Abstract
Tobacco-related health disparities disproportionately affect smokers with major depression (MD). Although tobacco simulation models have been applied to general populations, to date they have not considered populations with a comorbid mental health condition. We developed and calibrated a simulation model of smoking and MD comorbidity for the US adult population using the 2005–2018 National Surveys on Drug Use and Health. We use this model to evaluate trends in smoking prevalence, smoking-attributable mortality and life-years lost among adults with MD, and changes in smoking prevalence by mental health status from 2018–2060. The model integrates known interaction effects between smoking initiation and cessation, and MD onset and recurrence. We show that from 2018–2060, smoking prevalence will continue declining among those with current MD. In the absence of intervention, people with MD will be increasingly disproportionately affected by smoking compared to the general population; our model shows that the smoking prevalence ratio between those with current MD and those without a history of MD increases from 1.54 to 2.42 for men and from 1.81 to 2.73 for women during this time period. From 2018–2060, approximately 484,000 smoking-attributable deaths will occur among adults with current MD, leading to 11.3 million life-years lost. Ambitious tobacco control efforts could alter this trajectory. With aggressive public health efforts, up to 264,000 of those premature deaths could be avoided, translating into 7.5 million life years gained. This model can compare the relative health gains across different intervention strategies for smokers with MD.
Keywords: smoking, major depression, depression, simulation model, systems dynamics, tobacco
Introduction
Each year 16.2 million people in the US experience a major depressive episode, approximately 6.7% of the population.1 People with major depression (MD) die up to 11 years earlier than those without MD, driven in part by their high smoking rates.2 In 2017, 23.5% of people with depression smoked compared to 15% of people without depression, and their daily smoking prevalence was nearly twice as high.3 International organizations and experts consider smokers with comorbid mental illness such as MD a high priority group for tobacco control intervention.4–7
The relationship between MD and smoking is bi-directional and causal, even after adjusting for demographic characteristics and other psychiatric disorders.8–11 MD is a risk factor for future smoking, and tobacco use predicts subsequent MD symptoms. Smoking cessation decreases depressive symptoms.12 These feedback loops imply that changes in MD or smoking could affect the other. To examine the implications of this relationship, we consider the potential for MD and smoking to reinforce each other.
Governments increasingly use simulation models to facilitate public health decision-making and long-term planning. Numerous models already estimate population tobacco use over time.13 To our knowledge, none have considered smoking disparities by mental health status. Similarly, existing MD models do not explicitly account for smoking comorbidity.14–19 A model of smoking and MD can evaluate the burden of smoking in populations with MD and changes to this disparity over time.
We developed a system dynamics model of smoking and MD comorbidity for the US adult population. System dynamics models include stocks of individuals within a health state (e.g., people with current MD) and flows characterizing individuals’ movement between health states (e.g., MD incidence or recovery). They are ideal for testing macro-level changes in systems with nonlinearity, feedback loops (e.g., interactions between smoking and MD), and time delays between events (e.g., smoking initiation) and future health consequences (e.g., disease and death). We projected smoking prevalence, smoking-attributable mortality, and life-years lost by MD status from 2018–2060. We also calculated health gains under an ideal tobacco control scenario.
Methods
We used data from the 2005–2018 National Surveys on Drug Use and Health (NSDUH). NSDUH is an annual nationally representative survey of the non-institutionalized population of US adults ages ≥18.20 NSDUH is the most historically comprehensive national survey to include measures for smoking behaviors and depressive episodes.
We calibrated separate smoking-only and MD-only sub-models to NSDUH data, and then combined both sub-models and their inputs into the full comorbidity model. We recalibrated the combined model to fit survey data on smoking prevalence by MD status, and vice versa. The full model integrated and estimated known and unknown interaction effects between smoking initiation and cessation, and MD onset, relapse and recovery. Analyses were conducted using R version 3.1.3.
Smoking sub-model
We considered three mutually exclusive smoking states in the smoking sub-model: never smoker, current smoker, and former smoker. Current smokers smoked at least 100 cigarettes in their lifetime and smoked anytime within the past year. Former smokers smoked at least 100 cigarettes in their lifetime but have not smoked at any point in the last year. This strict definition for current smokers includes those who have quit smoking less than one year prior to survey assessment, avoiding the problem of modeling cessation relapse by categorizing as former smokers only those who likely have permanently quit. Never smokers have smoked fewer than 100 cigarettes in their lifetime. These definitions enable the model to be more consistent with input data sources and to simulate permanent smoking cessation without relapse. (See Appendix Table S1.)
