Abstract
Background:
AMPA receptors are ionotropic glutamate receptors that have been investigated for their role in modulating alcohol consumption. However, little is known about the role of AMPA receptors in the control of binge-like or free-access alcohol drinking in C57BL/6J or in selectively bred high alcohol preferring (HAP) mice. The purpose of this experiment was to assess the role of systemic administration of the AMPA receptor antagonist, NBQX, on alcohol consumption using a model of binge-like drinking, Drinking-in-the-Dark (DID) and free-access two-bottle choice (2BC) in male and female C57BL/6J and HAP mice
Methods:
C57BL/6J mice were allowed free access to 20% (v/v) alcohol for two hours each day beginning three hours into the dark cycle for four days. On day five mice were intraperitoneally injected with one of four doses of NBQX (0, 3, 10, or 30 mg/kg; n=10) 15-minutes before alcohol presentation and were given four-hour alcohol access (extended DID). HAP mice were given 24-hour free access to 10% (v/v) alcohol and water for 19 days. On day 20 mice were intraperitoneally injected with one of four doses of NBQX (0, 3, 10, 30 mg/kg; n=9) 15-minutes before alcohol and water presentation.
Results:
In the first two-hours of DID, at 30 mg/kg, male, but not female C57BL/6J or HAP, mice drank significantly less alcohol compared to controls and 30 mg/kg NBQX did not alter saccharin intake in the males. Although male HAP mice drank significantly less alcohol than female mice following 10 mg/kg NBQX, neither sex exhibited drinking that differed significantly from controls. NBQX did not reduce locomotor behavior at any dose, sex, or genotype.
Conclusions:
These data suggest that AMPA receptors play a key role in modulating binge-like alcohol consumption without altering saccharin consumption or general locomotion, and that this effect is specific to sex and genotype.
Keywords: Alcohol, Binge, AMPA, NBQX
Introduction
Harmful alcohol use resulted in over 3 million deaths in 2016 worldwide, accounting for 5.3% of all deaths that year (World Health Organization, 2018). Over half of all alcohol attributable deaths are due to excessive alcohol consumption in which binge-drinking is the most common form (Stahre et al., 2014). The NIAAA defines binge drinking as 4 or more drinks for females and 5 or more drinks for males within 2 hours, resulting in blood alcohol levels at or above .08 g/dL (NIAAA, accessed July 2019). Additionally, large numbers of people drink to the level of a binge with nearly 25 percent of people aged 12 and older self-reporting binge drinking within the past month (SAMHSA, 2015). These numbers are alarming, especially considering that the exact neurobiological mechanisms that underlie the actions of alcohol, binge-drinking, and alcohol use disorder (AUD) are incompletely understood. Further, because AUD is hereditable it becomes increasingly important to understand the genetic contribution to AUD (Kendler et al., 1995; Verhulst et al., 2015).
AMPA receptors are a subtype of ionotropic glutamate receptors that are vital for fast synaptic neurotransmission and long-term potentiation (LTP) within the central nervous system and have recently been under investigation for their role in modulating alcohol consumption (Wang et al., 2012; Corbit et al., 2014; Henley & Wilkinson, 2016; Ruda-Kucerova et al., 2018; Ueno et al., 2019; Dannenhoffer & Spear, 2019). LTP is dependent on the insertion of AMPA receptors into the post-synaptic membrane where glutamate binding to AMPA receptors is necessary to cause post-synaptic depolarization of the membrane ultimately leading to an upregulation of LTP dependent AMPA receptors. Furthermore, because alcohol acts on the glutamate system in a variety of ways (for example acute alcohol reduces extracellular glutamate concentrations and chronic ethanol exposure increases extracellular glutamate concentrations) investigating the role of AMPA receptors in modulating alcohol intake is pertinent (Roberto et al., 2004; Ding et al., 2013; Tiwari et al., 2014; for further review of alcohols actions on glutamate please see Banerjee, 2014 & Goodwani et al., 2017).
Previously, researchers have used the competitive AMPA/Kainate receptor antagonist, NBQX, to investigate AMPA receptors. NBQX has a relatively high binding affinity for AMPA receptors (KD = 47 nM; Dev et al., 1996) and is more selective for AMPA receptors by 30 fold than it is for kainate receptors, making it a useful pharmacological tool to assess the role of AMPA receptors in the control of alcohol drinking (Sheardown et al., 1990). Wang et al. (2012) demonstrated that either repeated systemic injection of alcohol or intermittent access two-bottle choice drinking upregulates synaptic AMPA receptors in the dorsomedial striatum (DMS), while systemic injection (Stephens & Brown, 1999; Ruda-Kucerova et al., 2018) and direct dorsolateral striatal (DLS) or DMS infusion of the AMPA receptor antagonist NBQX reduces operant responding for alcohol in rats (Wang et al., 2012; Corbit et al., 2014). However, the aforementioned experiments were all done in male rats and either did not assess binge-drinking or used procedures that did not result in intake that is of the level of a binge. Additionally, none of these experiments investigated the effect of NBQX on AMPA receptors in a genetic mouse model of high excessive alcohol drinking.
To expand upon this literature, the current experiments sought to assess whether systemic administration of NBQX reduces alcohol drinking. First, we assessed whether NBQX reduces binge-like alcohol drinking using a limited access model of alcohol drinking, Drinking-in-the-Dark (DID; Thiele, Crabbe, & Boehm, 2014) in both male and female C57BL/6J mice. We next assessed whether NBQX reduces free-access two-bottle choice (2BC) alcohol preference drinking in male and female replicate High Alcohol Preferring (HAP3) mice. We utilized two different drinking paradigms because C57BL/6J mice readily consume high amounts of alcohol in DID (Fuller, 1964; Belknap, Crabbe, & Young, 1993; Thiele, Crabbe, & Boehm, 2014) and HAP3 mice are selectively bred to consume high amounts of alcohol under 2BC procedures (Oberlin et al., 2011). We also sought to determine if the effect of NBQX is specific to the drinking paradigm and/or mouse genotype. We determined this by testing replicate HAP3 mice in DID in the same manner as we previously had with the C57BL/6J mice, by replicating the NBQX effects in male C57BL/6J mice, and by demonstrating the NBQX does not alter saccharin consumption.
