Abstract
BACKGROUND:
Myopathy affects nearly half of individuals with alcohol use disorder (AUD), and impaired skeletal muscle regenerative potential is a probable contributing factor. Previous findings from our laboratory indicate that chronic in vivo and in vitro ethanol treatment decrease myogenic potential of skeletal muscle myoblasts. Myogenesis, a highly coordinated process, requires shifts in cellular metabolic state allowing for myoblasts to proliferate and differentiate into mature myotubes. The objective of this study was to determine whether alcohol interferes with myoblast mitochondrial and glycolytic metabolism and impairs myogenic differentiation.
METHODS:
Myoblasts were isolated from vastus lateralis muscle excised from alcohol-naïve adult male (n=5) and female (n=5) rhesus macaques. Myoblasts were proliferated for 3 days (day 0 differentiation; D0) and differentiated for 5 days (D5) with or without 50 mM ethanol. Metabolism was assessed using a Mitochondrial Stress Test to measure oxygen consumption (OCR) and extracellular acidification (ECAR) rates at D0. Differentiation was examined at D5. Expression of mitochondrial and glycolytic genes and mitochondrial DNA (mtDNA) were measured at D0 and D5.
RESULTS:
Ethanol significantly (p<0.05) increased myoblast maximal OCR and decreased ECAR at D0, and decreased fusion index, myotubes per field, and total nuclei at D5. The ethanol-induced decrease in ECAR was associated with the ethanol-mediated decreases in fusion index and myotubes per field. Ethanol did not alter the decrease in glycolytic gene expression and increase in mtDNA from D0 to D5.
CONCLUSION:
During myoblast proliferation, ethanol decreased glycolytic metabolism and increased maximal OCR, suggesting that myoblast metabolic phenotype was dysregulated with ethanol. The ethanol-induced decrease in ECAR was associated with decreased differentiation. These findings suggest that ethanol-mediated shifts in metabolic phenotype may underlie impaired differentiation, which has important clinical implications for myogenesis in those affected by alcoholic myopathy.
Keywords: Myogenesis, mitochondria, glycolysis, alcohol, skeletal muscle
INTRODUCTION
Over 15 million adults in the U.S. have an Alcohol Use Disorder (AUD), and more than a quarter of adults in the U.S. reported engaging in unhealthy alcohol use (binge or heavy drinking) in the past month (Substance Abuse and Mental Health Services Administration, 2016). Chronic at-risk alcohol use presents significant healthcare challenges including myopathy, defined as reduced skeletal muscle (SKM) mass and function, which is highly prevalent among this population (Estruch et al., 1993; Urbano-Márquez et al., 1995). Furthermore, SKM mass and function are inversely associated with morbidity (Jackson et al., 2010; Jurca et al., 2004; Ruiz et al., 2009) and all-cause mortality (Rantanen et al., 2000; Ruiz et al., 2008), emphasizing the importance of maintaining SKM health among people with at-risk alcohol use. SKM energetic dysfunction resulting from impaired mitochondrial and glycolytic metabolism is an important and potentially treatable contributing factor (Romanello and Sandri, 2015). Although mature muscle differentially relies upon these energy systems based on energetic demands, these bioenergetic pathways are particularly important during myogenesis (Rochard et al., 2000; Tixier et al., 2013).
Maintenance and repair of skeletal muscle involves activation and proliferation of myoblasts (skeletal muscle stem cells) followed by their differentiation and fusion into multinucleated myotubes. We previously observed that in vivo chronic binge alcohol administration decreases the in vitro differentiation potential of myoblasts isolated from simian immunodeficiency virus (SIV)-infected (Simon et al., 2017) or SIV-naïve male rhesus macaques (Simon et al., 2014), and that in vitro ethanol treatment (50mM) decreases the differentiation potential of myoblasts from male macaques assessed at 5 days of differentiation (Adler et al., 2019). Furthermore, our studies in a rodent model of hindlimb immobilization demonstrated that chronic alcohol feeding suppressed SKM expression of myogenic genes at 3 days after remobilization following one week of immobilization (Levitt et al., 2020). However, whether alcohol similarly impairs in vitro myoblast differentiation potential in females is unknown. A recent study suggests sex differences in expression of myogenic regulatory factors in men and women after a mechanical stimulus (Luk et al., 2019), and clinical evidence suggests sex differences in alcoholic myopathy (Urbano-Márquez et al., 1995); hence, it is possible that alcohol might alter myoblast differentiation in a sex-specific manner, contributing to sex differences in alcoholic myopathy.
Changes in metabolic programming support changes in stem cell fate and growth state (McGraw and Mittal, 2010; Wanet et al., 2015). Current evidence suggests that energetic demands of quiescent myoblasts are supported largely by mitochondrial oxidative metabolism, which shifts to a more glycolytic phenotype upon activation, proliferation, and early differentiation (Bhattacharya and Scimè, 2020; Pala et al., 2018; Webster et al., 1990). At the onset of differentiation, increased autophagy supports myoblast mitochondrial remodeling and subsequent biogenesis (Sin et al., 2016). Greater reliance on glycolysis to meet ATP demands during this early differentiation appears essential. RNAi silencing of glycolytic genes during embryogenesis decreased subsequent myoblast fusion (Tixier et al., 2013) and myoblast-specific knockout of AMP-activated protein kinase (AMPK) α1, which stimulates glycolysis in myoblasts, impaired proliferation in vitro and prevented muscle regeneration after damage in vivo (Fu et al., 2015). As cells progress through differentiation, expression of mitochondrial enzymes increases suggesting that oxidative metabolism supports a greater proportion of metabolic demands (Webster et al., 1990). Underscoring the importance of mitochondria in the process of myogenesis, inhibiting or promoting mitochondrial protein synthesis during proliferation alters the balance of proliferating and post-mitotic myoblasts (Seyer et al., 2006). Furthermore, inhibiting mitochondrial metabolism or mitochondrial protein synthesis under differentiation conditions severely limits myoblast fusion (Hamai et al., 1997; Rochard et al., 2000; Seyer et al., 2006).
