Abstract
Background:
Chronic energy deficiency observed in women that exercise strenuously affects reproductive function, often leading to hypothalamic amenorrhea (HA). In such conditions, hypoleptinemia and robust changes in the Activin-Follistatin-Inhibin Axis (AFI) are observed. Treatment with leptin restores menstruation in many (60% responders) but not all (40% non-responders) women, suggesting that leptin is not the only regulator of reproductive function related to energy balance. In this work, we aimed to identify differences in hormonal profiles between leptin responders and non-responders among women with HA, with particular focus on the AFI axis.
Methods:
AFI axis and reproductive hormones (LH, FSH, Estradiol, ΑΜΗ) were measured in blood in: a) An open-label interventional study, b) a randomized placebo-controlled trial, both investigating responders versus non-responders/women with HA treated with leptin.
Results:
Women with HA that responded to leptin treatment have higher circulating levels/peak values of Inhibin A, Estradiol (E2), higher LH/FSH ratio and a trend to lower AMH compared with non-responders.
Conclusions:
Components of the AFI axis are associated with improvement of reproductive function in women with HA treated with leptin. ΑΜΗ may serve as a marker of ovarian recovery under HA treatment.
Keywords: reproduction, fasting, infertility, energy, anorexia, exercise
1. Introduction
Human reproduction is an energy consuming process that relies on energy sufficiency. In conditions of chronic energy deficiency, such as anorexia nervosa or in lean women that exercise strenuously, hypothalamic amenorrhea (HA) often occurs, and reproductive function is thus attenuated. We have previously demonstrated that in these conditions, a profound hypoleptinemia and robust changes in the circulating hormones of the Activin-Follistatin-Inhibin Axis (AFI axis) are observed [1–5]; i.e. women with HA demonstrate lower circulating levels of Activins and FSTL3, and higher levels of FST compared to healthy women. We have also shown that leptin administration in women with HA can restore menstrual cycles and the circulating levels of reproductive hormones [2, 5]. However, the exact mechanism of action has not been fully elucidated. Taken into consideration, that approximately 60% of women with HA responded to leptin treatment (responders) and 40% did not (non-responders), leptin seems to be an important, but not the only regulator of reproductive function in relation to energy balance [2, 4, 5]. Whether established hormonal factors, with particular focus on the AFI axis, play an additional role, and could potentially predict leptin response, remains unclear.
The AFI axis is well-known to have a regulatory role not only on muscle growth, lipid metabolism, glucose metabolism, and metabolic diseases [4, 6–10], but also on many aspects of reproductive and developmental biology [11–20]. Activins (A, B), and Inhibins (A, B), belong to the transforming growth factor beta (TGF-β) superfamily, and are highly expressed in the pituitary gland, gonads, placenta, and corpus luteum [14, 20]. Activins enhance pituitary Follicle stimulating hormone (FSH) secretion, whereas Inhibins have an opposite action as negative feedback regulators of FSH secretion [20–22]. Hence, they both have a role in the regulation of menstrual cycle. At the same time, they have paracrine effects associated with ovarian follicular development and steroidogenesis, which are important processes of reproductive function [14, 17–20]. Particularly, during folliculogenesis, in normal cycles, Inhibin A is primarily secreted from dominant follicles, along with E2, and Progesterone from the corpus luteum [21, 22]. Its levels in follicular fluid increase with follicular maturation [23]. Inhibin B on the other hand, is secreted from small antral follicles [14, 24, 25] and in contrast to Inhibin A, its concentration does not correlate with follicular size [23]. During the primary follicle to antral stage, the exact role of Inhibins remains unclear [14]. During the antral follicle development stage, Inhibin A is reported to be associated with LH-dependent androgen production by theca cells [26, 27], and FSH-induced E2 secretion by granulosa cells [26]. Some studies also suggest that Inhibin A may promote LH-induced Progesterone secretion [28, 29]. However, the exact local role of Inhibin A in the corpus luteum remains unclear. In addition, many studies have demonstrated an inverse relationship between increasing FSH and decreasing Inhibin A and Inhibin B in association with reproductive aging [20, 30–32].The importance of Inhibins in folliculogenesis has also been highlighted in several knock out/knock in mouse studies [33–35]. Finally, Follistatins are glycoproteins that neutralize many of the biological actions of Activins [36]. Specifically, Follistatin (FST) is primarily secreted by the liver, pituitary, and ovaries and is the main inhibitor of Activin A [37]. Follistatin-like 3 (FSTL3) is primarily expressed in the placenta, testis, endometrium, adrenal glands, and skeletal muscle, has similar structure and function to FST and inhibits Activins but less potently [14]. Concerning their role in reproduction, Follistatins antagonize local actions of Activins, mainly on FSH secretion in pituitary glands, and stimulate luteinization [14, 17, 18]. Of note, during pregnancy the feto-placental unit is the main source of serum Activin A [38, 39], while Inhibins, and Follistatins are also highly expressed by the placenta [40–42] and fetal membranes [40, 41].
In the current study we aimed to investigate hormonal differences between women with HA that responded to leptin treatment (responders) vs women that did not respond (non-responders) in terms of reproductive function, i.e. restoration of menses, with a particular focus on the AFI axis.
2. Material and Methods
2.1. Study population-Study design
2.1.1. Study 1:
This was an open label pilot study of 8 women with hypothalamic amenorrhea (HA) due to increased exercise or low body weight, who were studied for up to 3 months under leptin treatment as previously described [2]. Inclusion-exclusion criteria, participants’ characteristics and outcomes have been previously reported [2].
