Abstract
Cigarette smoking during pregnancy increases risk for pregnancy complications, growth restriction, and other adverse health outcomes. The most effective intervention for reducing smoking during pregnancy is financial incentives contingent on biochemically-verified smoking abstinence. The present study examined the efficacy of a smartphone-based intervention whereby smoking monitoring and incentive delivery occurred remotely using a mobile app. If efficacious, this remote intervention would allow pregnant women residing in geographically remote areas to benefit from incentives-based cessation interventions. Sixty U.S. pregnant smokers were recruited between May 2018 to May 2019 via obstetrical clinics, Women, Infants, and Children (WIC) offices, and Facebook. Participants were assigned sequentially to one of two treatments: best practices alone (N = 30) or best practices plus financial incentives (N = 30). Outcomes were analyzed using repeated measures analysis based on generalized estimating equations (GEE). Seven-day point prevalence abstinence rates were greater in the incentives versus best practices arms early- (46.7% vs 20.0%, OR=3.50, 95%CI=1.11,11.02) and late-antepartum (36.7% vs 13.3%, OR=3.76, 95%CI=1.04,13.65), and four- (36.7% vs 10.0%, OR=5.21, 95%CI=1.28,21.24) and eight-weeks postpartum (40.0% vs 6.7%, OR= 9.33, 95%CI=1.87,46.68), although not at the 12- (23.3% vs 10.0%, OR=2.74, 95%CI=0.63,11.82) or 24-week (20.0% vs 6.7%, OR=3.50, 95%CI=0.65,18.98) postpartum assessments likely due to this pilot study being underpowered for discerning differences at the later assessments, especially 24-weeks postpartum which was three months after treatment completion. These results support the efficacy of this remote, incentives-based intervention for pregnant smokers. Further research evaluating its efficacy and cost-effectiveness in a well-powered, randomized controlled trial appears warranted.
Keywords: pregnancy, cigarette smoking, smoking cessation, smartphone, financial incentives, mHealth
Introduction
Smoking during pregnancy is the leading preventable cause of poor pregnancy outcomes in the U.S. and other developed countries, increasing risk for pregnancy complications, preterm birth, childhood behavioral problems, and development of cardiovascular disease, obesity, and metabolic syndrome in adulthood (Baba et al., 2012; Barker, 2004; Cohen et al., 2010; Dietz et al., 2010; Leslie, 2013; Rogers, 2009; Services, 2014; Thompson et al., 2009). Smoking during pregnancy also has substantial economic impacts, with recent U.S. estimates placing annual costs of maternal smoking during pregnancy at $370 million in neonatal costs alone (Mohlman and Levy, 2016). Unfortunately, prevalence of smoking among national samples of U.S. pregnant women has largely remained stagnant at ~ 13% over the past decade (Alshaarawy and Anthony, 2015; Kurti et al., 2017; Nighbor et al., 2020). Moreover, maternal smoking during pregnancy is overrepresented among socioeconomically disadvantaged women and exacerbates the glaring problem of health disparities that demands greater action (Higgins and Chilcoat, 2009; Kandel et al., 2009).
Meta-analyses indicate that interventions using financial incentives produce the largest effect sizes of any psychosocial or pharmacological interventions for promoting smoking cessation during pregnancy (Chamberlain et al., 2017; Lumley et al., 2009). A series of randomized controlled trials conducted at the University of Vermont supported the efficacy of financial incentives delivered contingent on biochemically-delivered smoking abstinence versus a control condition in which incentives of comparable monetary value were provided independent of smoking status (Heil et al., 2008; Higgins et al., 2004; Higgins et al., 2012). Across trials, late-pregnancy smoking abstinence rates were nearly five-fold greater among women assigned to the contingent incentives versus control conditions (34.1% vs. 7.4%), with treatment effects remaining discernible at a final, 24-weeks postpartum assessment (14.1% vs. 1.2%) conducted 12 weeks after incentives were discontinued (Higgins et al., 2012). Infants born to mothers treated in the abstinence-contingent incentives condition also had greater mean birth weight, and a lower proportion was categorized as low birth-weight relative to those born to controls. Subsequent research conducted in the U.K. demonstrated the efficacy of financial incentives for reducing smoking during pregnancy when delivered in a clinical rather than a research setting (Ierfino et al., 2015), and also supported the cost-effectiveness of this approach (Boyd et al., 2016).
One limitation of incentives-based treatments to promote smoking cessation during pregnancy can be the frequent clinic visits often involved in obtaining biochemical verification of smoking status, meaning only women living in close geographical proximity of clinics are likely to access the intervention. That situation precludes access to pregnant women residing in more rural areas or those without reliable transportation. Also, effective implementation of an incentives program requires accurate delivery and withholding of the incentive conditional on the test results for smoking status, as well as accurate record keeping of earnings. Managing these contingencies, associated record keeping, and biochemical verification methods can be challenging for personnel without training to implement with fidelity. In addition to surmounting geographic barriers to treatment, capitalizing on mobile phone technology to deliver the financial incentives intervention remotely has the potential to allow for automation of these intervention procedures assuring greater treatment fidelity. Various previous studies have examined smartphone apps for promoting smoking cessation during pregnancy (Tombor et al., 2019; Tombor et al., 2016; Wu et al., 2017), however no prior studies to our knowledge have deployed an app designed explicitly to support remote monitoring of smoking status and delivery of incentives. Thus, the purpose of the current pilot study was to examine the efficacy of leveraging a smartphone app to deliver the financial incentives intervention remotely to a sample of pregnant women recruited from throughout the U.S.