Early research into the consequences of at-risk alcohol use on SKM demonstrated changes in mitochondrial morphology after 28 days of alcohol ingestion in human volunteers (Song and Rubin, 1972), decreased aerobic metabolism during exercise in chronic alcoholic subjects (Haida et al., 1998), and decreased activity of select glycolytic enzymes in chronic alcohol-fed rats (Trounce et al., 1990). More recently, impaired mitochondrial dynamics and enhanced fatigability in SKM from alcohol-fed rats has been reported (Eisner et al., 2014). Together, these results suggest alcohol-mediated SKM energetic dysregulation, which could impair myogenesis and have deleterious health consequences for individuals with at-risk alcohol use. Previous findings from our group show that chronic binge alcohol administration dysregulates SKM expression of genes involved in bioenergetics and mitochondrial function at end-stage SIV disease (Duplanty et al., 2017; Simon et al., 2015) and reduces maximal OCR in myoblasts and succinate dehydrogenase activity in SKM of asymptomatic SIV-infected male rhesus macaques (Duplanty et al., 2018). However, whether alcohol directly impairs myoblast metabolic function contributing to decreased differentiation potential and whether there are sex differences in myoblast differentiation is unknown. Therefore, we sought to determine effects of alcohol on myoblast differentiation potential, bioenergetics, and expression of related genes, and to examine whether changes occur in in a sex-specific manner.
MATERIALS AND METHODS
Muscle samples used for isolation of myoblasts used in the present study were obtained from animals included in two parent longitudinal studies. The detailed experimental design and data collected from the parent study for the male cohort have been previously published (Ford et al., 2018, 2016; Molina et al., 2014; Simon et al., 2018, 2017). The parent study for the female cohort is ongoing. All animal experiments used in this study were approved by the Institutional Animal Care and Use Committees at Louisiana State University Health Sciences Center (LSUHSC) in New Orleans, Louisiana and Tulane National Primate Research Center (TNPRC) in Covington, Louisiana and adhered to the National Institutes of Health guidelines for the care and use of experimental animals. Adult (4–9 years old) male and female rhesus macaques (Macaca mulatta) were acclimated for one week and maintained on a normal chow diet before undergoing a baseline (i.e., alcohol-naïve) muscle biopsy (described below). Samples from a subset of 5 male and 5 female macaques were selected for use in the present study.
Muscle biopsy
Macaques were fasted for 16 hours before administration of an anesthetic (ketamine/xylazine) in their home cage and transported to the surgical suite. General anesthesia was maintained with isoflurane and oxygen during the procedure. The surgical site on the anterior aspect of one rear limb distal to the coxofemoral joint and proximal to the stifle was scrubbed with chlorhexidine. The limb was covered with a fenestrated sterile drape exposing only the surgical site, and a small incision was made through the skin using a sterile scalpel. Approximately 50 mg of the vastus lateralis muscle was excised for myoblast isolation. The incision was then closed using absorbable sutures.
Myoblast isolation and expansion
Primary myoblasts were isolated from vastus lateralis muscle samples as previously described (Levitt et al., 2019; Simon et al., 2017, 2014). Samples were washed three times in Ham’s F-12 nutrient mixture (GE Healthcare Life Sciences, Marlborough, MA), minced, and trypsin digested (0.25% trypsin EDTA diluted 1:4 in Ham’s F-12). Digested muscle tissue was plated for 5 hrs in growth media [Ham’s F-12 nutrient mixture with 10% fetal bovine serum, 2% L-glutamine, and 2.5 ng/ml recombinant human fibroblast growth factor (R&D systems, Minneapolis, MN)] to allow fibroblasts to adhere to the plate. Supernatant containing muscle tissue and myoblasts was transferred to a fresh plate with growth media, and myoblast colonies were allowed to grow for 1 week. Thereafter, media was changed every other day. Myoblasts were passaged at 80–90% confluence and cryopreserved after each passage. Cells were taken to passage 4 for use in experiments.
In vitro alcohol cell culture
Myoblast cell culture was performed according to our previously published detailed methods (Adler et al., 2019; Levitt et al., 2019). Myoblasts were seeded in growth media at a density of 2.8×103 cells per cm2 on collagen-coated 6-well plates containing either 0 or 50 mM ethanol and maintained in cell culture incubators under standard culture conditions (5% CO2, 37°C) until reaching 70–80% confluence (3 days). Ethanol-treated myoblasts were maintained in a separate incubator in which the water bath was supplemented with 75 mM ethanol every 48 hours to maintain 50mM ethanol in culture media (Adler et al., 2019). Thereafter, cells were differentiated in low-serum media (2% horse serum) for 5 days. Cells were harvested at day 0 (i.e., after 3 days of proliferation) and day 5 of differentiation.
HEMA 3 staining for analysis of differentiation
At day 5 of differentiation, cells were fixed in ice-cold 100% methanol for 10 minutes. Thereafter, methanol was removed and cells were dried inside a laminar flow biosafety cabinet for 10 minutes. Fixed cells were stored covered at room temperature until staining. Fixed cells were stained using our previously published HEMA 3 method (Levitt et al., 2019). Cells were treated with HEMA 3 solution I (Fisher HealthCare, Inc., Waltham, MA) for 10 min at room temperature. Cells were then washed with distilled water and treated with HEMA 3 solution II (Fisher HealthCare, Inc.) for 10 min at room temperature, washed twice, and dried. Fifteen images per well of stained cells were captured at 20x magnification using bright field microscopy. For each image, total nuclei and nuclei fused into myotubes were counted using ImageJ. Myotubes per field were calculated as the number of cells with 3 or more nuclei and fusion index was calculated as the percentage of all nuclei fused into myotubes (Adler et al., 2019; Levitt et al., 2019; Simon et al., 2014). Final calculations of fusion index, myotubes per field, and total nuclei across the 15 images per well were averaged for analysis.