Materials and Methods
Animals
Naïve adult male and adult female C57BL/6J mice (PND 61-70 at drinking start) were acquired from The Jackson Laboratory (Bar Harbor, ME). Naïve adult male and female HAP3 mice (PND 61-95 at drinking start) were bred on site at the IUPUI School of Science, Indianapolis, IN (for more information on the selection and characterization of HAP3 mice, please see Oberlin et al., 2011). Animals were individually housed in a vivarium with 12h:12h reverse light-dark cycle for at least one week prior to the start of experiments. Mice were housed in non-filtered wired top standard shoe box mouse cages (18.4 cm wide, 29.2 cm long, 12.7 cm tall), and were given food (Lab Diet 5001, Rodent Diet) and tap water ad libitum with the exception of water bottle removal during the DID sessions. Procedures were approved by the IUPUI School of Science Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals (The National Academic Press, 2003).
Drugs
190 proof alcohol was purchased from Pharmco, Inc (Brookfield, CT) and was added to tap water to create a 20% v/v alcohol solution for use in the DID experiments. 200 proof alcohol was purchased from Pharmco, INC (Brookfield, CT) and was added to tap water to create a 10% v/v alcohol solution for use in 2BC experiments. 200 proof alcohol was used for the 2BC experiments instead of 190 proof because the HAP mice are bred for alcohol drinking on 200 proof alcohol solutions. Saccharin (≥99%) was purchased from Sigma Aldrich (St. Louis, MO) and was added to tap water to create a 0.2% saccharin concentration, as previously done in our lab (Kasten & Boehm, 2014). NBQX disodium salt hydrate (≥98%) was purchased from Sigma Aldrich (St. Louis, MO). The NBQX was dissolved into saline purchased from Teknova (Newark, DE) to make drug doses of 0, 3, 10 and 30 mg/kg and injected at volumes of 10 ml/kg. Drug doses and pretreatment timing was chosen based on available literature (Stephens & Brown, 1999; Ruda-Kucerova et al., 2018) and our own pilot data.
Drinking-in-the-Dark (DID)
DID is a limited-access model of binge-like alcohol consumption (Thiele, Crabbe, & Boehm, 2014). 80 C57BL/6J (40 male and 40 female) mice received one 10 mL ball-bearing sipper tube of 20% v/v alcohol in tap water into their home cages in place of the regular water bottle. This occurred three hours into the dark cycle for two-hours on days 1-4 and at two and four-hours on day five. C57BL/6J mice demonstrate binge-like alcohol consumption during DID, achieving two-hour intakes approaching 3.0 g/kg and blood alcohol levels surpassing 0.08 g/dL (Kasten & Boehm, 2014). Consumption was measured by reading the sipper tubes to the nearest 0.025 mL, and volumes were adjusted for leak based on of volumes leaked from sipper tubes in empty cages on the same drinking rack. A saline vehicle injection (IP) was given 15 min prior to alcohol access on day 4 to habituate animals to the injection procedure; body weights were taken the day prior to day 1 of DID to determine g/kg consumption and on day 4 to determine injection volume. Then on day 5, animals received an injection of NBQX at 0, 3, 10, or 30 mg/kg (IP) 15 min prior to alcohol access (n=10/dose/sex). Drug doses, group sizes, and pretreatment timing was chosen based on available literature (Stephens & Brown, 1999; Ruda-Kucerova et al., 2018) and our own pilot data.
Based on the results of our initial experiments, we conducted a follow-up experiment to determine whether the main finding is specific to the DID model, male C57BL/6J mice, and to alcohol drinking. 80 HAP3 (40 male and 40 female) mice were used to assess the effect of NBQX on DID, and 20 male C57BL/6J mice were used to replicate our main finding and to show that saccharin consumption is not affected by 30 mg/kg NBQX. The DID occurred for 5 days total as previously described above. The saccharin drinking occurred in the same C57BL/6J mice. Following DID the mice were given two days of saccharin (0.2%) drinking total, with a saline injection (IP) proceeding the first day drinking, and a 30 mg/kg NBQX injection proceeding the second day drinking
Two-Bottle Choice (2BC)
2BC is a free-access model of alcohol consumption whereby animals get unlimited access to both alcohol (10% v/v) and tap water in their home cages 24-hours a day. Animals were allowed free access for a total of 20-days, as 2-3 weeks is the general timeline in which HAP mice establish stable drinking (Oberlin et al., 2011). In this experiment, 36 male and 36 female HAP3 mice underwent the 2BC drinking paradigm. Weights were taken the day before drinking on day 1, and weekly thereafter, and on day 19. On day 19 mice were injected (IP) with saline to habituate them to the stress of injection. Two-hours and 45-minutes into the dark cycle on day 20, mice were injected (IP) with one of four doses of NBQX (0, 3, 10, or 30 mg/kg) and bottles were read two-, four- and 24-hours later (the two and four hour readings were done as to not miss the potential effect of NBQX on alcohol drinking; n=9/dose/sex). Injections were given 2 hours and 45-minutes into the dark cycle, as to stay consistent with the timing of injections relative to the dark cycle for the DID experiment. Consumption was measured by reading the sipper tubes to the nearest 0.025 mL. 2BC preference scores were calculated by dividing alcohol consumed by total alcohol and water consumed.
Locomotor Monitoring
Home cage locomotor activity was monitored during all initial drinking sessions (DID in C57BL/6J male and female mice and 2BC in HAP3 male and female mice) to capture any hypolocomotor effects of NBQX on alcohol drinking. Data were collected using the Opto M-3 system (Columbus Instruments) which collects locomotor activity by totaling beam breaks over a set time-interval from infrared beams that surround the perimeter of the homecage. Activity was detected by the interruption of photocell beams (0.32 cm diameter; 875 nanometer wavelength; 160 hertz scan rate) positioned along the walls of standard shoebox mouse cages (18.4 cm wide, 29.2 cm long, 12.7 cm tall). For each cage, two 33 cm long photocell sensor units, containing 12 recording photocell detector or emitters each spaced 2.54 cm apart, were positioned 27 cm apart along the long walls of the mouse cage. Two 24 cm long photocell sensor units (containing 8 recording photocell detector or emitters each spaced 2.54 cm apart) were positioned 32 cm apart along the short walls of the mouse cage. Ambulatory data were collected every 5-minutes.