Briefly, subjects self-administered leptin, in replacement dose, i.e. 0.08 mg/kg/d, with 40% of the daily dose at 8 a.m. and 60% at 8 p.m. They were evaluated weekly with physical examination, transvaginal or transabdominal pelvic ultrasonography and biochemical controls. In women that ovulation occurred, according to specific ultrasound, and laboratory criteria, that have been previously reported [2], study was completed at 2 months; in the rest, leptin dose was increased to 0.2 mg/kg/d (divided as described above) for one more month [2].
In the current analysis, we excluded one subject, that withdrew from study after one month for reasons unrelated to the study. In addition, we divided the subjects into two groups, responders (n=3) and non-responders (n=4), based on whether they responded to leptin treatment or not, in terms of ovulation, and menstruation. More specifically, subjects were classified as responders, if they met both of the following criteria: a) had ultrasound findings of ovulation, as already described [2], and b) had at least one menstrual bleeding during treatment. The rest of the subjects were classified as non-responders.
2.1.2. Study 2:
This was a randomized, double-blinded, placebo-controlled trial of 20 women with HA, who were studied over 9 months, as previously described. The trial was registered at the US National Institutes of Health (ClinicalTrials.gov) #NCT00130117 [5]. 11 subjects were randomly assigned to receive leptin, and 9 received placebo. Inclusion-exclusion criteria, participants’ characteristics and outcomes have been previously reported [5].
In brief, subjects self-administered leptin or matching placebo s.c, once daily between 7 p.m., and 11 p.m. for 9 months. Leptin in replacement dose (0.08mg/kg/d) or placebo was initially administered to all subjects over 3 months. At the end of this time, subjects, that had menstruated at least one time, continued with the same dose for 6 more months, whereas subjects, that had not menstruated, had a dose increase to 0.12 mg/kg/d. During the study, body weight was regularly monitored, and there was a leptin dose adjustment to maintain stable body weight, as already reported [5]. If body weight decreased <8% of baseline for more than one visit, or to <80% of ideal body weight, subjects were withdrawn from the study. Subjects were evaluated every 4 weeks, with physical examination, and laboratory controls.
In the current analysis we included only the leptin treated group and excluded one subject that withdrew from the study soon after the first visit, because of injection-site reactions. Three participants that were discontinued, i.e. one non-responder, at week 24 due to traveling, and two responders, at week 24, and week 28 due to pregnancy, and persistent weight loss respectively, were included in the analysis.
Similarly to study 1, we divided subjects into two groups, responders (n=7) and non-responders (n=3), based on whether they responded to leptin treatment or not, in terms of ovulation, and menstruation. Since this study did not include ultrasound scans to directly confirm ovulation, we used Progesterone levels as an indication of ovulation [43]. Thus, subjects were classified as responders, if they met both of the following criteria: a) had at least one Progesterone level ≥ 6ng/ml in the midluteal phase, indicative of normal corpus luteum function [43], b) had at least one menstrual bleeding during treatment. The rest of the subjects were classified as non-responders.
Protocols of study 1 and study 2 were approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center (BIDMC), which comply with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. All participants signed written informed consent. Amgen, Inc. (Thousand Oaks, CA) provided leptin.
2.2. Hormone Measurements
All hormones, and body composition parameters were measured previously [2, 4, 5]. Results are presented here, after analyzing, and comparing, data between responders, and non-responders.
Additionally, in study 2, Inhibin A was measured here for the first time, and Activin A was re-measured in order to include all timepoints/visits of the study for the current analysis, by enzyme linked immunosorbent assay (ELISA), with commercially available kits (Ansh Labs, Webster, TX, USA). More specifically, Inhibin A (AL-123; Intra-assay Variability:<5.6%, Inter-assay Variability: <4.3%, sensitivity 5.45 pg/mL), and Activin A (AL-110; Intra-assay Variability:<4.25%, Inter-assay Variability: <3.83%, sensitivity 0.065 ng/mL). Kits, were used according to manufacturer’s instructions.
In both study 1 and study 2, samples of the same subject were run in duplicates, within the same assay, to decrease inter-assay variability. They were repeated, if coefficient of variation was >20% and the new measurements were considered the valid ones.
2.3. Statistical Analysis
Statistical analysis was performed with SPSS v26.0 (SPSS, Inc., Chicago, IL) and with GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA). Values measured below assay sensitivity were replaced with half the value of the lowest standard of the assay for the analysis [44]. Data are reported as mean ± standard error of mean (SEM). The level of statistical significance was set at p≤0.05 (two tailed or one tailed, only where appropriate, and noted as such in results and figures).
In study 1, an intention-to-treat analysis (ITT) with last observation carried forward approach was performed. In study 1 and study 2 we investigated the potential effect of group (responders versus non-responders), time, and interaction of group by time by using mixed models. For each molecule, analysis was performed with and without adjustment for baseline, in order to adjust for baseline group differences; post-hoc Fischer LSD test was performed to compare responders vs non-responders, for each timepoint. In study 1, we further adjusted for BMI since it was significantly different between responders vs non-responders to leptin treatment. No data were available to perform formal power calculations based on specific prior changes of the molecules of interest.
3. Results
Baseline characteristics have been previously reported [2, 5]. Here, we compare responders vs non-responders to leptin treatment.
3.1. Responders to leptin treatment may have a higher BMI before treatment initiation but they show no substantive differences in body composition and leptin levels both before and during leptin treatment compared to non-responders.