Oxygen consumption and extracellular acidification rates
Mitochondrial oxygen consumption rate (OCR) was measured using Seahorse technology (Agilent Technologies, Santa Clara, CA) as previously described (Duplanty et al., 2018) with slight modifications. Simultaneous measurement of myoblast extracellular acidification rate (ECAR) was performed to assess the glycolytic contribution to myoblast energy metabolism (Pala et al., 2018; Tixier et al., 2013). Myoblasts at P4 were maintained in standard cell culture conditions (5% CO2, 37°C) with 0 or 50 mM ethanol for 48 hours. Cells were then seeded in triplicate at a density of 75,000 cells per well in a 24-well Seahorse plate in growth media with 0 or 50 mM ethanol. After 24 hrs, growth media was replaced with ethanol-free XF Assay Medium with sodium pyruvate (1 mM), L-glutamine (2 mM), and glucose (10 mM) and incubated at 37°C without CO2 for 1 hr before measuring myoblast mitochondrial respiration and extracellular acidification rates with an XFe24 Extracellular Flux Analyzer (Agilent) according to manufacturer’s instructions. OCR and ECAR were measured and respiratory parameters assessed using the Mito Stress Test Kit (Agilent) by the sequential addition of oligomycin (1 μM), carbonyl cyanide‐p‐trifluoromethoxyphenylhydrazone (FCCP; 3 μM), and rotenone/antimycin A (0.5 μM). Resulting OCR and ECAR measurements were corrected for cell count obtained by staining nuclei with Hoescht dye (2 μM; ThermoFisher Scientific, Waltham, MA) and visualizing on a BioTek Cytation 5 cell imaging multi-mode reader (BioTek, Winooski, VT). Results for each of the three replicates per animal were averaged for analyses. We used myoblasts from 2 animals (one male and one female) as inter-assay controls and results showed strong reliability of the assay.
RNA & DNA isolation and quantitative real-time polymerase chain reaction (qPCR)
To assess expression of mitochondrial and glycolytic genes, total RNA was extracted from cells using the miRNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. cDNA was synthesized from 0.5 μg of RNA using the Quantitect reverse transcription kit (Qiagen) according to manufacturer’s instructions and diluted 1:2 in DNase RNase-free water. To assess mitochondrial content using mitochondrial DNA (mtDNA), total DNA was extracted using DNeasy Blood & Tissue kit (Qiagen). Custom primers designed to span exon-exon junctions were purchased from Integrated DNA Technologies (Coralville, IA; Table 1). Final reactions contained cDNA (50 ng) or gDNA (25 ng), primers (500 nM), SyBr green (Quantitect SyBr Green PCR kit, Qiagen), and DNase RNase-free water to 20 ul. qPCR reactions were carried out in duplicate using a CFX96 thermal cycler (Bio-Rad, Hercules, CA) with ribosomal protein S13 (RPS13) as the endogenous control for mRNA assessment (Adler et al., 2019; Duplanty et al., 2017; Robichaux et al., 2016) and B2M for mtDNA assessment (Duplanty et al., 2018; Jackson et al., 2012). Data were then analyzed using the 2−ΔΔCt method. Results for target genes are expressed as fold change versus the control group (0 mM ethanol) at 0 days of differentiation.
Table 1.
List of primers for qPCR analysis (primers from IDT technologies).
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Peroxisome proliferator-activated receptor gamma coactivator-1A (PGC1A) | GAGCGCCGTGTGATTTAT | CATCATCCCGCAGATTTACT |
| Peroxisome proliferator-activated receptor gamma coactivator-1B (PGC1B) | CTCATTCGGTGCTGTGTATT | TCTGGGTTACTCCTCAGTTT |
| Mitochondrial transcription factor A (TFAM) | GATTTCCCATAGTGCCTCAC | GAGAACCTCCTGACACTACA |
| Hexokinase (HK) | TCTGCTCGCCTACTTCTT | ATCTCCAAGAGGGTCTCATC |
| Aldolase (ALDO) | CTCTTCCACGAGACACTCTA | ACGCCCTTGTCTACCTT |
| Pyruvate kinase (PK) | CACCCAATCAAGGGAAGAA | GACGGAGTATACACAGGAAAG |
| Phosphofructokinase (PFK) | CTGTGGTTCGAGTTGGTATT | GGTGGCTTCCTTGATGTTAT |
| Ribosomal protein S 13 (RPS13) | CCCACTTGGTTGAAGTTGA | CAGGATCACACCGATTTGT |
| Displacement loop (DLOOP) | CAAGATCGCCCACACGTTC | AAATCTCCCGTGACTGGTTA |
| Beta-2-microglobulin (B2M) | TGTAAGCAGCATCATGGAGGT | TGTTCTCCACATAGTGAGGGT |
Statistical analyses
Data were checked for the assumptions of parametric statistics. Differentiation indices, OCR, and ECAR data were analyzed using a 2 (sex) × 2 (condition) analysis of variance (ANOVA) with repeated measures on condition. When appropriate, post-hoc pairwise comparisons were conducted using Tukey’s HSD. To analyze the relationship between ethanol-mediated suppression of differentiation and changes in metabolic function, the percent differences (ethanol versus control) in fusion index and myotubes per field were calculated and correlated with the differences in OCR and ECAR using Pearson product moment correlations. Gene expression data were collapsed across sex and analyzed using a 2 (condition) × 2 (day) ANOVA with repeated measures on both factors. The gene expression data violated the assumption of normality; thus, the data were log10 transformed for normalization prior to analysis. Data in the figures are presented as raw values. All analyses were performed using GraphPad Prism 8.3.0 (Dan Diego, CA). The alpha level of significance was set at 0.05.
RESULTS
Myoblast differentiation
Ethanol significantly decreased fusion index (p=0.01, Figure 1A), myotubes per field (p<0.001, Figure 1B), and total nuclei (p<0.001, Figure 1C). No significant differences between sexes were observed for fusion index, myotubes, or total nuclei. Representative images of HEMA 3-stained myotubes are pictured in Figure 1D–G.
Figure 1.