Individuals enter the model at birth as never smokers who can become current smokers, and then former smokers, based on initiation and cessation probabilities developed by the Cancer Intervention and Surveillance Modeling Network (CISNET) lung consortium.21 Individuals exit the model through death or after age 99. CISNET provides age, gender, and birth cohort-specific mortality rates for never smokers, former smokers, and current smokers.22 For future projections, CISNET smoking initiation and cessation probabilities are held constant going forward, consistent with previous applications.23
MD sub-model
NSDUH screens adults for lifetime and past year experience of an MD episode using criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV); an individual must report at least five of nine common depression symptoms for a two-week or longer period.24 NSDUH does not exclude MD episodes caused by illness, bereavement, substance use or other psychiatric disorders, such as bipolar disorder.25
For this model, people with current MD have had an MD episode in the past year. Those with former MD have had an episode in their lifetime but not in the past year. Those with never MD have never had an MD episode. Cross-sectional surveys of depression that rely on retrospective evaluations can lead to substantial underestimation of lifetime history of depression.26 Therefore, the model explicitly accounts for the probability of recall error and misclassification of individuals as some never MD are modeled as former MD. This sub-model has been used to analyze the impact of recall error on US patterns of lifetime MD.19
Individuals are born as never MD, and may transition to a first MD episode based on incidence data from the Baltimore Epidemiological Catchment Area (ECA) Study,27 the nation’s longest-running psychiatric epidemiological cohort study. Since MD incidence data for females ages <22 and males ages <29 do not exist, we estimated the annual probability of a first MD episode at younger ages as part of calibration using cubic natural splines to fit the sub-model to NSDUH data. Individuals may recover from a first MD episode and shift into a former MD category. Former MD individuals may also have a recurrent MD episode and subsequently recover.28 MD age-specific incidence rates are assumed to remain constant into the future, as NSDUH data show no visible trends in depression patterns by age for the 2005–2015 period. Due to recall error among those with former MD, we report findings for the current and ‘true’ never MD population only; results reported for the never MD population are adjusted for recall error by removing those who incorrectly self-report as never MD.19 Because recent NSDUH data show that MD prevalence has increased among young adults ages 18–25,29 MD incidence parameters were increased for this age group in 2016 and held constant thereafter to be conservative. We present an alternative scenario in which MD incidence probabilities return to pre-2016 levels in the Appendix.
MD and smoking comorbidity model
The combined MD and smoking (MDS) model includes 15 mutually exclusive smoking and depressive states (Figure 1) and projects future smoking and MD prevalence for the US population ages ≥18. We modeled males and females separately, as females have higher risk for depression and earlier ages at onset than males, and males have higher smoking and mortality rates compared to females.30,31
Figure 1. Major depression and smoking model diagram.

Overview of the major depression and smoking model with 15 mutually exclusive smoking and MD compartments. Dotted outline = never smoker; solid outline = current smoker; dashed outline = former smoker; white fill = no history of MD episode in lifetime; black fill = MD episode within the last year; gray fill = lifetime history of at least 1 MD episode but none in the past year; diagonal cross-hatch = history of at least 1 MD episode.
Each cohort is born a never smoker and never MD state using Census Bureau projected population sizes.32 We simulated the population through 2060, the final year of population estimates available from the Census Bureau, as done in previous simulation studies.32–34 We initialize the model in year 1900 with the 1900 birth cohort, and new birth cohorts are added each year such that by 1999, the model includes the entire population ages 0–99. We apply a relative risk of mortality for individuals with histories of MD estimated during model calibration to smoking status-specific mortality rates.
Model calibration
To calibrate the sub-models and full model, we minimized the sum of squared differences between the proportions of individuals in age, smoking, and MD categories observed in the NSDUH and the model predictions using the Davidon-Fletcher-Powell optimization algorithm in the Bhat package in R.35 Given initial model parameter values and their corresponding upper and lower limits, the algorithm searches the parameter space for estimates of scaling factors and interaction effects between smoking and depression that enable the model to reproduce observed smoking and depression patterns by age group. The calibration process refined existing parameter estimates and generated plausible values that do not otherwise exist in the literature, such as depressive episode recovery rates by smoking status. Where possible, we drew initial values used in the optimization from the literature.
Input parameters used in the model, including the smoking and MD sub-models appear in Appendix Table S2. Parameters for interaction effects assumed that current and former smokers have equal or higher MD onset and recurrence rates and equal or lower recovery rates compared to never smokers. We re-estimated effects of depression on smoking behaviors such that those with current MD had increased probability of smoking initiation and lower odds of cessation. To evaluate the sensitivity of model outcomes to each of the interaction effect estimates derived from calibration, we conducted Latin Hypercube Sampling (LHS) and report these results as uncertainty ranges. See Appendix.