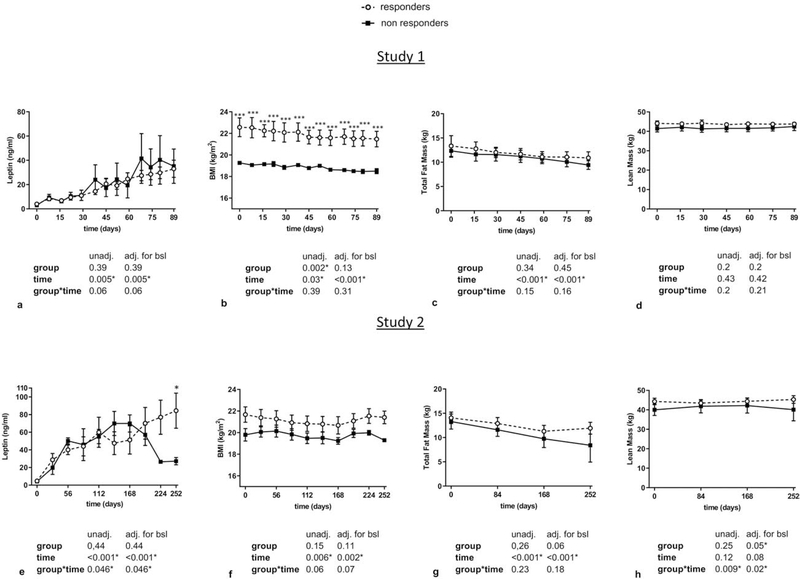
Regarding leptin levels, in study 1 there was no difference at start and levels were increased continuously and equally both in responders and non-responders to leptin treatment (Figure 1a). BMI was higher at the beginning and throughout the study in responders compared to non-responders, but both groups lost similar amounts of weight during leptin treatment (Figure 1b). Weight loss was mainly fat mass and not muscle mass, and was similar both in responders and non-responders (Figure 1c–d). In study 2, leptin levels were similar at baseline prior to treatment and increased appropriately in both responders, and non-responders in the initial phase of treatment. Subsequently, they decreased, mainly in non-responders, due to dose adjustments because of weight loss, which was slightly more in non-responders than in responders (Figure 1e). BMI tended to be higher at the beginning and throughout the study in responders compared to non-responders (Figure 1f). In agreement with study 1, body fat mass loss and not lean mass loss was observed during leptin treatment in both responders and non-responders (Figure 1g–h).
Figure 1: Circulating leptin levels and body composition parameters in responders vs non-responders to leptin treatment (study 1 and study 2).
Leptin levels, BMI, total fat mass, and lean mass of responders vs non-responders during leptin treatment in study 1 (a-d) and study 2 (e-h) are demonstrated. Data are reported as mean ± SEM. P-values one-tailed for group, time, and group by time effect were calculated with mixed models and are shown below the corresponding graphs (unadjusted and adjusted for baseline). For all molecules post-hoc Fisher’s LSD test was performed to compare responders vs non-responders for each timepoint. *, **, *** correspond to p≤0.05, 0.01, 0.001 respectively. adj.: adjusted; BMI: Body Mass Index; bsl: baseline; SEM: Standard Error of the Mean; unadj.: unadjusted
3.2. Responders to leptin treatment show higher LH to FSH ratio and higher peaks of E2, Inhibin A and Progesterone during leptin treatment compared to non-responders.
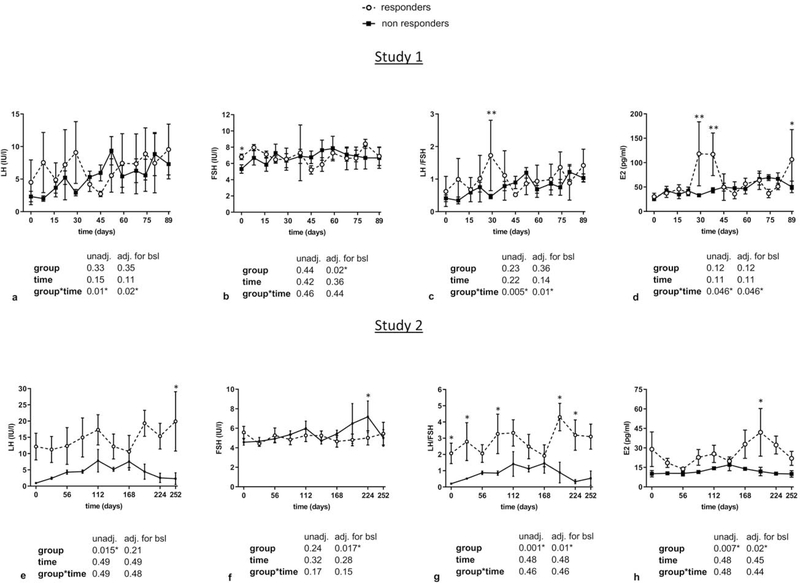
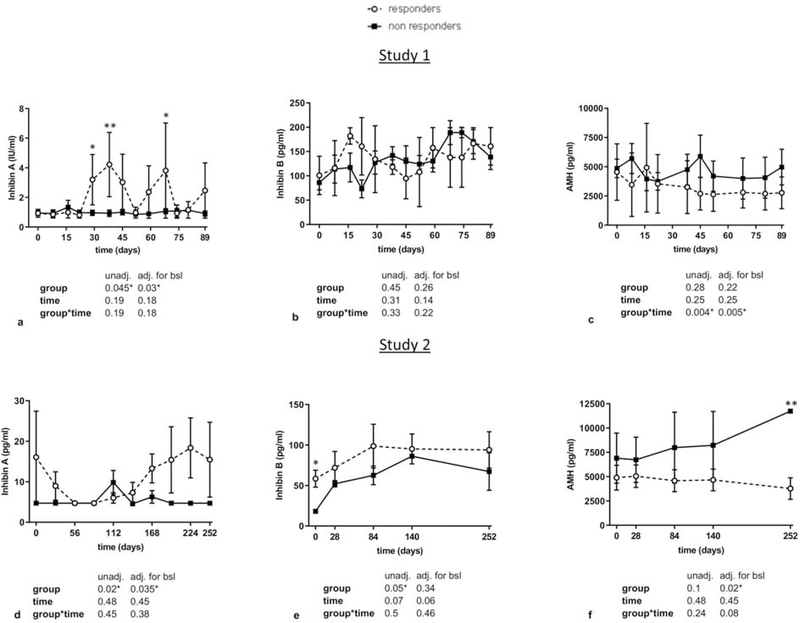
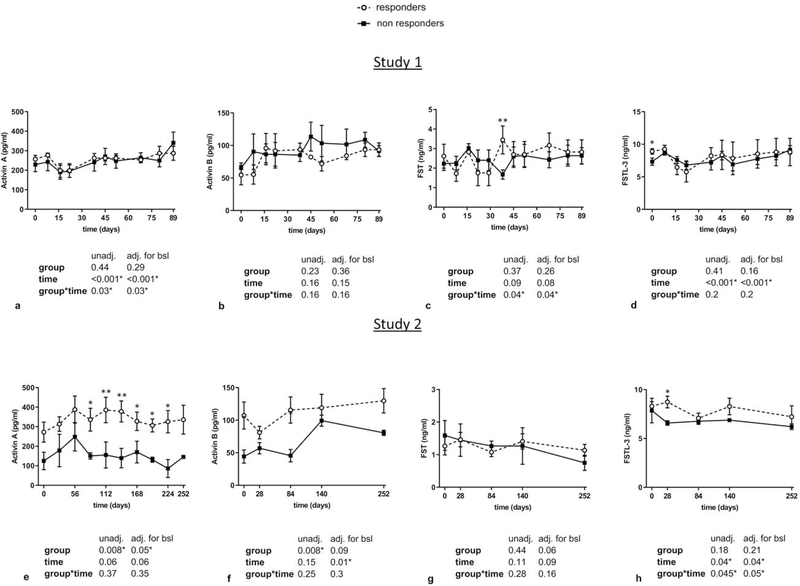
Regarding gonadotropins, LH levels tended to be higher in study 1 and were significantly higher in study 2 in responders compared to non-responders, whereas no significant differences were observed in FSH levels in both studies (Figure 2a–b and 2e–f). In addition, LH to FSH ratios were higher after 30 days of treatment in study 1, and from start to almost completion of treatment in study 2 in