Method
Participants
Participants were recruited from Facebook advertisements deployed nationwide and obstetric practices and Women, Infants, and Children (WIC) offices throughout Vermont. Recruitment was completed in a one-year period between May 2018 and 2019. Study inclusion criteria were being ≥ 18 years, biochemically confirmed smoking in the past seven days, gestational age ≤ 25 weeks, English speaking, and owning a smartphone. Exclusion criteria were incarceration, endorsing a psychological or physical condition that may interfere with study participation, smoking marijuana more than once per week and not willing to quit during the intervention (marijuana can increase breath CO, thereby potentially preventing women from earning incentives), current opioid use disorder, and use of opioid, psychomotor stimulant, or antipsychotic medications.
All women receiving prenatal care at referring clinics in Vermont completed a brief questionnaire on smoking status, whereas women recruited via Facebook completed questions assessing smoking and pregnancy status online. In both cases, women who were pregnant and endorsed current cigarette smoking had the opportunity to provide their contact information, permitting researchers to contact them for further eligibility screening. Smokers who provided their contact information on either the brief questionnaire administered in person, or online, received a link to complete an online preliminary eligibility screening administered via REDcap. Algorithms programmed into REDcap were used to identify those women who met preliminary eligibility criteria, and these women were provided the opportunity to complete the full intake assessment battery. For women recruited via Facebook, this intake assessment battery also included signing a medical release form allowing the researchers to verify their pregnancy status with a physician or other healthcare provider before proceeding with their enrollment. This requirement was not necessary for women recruited at obstetrical clinics or WIC offices, as receptionists at these sites only administered the brief screening forms to pregnant women.
Women who completed the full intake assessment battery and remained eligible were contacted by phone for further orientation to the research study. During this orientation, women received detailed information about the study (e.g., a description of the two treatment arms, the possibility that they could be randomly assigned to either treatment condition, maximum compensation possible in each condition, study duration), and decided whether they wished to participate. Those women who remained interested in participation following this orientation session were mailed an initial saliva cotinine test kit, and those who completed the remote saliva test and were biochemically verified to be smokers were assigned sequentially (incentives first) to one of the two treatment conditions described below. The rationale for assigning women to treatment sequentially is that the study was initially conducted to pilot test recruitment procedures and practical components of delivering financial incentives remotely. As no substantive recruitment challenges emerged nor practical problems with delivering incentives remotely, we opted to include a control condition as a first step in efficacy testing. The University of Vermont Institutional Review Board approved this study and all participants provided verbal informed consent.
Treatment Interventions
Best Practices.
Participants in the Best Practices only control condition received brief smoking cessation counseling based on the 5 As (Fiore et al., 2008), and study staff submitted referrals on participants’ behalf to their state tobacco quit lines. The brief smoking cessation counseling (~10 mins) consisted of asking participants about their current cigarette smoking, advising them to quit, assessing their willingness to make a quit attempt, assisting them in selecting a quit date, and arranging for a follow-up at their subsequent study assessment (see Assessments). Counseling was conducted by phone immediately after participants were assigned to a treatment condition, and at both the early- and late-antepartum assessments. In addition to receiving the 5As and a quit line referral, women also received any smoking cessation advice that was provided at their obstetrical clinics.
Best Practices Plus Financial Incentives.
Participants in this condition, hereafter referred to as the Incentives condition, received all of the best practices procedures described above plus the remote financial-incentives intervention. Smoking monitoring and the delivery of incentives were completed remotely using the DynamiCare Rewards smartphone app (DynamiCare Health Inc., Newton, MA). Use of this app permitted the intervention to be largely automated, with participants receiving push notifications when biochemical tests were due, as well as notifications about earnings associated with each test. Breath and saliva tests were submitted remotely in the form of videos of participants completing the tests. Afterwards, the study app created text files containing a timestamp and video file. Participant videos were reviewed within 1-2 hours after submission, after which participants received auto-generated notifications from the app informing them of their test results and associated earnings, and the value of their next incentive. Incentives were delivered remotely in the form of money loaded onto a PEX debit card (provided by the researchers) following video validation.
Upon being assigned to the Incentives condition, participants set a quit date for either the first or second Monday following treatment assignment. Beginning on the quit date and extending for one week, participants were required to submit twice daily CO samples, separated by a minimum of 8 hours from one another. Participants received their first test request at 8:00 AM in their respective time zone. In order to allow 8 hours to pass between samples and still complete the second test before midnight, participants had to submit their first breath test before 4:00 PM each day. All breath samples ≤ 6 ppm were considered negative (abstinent) during week one. Although less stringent than the recommended cut point of ≤ 4 ppm for determining smoking status among pregnant women (Bailey, 2013; Bauld et al., 2012; Higgins et al., 2007), a cut point of ≤ 6 ppm minimizes the likelihood of false positives, thereby maximizing opportunities to deliver incentives early in the intervention. Previous research with pregnant women indicates that abstinence during the first two weeks of incentives interventions is highly predictive of late-pregnancy smoking abstinence (Higgins et al., 2006). The ≤ 6 ppm cut point was only used for the purposes of providing incentives during Week one in this treatment group.
Following the initial quit week, the schedule of monitoring was reduced and incentives were contingent on a negative salivary-cotinine test result. More specifically, participants submitted videos of themselves completing saliva tests twice weekly (Mondays and Thursday) during Weeks two through six, then once weekly from Week seven until delivery (test day determined randomly). The schedule of potential earnings during Weeks two through delivery was a continuation of the escalating pay schedule from the initial quit week. To protect against relapse following delivery, the frequency of monitoring was increased to twice weekly for the initial four weeks postpartum. After the first month postpartum, monitoring was returned to once weekly for the remaining eight weeks, with the opportunity to earn incentives being terminated at the end of postpartum Week 12. Each negative sample postpartum was worth $33.25.