Statistical Analyses
Statistical analyses were conducted separately by time in the C57BL/6J and HAP3 experiments for alcohol drinking, saccharin drinking, and locomotor activity. Baseline consumption (days 1-4 for DID; days 1-19 for 2BC) was analyzed by three-way repeated measures ANOVA (day X dose X sex; RM by day). The effect of NBQX on alcohol drinking (day 5 for the DID experiments; day 20 for 2BC) was assessed with two-way ANOVA (dose X sex). Locomotor activity was assessed by two-way ANOVA (dose X sex). Post-hoc one-way ANOVA and Dunnett’s multiple comparison tests were performed on the male alcohol consumption group for the C57BL/6J mice. Post-hoc unpaired t-test were performed to follow-up on the interaction of dose and sex for the 24-hour HAP3 consumption. In our follow up experiments, baseline consumption (days 1-4 for DID) was analyzed with repeated measures two-way ANOVA (day X dose for C57BL/6J males and day X dose X sex for HAP3; RM by day). The effect of NBQX on alcohol drinking (day 5 for DID) was assessed with two-way ANOVA for HAP3 (dose X sex) at each time point and with unpaired t-tests (saline vs 30 mg/kg NBQX) in the C57B/6J mice at each timepoint. In the same C57BL/6J mice that underwent the replication of NBQX on DID in C57BL/6J mice, the effect of NBQX on saccharin drinking (day 7, where days 1-5 were alcohol DID, and days 6 and 7 were saccharin DID) was assessed with unpaired t-tests (saline vs 30 mg/kg) at each time point. Differences were considered significant at p < 0.05.
Results
Drinking-in-the-Dark (DID) in C57BL/6J Mice
Baseline alcohol drinking (days 1-4) for male and female C57BL/6J mice are shown in figure 1. Alcohol consumption is displayed in grams consumed per kilogram of body weight. Three-way repeated measures ANOVA of baseline drinking revealed a significant main effect of day [F(3,219) = 5.476, p < 0.0001] and sex [F(1,73) = 122.85, p < 0.0001], but no significant main effect of dose or significant interaction of day, sex, and dose. On day four, all drug groups were given a saline injection 15-minutes prior to the DID session. Drug group assignments were chosen based on day four alcohol consumption so that baseline drinking was similar across drug groups.
Figure 1. Baseline Limited-Access Alcohol Consumption Across Dosing-Groups in C57BL/6J Mice.

Average daily consumption per dose across the four days prior to NBQX injection in male (A) and female (B) C57BL/6J mice. Main effect of day and sex, (****p’s < 0.0001).
The effect of NBQX on alcohol consumption during DID in male and female C57BL/6J mice is shown in figure 2. Two-way ANOVA revealed a significant main effect of dose [F(3,73) = 8.428, p < 0.0001], sex [F(1,73) = 109.7, p < 0.0001], and an interaction of dose and sex [F(3,73) = 4.811, p = 0.0041] on consumption during the first two-hours of DID. Simple effects ANOVA in the first two-hours revealed a main effect of dose [F(3,36) = 11, p < 0.0001] in male, but not female, C57BL/6J mice. Dunnett’s multiple comparisons indicate that the average consumption was significantly less for male mice in the 30 mg/kg group (M= 0.837, SD = 0.7751) than in the saline control group (M = 2.438, SD = 1.143), [F(3, 36) = 11, p < 0.0001], figure 2A. Two-way ANOVA of hours 3-4 of DID revealed a significant main effect of sex [F(1, 73) = 13.15, p < 0.001], but no significant main effect of dose or significant interaction of sex and dose on consumption; figure 2B. Two-way ANOVA at hours 1-2 revealed a significant main effect of sex [F(1,68) = 53.05 p < 0.0001] on locomotor activity, but no significant effect of dose or interaction of sex and dose; figure 2C. Two-way ANOVA at hours 3-4 revealed a significant main effect of sex [F(1,68) = 35.44, p < 0.0001], but no significant main effect of dose or significant interaction of sex and dose on locomotor activity; figure 2D. Thus, NBQX reduced binge-like alcohol drinking at the highest dose in male C57BL/6J mice, without altering locomotor activity. NBXQ did not alter binge-like alcohol drinking or locomotion in female C57BL/6J mice at any dose tested.
Figure 2. Effect of NBQX on Limited-Access Alcohol Consumption and Locomotor Activity in C57BL/6J mice.

Male and female C57BL/6J mice were treated with four doses of NBQX (n=10/dose/sex) and alcohol consumption and locomotor activity were monitored during a four-hour DID session on day five of DID. A. Main effect of dose and sex (****p < 0.0001). 30 mg/kg significantly reduced alcohol drinking in males relative to saline (**p < 0.01). Females consumed significantly more alcohol than males at each dose (#p < 0.05, ###p < 0.001, ####p < 0.0001). B. Main effect of sex on alcohol consumption (***p < 0.001). C. Main effect of sex at hours 1-2 on locomotor activity (****p < 0.0001). D. Main effect of sex at hours 3-4 on locomotor activity (****p < 0.0001.
Two-Bottle Choice (2BC) in HAP3 Mice
Baseline alcohol drinking (days 1-19) for male and female HAP3 mice are shown in figure 3. Alcohol consumption is displayed in grams consumed per kilogram of body weight. Three-way repeated measures ANOVA of baseline drinking revealed a significant main effect of day [F(1,59) = 27.704, p < 0.0001], and a significant main effect of sex [F(1, 59) = 8.99, p = 0.004], but no significant effect of dose, or interaction of day, sex, or dose. Thus, although alcohol intake differed across days, and between sexes, the groups did not differ based on dose assignment prior to day 20 NBQX treatment.
Figure 3. Baseline Continuous-Access Alcohol Consumption Across Dosing-Groups in HAP3 Selectively Bred Mice.

Average daily consumption per dose across the 19 days prior to NBQX injection in male (A) and female (B) HAP3 mice. Main effect of day and sex, (****p < 0.0001).