responders (Figure 2c and 2g). Similarly, transient significant increases in different timepoints of leptin treatment in study 1 and study 2 were observed in E2 and Inhibin A (but not in Inhibin B) in responders compared to non-responders (Figure 2d, 2h, and Figure 3a–b and 3d–e). These significant changes were maintained after adjusting for BMI in study 1. For Activin A and Activin B, we observed significantly higher levels in responders in study 2 but not in study 1 (Figure 4a–b, 4e–f), whereas no differences between the two groups were observed in FST and FSTL3 levels in both studies (Figure 4c–d, 4g–h). For AMH, we observed in both studies a tendency to lower levels in responders compared to non-responders (Figure 3c, 3f). We hypothesized that the large variations observed in the concentrations of gonadotropins, E2 and Inhibin A, particularly in responders, may simply reflect an heterochronic response to treatment between subjects. Thus, we additionally compared peak values of all hormones during leptin treatment between responders and non-responders (Supplemental Table 1 and 2) and evaluated the hormonal profile of each responder individually in relation to occurrence of ovulation/menstruation (Supplemental Figure 1 and Figure 2). We observed that peak values of E2, Inhibin A, as well as Progesterone in study 1 were significantly higher in responders compared to non-responders. Notably, the majority of peaks/higher values were documented around or at the timepoints that ovulation occurred, and menstrual bleeding followed. These results were validated in study 2, where additionally peak values of LH/FSH ratio, Activin A, and Activin B were higher in responders, compared to non- responders.
Figure 2. Circulating profile of gonadotropins and E2 in responders vs non-responders to leptin treatment (study 1 and study 2).
Blood concentrations of responders vs non-responders of LH, FSH, LH to FSH ratio, and E2 in study 1 (a-d) and study 2 (e-h) are demonstrated. Data are reported as mean ± SEM. P-values one-tailed for group, time, and group by time effect were calculated with mixed models and are shown below the corresponding graphs (unadjusted and adjusted for baseline). For all molecules post-hoc Fischer LSD test was performed to compare responders vs non-responders for each timepoint. *, **, *** correspond to p≤0.05, 0.01, 0.001 respectively.
adj.: adjusted; bsl: baseline; E2: Estradiol; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; SEM: Standard Error of the Mean; unadj.: unadjusted
Figure 3. Circulating profile of Inhibins and AMH in responders vs non-responders to leptin treatment (study 1 and study 2).