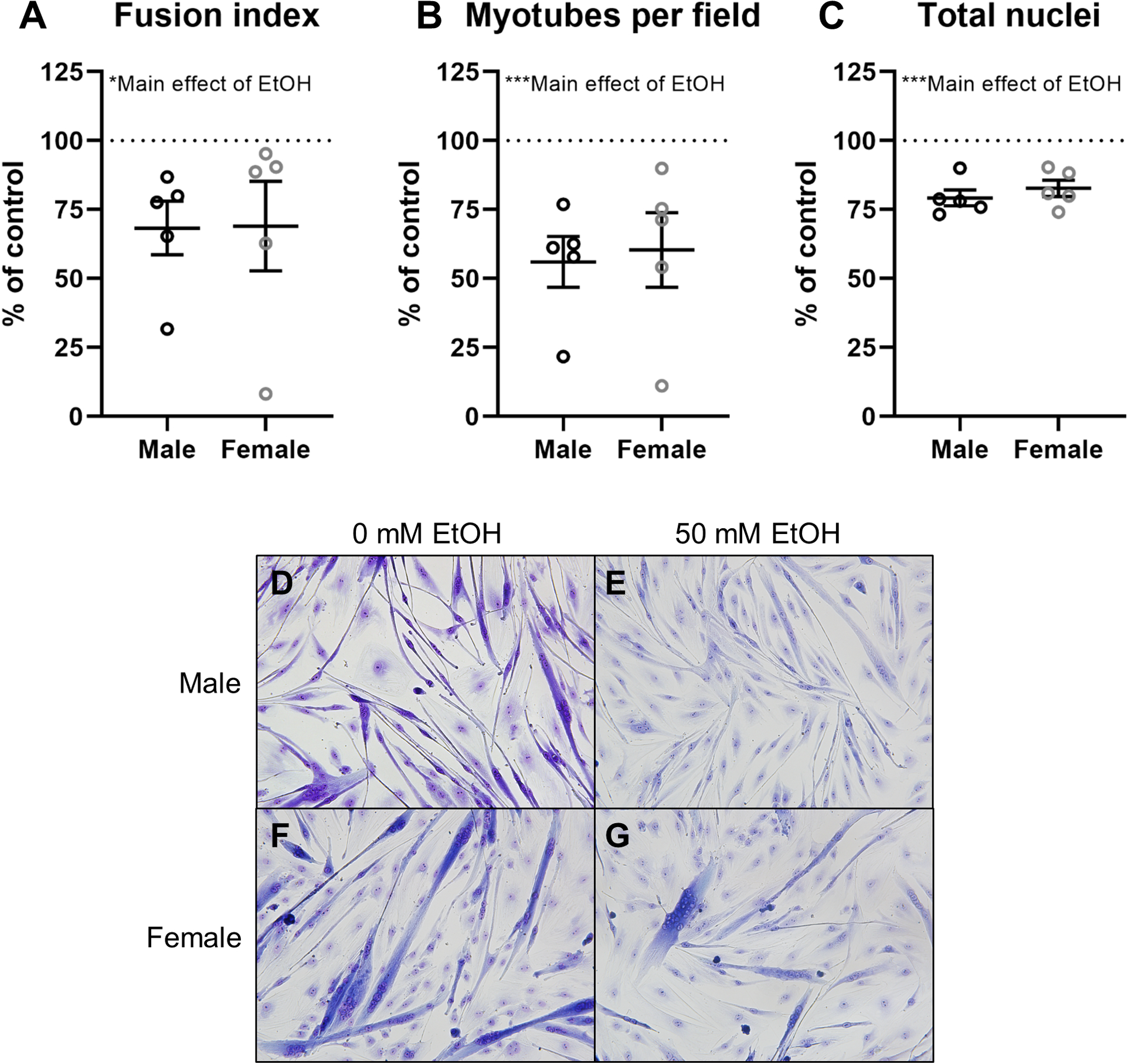
Analysis of differentiation indices in myoblasts from male (n=5; black) and female (n=5; grey) rhesus macaques after 5 days of differentiation with or without 50mM ethanol. Myoblast fusion index (A), myotubes per field (B), and total nuclei (C) for ethanol-treated myoblasts expressed as percent of control (0 mM ethanol; dashed line); representative images (20x) of HEMA 3-stained myoblasts (D-G). Values calculated from the average of 10–15 images. *p<0.05, ***p<0.001 analyzed by two-way (sex × condition) ANOVA with repeated measures on condition; mean ± SE.
Mitochondrial stress test
Oxygen consumption rate
OCR measures of the mitochondrial stress test for males and females are shown in Figure 2A and 2B, respectively. No significant differences due to sex or ethanol were observed for basal (baseline minus non-mitochondrial OCR) or baseline OCR, but there was a non-significant trend for myoblasts from females to have higher basal (p=0.07, Figure 2C) and baseline (p=0.09, Figure 2D) OCR than those from males. After oligomycin treatment, myoblasts from females had non-significantly higher ATP-linked OCR than those from males (p=0.08, Figure 2E) and ethanol-treated cells had non-significantly higher proton leak (p=0.06, Figure 2F) and total non-ATP-linked OCR (p=0.05, Figure 2H). After the addition of FCCP, we observed significantly higher maximal OCR (p=0.01, Figure 2I) and spare capacity (p<0.001, Figure 2J) in ethanol-treated cells. After the addition of rotenone/antimycin A, no significant differences in non-mitochondrial OCR due to sex or ethanol were observed (Figure 2G).
Figure 2.