The effect of NBQX on 2BC alcohol consumption in male and female HAP3 mice is shown in figure 4. Two-way ANOVA did not reveal a significant main effect of dose, sex, or interaction of dose and sex during hours 1-2 of 2BC consumption; figure 4A. Two-way ANOVA of hours 3-4 of 2BC revealed a significant main effect of sex [F(1, 64) = 21.14, p < 0.0001] but no significant effect of dose, or interaction of dose and sex on consumption, figure 4B. Two-way ANOVA at 24-hours did not reveal a main effect of sex or dose, but there was a significant interaction of sex and dose [F(3, 64) = 2.753, p = 0.0497] on consumption. Simple main effect ANOVA did not reveal a main effect of dose in either males or females. Post-hoc unpaired t-test revealed a significant difference in consumption for male (M = 18.6, SD = 3.734) and female (M = 27.36, SD = 9.282) mice at 10 mg/kg at the 24-hour consumption timepoint; t(16) = 2.628, p = 0.0185; figure 4C. Two-way ANOVA at hours 1-2 revealed a significant main effect of sex [F(1,62) = 18.77 p < 0.0001], but no significant main effect of dose, or interaction of sex and dose on locomotor activity; figure 4D. Two-way ANOVA at hours 3-4 revealed a main effect of sex, [F(1,62) = 16.24, p < 0.001], but no significant main effect of dose, or interaction of sex and dose on locomotor activity; figure 4E. Two-way ANOVA at 24-hours revealed a main effect of sex [F(1,62) = 13.71, p = 0.0005], but no significant main effect of dose, or interaction of sex and dose on locomotor activity; figure 4F. Thus, NBQX did not alter excessive alcohol drinking, or locomotion, in male or female HAP3 mice, regardless of dose.
Figure 4. Effect of NBQX on Continuous Alcohol Consumption and Locomotor Activity in Selectively Bred HAP3 mice.
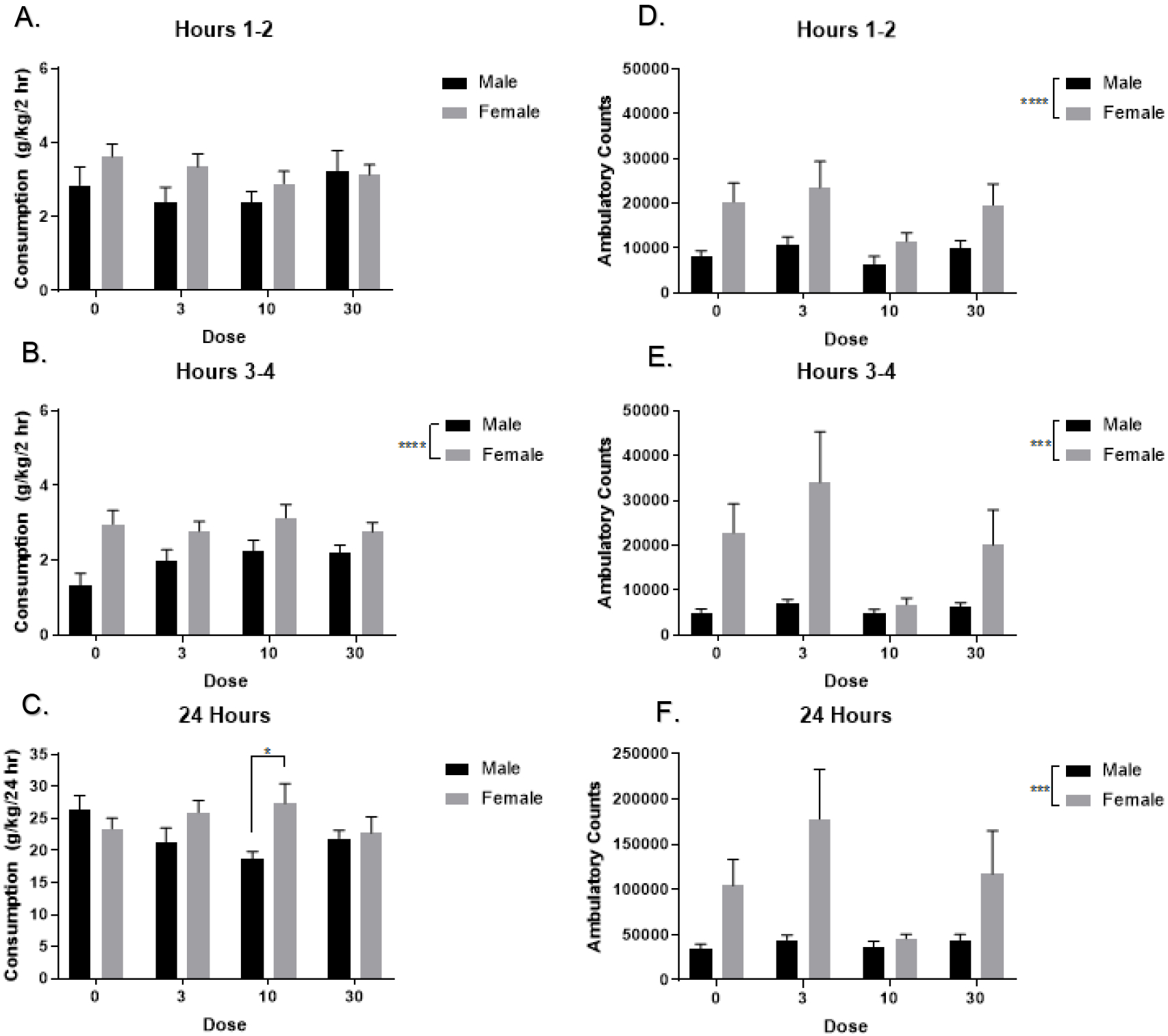
Male and female HAP3 mice were treated with one of four doses of NBQX (n=9/dose/sex) and alcohol consumption and locomotor activity was monitored during two, four, and 24 hours into a 24-hour two-bottle choice drinking session on day 20 of DID. A. Neither sex nor NBQX altered alcohol drinking during hours 1-2 (p’s > 0.05). B. Main effect of sex on alcohol drinking during hours 3-4 (****p < 0.0001). C. 24-hours later, female mice consumed significantly more than male mice at 10 mg/kg (*p < 0.05). D. Main effect of sex on locomotor activity at hours 1-2 (****p < 0.0001). E. Main effect of sex on locomotor activity at hours 3-4 (***p < 0.001). F. Female mice had significantly more locomotor activity than male mice 24-hours later (***p < 0.001).
Preference drinking data were collected on day 20. Mice that were treated with one of four doses of NBQX (n=9/dose/sex) received both water and alcohol bottles during the 2BC session. NBQX also did not alter alcohol preference in male and female HAP3 mice (table 1). Two-way ANOVAs at the 1-2, 3-4, and 24-hour intervals did not reveal any significant main effects of dose, sex, or their interact on alcohol preference drinking.