The incentives intervention was offered from the start of the study (14.9 ± 4.9 weeks gestational age among women assigned to incentives condition) through 12-weeks postpartum. Maximum earnings potential was approximately $1,620 (2017 inflation-adjusted equivalent of maximum $1,200 earnings used in initial studies on this topic at the University of Vermont beginning in 2002) (Heil et al., 2008; Higgins et al., 2004; Higgins et al., 2012). Because incentives are only paid when women meet the abstinence criterion and not all women abstained, the average earning in incentives in the present study was $411.47 ± $464.23. The schedule of potential earnings started at $6.25 for the first negative sample and increased by $1.00 for each consecutive negative sample until reaching $33.25, where it remained throughout the intervention period. Positive or missing samples reset the value of the incentive to $6.25, although submitting two negative samples following a reset restored the incentive to its value prior to the lapse.
Assessments
Participants in both treatment conditions completed a total of seven assessments (including their initial intake assessment) during study participation. Each assessment consisted of questionnaires and biochemical verification of participants’ smoking status via breath and saliva specimens submitted remotely using a smartphone. Breath specimens were submitted using the handheld, smartphone-compatible iCO™ Smokerlyzer® (coVita, Inc.) CO monitor (Wong et al., 2019), and saliva cotinine levels were measured using the Alere iScreen® OFD Test (New Line Medical) (Moore et al., 2018). With the exception of sociodemographic characteristics that were assessed at intake only, the questionnaires completed at each assessment were largely identical, and queried participants about various secondary outcomes (e.g., nicotine dependence, smoking behavior, depression levels) that will be examined in our ongoing, fully randomized trial examining the current intervention (ClinicalTrials.gov Identifier: NCT03922360). As the current study purpose was to examine the feasibility and efficacy of a remote financial incentives intervention, our primary outcome of interest from each assessment was participants’ biochemically verified smoking status.
Assessments were completed three times antepartum (i.e., the initial study-intake assessment before treatment assignment; an early pregnancy assessment ~ one month after treatment assignment; and a late pregnancy assessment at ≥ 28-weeks gestation), and at 4-, 8-, 12-, and 24-weeks postpartum (the final assessment being 3-months following treatment termination. Smoking abstinence at these assessments was defined as self-reported smoking abstinence in the past seven days in combination with a cotinine-negative saliva test result.
The procedure for collecting saliva samples using the Alere iScreen® OFD Test involved inserting the sponge end of a collector tool into the mouth, and actively swabbing the inside of the mouth and tongue with the sponge for ≥ 3 minutes. After saturating the sponge, the collector was inserted vertically into a cap, and placed horizontally on a level surface until a result indicating smoking status appeared on the collector’s result window. The Alere iScreen® permits qualitative detection of cotinine in oral fluids at 30 ng/ml. This cut-off has been found to differentiate pregnant smokers from pregnant non-smokers with 95% sensitivity and 100% specificity (Hegaard et al., 2007), and has been used to determine smoking status in previous studies with pregnant women (Hegaard et al., 2003; Windsor et al., 1993). The procedure takes ~ 5 to 10 minutes, and participants submitted videos of themselves completing the saliva tests using the DynamiCare Rewards app.
For women who reported using nicotine replacement therapy (NRT) or e-cigarettes in the absence of cigarettes, abstinence at formal assessments was defined as self-reported no smoking in the past week and biochemically verified by providing a breath carbon monoxide (CO) sample ≤ 4 ppm.
Statistical Methods
The primary analysis of smoking status was based on all participants who were assigned to treatment with the exception of one woman withdrawn due to pregnancy termination/fetal demise prior to their late pregnancy assessment (N = 60) (Friedman et al., 1998). Treatment conditions were compared on participant characteristics using t tests for continuous measures and chi-square tests for categorical measures. Repeated measures analysis for categorical data based on generalized estimating equations (GEE) utilizing a logistic link function (SAS PROC GENMOD) was used to compare treatment conditions on point prevalence smoking abstinence over the antepartum and postpartum assessments. To determine predictors of smoking abstinence at late pregnancy, smokers and abstainers at late pregnancy were compared on all sociodemographic variables. All variable that differed between the two groups (p < .05) were included in a logistic regression model to predict smoking abstinence at late pregnancy. All analyses were performed using SAS Version 9 statistical software (SAS Institute, Cary NC). Statistical significance was determined based on p < .05. Study results were not analyzed blinded to group assignment as our system of assigning participant ID numbers prohibited us from doing so.
Results
Recruitment and Participant Characteristics
Of the 1,034 women who initiated the study screening process, 973 women were either ineligible based on their responses to the preliminary eligibility screening or intake assessment (n = 691), or were eligible but did not complete the study enrollment process (n = 282) (Figure 1). The remaining 61 women (5.90%) who enrolled in the study were assigned sequentially first to a financial incentives condition (N = 31), and then to a control condition whereby women received current best practices for promoting smoking cessation during pregnancy (N = 30).
Figure 1.