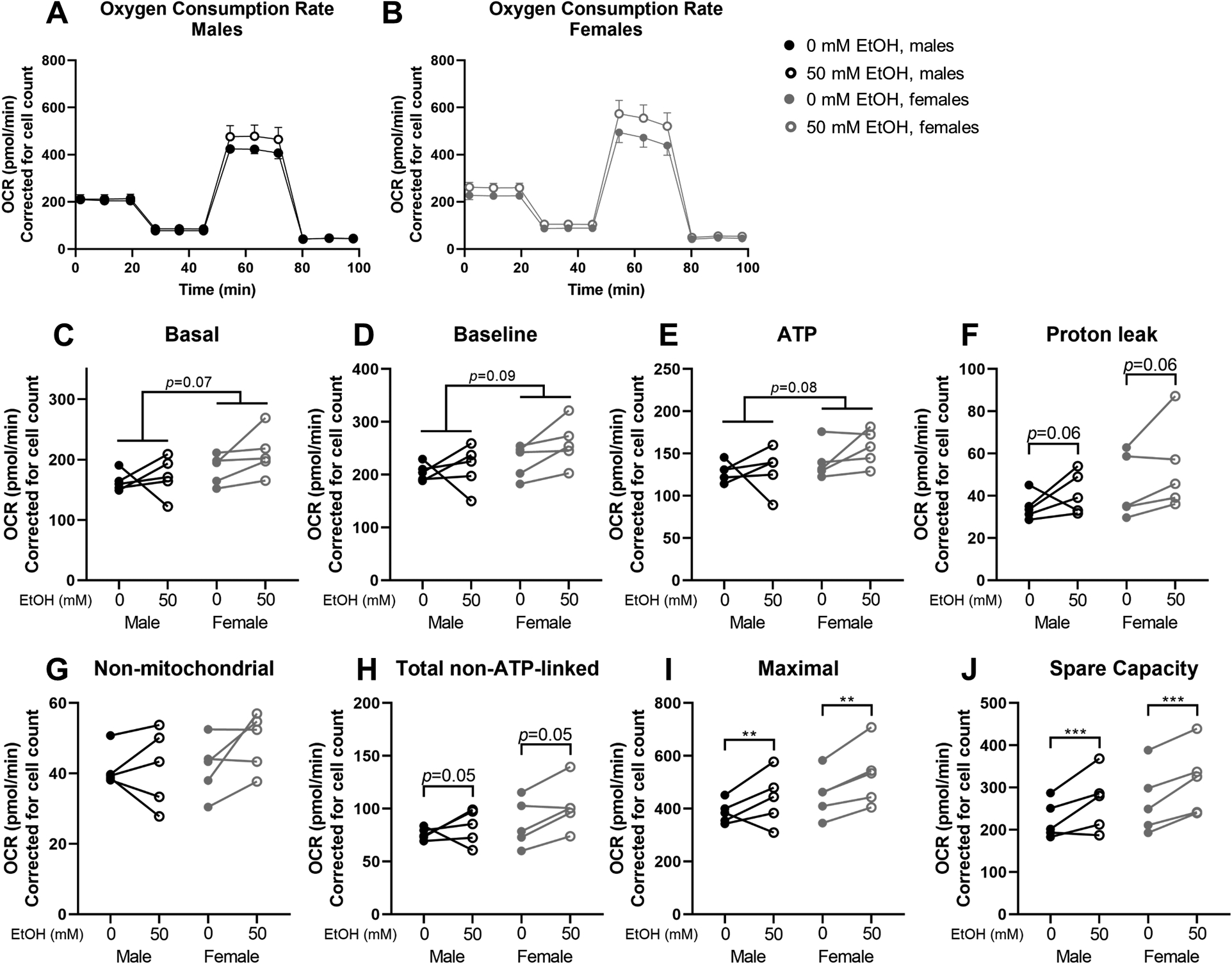
Oxygen consumption rate (OCR) parameters from Mitochondrial Stress Test using Seahorse XFe24 technology. Overall oxygen consumption rates (OCR) in myoblasts from male (A; n=5; black) and female (B; n=5; grey) rhesus macaques after 3 days of proliferation with (open circles) or without (closed circles) 50mM ethanol. Parameters include: basal (C), baseline (D), ATP-linked (E), proton leak-linked (F); non-mitochondrial (G), total non-ATP-linked (H), maximal (I), and spare capacity (J). Cells were seeded at 75,000 cells/well and counted after the assay using a Cytation 5 after staining nuclei using Hoescht dye. OCR values are adjusted for cell count. **p<0.01, ***p<0.001 analyzed by two-way (sex × condition) ANOVA with repeated measures on condition; A and B expressed as mean ± SE; C-J expressed as individual changes.
Extracellular acidification rate and OCR/ECAR ratio
Baseline ECAR was significantly lower in ethanol-treated cells (p=0.005, Figure 3A). The OCR/ECAR ratio was significantly higher in ethanol-treated cells at baseline (p=0.006, Figure 3B). After the addition of oligomycin, ethanol-treated cells had significantly lower ECAR (p=0.001, Figure 3C) and higher OCR/ECAR ratio (p=0.003, Figure 3D). No sex differences in ECAR or OCR/ECAR ratio were observed.
Figure 3.
