Abstract
Recombinant human bone morphogenetic proteins (BMPs) have shown clinical success in promoting bone healing, but they are also associated with unwanted side effects. The development of improved BMP carriers that can retain BMP at the defect site and maximize its efficacy would decrease the therapeutic BMP dose and thus improve its safety profile. In this review, we discuss the advantages of using self-assembling peptides, a class of synthetic supramolecular biomaterials, to deliver recombinant BMPs. Peptide amphiphiles (PAs) are a broad class of self-assembling peptides, and the use of PAs for BMP delivery and bone regeneration has been explored extensively over the past decade. Like many self-assembling peptide systems, PAs can be designed to form nanofibrous supramolecular biomaterials in which molecules are held together by non-covalent bonds. Chemical and biological functionality can be added to PA nanofibers, through conjugation of chemical moieties or biological epitopes to PA molecules. For example, PA nanofibers have been designed to bind heparan sulfate, a natural polysaccharide that is known to bind BMPs and potentiate their signal. Alternatively, PA nanofibers have been designed to synthetically mimic the structure and function of heparan sulfate, or to directly bind BMP specifically. In small animal models, these bio-inspired PA materials have shown the capacity to promote bone regeneration using BMP at doses 10 – 100 times lower than established therapeutic doses. These promising results have motivated further evaluation of PAs in large animal models, where their safety and efficacy must be established before clinical translation. We conclude with a discussion on the possiblity of combining PAs with other materials used in orthopedic surgery to maximize their utility for clinical translation.
CLINICAL CHALLENGES IN BONE HEALING
Despite the relatively robust capacity for bone to regenerate after injury, delayed healing or non-union (failure to heal) is a significant clinical challenge in orthopaedic surgery. Non-union rates of >10% are commonly reported, with much higher rates in at-risk populations such as smokers, diabetics, and osteoporotic patients1–11. Treatment of the nearly 8 million fractures that occur in the U.S. annually is estimated to cost $21 billion/year1.
Autologous bone grafting is the historical “gold standard” for surgical bone repair, delivering the three requirements for successful bone regeneration: 1) osteoinductive signals; 2) an osteoconductive matrix; and 3) osteogenic cells12–14. However, the procedure requires a longer operative time and is associated with greater blood loss, increased infection risk, and donor site pain15–21. Morbidity (e.g., bleeding and hematoma) is not uncommon, and significant acute and chronic donor site pain at the iliac crest have been reported15, 22, 23, although the incidence rates of these complications have more recently been disputed24, 25. Graft volume/availability is also a limitation, and despite its historical use, autologous bone grafting still results in non-unions (failed healing), reportedly in 5–30 % of patients, depending on the injury or defect as well as patient-specific conditions2, 10, 26–29. As a result, the use of iliac crest autograft bone (ICBG) has dropped dramatically in the past two decades7, 16, 30, and interest has risen in developing alternative approaches to augment bone healing. An ideal solution would be a synthetic bone graft substitute that safely promotes bone regeneration and healing while obviating the need to harvest iliac crest or local vertebral bone.
THE BONE HEALING CASCADE
Bone healing occurs through a highly orchestrated sequence of events involving both biochemical and biomechanical cues31. The initial disruption of blood vessels after injury results in the formation of a clot, which provides hemostasis. In closed fractures, a non-infectious inflammatory response is then initiated, whereupon inflammatory cells are recruited to the defect32. This is followed by a fibrovascular phase involving recruitment of mesenchymal stem cells (MSCs) via chemotactic signals. These cells undergo proliferation for several days, and then condense and differentiate into chondroblasts or osteoblasts, depending on the microenvironment and growth factor signals received33, 34. In the case of endochondral ossification, a cartilaginous intermediate first forms, which undergoes hypertrophy after 10–14 days35. The cartilage is gradually replaced by bone, which becomes ossified after several weeks. Finally, osteoclasts are recruited to the ossified bone, which then undergoes remodeling to produce mature, lamellar bone32. Fracture healing is ultimately considered complete with the restoration of functional and biomechanical properties36.
Despite the generally robust capacity for skeletal tissue to regenerate, delayed healing or failure to heal (non-union) are not uncommon. The FDA defines a non-union as a fracture that has not healed by 9 months after injury, with radiography showing no improvement over the final 3 months37. The risk for non-union is determined by many patient-specific factors. Smokers, diabetic patients, and elderly patients with or without osteoporosis are at increased risk for delayed healing and non-union1–8, 11, 37. Other factors include the type of bone involved, mechanics of the fracture, degree of initial bone loss, time since initial injury, and the extent to which the adjacent soft tissue is disrupted. Consideration of these individual factors is important in determining the ideal approach for the initial treatment or in addressing delayed healing and non-unions.
THE HISTORICAL ROLE OF BMPs IN BONE REGENERATION
The three major mechanisms of osteoconductivity, osteoinductivity, and osteogenesis are well-established requirements for bone healing. The first refers to the capacity of a material to serve as a scaffold that allows for bone ingrowth and mineral deposition. Examples of osteoconductive materials include hydroxyapatite and other calcium phosphate ceramics, allograft, and to a lesser degree, demineralized bone matrix and other collagen-based scaffolds. Osteoinductivity refers to factors with the ability to signal progenitor cells to differentiate into bone-forming cells (osteoblasts). Classic examples of osteoinductive factors are BMPs, reviewed in this section35, 38–40. Finally, osteogenic materials are those that contain cell types capable of forming new bone, or osteoprogenitor cells. Examples of osteogenic materials include autologous bone and bone marrow.
Bone morphogenetic protein (BMP) was first discovered in 1965 by Marshall Urist, a bone biologist, orthopaedic surgeon, and pioneer in the field of bone regenerative medicine39–41. In his original 1965 publication, Dr. Urist showed that demineralized bone matrix (DBM)—prepared from human cadaveric bone and processed to remove mineral—could induce new bone formation when implanted at non-bony sites in animal models39. Urist postulated that this result was indicative of a previously unknown substance present in bone, which he termed, bone morphogenetic protein. He and others then undertook the challenge over the next 30 years of isolating BMPs and determined that these proteins play a critical role in several steps of the bone healing cascade, including mitogenic, chemotactic, and osteoinductive actions, as well as promoting callus formation and mineralization33, 34, 42. Since then, many studies have validated the role of BMPs in the growth, recruitment, attachment, proliferation, and differentiation of mesenchymal progenitor cells, ultimately resulting in new bone formation41, 43.
BMP-2
Although eight of the BMPs have established osteochondral functions44, BMP-2 appears to be the most potently osteoinductive and has therefore garnered the most interest. BMP-2 was first cloned in 1988 by a team led by John Wozney, which led to its classification as a member of the TGF-β superfamily34. The clinical use of the growth factor for augmentation of bone healing soon followed45. Over the next several decades, the newly available recombinant human BMPs (rhBMPs) were evaluated in pre-clinical animal models for safety and efficacy35, 41, 46–56. With the ability to induce progenitor cells to both the chondrogenic and osteogenic lineages, BMP-2 thus has utility in both endochondral and intramembraneous ossification applications32, 57. Initial in vivo studies typically evaluated the efficacy of rhBMP-2 using simple carriers, such as an absorbable type 1 collagen sponge, although advances in the past two decades have enabled more sophisticated delivery modalities. BMP-2 shows osteoinductivity in vivo, as evidenced by the formation of ectopic bone after implantation in non-bony sites (i.e., subcutaneous and intramuscular implantation)58–64. Bony defect models in which rhBMP-2 has shown pre-clinical efficacy include spinal fusion (rats, rabbits, goats, sheep, non-human primates)65–75, extremity segmental defects (rats, rabbits, dogs, non-human primates)62, 76–83, and cranial defects (mice, rats, rabbits, dogs, and goats)84–90, among others. Dosing for these studies is highly variable, and is dependent upon not only the carrier, but also the species, anatomy of the defect, and implant size57.
Clinical studies evaluating the efficacy of BMP-2 have been performed in several orthopaedic settings, including open tibial shaft and other traumatic extremity fractures91, 92. The growth factor has also been extensively utilized in interbody and posterolateral spine fusion procedures93–100. In 2002, rhBMP-2 (Infuse™) was approved for open tibial shaft fractures as well as in anterior lumbar fusions in the setting of degenerative disc disease91. Many of the clinical studies leading up to and following FDA approval suggest that rhBMP-2 performs comparably to autogenous bone graft, and in some cases promotes more efficient bone healing while obviating the need for graft harvest94, 96, 100. However there are a number of more recent studies which suggest that initial reports of efficacy were exaggerated, and adverse events associated with its use underreported97, 101, 102. Complications which are well-established to result from supraphysiologic rhBMP-2 dosing include ectopic and heterotopic bone formation, exacerbated inflammation and seroma formation, bone resorption, urogenital complications, and dysphagia when used in the cervical spine97, 103. Induction of cancer was also a concern by some, although this has been much debated97, 104, 105. Although more judicious use of the growth factor—in terms of both dose and clinical indications—has now been adopted by many surgeons, the delivery of rhBMP-2 using a more efficient carrier would both reduce the necessary dose to achieve high rates of union and reduce or potentially even eliminate the complications associated with its clinical use.
BMP-7
When delivered using a collagen carrier, rhBMP-7 (originally referred to as Osteogenic Protein-1, OP-1) was originally shown in 1992 to induce ectopic bone formation in intramuscular and subcutaneous models106. Following that discovery, BMP-7 was investigated extensively to assess its capacity to promote bone and cartilage regeneration, resulting in a large body of pre-clinical research that has validated its use as a means to augment bone healing in orthopaedic applications. The growth factor is sufficiently osteoinductive to promote healing of critical sized defects in rabbits, dogs, and non-human primates, and could promote spinal fusion in large animals after delivery using collagen matrix and other similar carriers54, 107–116. Some of these studies found that BMP-7 delivery surpassed even autogenous bone graft in some functional outcomes, such as biomechanical strength109, 116.
The promise of these pre-clinical results led to the first prospective randomized controlled trial on a BMP, which compared OP-1 Device (rhBMP-7) to autograft bone in the capacity to heal established nonunions of the tibiae117. The experimental group, which received 3.5 mg rhOP-1 with 1g bovine type I collagen matrix (“OP-1 Device”), achieved comparable clinical outcomes to the autograft control group, while obviating the morbidity associated with autograft harvest (donor site pain, increased blood loss, and increased infection risk).
In 2001, rhBMP-7 (rhOP-1) was approved by the FDA for the treatment of tibial nonunions, where it performed similarly to autogenous bone graft117. RhBMP-7 was also investigated as an adjunct to allograft bone as well as hydroxyapatite, where it enhanced osseointegration and new bone formation118, 119. Although rhBMP-7 is not approved generally for spinal fusion indications and requires a humanitarian device exemption in that setting, based on equivalence in clinical and radiographical outcomes, the combined use of rhBMP-7 and local autograft has been recommended as a viable alternative to iliac crest autologous bone graft (ICBG) for single-level instrumented spinal fusion procedures93. However, due to conflicting evidence for the use of rhBMP-7 delivered with an absorbable collagen sponge as a bone graft substitute for spine fusion, no recommendation has been made for its use in this clinical application93. Furthermore, similar to the supraphysiologic dosing of rhBMP-2, bone resorption associated with rhBMP-7 use has been reported120, 121.
BMP-6
Although not yet FDA-approved, BMP-6 is another osteoinductive BMP that has received significant attention for its potential for clinical translation. The capacity to promote osteogenic differentiation is well-established, and its ability to promote bone formation and healing have been tested in a number of pre-clinical models122–126. Studies have been performed to compare the osteoinductivity and bone forming capacity of rhBMP-6 with that of either rhBMP-7 or rhBMP-2, with conflicting results125, 127–129. Despite this, continued investigations exploring the utility of rhBMP-6 in combination with a variety of carriers for bone regenerative medicine are ongoing125, 126, 130–132.
RhBMP-6 is the biologic component of a product currently under development for clinical translation for bone regenerative purposes. The carrier in this OSTEOGROW device, is autologous blood coagulum, which has a greater affinity for rhBMP-6 than does the FDA-approved rhBMP-2 and rhBMP-7 carriers for their respective growth factors. This property is expected to eliminate the burst release and enable delivery of a significantly lower therapeutic dose of the growth factor133. OSTEOGROW is currently being evaluated in GLP and GMP studies for safety and efficacy in both acute radial fracture and high tibial osteotomy (HTO) indications, and in the first report of Phase I results from the HTO trial, no serious side effects were reported125.
SYNTHETIC CARRIERS FOR BMP
The safety of BMPs for clinical use came into question after serious side effects were noted with use of rhBMP-2 in spine surgery applications97, 105, and its use subsequently dropped dramatically. The adverse outcomes are attributed to the supraphysiologic dosing required for successful healing, and the need for such high doses is due to the inefficiency of growth factor delivery with the use of the FDA-approved carriers. This understanding highlights the importance of the carrier in delivering GF for optimal efficacy while maintaining an acceptable safety profile. In recent years, a wide variety of delivery vehicles have been investigated for improved efficiency in growth factor delivery. One such approach is the use of self-assembling peptides.
To obviate the requirement for supraphysiologic doses of recombinant growth factor, improved carriers for BMP should more efficiently bind and retain the growth factor. This enables the maintenance of local concentrations sufficient to induce signaling while preventing unwanted diffusion and off-target effects such as heterotopic/ectopic bone growth or uncontrolled inflammation. In addition to slowing BMP release, the ideal carrier would also provide an osteoconductive scaffold that can support the infiltration of bone-forming cells and bony ingrowth. Researchers have loaded BMP onto a wide variety of materials, including minerals such as hydroxyapatite134 and tricalcium phosphate135, proteins such as collagen136 and fibrin137, and natural polymers such alginate138, hyaluronic acid,139 and chitosan140. BMP carriers have also been crafted from synthetic polymers, usually covalent polymers such as polyethylene glycol (PEG)141, poly(ethyl acrylate) (PEA),142 and polylactic-co-glycolic acid (PLGA)143.
