Abstract
Physical activity (PA) and exercise are among the most important determinants of health. However, PA is a complex and heterogeneous behavior and the biological mechanisms through which it impacts individuals and populations in different ways are not well understood. Genetics and environment likely play pivotal roles but further work is needed to understand their relative contributions and how they may be mediated. Metabolomics offers a promising approach to explore these relationships.
In this review, we provide a comprehensive appraisal of the PA-metabolomics literature to date. This overwhelmingly supports the hypothesis of a metabolomic response to PA, which can differ between groups and individuals. It also suggests a biological gradient in this response based on PA intensity, with some evidence for global longer-term changes in the metabolome of highly active individuals. However, many questions remain and we conclude by highlighting future critical research avenues to help elucidate the role of PA in the maintenance of health and the development of disease.
Keywords: Physical activity, Exercise, Metabolomics, The Metabolome, Health, Health promotion
1. Physical activity and health
Physical inactivity is a worldwide health problem, and is ranked as the fourth leading behavioral risk factor for global mortality [1]. The World Health Organization (WHO) recommends adults undertake at least 150 min moderate or 75 min vigorous intensity physical activity (PA) per week [2], conduct muscle strengthening activities twice a week, and minimize time spent being sedentary, with similar guidelines for under 18 s and older adults. Regular PA can reduce the risk of over 20 chronic conditions including coronary heart disease, stroke, type 2 diabetes, some cancers, obesity, mental health problems such as depression, and certain neurological conditions including dementia. Importantly, as well as reducing disease risk, it can also promote well-being and quality of life [3]. Consequently, harnessing the power of PA could have far-reaching preventive public health impacts. However, to do so is complicated by the fact that PA is not a single behavior but rather a complex and heterogeneous set of actions. It can be classified by domain (occupational, leisure-time, travel, home), dimension (frequency, intensity, duration), context (access to greenspace, facilities, and resources like money and social support), motivation (reasons for undertaking PA) and type (which can range from aerobic treadmill exercise through yoga to housework and gardening) [4].
As with many areas in population health the associations between risk (low PA) and health are well documented but the mechanisms involved and their complex interactions are not yet fully elucidated [5]. Despite growing epidemiological evidence for a relationship, how different dimensions and types of PA, and the domains and contexts in which they are undertaken, impact the health of individuals and populations in different ways is not well understood. Similarly, the reasons and mechanisms explaining why the same PA behaviors appear to have differential impacts within a population remain elusive. Genetics and environment likely play pivotal roles but further work is needed to understand their relative contributions and how they may be mediated [6]. Metabolomics offers a promising approach to explore these relationships.
2. Metabolomics
Metabolomics is the systematic study of all the metabolites (i.e. small molecules < 10 kDa, including carbohydrates, amino acids, organic acids, nucleotides and lipids) in a biological sample utilizing either Mass-Spectrometry (MS) or Nuclear Magnetic Resonance Spectroscopy (NMR) [7]. It provides a means of characterizing metabolic differences between groups and individuals, while informing understanding of the biology and biological mechanisms underlying these differences. The metabolome reflects the genome, the transcriptome and the proteome as well as their interactions with the environment. In this way, it is a means of studying the life course experiences of an individual [8]. The metabolome can capture past exposures (both short and longer term), indicate likelihood of future phenotypes and reflect current-status and responses [7]. As such, metabolomics is ideally suited to the study of PA. Its potential role in this field is evidenced, both by the increasing body of literature as well as by initiatives such as MoTrPAC [9] (The Molecular Transducers of Physical Activity Consortium, https://www.motrpac.org). This is a nationwide US research consortium funded by the US National Institutes of Health, that aims to study molecular, including metabolomic, changes relating to PA to improve the understanding of how PA influences health [10].
Widespread alterations in metabolite levels are known to occur when a biological system is in a dysregulated or exacerbated state. Accordingly, changes in components of the metabolome induced by intense and strenuous exercise regimes have been noted for many decades [11,12]. In fact, as early as 1975, Wahren et al. [13] argued that the metabolic status following 40–60 min of upright continuous bicycle exercise can be comparable to a few days starvation, with changes relating to gluconeogenesis, increased protein catabolism, increased urea excretion and the metabolism of nitrogenous substances [12,13]. Despite this, PA is known to induce numerous positive short and long health effects and can lead to beneficial long-term physiological adaptations [14].
This narrative review describes currently published literature on the metabolomics of PA (and exercise) in humans. Our aim is to understand the breadth and depth of the current evidence base, expanding upon and updating previous reviews in this area [15–17]. We highlight current understanding and potential novel research priorities by mapping the themes in the literature base, which continues to grow rapidly. Critical analysis of this literature will afford an improved understanding of the status of PA metabolomics, help to identify critical evidence gaps, and inform future studies [18].
The metabolomics of PA literature falls into two general groups based on their aims and study designs and populations: (i) observational studies that compare the average PA behaviors or overall fitness levels (a well-established outcome of PA), as assessed via questionnaire, fitness testing or participation in certain elite sports, and the corresponding metabolomes between groups (Table 1); and, (ii) experimental intervention studies that measure metabolites before, after and/ or during PA or exercise interventions. The intervention trials can be further subdivided into two categories; (ii.a) short-term interventions that focus on a discrete bout or set of bouts of PA/exercise within short time frame (< 1 week) (Table 2) and (ii.b) long-term interventions that assign individuals to training or PA/exercise interventions lasting > 1 week and compare individuals before and after this training (Table 3). For both short- and long-term interventions, a majority of studies compare the same individuals pre- and post, while the remainder compare different groups subject to the same or to differing interventions. There are also subsets of the literature that focus specifically on “at-risk” populations including overweight/obese individuals and those with certain diseases, and those that focus on elite athletes.
Table 1.
Observational studies included in this review.
| Author (year) | Primary aim | Metabolomic profiling platform | Biosample | Study population | Assessment of PA intensity/duration or fitness level | Sample collection timepoint | Primary findings |
|---|---|---|---|---|---|---|---|
| Kujala et al. (2013) | To explore whether persistent PA has a global effect on the serum metabolome that reduces cardiometabolic disease risk. | NMR | Serum. | Discovery: 16 same-sex twin pairs discordant for PA; Men and Women; 50–74 years; Follow-up: 1037 age- and sex-matched pairs that were either persistently active or persistently inactive; Men and Women; 41–62 years. | Validated questionnaires and MET index. | One sample concurrent with estimate of active/inactive status. | Persistently physically active individuals have a coherently healthier circulating metabolite profile than their inactive counterparts. Larger differences were noted with more discordant activity profiles and with increased length of discordant activity. |
| Morris et al. (2013) | To explore differences between adults by fitness level. | GC-MS | Urine and plasma. | 65 Men and Women; 18–60 years. | Cycle Ergometer based fitness test to assess maximal oxygen consumption levels (mL/kg/min). | One sample concurrent to fitness assessment. | Metabolomic profiles differed between fitness groups, specifically with respect to amino acids. Higher levels of branched chain amino acids were associated with lower fitness levels and higher insulin resistance. Most pronounced in females. |
| Floegal et al. (2014) | To investigate associations between diet, PA, cardiorespiratory fitness and obesity with serum metabolite networks. | MS | Serum. | 100 Men and Women; 35–65 years. | Combined heart rate and movement sensor. | Two samples 4 months apart. | Cardiorespiratory fitness was more strongly correlated with metabolic networks than measures of physical activity. |
| Fukai et al. (2016) | To investigate relationship between daily PA and metabolomic profile. | MS | Plasma. | 1193 Men; 35–74 years. | Self-reported questionnaires. | One sample concurrent to questionnaire. | PA is related to changes to the plasma metabolome, including known biomarkers for future insulin resistance and type 2 diabetes. |
| Xiao et al. (2016) | To identify a metabolic signature of PA. | LC-MS and GC-MS | Plasma. | 277 Men and Women, aged 40–65 + | Accelerometer-measured PA for 7 days on four occasions over 1 year. | Two samples; beginning and end of 1-year study period. | Differing patterns were observed for light versus moderate to vigorous PA. Overall volume of PA was the biggest driver of metabolomic profile. |
| Barton et al. (2017) | To explore the impact of extreme PA. | NMR, UPLC-MS, GC-MS | Urine and feces. | 46 male professional international rugby union players (mean (SD) age 29 (4) years) and 46 controls (mean (SD) age 29 (6)). | Adapted version of the EPIC-Norfolk physical activity questionnaire. | One sample concurrent to questionnaire. | Strong metabolomic differences observed between athletes and controls in urine, and to a lesser extent in fecal samples. |
| Al-Khelaifi et al. (2018a) | To compare blood metabolic profiles between moderate- and high-power and endurance elite athletes. | LC-MS | Serum. | 191 elite athletes (171 males and 20 females) from different sporting disciplines (anonymized data, age not reported). | Categorized according to sports which are classified by level of power, endurance and whether they are dynamic or static. | One sample concurrent to categorization. | High-power and high-endurance athletes exhibit a distinct metabolic profile that reflects steroid biosynthesis, fatty acid metabolism, oxidative stress, and energy-related metabolites. |
| Al-Khelaifi et al. (2018b) | To analyze the presence of various xenobiotics in serum samples from elite athletes of different sports. | LC-MS | Serum. | 478 elite athletes (anonymized data, age not reported). | Classified according to sport; football, athletics, cycling, rugby, swimming, boxing and rowing. | One sample concurrent to categorization. | Athletes from different sports exhibit a distinct xenobiotic profile that may reflect their drug/supplement use, diet and exposure to various chemicals. |
| Bell et al. (2018) | To determine metabolomic associations with total activity, moderate-to-vigorous PA and sedentary time. | NMR | Blood. | 1826 boys and girls; mean (SD) age 15.4 years (0.2 years). | Accelerometry assessed PA average over three days at age ~12 years, ~14 years and ~15 years. | One sample at age 15 years visit. | Higher total activity associates with a range of metabolites, and associations are strongest for moderate-to-vigorous activity. Associations of current activity with most metabolic traits do not differ by previous activity. |