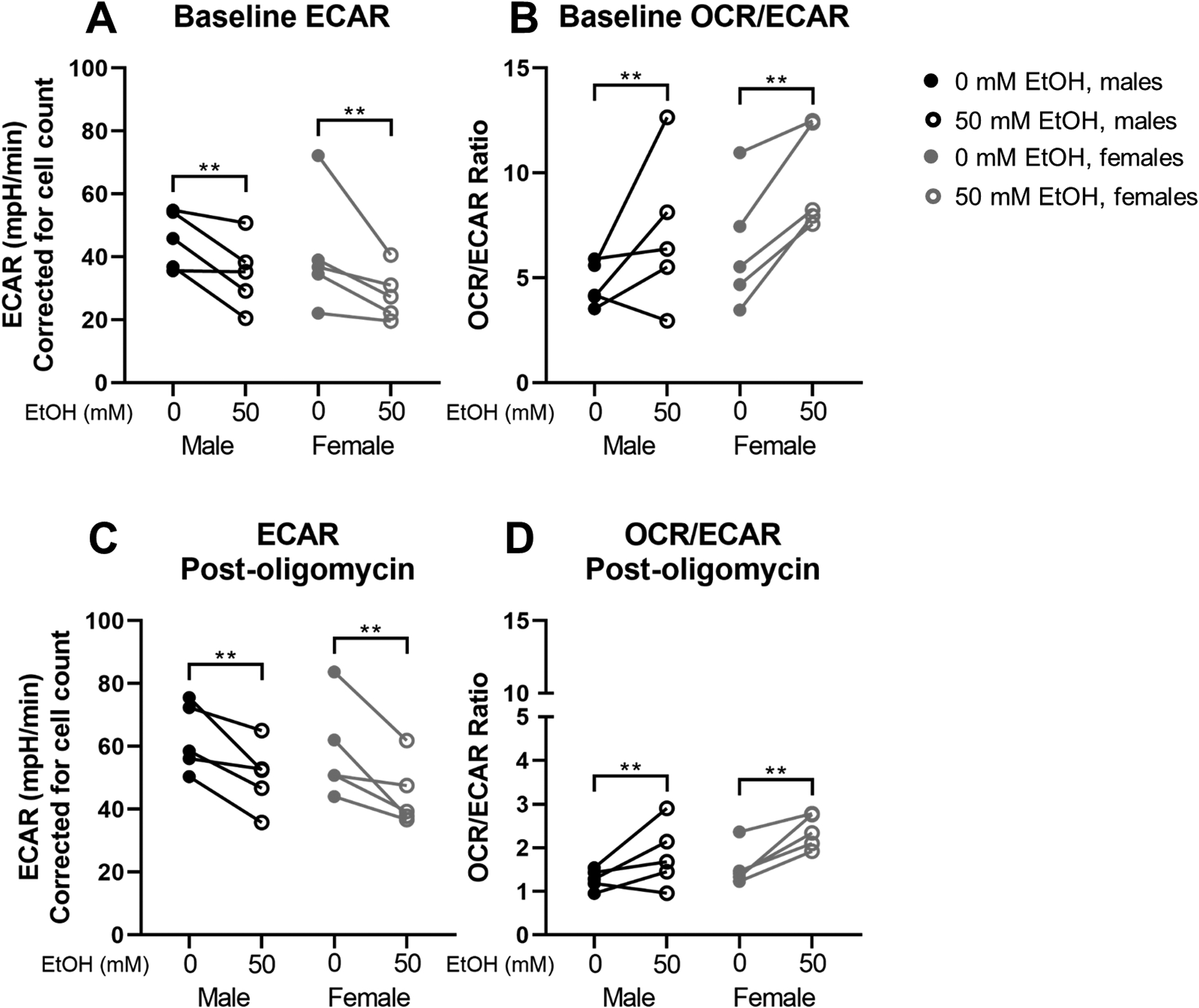
Extracellular acidification rate (ECAR) and ECAR/oxygen consumption rate (OCR) ratio results from Mitochondrial Stress Test using Seahorse XFe24 technology. Baseline ECAR (A) and OCR/ECAR (B); post-oligomycin ECAR (C) and OCR/ECAR (D) in myoblasts from male (n=5; black) and female (n=5; grey) rhesus macaques after 3 days of proliferation with (open circles) or without (closed circles) 50mM ethanol. Cells were seeded at 75,000 cells/well and counted after the assay using a Cytation 5 after staining nuclei using Hoescht dye. OCR and ECAR values are adjusted for cell count. **p<0.01 analyzed by two-way (sex × condition) ANOVA with repeated measures on condition; expressed as individual changes.
Relationships between myoblast metabolic and differentiation parameters
The ethanol-mediated decrease in ECAR was significantly associated with the ethanol-mediated decreases in fusion index (r=0.78, p=0.01, Figure 4A) and myotubes per field (r=0.75, p=0.02, Figure 4B). No significant relationship was found between changes in OCR and changes in differentiation indices with ethanol (not shown).
Figure 4.
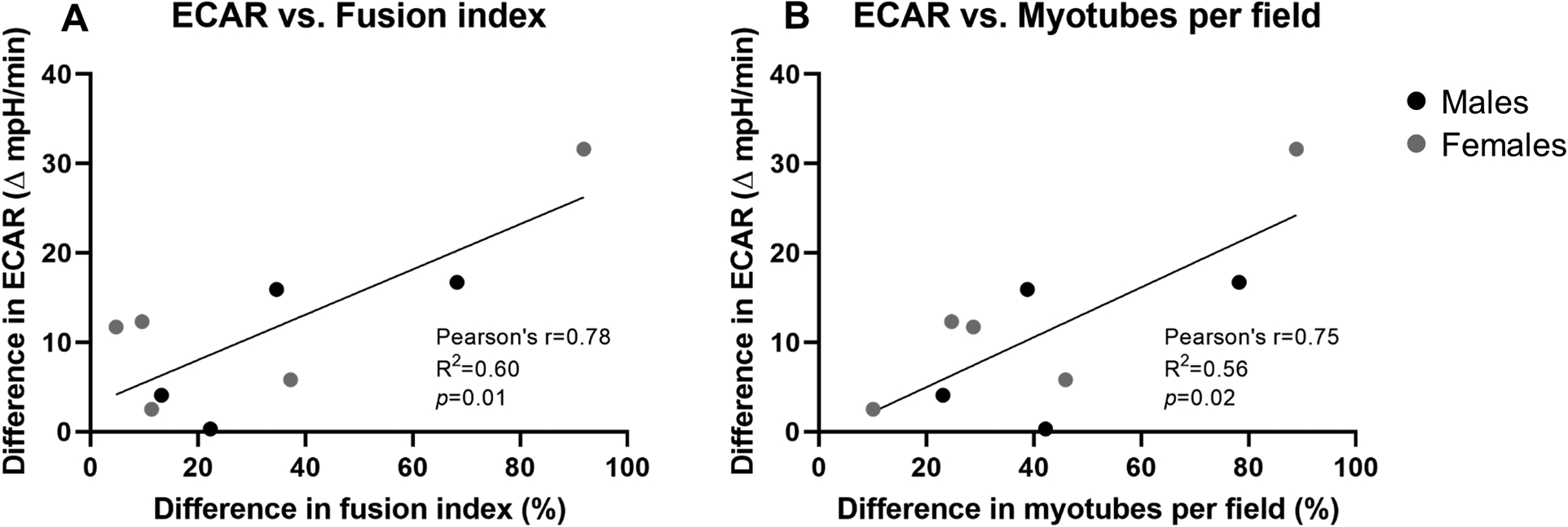
Relationship between the ethanol-mediated difference (│50mM – 0mM│) in baseline extracellular acidification rate (ECAR) after 3 days of proliferation and the ethanol-mediated percent difference in fusion index (A) and myotubes per field (B) after 5 days of differentiation in myoblasts from male (n=4; black) and female (n=5; grey). Analyzed by Pearson product moment correlation. One male was not included because insufficient cells from the same animal existed for Seahorse and differentiation analyses.