The use of completely synthetic materials can offer improved control over materials properties, since the chemical structures can be rationally tuned. Historically, synthetic carriers for BMPs and other growth factors have been traditional polymers in which structural units are linked through covalent bonds. However, polymers may take a long time to biodegrade or be cleared, and thus compromise biocompatibility of the carriers. At the same time, formation of ordered structures designed to optimize function of carriers, particularly for bioactivity, is rather challenging with covalent polymers. Over the past two decades supramolecular materials have emerged144, in which structural units or monomers interact through non-covalent bonds. An early example of a supramolecular biomaterial with liquid crystalline properties was reported by the Stupp laboratory145, and many of these materials are known as supramolecular polymers.146 Thus, the use of supramolecular biomaterials based on formation of non-covalent bonds among their constituent molecules may offer distinct advantages as osteoconductive carriers for BMP delivery. In supramolecular biomaterials, the individual molecular building blocks are not tethered together by covalent bonds, but instead self-assemble through non-covalent interactions such as hydrogen bonds, hydrophobic interactions, metal chelation, van der Waals forces, or π-π stacking.
SELF-ASSEMBLING PEPTIDES FOR BMP DELIVERY
Within supramolecular biomaterials, self-assembling peptides have gained attention and shown promise in a variety of applications147. Since signal transduction is largely mediated by proteins, which requires peptide chains to engage in non-covalent interactions, self-assembling peptides are an attractive platform for designing bioactive materials. The non-covalent connections among the monomers facilitate the formation of ordered structures that may be important for bioactivity and biodegradation rates might be much faster than those associated with covalent polymers148. The library of natural and unnatural amino acids offers great combinatorial diversity, and many amino acids have hydrogen bonding or hydrophobic regions that can participate in non-covalent self-assembly.
PuraMatrix™ is a hydrogel scaffold comprised of a 16 amino acid peptide sequence called RADA16, which forms non-covalent β-sheeted structures to self-assemble into peptide nanofibrils149 (Figure 1A). In one study, PuraMatrix™ mixed with recombinant human BMP-2 successfully regenerated calvarial bone in New Zealand white rabbits, while PuraMatrix™ alone and BMP-2 alone did not150. In another study, researchers created an injectable hydrogel of RADA16 mixed with BMP-2, which transitions from a solution to a gel in an ex-vivo pig femoral head model151. The RADA16 hydrogel successfully slowed BMP-2 release, and in vitro experiments demonstrated that the BMP-2 released retained its bioactivity151. The RADA16 peptide may also be modified to incorporate biological motifs, in particular short peptide sequences that can signal cells152 (Figure 1B). One study found that the incorporation of cell adhesion motifs into RADA16 hydrogels improved osteoblast cell adhesion and migration into the scaffolds152 (Figure 1B). Another self-assembling peptide system is SPG-178, a 13-amino acid sequence that forms nanofibrous structures153 (Figure 2A). Similar to RADA16, SPG-178 self-assembles into nanofibers due to β-sheet structures among the peptide monomers153 (Figure 2A). A network of SPG-178 nanofibers can form hydrogels, which have been shown to have some inherent osteoinductive properties (Figure 2B)154, 155. While RADA16 and SPG-178 are both relatively short peptide sequences (16 and 13 amino acids long, respectively), longer peptide chains can also be the basis for self-assembling peptide materials. For example, Poly(VPAVG)220 is an thermoresponsive elastin-like polymer with the sequence VPAVG repeated 220 times156. Poly(VPAVG)220 self-assembles into spherical nanoparticles (Figure 3), which are capable of encapsulating and delivering both BMP-2 and BMP-14156. These examples show the potential of self-assembling peptides for BMP delivery and bone regeneration as well as the diversity that is possible with the use of self-assembling peptide biomaterials. However, peptides can be further improved by modification with other types of molecules, similar to the post-translational modification of proteins in biology. One example is peptide amphiphiles (PAs), a class of synthetic molecules that contain a short peptide chain conjugated to an aliphatic tail.148, 157–159
Figure 1.
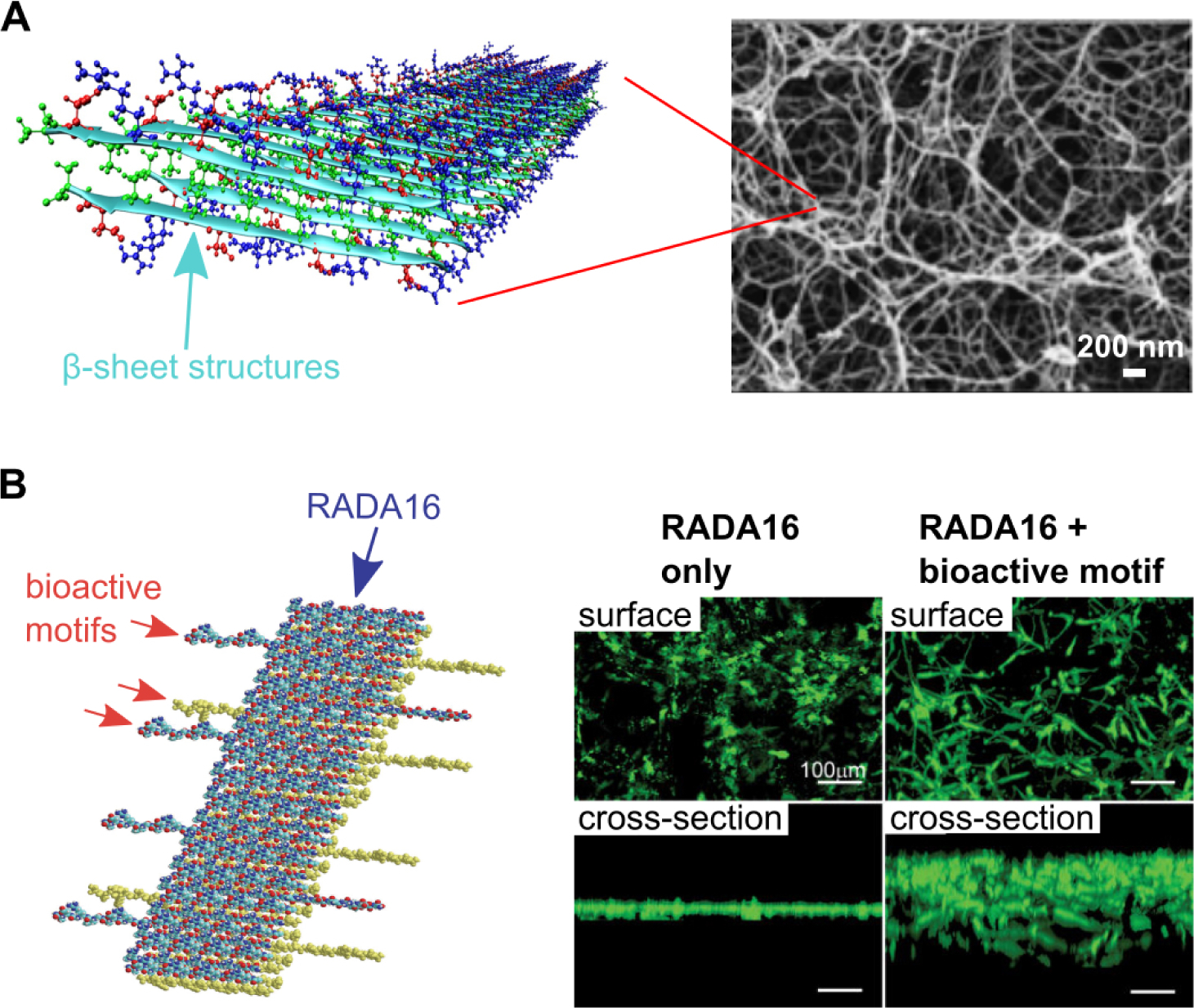
(A) Molecular graphics representation of RADA16 nanofibrils, and scanning electron micrograph (SEM) of a RADA16 hydrogel. Adapted with permission.190, 191 Copyright 2013, American Chemical Society; Copyright 2011, Royal Society of Chemistry (B) Molecular graphics representation of RADA16 nanofibrils with a bioactive motif incorporated, and calcein-stained cells cultured on RADA16 hydrogels with and without bioactive motifs (in this case, a cell adhesion ligand). The bioactive cell adhesion motif improves cell spreading on and infiltration into RADA16 hydrogels. Adapted with permission under the terms of the Creative Commons Attribution License.152 Copyright 2007, S. Zhang, published by PLOS.
Figure 2.
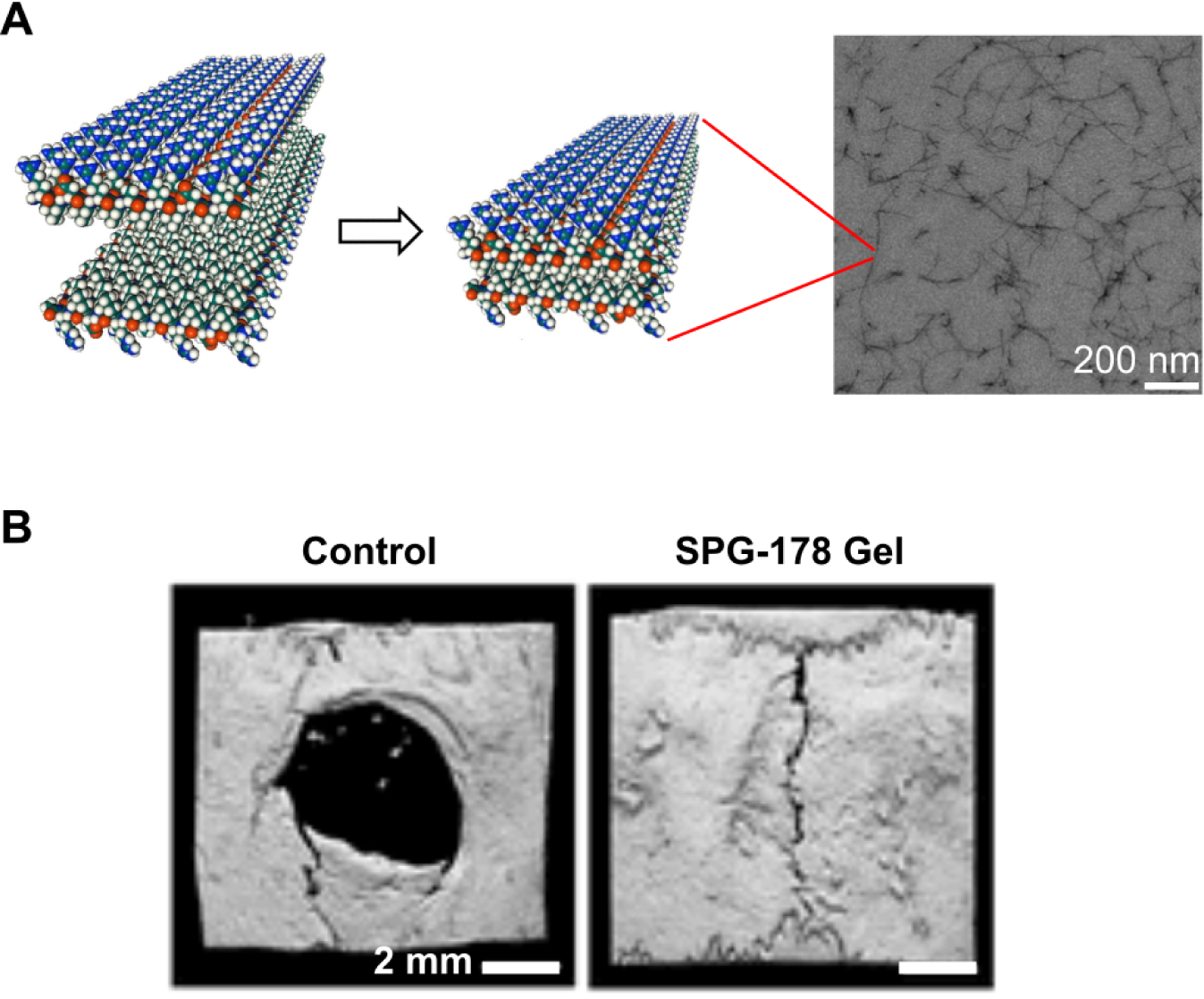
(A) Molecular graphics representation of SPG-178 peptide nanofiber assemblies, and transmission electron micrograph (TEM) of a SPG-178 hydrogel. Adapted with permission.153 Copyright 2012, Elsevier (B) Representative microCT (computerized tomography) reconstructions of rat calvarial defects, showing the degree of bone healing after 3 weeks when untreated (Control) or treated with SPG-178 hydrogels. Reproduced with permission.155 Copyright 2017, Mary Ann Liebert, Inc.
Figure 3.
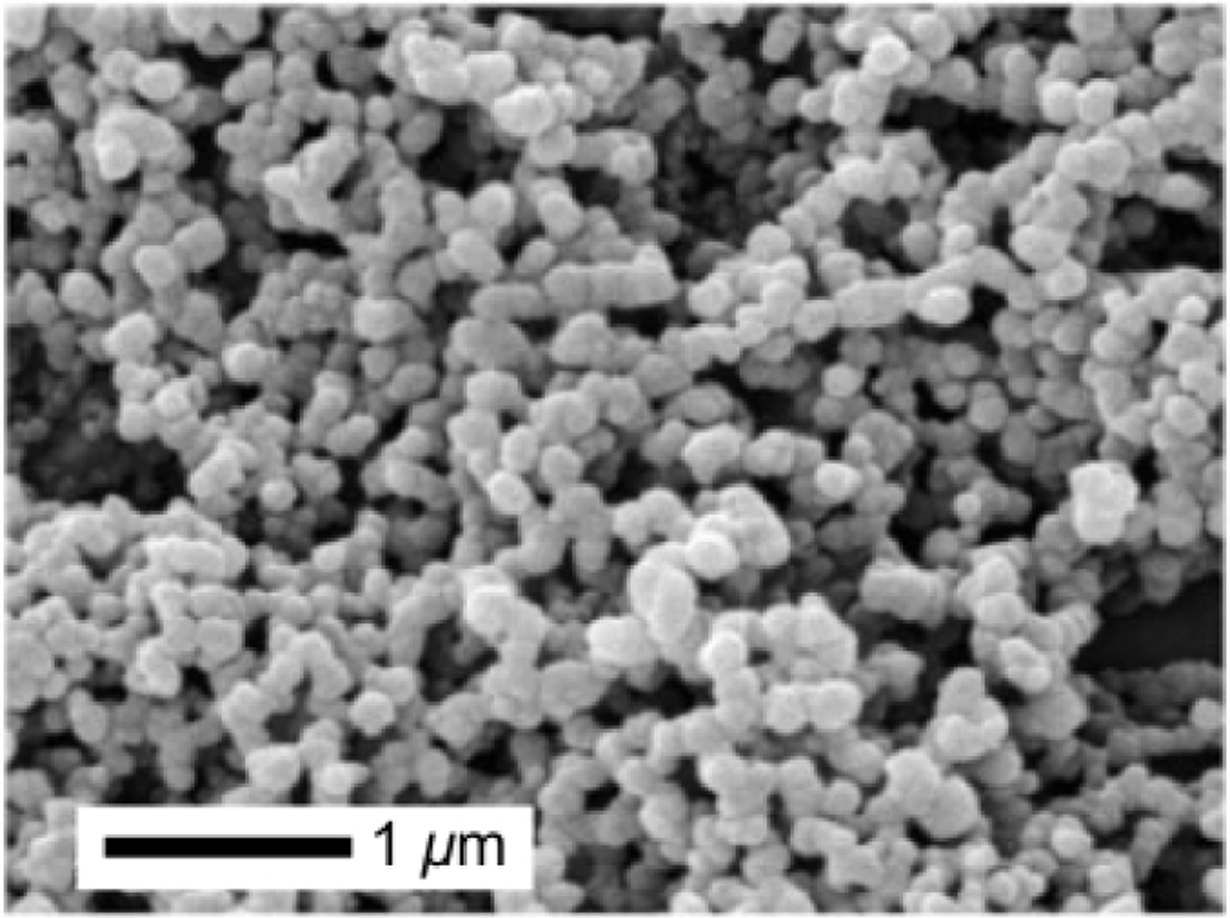
Scanning electron micrograph (SEM) of elastin-like Poly(VPAVG)220 supramolecular assemblies. Reproduced with permission.156 Copyright 2010 Elsevier.