Smoking-attributable mortality
To assess smoking-attributable mortality, we sum smoking-attributable deaths (SAD) for former smokers and current smokers across all ages and both genders. We calculate total deaths by multiplying the current and former smoker prevalences (prevcs,fs) by the corresponding population sizes (P) for each age group and gender, and by the difference in mortality rates between current or former smokers and never smokers (μcs,fn,ns) as follows36:
This equation determines the number of deaths attributable to current and former smoking for a given population. Total years of life lost (YLL) take each former or current smoker death at a given age, and apply remaining never smoker life expectancy at those ages (ens,a,g×SADa,g).36 We report prevalence data comparing the population with current and never MD (excluding recall error) to assess differences between the two most disparate groups. This calculation excludes deaths due to secondhand smoke exposure. Of all smoking-attributable deaths and years of life lost among the comorbid population, we furthermore calculate the number that could be completely avoided under a maximum potential reduction in premature mortality (MPRPM) scenario37, in which all smokers with MD across all ages immediately quit in 2018 and no new smoking initiation occurs from 2018–2060.
Results
Following calibration, the full MDS model demonstrated a close fit with corresponding NSDUH 2005–2018 estimates of smoking in the adult current and never MD populations (Figure 2). MDS model estimates generally fell within annual NSDUH 95% confidence intervals. Both NSDUH and MDS model data show expected declines in smoking prevalence over this time period. The 2016–2018 increase in current MD incidence among young adults is reflected by the change in slope shown during those years in Figure 2.
Figure 2. Smoking prevalence among US adults with current and never MD, calibrated model vs. NSDUH of 2005–2018.

Comparison of adult smoking prevalence estimates between survey data and the calibrated model. Dots with vertical lines = NSDUH estimates of current smoking among adults with current MD with confidence intervals; triangles with vertical lines = NSDUH estimates of current smoking among adults with never MD with confidence intervals; solid lines = model estimates for adults with current MD; dashed lines = model estimates for adults with never MD.
Under a status quo scenario, all populations experience decreasing smoking prevalence over time (Table 1). Among those in the model who have no history of MD, smoking prevalence is projected to decrease from 16.2% to 7.5% for females and from 22.3% to 11.0% for males between 2018 and 2060, representing a 56% and 51% relative decrease respectively (−8.7 and −11.3 percentage points in absolute terms). By comparison, adults with current MD have consistently higher smoking rates during this period, with female prevalence declining from 29.3% to 19.6% (33% relative decrease; −9.7 percentage points) and male prevalence from 34.3% to 26.6% (22% relative decrease; −7.7 percentage points).
Table 1.
Smoking-attributed mortality and life-years lost among US adults with current and never MD, 2018–2060
| Males | Current MD | Never MD | Total population | |||
|---|---|---|---|---|---|---|
| 2018 | 2060 | 2018 | 2060 | 2018 | 2060 | |
| Smoking prevalence (%) | 34.3 | 26.6 | 22.3 | 11.0 | 23.4 | 13.9 |
| Proportion of all deaths attributed to smoking (%) | 27.7 | 15.2 | 24.8 | 11.6 | 25.0 | 12.0 |
| Annual number of deaths | 9,339 | 6,264 | 327,807 | 148,555 | 357,628 | 169,653 |
| Cumulative number of deaths | 9,339 | 285,238 | 327,807 | 10,082,538 | 357,628 | 11,021,055 |
| Annual years of life lost | 221,026 | 158,512 | 4,817,050 | 1,708,241 | 5,498,403 | 2,224,591 |
| Cumulative years of life lost | 221,026 | 6,764,096 | 4,817,050 | 129,461,482 | 5,498,403 | 150,959,311 |
| Females | Current MD | Never MD | Total population | |||
| 2018 | 2060 | 2018 | 2060 | 2018 | 2060 | |
| Smoking prevalence (%) | 29.3 | 19.6 | 16.2 | 7.2 | 18.6 | 10.4 |
| Proportion of all deaths attributed to smoking (%) | 16.5 | 7.6 | 9.8 | 5.6 | 10.5 | 6.0 |
| Annual number of deaths | 6,828 | 3,143 | 101,783 | 55,352 | 123,205 | 65,847 |
| Cumulative number of deaths | 6,828 | 198,341 | 101,783 | 3,599,059 | 123,205 | 4,240,851 |
| Annual years of life lost | 164,503 | 71,910 | 1,488,729 | 526,912 | 1,994,722 | 760,031 |
| Cumulative years of life lost | 164,503 | 4,497,855 | 1,488,729 | 43,444,689 | 1,994,722 | 57,644,928 |
Summary table of model projections for adult males and females with a past year major MD episode (current MD), no lifetime history of an MD episode (never MD), and the total population. Current smoking is defined as smoking at all within the past year. See Appendix Table S5 for uncertainty distributions for the current and never MD populations.