Gene expression
Mitochondrial gene expression
No significant ethanol-mediated differences in mRNA expression of peroxisome proliferator-activated receptor gamma coactivator (PGC)1A, PGC1B, or mitochondrial transcription factor A (TFAM) were observed. Similarly, no significant differences in gene expression were observed from day 0 to day 5 of differentiation (all p>0.05, Figure 5A–C).
Figure 5.
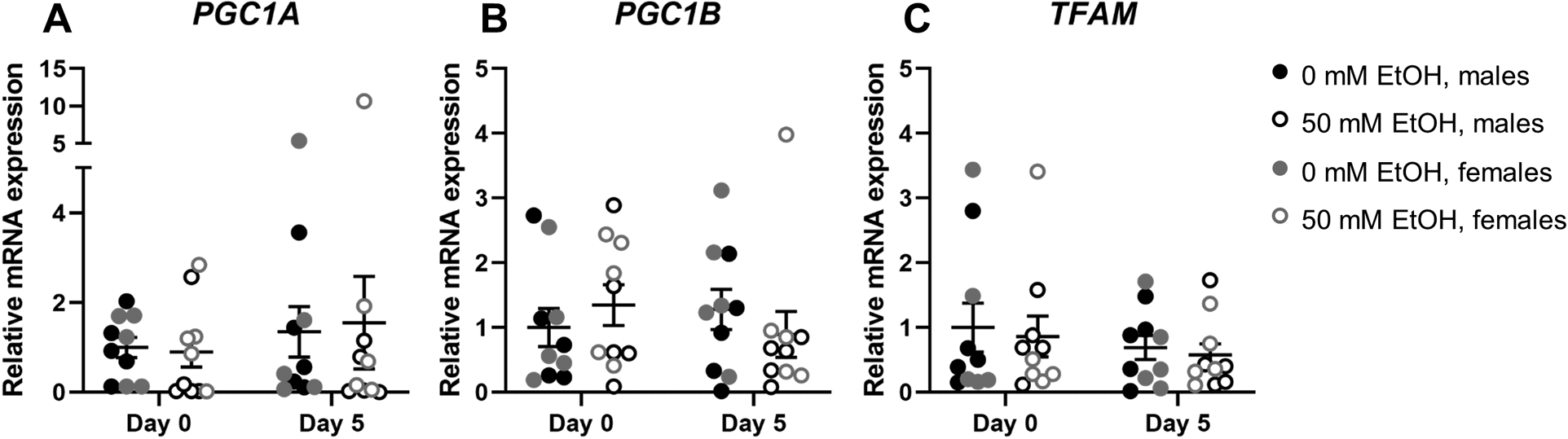
Expression of peroxisome proliferator-activated receptor gamma coactivator (PGC)1A (A), PGC1B (B), and mitochondrial transcription factor A (TFAM) (C) mRNA relative to RPS13 in myoblasts from male (n=5; black) and female (n=5; grey) rhesus macaques after 0 days (i.e., after 3 days of proliferation) or 5 days of differentiation with (open circles) or without (closed circles) 50mM ethanol. Analyzed by two-way (condition × day) ANOVA with repeated measures on both factors; expressed as mean ± SE.
Glycolytic gene expression
No significant ethanol-mediated effects on mRNA expression of hexokinase (HK), aldolase (ALDO), pyruvate kinase (PK), or phosphofructokinase (PFK) were observed (all p>0.05, Figure 6A–D). Expression of HK (p<0.001, Figure 6A), ALDO (p=0.002, Figure 6B), and PK (p<0.001, Figure 6C), but not PFK (Figure 6D) mRNA were significantly decreased at day 5 of differentiation compared to day 0. Data were log10 transformed for analysis and are presented as raw values.
Figure 6.
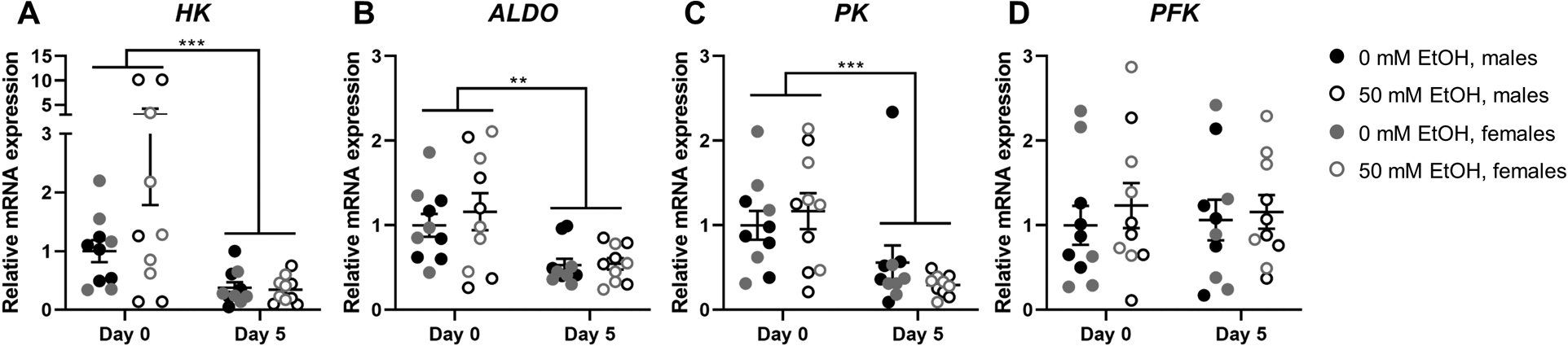
Expression of hexokinase (HK; A), aldolase (ALDO; B), pyruvate kinase (PK; C), and phosphofructokinase (PFK; D) mRNA relative to RPS13 in myoblasts from male (n=5; black) and female (n=5; grey) rhesus macaques after 0 days (i.e., after 3 days of proliferation) or 5 days of differentiation with (open circles) or without (closed circles) 50mM ethanol. **p<0.01, ***p<0.001, analyzed by two-way (condition × day) ANOVA with repeated measures on both factors; expressed as mean ± SE.