| Ding et al. (2019) | To identify metabolites associated with habitual PA. | LC-MS | Plasma | 5197 Men and women; mean (SD) age ranged from 44.7 (4.5) to 64.0 (8.2) years across the cohorts. | Average of two questionnaires within two years of blood draw. | One sample within two years of both questionnaires. | 10 habitual PA associated metabolites were replicated in two large scale cohort studies. |
| Author (year) | Primary aim | Metabolomic profiling platform | Biosample | Study population | Exercise/physical activity intervention or test | Sample collection timepoint | Primary findings |
| Sun et al. (2017) | To identify changes in metabolomic pathways in urine after an 800-meter run. | NMR | Urine. | 19 athletes, men mean (SD) age 19.2 (0.7) years. | 800-meter run. | Two samples; before and 35 min after run. | Results indicated changes in the urinary metabolomic profile after exertion indicating increase in oxidative stress. |
| Davison et al. (2018) | To examine the role of exercise in hypoxia using metabolomics profiling. | LC-MS | Serum. | 24 men mean (SD) age 28 (5). | 1-hour exercise in hypoxia and in normoxia. | Three samples; pre-exercise and immediately and 3 h post exercise. | There are small differences in the metabolomic changes associated with exercise and recovery in hypoxia and normoxia, but both affect pathways of purine and pyrimidine metabolism. |
| Howe et al. (2018) | To investigate the physiological responses to prolonged physical exertion. | LC-MS | Plasma. | 9 ultramarathon runners, men mean (SD) age; 34 (7) years. | 80.5 km self-paced treadmill-based time trial. | Two samples pre and immediately post exercise. | The findings provide a potential explanation for the cardio-protective effects of ultramarathon running. |
| Stander et al. (2018) | To characterize the acute metabolic changes induced by a marathon. | GC-MS | Serum. | 31 runners; men mean (SD) age 41 (12) years. | Marathon running. | Two samples: pre and post marathon. | Running a marathon places immense strain on the energy-producing pathways of the athlete, leading to extensive protein degradation, oxidative stress and autophagy. |
| Valerio et al. (2018) | To compare the metabolomic response to high load versus low load resistance exercise. | NMR | Serum. | 9 well trained men, mean (SD) age 26.4 (4.4) years. | Resistance exercise: Leg presses at difference loads. | Two samples pre and 5 min post each trial. | Both high and low load resistance training elicited changes in the metabolome relative to controls; and there were additional differences by load. |
| Manaf et al. (2019) | To identify changes in the plasma-metabolome associated with the onset of fatigue during prolonged cycling. | LC-MS | Plasma. | 18 men; aged 18–24 years. | Cycling to exhaustion. | Four samples; 10 min after exercise onset, pre-fatigue, post-fatigue and 20 min post fatigue. | There was a unique metabolomic trajectory associated with time to fatigue and recovery. |
| Manaf et al. (2019) | To identify changes in the plasma-metabolome associated with the onset of fatigue during prolonged cycling. | LC-MS | Plasma. | 18 men; aged 18–24 years. | Cycling to exhaustion. | Four samples; 10 mins after exercise onset, pre-fatigue, post-fatigue and 20 min post fatigue. | There was a unique metabolomic trajectory associated with time to fatigue and recovery. |
| Pitti et al. (2019) | To compare the saliva metabolome before and after a soccer match. | NMR | Saliva. | 17 professional team soccer players, women mean (SD) age across groups ranged from 18 (1) to 23 (5) years. | Soccer/Football match. | Two samples; pre and postgame. | PCA demonstrated a separation in the metabolomic profile of players before and after the game. Changes were driven by amino acids and energy metabolites. |
| Siopi et al. (2019) | To investigate whether the response of the human serum metabolic fingerprint to exercise depends on exercise mode. | LC-MS | Serum. | 23 sedentary men; age not reported. | Four trials: resting, high-intensity interval exercise (HUE), continuous moderate-intensity exercise (CME), and resistance exercise (RE). | Three samples; pre-exercise, immediately post-exercise and 1-hour post-exercise. | The largest changes from baseline were found in the immediate post-exercise samples. RE caused the strongest responses overall, followed by HUE, while CME had minimal effect. |
| Schader et al. (2020) | To compare training induced metabolic shifts across fitness levels. | LC-MS | Plasma. | 76 runners; men mean (SD) age between groups ranged from 32.8 (7.9) to 50.5 (7.9) years. | Marathon running. | Five samples; five weeks and one week before the race and immediately, 24 h, and 72 h after the race. | Prolonged intense exercise is associated with an extensive and prolonged perturbation in plasma metabolite concentrations that is greater in the slower, less aerobically fit runners. |
All Individuals were ‘healthy’ at blood draw unless otherwise stated.
GC–MS – Gas Chromatography–Mass Spectrometry.
LC-MS - Liquid Chromatography-Mass Spectrometry.
MET - metabolic equivalent of task.
MS – Mass Spectrometry.
NMR - Nuclear Magnetic Resonance Spectroscopy.
PA – Physical Activity.
SD – Standard Deviation.
UPLC-MS – Ultra Performance Liquid Chromatography- Mass Spectrometry.
BM – Boston Marathon.
CE-TOFMS – Capillary Electrophoresis Time of flight mass spectrometry.
CME – Continuous moderate exercise.
ETT – Exercise Testing Protocol.
HIIE – High intensity interval exercise.
LC/TOF-MS – Liquid chromatography/Time of flight Mass spectrometry.
MS/MS – tandem Mass Spectrometry.
OPLS - Orthogonal partial least squares.
PCA – Principal components analysis.
RE – Resistance exercise.
Table 2.
Short-term intervention studies included in this review.
| Author (year) | Primary aim | Metabolomic profiling platform | Biosample | Study population | Exercise/physical activity intervention or test | Sample collection timepoint | Primary findings |
|---|---|---|---|---|---|---|---|
| Sabatine et al. (2005) | To compare metabolomic pathways activated with exercise in myocardial ischemia cases versus controls. | LC-MS | Plasma | 18 ischemia cases (mean (SD) age 64 (10) years) and 18 controls (mean (SD) age 65 (11) years); men and women. | Exercise testing with standard Bruce protocol. | Two samples before and after exercise testing. | Demonstrated significant changes after exercise stress testing in circulating levels of multiple metabolites; identified clusters of metabolites that were altered in all individuals, and metabolites that showed discordant effects after exercise in ischemia cases versus controls. |
| Enea et al. (2009) | To investigate biochemical changes due to short-term and prolonged PA. | NMR | Urine | 22 trained and untrained women; mean (SD) age between 20.7 (0.7) years to 23.0 (1.9) years. | Prolonged exercise test until exhaustion and short-term intensive test. | Two samples before and after each exercise test. | Metabolic changes were observed in urine after a short-term intensive exercise test; the extent of the changes depended on prior training status. |
| Kirwan et al. (2009) | To demonstrate the potential for 1H NMR analysis in exercise biochemistry. | NMR | Plasma | 7 endurance-trained cyclists/ triathletes, men, age not reported. | Intermittent exhaustive cycling. | Eight samples, prior to and immediately following exercise and at 30, 60, 90, 120, 140 and 280 min during the 4-h passive recovery period. | Differences in the response to exhaustive exercise and subsequent recovery varied according to baseline metabolic substrate levels. |
| Lehmann et al. (2010) | To capture the network of metabolites regulating the beneficial effects of exercise. | LC/TOF-MS | Plasma | 21 men in two groups, mean (SD) age 32.6 (6.1) years in first group, 30.9 (5.8) years in second group. | 60 or 120-min treadmill run. | Four samples; before and after running and 3 and 24 h into recovery. | An increase in acylcarnitines during exercise supports fat oxidation and may exert beneficial biological functions. |
| Lewis, et al. (2010) | To explore exercise-induced metabolic responses. | LC-MS | Plasma | Exercise testing protocol (ETT) - 78 men and women, mean (SD) age ranged between 48 (14) and 59 (12) years; Boston Marathon (BM) Cohort- 25 healthy amateur runners men and women mean (SD) age 42 (9) years. | Diagnostic treadmill ETT or bicycle ergometry cardiopulmonary exercise testing. | ETT; three samples at baseline, peak exercise and 60 min after completion; BM - two samples before and after marathon. | Acute and long-term exercise elicited a metabolic response. Metabolic responses differed by BMI by and baseline fitness level, and in those with ischemia. |
| Pechlivanis et al. (2010) | To investigate changes in urine metabolome elicited by two differing exercise sessions. | NMR | Urine | 12 men in two groups; mean (SD) age in exercise group 1; 21 (2) years, mean (SD) age in exercise group 2; 20 (1) years. | Three sets of two 80 m maximal runs, separated by 10 s or 1 min of rest. | Two samples: before and 35 min after exercise session. | Urine metabolomic profiles could distinguish between pre- and post-exercise and between the two different exercise groups; perturbations were more pronounced when the time between exertions was shorter. |
| Krug et al. (2012) | To explore the response of the metabolome to a PA challenge. | MS/MS and NMR | Plasma, Urine, Exhaled air, Breath condensate. | 15 men; mean (SD) age 27.8 (2.9) years. | 30-min bicycle ergometer. | Twelve samples: Plasma and exhaled air was collected before the physical activity test (PAT), during the PAT at 15 and 30 min, and after the PAT at 45, 60, 90, and 120 min. EBC was collected at 0, 60- and 120-min. Urine was collected at 0 and 120 min. | Changes in the metabolomic profile were observed immediately follow the initiation of the challenge but returned to almost normal within 2 h of completion. Inter-subject variation increased even more during the challenges. |
| Nieman et al. (2013) | To investigate changes in serum metabolome elicited by a 3-day period of intensified training. | GC-MS, LC-MS | Serum | 15 men (mean (SD) age 35.5 (9.2) years) and women (mean (SD) age 35.1 (8.9) years) runners. | 3-day intense running (2.5 h on treadmill/day). | Three samples; pre-exercise, immediately post-exercise and 14 h post-exercise. | Runners experienced a profound systemic shift in blood metabolites related to energy production especially from the lipid super pathway following 3 days of heavy exertion that was not fully restored to pre-exercise levels after 14 h recovery. |
| Mukherjee et al. (2014) | To understand molecular mechanisms mediating the beneficial adaptations of exercise in older adults. | NMR | Urine | Competitive cyclists (n = 9) and untrained, minimally active controls (n = 8); men aged 50–60 years old. | Submaximal endurance cycle. | Pre-exercise, immediately post exercise and 24 h post exercise. | Post exercise, highly trained competitive athletes have a characteristic metabolic footprint that differs from non-trained, minimally active older individuals. |
| Nieman et al. (2014) | To identify metabolomic correlates of oxidative stress during endurance exercise. | LC-MS and GC-MS | Plasma | 19 cyclists, men aged 27–49 years old. | 75-km cycling on an ergometer. | Four samples; before and immediately, 1.5 and 21 h after exercise. | Metabolomics confirms the role of oxidative stress and fatty acid metabolism with endurance exercise. |
| Peake et al. (2014) | To assess effects of high-intensity interval training (HUT) vs. work-matched moderate-intensity continuous exercise (MOD) on metabolism. | GC-MS | Plasma. | 10 well-trained cyclists and triathletes; men mean (SD) age 33.2 (6.7) years. | Electromagnetically braked cycle ergometer until exhaustion. | Four samples: before, immediately-post and 1- and 2- hours post exercise. | Although many metabolic changes were the same, there were also some distinct differences in specific metabolites following HUT vs. MOD. |