The model estimates that 483,579 SADs will occur among people with current MD from 2018 to 2060, 59% among men. The number of annual SADs is expected to decline from 16,167 in 2018 to 9,407 in 2060. In 2018, smoking-attributable deaths represent 16.5% and 27.7% of all deaths among women and men with MD, higher than for women and men without a history of MD for whom SADs represent 9.8% and 24.8% of all population deaths. By 2060, SADs will represent 7.6% and 15.2% of all deaths among women and men with MD, compared to 5.6% and 11.6% among women and men without a history of MD. However, uncertainty analyses for this metric show that estimates overlap with each other (Appendix Table S5). From 2018–2060, approximately 11.3 million cumulative life years would be lost among adults with current MD, even as smoking declines and the estimated number of life years lost drops each year. Under an MPRPM scenario in which no new initiation occurs and 100% of smokers quit in 2018, including those at younger ages, 7.5 million life years would be gained, representing 66% of those lost under current conditions. Of all SADs expected, 264,000 (55%) could be avoided under this best-case scenario (Figure 3).
Figure 3. Cumulative smoking-attributable deaths and years of life lost among US adults with MD, 2018–2018.
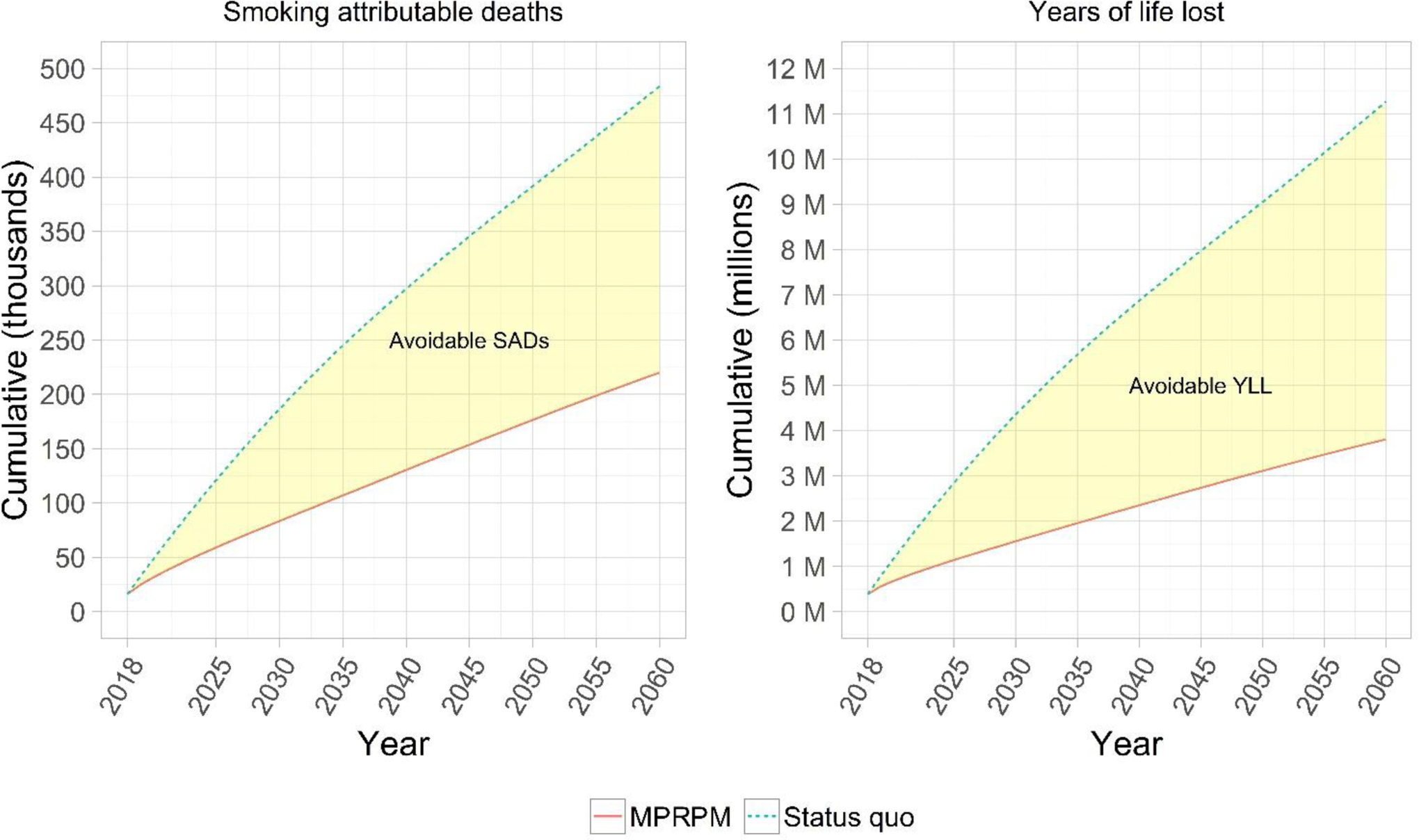
Model projections of the cumulative number of smoking-attributable mortality and life years lost among the adult population with a past year MD episode. SAD = Smoking-attributable death; YLL = years of life lost; MPRPM = Maximum potential reduction in premature mortality scenario in which all smokers with MD quit smoking and no new smoking initiation occurs starting in 2020. Shaded yellow ribbons represent SADs and YLL that could be completely avoided under the MPRPM scenario.