PEPTIDE AMPHIPHILE SCAFFOLDS FOR BMP DELIVERY
In canonical form, peptide amphiphile (PA) molecules that can self-assemble into supramolecular nanofibers and form hydrogels contain a single aliphatic tail covalently bonded to a peptide sequence that induces hydrophobic collapse. The peptide segment commonly is one that leads to the formation of β-sheets with high densities of intermolecular hydrogen bonding148, 157–161. These non-covalent interactions can be tuned to enable self-assembly into a variety of structures, making PAs a versatile platform for biomaterials design.148, 157–159 Since the first report in 2001157, work on PA nanofibers over the past two decades has demonstrated their ability to regenerate a variety of tissues including bone162–165, cartilage166, muscle167, vasculature168, and neural tissue169. For bone regeneration specifically, PA-based biomaterials have successfully healed bone defects with low doses of recombinant BMP-2.163–165.
In 2008 the authors’ laboratory developed a “heparin-binding PA,” which contained a short peptide sequence that binds heparan sulfate170, a highly sulfated polysaccharide that regulates growth factor activity. In addition to their heparan sulfate-binding activity, the PA molecules were designed to self-assemble into nanofibrous structures reminiscent of the natural extracellular matrix (ECM)170. Heparan sulfate naturally exists as a glycosaminoglycan bound to fibrillar proteins within the ECM, from where it binds a multitude of growth factors and controls their interactions with cell receptors171. Since one of these growth factors is BMP-264, 172, the ability of the heparin-binding PA to regenerate bone was explored in 2013163. In that study, it was postulated that PA nanofibers would bind heparan sulfate, which would in turn bind BMP-2 and present it to cell receptors163 (Figure 4A). The heparin-binding PA nanofibers were combined with low-dose (1 μg) BMP-2, loaded onto a porous collagen sponge to improve surgical handling properties, and then heparan sulfate was added to this scaffold (Figure 4B–C)163. This synthetic biomaterial was implanted in rat critical sized femoral defects, where the combination of PA, BMP-2, and heparan sulfate achieved full bony bridging in over half of animals (Figure 4D)163. Both the PA and heparan sulfate were required to achieve this healing rate with use of the low-dose BMP-2 (Figure 4D)163. Since heparan sulfate is a natural glycosaminoglycan that can potentiate BMP signaling, its co-delivery with PA nanofibers is an attractive strategy for bone regeneration. However, to avoid the need to source the highly diverse heparan sulfate, supramolecular polymers with “built-in” synthetic ability to potentiate BMP-2 signal would be desirable.
Figure 4.
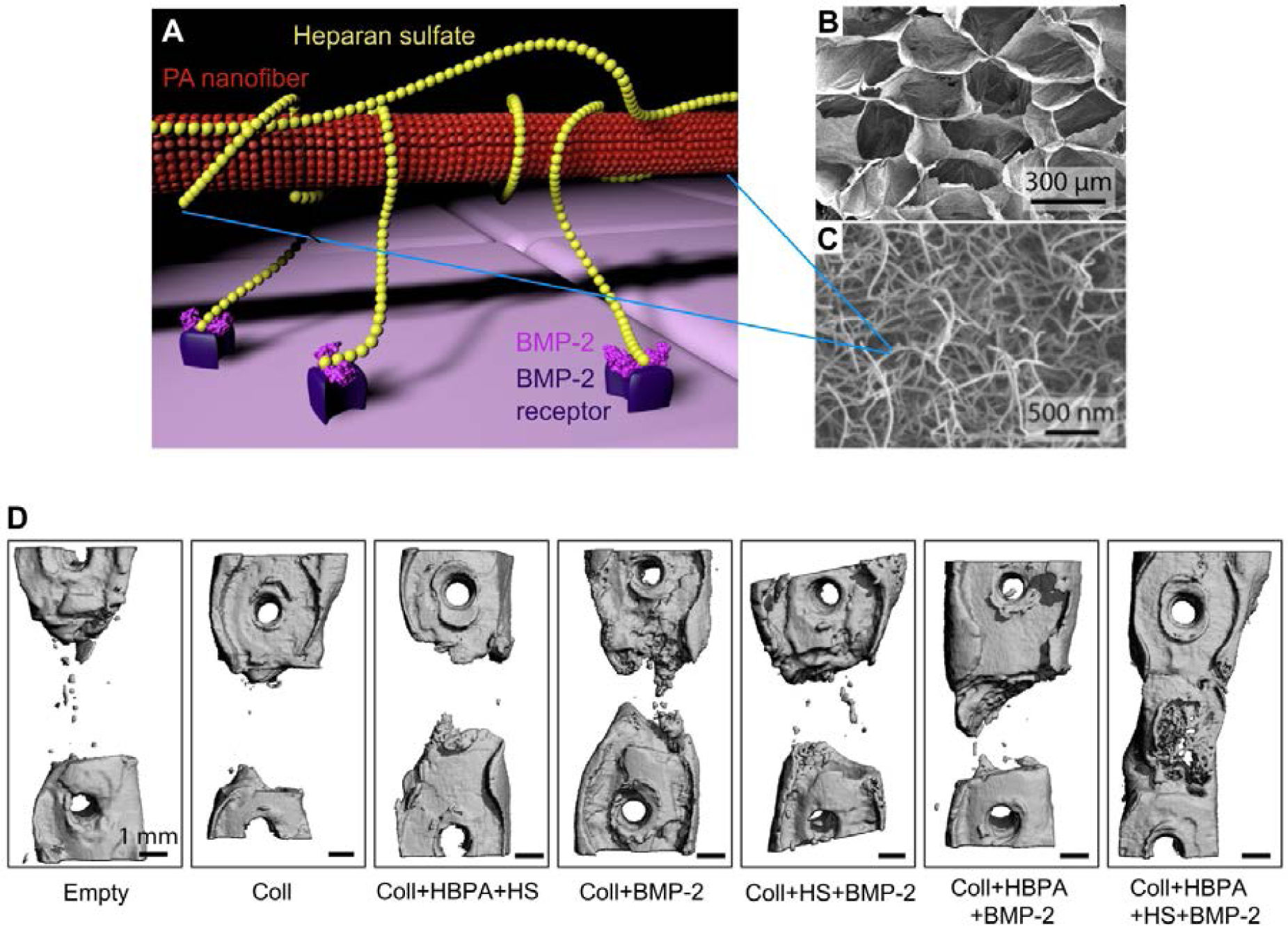
(A) Molecular graphics representation of heparin-binding PA structure and function. The heparin-binding PA nanofiber binds heparan sulfate, which is a lengthy polysaccharide. The heparan sulfate in turn binds BMP-2 growth factor and presents it to receptors on the cell membrane, thus potentiating the signal of BMP-2. (B) Empty porous collagen sponge, which heparin-binding PA nanofibers were loaded onto to improve surgical handling properties. (C) Scanning electron micrograph (SEM) of heparin-binding PA nanofibers, mixed with heparan sulfate and loaded onto a porous collagen sponge. (D) Representative microCT (computerized tomography) reconstructions of rat femur defects, showing the degree of bone healing after 6 weeks when treated with the indicated materials. Abbreviations: Coll – collagen sponge; HBPA – heparin-binding PA; HS – heparan sulfate. A) Adapted B-D) Reproduced with permission.163 Copyright 2013, Elsevier.
PA nanostructures can be synthetically designed with biological function by adding bioactive epitopes, usually short amino acid sequences that comprise the bioactive portion of natural proteins (Figure 5A)168, 169, 173, 174. Compared to natural proteins which have short half lives, these synthetic epitopes that mimic proteins are more stable when embedded in supramolecular assemblies and can remain bioactive for longer periods of time. Furthermore, the presentation of epitopes on PA nanofibers can result in higher bioactivity than the soluble peptides, given the stability of the supramolecular construct with all of its internal cohesive energy relative to the soluble peptide174 (Figure 5B–C). In addition, since supramoleciular polymers are dynamic175, 176, the internal structure of bioactive signals may rearrange to optimize effective binding with cells receptors146 (Figure 5D). The chemical sequence and structural diversity of heparan sulfate allows this natural polysaccharide to interact with many different proteins – one report suggests up to 435 unique proteins177 - using the proteins’ heparin-binding domains that recognize the sulfated moieties with specific chemical sequences. Due to this structural diversity, capturing the function of heparan sulfate presents unique challenges compared to proteins on which short bioactive sequences have been clearly identified.
Figure 5.
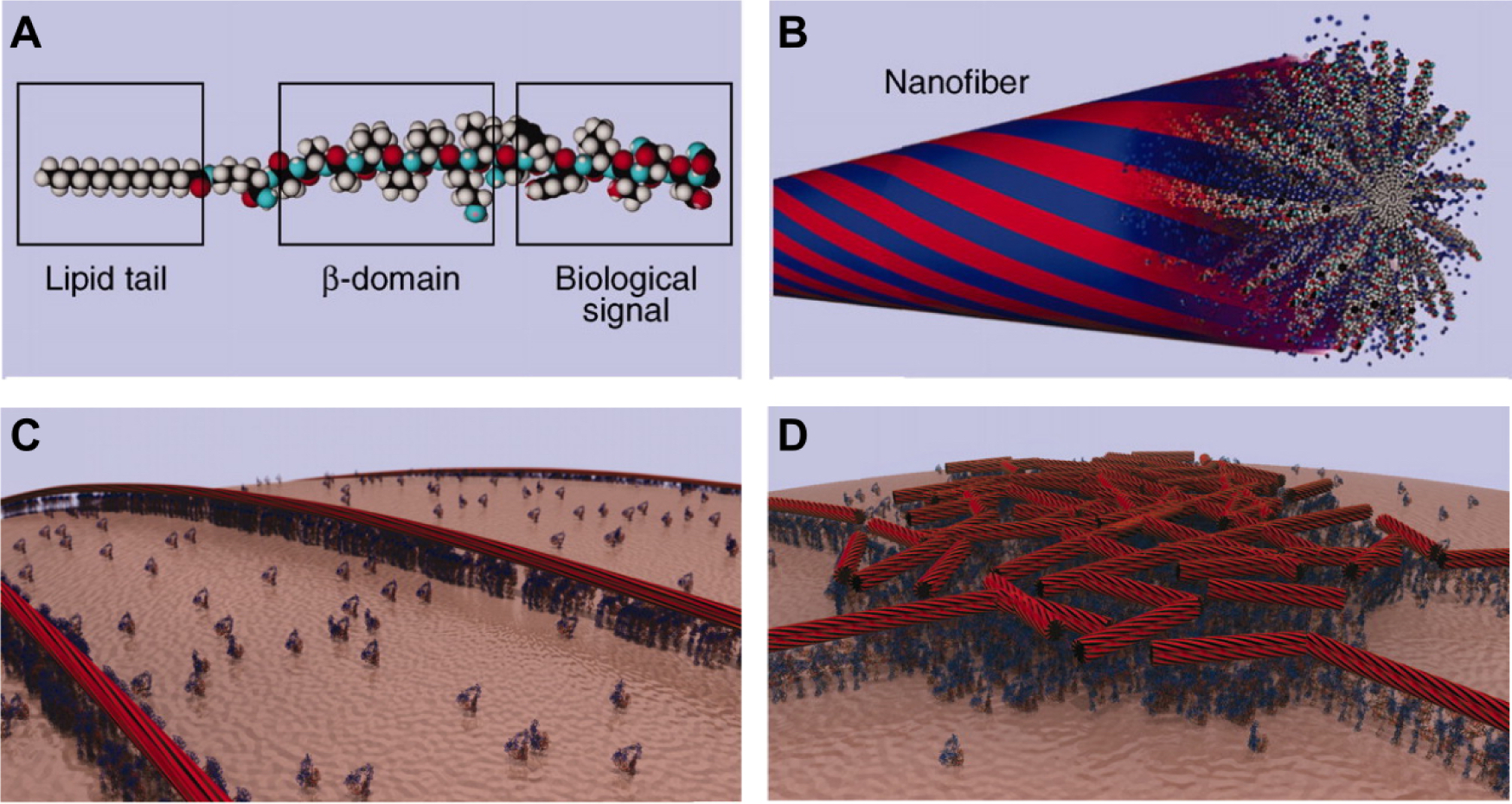
Molecular graphics representations showing (A) a PA molecule incorporating a bioactive peptide epitope and (B) the supramolecular nanofiber formed by these PA molecules. Due to hydrophobic collapse of the lipid tail in aqueous environments, the bioactive signals are displayed at high density of the surface of the nanofiber (idealized in red portions). The blue portions represent idealized water domains. (C) Molecular graphics representation of PA nanofibers concentrating and presenting biological signals to cell membranes. (D) Due to their non-covalent nature, PA nanofibers may dynamically rearrange over time as they interact with cells. In this molecular graphics representation, PA nanofibers have concentrated around a lipid raft structure where cell signaling activity is centered. Reproduced with permission.146 Copyright 2012, AAAS.