| Ra et al. (2014) | To identify salivary fatigue markers. | CE-TOFMS | Saliva. | 37 fatigued soccer players, mean (SD) age 20.6 (0.04) years. | 3 consecutive days of soccer. | Two samples; before and after 3 days of soccer. | Salivary metabolites increased after three days of soccer may represent novel biomarkers of fatigue. |
| Zheng et al. (2014) | To investigate the effects of PA on the adolescent metabolome. | NMR | Urine, plasma. | 192 overweight adolescents; 12–15 years old. | Pedometer for 7 consecutive days. | Two samples: at baseline and twelve weeks later. | No strong correlation could be identified neither between the plasma nor the urine metabolome and daily PA. |
| Wang et al. (2015) | To determine the effects of different levels of training exercises on the urine metabolome. | NMR | Urine. | 12 professional half-pipe snowboarders, men and women age 25–25 years. | Strength, endurance, and trampoline exercises at three different intensity levels. | Three samples; at three timepoints throughout. | A PCA plot was able to distinguish between the different intensity levels. Results show that organisms reach a relatively stable physical state to adapt to the training load after long-term training. |
| Danaher et al. (2016) | To compare the metabolomic effects of workload matched high intensity trials. | GC-MS | Plasma. | 7 untrained men, mean (SD) age 22.9 (5) years. | 30 min cycling at two different intensities. | Four samples; at rest (preexercise), 10 min into exercise, immediately after exercise and after 60 min recovery. | The high intensity protocol elicited greater metabolic changes relating to lipid metabolism and glycolysis than the moderate intensity. The changes were more pronounced throughout the recovery period than during the exercise. |
| Muhsen et al. (2016) | To explore the metabolomic effects of submaximal exercise. | LC-MS | Urine. | 10 men and women, 23–48 years. | Braked cycle ergometer at light and moderate intensities for 45 min. | Three samples: Day before, day of and day after exercise. | PCA could separate between pre- and post-exercise samples. |
| Zafeiridis et al. (2016) | To compare the metabolic profile of three aerobic exercises matched for effort/strain. | NMR | Plasma. | 9 active men; mean (SE) age 20.5 (0.7) years. | Continuous, long-interval (3 min), and short-interval (30 s) bouts of exercise. | Two samples for each protocol; pre and post exercise. | OPLS demonstrated a distinct separation in metabolomic profiles between pre and post exercise samples, but no differences between the three different regimens. |
| Berton et al. (2017) | To explore the metabolomic response to resistance exercise. | NMR | Serum. | 10 men, mean (SD) age 24 (2) years. | Resistance exercise: leg press and knee extension exercises. | Six samples: 1-hour pre and immediately post exercise and 5, 15, 30- and 60-minutes post exercise. | Resistance exercise induced changes in the serum metabolome. |
| Couto et al. (2017) | To investigate the effect of a swimming training session on oxidative stress markers of asthmatic compared to non-asthmatic elite swimmers using exhaled breath metabolomics. | GC-MS | Exhaled breath. | 20 elite swimmers (including 9 with asthma); men and women aged 13–24 years old. | 1-hour Swimming training session. | Two samples; before and after exercise. | In well-trained athletes, swimming is associated with a decrease in oxidative stress markers independently of the presence of asthma, although a more pronounced decrease was seen in controls. |
| Karl et al. (2017) | To explore metabolomic changes associated with strenuous exercise. | LC-MS | Plasma. | 25 army Soldiers, men mean (SD) age 19 (1) years. | 4-day, 51-km cross-country ski march. | Two samples before and after march. | Observed increases in energy metabolism, lipolysis, fatty acid oxidation, ketogenesis, and branched-chain amino acid catabolism. |
| Messier et al. (2017) | To determine whether the metabolomic pathways used during endurance exercise differ according to whether the effort is performed at sea level or at moderate altitude. | NMR | Plasma. | 20 men, mean (SD) age 39 (4.3) years. | 60 min cycle ergometer. | Two samples before exercise and after 60 min (when participants were still peddling). | At similar exercise intensity, substrate use during endurance exercise differed by altitude level. |
| Nieman et al. (2017) | To determine the relationship between exercise-induced increases in IL-6 and lipid-related metabolites. | LC-MS and GC-MS | Plasma. | 24 runners, men aged 22–55 years old. | Treadmill to exhaustion. | Two samples before and after exercise testing. | There was a clear separation in metabolomic profiles pre and post exercise. |
| Prado et al. (2017) | To understand exercise-induced changes during a soccer match. | MS | Urine and blood. | 30 professional soccer players, men aged 18–20 years old. | Soccer/Football match. | Two samples pre-match and postmatch. | Hypoxanthine and related metabolites were upregulated in urine after a soccer match, suggesting adenosine monophosphate deamination was increased. |
Table 3.
Long-term intervention studies included in this review.
| Author (year) | Primary aim | Metabolomic profiling platform | Biosample | Study population | Exercise/physical activity intervention or test | Sample collection timepoint | Primary findings |
|---|---|---|---|---|---|---|---|
| Yan et al. (2009) | To investigate alterations in metabolic phenotype of professional athletes induced by long-term training. | GC/TOF-MS | Venous blood | 28 men: 16 professional rowers (mean age (SD) 23 (2.7) years) and twelve controls (mean age (SD) 23.8.(1.5) years) | 2 weeks of technical and aerobic exercise. | Three samples: before training and after one- and two-weeks training. | Significant metabolomic differences observed between professional athletes and controls. Long-term strength and endurance training induced distinct separation between athletes by seniority, training stage and training |
| Huffman et al. (2011) | To profile the metabolome at baseline, after 6 months of exercise training, and 2 weeks after exercise training cessation. | Targeted MS | Plasma | 53 middle-aged, overweight and moderately obese, inactive participants, men and women (age not reported) | 6 months supervised aerobic exercise training. | Three samples; baseline, after 6 months of exercise training and two weeks after exercise cessation. | Improvements in insulin sensitivity with the intervention was associated with reductions in circulating free fatty acids and increased levels of glycine and proline. These changes persisted two weeks post intervention cessation. |
| Neal et al. (2012) | To investigate physiological adaptation with two endurance-training periods differing in intensity distribution. | NMR | Urine | 12 cyclists, men, mean age (SD) 37 (6) years | Two 6-week training periods following two models in a crossover design. 1. polarized training model (—75–80% low intensity, 5–10% moderate intensity, 15–20% at high intensity); and 2. threshold training model (40–50% moderate intensity, little high intensity and the remainder at low intensity). | Two samples; before and after exercise. | Some markers of cellular energy stress were modified with low intensity but not with high intensity training. |
| Pechlivanis et al. (2013) | To investigate changes in the human serum metabolome elicited by two differing exercise sessions. | NMR | Serum | 14 men in two groups; group A mean (SD) age 21 (2) years; group B mean (SD) age 20 (2) years. | 8-week training program involving sets of two 80 m maximal runs, separated by (group A) 10 s or (group B) 1 min of rest. | Two samples: during a training session in week 1 and during a training session in week 8. | Serum metabolomic profiles could distinguish between pre- and post-exercise and between the two different exercise groups; there was greater dispersion among the post exercise samples, indicating greater variation in the response to exercise. |
| Huffman et al. (2014) | To evaluate the effect of exercise training on muscle metabolic signatures. | MS/MS GC-MS | Muscle | 112 men and women at risk of metabolic disease. | Six months following one of the six interventions: (i) low-amount moderate-intensity exercise; (ii) low-amount vigorous-intensity exercise; (iii) high-amount vigorous-intensity exercise; (iv) resistance training; (v) linear combination of low-amount vigorous-intensity exercise and resistance training; (vi) continued inactivity, age 18–70 years. | Two samples; before and 16–25 h after the last exercise bout. | Dose response relationship between exercise regime energy expenditure and increase in muscle concentrations of acyl carnitines. The smallest changes were seen in the resistance training group. |
| Felder et al. (2017) | To identify endogenous metabolites that distinguish the trained from the untrained state. | LC-MS | Serum | 37 men, 45–65 years. | Electronically braked cycle ergometer before and after completion of a ten-week exercise program. | Two samples: before and after exercise program. | Detected metabolites that differed between the trained and untrained state. |
| Meucci et al. (2017) | To explore metabolomic changes associated with PA. | GC/TOF-MS | Urine | 22 recreationally active overweight preadolescents, boys and girls 8–12 years old. | 4 week or 8 week supervised, play-based PA for 6 h per day, 5 h per week, and one group with unsupervised play. | Two samples pre and post intervention. | After an eight-week PA intervention there were significant metabolomic differences between a control group who did not undergo the intervention, and between the pre- and post-exercise samples of the children who underwent the intervention. No change was evident after four weeks. |
| Brennan et al. (2018a) | To explore metabolomic changes associated with varying exercise interventions. | LC-MS | Plasma | 216 middle-aged abdominally obese men and women; age 52.5 (8.0) years. | Four intervention groups varying in exercise amount and intensity for 6 months. | Three samples; baseline and at 48 h after the last session at 16 and 24 weeks. | There were no differences between specific intervention groups, or in the exercise group compared to the control group. |
| Brennan et al. (2018b) | To examine changes in the metabolome following chronic aerobic exercise. | LC-MS/MS | Plasma | Three hundred abdominally obese men and women, mean (SD) age ranged between groups from 51.8 (8.3) to 55.1 (6.6) years. | Four intervention groups varying in exercise amount and intensity for 6 months. | Three samples; baseline and at 48 h after the last session at 24 weeks. | Few metabolites changed with exercise compared to controls, therefore associations between adipose tissue deposits and metabolites where not sue to exercise induced adipose tissues reduction. |
| Grapov et al. (2020) | To investigate how the effects of insulin resistance on the metabolome are altered by exercise and fitness. | GC-MS | Plasma | 12 insulin resistant obese women aged 30–50 years. | Cycle Ergometer before and after a 14-week training and weight loss intervention. | 11 samples at 5 min increments during 30 min cycling and postcycling cool down. The same 11 sample procedure was performed before and after the intervention. | The long-term intervention had little impact on the global metabolome, however the excise itself strongly impacted the metabolome. |
| Sakaguchi et al. (2020) | To investigate the chronic effect of inspiratory muscle training (IMT) on the metabolome. | NMR | Serum | 28 cyclists randomized to 3 levels of IMT intensity for 11 weeks or a control group, men aged 20–40 years old. | Inspiratory Muscle Training for 11 weeks. | Two samples; one week before and 11 weeks after IMT. | Metabolites shifts did not differ by IMT intensity level or in comparison to controls, indicating physical training has negligible effects on the serum metabolome. |
All Individuals were ‘healthy’ at blood draw unless otherwise stated.
GC–MS – Gas Chromatography–Mass Spectrometry.
GC/TOF-MS – Gas Chromatography/Time of Flight-Mass Spectrometry.
IMT – Inspiratory Muscle Training.
LC-MS - Liquid Chromatography-Mass Spectrometry.
MS – Mass Spectrometry.
MS/MS – Tandem Mass Spectrometry.
NMR - Nuclear Magnetic Resonance Spectroscopy.
PA – Physical Activity.
SD – Standard Deviation.