Blood concentrations of responders vs non-responders of Inhibin A, Inhibin B, and AMH in study 1 (a-c), and study 2 (d-f) are demonstrated. Data are reported as mean ± SEM. P-values one-tailed for group, time, and group by time effect were calculated with mixed models and are shown below the corresponding graphs (unadjusted and adjusted for baseline). For all molecules post-hoc Fischer LSD test was performed to compare responders vs non-responders for each timepoint. *, **, *** correspond to p≤0.05, 0.01, 0.001 respectively.
adj.: adjusted; bsl: baseline; AMH: Anti-mullerian hormone; SEM: Standard Error of the Mean; unadj.: unadjusted
Figure 4. Circulating profile of Activins and Follistatins in responders vs non-responders to leptin treatment (study 1 and study 2).
Blood concentrations of responders vs non-responders of Activin A, Activin B, FST, and FSTL-3 in study 1 (a-d), and study 2 (e-h) are demonstrated. Data are reported as mean ± SEM. P-values one-tailed for group, time, and group by time effect were calculated with mixed models and are shown below the corresponding graphs (unadjusted and adjusted for baseline). For all molecules post-hoc Fischer LSD test was performed to compare responders vs non-responders for each timepoint. *, **, *** correspond to p≤0.05, 0.01, 0.001 respectively.
adj.: adjusted; bsl: baseline; FST: Follistatin; FSTL-3: Follistatin-like 3; SEM: Standard Error of the Mean; unadj.: unadjusted
4. Discussion
We demonstrate herein, that women with HA that responded to leptin treatment and restored their reproductive function show a distinct hormonal profile compared to non-responders. This hormonal profile is characterized by higher LH/FSH ratio, driven mainly by higher LH levels and repeated high peaks of E2 and Inhibin A (Figure 5).
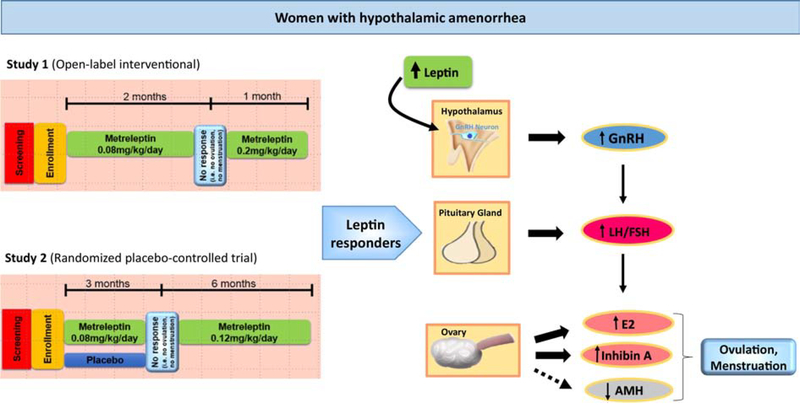
Figure 5: Mechanism of restoration of reproductive function in women with hypothalamic amenorrhea by leptin.
Women with hypothalamic amenorrhea that respond to leptin treatment and have their reproductive function restored show a distinct hormonal profile when compared to non-responders. This hormonal profile is characterized by higher LH/FSH ratio, apparently due to the central effects of leptin on GnRH secretion in hypothalamus. Responders also have higher peaks of E2 and Inhibin A in blood, which indicates improved ovarian function and restoration of normal regular menstrual cycles. Additionally, circulating AMH, which serves as an additional marker of ovarian recovery, is reduced.
AMH: Anti-mullerian hormone; E2: Estradiol; FSH: Follicle-stimulating hormone; GnRH: Gonadotropin-releasing hormone; LH: Luteinizing hormone
Our study significantly expands on our previous findings regarding the impact of leptin on human reproductive physiology. Specifically, we have previously demonstrated that short-term leptin replacement during acute, fasting-induced hypoleptinemia, restores LH pulsatility without affecting E2, AFI hormones or AMH [4, 45]. In the long-term studies, LH pulsatility has not been assessed but morning LH levels were increased during leptin treatment in the open-label study (study 1) and were not significantly different in the placebo-controlled study (study 2). E2 levels were increased in both long-term studies [2, 5]. Regarding AFI hormones, although their levels showed robust differences in women with HA compared to healthy controls (lower circulating levels of Activins and FSTL3, and higher levels of FST), they demonstrated only minor changes during long-term leptin treatment, i.e. only a slight increase of Activin B [4].
Here, we show that women with HA that responded to leptin treatment, i.e. ovulated, and developed menstrual bleeding, have a higher LH/FSH ratio and demonstrate repeated peaks in E2 and Inhibin A levels during treatment compared to women that were treated with leptin but did not ovulate and did not restore menstruation. Thus, our findings confirm that the main effect of leptin on reproductive function is the improvement of gonadotropin secretion, resulting in higher E2 and ovulation. Additionally, they reveal for the first time an important role of Inhibin A in restoration of menstruation with leptin treatment.
Regarding the effects of leptin on gonadotropin secretion, we have previously suggested that it is most probably related to restoration of GnRH secretion. Interestingly, GnRH secretion increases the LH/FSH ratio more robustly than LH alone [46], which may explain why in our study the LH/FSH ratio shows more significant differences than LH between responders vs non-responders.