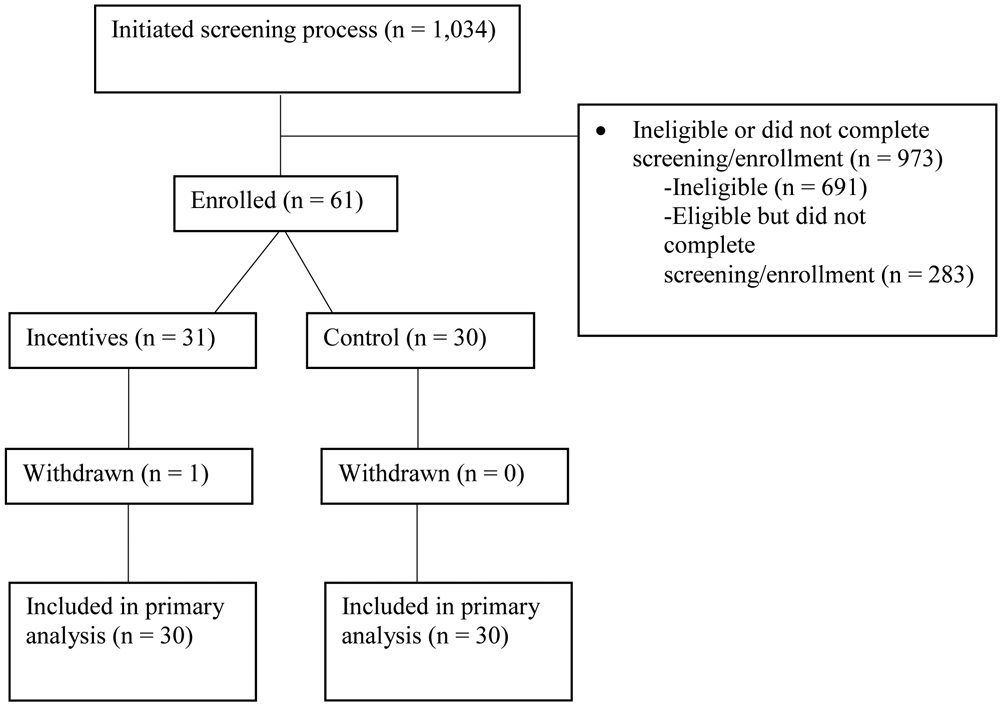
The flow of participants through the study. Participants were pregnant smokers recruited locally in greater Burlington, VT, and nationally via Facebook, between 2018-2019.
The only criterion for withdrawing someone from the study following treatment assignment was pregnancy termination/fetal demise prior to participants’ late pregnancy assessment (≥ 28 weeks gestational age). One participant was withdrawn due to pregnancy termination, leaving 60 remaining women (30 Best Practices, 30 Incentives) who were enrolled in the present study and included in all data analyses. Six women (10.0%) were recruited from WIC offices and obstetrical clinics in Vermont, with the remaining fifty-four women (90.0%) being recruited via Facebook advertising.
Figure 2 shows the reach of the intervention achieved with these combined local and national recruitment strategies, and participant characteristics are displayed in Table 1. The only baseline characteristic that differed significantly between the two treatment conditions is that a higher proportion of participants in the Best Practices condition reported working for pay outside the home (53%) compared to the Incentives condition (27%) (Table 1). This characteristic was not significantly associated with abstinence outcomes and thus not include as a covariate in analyses.
Figure 2.
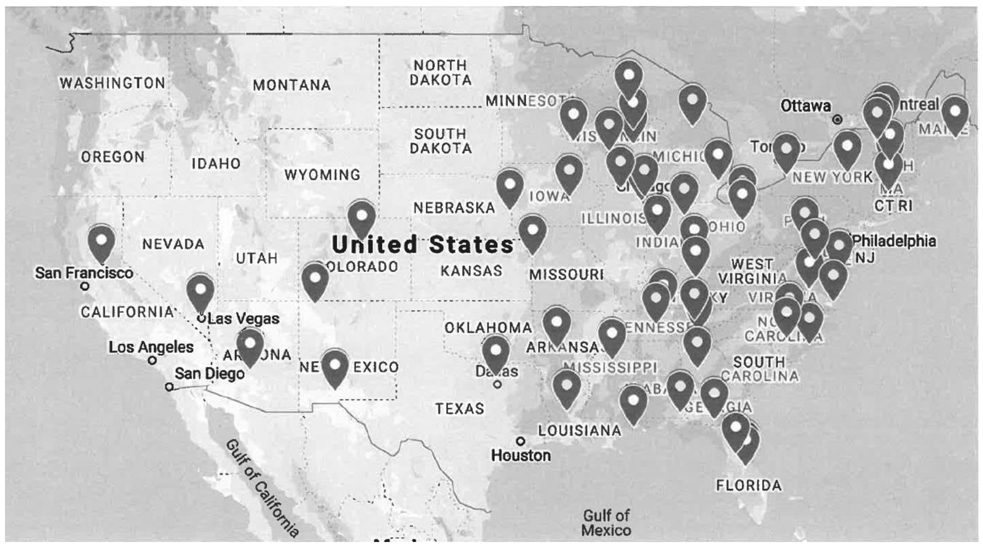
Map showing the reach of the intervention. Each dot on the map denotes the hometown of an individual participant.
Table 1.
Participant characteristics overall and examined separately among women assigned sequentially to the Incentives vs Best Practices treatment groups.