To mimic the structure and function of natural heparan sulfate, the authors’ laboratory synthesized an abiotic tri-sulfated monosaccharide and conjugated it to a PA molecule, thus creating a “glycopeptide PA”165 (Figure 6A). When the glycopeptide PA self-assembles into nanofibers, the tri-sulfated monosaccharides are presented at the surface of those nanofibers (Figure 6B). Because non-covalent bonds within supramolecular structures can dynamically rearrange,175, 176 the tri-sulfated monosaccharides can access different configurations and thus adapt to the heparin-binding domains of different proteins.165 Interestingly, it was discovered that supramolecular assemblies of the glycopeptide PA were able to bind five different important proteins in biological development and regenerative medicine, BMP-2, BMP-4, FGF-1, FGF-1, and VEGF, which demonstrates its potential for multipotent protein activation165. When the tri-sulfated monosaccharide was replaced with a non-sulfated counterpart (Figure 6A), protein binding to PA assemblies did not occur165. Furthermore, in-vitro experiments measuring alkaline phosphatase (ALP) expression showed that supramolecular nanofibers of the glycopeptide PA enhanced signaling of wild type BMP-2, but not of a mutant BMP-2 lacking its heparin-binding domain165.
Figure 6.
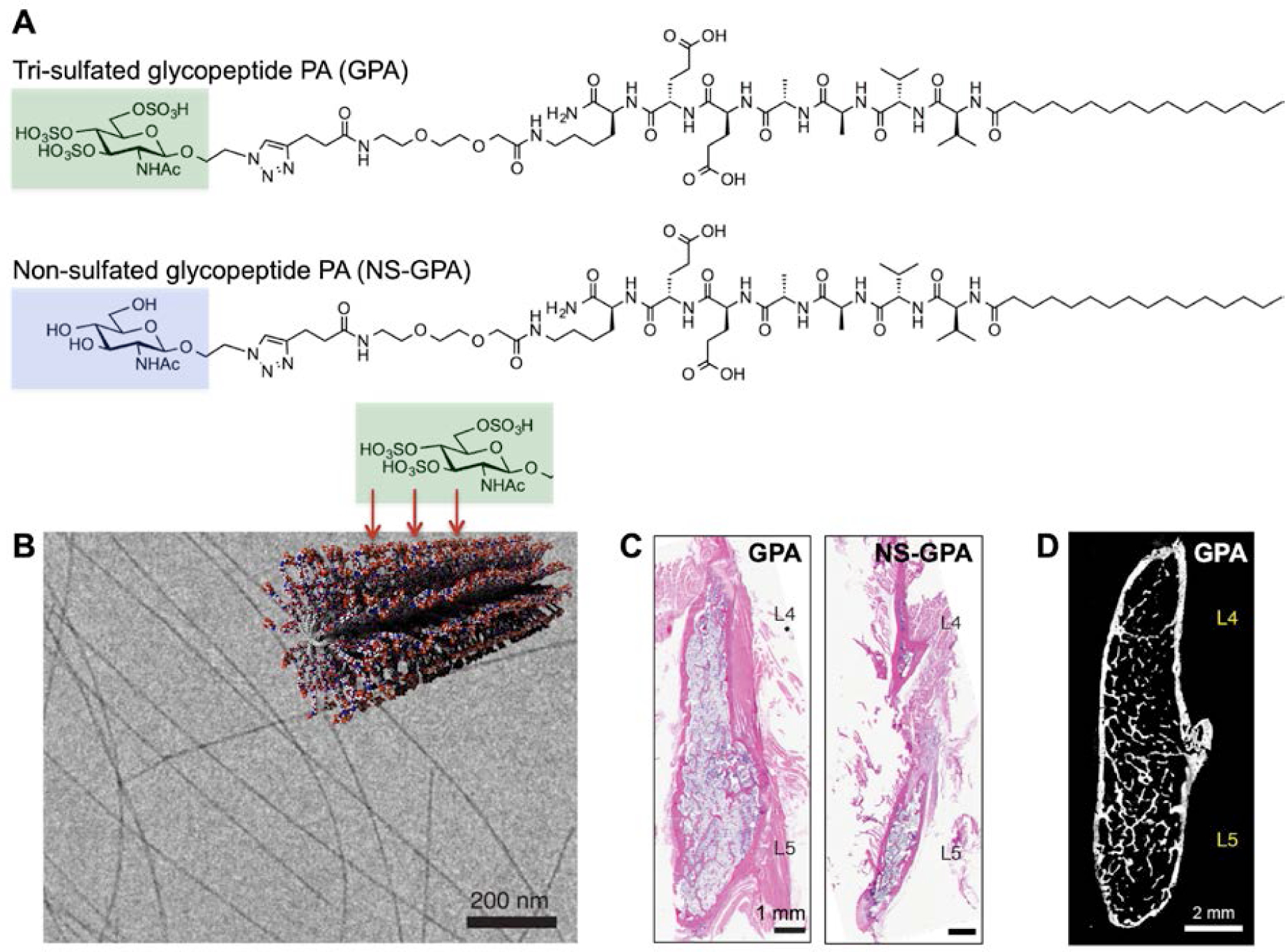
(A) Chemical structure of PA containing a tri-sulfated monosaccharide, highlighted in green, and the same PA bearing a non-sulfated version of the monosaccharide, highlighted in blue. (B) Cryogenic transmission electron micrograph (cryoTEM) of the tri-sulfated glycopeptide PA and molecular graphics representation of the PA nanofibers. The tri-sulfated monosaccharide moities are displayed on the surface of supramolecular nanofibers. (C) Representative sagittal cross-sectional images of the fusion bed in rat spines, visualized with hematoxylin and eosin (H&E) staining. Rats received 0.1 μg of BMP-2 and the indicated PAs. (D) High-resolution microCT (computerized tomography) reconstruction from a fused rat treated with tri-sulfated glycopeptide PA and 0.1 μg BMP-2. A) Adapted B-D) Reproduced with permission.165 Copyright 2017, Springer Nature.
The glycopeptide PA supramolecular polymers were tested for in vivo bioactivity by absorbing them into porous collagen sponges, and interestingly these biomaterials were found to reduce by a factor of 100 the necessary therapeutic dose to achieve rat spinal fusion. This extremely low dose was sufficient to achieve a fusion rate of 100% when pre-loaded with only 0.1 μg BMP-2/rat165 (Figure 6C). High-resolution microCT (computerized tomography) revealed the robust formation of bone throughout the entire fusion bed165 (Figure 6D). PA nanofibers bearing a non-sulfated version of the same monosaccharide achieved minimal fusion (10%) at the sub-therapeutic dose of 0.1 μg rhBMP-2/rat165 (Figure 6C). Similar to the heparin-binding PA supramolecular polymers, the glycopeptide PA ones potentiate BMP-2 signal, but the bioactivity derives from the PA assemblies themselves and not from bound heparan sulfate. Given the experiment mentioned above about the system with a mutated heparin-binding domain, it is reasonable to conclude that the glycopeptide PA supramolecular nanofibers orient BMP-2 protein molecules in the correct spatial orientation to signal their receptor. This may be the basis of the biomaterial’s remarkable bioactivity toward bone regeneration in spinal fusion165.
Motivated by the clinical translation of BMP-2 to promote bone regeneration, a PA supramolecular polymer with capacity to directly bind this protein was developed in the authors’ laboratory164, 178. The monomer of this supramolecular system is known as the BMP-2- binding PA which includes a bioactive peptide sequence discovered by phage display178 (Figure 7A–B). While the glycopeptide PA utilizes a tri-sulfated monosaccharide to emulate sequences in heparan sulfate that would recognize BMP-2’s heparin binding domain, the BMP-2 binding PA is functionalized instead with a short peptide sequence capable of binding the protein. The BMP-2 binding PA was tested in a pre-clinical rat model of spinal fusion, where the established positive control—rhBMP-2 delivered using a collagen sponge (similar to the clinical product Infuse™)—requires a dose of 10 μg/rat in order to achieve a fusion rate of 100%164. When deployed in this model, the BMP-2-binding PA reduced the therapeutic BMP-2 requirement 10-fold, achieving successful fusion in 100% of the animals, using a dose of 1 μg BMP-2/rat (0.5 μg/implant)164 (Figure 7C–D). Interestingly, when this PA was delivered alone (i.e. without any rhBMP-2), successful fusion was noted in 42% of animals, which was assumed to be a result of the capacity for the supramolecular PA assembly to bind endogenous BMP-2 and potentiate signaling (Figure 7C–D)164.
Figure 7.
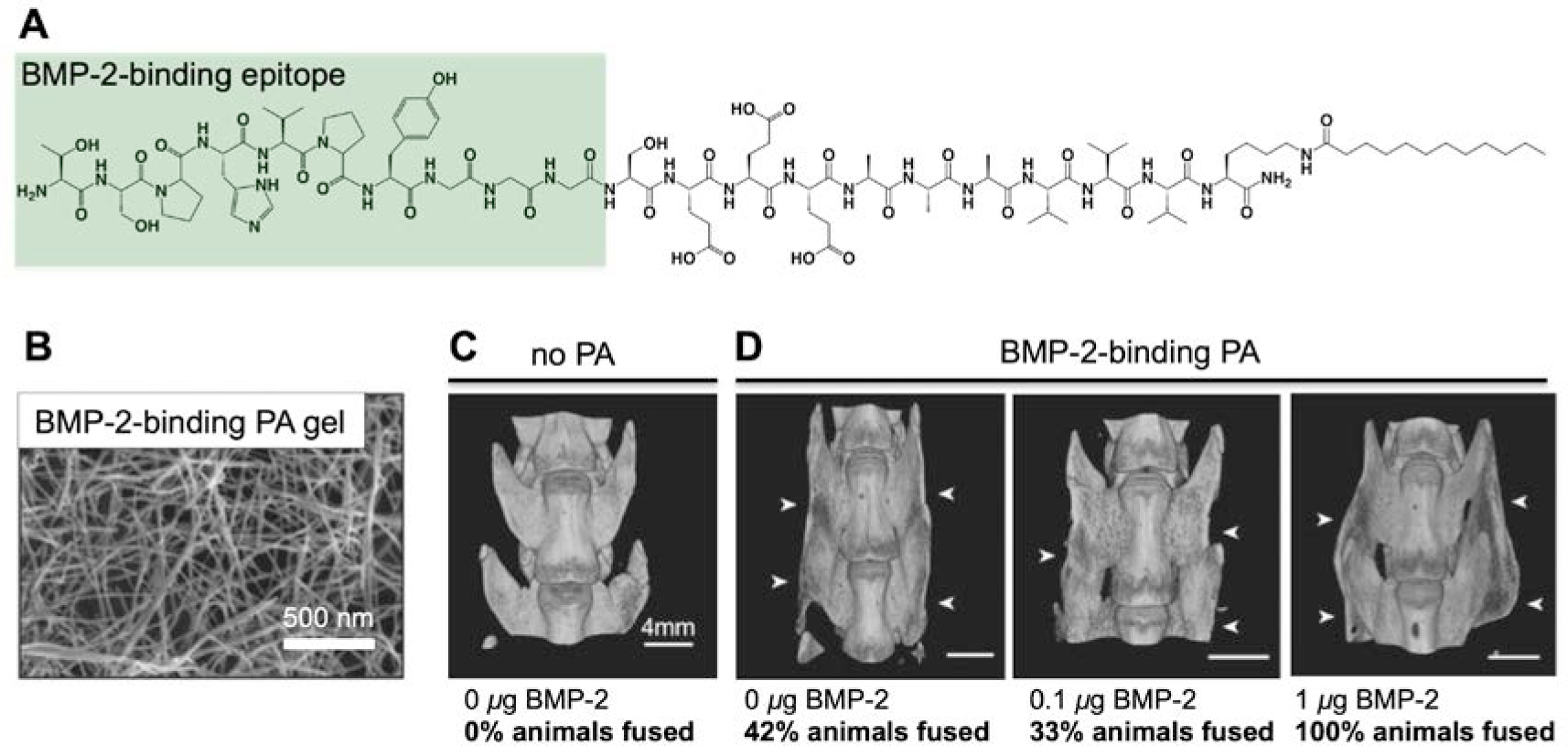
(A) Chemical structure of a PA containing a short peptide sequence identified to bind BMP-2 via phage display. The bioactive peptide portion is highlighted in green. (B) Scanning electron micrograph (SEM) of a hydrogel containing the BMP-2 binding PA. (C) MicroCT (computerized tomography) reconstruction of an unfused animal treated with no PA and no BMP-2, included for comparison with (D) microCT (computerized tomography) reconstructions of fused animals treated with BMP-2 bindign PA and indicated BMP-2 dosages. The images are specifically of fused animals in the groups; the overall fusion rates164 are indicated. The white arrows indicate the fusion bed. Adapted with permission.164 Copyright 2015, Wiley VCH.
In summary, all three of the PA-based bone regenerative materials described here have demonstrated ability to lower the therapeutic BMP dose in animal models. Osteoinductive signals were incorporated into these PA supramolecular polymers not only by encapsulating BMP-2, but also by rational chemical design to potentiate its signal. All three PA systems formed nanofibrous structures reminiscent of natural ECM, which likely contributed to the ability of cells to infiltrate the scaffolds containing PA assemblies. In surgical settings, the periosteum and bone marrow space are typically exposed and serve as a source of osteoprogenitor cells that can infiltrate the scaffold. Although obviating the need to source live cells is ideal, given that PA nanofibers can present bioactive signals, PA-based materials for cell delivery may also lead to promising therapies. In particular, bone marrow aspirate contains self-renewing cells with the capacity to synthesize growth factors such as BMPs and VEGF179, 180. Thus, if these cells were delivered with a PA vehicle, the PA scaffold may increase cell survival as well as potentiate the signal of the synthesized growth factors.