A brief methods description is provided in Supplementary Methods, and the included studies are described in Tables 1, 2 and 3. When reviewing the literature, “Physical activity” and “exercise” are important terms to distinguish. As mentioned above, exercise is a sub-type of PA generally more structured and repetitive, and more strongly associated with health and fitness [19]. Laboratory studies employing treadmills, ergometers, etc. are more likely to be using “exercise” as their independent variable. However, exercise has been shown to be a small contributor to overall population PA levels [20,21], and as such differentiating is important to understanding the implications of PA metabolomics evidence. We found that the terms PA and exercise have been variously and inconsistently defined throughout these studies, and at times used synonymously, although the majority of studies focus on structured exercise. As such we have used the term “PA/exercise” where appropriate so as not to miss-represent the evidence base, and where necessary we have employed the wording used in the cited study. Despite these inconsistencies in definitions, there are a number of overarching themes that are consistent throughout the existing literature as we report below.
3. The PA/exercise metabolome
The literature to date, with some notable exceptions [22–25], overwhelmingly concludes that varying degrees of PA or exercise induce, or are associated with, quantifiable changes in the metabolome, as measured by MS and NMR spectroscopy in biological samples [15–17]. The metabolomic alterations associated with overall PA/exercise are most commonly characterized by changes in fatty acid metabolism, mobilization and lipolysis, the TCA cycle, glycolysis, amino acid metabolism, carnitine metabolism, purine metabolism, cholesterol metabolism and insulin sensitivity among others [26–69] (Fig. 1). These findings broadly reflect the known physiological adaptations to PA/exercise. The reported pathways and metabolites are inextricably linked through their roles in increased anaerobic and aerobic metabolism, increased Adenosine Triphosphate (ATP) turnover, changes in substrate utilization energy metabolism, lipolysis, fatty acid oxidation, ketogenesis, branched-chain amino acid catabolism, glutathione metabolism and oxidative stress [31,37,44,47,60,62,70].
Fig. 1.
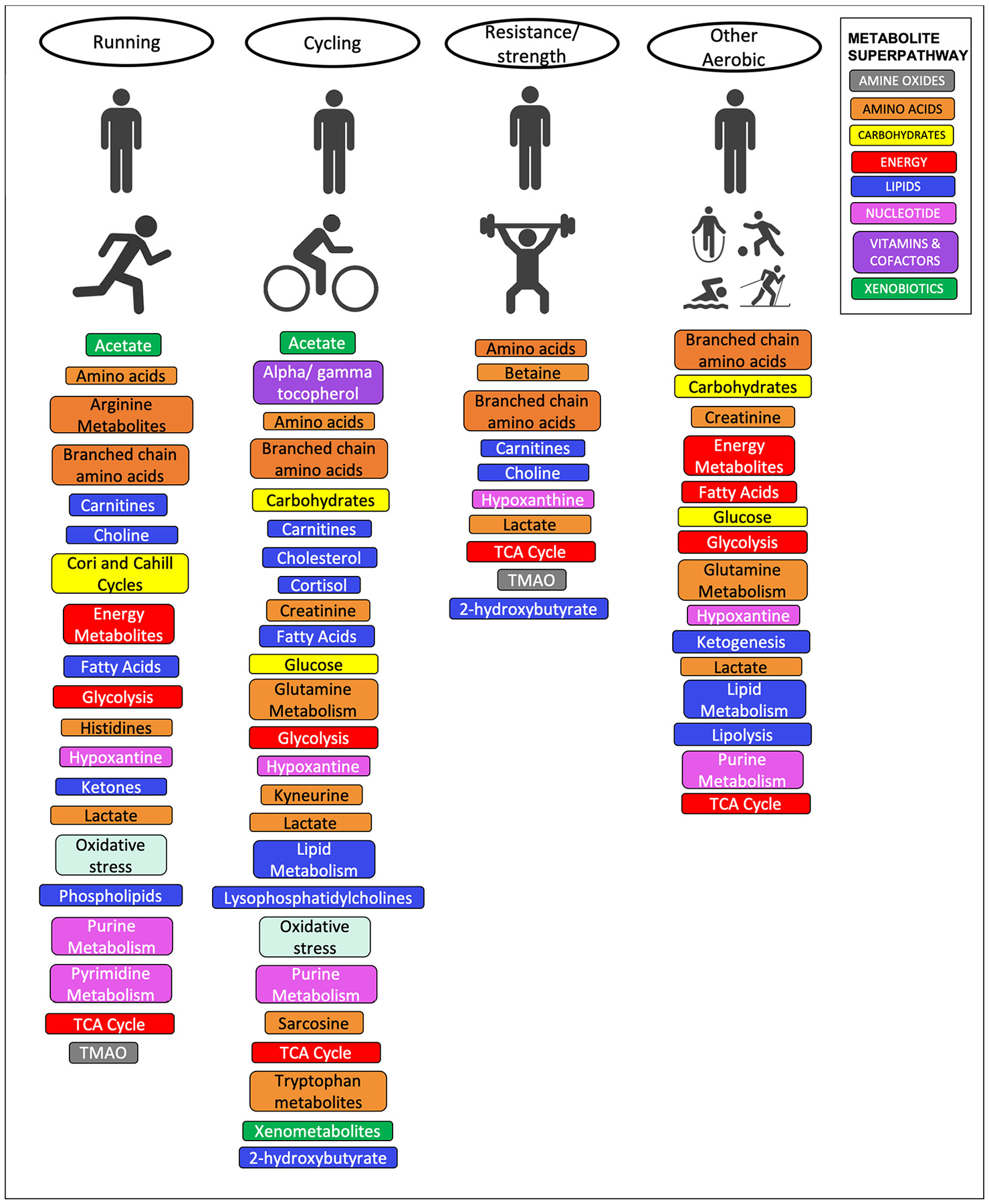
Metabolites, metabolomics pathways and biological processes reported in the literature for different categories of physical activity interventions. Metabolites and pathways are colored according to Metabolon Superpathway [71] where available, or else are denoted according to their biological process.
There is some debate in the literature as to which components of PA/exercise lead to the most pronounced changes. Some of the strongest reported findings related to the most “intense exercise”, as defined by variously by kJ expenditure, peak and type [38,39,51,53,61], and among those studies that did not see a PA/exercise associated change in the metabolome, Zheng et al., postulated that this may be due to low intensity levels in their study [22]. However, a number of other studies considering only moderate and low intensity PA/exercise have reported significant findings, and those studies directly comparing metabolomic patterns associated with light, moderate and vigorous PA/exercise patterns generally conclude that while the metabolomic patterns may differ slightly between these groups, overall volume was the biggest driver of the metabolomic profile [27,39,41,72]. This points to a multifactorial dose response relationship between PA/exercise and its effect on the metabolome, incorporating intensity, bout length, frequency and timeframe of exposure (hours, days, weeks, years).
4. Acute versus long-term effects of PA/exercise on the metabolome
The evidence therefore suggests PA/exercise affects the metabolome, but the question of when these changes occur and for how long they persist, is more complex. However, it is crucial to understand the effects of long-term and sustained PA/exercise as they may have important implications for weight loss or training and for public health more generally. It has been reported in several studies that PA/exercise associated metabolic alterations were evident almost as soon as the activity begins [15,34,39]. However, the studies differed in their findings during the post-activity and recovery phases; some reported a return to a pre-activity metabolic state almost immediately (within minutes) while others reported the effects of acute PA/exercise was evident in the levels of some metabolites for hours [31,34,35,67] or even days [54,66]. Importantly, it was also noted that the timing of the change in metabolite levels and how long they persisted differed depending on the length and intensity of the exertion [35].
Differences were further noted between metabolites and metabolite classes [35]. For example, metabolic pathways responsible for skeletal muscle substrate utilization were shown to be changed immediately after “acute exercise”, while changes in metabolites of the TCA cycle were apparent 60 min later [31,55]. These differences likely reflect the timing of the physiological adaptations to the onset of the activity and the metabolites corresponding to this physiology. Amino acids including leucine, isoleucine, asparagine, methionine, lysine, glutamine, alanine and lipids have been reported to remain significantly decreased after 14 h of post-activity recovery while changes in plasma medium-and long-chain fatty acids, ketone bodies, bile acids and triacylglycerol esters, have also been shown to persist for many hours after activity [35], before eventually returning to pre-activity levels [15].
While the literature undoubtedly supports the existence of acute metabolomic effects of PA/exercise, many critical public health outcomes manifest over timeframes of weeks, months, years and decades. Therefore, it is important to also study and understand the longer term metabolomic and health changes associated with PA/exercise. Observational studies provide further insights into these longer-term effects by directly comparing groups of individuals with differing levels of previous exposure in a cross-sectional manner to determine whether their metabolomes differed (Fig. 2) [46,70,72–76]. Overall, the findings we present in this paper suggest that there may also be a chronic adaptation in the metabolome in response to a long-term PA/exercise regime that is characterized by changes in lipids and amino acids. A large study of men in Japan that used self-reported questionnaires to assess metabolomic changes with long-term PA, observed lower levels of amino acids and higher levels of pipecolate in more active men [70], while a study of over 5000 individuals from four cohort studies replicated a number of long term PA associated metabolites including several lipids [76]. However, the underlying metabolic variability between individuals due to the inherently dynamic nature of the metabolome can render the comparison of groups based on a single metric complex, particularly when the actual changes in metabolite levels have been shown to be small [75]. To overcome this, Kujala et al. compared sets of persistently discordantly active twins over multiple decades. They determined that with persistent PA over time the fatty acid composition of the metabolome shifted from a saturated to a more polyunsaturated profile, with lower levels of glucose, and isoleucine among the active [73]. Importantly, they were able to validate these findings in an independent population-based cohort. Among the intervention studies, while some studies comparing metabolomic profiles of individuals before and after a long-term or PA/exercise intervention observed a clear distinction in the pre- and post-metabolomics profiles [36,43,66,68], several others have reported no differences [22–25,69]. Consequently, further work is needed to disentangle whether long-term PA/exercise can shift the metabolome, and by how much.
Fig. 2.