| Characteristic | Overall (N = 60) |
Incentives (N = 30) |
Best Practices (N = 30) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 30.4 (5.2) | 30.8 (5.0) | 30.0 (5.4) | .54 |
| Race/Ethnicity | ||||
| % White | 72 | 67 | 77 | .39 |
| Education | ||||
| % < 12 years of education | 7 | 3 | 10 | .10 |
| % = 12 years of education | 53 | 67 | 40 | |
| % > 12 years of education | 40 | 30 | 50 | |
| % Participating in WIC | 47 | 53 | 40 | .30 |
| % Working for pay outside of home | 40 | 27 | 53 | .03 |
| Smoking Characteristics | ||||
| Cigarettes per day pre-pregnancy | 18.6 (5.2) | 19.0 (4.8) | 18.2 (5.5) | .56 |
| Cigarettes per day at intake | 10.6 (6.2) | 11.4 (6.0) | 9.9 (6.5) | .36 |
| Age first started smoking cigarettes | 15.5 (3.1) | 15.4 (3.3) | 15.6 (3.0) | .82 |
| % Living with another smoker | 72 | 73 | 70 | .77 |
| % With no smoking allowed in home | 73 | 70 | 77 | .56 |
| Cigarette Type | 0 | 0 | 0 | .07 |
| % Light | 0 | 0 | 0 | .07 |
| % Medium | 13 | 3 | 23 | |
| % Full Flavor | 17 | 17 | 17 | |
| % Contains Menthol | 58 | 63 | 53 | .43 |
| % Tried quitting pre-pregnancy | 69 | 72 | 67 | .63 |
| % Tried quitting during pregnancy | 54 | 52 | 57 | .70 |
| Pregnancy Characteristics | ||||
| Gestational age (weeks) | 14.4 (4.6) | 14.9 (4.9) | 13.9 (4.3) | .41 |
| Pregnancy Intention | ||||
| % Now | 13 | 17 | 10 | .13 |
| % Later | 40 | 30 | 50 | |
| % Sooner | 8 | 17 | 0 | |
| % Never | 23 | 23 | 23 | |
| % Don’t know | 15 | 13 | 17 | |
Smoking Abstinence
The repeated measures analysis based on generalized estimating equations (GEE) indicated significant main effects of both treatment (χ2 = 5.63, p < 0.05) and assessment time (χ2 = 15.02, p < 0.05), and a significant interaction between treatment condition and assessment time (χ2 = 11.38, p < 0.05). With respect to the interaction, seven-day point prevalence abstinence rates were greater in the incentives versus best practices arms at early- (46.7% vs 20.0%, OR = 3.50, 95% CI = 1.11, 11.02) and late-antepartum assessments (36.7% vs 13.3%, OR = 3.76, 95% CI = 1.04, 13.65), and at four- (36.7% vs 10.0%, OR = 5.21, 95% CI = 1.28, 21.24) and eight-weeks postpartum (40.0% vs 6.7%, OR = 9.33, 95% CI = 1.87, 46.68), although not at the 12- (23.3% vs 10.0%, OR = 2.74, 95% CI = 0.63, 11.82) and 24-week (20.0% vs 6.7%, OR = 3.50, 95% CI = 0.65, 18.98) assessments despite abstinence levels in the Incentives condition being between 2.0- and 4.0-fold above Best Practices alone at those two latter assessments (Figure 3).
Figure 3.
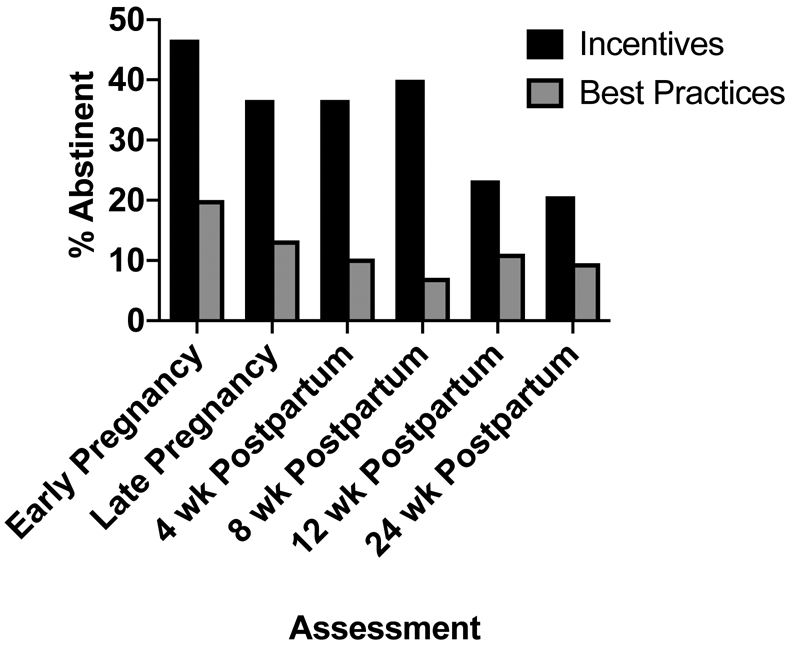
7-day point-prevalence abstinence rates for each treatment condition across antepartum and postpartum assessments. Participants were 60 pregnant smokers recruited locally in greater Burlington, VT, and nationally via Facebook, between 2018-2019.
Results of the adjusted logistic regression models examining predictors of late pregnancy smoking abstinence identified two independent predictors: Treatment Condition and Time to First Cigarette (TTFC) upon waking item from the FTND. More specifically, participants in the Incentives condition had 4.22 times greater odds (95% CI = 1.06, 16.77) of achieving biochemically verified smoking abstinence than Best Practices participants. Regarding TTFC, those who smoked their first cigarette > 30 mins after waking had 5.42 times greater odds (95% CI = 1.44, 20.37) of achieving late pregnancy smoking abstinence relative to those who smoked within ≤ 30 mins upon waking.
Discussion
The present study is the first to our knowledge to demonstrate the efficacy of a smartphone-based financial incentives treatment to promote smoking cessation during pregnancy. Indeed, comparable treatment effects were observed in the present study relative to prior trials conducted at the University of Vermont that utilized the conventional, in-person model of treatment delivery (Heil et al., 2008; Higgins et al., 2004; Higgins et al., 2012). More specifically, late pregnancy abstinence rates collapsed across those preceding randomized controlled clinical trials were ~35% (Incentives) versus ~7% (Control), which aligns quite well with the 36.7% versus 13.3% differences observed with this remote implementation method as well as outcomes observed in meta-analyses that included controlled trials on incentives-based interventions with pregnant smokers conducted at other institutions (Cahill et al., 2015). While encouraging, the efficacy of this intervention should be further tested in a well-powered, randomized controlled trial to rule-out any potential confounding influence from sequential assignment and to refine the estimates of treatment effect sizes.