Regarding Inhibin A, in women with regular menstrual cycle, circulating levels increase progressively and peak twice, once in the end of follicular phase concomitantly with E2, and once in the midluteal phase almost concomitantly with Progesterone [20, 47]. During the follicular phase, Inhibin A is mainly considered a product of granulosa cells, reflects the size of the dominant follicle and contributes together with E2 to the gonadotropin surge leading to ovulation. In the luteal phase, Inhibin A is the product of corpus luteum and it may contribute to the negative feedback control of FSH secretion [20]. Although due to the long-duration of our studies and the special characteristics of our study population (women with HA), we did not perform more regular measurements than every 2 to 4 weeks, we were still able to capture peaks in E2 and Inhibin A that were synchronous with ovulation-periovulatory phase in study 1 or were approximately two weeks before the observed menstruation in study 2. Thus, in contrast to non-responders, responders to leptin treatment have reproductive hormonal profiles that are very similar to the ones observed during normal regular menstrual cycles.
Regarding AMH, a hormone with similar structure to Inhibins,and Activins, secreted in females by granulosa cells of the ovary, it is widely accepted that it is a biomarker for the relative size of the ovarian reserve [48, 49]. Several prior studies related to HA support, that in this condition AMH circulating levels may be elevated or within the normal high range [50–53], and may reverse after restoration of menses [51, 52]. In our studies, we observed generally lower circulating levels in responders during treatment compared to non-responders (Figure 5). This, however, reached clear significance only at the end of study 2, where circulating levels in responders were within normal range, compared to non-responders, that had elevated levels [54]. Thus, our findings in combination with the results from previous studies indicate that AMH may serve as a marker of ovarian recovery under HA treatment. Larger studies however are needed to confirm, and extend this assumption.
We have also investigated whether the reproductive response to leptin treatment among women with HA can be explained by differences in their metabolic and hormonal status at baseline, before treatment initiation. We could not identify a hormonal factor that was consistently different between responders and non-responders at baseline in both studies. However, we do observe significantly higher BMI levels in the open-label study and a trend to higher levels in the placebo-controlled study in responders compared to non-responders. BMI levels were similarly reduced in both groups during leptin treatment in both studies. The differences in BMI, however,were not supported by significant differences in leptin levels due to higher fat mass or by robust changes in muscle mass. In addition, in study 1, including BMI as a covariate in the model did not alter significance, indicating that this parameter does not influence the currently investigated hormones. Whether BMI can affect the outcome, i.e.restoration of reproductive function, by regulation of other hormones than the ones investigated herein remains to be clarified in future studies.
Limitations of the studies include the small number of participants and that the clinical trials included in our analyses were designed to address other primary, and secondary outcomes than the ones reported here. The results of the study were statistically significant, however, this study focused on mechanisms underlying the primary and secondary outcomes of the original study. Additionally, in study 2, ultrasound findings were not included in the study design, to directly confirm ovulation. The strength of these studies is their uniqueness, since this type of intervention cannot be easily replicated, as leptin is currently approved only for complications of congenital or acquired lipodystrophy.
Conclusions
The profile of six hormones of the AFI axis was investigated in women with HA treated with leptin. Response to leptin treatment is characterized by an increase in LH/FSH that upregulates E2 and Inhibin A levels, resulting in ovulation and restoration of menstruation in women with HA, and eventually a normalization of the elevated AMH circulating levels.
Our findings expand the role of Inhibin A, member of the AFI axis, in human reproductive physiology and additionally show the potential of AMH as a marker of ovarian recovery under HA treatment.
Supplementary Material
Supplemental Figure 1: Circulating profiles of LH to FSH ratio, E2 and Inhibin A of every responder and non-responder in study 1.
Blood concentrations of LH to FSH ratio, E2, and Inhibin A of every responder (a-c), and non-responder (d-g) during leptin treatment in study 1 are demonstrated. Data are reported as mean ± SEM.
↑: timepoints of ovulation, followed by menstrual bleeding
E2: Estradiol; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; SEM: Standard Error of the Mean
Supplemental Figure 2: Circulating profiles of LH to FSH ratio, E2 and Inhibin A of every responder and non-responder in study 2.
Blood concentrations of LH to FSH ratio, E2, and Inhibin A of every responder (a-g), and non-responder (h-j) during leptin treatment in study 2 are demonstrated. Data are reported as mean ± SEM.
↑: timepoints of menstrual bleeding
E2: Estradiol; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; SEM: Standard Error of the Mean
Highlights.
Women with HA that responded to leptin treatment, i.e. ovulated and restored menstruation, show a distinct hormonal profile compared to non-responders.
This hormonal profile is characterized by higher LH/FSH ratio and higher circulating levels/peak values of Inhibin A and E2 compared to non-responders.
AMH may serve as a marker of ovarian recovery under HA treatment.
Acknowledgments
We are thankful to Amgen Inc for providing clinical-quality r-metHuLeptin for study 1, and study 2.
Funding
The current study was supported by NIH K24DK081913 to CSM. NP was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - PE2431/3-1:1.
Footnotes
Declaration of interest
EB and NP have nothing to declare. CSM is advisor of Ansh Labs LLC.