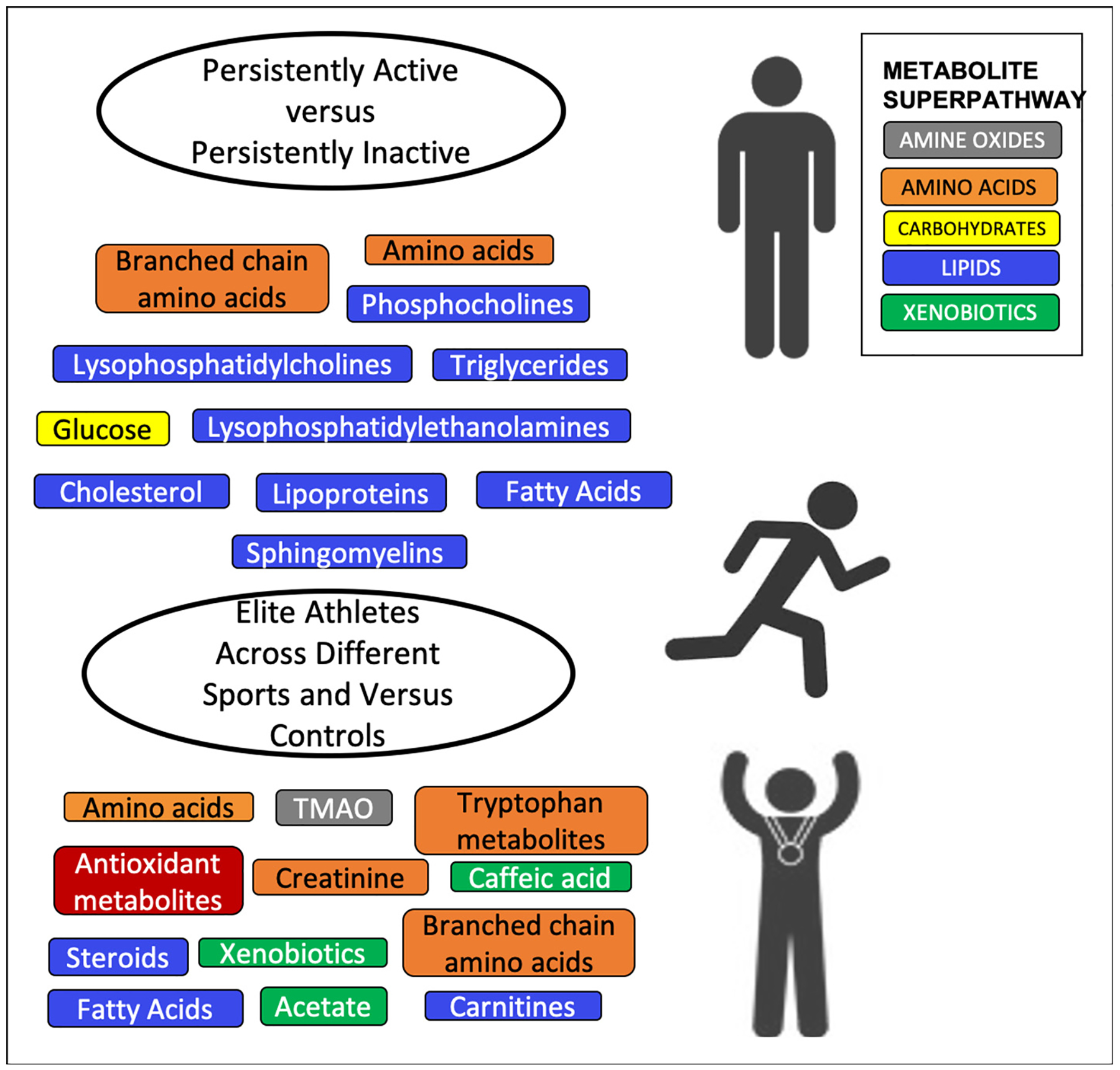
Metabolites, metabolomics pathways and biological processes reported in the literature in observation PA studies. Metabolites and pathways are colored according to Metabolon Superpathway [71] where available, or else are denoted according to their biological process.
With the assumption that there are metabolic differences between persistently physically active versus persistently inactive people, several studies have suggested these differences may be even greater when undertaking PA/exercise, than when at rest [29,32,34,36]. This indicates that the adaptations in the metabolome induced by a PA/exercise regime may continue to influence an individual’s response to acute activity [29,56]. Interestingly, Schader et al. noted that among men running a marathon, it is the slower, less aerobically fit runners who experience the greatest perturbation in metabolite levels, specifically phospholipids and amino acids [54] The hypothesis of a possible metabolomic adaption to PA/exercise is further supported by evidence that metabolic signatures of “exercise-trained” skeletal muscle reflected reprogramming of mitochondrial function and intermediary metabolism [67]. However, not all studies are in agreement with it being argued by some that “associations of current activity with most metabolic traits do not differ by previous activity” [75].
A subset of the literature is specifically focused on elite athletes, in whom it may be assumed that any metabolomic adaptations would be most pronounced. It is well known that the physiology of athletes differs from that of the normal population in a variety of ways. Depending on the type of athlete these may include greater heart function (e.g. stroke volume, left ventricular volume), higher maximal oxygen utilization (VO2 max), greater lung volume, lower body fat percentage and differing fiber-type compositions of the muscles [16]. What the metabolomics literature now indicates, is that athletes may also differ in terms of an altered metabolome (Fig. 2). In particular, differing levels of lactate, β-d-methylglucopyranoside, pyroglutamic acid, free fatty acids, amino acids, carnitines, trimethylamine-N-oxide (TMAO), and carboxylic acid derivatives, among others, have all been observed between athletes and untrained individuals. Taken together, this points to underlying differences in intermediary metabolism, fuel substrate utilization, glucose transport, fatty acid oxidation, oxidative stress, steroid biosynthesis, insulin signaling and microbial density between these groups [26,27,55,56,58].
There is also a body of literature investigating whether the type of PA/exercise influences the metabolomic response. Metabolomic differences between sporting groups, including athletics, boxing, cycling, football, rowing, rugby and swimming, have been reported [27]. One study identified differences between the goal keeper and the field players participating in the same game [65]. Additional differences have been noted between athletes competing in dynamic (e.g. marathon running) as opposed to static sports (e.g. weightlifting) and those competing in sports considered to be both dynamic and static such as rowing or boxing [27]. In particular, elevated levels of metabolites involved in sex steroid hormone biosynthesis and rapid changes in whole-body substrate utilization characterized by upregulated lipolysis, fatty acid oxidation, amino acid oxidation, ketogenesis, and glycolysis have been observed [27,30,35,44]. Again, these differences are most likely explained by the intensity as a function of the effort, strain and duration of a given activity rather than the protocol itself [38,41,53,66]. This would provide a rationale for the finding of an increased augmentation of pantothenate and fumarate in individuals who had just completed a marathon, which were not observed after a short-term treadmill intervention, as well as metabolomic differences between faster versus slower marathon runners [31]. Consequently, it has been argued that when comparing different training modalities, it is vital to match on overall effort [41].
5. Metabolomics and fitness
It should further be noted that, some of these differences may be related to pre-existing fitness levels rather than the PA/exercise dose. As well as differences in age, and geography between the cohorts, there are strong socioeconomic differences in participation in different sports that could (at least in part) explain metabolomic differences [77]. Similarly, some sports may require very specific dietary regimes that impact the metabolome, while others such as weight-lifting, may both promote specific diets as well as select for people with a certain underlying physiology or phenotype. Furthermore, it has been shown that athletes from different sports may exhibit a distinct xenobiotic profile (i.e. exogenous metabolites not naturally produced in the human body) reflecting their drug/supplement use, diet and exposure to various chemicals, such as Trichloramine in swimmers [62] or grass exposure in footballers [78]. Baseline levels of metabolites, BMI and even the altitude at which the exercise is undertaken may also influence the metabolomic response [31,45,55]. All of these factors further complicate the comparisons of the metabolomic response to different PA/sport/exercise regimes. In addition, there is increasing evidence that social stimuli during PA/exercise can influence health outcomes due to psychosocial processes [79] as well as social cohesion and adherence [80].
The study of the metabolomic response to PA/exercise is, therefore, inextricably linked to the ‘metabolome of fitness’ (by which we mean exercise related fitness such as aerobic fitness, muscle strength, muscle endurance, and flexibility). Indeed, it has been reported that cardiorespiratory fitness is more strongly correlated with metabolic networks than with measures of PA/exercise [23,74]. A study by Morris et al. noted that individuals could be distinguished into fitness levels on the basis of their metabolome [46], while Castro et al. similarly reported a dose-response correlation between cardiorespiratory fitness and the metabolome [51]. As with the findings observed in the elite athletes, overall, fitter individuals (as defined by maximal oxygen consumption) have been shown to have lower levels of amino acids particularly branched chain amino acids, likely due to increased gluconeogenesis, more efficient utilization of fat stores [46,74], and a metabolomic profile that enables a greater capacity to activate lipolysis, facilitate entry of fatty acids into the TCA cycle, and to expand the TCA cycle intermediate pool during PA/exercise [31]. The antioxidant and cardiorespiratory inflammatory defense systems have also been shown to improve with exercise training. Perhaps not unsurprisingly lower levels of amino acids have also been associated with improved “post-exercise” recovery times [55]. Recovery is a critically important component of PA/exercise, and a number of studies have specifically explored this aspect. The results suggest that recovery may also exhibit unique metabolomic changes between individuals [52] that again depend on baseline metabolic profile [55], and which according to one study, may be even greater than the changes associated with the exercise itself [39].
6. Beneficial health effects
One of the primary reasons that individuals undertake many physical activities is for the purported health benefits. Based on the unique properties of the metabolome as the “ome” closest to phenotype and the ability to inform on past exposures, it can therefore be hypothesized that the beneficial health effects of PA/exercise can be tracked via the metabolic alterations it induces. Indeed, evidence suggests higher overall volume of habitual PA/exercise is associated with metabolomic signatures that are consistent with better cardiometabolic health [72], and physically active individuals have a “coherently healthier metabolic profile than their inactive counterparts” that helps to reduce the increased cardiometabolic risk associated with a sedentary/inactive lifestyle [73]. Although it should be noted here being active and having high levels of sedentary behavior are not mutually exclusive and there is some debate in the literature regarding the different risks and benefits attached to these two states [81].
The “coherently healthier metabolic profile” has been variously defined as lower levels of isoleucine, α1-acid glycoprotein, VLDL cholesterol, triglycerides and glucose, an overall less saturated fatty acid profile and a shift toward large high-density lipoproteins [26,72,75]. The impact of PA/exercise on amino acids, particularly branched chain amino acids, has been suggested to result from the activation of key enzymes involved in BCAA degradation, and these lower levels reflect an increase in fat oxidation efficient utilization of fat stores, more efficient lipid metabolism and fatty acid oxidation and more efficient adjustment of energy expenditure during recovery which may lead to improved insulin sensitivity [30,46,53,64,72]. It has further been suggested that high fitness status induces an increased cardiorespiratory inflammatory and antioxidant defense system, more prone to deal with the inflammatory response following PA/exercise [31,33,62]. Metabolomics can also help understand the potential negative effects of strenuous PA/exercise, including elucidating the mechanism underlying disorders induced by strength-endurance activities, such as overtraining and fatigue [52,56,59].
Interestingly, it has been observed in a number of studies focusing on diseased populations that although the majority of PA/exercise-associated metabolic changes are the same in all people, including alterations in lactic acid, hypoxanthine, inosine and alanine levels, which were identified in multiple studies [23,31,33,40–42,48,50,51,53,55–58,60,61,63,65,75], the metabolomic response can differ between healthy and diseased population in a metabolite specific manner [28,31,62]. This leads to the question as to whether PA/exercise has a differential impact on the metabolome in those who are susceptible to disease, or whether the disease associated metabolomic changes outweigh those due to PA/exercise. Additional work in more diverse populations, with a focus on the magnitude of absolute changes in metabolite levels, is required to disentangle this further.