Table 1. Effect of NBQX on Continuous Access Alcohol Preference in Selectively Bred HAP3 Mice.
Alcohol and water drinking data were calculated for day 20. Male and female mice that were treated with one of four doses of NBQX (n=9/dose/sex) received both water and alcohol bottles during the two-bottle choice session and alcohol preference was calculated. Preference was calculated as follows: (((alcohol consumption) / (alcohol + water consumption)) X 100), where a score of 100 would indicate 100% alcohol preference. Hours 1-2, 3-4, and 24 did not reveal an effect of sex, dose, or an interaction of sex and dose (p’s > 0.05). Data are displayed as mean ± SEM.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| NBQX Dose mg/kg | Hours 1-2 | Hours 3-4 | 24-hours | Hours 1-2 | Hours 3-4 | 24-hours |
| 0 | 88.8 ± 6.0 | 92.6 ± 15 | 89.6 ± 2.7 | 91.4 ± 4.4 | 93.1 ± 3.5 | 93.3 ± 2.4 |
| 3 | 79.7 ± 9.4 | 70 ± 9.6 | 84.3 ± 5.8 | 84.9 ± 7.0 | 85.3 ± 7.6 | 88.0 ± 4.0 |
| 10 | 88.3 ± 4.7 | 73 ± 10.4 | 82.3 ± 5.5 | 93.4 ± 3.3 | 86.4 ± 6.8 | 90.2 ± 5.0 |
| 30 | 85.0 ± 7.2 | 92.6 ± 7.4 | 91.1 ± 2.2 | 94.7 ± 3.5 | 83.1 ± 7.4 | 89.4 ± 5.8 |
Follow-up Experiments
To further investigate whether the effect of NBQX on consumption was specific to DID, we assessed the effect of NBQX in HAP3 mice during DID. Baseline alcohol drinking (days 1-4) for male and female HAP3 mice is shown in figure 5. Alcohol consumption is displayed in grams consumed per kilogram of body weight. Three-way repeated measures ANOVA of baseline drinking revealed a significant main effect of day [F(3,207) = 3.1, p = 0.03] and sex [F(1,69) = 22.66, p < 0.0001], but no significant main effect of dose or significant interaction of day, sex, and dose. On day four, all drug groups were given a saline injection 15-minutes prior to the DID session. Drug group assignments were chosen based on day four alcohol consumption so that baseline drinking was similar across drug groups. Importantly, while the daily pattern of DID differed by day and between males and females, there were no group differences for drug dose on drinking on any of the days or on day four, or immediately prior to drug treatment on day five, for either sex.
Figure 5. Baseline and Effect of NBQX on Limited-Access Alcohol Consumption Across Dosing-Groups in HAP3 Selectively Bred Mice.

Average daily consumption per dose across the 4 days prior to NBQX injection in male (A) and female (B) HAP3 mice. Male and female HAP3 mice were treated with one of four doses of NBQX (n=9/dose/sex) and alcohol consumption was monitored during two and four hours on day 5 of DID. A and B. No effect of day on alcohol consumption (p > 0.05). C and D. Main effect of sex on alcohol consumption (***p < 0.001).
The effect of NBQX on alcohol consumption is shown in figure 5. HAP3 mice were treated with four doses of NBQX (n=9-10/dose/sex) and alcohol consumption and locomotor activity were monitored across two and four hours during a four-hour DID session on day five of DID. Two-way ANOVA revealed a significant main effect of sex [F(1,69) = 15.27, p > 0.001], but no significant effect of dose or interaction of dose and sex on consumption during the first two-hours of DID; figure 5C. Two-way ANOVA of hours 3-4 of DID revealed a significant main effect of sex [F(1,69) = 15.20, p < 0.001], but no significant main effect of dose or significant interaction of sex and dose on consumption; figure 5D. Thus, NBQX did not affect binge-like alcohol drinking in male or female HAP3 mice.
To further investigate the robustness of our main finding, we sought to replicate the effect of NBQX on alcohol consumption during DID in male C57BL/6J mice. Baseline alcohol drinking (days 1-4) are shown in figure 6. Alcohol consumption is displayed in grams consumed per kilogram of body weight. Two-way repeated measures ANOVA of baseline drinking did not reveal a significant main effect of day, dose, or interaction of day and dose. On day four, all drug groups were given a saline injection 15-minutes prior to the DID session. Drug group assignments were chosen based on day four alcohol consumption so that baseline drinking was similar across drug groups. Importantly, there were no group differences for drug dose on drinking on any of the days or on day four, or immediately prior to drug treatment on day five, for either sex.
Figure 6. Replication of 30 mg/kg NBQX in Male C57BL/6J Mice during DID on Alcohol Drinking and Effect of 30 mg/kg NBQX on Saccharin Drinking in Male C57BL/6J Mice.
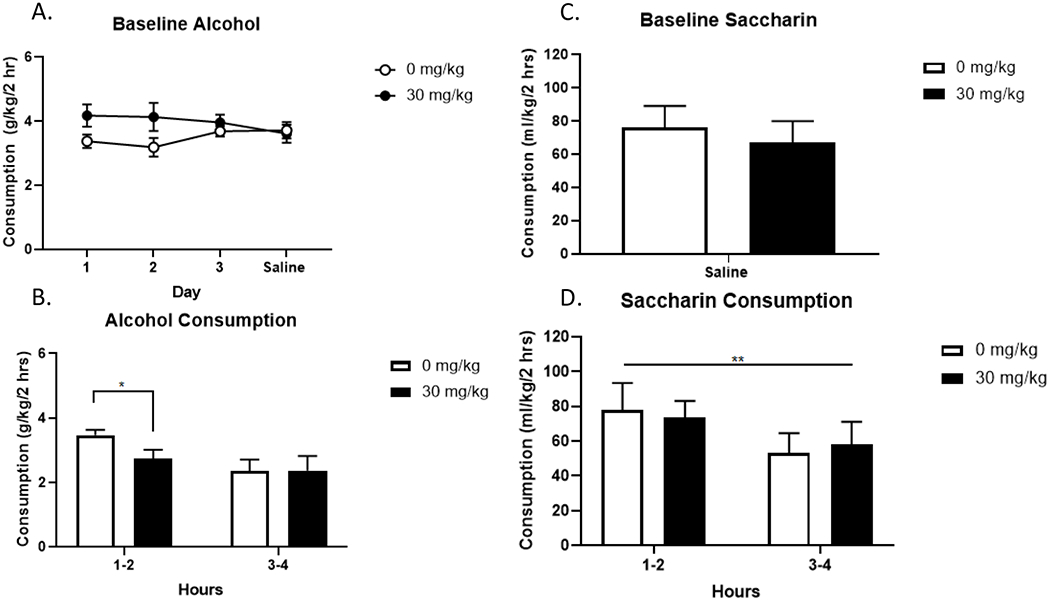
Average daily consumption per dose across the 4 days prior to NBQX injection in male C57BL/6J mice (A). Male C57BL/6J mice were treated with one of four doses of NBQX (n=10/dose) and alcohol consumption was monitored during two and four hours on day 5 of DID. On day 6 and 7 the same mice were given saccharin during DID instead of alcohol. On day 6 all animals were given a saline injection. Groups were assigned based on day 6 consumption (C). Male C57BL/6J mice were treated with either saline or 30 mg/kg NBQX (n=9-10/dose) and alcohol saccharin consumption was monitored during two and four hours on day 7 of saccharin DID. A. No effect of dose or day on alcohol consumption (p > 0.05). B. At two hours, male C57BL/6J mice given NBQX drank significantly less alcohol than mice given saline (*p < 0.05). C. No effect of dose on baseline saccharin consumption (p > 0.05) D. Main effect of time on saccharin consumption (**p < 0.01).