Clinical Trial Information: ClinicalTrials.gov no., NCT00130117
Data availability
All data used for the analysis in this article are available on request from the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bluher S and Mantzoros CS, Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes, 2007. 14(6): p. 458–64. [DOI] [PubMed] [Google Scholar]
- 2.Chou SH, et al. , Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A, 2011. 108(16): p. 6585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou SH and Mantzoros C, 20 years of leptin: role of leptin in human reproductive disorders. J Endocrinol, 2014. 223(1): p. T49–62. [DOI] [PubMed] [Google Scholar]
- 4.Perakakis N, et al. , Regulation of the activins-follistatins-inhibins axis by energy status: Impact on reproductive function. Metabolism, 2018. 85: p. 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welt CK, et al. , Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med, 2004. 351(10): p. 987–97. [DOI] [PubMed] [Google Scholar]
- 6.Perakakis N, et al. , Metabolic regulation of activins in healthy individuals and in obese patients undergoing bariatric surgery. Diabetes Metab Res Rev, 2020: p. e3297. [DOI] [PubMed] [Google Scholar]
- 7.Perakakis N, et al. , Follistatins in glucose regulation in healthy and obese individuals. Diabetes Obes Metab, 2019. 21(3): p. 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perakakis N, et al. , Physiology of Activins/Follistatins: Associations With Metabolic and Anthropometric Variables and Response to Exercise. J Clin Endocrinol Metab, 2018. 103(10): p. 3890–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perakakis N, et al. , Non-invasive diagnosis of non-alcoholic steatohepatitis and fibrosis with the use of omics and supervised learning: A proof of concept study. Metabolism, 2019. 101: p. 154005. [DOI] [PubMed] [Google Scholar]
- 10.Polyzos SA, et al. , Targeted Analysis of Three Hormonal Systems Identifies Molecules Associated with the Presence and Severity of NAFLD. J Clin Endocrinol Metab, 2020. 105(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brun T, et al. , The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J Cell Biol, 2004. 167(6): p. 1123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florio P, et al. , Activin A stimulates insulin secretion in cultured human pancreatic islets. J Endocrinol Invest, 2000. 23(4): p. 231–4. [DOI] [PubMed] [Google Scholar]
- 13.Li L, et al. , Activin A and betacellulin: effect on regeneration of pancreatic beta-cells in neonatal streptozotocin-treated rats. Diabetes, 2004. 53(3): p. 608–15. [DOI] [PubMed] [Google Scholar]
- 14.Makanji Y, et al. , Inhibin at 90: from discovery to clinical application, a historical review. Endocr Rev, 2014. 35(5): p. 747–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijayarathna R and de Kretser DM, Activins in reproductive biology and beyond. Hum Reprod Update, 2016. 22(3): p. 342–57. [DOI] [PubMed] [Google Scholar]
- 16.Zaragosi LE, et al. , Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes, 2010. 59(10): p. 2513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Kretser DM, et al. , Inhibins, activins and follistatin in reproduction. Hum Reprod Update, 2002. 8(6): p. 529–41. [DOI] [PubMed] [Google Scholar]
- 18.Gregory SJ and Kaiser UB, Regulation of gonadotropins by inhibin and activin. Semin Reprod Med, 2004. 22(3): p. 253–67. [DOI] [PubMed] [Google Scholar]
- 19.Luisi S, et al. , Inhibins in female and male reproductive physiology: role in gametogenesis, conception, implantation and early pregnancy. Hum Reprod Update, 2005. 11(2): p. 123–35. [DOI] [PubMed] [Google Scholar]
- 20.Hall JE, Yen and Jaffe’s Reproductive Endocrinology (Eighth Edition) Physiology, Pathophysiology, and Clinical Management; Chapter 7 - Neuroendocrine Control of the Menstrual Cycle. 2019: Elsevier; Pages 149–166.e5. [Google Scholar]
- 21.Muttukrishna S, et al. , Serum concentrations of dimeric inhibin during the spontaneous human menstrual cycle and after treatment with exogenous gonadotrophin. Hum Reprod, 1994. 9(9): p. 1634–42. [DOI] [PubMed] [Google Scholar]
- 22.Welt CK, et al. , Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab, 1999. 84(1): p. 105–11. [DOI] [PubMed] [Google Scholar]
- 23.Strauss JF III, W.C.J., Yen and Jaffe’s Reproductive Endocrinology (Eighth Edition) Physiology, Pathophysiology, and Clinical Management; Chapter 8 - Ovarian Life Cycle. 2019: Elsevier. [Google Scholar]
- 24.Burger HG, Groome NP, and Robertson DM, Both inhibin A and B respond to exogenous follicle-stimulating hormone in the follicular phase of the human menstrual cycle. J Clin Endocrinol Metab, 1998. 83(11): p. 4167–9. [DOI] [PubMed] [Google Scholar]
- 25.Fraser HM, Groome NP, and McNeilly AS, Follicle-stimulating hormone-inhibin B interactions during the follicular phase of the primate menstrual cycle revealed by gonadotropin-releasing hormone antagonist and antiestrogen treatment. J Clin Endocrinol Metab, 1999. 84(4): p. 1365–9. [DOI] [PubMed] [Google Scholar]
- 26.Campbell BK and Baird DT, Inhibin A is a follicle stimulating hormone-responsive marker of granulosa cell differentiation, which has both autocrine and paracrine actions in sheep. J Endocrinol, 2001. 169(2): p. 333–45. [DOI] [PubMed] [Google Scholar]
- 27.Wrathall JH and Knight PG, Effects of inhibin-related peptides and oestradiol on androstenedione and progesterone secretion by bovine theca cells in vitro. J Endocrinol, 1995. 145(3): p. 491–500. [DOI] [PubMed] [Google Scholar]