The prevalence ratio comparing smoking among adults with current MD with adults who have never had MD is projected to increase over time (Figure 4). Sensitivity analyses show that this general increase in the prevalence ratio is consistent across parameter combinations. In 2018, women with MD were 1.81 (LHS range: 1.64 – 2.09) times as likely to be smokers compared to those without a history of MD; by 2060, the female smoking prevalence ratio reaches 2.73 (LHS range: 2.43 – 3.37). For men the ratio is 1.54 (LHS range: 1.38 – 1.71), rising to 2.42 (LHS range: 2.13 – 2.73) by 2060.
Figure 4. Smoking prevalence ratio between adults with current and never MD, 2018–2060.

Female (red) and male (blue) model projections for the smoking prevalence ratio between adults with a past year MD episode (current MD) and adults who have never had an MD episode in their lifetime (never MD). Current smoking is defined as smoking at all within the past year. Transparent red and blue ribbons represent optimum range values generated from Latin hypercube sampling sensitivity analysis (See Appendix for details).
Discussion
We developed a simulation model of smoking and MD comorbidity to evaluate smoking prevalence, smoking-attributable mortality and life-years lost among US adults with MD. In the absence of intervention, our model projects that 484,000 adults with current MD will die prematurely as a result of their smoking with 11.3 million life years lost from 2018–2060. Although people with current MD make up a small segment of the US adult population (females: 8.9%; males: 5.4%), MD is the most prevalent mental disorder—thus they constitute a large segment of smokers with any mental illness. Depression remains one of the largest contributors to the global burden of disease and disability.38 Among those with MD, a large proportion smoke, which further increases risk of disability and disease.
Despite overall decreases in smoking prevalence, our model shows that the smoking prevalence ratio by MD status will widen over time, even under optimistic parameter value assumptions. Though this metric suggests a widening of the disparity with time, adults with MD would still experience larger absolute declines in smoking prevalence compared to those who have never had MD—a reflection of their higher initial starting point. For instance, from 2018–2060 smoking prevalence among women with current MD would decrease by 9.7 percentage points compared to 8.7 percentage points among women with never MD.
Although the smoking prevalence ratio between women with and without MD is greater than for men, the proportion of smoking-attributable deaths is still larger for men. Gender differences in attributable deaths are driven by both the higher prevalence of smoking among men and the higher prevalence of depression among women. More than 1 in 4 deaths among men and approximately 1 in 6 among women with MD are smoking-attributable. This statistic is more stark for adults with serious psychological distress, who smoke at even higher rates than people with MD—roughly 1 in 3 of their deaths is smoking-attributable.39
Study limitations and strengths
This is the first joint model of smoking and MD comorbidity in the U.S. It integrates the best available data sources on US smoking and depression patterns. First, the underlying sub-model uses re-scaled CISNET smoking initiation and cessation inputs that reflect historical patterns by age, gender, and birth cohort. Second, for trend assessment we use the NSDUH, the only nationally representative dataset with consecutive years of data on depression and smoking. While we calibrated the model to 14 years of observed data, the CISNET smoking inputs were generated using the National Health Interview Surveys for 1965–2018. We leverage over five decades worth of historical patterns from the NHIS, while fitting the model to contemporary NSDUH data specific to people with MD. Third, depression parameters are from the nation’s longest-running psychiatric epidemiological survey, the Baltimore Epidemiological Catchment Area (ECA) Follow-up Study, re-scaled and calibrated to the NSDUH.19
This model does not consider e-cigarette use, which has risen dramatically, especially among youth and young adults.40–42 Unfortunately the NSDUH does not include questions about use of e-cigarettes or other electronic nicotine delivery systems (ENDS). This model offers a baseline scenario for comparison with future scenarios in which patterns of ENDS use influence smoking use and disparities by mental health status. The rising popularity of ENDS among smokers could have a positive impact on the population with mental health conditions if ENDS facilitate quitting, and on disparities if they do so at higher levels for this group compared to those without mental health conditions. This model also assumes smoking rates remain constant by age, period, and birth cohort going forward. However they may decline even more, which would further decrease expected smoking-attributed mortality in the population.
Larger trends beyond the 14-year period of calibration may not be captured by the current model. A recent study found depression prevalence rising for specific subgroups, including youth ages 12–17.43 The NSDUH data in our analysis show increasing MD among young adults ages 18–25.29 If MD incidence rates are increasing over time, our results are likely to underestimate the true burden of depression in the population, and thereby the extent of smoking-attributable mortality among those with depression. Changing depression patterns could affect our estimates of smoking-attributable mortality in the model; we assume that MD incidence rates for young adults remain constant at their 2018 levels, but if they revert back to their pre-2016 levels, the mortality burden of MD would decrease (See Appendix Table S6).
