883 DOI: 10.3390/ijms18091883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Peracchio C; Alabiso O; Valente G; Isidoro C Involvement of autophagy in ovarian cancer: a working hypothesis. J Ovarian Res. 2012; 5:22 DOI: 10.1186/1757-2215-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou J; Liao W; Yang J; Ma K; Li X; Wang Y; Wang D; Wang L; Zhang Y; Yin Y; Zhao Y; Zhu WG FOXO3 induces FOXO1-dependent autophagy by activating the AKT1 signaling pathway. Autophagy. 2012. 8(12): 1712–23. DOI: 10.46161/auto.21830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Avivar-Valderas A; Salas E; Bobrovnikova-Marjon E; Diehl JA; Nagi C; Debnath J; Aguirre-Ghiso JA PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011; 31(17): 3616–29. DOI: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen YF; Liu H; Luo XJ; Zhao Z; Zou ZY; Li J; Lin XJ; Liang Y The roles of reactive oxygen species (ROS) and autophagy in the survival and death of leukemia cells. Crit Rev Oncol Hematol. 2017; 112: 21–30. DOI: 10.1016/j.critevonc.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 216.Li ZY; Yang Y; Ming M; Liu B Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem Biophys Res Commun. 2011; 414(1): 5–8. DOI: 10.1016/j.bbrc.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 217.Zou P; Xia Y; Chen T; Zhang J; Wang Z; Chen W; Chen M; Kanchana K; Yang S; Liang G Selective killing of gastric cancer cells by small molecule targeting ROS-mediated ER stress activation. Molecular Carcinogenesis. 2015. DOI: 10.1002/mc.22351. [DOI] [PubMed] [Google Scholar]
- 218.Wang T; Gao J; Yu J; Shen L Synergistic inhibitory effect of wogonin and low-dose paclitaxel on gastric cancer cells and tumor xenographs. Chin J Cancer Res. 2013; 25(5): 505–513. DOI: 10.3978/j.issn.1000-9604.2013.08.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li SJ; Sun SJ; Gao J; Sun FB Wogonin induces Beclin-1/PI3K and reactive oxygen species-mediated autophagy in human pancreatic cells. Oncology Letters. 2016; 12(6): 5059–67. DOI: 10.3892/ol.2016.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Aviles-Jimenez F; Vazquez-Jimenez F; Medrano-Guzman R; Mantilla A; Torres J Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci Rep. 2014; 4: 4202 DOI: 10.1038/srep04202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hu YL; Pang W; Huang Y; Zhang Y; Zhang CJ The Gastric Microbioe is Perturbed in Advanced Gastric Adenocarcinoma Identified Through Shotgun Metagenomics. Frontiers. 2018; 8: 433 DOI: 10.3389/fcimb.2018.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu X; Nie W; Liang J; Li Y Interaction of Helicobacter pylori with other microbiota species in the development of gastric cancer. Clinical Microbiology. 2017. [Google Scholar]
- 223.Dias, Jacome E; Libanio D; Borges-Canha M; Galaghar A; Pimentel-Nunes P Gastric microbiota and carcinogenesis: the role of non-Helicobacter pylori bacteria- A systematic review. Revistia Espanola de Enfermedades Digestivas. 2016. DOI: 10.17235/reed.2016.4261/2016. [DOI] [PubMed] [Google Scholar]
- 224.Salazar CR; Sun J; Li Y; Francois F; Corby P; Perez-Perez G; Dasanayake A; Pei Z; Chen Y Association between Selected Oral Pathogens and Gastric Precancerous lesions. PLoS One. 2013; 8(1): e51604 DOI: 10.1371/journal.pone.0051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hsieh YY; Tung SY; Pan HY; Yen CW; Xu HW; Lin Y; Deng YF; Hsu WT; Wu CS; Li C Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan. Sci Rep. 2018; 8(158). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Roberts PJ; Dickinson RJ; Whitehead A; Laughton CR; Foweraker JE The culture of lactobacilli species in gastric carcinoma. Journal of Clinical Pathology. 2002; 55: 477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Weng MT; Chiu YT; Wei PY; Chiang CW; Fang HL; Wei SC microbiota and gastrointestinal cancer. Journal of the Formosan Medical Association. 2019; 118(1): S32–S41 DOI: 10.1016/j.fma.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 228.Wang LL; Yu XJ; Zhan SH; Jia SJ; Tian ZB; Dong QJ Participation of microbiota in the development of gastric cancer. World J Gastroenterol. 