7. Obesity and weight management
A number of studies have been conducted in populations that included individuals who were overweight or obese [22,23,66–69]. Obesity and overweight are whole system disorders known to elicit a coordinated metabolic response [82,83], and therefore to impact the metabolome. There is some contention regarding the role of “exercise” in BMI and weight management [84]. However, the underlying metabolomic hypothesis is that PA/exercise induces AMP-activated protein kinase pathway in muscle and other tissues, which increases fat oxidation and glucose transport [83], and additionally acts upon insulin sensitivity to increase fat loss. Given the involvement of these pathways, changes in weight will also likely influence the metabolome. Therefore, the interconnected relationships between PA/exercise, weight, weight changes and the metabolome, complicate the interpretation of metabolomics studies of PA/exercise that are specifically targeted at weight loss. It may be hard to disentangle those metabolomic changes that are due to a decrease in BMI, from those that are due to the PA/exercise that precipitated that change in BMI. Indeed, many metabolites previously reported in the literature to be associated with BMI, were also identified by studies in this review as associated with PA/exercise, including branched-chain and aromatic amino acids, long-chain polyunsaturated fatty acids, phospholipids, lysophosphatidylcholines, sphingomyelins, carbohydrates, nucleotides and carnitines [27,29,30,34,36,40,42,43,47,53,54,60,67–70,85].
PA/exercise has been shown in one study to influence the metabolome independently of weight loss. Meucci et al. tracked the urinary metabolome across an eight-week exercise intervention. Although this intervention resulted in a 7% decrease in body fat that did induce metabolic changes, they were also able to identify independent metabolic alterations that related to the activity itself [68]. However, it should also be noted that, three other studies of medium to long term PA/exercise interventions in overweight/obese individuals, observed no change in the metabolome at all [22,23,69]. Furthermore Brennan et al. noted that the levels of certain metabolites did change with a change in adipose tissue deposits, but that these changes could not be attributed to the long-term PA/exercise intervention [25]. Consequently, further work is necessary to determine the role of BMI in the PA/exercise-metabolomics relationship.
8. Sex and PA/exercise metabolomics
Several metabolomics studies have shown baseline differences in the metabolome of males and females [86]. Similarly, due to hormonal and muscle mass differences between the sexes, men and women tend to show differences in their physiological responses and adaptations to PA/exercise [87]. Therefore, it can be hypothesized that they may also differ in their metabolic response to PA/exercise, however, there is some disagreement in the literature as to whether this is actually the case. Sex differences in the metabolomic responses to activity in the literature have been noted, with differential directions of effect for certain metabolites [27] and metabolite sex interactions for others [46]. In this latter study, the beneficial effects of PA/exercise, including more efficient utilization of fat stores during PA/exercise, more efficient adjustment of energy expenditure during recovery, and improved insulin sensitivity were shown to be most pronounced in females [46]. This may result from the fact that females are likely to utilize more fat and less protein as an energy source during PA/exercise [22]. Similarly, it has been reported that the observed metabolite-PA/exercise energy expenditure associations, including for valine, alpha-hydro-xyisovalerate, 2-hydroxybutyrate (AHB), mannose, glucose and threonate were stronger in men than in women, and additionally that some metabolites were associated with PA/exercise energy expenditure in women only [72]. However, often where any differences were reported, the direction of effect was the same between both sexes, it was just the magnitude of effect that different slightly [46,72], which may be a function of power and sample size rather than biology.
Furthermore, the studies that did report differences are relatively small, and it should be noted that a number of other studies have reported no differences by sex in the response to the same intervention or sex-specific differences in the PA/exercise associated metabolome [35,76]. Additionally, among the studies selected for this review there is no evidence of a systematic difference in the results between the all-male and the all-female populations. Perhaps even more importantly, a large number of studies have either not reported sex specific results at all or have only included men in their study populations.
9. Biological sample
A common criticism of metabolomics studies is that they often profile blood or urine rather than the tissue most proximal or relevant to the outcome of interest. For PA/exercise, it is debatable which tissues this would be. Skeletal muscle is one of the most important organs in terms of the metabolic adaptations accompanying PA/exercise. Consequently, it is here that you may expect to see some of the greatest metabolic changes. Indeed, Huffman reported that the metabolic signatures of “exercise-trained skeletal muscle” reflected reprogramming of mitochondrial function, intermediary metabolism and whole body insulin sensitivity, which in turn augments oxidative capacity and “exercise tolerance” [67]. Nevertheless, blood is the most commonly used biologic media reported in the existing literature, with the remainder comprising a combination of muscle, urine, exhaled air, breath condensate, saliva and feces. [22,26,32,34,40,46,51,57–63,65,67,68].
There is some inconsistency in the literature regarding whether the same metabolomic changes can be observed in different tissue types. Many studies report similar results regardless of biosample, including plasma, urine, exhaled air, breath condensate, saliva and stool [26,34,46,65], and in blood samples from both “exercising and non-exercising” parts of the body [31]. Conversely, in a comparison of urine and stool, Barton et al. observed that the metabolomic differences between elite rugby players and controls was greatest in the urine [26], while Castro et al. also noted some difference between serum and skeletal muscle [51].
10. Other omics
It is increasingly recognized that metabolomic data alone may be insufficient to fully characterize complex pathologies [88]. The metabolome represents the most downstream ‘ome’, therefore it is also of biological interest to characterize the upstream ‘omes’ (i.e. the genome, epigenome, transcriptome and proteome) that may play a role in driving the metabolic changes. The integration of metabolomics with other omics data to identify the interactions and synergies between the different hierarchical components of the ‘central biological dogma’ represents a potential strategy that will allow the visualization of a biological system on the most global level [6]. Accordingly, several studies [26,31,58,67] also reported additional omics data or integrative omics analyses.
Overall these results provided a measure of validation of the metabolomics findings. In general, alterations in metabolite levels were accompanied by an up or down regulation of the gene sets encoding the enzymes catalyzing the relevant metabolome reactions e.g. acylcarnitines with lipid metabolic genes involved in muscle uptake and oxidation of fatty acids and succinate with genes involved in oxidative phosphorylation [58,67]. Similarly, upregulation of nur77 a transcriptional regulator of glucose utilization and lipid metabolism genes in skeletal muscle was shown to be driven by a combination of glycerol, niacinamide, glucose-6-phosphate, pantothenate, and succinate that increased in plasma in response PA/exercise [31]. The microbiome has also been explored in relation to the metabolome of PA/exercise. Again, the additional omic results expanded upon and provided a further rationale for the observed metabolomics findings, with observed increases in both metabolites and microbiota associated with enhanced muscle turnover [26].
11. Discussion
The associations between PA/exercise and health are well described in the epidemiological literature [2] as are the physiological processes of adaptation to PA/exercise [89]. However, the metabolomic biology underlying the differential effects within and between individuals in terms of improvements in fitness and in health is less well understood. Our review has demonstrated that PA-metabolomics has the potential to address some of the biggest mechanistic questions in the study of PA, exercise and health. This includes the metabolic adaptations that occur in response to PA/exercise, and crucially how these adaptations may influence the maintenance of health, the development of disease, the regulation of a healthy weight, rehabilitation or other physical and psychological health related outcomes. Furthermore, it also provides the potential for the identification of clinically useful biomarkers.
The metabolomics literature to date overwhelmingly supports the hypothesis of a metabolomic response to PA/exercise, and a differential response between individuals. It also suggests a biological gradient in this response based on the duration or intensity of the activity, with some potential evidence for global longer-term changes in the metabolome of highly active individuals. Several common themes emerged throughout the studies relating to, branched chain amino acids, the TCA cycle glycolysis, oxidative stress, insulin sensitivity and fatty acid mobilization, as well as specific metabolites such as glucose, pyruvate, succinate and alanine, serine, glutamate, sarcosine, carnitines and kynurenine.
Nevertheless, there is substantial heterogeneity in the PA metabolomics literature, in terms of PA/exercise exposure and how it is measured, study design, intervention characteristics, sample type, timing of collection and the underlying study population. The results from the literature to date suggest this latter point may be of particular importance, and as such it is unclear to what extent differences in results between studies reflect true biology and to what extent they are merely a function of the heterogeneity between the studies, including sex, age, geography, fitness level, nutrition and disease status. The vast majority of studies have been conducted in small populations, making it difficult to further interrogate these questions with the data available. Of the 54 studies detailed in Tables 1, 2 and 3, 43 (80%) included < 100 participants and 31 (57%) included < 25 participants. Although the nature of many of these studies, particularly the intervention studies which require specialized equipment, continuous monitoring, suitable and willing participants and repeat sampling, renders them unfeasible for large scale investigations, these numbers are still strikingly small in the –omics field. Highly dimensional omic studies typically employ hundreds or thousands of subjects, and as such some of these results should be viewed with some caution, particularly as today, very few of the studies to date have attempted to directly replicate their studies in an independent cohort [76]. It should also be noted, the vast majority of studies have been conducted in all-male or majority male populations, and the question of the role of sex in the response to PA is still to be definitively determined. The difficulty of assimilating the data from the multiple studies with vastly different designs, populations, PA/exercise context, and measures classifying and defining the activity, remains a critical challenge to understanding the impact of exercise on the metabolome. Finally, many of the metabolites identified in the PA/exercise literature have also been reported to be important in metabolomic studies of other phenotypes and many pathologies. Determining to what extent, this is a reflection of these phenotype’s relationships with PA/ exercise, and isolating what is specific and unique to PA/exercise over other exposures is an ongoing challenge.
A further role for metabolomics, not covered here, is as an unbiased means of assessing how nutritional interventions aimed at improving PA/exercise effects and recovery may be acting at a molecular level. Metabolomic studies of nutritional supplementation and exercise, represent a fast growing sub-specialty within this field. Such studies are outside the scope of this current review, but as the evidence suggests that the metabolic changes associated with PA/exercise can be manipulated through the use of nutritional supplements [90–94], depending on their content, purpose and timing of administration, nutrition will play a crucial role in the understanding of the metabolomics of PA/exercise moving forward.
A better understanding of the differential effects of PA/exercise could help begin to explain what types of PA work best, for whom and why. It can also inform on why some people respond better to general activity versus structured training, and vice versa. Ultimately it can help to inform viable solutions for those who do not seem to benefit from PA (i.e. “non-” or “low-responders”) [95–97]. One of the biggest challenges in PA/exercise promotion is supporting long-term behavior change. It is assumed that many people do not maintain long term PA/exercise behavior as they do not enjoy it or see the hoped-for health changes in the expected timeframes. It may be that PA-metabolomics could be harnessed to identify the most effective and rewarding types of PA/exercise (aerobic, muscle strengthening, dose, and intensity) and support more long-term health behavior change.