We do not account for other sociodemographic factors associated with both smoking and MD. For example, disparities in smoking by MD status are even more prominent when considering differences by socioeconomic status.44 The current model does not disaggregate the population beyond age and gender, because introducing too much population heterogeneity can dramatically increase the number of unique states in the model, leading to ‘state explosion’.45 It would be problematic to calibrate a model with a large number of states to survey data when small numbers in specific subgroups would also lead to unstable estimates (e.g., wide confidence intervals).
Moreover, the model simplifies aspects of the individual history of depression to capture patterns at the population-level. For example, we do not examine duration or frequency of depressive episodes at the individual-level.
Our data sources do not survey the homeless, imprisoned, or institutionalized populations, where both smoking and MD are highly prevalent.46 While absolute projections from the model cannot be generalized to these groups, the relative trends may still be applicable for people with other behavioral health conditions. MD is often comorbid with other psychiatric disorders; this analysis does not evaluate MD effects independent of other mental disorders. If these groups were included, the burden of both smoking and MD in the US population, and the smoking disparity by MD status, would likely be much larger.
The absence of other data sources that assess both smoking and MD prevents comparison of the model predictions with external historical data; external model validation would increase confidence in our results. Still, the model outputs corroborate existing research showing the potential for the burden of tobacco to continue disproportionately affecting people with mental illness even as the population experiences declines in smoking prevalence.47–49
Conclusions
Disparities in smoking by mental health status are likely to persist unless major changes to the policy and treatment environment are implemented. We modeled an ideal MPRPM scenario, the benefits of which will not come close to being realized unless bold, comprehensive tobacco control policies are pursued in tandem. Despite long-standing recommendations, only 38% of US mental health treatment facilities offer tobacco cessation counseling.50 Evidence shows that public education campaigns that feature smokers with depression can increase quit attempts among smokers with mental health conditions as well.51,52 Tobacco product regulations that reduce the nicotine content in cigarettes to non-addictive levels could help smokers with mental health conditions to quit.53,54 ENDS present a reduced-risk alternative that could improve the health of comorbid smokers if used instead of cigarettes by those who are otherwise unwilling or unable to quit.55 While these strategies could collectively narrow the cessation disparity,3 interventions that address smoking initiation among people with mental health conditions would lead to even larger mortality reductions in the long-run.
This simulation model offers decision-makers a potential view of the future in the absence of meaningful intervention. The model can also be adapted to evaluate and compare the potential public health impacts of various tobacco control strategies on the MD population. This study advances MD research by focusing on its comorbidity with smoking, and placing it in the context of life expectancy. Given the bidirectional nature of this relationship, if smoking cessation can improve depressive symptoms,8,12,56 this could furthermore reduce MD prevalence; improving prevention and treatment of MD could likewise reduce harms from smoking—areas for future research.
The US is not alone in having high smoking rates among those with mental health conditions;57 other countries with different environments for mental health care and tobacco control could similarly benefit from similar models.58 Future research can explore the population-level impact of large-scale tobacco control interventions that promote quitting or prevent initiation among people with MD, and the health gains that have yet to be achieved.
Supplementary Material
Highlights:
This is the first tobacco simulation model to consider disparities by mental health status.
Model projections show that the smoking disparity by depression status will persist over time.
Smoking prevalence will continue to decline for adults with major depression (MD).
484,000 smoking-related deaths will occur among adults with MD from 2018–2060, with 11.3 million life years lost.
At most, interventions could prevent up to 264,000 of these premature deaths, with 7.5 million life years gained.
Funding:
This work was supported by the National Institute of Drug Abuse of the National Institutes of Health [grant number F31DA041083, 2016–2018]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Taylor is funded by a Cancer Research UK Postdoctoral Fellowship (C56067/A21330). Drs. Meza and Warner acknowledge support from the National Cancer Institute [Meza/Warner; grant number U54CA229974, 2018–2020. Meza; grant number U01CA199284, 2015–2020]. Funding sources had no involvement in the study design, analysis, writing or submission of this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.National Institute of Mental Health. Major Depression. 2019; https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Accessed June 24, 2018.