There are at least four additional points that merit mention. First, study results demonstrating the efficacy of smartphone-based financial incentives highlight the capacity for technology to expand the reach of this efficacious, evidence-based treatment, as well as the potential public health impact of scaling up this intervention and disseminating it more widely. The 60 women enrolled in the present study were recruited across 33 states, with only five of the 1,034 (0.48%) women who completed an initial preliminary eligibility screening being excluded for not owning a smartphone. Although this may be attributable in part to the fact that a majority of participants were recruited online via Facebook, this finding that < 1.0% of pregnant women reported not owning a smartphone indicates that a larger-scale study of remote financial incentives to reduce smoking during pregnancy is feasible.
Second, although results are generally consistent with our prior trials, the magnitude of the treatment effect was diminished slightly in the present study. As late pregnancy abstinence rates in the incentives conditions in the prior in-person studies versus the current remote study were essentially identical, the lower-magnitude treatment effect that we observed appears to be attributable to quit rates in the control condition. More specifically, late pregnancy abstinence in the present control group was ~ two-fold greater than the prior, in-person studies (i.e., ~13% vs ~7%, respectively). One possibility is that the online recruitment methods used in the current study attract more affluent participants than the recruitment methods used in prior, in-person incentives trials (e.g., referrals from WIC offices and obstetrical clinics). Research conducted among a U.S. national sample of pregnant smokers indicated that while they reported comparable use of various digital forms (e.g., smartphone apps, social media) than their non-smoking counterparts, some socioeconomic disparities in technology use persisted (Kurti et al., 2019). Thus, recruitment methods that leverage digital forms to reach pregnant smokers may inadvertently target a narrower, more affluent segment of this population than intended. This possibility is further supported by comparisons between participants in the present, remote study versus one of the prior, clinic-based studies conducted at University of Vermont (Heil et al., 2008). More specifically, women in the present study were older (30.4 vs 24.6 years), and 40% reported having > 12 years educational attainment versus 19% in the clinic-based intervention. Additionally, somewhat fewer women in the present study reported living with other smokers (72% vs 81.3%), and were more likely to have rules against smoking in the home (73% vs 61%) relative to the prior, clinic-based study. It will be important for future research and treatment leveraging online recruitment strategies to identify methods for reaching the broadest swath of this population possible, as well as identifying methods to facilitate participation among women without access to technology (e.g., directing women to locations in their community that offer free computer and Internet access [Komando, 2012], and/or programs that distribute free mobile phones to low-income persons) [Bagchi et al., 2008].
Third, although participants in the Incentives condition had 2.74- and 3.50-times greater odds of being abstinent than those in Best Practices alone at 12- and 24-weeks postpartum, respectively, women in the Incentives condition exhibited substantially greater quit rates antepartum (~42%) relative to their 12- and 24-week postpartum assessments (~22%). This discrepancy between quit rates antepartum versus at the last two follow-up assessments indicates that increased efforts are needed to sustain antepartum abstinence levels during the postpartum period by planning for maintenance in incentives-based treatments, as secondhand smoke following delivery can have detrimental health consequences for the infant (e.g., respiratory infections, asthma exacerbation, sudden infant death syndrome). Importantly, technology-based treatment delivery platforms offer a way to provide ongoing support for sustaining abstinence. For example, prior research has leveraged technology to provide ongoing support (e.g., telephone counseling, tailored motivational messages) to smokers following hospital discharge (Japuntich et al., 2012), and other effective methods for promoting sustained drug abstinence (e.g., community reinforcement approach; Secades-Villa et al., 2011) also lend themselves to remote treatment delivery platforms (Christensen et al., 2014). Approaches that target behavioral mechanisms associated with persistent tobacco use (e.g., delay discounting, or the rapid devaluing of delayed rewards; MacKillop et al., 2011; Stein et al., 2018) may represent other promising adjuncts to financial incentives. For example, episodic future thinking (EFT) is an intervention that requires participants to engage in vivid mental simulation of future events (Hollis-Hansen et al., 2019; Schacter et al., 2017), thereby potentially promoting a more future-oriented time perspective and diminishing sensitivity to smaller, immediate rewards at the expense of larger, delayed ones. EFT is compatible with technology-based treatment delivery platforms (Sze et al., 2015), and has been shown to reduce both impulsivity and cigarette self-administration (Stein et al., 2018).
Fourth, although studies conducted in the U.K. have demonstrated financial incentives for reducing smoking during pregnancy in clinic-based settings to be cost-effective (Boyd et al., 2016), this question has not been examined when the treatment is delivered remotely via smartphone. Demonstrating the cost-effectiveness of leveraging mobile technology to deliver incentives-based treatments represents an important contribution to the current evidence base, as that may be the final arbiter of whether state Medicaid programs will support incentives as the standard of care for treating smoking during pregnancy, thereby eliminating the cost of such programs as a barrier to implementing this evidence-based treatment more widely. A fully randomized controlled clinical trial is currently underway to further assess the feasibility, acceptability, and cost-effectiveness of the present intervention.