- 28.Smith KB and Fraser HM, Control of progesterone and inhibin secretion during the luteal phase in the macaque. J Endocrinol, 1991. 128(1): p. 107–13. [DOI] [PubMed] [Google Scholar]
- 29.Webley GE, Marsden PL, and Knight PG, Differential control of immunoreactive alpha-inhibin and progesterone production by marmoset luteal cells in vitro: evidence for a paracrine action of alpha-inhibin on basal and gonadotropin-stimulated progesterone production. Biol Reprod, 1994. 50(6): p. 1394–402. [DOI] [PubMed] [Google Scholar]
- 30.Burger HG, et al. , Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clin Endocrinol (Oxf), 1998. 48(6): p. 809–13. [DOI] [PubMed] [Google Scholar]
- 31.Klein NA, et al. , Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab, 1996. 81(3): p. 1038–45. [DOI] [PubMed] [Google Scholar]
- 32.Santoro N, et al. , Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am J Obstet Gynecol, 1998. 178(4): p. 732–41. [DOI] [PubMed] [Google Scholar]
- 33.Brown CW, et al. , Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet, 2000. 25(4): p. 453–7. [DOI] [PubMed] [Google Scholar]
- 34.Kumar TR, Wang Y, and Matzuk MM, Gonadotropins are essential modifier factors for gonadal tumor development in inhibin-deficient mice. Endocrinology, 1996. 137(10): p. 4210–6. [DOI] [PubMed] [Google Scholar]
- 35.McMullen ML, et al. , Gonadal pathologies in transgenic mice expressing the rat inhibin alpha-subunit. Endocrinology, 2001. 142(11): p. 5005–14. [DOI] [PubMed] [Google Scholar]
- 36.Sidis Y, et al. , Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology, 2006. 147(7): p. 3586–97. [DOI] [PubMed] [Google Scholar]
- 37.Flanagan JN, et al. , Role of follistatin in promoting adipogenesis in women. J Clin Endocrinol Metab, 2009. 94(8): p. 3003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Florio P, et al. , Inhibins and activins in pregnancy. Mol Cell Endocrinol, 2004. 225(1–2): p. 93–100. [DOI] [PubMed] [Google Scholar]
- 39.Muttukrishna S, et al. , Activin and follistatin in female reproduction. Mol Cell Endocrinol, 2004. 225(1–2): p. 45–56. [DOI] [PubMed] [Google Scholar]
- 40.Ciarmela P, et al. , Human placenta and fetal membranes express follistatin-related gene mRNA and protein. J Endocrinol Invest, 2003. 26(7): p. 641–5. [DOI] [PubMed] [Google Scholar]
- 41.Petraglia F, Inhibin, activin and follistatin in the human placenta--a new family of regulatory proteins. Placenta, 1997. 18(1): p. 3–8. [DOI] [PubMed] [Google Scholar]
- 42.Tortoriello DV, et al. , Human follistatin-related protein: a structural homologue of follistatin with nuclear localization. Endocrinology, 2001. 142(8): p. 3426–34. [DOI] [PubMed] [Google Scholar]
- 43.Welt CK, UptoDate; Physiology of the normal menstrual cycle. 2019. [Google Scholar]
- 44.Beal SL, Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn, 2001. 28(5): p. 481–504. [DOI] [PubMed] [Google Scholar]
- 45.Chan JL, et al. , Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A, 2006. 103(22): p. 8481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaiser UB, et al. , A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc Natl Acad Sci U S A, 1995. 92(26): p. 12280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groome NP, et al. , Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab, 1996. 81(4): p. 1401–5. [DOI] [PubMed] [Google Scholar]
- 48.Dewailly D, et al. , The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update, 2014. 20(3): p. 370–85. [DOI] [PubMed] [Google Scholar]
- 49.Visser JA, et al. , Anti-Mullerian hormone: a new marker for ovarian function. Reproduction, 2006. 131(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 50.Bradbury RA, Lee P, and Smith HC, Elevated anti-Mullerian hormone in lean women may not indicate polycystic ovarian syndrome. Aust N Z J Obstet Gynaecol, 2017. 57(5): p. 552–557. [DOI] [PubMed] [Google Scholar]
- 51.Carmina E, Fruzzetti F, and Lobo RA, Features of polycystic ovary syndrome (PCOS) in women with functional hypothalamic amenorrhea (FHA) may be reversible with recovery of menstrual function. Gynecol Endocrinol, 2018. 34(4): p. 301–304. [DOI] [PubMed] [Google Scholar]
- 52.Panidis D, et al. , Serum anti-Mullerian hormone (AMH) levels are differentially modulated by both serum gonadotropins and not only by serum follicle stimulating hormone (FSH) levels. Med Hypotheses, 2011. 77(4): p. 649–53. [DOI] [PubMed] [Google Scholar]
- 53.Robin G, et al. , Polycystic Ovary-Like Abnormalities (PCO-L) in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab, 2012. 97(11): p. 4236–43. [DOI] [PubMed] [Google Scholar]
- 54.Mayo Medical Laboratories. retrieved April 2020; Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/89711.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Circulating profiles of LH to FSH ratio, E2 and Inhibin A of every responder and non-responder in study 1.
Blood concentrations of LH to FSH ratio, E2, and Inhibin A of every responder (a-c), and non-responder (d-g) during leptin treatment in study 1 are demonstrated. Data are reported as mean ± SEM.
↑: timepoints of ovulation, followed by menstrual bleeding
E2: Estradiol; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; SEM: Standard Error of the Mean
Supplemental Figure 2: Circulating profiles of LH to FSH ratio, E2 and Inhibin A of every responder and non-responder in study 2.
Blood concentrations of LH to FSH ratio, E2, and Inhibin A of every responder (a-g), and non-responder (h-j) during leptin treatment in study 2 are demonstrated. Data are reported as mean ± SEM.
↑: timepoints of menstrual bleeding
E2: Estradiol; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; SEM: Standard Error of the Mean