The effect of 30 mg/kg NBQX on alcohol consumption is shown in figure 6 (n=10/dose). Unpaired t-test at the two-hour timepoint revealed a significant effect of 30 mg/kg NBQX (M = 2.73, SD = 0.89) at reducing alcohol drinking compared to saline (M = 3.67, SD = 0.50), t(18) = 2.27, p = 0.035; figure 6B. Unpaired t-test at the four-hour timepoint did not reveal a significant effect of NBQX; figure 6B. Thus, we were able to replicate our primary finding that 30 mg/kg NBQX significantly reduces binge-like alcohol drinking in the first two-hours of DID in male C57BL/6J mice.
To further investigate the robustness of our main finding, we sought to investigate whether our main effect of NBQX in male C57BL/6J mice is specific to alcohol. In the same male C57BL/6J used to replicate the effect of NBQX on alcohol drinking, we assessed the effect of NBQX on 0.2% saccharin drinking. Baseline saccharin drinking (i.e. day 6) is shown in figure 6C where drug groups were assigned based on average intakes on day 6. Figure 6D shows 0.2 % saccharin drinking in the males following 30 mg/kg NBQX or saline (n=9-10/dose). Consumption data are shown in milliliter of solution consumed per kilogram of mouse across hours 1-2 and hours 3-4. Unpaired t-tests at hours 1-2 and hours 3-4 of saccharin drinking following 30 mg/kg NBQX demonstrated that the drug did not alter saccharin intake in male C57BL/6J mice; figures 6D.
Discussion
The goal of the present experiment was to understand whether there is a role for AMPA receptors in the modulation of binge-like alcohol DID drinking in C57BL/6J and HAP3 mice, and 2BC alcohol preference drinking in HAP3 mice, and to address gaps in the literature with the inclusion of both male and female mice. The results from this experiment demonstrate that NBQX at 30 mg/kg reduces binge-like alcohol drinking in male C57BL/6J mice, but not female C57BL/6J mice or HAP3 mice. Additionally, NBQX reduced drinking for the male C57BL/6J mice in hours 1-2 but not in hours 3-4 during the extended DID session which is congruent with the half-life of NBQX (30 minutes; Gill et al., 1992; Shimizu-Sasamata et al., 1996). Importantly, the reduction in consumption does not appear to be due to a sedentary effect as NBQX did not reduce locomotor activity at any dose. We were able to replicate our main finding and the drug effect observed appears specific to alcohol as 0.2% saccharin consumption was not affected by NBQX at 30 mg/kg when assessed in a separate group of males. These data differ from previous findings from Stephens and Brown (1999), who demonstrated an NBQX-induced reduction in operant pressing for alcohol, sucrose, and saccharin, as well as a significant suppression of locomotor activity when administered IP in rats at 3 and 6 mg/kg. Importantly, our data align with a recent publication in that NBQX (5 and 10 mg/kg) reduced alcohol consumption in male Wistar rats without affecting overall locomotor activity (Ruda-Kucerova et al., 2018).
The contrasting findings of NBQX on locomotor activity in this experiment and previous experiments, could be due to differences in alcohol consumption model and species. Our experimental findings were specific to C57BL/6J mice and to DID, whereas locomotor suppression has been reported during operant responding in rats (Stephens and Brown, 1999). Although, the suppressed locomotor response in Stephens and Brown (1999) appears to be the exception, rather than the rule, as numerous other studies utilizing IP administration of NBQX have reported a specific behavioral response without locomotor suppression at doses similar to what we used. For example, 10, 20, and 30 mg/kg NBQX impaired Morris Water Maze function in rats without altering locomotion (Filliat et al., 1998), 12.5 and 25 mg/kg NBQX reduced cocaine and amphetamine induced ambulatory sensitization but did not alter general locomotion in rats (Li et al., 1997), and 6 and 8 mg/kg reduces play behavior, social investigation, and contact behavior without altering general locomotion (Dannenhoffer et al., 2018). Locomotor activity and alcohol drinking in female C57BL/6J mice were unaffected by NBQX at any dose. The sex difference seen here may be the result of female C57BL/6J mice consuming higher amounts of alcohol, thus possibly creating a higher dose needed to antagonize the AMPA receptors with NBQX. Additionally, recent data from Finn et al. (2018) demonstrated that sex influences response to binge-like alcohol drinking such that nucleus accumbens transcriptional properties between male and female mice differ in response to alcohol. Further understanding of the mechanistic differences of binge-like alcohol drinking between male and female mice is needed.
2BC alcohol drinking, alcohol preference, and locomotor activity in the HAP3 mice was not affected by NBQX at any dose. Because HAP3 mice have continuous alcohol access and drink to high blood alcohol concentrations over the course of 24-hours (Matson & Grahame, 2013), the doses of NBQX used here may be not be high enough to produce a behavioral effect, particularly if the AMPA receptor structure and composition is altered as a function of alcohol intake or genetic predisposition. Recently, Cannady et al. (2017) demonstrated that P-rats, a selectively bred alcohol preferring rat, that self-administered low alcohol doses exhibited increased GluA1 AMPAR subunit phosphorylation in the central amygdala, basolateral amygdala, and the nucleus accumbens core compared to sucrose drinking control P-rats. Because NBQX is a competitive antagonist and chronic ethanol has been shown to increase extracellular glutamate concentrations, it is plausible that a more potent dose of NBQX is needed to reduce consumption.