- 2.Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One. 2011;6(5):e19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberger AH, Chaiton MO, Zhu J, Wall MM, Hasin DS, Goodwin RD. Trends in the Prevalence of Current, Daily, and Nondaily Cigarette Smoking and Quit Ratios by Depression Status in the U.S.: 2005–2017. Am J Prev Med. 2020. [DOI] [PubMed] [Google Scholar]
- 4.Williams JM, Steinberg ML, Griffiths KG, Cooperman N. Smokers With Behavioral Health Comorbidity Should Be Designated a Tobacco Use Disparity Group. Am J Public Health. 2013;103(9):1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legacy Foundation. A Hidden Epidemic: Tobacco Use and Mental Illness. Washington, DC: June 2011. [Google Scholar]
- 6.Prochaska JJ, Das S, Young-Wolff KC. Smoking, Mental Illness, and Public Health. Annu Rev Public Health. 2017;38:165–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroeder SA, Morris CD. Confronting a neglected epidemic: tobacco cessation for persons with mental illnesses and substance abuse problems. Annu Rev Public Health. 2010;31:297–314. [DOI] [PubMed] [Google Scholar]
- 8.Taylor GMJ, Munafo MR. Does smoking cause poor mental health? Lancet Psychiatry. 2019;6(1):2–3. [DOI] [PubMed] [Google Scholar]
- 9.Wootton RE, Richmond RC, Stuijfzand BG, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychological Medicine. 2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fluharty M, Taylor AE, Grabski M, Munafò MR. The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nicotine & Tobacco Research. 2016;19(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaiton MO, Cohen JE, O’Loughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health. 2009;9:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ. 2014;348:g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feirman SP, Glasser AM, Rose S, et al. Computational Models Used to Assess US Tobacco Control Policies. Nicotine Tob Res. 2017. [DOI] [PubMed] [Google Scholar]
- 14.Department of Health. Mental health promotion and mental illness prevention - the economic case. London: Personal Social Services Research Unit, London School of Economics and Political Science; April 2011. [Google Scholar]
- 15.Soeteman DI, Miller M, Kim JJ. Modeling the risks and benefits of depression treatment for children and young adults. Value Health. 2012;15(5):724–729. [DOI] [PubMed] [Google Scholar]
- 16.Both F, Hoogendoorn M, Klein MC, Treur J. Modeling the Dynamics of Mood and Depression. Paper presented at: ECAI; June, 2008. [Google Scholar]
- 17.Vos T, Haby MM, Barendregt JJ, Kruijshaar M, Corry J, Andrews G. The burden of major depression avoidable by longer-term treatment strategies. Arch Gen Psychiatry. 2004;61(11):1097–1103. [DOI] [PubMed] [Google Scholar]
- 18.Patten SB. Markov models of major depression for linking psychiatric epidemiology to clinical practice. Clin Pract Epidemiol Ment Health. 2005;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam J, Mezuk B, Zivin K, Meza R. U.S. Simulation of Lifetime Major Depressive Episode Prevalence and Recall Error. American Journal of Preventive Medicine. 2020;59(2):e39–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Substance Abuse and Mental Health Data Archive. National Survey on Drug Use and Health (NSDUH). 2020; https://www.datafiles.samhsa.gov/study-series/national-survey-drug-use-and-health-nsduh-nid13517. Accessed March 1, 2020.
- 21.Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort–specific smoking histories, 1965–2009. Am J Prev Med. 2014;46(2):e31–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg MA, Feuer EJ, Yu B, et al. Chapter 3: Cohort life tables by smoking status, removing lung cancer as a cause of death. Risk analysis : an official publication of the Society for Risk Analysis. 2012;32 Suppl 1:S25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon J, Holford TR, Levy DT, et al. Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach. Annals of internal medicine. 2018;169(10):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th edition). Washington, DC: 2000. [Google Scholar]
- 25.Center for Behavioral Health Statistics and Quality. Impact of the DSM-IV to DSM-5 Changes on the National Survey on Druge Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration;2016. [PubMed] [Google Scholar]
- 26.Takayanagi Y, Spira AP, Roth KB, Gallo JJ, Eaton WW, Mojtabai R. Accuracy of reports of lifetime mental and physical disorders: results from the Baltimore Epidemiological Catchment Area study. JAMA Psychiatry. 2014;71(3):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton WW, Anthony JC, Gallo J, et al. Natural History of Diagnostic Interview Schedule / DSM-IV Major Depression: The Baltimore Epidemiologic Catchment Area Follow-up. Arch Gen Psychiatry. 1997;54:993–999. [DOI] [PubMed] [Google Scholar]
- 28.Eaton WW, Shao H, Nestadt G, Lee BH, Bienvenu OJ, Zandi P. Population-Based Study of First Onset and Chronicity in Major Depressive Disorder. Arch Gen Psychiatry. 2008;65(5):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Twenge JM, Cooper AB, Joiner TE, Duffy ME, Binau SG. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005–2017. J Abnorm Psychol. 2019;128(3):185–199. [DOI] [PubMed] [Google Scholar]
- 30.Husky M, Mazure C, Paliwal P, McKee S. Gender differences in the comorbidity of smoking behavior and major depression. Drug Alcohol Depend. 2008;93(1–2):176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jha P Avoidable global cancer deaths and total deaths from smoking. Nature Reviews Cancer. 2009;9(9):655–664. [DOI] [PubMed] [Google Scholar]
- 32.United States Census Bureau. 2017. National Population Projections Datasets, Projections for the United States: 2017 to 2060. 2020; https://census.gov/data/datasets/2017/demo/popproj/2017-popproj.html. Accessed April 15, 2020.