In addition to BMP delivery, the PA platform offers many other advantages for bone regeneration applications. Although PA nanofibers can support cell adhesion without added biological functionality160, 181, 182, cell adhesion to PA nanofibers can be improved by adding the fibronectin cell adhesion motif, RGD183, and the chemical structure of RGD-bearing PAs can be tuned to optimize biological response173. Furthermore, PA nanofibers can support mineral deposition, a critical process in new bone formation. In particular, PAs with phosphoserine residues show the ability to nucleate hydroxyapatite crystallization157, as well as the ability to promote the expression of early osteogenic markers in vitro184. In one report, a composite of RGD-bearing PA and phosphoserine-bearing PA was implanted in a rat femoral defect model, where the combination of both PAs led to more bone growth than either PA alone162. Furthermore, the morphology of PA assemblies can be tuned to template macroscopic alignment of hydroxyapatite across length scales, thus orienting hydroxyapatite in ways that emulate the structure of bone.185
Beyond direct BMP-2 delivery, PAs can also potentiate BMP-2 signaling via modulation of lipid raft mobility186. A PA molecule lacking a bioactive epitope was shown to significantly enhance both BMP-2 and Wnt signaling in vitro; this was attributed to the ability of PA assemblies to associate with the cell membrane and affect lipid raft structures186. More specifically, this PA had positive charge, opposite to negatively charged cell membranes, and its supramolecular assemblies also had weak internal cohesion186. This allowed the PA molecules to interact with the cell membrane and increase diffusion within its lipid rafts, which interestingly led to an increase in signaling186. An analogous PA with similar charge but strong internal cohesion in fact did not enhance BMP-2 signaling186. While many studies have examined how PAs can influence BMP-2 signaling, other signaling pathways are of course integral in the bone healing process. PAs with biomimetic epitopes for VEGF168 and FGF-2187 have been developed, which could promote angiogenic, mitogenic, and chemotactic activities that are all required for successful bone regeneration.
Comprised of the same amino acids that build natural proteins, self-assembling peptides are uniquely qualified as biomaterials with cell-signaling capabilities. Compared to covalent polymers, supramolecular polymers based upon dynamic non-covalent bonds more closely mimic the nature of biological tissues and are therefore often more biocompatible and biodegradable. With the potential for further modification of peptides, particularly through conjugation of an aliphatic tail or monosaccharides, the PA platform can achieve physical and biological properties not possible with self-assembling peptides alone. The ability of PAs to regenerate bone with low BMP-2 doses was first observed in rodents and the next challenge is to establish their safety and efficacy in large animal models, which will clearly be necessary for these systems to advance toward clinical translation. Large animals will require higher BMP doses to promote bone regenearation, so the ability of PAs to potentiate BMP signaling safely on scales closer to the ones required in humans will be investigated in these models. At the same time, in large animals greater amounts of PA will be required to fill defects or achieve spinal fusion. On these larger scales, the kinetics of biodegradation, overall biocompatibility, as well as mechanical and rheological properties will have to be tested as well. PA nanofibers have a high charge density by design in order to promote solubility in aqueous media159, and interactions of these charges with cells and proteins might be different in large versus small animals. In this regard it is encouraging that there have been reports where self-assembling peptides were used effectively in in large animals such as rabbits166 and pigs188. Also, we are greatly encouraged by the fact that preliminary work in the authors’ laboratory and elsewhere has demonstrated great efficacy and safety in rabbit models of spinal fusion with low dose BMP-2 and PAs189.
CONCLUSIONS AND OUTLOOK
Given the many requirements for successful bone healing, the ideal synthetic biomaterial to support the regeneration of bone will likely require the integration of multiple components. Although recombinant BMPs provide a highly effective osteoinductive signal, this family of growth factors cannot be used effectively without a self-supporting, implantable carrier. Absorbable collagen sponges have been used as carriers for recombinant human BMP-2 and BMP-7 with clinical efficacy, but not without side effects, which are attributed to the supraphysiological BMP dosages required. Thus, the development of carriers that can reduce the effective BMP dose by slowing BMP release and/or potentiating BMP signal would be beneficial. In this review, we have discussed the use of self-assembling peptides to fulfill these important functional features. Bio-inspired strategies based upon our understanding of the composition and function of natural extracellular matrices, as well as osteoinductive growth factor signaling, have led to the design of PA structures which not only slow BMP-2 release, but may also to recruit and potentiate endogenous growth factor activity. Beyond BMP, we discussed the notion of multipotent protein activation by heparan sulfate-mimetic PA systems, which are capable of binding a multitude of growth factors involved in bone regeneration, including VEGF, and FGF. In addition to binding and delivering growth factors, the filamentous structures that result from PA self-assembly are highly mimetic of the natural extracellular matrix, which can support cell adhesion as well as mineralization.
Self-assembling peptides such as PA-based supramolecular polymers have demonstrated potential to become effective BMP carriers, and can complement the large variety of existing materials in orthopedic surgery. In this respect, since PAs are relatively soft materials, the combination of PAs with materials that have load-bearing capacity would be beneficial. The heparin-binding PA and glycopeptide PA discussed in this review were applied to collagen sponges, similar to how recombinant BMP-2 is currently used. PA liquid solutions may also be loaded onto metal, ceramic, or 3D-printed scaffolds, creating completely synthetic composites that have both load-bearing capabilities and bioactive functions. A composite of PA and demineralized bone matrix (DBM) represents an alternative strategy to PA-based recombinant growth factor delivery. Furthermore, cells can be suspended in a PA solution, which could then be used to coat a scaffold. With the capacity to safely harness growth factor-based bioactivity, PA-functionalized composite materials could potentially overcome the limitations associated with many currently available bone regenerative products. We anticipate that self-assembling peptides combined with BMPs as well as load-bearing materials for structural integrity could generate highly effective systems for bone regeneration and completely novel clinical opportunities.
ACKNOWLEDGEMENTS
The authors are grateful for support of the work discussed herein by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01AR072721 and R01AR069580, and the Simpson Querrey Institute at Northwestern University.
References
- 1.Buza JA 3rd; Einhorn T. Bone healing in 2016. Clin. Cases Miner. Bone Metab 2016. 13(2), 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glassman SD; Anagnost SC; Parker A; Burke D; Johnson JR; Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine (Phila Pa 1976). 2000. 25(20), 2608–15. [DOI] [PubMed] [Google Scholar]
- 3.Porter SE; Hanley EN Jr. The musculoskeletal effects of smoking. J. Am. Acad. Orthop. Surg 2001. 9(1), 9–17. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein P. Smoking and wound healing. Am. J. Med 1992. 93(1A), 22S–24S. [DOI] [PubMed] [Google Scholar]
- 5.Sloan A; Hussain I; Maqsood M; Eremin O; El-Sheemy M. The effects of smoking on fracture healing. Surgeon. 2010. 8(2), 111–6. [DOI] [PubMed] [Google Scholar]
- 6.Guo S; Dipietro LA. Factors affecting wound healing. J. Dent. Res 2010. 89(3), 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ondra SL; Marzouk S. Revision strategies for lumbar pseudarthrosis. Neurosurg. Focus 2003. 15(3), E9. [DOI] [PubMed] [Google Scholar]
- 8.Fini M; Giavaresi G; Salamanna F; Veronesi F; Martini L; De Mattei M; Tschon M. Harmful lifestyles on orthopedic implantation surgery: a descriptive review on alcohol and tobacco use. J. Bone Miner. Metab 2011. 29(6), 633–44. [DOI] [PubMed] [Google Scholar]
- 9.Hollinger JO; Onikepe AO; MacKrell J; Einhorn T; Bradica G; Lynch S; Hart CE. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-BB and an injectable beta-tricalcium phosphate/collagen matrix. J. Orthop. Res 2008. 26(1), 83–90. [DOI] [PubMed] [Google Scholar]
- 10.Phan K; Fadhil M; Chang N; Giang G; Gragnaniello C; Mobbs RJ. Effect of Smoking Status on Successful Arthrodesis, Clinical Outcome, and Complications After Anterior Lumbar Interbody Fusion (ALIF). World Neurosurg. 2018. 110, e998–e1003. [DOI] [PubMed] [Google Scholar]
- 11.Castillo RC; Bosse MJ; MacKenzie EJ; Patterson BM; Group LS. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J. Orthop. Trauma 2005. 19(3), 151–7. [DOI] [PubMed] [Google Scholar]
- 12.Sohn HS; Oh JK. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res 2019. 23, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghodasra JH; Daley EL; Hsu EL; Hsu WK. Factors influencing arthrodesis rates in a rabbit posterolateral spine model with iliac crest autograft. Eur. Spine J 2014. 23(2), 426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Long WG Jr.; Einhorn TA; Koval K; McKee M; Smith W; Sanders R; Watson T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. 2007. 89(3), 649–58. [DOI] [PubMed] [Google Scholar]
- 15.Summers BN; Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J. Bone Joint Surg. Br 1989. 71(4), 677–80. [DOI] [PubMed] [Google Scholar]
- 16.Myeroff C; Archdeacon M. Autogenous bone graft: donor sites and techniques. J. Bone Joint Surg. Am 2011. 93(23), 2227–36. [DOI] [PubMed] [Google Scholar]
- 17.Goulet JA; Senunas LE; DeSilva GL; Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin. Orthop. Relat. Res 1997. (339), 76–81. [DOI] [PubMed] [Google Scholar]
- 18.Pollock R; Alcelik I; Bhatia C; Chuter G; Lingutla K; Budithi C; Krishna M. Donor site morbidity following iliac crest bone harvesting for cervical fusion: a comparison between minimally invasive and open techniques. Eur. Spine J 2008. 17(6), 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seiler JG 3rd; Johnson J. Iliac crest autogenous bone grafting: donor site complications. J. South. Orthop 2000. 9(2), 91–7. [PubMed] [Google Scholar]
- 20.Geideman W; Early JS; Brodsky J. Clinical results of harvesting autogenous cancellous graft from the ipsilateral proximal tibia for use in foot and ankle surgery. Foot Ankle Int. 2004. 25(7), 451–5. [DOI] [PubMed] [Google Scholar]
- 21.St John TA; Vaccaro AR; Sah AP; Schaefer M; Berta SC; Albert T; Hilibrand A. Physical and monetary costs associated with autogenous bone graft harvesting. Am. J. Orthop 2003. 32(1), 18–23. [PubMed] [Google Scholar]
- 22.Silber JS; Anderson DG; Daffner SD; Brislin BT; Leland JM; Hilibrand AS; Vaccaro AR; Albert TJ. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003. 28(2), 134–9. [DOI] [PubMed] [Google Scholar]
- 23.Robertson PA; Wray AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine (Phila Pa 1976). 2001. 26(13), 1473–6. [DOI] [PubMed] [Google Scholar]
- 24.Loeffler BJ; Kellam JF; Sims SH; Bosse MJ. Prospective observational study of donor-site morbidity following anterior iliac crest bone-grafting in orthopaedic trauma reconstruction patients. J. Bone Joint Surg. Am 2012. 94(18), 1649–54. [DOI] [PubMed] [Google Scholar]
- 25.Delawi D; Dhert WJ; Castelein RM; Verbout AJ; Oner FC. The incidence of donor site pain after bone graft harvesting from the posterior iliac crest may be overestimated: a study on spine fracture patients. Spine (Phila Pa 1976). 2007. 32(17), 1865–8. [DOI] [PubMed] [Google Scholar]
- 26.Park DK; Roberts R; Arnold P; Kim DH; Sasso R; Baker KC; Fischgrund JS. Lumbar Spine Fusion Rates With Local Bone in Posterolateral and Combined Posterolateral and Interbody Approaches. J. Am. Acad. Orthop. Surg. Glob. Res. Rev 2019. 3(11), e018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariscal G; Nunez JH; Barrios C; Domenech-Fernandez P. A meta-analysis of bone morphogenetic protein-2 versus iliac crest bone graft for the posterolateral fusion of the lumbar spine. J. Bone Miner. Metab 2020. 38(1), 54–62. [DOI] [PubMed] [Google Scholar]
- 28.Haddad SL; Coetzee JC; Estok R; Fahrbach K; Banel D; Nalysnyk L. Intermediate and long-term outcomes of total ankle arthroplasty and ankle arthrodesis. A systematic review of the literature. J. Bone Joint Surg. Am 2007. 89(9), 1899–905. [DOI] [PubMed] [Google Scholar]