DID in HAP3 mice was also not affected by NBQX at any dose. We initially chose to test the effects of NBQX on alcohol consumption in HAP3 mice with 2BC drinking because HAP3 mice are selectively bred to drink alcohol under the 2BC procedure. Our finding showing that HAP3 mice are not affected by NBQX at any dose in 2BC caused us to consider how the alcohol intake model may affect susceptibility to reduced alcohol consumption via NBQX. To assess this, we chose to test the effect of NBQX in HAP 3 mice during DID, finding the DID model is not better suited for NBQX’s effects on alcohol drinking as compared to the 2BC procedure. One limitation to interpretation of this finding is that comparison between the 2BC procedure and DID model for HAP3 mice is difficult because of the differences in alcohol drinking history and access schedule. If the pharmacological effect of alcohol were to cause changes in AMPA receptor expression and/or phosphorylation, the differences in drinking history and schedule muddle potential for interpretation of NBQX’s effect on AMPA receptors between the two drinking histories and consumption models. Importantly, the lack of effect of NBQX in HAP3 mice in either DID or 2BC provides evidence that selectively bred high alcohol consuming mice may be less reliant on AMPA receptors for alcohol drinking than C57BL/6J mice. Additionally, HAP mice are a genetic mouse model of high alcohol drinking and therefore may be less susceptible to manipulations that reduce alcohol drinking. Indeed, other drugs administered IP have reduced HAP alcohol drinking at higher doses than those effective in C57BL/6J mice. For example, naltrexone at either 3 or 10 mg/kg reduced 30-minute home cage drinking in HAP 1 and HAP 2 mice without affecting overall consumption levels (Oberlin et al., 2010). Whereas, in C57BL/6J mice, lower doses (0.5, 1, and 2 mg/kg) of naltrexone administered IP have effectively reduced binge-like alcohol drinking during DID without effecting sugar water consumption (Kamdar et al., 2007), 1.5 and 2 g/kg naltrexone administered IP in C57BL/6J mice reduced binge-like alcohol drinking during DID (with ineffective doses at 4 and 8 mg/kg; Phillips et al., 1997) and, at somewhat higher doses, naltrexone (1 and 6 mg/kg) administered subcutaneously reduced 24-hour alcohol consumption in C57BL/6J mice (Middaugh & Bandy, 2000). Future research should investigate the relationship between genetically high alcohol preferring rodent models of alcohol consumption, susceptibility to pharmacological manipulation on alcohol drinking, and AMPA receptors to further disentangle this relationship.
Limitations to these experiments exist. The drug effect demonstrated in our experiment cannot be completely attributed to AMPA receptor antagonism, as NBQX is a competitive AMPA/kainate receptor antagonist, despite being 30 times more selective for AMPA than kainate receptors (Sheardown et al., 1990). Due to the design of the experiments (i.e. IP injections) the exact location of the drug action is unknown and greatly limits the interpretability of the results, as AMPA receptors are ubiquitously expressed in the central and peripheral nervous system. However, it is known that NBQX readily crosses the blood brain barrier (Sheardown et al.,1990; Gill et al., 1992; Libbey et al., 2016).
Previous research has demonstrated that repeated alcohol exposure can upregulate AMPA receptors in the dorsomedial striatum (DMS, Wang et al., 2012), and that direct dorsolateral striatum (DLS) or DMS infusion of NBQX reduces operant responding for alcohol in rats (Wang et al., 2012; Corbit et al., 2014). These experiments provide further evidence in support of the importance of AMPA receptors in the control of alcohol drinking, particularly limited-access binge-like drinking. Given the importance of AMPA receptors in learning, memory, and LTP, further research to understand the site-specific effects of NBQX on alcohol drinking, AMPA receptor (glutamatergic) circuitry and alcohol, and more direct investigation of AMPA, LTP, and alcohol would be of great benefit.
In summary, we have shown that NBQX at 30 mg/kg reduces binge-like alcohol drinking, but not saccharin drinking, without affecting overall locomotor behavior in male C57BL/6J mice. Female C57BL/6J mice, and both male and female HAP3 mice were unaffected by NBQX at any dose. The findings from this experiment suggest that AMPA receptors are important for the modulation of binge-like alcohol drinking and that AMPA receptors may be differentially affected by sex and genetic background. To our knowledge, this is the first experiment in which AMPA receptors have been investigated in the context of binge-like alcohol drinking and 2BC preference drinking using NBQX in male and female C57BL/6J and HAP3 mice.
Acknowledgments
This work was supported by the Indiana Alcohol Research Center and NIH/NIAAA grant AA07611.
Footnotes
The authors report no conflicts of interest.