The homogenous nature of the recommendations in PA/exercise guidelines may not be eliciting the optimum health effects across the heterogeneous populations they are aimed at. The recommendations appear to have been based on the assumption that because in an epidemiological sense the benefits of PA/exercise are so clear-cut that the physiological processes are broadly homogeneous. This review suggests that they may not be and that in these different arenas different biological mechanisms are at work. Given the complexity of human biology (and behavior) this is hardly surprising, but its implications for public health policies around the world may be profound.
Based on our review, we have summarized some research priority areas in Table 4.
Table 4.
Suggestions for future research areas.
| Area | Recommendation |
|---|---|
| Conceptual mapping | Conceptual mapping of the area of PA and metabolomics to outline key thematic areas to guide more in depth study. Themes highlighted in this current review include |
| |
| Novel human studies | Generation of novel observational and intervention studies in larger more diverse populations. Based on the findings of this review this should include |
| |
| Biological mapping | Construction of a biological systems map linking metabolites and metabolomics pathways with PA/exercise exposures, metabolomics responses and acute and chronic health effects |
| Biomarker development | Development of studies designed to specifically assess the utility of metabolomics biomarkers in the field of PA/exercise. As a first step, studies should move toward absolute quantification of metabolite levels. Special consideration should be given to the specificity of PA/exercise biomarkers. |
| Expert consensus | Stakeholder and consensus seeking work to describe how better understanding in this area can be harnessed to improve both health promotion, health protection and exercise training |
Metabolomic studies of PA/exercise help us to answer many questions, but they also raise many more that are worthy of further investigation. Accordingly, we are seeing an exponential growth of the use of metabolomics in the study of PA [17] as outlined in this review. Encouragingly, governments and research institutions are also recognizing the importance of PA-metabolomics research and are willing to make huge investments to the field, such as the United States NIH Common Fund MoTrPAC initiative [9,10]. Thus, this field will continue to grow, and with time we may be able to answer these remaining questions and more fully understand the metabolomics response to exercise, and how this influences health and disease in populations and individuals.
Supplementary Material
Funding
RSK is supported by K01 HL146980 from the US National Heart, Lung and Blood Institute and MPK by AH/M005917/1 from the UK Arts and Humanities Research Council, and PR-PRU-1217-20501 and PHR 15/82/12 from the National Institute for Health Research (NIHR) UK.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Kohl HW 3rd et al. , The pandemic of physical inactivity: global action for public health, Lancet 380 (9838) (2012) 294–305. [DOI] [PubMed] [Google Scholar]
- [2].Sallis JF, et al. , Progress in physical activity over the Olympic quadrennium, Lancet 388 (10051) (2016) 1325–1336. [DOI] [PubMed] [Google Scholar]
- [3].Booth FW, Roberts CK, Laye MJ, Lack of exercise is a major cause of chronic diseases, Compr Physiol 2 (2) (2012) 1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kelly P, Fitzsimons C, Baker G, Should we reframe how we think about physical activity and sedentary behaviour measurement? Validity and reliability reconsidered. Int J Behav Nutr Phys Act 13 (2016) 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prisoners of the Proximate, Loosening the constraints on epidemiology in an age of change, Am J Epidemiol 185 (11) (2017) 1206–1216. [DOI] [PubMed] [Google Scholar]
- [6].Sarzynski MA, et al. , Advances in exercise, fitness, and performance genomics in 2015, Med Sci Sports Exerc 48 (10) (2016) 1906–1916. [DOI] [PubMed] [Google Scholar]
- [7].Beger RD, et al. , Metabolomics enables precision medicine: “A white paper, community perspective”, Metabolomics 12 (10) (2016) 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kelly MP, Kelly RS, Quantifying social influences throughout the lifecourse: Action, structure and ‘omics’, in The Palgrave Handbook of Biology & Society, Meloni M, Cromby J, Fitzgerald D, Lloyd S, Editor. 2018, Palgrave Macmillan UK; p. 587–609. [Google Scholar]
- [9].Sanford JA, et al. , Molecular transducers of physical activity consortium (MoTrPAC): mapping the dynamic responses to exercise, Cell 181 (7) (2020) 1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sparks LM, Exercise training response heterogeneity: physiological and molecular insights, Diabetologia 60 (12) (2017) 2329–2336. [DOI] [PubMed] [Google Scholar]
- [11].Decombaz J, et al. , Biochemical changes in a 100 km run: free amino acids, urea, and creatinine, Eur J Appl Physiol Occup Physiol 41 (1) (1979) 61–72. [DOI] [PubMed] [Google Scholar]
- [12].Calles-Escandon J, et al. , Influence of exercise on urea, creatinine, and 3-methylhistidine excretion in normal human subjects, Am J Physiol 246 (4 Pt 1) (1984) E334–E338. [DOI] [PubMed] [Google Scholar]
- [13].Wahren J, Felig P, Hagenfeldt L, Hendler R, Ahlborg G, Splanchnic and leg metabolism of glucose, free fatty acids and amino acids during prolonged exercise in man, in Metabolic adaptation to prolonged physical exercise, Howald H, Poortmans JR, Editor. 1975, Birkhäuser: Basel: p. 144–153. [Google Scholar]
- [14].Warburton DE, Nicol CW, Bredin SS, Health benefits of physical activity: the evidence, CMAJ 174 (6) (2006) 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sakaguchi CA, et al. , Metabolomics-Based Studies Assessing Exercise-Induced Alterations of the Human Metabolome: A Systematic Review. Metabolites, 2019. 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].San-Millan I, Blood biomarkers in sports medicine and performance and the future of metabolomics, Methods Mol Biol 1978 (2019) 431–446. [DOI] [PubMed] [Google Scholar]
- [17].Duft RG, Castro A, Chacon-Mikahil MPT, Cavaglieri CR, Metabolomics and exercise: possibilities and perspectives, Motriz 23 (2) (2017) e101634. [Google Scholar]
- [18].Kelly MP, et al. , AHRQ series on complex intervention systematic reviews-paper 2: defining complexity, formulating scope, and questions, J Clin Epidemiol 90 (2017) 11–18. [DOI] [PubMed] [Google Scholar]
- [19].Caspersen CJ, Powell KE, Christenson GM, Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research, Public Health Rep 100 (2) (1985) 126–131. [PMC free article] [PubMed] [Google Scholar]
- [20].Strain T, et al. , Age-related comparisons by sex in the domains of aerobic physical activity for adults in Scotland, Prev Med Rep 3 (2016) 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Belanger M, Townsend N, Foster C, Age-related differences in physical activity profiles of English adults, Prev Med 52 (3–4) (2011) 247–249. [DOI] [PubMed] [Google Scholar]
- [22].Zheng H, et al. , NMR-based metabolomic profiling of overweight adolescents: an elucidation of the effects of inter-/intraindividual differences, gender, and pubertal development, Biomed Res Int 2014 (2014) 537157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brennan AM, et al. , Plasma metabolite profiles in response to chronic exercise, Med Sci Sports Exerc 50 (7) (2018) 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sakaguchi CA, et al. , Chronic Influence of Inspiratory Muscle Training at Different Intensities on the Serum Metabolome. Metabolites, 2020. 10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brennan AM, et al. , Depot-specific adipose tissue metabolite profiles and corresponding changes following aerobic exercise, Front Endocrinol (Lausanne) 9 (2018) 759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Barton W, et al. , The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level, Gut 67 (4) (2018) 625–633. [DOI] [PubMed] [Google Scholar]
- [27].Al-Khelaifi F, et al. , A pilot study comparing the metabolic profiles of elite-level athletes from different sporting disciplines, Sports Med Open 4 (1) (2018) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sabatine MS, et al. , Metabolomic identification of novel biomarkers of myocardial ischemia, Circulation 112 (25) (2005) 3868–3875. [DOI] [PubMed] [Google Scholar]
- [29].Enea C, et al. , (1)H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise, Anal Bioanal Chem 396 (3) (2010) 1167–1176. [DOI] [PubMed] [Google Scholar]
- [30].Lehmann R, et al. , Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation, PLoS One 5 (7) (2010) e11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lewis GD, et al. , Metabolic signatures of exercise in human plasma. Sci Transl Med, 2010. 2(33): p. 33ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pechlivanis A, et al. , (1)H NMR-based metabonomic investigation of the effect of two different exercise sessions on the metabolic fingerprint of human urine, J Proteome Res 9 (12) (2010) 6405–6416. [DOI] [PubMed] [Google Scholar]
- [33].Chorell E, et al. , Physical fitness level is reflected by alterations in the human plasma metabolome, Mol Biosyst 8 (4) (2012) 1187–1196. [DOI] [PubMed] [Google Scholar]
- [34].Krug S, et al. , The dynamic range of the human metabolome revealed by challenges, FASEB J 26 (6) (2012) 2607–2619. [DOI] [PubMed] [Google Scholar]
- [35].Nieman DC, et al. , Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners, J Proteome Res 12 (10) (2013) 4577–4584. [DOI] [PubMed] [Google Scholar]
- [36].Pechlivanis A, et al. , 1H NMR study on the short- and long-term impact of two training programs of sprint running on the metabolic fingerprint of human serum, J Proteome Res 12 (1) (2013) 470–480. [DOI] [PubMed] [Google Scholar]
- [37].Nieman DC, et al. , Metabolomics approach to assessing plasma 13- and 9-hydroxyoctadecadienoic acid and linoleic acid metabolite responses to 75-km cycling, Am J Physiol Regul Integr Comp Physiol 307 (1) (2014) R68–R74. [DOI] [PubMed] [Google Scholar]
- [38].Peake JM, et al. , Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise, Am J Physiol Endocrinol Metab 307 (7) (2014) E539–E552. [DOI] [PubMed] [Google Scholar]
- [39].Danaher J, Gerber T, Wellard R, Stathis C, Cooke M, The use of metabolomics to monitor simultaneous changes in metabolic variables following supramaximal low volume high intensity exercise, Metabolomics 12 (7) (2015) s11306 −015–0883-7. [Google Scholar]
- [40].Muhsen Ali A, et al. , Metabolomic Profiling of Submaximal Exercise at a Standardised Relative Intensity in Healthy Adults. Metabolites, 2016. 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zafeiridis A, et al. , Global Metabolic Stress of Isoeffort Continuous and High Intensity Interval Aerobic Exercise: A Comparative (1)H NMR Metabonomic Study, J Proteome Res 15 (12) (2016) 4452–4463. [DOI] [PubMed] [Google Scholar]
- [42].Berton R, et al. , Metabolic time-course response after resistance exercise: a metabolomics approach, J Sports Sci 35 (12) (2017) 1211–1218. [DOI] [PubMed] [Google Scholar]