- 29.Kitaoka HB. Arthrodesis of the ankle: technique, complications, and salvage treatment. Instr. Course Lect 1999. 48, 255–61. [PubMed] [Google Scholar]
- 30.Raizman NM; O’Brien JR; Poehling-Monaghan KL; Yu WD. Pseudarthrosis of the spine. J. Am. Acad. Orthop. Surg 2009. 17(8), 494–503. [DOI] [PubMed] [Google Scholar]
- 31.Dimitriou R; Tsiridis E; Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005. 36(12), 1392–404. [DOI] [PubMed] [Google Scholar]
- 32.Hankenson KD; Gagne K; Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev 2015. 94, 3–12. [DOI] [PubMed] [Google Scholar]
- 33.Wang EA; Rosen V; Cordes P; Hewick RM; Kriz MJ; Luxenberg DP; Sibley BS; Wozney JM. Purification and characterization of other distinct bone-inducing factors. Proc. Natl. Acad. Sci. USA 1988. 85(24), 9484–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wozney JM; Rosen V; Celeste AJ; Mitsock LM; Whitters MJ; Kriz RW; Hewick RM; Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988. 242(4885), 1528–34. [DOI] [PubMed] [Google Scholar]
- 35.Chen D; Zhao M; Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004. 22(4), 233–41. [DOI] [PubMed] [Google Scholar]
- 36.Augat P; Faschingbauer M; Seide K; Tobita K; Callary SA; Solomon LB; Holstein JH. Biomechanical methods for the assessment of fracture repair. Injury. 2014. 45 Suppl 2, S32–8. [DOI] [PubMed] [Google Scholar]
- 37.Bishop JA; Palanca AA; Bellino MJ; Lowenberg DW. Assessment of compromised fracture healing. J. Am. Acad. Orthop. Surg 2012. 20(5), 273–82. [DOI] [PubMed] [Google Scholar]
- 38.Lindholm TS; Urist MR. A quantitative analysis of new bone formation by induction in compositive grafts of bone marrow and bone matrix. Clin. Orthop 1980. (150), 288–300. [PubMed] [Google Scholar]
- 39.Urist MR. Bone: formation by autoinduction. Science. 1965. 150(698), 893–9. [DOI] [PubMed] [Google Scholar]
- 40.Urist MR; Silverman BF; Buring K; Dubuc FL; Rosenberg JM. The bone induction principle. Clin. Orthop 1967. 53, 243–83. [PubMed] [Google Scholar]
- 41.Grgurevic L; Pecina M; Vukicevic S. Marshall R. Urist and the discovery of bone morphogenetic proteins. Int. Orthop 2017. 41(5), 1065–1069. [DOI] [PubMed] [Google Scholar]
- 42.Urist MR; Strates BS. Bone morphogenetic protein. J. Dent. Res 1971. 50(6), 1392–406. [DOI] [PubMed] [Google Scholar]
- 43.Martinovic S; Mazic S; Kisic V; Basic N; Jakic-Razumovic J; Borovecki F; Batinic D; Simic P; Grgurevic L; Labar B; Vukicevic S. Expression of bone morphogenetic proteins in stromal cells from human bone marrow long-term culture. J. Histochem. Cytochem 2004. 52(9), 1159–67. [DOI] [PubMed] [Google Scholar]
- 44.Even J; Eskander M; Kang J. Bone morphogenetic protein in spine surgery: current and future uses. J. Am. Acad. Orthop. Surg 2012. 20(9), 547–52. [DOI] [PubMed] [Google Scholar]
- 45.Johnson EE; Urist MR; Finerman GA. Bone morphogenetic protein augmentation grafting of resistant femoral nonunions. A preliminary report. Clin. Orthop 1988. (230), 257–65. [PubMed] [Google Scholar]
- 46.Dumic-Cule I; Brkljacic J; Rogic D; Bordukalo Niksic T; Tikvica Luetic A; Draca N; Kufner V; Trkulja V; Grgurevic L; Vukicevic S. Systemically available bone morphogenetic protein two and seven affect bone metabolism. Int. Orthop 2014. 38(9), 1979–85. [DOI] [PubMed] [Google Scholar]
- 47.Flouzat-Lachaniette CH; Ghazanfari A; Bouthors C; Poignard A; Hernigou P; Allain J. Bone union rate with recombinant human bone morphogenic protein-2 versus autologous iliac bone in PEEK cages for anterior lumbar interbody fusion. Int. Orthop 2014. 38(9), 2001–7. [DOI] [PubMed] [Google Scholar]
- 48.Hinsenkamp M; Collard JF. Growth factors in orthopaedic surgery: demineralized bone matrix versus recombinant bone morphogenetic proteins. Int. Orthop 2015. 39(1), 137–47. [DOI] [PubMed] [Google Scholar]
- 49.Cheng H; Jiang W; Phillips FM; Haydon RC; Peng Y; Zhou L; Luu HH; An N; Breyer B; Vanichakarn P; Szatkowski JP; Park JY; He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Joint Surg. Am 2003. 85-A(8), 1544–52. [DOI] [PubMed] [Google Scholar]
- 50.Cook SD; Barrack RL; Shimmin A; Morgan D; Carvajal JP. The use of osteogenic protein-1 in reconstructive surgery of the hip. J. Arthroplasty 2001. 16(8 Suppl 1), 88–94. [DOI] [PubMed] [Google Scholar]
- 51.Helm GA; Alden TD; Beres EJ; Hudson SB; Das S; Engh JA; Pittman DD; Kerns KM; Kallmes DF. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J. Neurosurg 2000. 92(2 Suppl), 191–6. [DOI] [PubMed] [Google Scholar]
- 52.Hidaka C; Goshi K; Rawlins B; Boachie-Adjei O; Crystal RG. Enhancement of spine fusion using combined gene therapy and tissue engineering BMP-7-expressing bone marrow cells and allograft bone. Spine. 2003. 28(18), 2049–57. [DOI] [PubMed] [Google Scholar]
- 53.Johnsson R; Stromqvist B; Aspenberg P. Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in clinical studies. Spine. 2002. 27(23), 2654–61. [DOI] [PubMed] [Google Scholar]
- 54.Magin MN; Delling G. Improved lumbar vertebral interbody fusion using rhOP-1: a comparison of autogenous bone graft, bovine hydroxylapatite (Bio-Oss), and BMP-7 (rhOP-1) in sheep. Spine. 2001. 26(5), 469–78. [DOI] [PubMed] [Google Scholar]
- 55.Salamon ML; Althausen PL; Gupta MC; Laubach J. The effects of BMP-7 in a rat posterolateral intertransverse process fusion model. J. Spinal Disord. Tech 2003. 16(1), 90–5. [DOI] [PubMed] [Google Scholar]
- 56.Vaccaro AR; Patel T; Fischgrund J; Anderson DG; Truumees E; Herkowitz H; Phillips F; Hilibrand A; Albert TJ. A pilot safety and efficacy study of OP-1 putty (rhBMP-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur. Spine J 2003. 12(5), 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gothard D; Smith EL; Kanczler JM; Rashidi H; Qutachi O; Henstock J; Rotherham M; El Haj A; Shakesheff KM; Oreffo RO. Tissue engineered bone using select growth factors: A comprehensive review of animal studies and clinical translation studies in man. Eur. Cell Mater 2014. 28, 166–207; discussion 207–8. [DOI] [PubMed] [Google Scholar]
- 58.Chung EJ; Chien KB; Aguado BA; Shah RN. Osteogenic potential of BMP-2-releasing self-assembled membranes. Tissue Eng. Part A 2013. 19(23–24), 2664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaito T; Morimoto T; Mori Y; Kanayama S; Makino T; Takenaka S; Sakai Y; Otsuru S; Yoshioka Y; Yoshikawa H. BMP-2/7 heterodimer strongly induces bone regeneration in the absence of increased soft tissue inflammation. Spine J. 2018. 18(1), 139–146. [DOI] [PubMed] [Google Scholar]
- 60.Kimura Y; Miyazaki N; Hayashi N; Otsuru S; Tamai K; Kaneda Y; Tabata Y. Controlled release of bone morphogenetic protein-2 enhances recruitment of osteogenic progenitor cells for de novo generation of bone tissue. Tissue Eng. Part A 2010. 16(4), 1263–70. [DOI] [PubMed] [Google Scholar]
- 61.Lee JH; Kim CS; Choi KH; Jung UW; Yun JH; Choi SH; Cho KS. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials. 2010. 31(13), 3512–9. [DOI] [PubMed] [Google Scholar]
- 62.Luca L; Rougemont AL; Walpoth BH; Boure L; Tami A; Anderson JM; Jordan O; Gurny R. Injectable rhBMP-2-loaded chitosan hydrogel composite: osteoinduction at ectopic site and in segmental long bone defect. J. Biomed. Mater. Res. A 2011. 96(1), 66–74. [DOI] [PubMed] [Google Scholar]
- 63.Luca L; Rougemont AL; Walpoth BH; Gurny R; Jordan O. The effects of carrier nature and pH on rhBMP-2-induced ectopic bone formation. J. Control. Release 2010. 147(1), 38–44. [DOI] [PubMed] [Google Scholar]
- 64.Zhao B; Katagiri T; Toyoda H; Takada T; Yanai T; Fukuda T; Chung UI; Koike T; Takaoka K; Kamijo R. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J. Biol. Chem 2006. 281(32), 23246–53. [DOI] [PubMed] [Google Scholar]
- 65.Akamaru T; Suh D; Boden SD; Kim HS; Minamide A; Louis-Ugbo J. Simple carrier matrix modifications can enhance delivery of recombinant human bone morphogenetic protein-2 for posterolateral spine fusion. Spine. 2003. 28(5), 429–34. [DOI] [PubMed] [Google Scholar]
- 66.Alden TD; Pittman DD; Beres EJ; Hankins GR; Kallmes DF; Wisotsky BM; Kerns KM; Helm GA. Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J. Neurosurg 1999. 90(1 Suppl), 109–14. [DOI] [PubMed] [Google Scholar]
- 67.Bae HW; Zhao L; Kanim LE; Wong P; Marshall D; Delamarter RB. Bone marrow enhances the performance of rhBMP-2 in spinal fusion: a rodent model. J. Bone Joint Surg. Am 2013. 95(4), 338–47. [DOI] [PubMed] [Google Scholar]
- 68.Cheng SL; Lou J; Wright NM; Lai CF; Avioli LV; Riew KD. In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif. Tissue Int 2001. 68(2), 87–94. [PubMed] [Google Scholar]
- 69.Dohzono S; Imai Y; Nakamura H; Wakitani S; Takaoka K. Successful spinal fusion by E. coli-derived BMP-2-adsorbed porous beta-TCP granules: a pilot study. Clin. Orthop. Relat. Res 2009. 467(12), 3206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu TS; Chang YH; Wong CB; Wang IC; Tsai TT; Lai PL; Chen LH; Chen WJ. Mesenchymal stem cells expressing baculovirus-engineered BMP-2 and VEGF enhance posterolateral spine fusion in a rabbit model. Spine J. 2015. 15(9), 2036–44. [DOI] [PubMed] [Google Scholar]
- 71.Fu TS; Chen WJ; Chen LH; Lin SS; Liu SJ; Ueng SW. Enhancement of posterolateral lumbar spine fusion using low-dose rhBMP-2 and cultured marrow stromal cells. J. Orthop. Res 2009. 27(3), 380–4. [DOI] [PubMed] [Google Scholar]
- 72.Sidhu KS; Prochnow TD; Schmitt P; Fischgrund J; Weisbrode S; Herkowitz HN. Anterior cervical interbody fusion with rhBMP-2 and tantalum in a goat model. Spine J. 2001. 1(5), 331–40. [DOI] [PubMed] [Google Scholar]
- 73.Kandziora F; Pflugmacher R; Scholz M; Knispel C; Hiller T; Schollmeier G; Bail H; Schmidmaier G; Duda G; Raschke M; Haas NP. Comparison of BMP-2 and combined IGF-I/TGF-ss1 application in a sheep cervical spine fusion model. Eur. Spine J 2002. 11(5), 482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kandziora F; Scholz M; Pflugmacher R; Krummrey G; Schollmeier G; Schmidmaier G; Schnake KJ; Duda G; Raschke M; Haas NP. [Experimental fusion of the sheep cervical spine. Part II: Effect of growth factors and carrier systems on interbody fusion]. Chirurg. 2002. 73(10), 1025–38. [DOI] [PubMed] [Google Scholar]
- 75.Sandhu HS; Toth JM; Diwan AD; Seim HB 3rd; Kanim LE; Kabo JM; Turner AS. Histologic evaluation of the efficacy of rhBMP-2 compared with autograft bone in sheep spinal anterior interbody fusion. Spine. 2002. 27(6), 567–75. [DOI] [PubMed] [Google Scholar]
- 76.Baltzer AW; Lattermann C; Whalen JD; Wooley P; Weiss K; Grimm M; Ghivizzani SC; Robbins PD; Evans CH. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000. 7(9), 734–9. [DOI] [PubMed] [Google Scholar]
- 77.Boerckel JD; Kolambkar YM; Stevens HY; Lin AS; Dupont KM; Guldberg RE. Effects of in vivo mechanical loading on large bone defect regeneration. J. Orthop. Res 2012. 30(7), 1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lieberman JR; Daluiski A; Stevenson S; Wu L; McAllister P; Lee YP; Kabo JM; Finerman GA; Berk AJ; Witte ON. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J. Bone Joint Surg. Am 1999. 81(7), 905–17. [DOI] [PubMed] [Google Scholar]
- 79.Lieberman JR; Le LQ; Wu L; Finerman GA; Berk A; Witte ON; Stevenson S. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998. 16(3), 330–9. [DOI] [PubMed] [Google Scholar]
- 80.Yasko AW; Lane JM; Fellinger EJ; Rosen V; Wozney JM; Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J. Bone Joint Surg. Am 1992. 74(5), 659–70. [PubMed] [Google Scholar]
- 81.Zabka AG; Pluhar GE; Edwards RB 3rd; Manley PA; Hayashi K; Heiner JP; Kalscheur VL; Seeherman HJ; Markel. Histomorphometric description of allograft bone remodeling and union in a canine segmental femoral defect model: a comparison of rhBMP-2, cancellous bone graft, and absorbable collagen sponge. J. Orthop. Res 2001. 19(2), 318–27. [DOI] [PubMed] [Google Scholar]
- 82.Minier K; Toure A; Fusellier M; Fellah B; Bouvy B; Weiss P; Gauthier O. BMP-2 delivered from a self-crosslinkable CaP/hydrogel construct promotes bone regeneration in a critical-size segmental defect model of non-union in dogs. Vet. Comp. Orthop. Traumatol 2014. 27(6), 411–21. [DOI] [PubMed] [Google Scholar]
- 83.Marukawa E; Asahina I; Oda M; Seto I; Alam M; Enomoto S. Functional reconstruction of the non-human primate mandible using recombinant human bone morphogenetic protein-2. Int. J. Oral Maxillofac. Surg 2002. 31(3), 287–95. [DOI] [PubMed] [Google Scholar]
- 84.Freilich M; C MP; Wei M; Shafer D; Schleier P; Hortschansky P; Kompali R; Kuhn L. Growth of new bone guided by implants in a murine calvarial model. Bone. 2008. 43(4), 781–8. [DOI] [PubMed] [Google Scholar]
- 85.Gohil SV; Wang L; Rowe DW; Nair LS. Spatially controlled rhBMP-2 mediated calvarial bone formation in a transgenic mouse model. Int. J. Biol. Macromol 2018. 106, 1159–1165. [DOI] [PubMed] [Google Scholar]
- 86.Quinlan E; Thompson EM; Matsiko A; O’Brien FJ; Lopez-Noriega A. Long-term controlled delivery of rhBMP-2 from collagen-hydroxyapatite scaffolds for superior bone tissue regeneration. J. Control. Release 2015. 207, 112–9. [DOI] [PubMed] [Google Scholar]