References
- Banerjee N (2014) Neurotransmitters in alcoholism: A review of neurobiological and genetic studies. Indian journal of human genetics, 20(1), 20–31. doi: 10.4103/0971-6866.132750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, & Young ER (1993). Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology, 112(4), 503–510. 10.1007/BF02244901 [DOI] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Graham C, Crayle J, Besheer J, Hodge C W (2017) Potentiation of amygdala AMPA receptor activity selectively promotes escalated alcohol self-administration in a CaMKII-dependent manner. Addiction Biology, 22(3): 652–664. doi: 10.1111/abd.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH (2014) Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Frontiers in Beh Neuro. 8(301):1–9. doi: 10.3389/fnbeh.2014.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenhoffer CA, Varlinskaya EI, Spear LP (2018). Effects of AMPA receptor antagonist, NBQX, and extrasynaptic GABAA agonist, THIP, on social behavior of adolescent and adult rats. Physiol Behav. 194:212–217. doi: 10.1016/j.physbeh.2018.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenhoffer DA, Spear LP (2019) Excitatory/inhibitory balance across ontogeny contributes to age-specific behavioral outcomes of ethanol-like challenge in conditioned taste aversion. Developmental Psychobiology, 61: 1157–1167. doi: 10.1002/dev.21864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Petersen V, Honoré T, Henley JM (1996) Pharmacology and regional distribution of the binding of 6-[3H]nitro-7-sulphamoylbenzo[f]-quinoxaline-2,3-dione to rat brain. J Neurochem. 67(6):2609–12. doi: 10.1046/j.1471-4159.1996.67062609.x [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ (2013) Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addiction Biology, 18(2): 297–306. doi: 10.1111.abd.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Hashimoto JG, Cozzoli DK, Helms M L, Nipper MAM, Kaufman MN, Wiren KM, Guizzetti M (2018) Front. Genet. 12. doi: 10.3389/fgene.2018.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliat P, Pernot-Marino I, Baubichon D, Lallement G. (1998) Behavioral effects of NBQX, a competitive antagonist of the AMPA receptors. Pharmacol Biochem Behav. 59(4):1087–1092. doi: 10.1016/s0091-3057(97)00518-2 [DOI] [PubMed] [Google Scholar]
- Fuller JL (1964). Measurement of alcohol preference in genetic experiments. Journal of comparative and physiological psychology, 57, 85–88. doi: 10.1037/h0043100 [DOI] [PubMed] [Google Scholar]
- Gill R, Nordholm L, & Lodge D (1992) The neuroprotective actions of 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline (NBQX) in rat focal ischaemia model. Brain Res. 580(1-2):35–43. doi: 10.1016/0006-8993(92)90924-x [DOI] [PubMed] [Google Scholar]
- Goodwani S, Saternos H, Alasmari F, Sari Y (2017) Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neuroscience and biobehavioral reviews, 77, 14–31. doi: 10.1016/j.neubiorev.2017.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JM, Wilkinson KA (2016) Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neuroscience. 17(6): 337–50. doi: 10.1038/nrn.2016.37. [DOI] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS (2007). Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl). 192(2):207–217. doi: 10.1007/s00213-007-0711-5 [DOI] [PubMed] [Google Scholar]
- Kasten CR, Boehm SL (2014) Intra-nucleus accumbens shell injections of R(+)- and S(−)-baclofen bidirectionally alter binge-like ethanol, but not saccharin, intake in C57Bl/6J mice. Behav Brain Res. 272: 238–247. doi: 10.1016/j.bbr.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ (1995) The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch Gen Psychiatry, 52, 374–383. [DOI] [PubMed] [Google Scholar]
- Li Y, Vartanian AJ, White FJ, Xue CJ, Wolf ME. (1997) Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacology (Berl). 134(3):266–276. doi: 10.1007/s002130050449 [DOI] [PubMed] [Google Scholar]
- Libbey JE, Hanak TJ, Doty DJ, Wilcox KS, Fujinami RS (2016) NBQX, a highly selective competitive antagonist of AMPA and KA ionotropic glutamate receptors, increases seizures and mortality following picornavirus infection. Exp Neurol., 280: 89–96. doi: 10.1016/j.expneurol.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ (2013) Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addiction Biology, 18(6): 921–9. doi: 10.1111/j.1369.1600.2011.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh L, Bandy A (2000) Naltrexone effects on ethanol consumption and response to ethanol conditioned cues in C57BL/6 mice. Psychopharmacology 151, 321–327. doi:org/10.1007/s002130000479 [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism (NIAAA). Drinking Levels Defined. Available at https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N (2011) Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behavior Genetics. 41:288–302. doi: 10.1007/s10519-010-9394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Bristow RE, Heighton ME, Grahame NJ (2010) Pharmacologic dissociation between impulsivity and alcohol drinking in high alcohol preferring mice. Alcohol Clin Exp Res. 2010 August;34(8):1363–75. doi: 10.1111/j.1530-0277.2010.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Wenger CD, Dorow JD. (1997) Naltrexone effects on ethanol drinking acquisition and on established ethanol consumption in C57BL/6J mice. Alcohol Clin Exp Res. 21(4):691–702. [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR (2004) Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. The Journal of Neuroscience, 24(7):1594–1603, doi: 10.1523/JNEUROSCI.5077-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Babinska Z, Luptak M, Getachew B, Tizabi Y (2018) Both ketamine and NBQX attenuate alcohol drinking in male Wistar rats. Neurosci Lett. 14(666):175–180. doi: 10.1016/j.neulet.2017.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honoré T (1990) 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 247(4942):571–4. doi: 10.1126/science.2154034 [DOI] [PubMed] [Google Scholar]
- Shimizu-Sasamata M, Kawasaki-Yatsugi S, Okada M, Sakamoto S, Yatsugi S, Togami J, Hatankaka K, Ohmori J, Koshikya K, Usuda S, Murase K (1996) YM90K: pharmacological characterization as a selective and potent alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor antagonist. Journal of Pharmacology and Experimental Therapeutics, 276(1), 84–92. [PubMed] [Google Scholar]
- Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X (2014) Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis 11:130293. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Brown G (1999) Disruption of operant oral self-administration of ethanol, sucrose, and saccharin by the AMPA/kainite antagonist, NBQX, but not the AMPA antagonist, GYKI 52466. ACER. 23(12)1914–20. doi: 10.111/j.1530-0277.1999tb04091.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) 2015. National survey on drug use and Health (NSDUH) Table 2.1B—Tobacco product and alcohol use in lifetime, past year, and past month among persons aged 12 or older: Percentages, 2014 and 2015. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab2-46b. Accessed 7/8/19.
- Tiwari V, Veeraiah P, Subramaniam V, Patel AB (2014) Differential effects of ethanol on regional glutamatergic and GABAergic neurotransmitter pathways in mouse brain. Journal of Neurochemistry, 128:628–640. doi: 10.1111/jnc.12508 [DOI] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, & Boehm SL 2nd (2014). “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Current protocols in neuroscience, 68, 9.49.1–9.49.12. 10.1002/0471142301.ns0949s68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno F, Suzuki T, Nakajima S, Matsushita S, Mimura M, Tomoyuki M, Takuya T, Uchida H (2019) Alteration in AMPA receptor subunit expression and receptor binding among patients with addictive disorders: A systemic review of human postmortem studies. Neuropsychopharmacology Reports; 39:148–155. doi: 10.1002/npr2.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS (2015) The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychol Med. 45(5):1061–1071. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hamida SB, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Dorit R (2012) Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: Implications for alcohol drinking behavior. J Neurosci. 32(43): 15124–15132. doi: 10.1523/JNERUOSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018). Global status report on alcohol and health 2018. Retrieved from https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/ [Google Scholar]