Finally, there are several limitations that merit mention. First, the present pilot study assigned participants sequentially to the two treatment conditions, potentially contributing to minor differences in participant characteristics across the two groups. Prior research conducted at the University of Vermont revealed limited differences in results between a financial incentives pilot study for pregnant smokers that lacked randomization (Higgins et al., 2004) versus subsequent fully randomized controlled trials (Heil et al., 2008; Higgins et al., 2012) and other researchers have suggested sequential assignment as a reasonable alternative to randomization in some cases (Staines et al., 1999). Nonetheless, it will be important to evaluate the present smartphone-based intervention in the context of a fully randomized trial, as we are currently doing. Second, our online recruitment strategy, as well as a somewhat lengthy and onerous enrollment process requiring remote verification of pregnancy and smoking status, may have biased the sample towards including women of higher socioeconomic status. Future research and treatment that minimizes the effort required to enroll (e.g., opt-out procedures where pregnant smokers are automatically enrolled in financial incentives programs as the standard of care) (Campbell et al., 2016; Campbell et al., 2017) may reach a broader and more representative sample of the target population. Related to this, excluding women who reported frequent marijuana use and being unwilling to quit may have also reduced the generalizability of study findings, although the relatively low proportion of women excluded for this reason (55 of 1,034 screened, or ~ 5%) suggests that impacts on generalizability were likely minimal. Last, the approximately 2-fold reduction in quit rates among women in the Incentives condition antepartum vs at their 12- and 24-week postpartum follow-ups indicates that enhanced efforts to sustain long-term smoking abstinence in this population are critical in order to protect infants against the potential adverse health impacts of secondhand smoke associated with relapse following delivery.
These limitations notwithstanding, results of the present pilot study are highly promising in terms of supporting the preliminary feasibility and efficacy of smartphone-based financial incentives for promoting smoking cessation during pregnancy. If this approach is successfully scaled up into a broadly applicable and sustainable remotely delivered treatment targeting this population, then it stands poised to make a substantial public health impact in terms of improving maternal and infant health outcomes, and reducing health disparities.
Highlights.
Examined feasibility and efficacy of remote financial incentives for pregnant smokers
Recruiting and retaining pregnant smokers in a remote cessation study was feasible
Financial incentives produced higher quit rates antepartum and postpartum vs best practices
Randomized controlled trials are needed to further examine feasibility and effectiveness
Acknowledgments
Funding: This project was supported in part by Centers of Biomedical Research Excellence P20GM103644 award from the National Institute on General Medical Sciences, and Research awards R01HD075669 from the National Institute of Child Health and Human Development (NICHD) and Centers for Disease Control and Prevention (CDC) and R01HD078332 from NICHD.
Footnotes
Conflicts of interest: None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alshaarawy O, Anthony JC, 2015. Month-wise estimates of tobacco smoking during pregnancy for the United States, 2002-2009. Matern Child Health J 19:1010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba S, Wikstrom AK, Stephansson O, Cnattingius S, 2012. Influence of smoking and snuff cessation on risk of preterm birth. Eur J Epidemiol 27:297–304. [DOI] [PubMed] [Google Scholar]
- Bagchi K, Kirs P, López F, 2008. The impact of price decreases on telephone and cell phone diffusion. Inf. Manage. 45:183–93. [Google Scholar]
- Bailey BA, 2013. Using expired air carbon monoxide to determine smoking status during pregnancy: preliminary identification of an appropriately sensitive and specific cut-point. Addict Behav 38:2547–50. [DOI] [PubMed] [Google Scholar]
- Barker DJ, 2004. The developmental origins of adult disease. J Am Coll Nutr 23:588S–95S. [DOI] [PubMed] [Google Scholar]
- Bauld L, Hackshaw L, Ferguson J, Coleman T, Taylor G, Salway R, 2012. Implementation of routine biochemical validation and an 'opt out' referral pathway for smoking cessation in pregnancy. Addiction 107 Suppl 2:53–60. [DOI] [PubMed] [Google Scholar]
- Boyd KA, Briggs AH, Bauld L, Sinclair L, Tappin D, 2016. Are financial incentives cost-effective to support smoking cessation during pregnancy? Addiction 111:360–70. [DOI] [PubMed] [Google Scholar]
- Cahill K, Hartmann-Boyce J, Perera R, 2015. Incentives for smoking cessation. Cochrane Database Syst Rev:CD004307. [DOI] [PubMed] [Google Scholar]
- Campbell KA, Bowker KA, Naughton F, Sloan M, Cooper S, Coleman T, 2016. Antenatal Clinic and Stop Smoking Services Staff Views on "Opt-Out" Referrals for Smoking Cessation in Pregnancy: A Framework Analysis. Int J Environ Res Public Health 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KA, Cooper S, Fahy SJ, Bowker K, Leonardi-Bee J, McEwen A, Whitemore R, Coleman T, 2017. 'Opt-out' referrals after identifying pregnant smokers using exhaled air carbon monoxide: impact on engagement with smoking cessation support. Tob Control 26:300–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain C, O'Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, McKenzie JE, 2017. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev 2:CD001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DR, Landes RD, Jackson L, Marsch LA, Mancino MJ, Chopra MP, Bickel WK, 2014. Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol 82:964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G, Jeffery H, Lagercrantz H, Katz-Salamon M, 2010. Long-term reprogramming of cardiovascular function in infants of active smokers. Hypertension 55:722–8. [DOI] [PubMed] [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM, 2010. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med 39:45–52. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, et al. , 2008. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services. [Google Scholar]
- Friedman LM, Furberg C, DeMets DL, 1998. Fundamentals of clinical trials, 3rd ed. Springer, New York. [Google Scholar]
- Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B, 2003. Multimodal intervention raises smoking cessation rate during pregnancy. Acta Obstet Gynecol Scand 82:813–9. [DOI] [PubMed] [Google Scholar]
- Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B, 2007. Determination of a saliva cotinine cut-off to distinguish pregnant smokers from pregnant non-smokers. Acta Obstet Gynecol Scand 86:401–6. [DOI] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, Badger GJ, Lynch ME, 2008. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction 103:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Chilcoat HD, 2009. Women and smoking: an interdisciplinary examination of socioeconomic influences. Drug Alcohol Depend 104 Suppl 1:S1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Mongeon JA, Solomon LJ, McHale L, Bernstein IM, 2007. Biochemical verification of smoking status in pregnant and recently postpartum women. Exp Clin Psychopharmacol 15:58–66. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM, 2006. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug Alcohol Depend 85:138–41. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, Lynch ME, Badger GJ, 2004. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 6:1015–20. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, Bernstein IM, 2012. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med 55 Supp1:S33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis-Hansen K, O'Donnell SE, Seidman JS, Brande SJ, Epstein LH, 2019. Improvements in episodic future thinking methodology: Establishing a standardized episodic thinking control. PLoS One 14:e0214397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ierfino D, Mantzari E, Hirst J, Jones T, Aveyard P, Marteau TM, 2015. Financial incentives for smoking cessation in pregnancy: a single-arm intervention study assessing cessation and gaming. Addiction 110:680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japuntich SJ, Regan S, Viana J, Tymoszczuk J, Reyen M, Levy DE, Singer DE, Park ER, Chang Y, et al. , 2012. Comparative effectiveness of post-discharge interventions for hospitalized smokers: study protocol for a randomized controlled trial. Trials 13:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Griesler PC, Schaffran C, 2009. Educational attainment and smoking among women: risk factors and consequences for offspring. Drug Alcohol Depend 104 Suppl 1:S24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komando K, 2012. Tapped? “LifeLine” program offers free phone, USA Today. [Google Scholar]
- Kurti AN, Bunn JY, Nighbor T, Cohen AH, Bolivar H, Tang KJ, Dallery J, Higgins ST, 2019. Leveraging technology to address the problem of cigarette smoking among women of reproductive age. Prev Med 118:238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurti AN, Redner R, Lopez AA, Keith DR, Villanti AC, Stanton CA, Gaalema DE, Bunn JY, Doogan NJ, et al. , 2017. Tobacco and nicotine delivery product use in a national sample of pregnant women. Prev Med 104:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie FM, 2013. Multigenerational epigenetic effects of nicotine on lung function. BMC Med 11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L, 2009. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev:CD001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR, 2011. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 216:305–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlman MK, Levy DT, 2016. Disparities in Maternal Child and Health Outcomes Attributable to Prenatal Tobacco Use. Matern Child Health J 20:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MR, Mason MJ, Brown AR, Garcia CM, Seibers AD, Stephens CJ, 2018. Remote biochemical verification of tobacco use: Reducing costs and improving methodological rigor with mailed oral cotinine swabs. Addict Behav 87:151–54. [DOI] [PubMed] [Google Scholar]
- Nighbor TD, Coleman SRM, Bunn JY, Kurti AN, Zvorsky I, Orr EJ, Higgins ST, 2020. Smoking prevalence among U.S. national samples of pregnant women. Prev Med 132:105994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JM, 2009. Tobacco and pregnancy. Reprod Toxicol 28:152–60. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Benoit RG, Szpunar KK, 2017. Episodic Future Thinking: Mechanisms and Functions. Curr Opin Behav Sci 17:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secades-Villa R, Garcia-Rodriguez O, Garcia-Fernandez G, Sanchez-Hervas E, Fernandez-Hermida JR, Higgins ST, 2011. Community reinforcement approach plus vouchers among cocaine-dependent outpatients: twelve-month outcomes. Psychol Addict Behav 25:174–9. [DOI] [PubMed] [Google Scholar]
- Staines GL, McKendrick K, Perlis T, Sacks S, De Leon G, 1999. Sequential assignment and treatment-as-usual. Alternatives to standard experimental designs in field studies of treatment efficacy. Eval Rev 23:47–76. [DOI] [PubMed] [Google Scholar]
- Stein JS, Tegge AN, Turner JK, Bickel WK, 2018. Episodic future thinking reduces delay discounting and cigarette demand: an investigation of the good-subject effect. J Behav Med 41:269–76. [DOI] [PubMed] [Google Scholar]
- Sze YY, Daniel TO, Kilanowski CK, Collins RL, Epstein LH, 2015. Web-Based and Mobile Delivery of an Episodic Future Thinking Intervention for Overweight and Obese Families: A Feasibility Study. JMIR Mhealth Uhealth 3:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD, 2009. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci 10:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombor I, Beard E, Brown J, Shahab L, Michie S, West R, 2019. Randomized factorial experiment of components of the SmokeFree Baby smartphone application to aid smoking cessation in pregnancy. Transl Behav Med 9:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombor I, Shahab L, Brown J, Crane D, Michie S, West R, 2016. Development of SmokeFree Baby: a smoking cessation smartphone app for pregnant smokers. Transl Behav Med 6:533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, 2006. The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general, Atlanta, GA. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, 2014. The health consequences of smoking—50 years of progress: A report of the Surgeon General, Atlanta, GA. [Google Scholar]
- Windsor RA, Lowe JB, Perkins LL, Smith-Yoder D, Artz L, Crawford M, Amburgy K, Boyd NR Jr., 1993. Health education for pregnant smokers: its behavioral impact and cost benefit. Am J Public Health 83:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HY, Subramaniyan M, Bullen C, Amer Siddiq AN, Danaee M, Yee A, 2019. The mobile-phone-based iCO(TM) Smokerlyzer((R)): Comparison with the piCO(+) Smokerlyzer((R)) among smokers undergoing methadone-maintained therapy. Tob Induc Dis 17:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Tombor I, Shahab L, West R, 2017. Usability testing of a smoking cessation smartphone application ('SmokeFree Baby'): A think-aloud study with pregnant smokers. Digit Health 3:2055207617704273. [DOI] [PMC free article] [PubMed] [Google Scholar]