- 33.Tam J, Levy DT, Jeon J, et al. Projecting the effects of tobacco control policies in the USA through microsimulation: a study protocol. BMJ Open. 2018;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherng ST, Tam J, Christine PJ, Meza R. Modeling the Effects of E-cigarettes on Smoking Behavior: Implications for Future Adult Smoking Prevalence. Epidemiology (Cambridge, Mass). 2016;27(6):819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leubeck G, Meza R. Bhat: General likelihood exploration. R package version 0.9–10. In:2013. [Google Scholar]
- 36.Holford TR, Meza R, Warner KE, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA. 2014;311(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner KE, Mendez D. How much of the future mortality toll of smoking can be avoided? Tobacco Control. 2020:tobaccocontrol-2019–055530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382(9904):1575–1586. [DOI] [PubMed] [Google Scholar]
- 39.Tam J, Warner KE, Meza R. Smoking and the Reduced Life Expectancy of Individuals With Serious Mental Illness. Am J Prev Med. 2016;51(6):958–966. [DOI] [PubMed] [Google Scholar]
- 40.Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students - United States, 2011–2018. MMWR Morbidity and mortality weekly report. 2018;67(45):1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cullen KA, Gentzke AS, Sawdey MD, et al. e-Cigarette Use Among Youth in the United States, 2019. JAMA. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai H, Leventhal AM. Prevalence of e-Cigarette Use Among Adults in the United States, 2014–2018. JAMA. 2019;322(18):1824–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberger AH, Gbedemah M, Martinez AM, Nash D, Galea S, Goodwin RD. Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychological Medicine. 2017:1–10. [DOI] [PubMed] [Google Scholar]
- 44.Weinberger AH, Bandiera FC, Leventhal AM, et al. Socioeconomic Disparities in Smoking Among U.S. Adults With Depression, 2005–2014. Am J Prev Med. 2018. [DOI] [PubMed] [Google Scholar]
- 45.Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force–3. Medical Decision Making. 2012;32(5):690–700. [DOI] [PubMed] [Google Scholar]
- 46.Greg A Greenberg PD, Robert A. Rosenheck MD. Jail Incarceration, Homelessness, and Mental Health: A National Study. Psychiatric Services. 2008;59(2):170–177. [DOI] [PubMed] [Google Scholar]
- 47.Zvolensky MJ, Jardin C, Wall MM, et al. Psychological distress among smokers in the United States: 2008 to 2014. Nicotine Tob Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talati A, Keyes K, Hasin D. Changing relationships between smoking and psychiatric disorders across twentieth century birth cohorts: clinical and research implications. Molecular psychiatry. 2016;21(4):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA. 2014;311(2):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marynak K, VanFrank B, Tetlow S, et al. Tobacco Cessation Interventions and Smoke-Free Policies in Mental Health and Substance Abuse Treatment Facilities - United States, 2016. MMWR Morbidity and mortality weekly report. 2018;67(18):519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.YouTube. CDC: Tips From Former Smokers - Rebecca: Vicious Cycle [video]. Tips from Former Smokers 2016; https://www.youtube.com/watch?v=XCibiAnJ6Ts. Accessed May 5, 2018. [Google Scholar]
- 52.Prochaska JJ, Gates EF, Davis KC, Gutierrez K, Prutzman Y, Rodes R. The 2016 Tips From Former Smokers(R) Campaign: Associations With Quit Intentions and Quit Attempts Among Smokers With and Without Mental Health Conditions. Nicotine Tob Res. 2019;21(5):576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaalema DE, Tidey JW, Davis DR, et al. Potential Moderating Effects of Psychiatric Diagnosis and Symptom Severity on Subjective and Behavioral Responses to Reduced Nicotine Content Cigarettes. Nicotine Tob Res. 2019;21(Suppl 1):S29–s37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgins ST, Heil SH, Sigmon SC, et al. Addiction Potential of Cigarettes With Reduced Nicotine Content in Populations With Psychiatric Disorders and Other Vulnerabilities to Tobacco Addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henningfield JE, Higgins ST, Villanti AC. Are we guilty of errors of omission on the potential role of electronic nicotine delivery systems as less harmful substitutes for combusted tobacco use? Prev Med. 2018;117:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor G, Girling A, McNeill A, Aveyard P. Does smoking cessation result in improved mental health? A comparison of regression modelling and propensity score matching. BMJ Open. 2015;5(10):e008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization Regional Office for Europe. Tobacco use and mental health conditions: a policy brief. Copenhagen, Denmark: 2020. [Google Scholar]
- 58.Taylor GMJ, Itani T, Thomas KH, et al. Prescribing Prevalence, Effectiveness, and Mental Health Safety of Smoking Cessation Medicines in Patients With Mental Disorders. Nicotine & Tobacco Research. 2019;22(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