- 87.Hong L; Tabata Y; Yamamoto M; Miyamoto S; Yamada K; Hashimoto N; Ikada Y. Comparison of bone regeneration in a rabbit skull defect by recombinant human BMP-2 incorporated in biodegradable hydrogel and in solution. J. Biomater. Sci. Polym. Ed 1998. 9(9), 1001–14. [DOI] [PubMed] [Google Scholar]
- 88.Deng L; Li D; Yang Z; Xie X; Kang P. Repair of the calvarial defect in goat model using magnesium-doped porous hydroxyapatite combined with recombinant human bone morphogenetic protein-2. Biomed. Mater. Eng 2017. 28(4), 361–377. [DOI] [PubMed] [Google Scholar]
- 89.Song SY; Yun IS; Kim CH; Woo DG; Kim YO. Transport distraction osteogenesis with recombinant human bone morphogenic protein-2 for large calvarial defect reconstruction. J. Craniofac. Surg 2014. 25(2), 502–8. [DOI] [PubMed] [Google Scholar]
- 90.Kinsella CR Jr.; Bykowski MR; Lin AY; Cray JJ; Durham EL; Smith DM; DeCesare GE; Mooney MP; Cooper GM; Losee JE. BMP-2-mediated regeneration of large-scale cranial defects in the canine: an examination of different carriers. Plast. Reconstr. Surg 2011. 127(5), 1865–73. [DOI] [PubMed] [Google Scholar]
- 91.Govender S; Csimma C; Genant HK; Valentin-Opran A; Amit Y; Arbel R; Aro H; Atar D; Bishay M; Borner MG; Chiron P; Choong P; Cinats J; Courtenay B; Feibel R; Geulette B; Gravel C; Haas N; Raschke M; Hammacher E; van der Velde D; Hardy P; Holt M; Josten C; Ketterl RL; Lindeque B; Lob G; Mathevon H; McCoy G; Marsh D; Miller R; Munting E; Oevre S; Nordsletten L; Patel A; Pohl A; Rennie W; Reynders P; Rommens PM; Rondia J; Rossouw WC; Daneel PJ; Ruff S; Ruter A; Santavirta S; Schildhauer TA; Gekle C; Schnettler R; Segal D; Seiler H; Snowdowne RB; Stapert J; Taglang G; Verdonk R; Vogels L; Weckbach A; Wentzensen A; Wisniewski T; B. M. P. E. i. S. f. T. T. S. Group. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Joint Surg. Am 2002. 84(12), 2123–34. [DOI] [PubMed] [Google Scholar]
- 92.Hagen A; Gorenoi V; Schonermark MP. Bone graft substitutes for the treatment of traumatic fractures of the extremities. GMS Health Technol. Assess 2012. 8, Doc04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaiser MG; Groff MW; Watters WC 3rd; Ghogawala Z; Mummaneni PV; Dailey AT; Choudhri TF; Eck JC; Sharan A; Wang JC; Dhall SS; Resnick DK. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: bone graft extenders and substitutes as an adjunct for lumbar fusion. J. Neurosurg. Spine 2014. 21(1), 106–32. [DOI] [PubMed] [Google Scholar]
- 94.Boden SD; Kang J; Sandhu H; Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002. 27(23), 2662–73. [DOI] [PubMed] [Google Scholar]
- 95.Boden SD; Martin GJ Jr.; Horton WC; Truss TL; Sandhu HS. Laparoscopic anterior spinal arthrodesis with rhBMP-2 in a titanium interbody threaded cage. J. Spinal Disord 1998. 11(2), 95–101. [PubMed] [Google Scholar]
- 96.Boden SD; Zdeblick TA; Sandhu HS; Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000. 25(3), 376–81. [DOI] [PubMed] [Google Scholar]
- 97.Fu R; Selph S; McDonagh M; Peterson K; Tiwari A; Chou R; Helfand M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann. Intern. Med 2013. 158(12), 890–902. [DOI] [PubMed] [Google Scholar]
- 98.Hoffmann MF; Jones CB; Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch. Orthop. Trauma Surg 2012. 132(8), 1105–10. [DOI] [PubMed] [Google Scholar]
- 99.Kraiwattanapong C; Boden SD; Louis-Ugbo J; Attallah E; Barnes B; Hutton WC. Comparison of Healos/bone marrow to INFUSE(rhBMP-2/ACS) with a collagen-ceramic sponge bulking agent as graft substitutes for lumbar spine fusion. Spine (Phila Pa 1976). 2005. 30(9), 1001–7; discussion 1007. [DOI] [PubMed] [Google Scholar]
- 100.Mulconrey DS; Bridwell KH; Flynn J; Cronen GA; Rose PS. Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine. 2008. 33(20), 2153–9. [DOI] [PubMed] [Google Scholar]
- 101.Aro HT; Govender S; Patel AD; Hernigou P; Perera de Gregorio A; Popescu GI; Golden JD; Christensen J; Valentin A. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J. Bone Joint Surg. Am 2011. 93(9), 801–8. [DOI] [PubMed] [Google Scholar]
- 102.Lyon T; Scheele W; Bhandari M; Koval KJ; Sanchez EG; Christensen J; Valentin A; Huard F. Efficacy and safety of recombinant human bone morphogenetic protein-2/calcium phosphate matrix for closed tibial diaphyseal fracture: a double-blind, randomized, controlled phase-II/III trial. J. Bone Joint Surg. Am 2013. 95(23), 2088–96. [DOI] [PubMed] [Google Scholar]
- 103.Garrison KR; Shemilt I; Donell S; Ryder JJ; Mugford M; Harvey I; Song F; Alt V. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst. Rev 2010. (6), CD006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Epstein NE. Basic science and spine literature document bone morphogenetic protein increases cancer risk. Surg. Neurol. Int 2014. 5(Suppl 15), S552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simmonds MC; Brown JV; Heirs MK; Higgins JP; Mannion RJ; Rodgers MA; Stewart LA. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann. Intern. Med 2013. 158(12), 877–89. [DOI] [PubMed] [Google Scholar]
- 106.Sampath TK; Maliakal JC; Hauschka PV; Jones WK; Sasak H; Tucker RF; White KH; Coughlin JE; Tucker MM; Pang RH; et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J. Biol. Chem 1992. 267(28), 20352–62. [PubMed] [Google Scholar]
- 107.Cook SD; Baffes GC; Wolfe MW; Sampath TK; Rueger DC. Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clin. Orthop 1994. (301), 302–12. [PubMed] [Google Scholar]
- 108.Cook SD; Baffes GC; Wolfe MW; Sampath TK; Rueger DC; Whitecloud TS 3rd. The effect of recombinant human osteogenic protein-1 on healing of large segmental bone defects. J. Bone Joint Surg. Am 1994. 76(6), 827–38. [DOI] [PubMed] [Google Scholar]
- 109.Cook SD; Wolfe MW; Salkeld SL; Rueger DC. Effect of recombinant human osteogenic protein-1 on healing of segmental defects in non-human primates. J. Bone Joint Surg. Am 1995. 77(5), 734–50. [DOI] [PubMed] [Google Scholar]
- 110.Cook SD; Salkeld SL; Patron LP; Sargent MC; Rueger DC. Healing course of primate ulna segmental defects treated with osteogenic protein-1. J. Invest. Surg 2002. 15(2), 69–79. [DOI] [PubMed] [Google Scholar]
- 111.Ripamonti U; Crooks J; Rueger DC. Induction of bone formation by recombinant human osteogenic protein-1 and sintered porous hydroxyapatite in adult primates. Plast. Reconstr. Surg 2001. 107(4), 977–88. [DOI] [PubMed] [Google Scholar]
- 112.Ripamonti U; Ramoshebi LN; Matsaba T; Tasker J; Crooks J; Teare J. Bone induction by BMPs/OPs and related family members in primates. J. Bone Joint. Surg. Am 2001. 83-A Suppl 1(Pt 2), S116–27. [PubMed] [Google Scholar]
- 113.Fukuroku J; Inoue N; Rafiee B; Sim FH; Frassica FJ; Chao EY. Extracortical bone-bridging fixation with use of cortical allograft and recombinant human osteogenic protein-1. J. Bone Joint Surg. Am 2007. 89(7), 1486–96. [DOI] [PubMed] [Google Scholar]
- 114.Blokhuis TJ; den Boer FC; Bramer JA; Jenner JM; Bakker FC; Patka P; Haarman HJ. Biomechanical and histological aspects of fracture healing, stimulated with osteogenic protein-1. Biomaterials. 2001. 22(7), 725–30. [DOI] [PubMed] [Google Scholar]
- 115.den Boer FC; Bramer JA; Blokhuis TJ; Van Soest EJ; Jenner JM; Patka P; Bakker FC; Burger EH; Haarman HJ. Effect of recombinant human osteogenic protein-1 on the healing of a freshly closed diaphyseal fracture. Bone. 2002. 31(1), 158–64. [DOI] [PubMed] [Google Scholar]
- 116.den Boer FC; Wippermann BW; Blokhuis TJ; Patka P; Bakker FC; Haarman HJ. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-1 or autologous bone marrow. J. Orthop. Res 2003. 21(3), 521–8. [DOI] [PubMed] [Google Scholar]
- 117.Friedlaender GE; Perry CR; Cole JD; Cook SD; Cierny G; Muschler GF; Zych GA; Calhoun JH; LaForte AJ; Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J. Bone Joint Surg. Am 2001. 83-A Suppl 1(Pt 2), S151–8. [PMC free article] [PubMed] [Google Scholar]
- 118.Lietman SA; Inoue N; Rafiee B; Deitz LW; Chao EY. The effect of recombinant human osteogenic protein-1 on allograft incorporation. J Bone Joint Surg Br. 2005. 87(9), 1292–7. [DOI] [PubMed] [Google Scholar]
- 119.Caterini R; Potenza V; Ippolito E; Farsetti P. Treatment of recalcitrant atrophic non-union of the humeral shaft with BMP-7, autologous bone graft and hydroxyapatite pellets. Injury. 2016. 47 Suppl 4, S71–S77. [DOI] [PubMed] [Google Scholar]
- 120.Ekrol I; Hajducka C; Court-Brown C; McQueen MM. A comparison of RhBMP-7 (OP-1) and autogenous graft for metaphyseal defects after osteotomy of the distal radius. Injury. 2008. 39 Suppl 2, S73–82. [DOI] [PubMed] [Google Scholar]
- 121.Laursen M; Hoy K; Hansen ES; Gelineck J; Christensen FB; Bunger CE. Recombinant bone morphogenetic protein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: preliminary results. Eur. Spine J 1999. 8(6), 485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kugimiya F; Kawaguchi H; Kamekura S; Chikuda H; Ohba S; Yano F; Ogata N; Katagiri T; Harada Y; Azuma Y; Nakamura K; Chung UI. Involvement of endogenous bone morphogenetic protein (BMP) 2 and BMP6 in bone formation. J. Biol. Chem 2005. 280(42), 35704–12. [DOI] [PubMed] [Google Scholar]
- 123.Sheyn D; Pelled G; Zilberman Y; Talasazan F; Frank JM; Gazit D; Gazit Z. Nonvirally engineered porcine adipose tissue-derived stem cells: use in posterior spinal fusion. Stem Cells. 2008. 26(4), 1056–64. [DOI] [PubMed] [Google Scholar]
- 124.Valdes M; Moore DC; Palumbo M; Lucas PR; Robertson A; Appel J; Ehrlich MG; Keeping HS. rhBMP-6 stimulated osteoprogenitor cells enhance posterolateral spinal fusion in the New Zealand white rabbit. Spine J. 2007. 7(3), 318–25. [DOI] [PubMed] [Google Scholar]
- 125.Vukicevic S; Oppermann H; Verbanac D; Jankolija M; Popek I; Curak J; Brkljacic J; Pauk M; Erjavec I; Francetic I; Dumic-Cule I; Jelic M; Durdevic D; Vlahovic T; Novak R; Kufner V; Bordukalo Niksic T; Kozlovic M; Banic Tomisic Z; Bubic-Spoljar J; Bastalic I; Vikic-Topic S; Peric M; Pecina M; Grgurevic L. The clinical use of bone morphogenetic proteins revisited: a novel biocompatible carrier device OSTEOGROW for bone healing. Int. Orthop 2014. 38(3), 635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vukicevic S; Grgurevic L; Erjavec I; Pecin M; Bordukalo-Niksic T; Stokovic N; Lipar M; Capak H; Maticic D; Windhager R; Sampath TK; Gupta M. Autologous blood coagulum is a physiological carrier for BMP6 to induce new bone formation and promote posterolateral lumbar spine fusion in rabbits. J. Tissue Eng. Regen. Med 2020. 14(1), 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taipaleenmäki H; Suomi S; Hentunen T; Laitala-Leinonen T; Säämänen AM. Impact of stromal cell composition on BMP-induced chondrogenic differentiation of mouse bone marrow derived mesenchymal cells. Exp. Cell Res 2008. 314(13), 2400–10. [DOI] [PubMed] [Google Scholar]
- 128.Mizrahi O; Sheyn D; Tawackoli W; Kallai I; Oh A; Su S; Da X; Zarrini P; Cook-Wiens G; Gazit D; Gazit Z. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther. 2013. 20(4), 370–7. [DOI] [PubMed] [Google Scholar]
- 129.Rico-Llanos GA; Becerra J; Visser R. Insulin-like growth factor-1 (IGF-1) enhances the osteogenic activity of bone morphogenetic protein-6 (BMP-6) in vitro and in vivo, and together have a stronger osteogenic effect than when IGF-1 is combined with BMP-2. J. Biomed. Mater. Res. A 2017. 105(7), 1867–1875. [DOI] [PubMed] [Google Scholar]
- 130.Gümüşderelioğlu M; Sunal E; Tolga Demirtaş T; Kiremitçi AS. Chitosan-based double-faced barrier membrane coated with functional nanostructures and loaded with BMP-6. J. Mater. Sci. Mater. Med 2019. 31(1), 4. [DOI] [PubMed] [Google Scholar]
- 131.Li X; Zhang R; Tan X; Li B; Liu Y; Wang X. Synthesis and Evaluation of BMMSC-seeded BMP-6/nHAG/GMS Scaffolds for Bone Regeneration. Int. J. Med. Sci 2019. 16(7), 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grgurevic L; Oppermann H; Pecin M; Erjavec I; Capak H; Pauk M; Karlovic S; Kufner V; Lipar M; Bubic Spoljar J; Bordukalo-Niksic T; Maticic D; Peric M; Windhager R; Sampath TK; Vukicevic S. Recombinant Human Bone Morphogenetic Protein 6 Delivered Within Autologous Blood Coagulum Restores Critical Size Segmental Defects of Ulna in Rabbits. JBMR Plus. 2019. 3(5), e10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grgurevic L. Osteogrow Development: Bmp6 Bone Device For Enhancing Bone Healing. Clin. Ther 2016. 38(10S), e9–e10. [DOI] [PubMed] [Google Scholar]
- 134.Rohanizadeh R; Chung K. Hydroxyapatite as a carrier for bone morphogenetic protein. J. Oral Implantol 2011. 37(6), 659–72. [DOI] [PubMed] [Google Scholar]
- 135.Sohier J; Daculsi S Fau - Sourice G; Sourice S Fau - de Groot K; de Groot K Fau - Layrolle P; Layrolle P. Porous beta tricalcium phosphate scaffolds used as a BMP-2 delivery system for bone tissue engineering. J. Biomed. Mater. Res. A 2010. 92(3), 1105–14. [DOI] [PubMed] [Google Scholar]
- 136.Lee KA-O; Lee JS; Jang JW; Shim YB; Lee KI. Tendon-bone interface healing using an injectable rhBMP-2-containing collagen gel in a rabbit extra-articular bone tunnel model. J. Tissue Eng. Regen. Med 2017. 11(5), 1435–41. [DOI] [PubMed] [Google Scholar]