2014; 20(17): 4948–52. DOI: 10.3748/wjg.v20.i17.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhao Y; Gao X; Guo J; Yu D; Xiao Y; Wang H; Li Y Helicobacter pylori infection alters gastric and tongue coating microbial communities. Helicobacter. 2019; 24: e12567 DOI: 10.1111/hel.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang L; Fu L; Zhang S; Zhang J; Zhao Y; Zheng Y; He G; Yang S; Ouyang L; Liu B Discovery of a small molecule targeting ULK1-modulated cell death of triple negative breast cancer in vitro and in vivo. Chem Sci. 2017. 8(4): 2687–01. DOI: 10.1039/c6sc05368h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fu X; Huang Z; Hong L; Lu JH; Feng D; Yin XM; Li M Targeting ATG4 in Cancer Therapy. Cancers. 2019; 11(5): 649 DOI: 10.3390/cancers11050649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Xie J; Wang X; Proud CG mTOR inhibitors in cancer therapy. F1000Res. 2016; 5:F1000 Faculty Rev-2078. DOI: 10.12688/f1000research.9207.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rebecca VW; Nicastri MC; Fennelly C; Chude CL; Barber-Rotenberg JS; Ronghe A; McAfee Q; McLaughlin NP; Zhang G; Goldman AR; et al. PPT1 Promotes Tumor growth and is the molecular target of chloroquine derivatives in cancer. AACR Journals. Published 2018. DOI: 10.1158/2159-8290.CD-18-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhou J; Li G; Zheng Y; Shen HM; Hu X; Ming QL; Huang S; Li P; Gao N A Novel Autophagy/Mitophagy Inhibitor Liensinine Sensitizes Breast Cancer Cells to Chemotherapy Through DNM1L-mediated Mitochondrial Fission. Autophagy. 2015; 11(8): 1259–79. DOI: 10.1080/15548627.2015.1056970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mahameed M; Wilhelm T; Darawshi O; Obiedat A; Tommy WS; Chintha C; Schubert T; Samali A; Chevet E; et al. The unfolded protein response modulators GSK2606414 and KIRA6 are potent KIT inhibitors. Cell Death & Disease. 2019; 10(300). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Axten JM; Romeril SP; Shu A; Ralph J; Medina JR; Feng Y; Li WHH; Grant SW; Heerding DA; et al. Discovery of GSK2656157: An Optimized PERK Inhibitor Selected for Preclinical Development. ACS Medicinal Chemistry Letters. 2013. DOI: 10.1021/ml400228e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li J; Tu HJ; Li J; Dai G; Dai YC; Wu Q; Shi QZ; Cao Q; Li ZJ N-acetyl cysteine inhibits human signet ring cell gastric cancer cell line (SJ-89) cell growth by inducing apoptosis and DNA synthesis arrest. Eur J Gastroenterol Hepatol. 2007; 19(9): 769–74. DOI: 10.1097/MEG.0b013e3292202bda. [DOI] [PubMed] [Google Scholar]
- 238.Klenke S; Akdeli N; Stelmach P; Heukamp L; Schulte JH; Bachmann HS The small molecule Bcl-2/mcl-1 inhibitor TW-37 shows single-agent cytotoxicity in neuroblastoma cell lines. BMC Cancer. 2019; 19(1): 243 DOI: 10.1186/s12885-019-5439-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chen X; Mao G; Chen H; Liu S; Wang S; Li X; Ye Y; Wu H; Liu J TW37 enhances the pro apoptosis and anti-migratory ability of gefitinib in Non-Small Cell Lung Cancer. Cell Mol Bio (Noisy-le-grand). 2018; 64(4): 6–10. [PubMed] [Google Scholar]
- 240.Krasniqi E; Pellicori S; Formica V Emerging role of S-1 in gastric cancer. Indian J Med Paediatr Oncol. 2015; 36(4): 219–228. DOI: 10.4103/0971-5851.171542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Manero F; Gautier F; Gallenne T; Cauquil N; Gree D; Catron PF; Geneste O; Gree R; Vallette FM; Juin P The small organic compound HA14–1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006; 66(5): 2757–64. [DOI] [PubMed] [Google Scholar]
- 242.Sun XP; Zhang X; He C; Qiao H; Jiang X; Jiang H; Sun X ABT-737 synergizes with arsenic trioxide to induce apoptosis of gastric carcinoma cells in vitro and in vivo. J Int Med Res. 2012; 40(4): 1251–64. [DOI] [PubMed] [Google Scholar]
- 243.Zhang H; Zhong X; Zhang X; Shang D; Zhou YI; Zhang C Enhanced anticancer effect of ABT-737 in combination with naringenin on gastric cancer cells. Exp Ther Med. 2016; 11(2): 669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