- [43].Felder TK, et al. , Specific circulating phospholipids, acylcarnitines, amino acids and biogenic amines are aerobic exercise markers, J Sci Med Sport 20 (7) (2017) 700–705. [DOI] [PubMed] [Google Scholar]
- [44].Karl JP, et al. , Military training elicits marked increases in plasma metabolomic signatures of energy metabolism, lipolysis, fatty acid oxidation, and ketogenesis. Physiol Rep, 2017. 5(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Messier FM, et al. , The impact of moderate altitude on exercise metabolism in recreational sportsmen: a nuclear magnetic resonance metabolomic approach, Appl Physiol Nutr Metab 42 (11) (2017) 1135–1141. [DOI] [PubMed] [Google Scholar]
- [46].Morris C, et al. , The relationship between aerobic fitness level and metabolic profiles in healthy adults, Mol Nutr Food Res 57 (7) (2013) 1246–1254. [DOI] [PubMed] [Google Scholar]
- [47].Nieman DC, Sha W, Pappan KL, IL-6 Linkage to Exercise-Induced Shifts in Lipid-Related Metabolites: A Metabolomics-Based Analysis, J Proteome Res 16 (2) (2017) 970–977. [DOI] [PubMed] [Google Scholar]
- [48].Davison G, et al. , Metabolomic response to acute hypoxic exercise and recovery in adult males, Front Physiol 9 (2018) 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stander Z, et al. , The altered human serum metabolome induced by a marathon, Metabolomics 14 (11) (2018) 150. [DOI] [PubMed] [Google Scholar]
- [50].Valerio DF, et al. , Early metabolic response after resistance exercise with blood flow restriction in well-trained men: a metabolomics approach, Appl Physiol Nutr Metab 43 (3) (2018) 240–246. [DOI] [PubMed] [Google Scholar]
- [51].Castro A, et al. , Association of skeletal muscle and serum metabolites with maximum power output gains in response to continuous endurance or high-intensity interval training programs: the TIMES study - a randomized controlled trial, PLoS One 14 (2) (2019) e0212115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Manaf FA, et al. , Characterizing the plasma metabolome during and following a maximal exercise cycling test, J Appl Physiol 2018 (1985). [DOI] [PubMed] [Google Scholar]
- [53].Siopi A, et al. , Comparison of the Serum Metabolic Fingerprint of Different Exercise Modes in Men with and without Metabolic Syndrome. Metabolites, 2019. 9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schader JF, et al. , Metabolite Shifts Induced by Marathon Race Competition Differ between Athletes Based on Level of Fitness and Performance: A Substudy of the Enzy-MagIC Study. Metabolites, 2020. 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kirwan GM, et al. , Spectroscopic correlation analysis of NMR-based metabonomics in exercise science, Anal Chim Acta 652 (1–2) (2009) 173–179. [DOI] [PubMed] [Google Scholar]
- [56].Yan B, et al. , Metabolomic investigation into variation of endogenous metabolites in professional athletes subject to strength-endurance training. J Appl Physiol (1985), 2009. 106(2): p. 531–8. [DOI] [PubMed] [Google Scholar]
- [57].Neal CM, et al. , Six weeks of a polarized training-intensity distribution leads to greater physiological and performance adaptations than a threshold model in trained cyclists. J Appl Physiol (1985), 2013. 114(4): p. 461–71. [DOI] [PubMed] [Google Scholar]
- [58].Mukherjee K, et al. , Whole blood transcriptomics and urinary metabolomics to define adaptive biochemical pathways of high-intensity exercise in 50–60 year old masters athletes, PLoS One 9 (3) (2014) e92031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ra SG, et al. , Metabolomics of salivary fatigue markers in soccer players after consecutive games, Appl Physiol Nutr Metab 39 (10) (2014) 1120–1126. [DOI] [PubMed] [Google Scholar]
- [60].Sun T, et al. , Metabolomic profiles investigation on athletes’ urine 35 minutes after an 800-meter race, J Sports Med Phys Fitness 57 (6) (2017) 839–849. [DOI] [PubMed] [Google Scholar]
- [61].Wang F, et al. , Applying (1)H NMR spectroscopy to detect changes in the urinary metabolite levels of chinese half-pipe snowboarders after different exercises, J Anal Methods Chem 2015 (2015) 315217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Couto M, et al. , Oxidative stress in asthmatic and non-asthmatic adolescent swimmers-a breathomics approach, Pediatr Allergy Immunol 28 (5) (2017) 452–457. [DOI] [PubMed] [Google Scholar]
- [63].Prado Eduardo, S. GHMF, Pegurier Marcelle, Vieira Camila, Lima-Neto Abelardo Barbosa Moreira, Assis Marcio, Guedes Maria Izabel Florindo, Koblitz Maria Gabriela Bello, Ferreira Mariana Simões Larraz, Macedo Andrea Furtado, Bottino Altamiro, Bassini Adriana, Cameron LC, Non-targeted sportomics analyses by mass spectrometry to understand exercise-induced metabolic stress in soccer players. International Journal of Mass Spectrometry, 2017. 418: p. 1–5. [Google Scholar]
- [64].Howe CCF, et al. , Untargeted Metabolomics Profiling of an 80.5 km Simulated Treadmill Ultramarathon. Metabolites, 2018. 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pitti E, et al. , Salivary Metabolome and Soccer Match: Challenges for Understanding Exercise induced Changes. Metabolites, 2019. 9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Huffman KM, et al. , Exercise-induced changes in metabolic intermediates, hormones, and inflammatory markers associated with improvements in insulin sensitivity, Diabetes Care 34 (1) (2011) 174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Huffman KM, et al. , Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness, Diabetologia 57 (11) (2014) 2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Meucci M, et al. , Metabolomic shifts following play-based activity in overweight preadolescents, Curr Pediatr Rev 13 (2) (2017) 144–151. [DOI] [PubMed] [Google Scholar]
- [69].Grapov D, et al. , Exercise plasma metabolomics and xenometabolomics in obese, sedentary, insulin-resistant women: impact of a fitness and weight loss intervention, Am J Physiol Endocrinol Metab 317 (6) (2019) E999–E1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fukai K, et al. , Metabolic profiling of total physical activity and sedentary behavior in community-dwelling men, PLoS One 11 (10) (2016) e0164877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Evans A, et al. , High Resolution Mass Spectrometry Improves Data Quantity and Quality as Compared to Unit Mass Resolution Mass Spectrometry in High-Throughput Profiling Metabolomics. Metabolomics, 2014. 4(2). [Google Scholar]
- [72].Xiao Q, et al. , Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study, Int J Epidemiol 45 (5) (2016) 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kujala UM, et al. , Long-term leisure-time physical activity and serum metabolome, Circulation 127 (3) (2013) 340–348. [DOI] [PubMed] [Google Scholar]
- [74].Floegel A, et al. , Linking diet, physical activity, cardiorespiratory fitness and obesity to serum metabolite networks: findings from a population-based study, Int J Obes (Lond) 38 (11) (2014) 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bell JA, et al. , Associations of device-measured physical activity across adolescence with metabolic traits: prospective cohort study, PLoS Med 15 (9) (2018) e1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ding M, et al. , Metabolome-wide association study with habitual physical activity in four prospective cohort studies, Am J Epidemiol 188 (11) (2019) 1932–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Oja P, et al. , Associations of specific types of sports and exercise with all-cause and cardiovascular-disease mortality: a cohort study of 80,306 British adults, Br J Sports Med 51 (10) (2017) 812–817. [DOI] [PubMed] [Google Scholar]
- [78].Al-Khelaifi F, et al. , Metabolomics profiling of xenobiotics in elite athletes: relevance to supplement consumption, J Int Soc Sports Nutr 15 (1) (2018) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lubans D, et al. , Physical Activity for Cognitive and Mental Health in Youth: A Systematic Review of Mechanisms. Pediatrics, 2016. 138(3). [DOI] [PubMed] [Google Scholar]
- [80].Howick J, Kelly P, Kelly M, Establishing a causal link between social relationships and health using the Bradford Hill Guidelines, SSM Popul Health 8 (2019) 100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ekelund U, et al. , Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women, Lancet 388 (10051) (2016) 1302–1310. [DOI] [PubMed] [Google Scholar]
- [82].Abu Bakar MH, et al. , Metabolomics - the complementary field in systems biology: a review on obesity and type 2 diabetes, Mol Biosyst 11 (7) (2015) 1742–1774. [DOI] [PubMed] [Google Scholar]
- [83].Greenberg AS, Obin MS, Obesity and the role of adipose tissue in inflammation and metabolism, Am J Clin Nutr 83 (2) (2006) 461S–465S. [DOI] [PubMed] [Google Scholar]
- [84].Kelly P, et al. , Critique of ‘the physical activity myth’ paper: discussion of flawed logic and inappropriate use of evidence, Br J Sports Med 50 (20) (2016) 1230–1231. [DOI] [PubMed] [Google Scholar]
- [85].Rauschert S, et al. , Early programming of obesity throughout the life course: a metabolomics perspective, Ann Nutr Metab 70 (3) (2017) 201–209. [DOI] [PubMed] [Google Scholar]
- [86].Krumsiek J, et al. , Gender-specific pathway differences in the human serum metabolome, Metabolomics 11 (6) (2015) 1815–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Woo JS, et al. , The influence of age, gender, and training on exercise efficiency, J Am Coll Cardiol 47 (5) (2006) 1049–1057. [DOI] [PubMed] [Google Scholar]
- [88].Wanichthanarak K, Fahrmann JF, Grapov D, Genomic, proteomic, and metabolomic data integration strategies, Biomark Insights 10 (Suppl. 4) (2015) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].McArdle WD, Katch FI, Katch VL, Exercise Physiology: Nutrition, Energy, and Human Performance, 7 ed., Wolters Kluwer/Lippincott Williams & Wilkins, Baltimore, 2010. [Google Scholar]
- [90].Hodgson AB, et al. , Metabolic response to green tea extract during rest and moderate-intensity exercise, J Nutr Biochem 24 (1) (2013) 325–334. [DOI] [PubMed] [Google Scholar]
- [91].Jacobs DM, et al. , Metabolic response to decaffeinated green tea extract during rest and moderate-intensity exercise, J Agric Food Chem 62 (40) (2014) 9936–9943. [DOI] [PubMed] [Google Scholar]
- [92].Miccheli A, et al. , The influence of a sports drink on the postexercise metabolism of elite athletes as investigated by NMR-based metabolomics, J Am Coll Nutr 28 (5) (2009) 553–564. [DOI] [PubMed] [Google Scholar]
- [93].Chorell E, et al. , Predictive metabolomics evaluation of nutrition-modulated metabolic stress responses in human blood serum during the early recovery phase of strenuous physical exercise, J Proteome Res 8 (6) (2009) 2966–2977. [DOI] [PubMed] [Google Scholar]
- [94].Yde CC, et al. , Metabonomic response to milk proteins after a single bout of heavy resistance exercise elucidated by 1H nuclear magnetic resonance spectroscopy, Metabolites 3 (1) (2013) 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].NICE, Physical Activity and the Environment (NG90), (2018).
- [96].NICE, Physical Activity: Exercise Referral Schemes, (2014).
- [97].NICE, Physical Activity for Children and Young People, (2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