- 137.van der Stok J; Koolen MPM de Maat Mk Fau; de Maat Mp Fau - Yavari SA; Yavari Sa Fau - Alblas J; Alblas J Fau - Patka P; Patka P Fau - Verhaar JAN; Verhaar Ja Fau - van Lieshout EMM; van Lieshout Em Fau - Zadpoor AA; Zadpoor Aa Fau - Weinans H; Weinans H Fau - Jahr H; Jahr H. Full regeneration of segmental bone defects using porous titanium implants loaded with BMP-2 containing fibrin gels. Eur. Cell Mater 2015. 29, 141–53. [DOI] [PubMed] [Google Scholar]
- 138.Kolambkar YM; Dupont JD Fau - Boerckel Km; Boerckel Jd Fau - Huebsch N; Huebsch N Fau - Mooney DJ; Mooney Dj Fau - Hutmacher DW; Hutmacher Dw Fau - Guldberg RE; Guldberg RE. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011. 32(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim HD; Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J. Biomed. Mater. Res 2002. 59(3), 573–84. [DOI] [PubMed] [Google Scholar]
- 140.Wang Q; Wang M; Li P; Wang K; Fang L; Ren F; Lu G; Lu X. The interaction of chitosan and BMP-2 tuned by deacetylation degree and pH value. J. Biomed. Mater. Res. A 2019. 107(4), 769–779. [DOI] [PubMed] [Google Scholar]
- 141.Lutolf MP; Weber HG Schmoekel Fe Fau; Schmoekel Hg Fau - Schense JC; Schense Jc Fau - Kohler T; Kohler T Fau - Muller R; Muller R Fau - Hubbell JA; Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003. 21(5), 513–8. [DOI] [PubMed] [Google Scholar]
- 142.Cheng ZA; Alba-Perez A; Gonzalez-Garcia C; Donnelly H; Llopis-Hernandez V; Jayawarna V; Childs P; Shields DW; Cantini M; Ruiz-Cantu L; Reid A; Windmill JFC; Addison ES; Corr S; Marshall WG; Dalby MJ; Salmeron-Sanchez MA-O. Nanoscale Coatings for Ultralow Dose BMP-2-Driven Regeneration of Critical-Sized Bone Defects. Adv. Sci 2018. 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y Fau - Miao W; Miao Y Fau - Zhou X; Zhou X Fau - Nie W; Nie W Fau - Chen L; Chen L Fau - Liu D; Liu D Fau - Du H; Du H Fau - He C; He C. Local Delivery of BMP-2 from Poly(lactic-co-glycolic acid) Microspheres Incorporated into Porous Nanofibrous Scaffold for Bone Tissue Regeneration. J. Biomed. Nanotechnol 2017. 13(11), 1446–1456. [DOI] [PubMed] [Google Scholar]
- 144.Stupp SI; LeBonheur V; Walker K; Li LS; Huggins KE; Keser M; Amstutz A. Supramolecular Materials: Self-Organized Nanostructures. Science. 1997. 276(5311), 384. [DOI] [PubMed] [Google Scholar]
- 145.Hwang JJ; Iyer SN; Li L-S; Claussen R; Harrington DA; Stupp SI. Self-assembling biomaterials: liquid crystal phases of cholesteryl oligo(L-lactic acid) and their interactions with cells. Proc. Natl. Acad. Sci. USA 2002. 99(15), 9662–9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aida T; Meijer EW; Stupp SI. Functional Supramolecular Polymers. Science. 2012. 335(6070), 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Webber MJ; Appel EA; Meijer EW; Langer R. Supramolecular biomaterials. Nat. Mater 2016. 15(1), 13–26. [DOI] [PubMed] [Google Scholar]
- 148.Cui H; Webber SI Fau – Stupp Mj; Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010. 94(1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yokoi H; Kinoshita S T Fau - Zhang; S. Zhang. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. USA 2005. 102(24), 8414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ikeno H Fau - Hibi M; Hibi H Fau - Kinoshita K; Kinoshita K Fau - Hattori H; Hattori H Fau - Ueda M; Ueda M. Effects of self-assembling peptide hydrogel scaffold on bone regeneration with recombinant human bone morphogenetic protein-2. Int. J. Oral Maxillofac. Implants 2013. 28(5). [DOI] [PubMed] [Google Scholar]
- 151.Phipps MC; Monte F; Mehta M; Kim HK. Intraosseous Delivery of Bone Morphogenic Protein-2 Using a Self-Assembling Peptide Hydrogel. Biomacromolecules. 2016. 17(7), 2329–36. [DOI] [PubMed] [Google Scholar]
- 152.Horii A; Wang X; Gelain F; Zhang S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PloS One. 2007. 2(2), e190–e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nagai Y; Yokoi H; Kaihara K; Naruse K. The mechanical stimulation of cells in 3D culture within a self-assembling peptide hydrogel. Biomaterials. 2012. 33(4), 1044–1051. [DOI] [PubMed] [Google Scholar]
- 154.Ando K; Imagama SA-O; Kobayashi K; Ito K; Tsushima M; Morozumi M; Tanaka S; Machino M; Ota K; Nishida K; Nishida Y; Ishiguro N. Effects of a self-assembling peptide as a scaffold on bone formation in a defect. PLoS One. 2018. 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tsukamoto J; Naruse K; Nagai Y; Kan S; Nakamura N; Hata M; Omi M; Hayashi T; Kawai T; Matsubara T. Efficacy of a Self-Assembling Peptide Hydrogel, SPG-178-Gel, for Bone Regeneration and Three-Dimensional Osteogenic Induction of Dental Pulp Stem Cells. Tissue Eng. Part A 2017. 23(23–24), 1394–1402. [DOI] [PubMed] [Google Scholar]
- 156.Bessa PC; Machado S Fau - Nurnberger R; Nurnberger S Fau - Dopler D; Dopler D Fau - Banerjee A; Banerjee A Fau - Cunha AM; Cunha Am Fau - Rodriguez-Cabello JC; Rodriguez-Cabello Jc Fau - Redl H; Redl H Fau - van Griensven M; van Griensven M Fau - Reis RL; Reis Rl Fau - Casal M; Casal M. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J. Control. Release 2010. 142(3), 312–8. [DOI] [PubMed] [Google Scholar]
- 157.Hartgerink JD; Beniash E Fau - Stupp SI; Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001. 294(5547), 1684–8. [DOI] [PubMed] [Google Scholar]
- 158.Hartgerink JD; Beniash E Fau – Stupp SI; Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. USA 2002. 99(8), 5133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hendricks MP; Sato K; Palmer LC; Stupp SI. Supramolecular Assembly of Peptide Amphiphiles. Acc. Chem. Res 2017. 50(10), 2440–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tantakitti F; Boekhoven J; Wang X; Kazantsev RV; Yu T; Li J; Zhuang E; Zandi R; Ortony JH; Newcomb CJ; Palmer LC; Shekhawat GS; Olvera M Cruz de la; Schatz GC; Stupp SI. Energy landscapes and functions of supramolecular systems. Nat. Mater 2016. 15(4), 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ortony JH; Newcomb CJ; Matson JB; Palmer LC; Doan PE; Hoffman BM; Stupp SI. Internal dynamics of a supramolecular nanofibre. Nat. Mater 2014. 13(8), 812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mata A; Geng Y; Henrikson KJ; Aparicio C; Stock SR; Satcher RL; Stupp SI. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials. 2010. 31(23), 6004–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee SS; Huang BJ; Kaltz SR; Sur S; Newcomb CJ; Stock SR; Shah RN; Stupp SI. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials. 2013. 34(2), 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee SS; Hsu EL; Mendoza M; Ghodasra J; Nickoli MS; Ashtekar A; Polavarapu M; Babu J; Riaz RM; Nicolas JD. Gel scaffolds of BMP‐2‐binding peptide amphiphile nanofibers for spinal arthrodesis. Adv. Healthc. Mater 2015. 4(1), 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee SS; Fyrner T; Chen F; Álvarez Z; Sleep E; Chun DS; Weiner JA; Cook RW; Freshman RD; Schallmo MS. Sulfated glycopeptide nanostructures for multipotent protein activation. Nat. Nanotechnol 2017. 12(8), 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shah RN; Shah NA; Lim MMDR; Hsieh C; Nuber G; Stupp SI. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc. Natl. Acad. Sci. USA 2010. 107(8), 3293–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sleep E; Cosgrove BD; McClendon MT; Preslar AT; Chen CH; Sangji MH; Pérez CMR; Haynes RD; Meade TJ; Blau HM. Injectable biomimetic liquid crystalline scaffolds enhance muscle stem cell transplantation. Proc. Natl. Acad. Sci. USA 2017. 114(38), E7919–E7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Webber MJ; Tongers J; Newcomb CJ; Marquardt K-T; Bauersachs J; Losordo DW; Stupp SI. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair (vol 108, pg 13438, 2011). Proc. Natl. Acad. Sci. USA 2012. 109(23), 9220–9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Silva GA; Czeisler C; Niece KL; Beniash E; Harrington DA; Kessler JA; Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004. 303(5662), 1352–1355. [DOI] [PubMed] [Google Scholar]
- 170.Rajangam K; Arnold MS; Rocco MA; Stupp SI. Peptide amphiphile nanostructure-heparin interactions and their relationship to bioactivity. Biomaterials. 2008. 29(23), 3298–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sarrazin S; Lamanna WC; Esko JD. Heparan sulfate proteoglycans. Csh. Perspect. Biol 2011. 3(7), a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bramono DS; Murali S; Rai B; Ling L; Poh WT; Lim ZX; Stein GS; Nurcombe V; van Wijnen AJ; Cool SM. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2). Bone. 2012. 50(4), 954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sur S; Tantakitti F; Matson JB; Stupp SI. Epitope topography controls bioactivity in supramolecular nanofibers. Biomater. Sci 2015. 3(3), 520–532. [DOI] [PubMed] [Google Scholar]
- 174.Edelbrock AN; Àlvarez Z; Simkin D; Fyrner T; Chin SM; Sato K; Kiskinis E; Stupp SI. Supramolecular Nanostructure Activates TrkB Receptor Signaling of Neuronal Cells by Mimicking Brain-Derived Neurotrophic Factor. Nano Lett. 2018. 18(10), 6237–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.da Silva RMP; van der Zwaag D; Albertazzi L; Lee SS; Meijer EW; Stupp SI. Super-resolution microscopy reveals structural diversity in molecular exchange among peptide amphiphile nanofibres. Nat. Commun 2016. 7(1), 11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Freeman R; Han M; Álvarez Z; Lewis JA; Wester JR; Stephanopoulos N; McClendon MT; Lynsky C; Godbe JM; Sangji H; Luijten E; Stupp SI. Reversible self-assembly of superstructured networks. Science. 2018. 362(6416), 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ori A; Wilkinson MC; Fernig DG. A systems biology approach for the investigation of the heparin/heparan sulfate interactome. J. Biol. Chem 2011. 286(22), 19892–19904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Behanna HA; Donners JJJM; Gordon AC; Stupp SI. Coassembly of Amphiphiles with Opposite Peptide Polarities into Nanofibers. J. Am. Chem. Soc 2005. 127(4), 1193–1200. [DOI] [PubMed] [Google Scholar]
- 179.Sacchetti B; Funari A; Michienzi S; Di Cesare S; Piersanti S; Saggio I; Tagliafico E; Ferrari S; Robey PG; Riminucci M; Bianco P. Self-Renewing Osteoprogenitors in Bone Marrow Sinusoids Can Organize a Hematopoietic Microenvironment. Cell. 2007. 131(2), 324–336. [DOI] [PubMed] [Google Scholar]
- 180.Buza JA 3rd; Einhorn T. Bone healing in 2016. Clin Cases Miner Bone Metab. 2016. 13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen CH; Palmer LC; Stupp SI. Self-repair of structure and bioactivity in a supramolecular nanostructure. Nano Lett. 2018. 18(11), 6832–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang S; Greenfield MA; Mata A; Palmer LC; Bitton R; Mantei JR; Aparicio C; Olvera M Cruz de la; Stupp SI. A self-assembly pathway to aligned monodomain gels. Nat. Mater 2010. 9(7), 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Webber MJ; Tongers M-A Renault J Fau; Renault Ma Fau - Roncalli JG; Roncalli Jg Fau - Losordo DW; Losordo Dw Fau - Stupp SI; Stupp SI. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater. 2010. 6(1), 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sargeant TD; Aparicio C; Goldberger JE; Cui H; Stupp SI. Mineralization of peptide amphiphile nanofibers and its effect on the differentiation of human mesenchymal stem cells. Acta Biomater. 2012. 8(7), 2456–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Newcomb CJ; Bitton R; Velichko YS; Snead ML; Stupp SI. Biomimetic Mineralization: The Role of Nanoscale Architecture in Supramolecular Templating of Biomimetic Hydroxyapatite Mineralization (Small 14/2012). Small. 2012. 8(14), 2194–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Newcomb CJ; Sur S; Lee SS; Yu JM; Zhou Y; Snead ML; Stupp SI. Supramolecular Nanofibers Enhance Growth Factor Signaling by Increasing Lipid Raft Mobility. Nano Lett. 2016. 16(5), 3042–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rubert Pérez CM; Álvarez Z; Chen F; Aytun T; Stupp SI. Mimicking the Bioactivity of Fibroblast Growth Factor-2 Using Supramolecular Nanoribbons. ACS Biomater-Sci. Eng 2017. 3(9), 2166–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oumrani S; Barret M; Bordaçahar B; Beuvon F; Hochart G; Pagnon-Minot A; Coriat R; Batteux F; Prat F. Application of a self-assembling peptide matrix prevents esophageal stricture after circumferential endoscopic submucosal dissection in a pig model. PloS One. 2019. 14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Unpublished Work.
- 190.Cormier AR; Pang X; Zimmerman MI; Zhou H-X; Paravastu AK. Molecular Structure of RADA16-I Designer Self-Assembling Peptide Nanofibers. ACS Nano. 2013. 7(9), 7562–7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ho D; Fitzgerald M; Bartlett CA; Zdyrko B; Luzinov IA; Dunlop SA; Swaminathan Iyer K. The effects of concentration-dependent morphology of self-assembling RADA16 nanoscaffolds on mixed retinal cultures. Nanoscale. 2011. 3(3), 907–910. [DOI] [PubMed] [Google Scholar]