Mitochondrial DNA
No significant ethanol-induced differences in expression of mtDNA were observed. Expression of mtDNA was significantly increased at day 5 of differentiation compared to day 0 (p=0.03, Figure 7). Data were log10 transformed for analysis and are presented as raw values.
Figure 7.
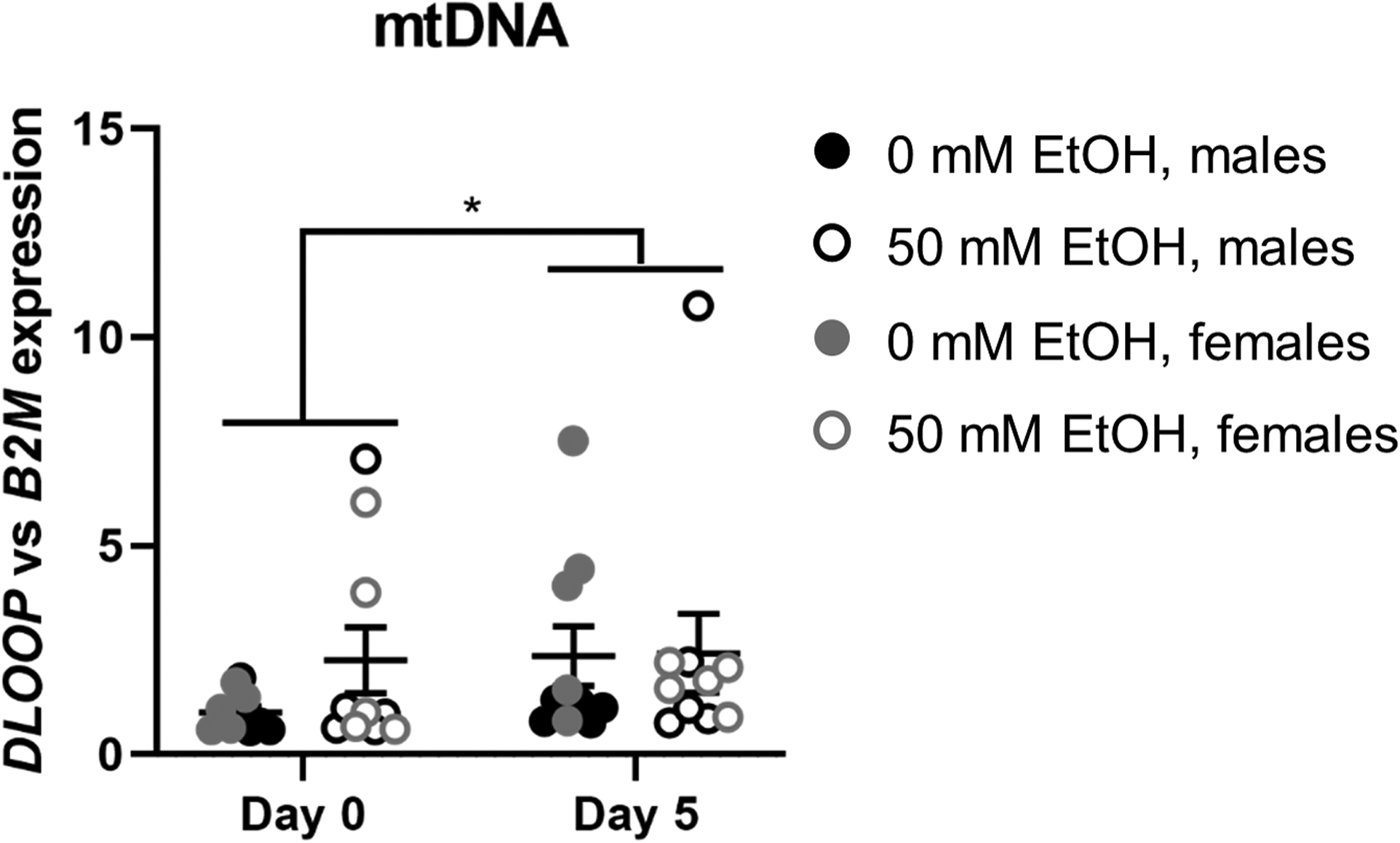
Expression of mitochondria-encoded DLOOP (mitochondrial DNA) relative to B2M (nuclear DNA) in myoblasts from male (n=5; black) and female (n=5; grey) rhesus macaques after 0 days (i.e., after 3 days of proliferation) or 5 days of differentiation with (open circles) or without (closed circles) 50mM ethanol. *p<0.05, analyzed by two-way (condition × day) ANOVA with repeated measures on both factors; expressed as mean ± SE.
DISCUSSION
In the present study, chronic in vitro ethanol treatment using a physiologically relevant concentration (50 mM) decreased myoblast differentiation potential and altered cellular metabolism irrespective of sex. Specifically, alcohol increased maximal mitochondrial respiration and impaired glycolytic metabolism during the proliferative phase (day 0 differentiation). This decrease in glycolytic metabolism during proliferation was significantly associated with myoblast fusion after five days of differentiation. However, no ethanol-mediated differences in expression of mitochondrial or glycolytic genes were observed. These findings have important implications for muscle health and metabolic function in those with at-risk alcohol use.
People with AUD are at high risk for alcoholic myopathy (Fernandez-Sola, 2000; Preedy et al., 2003), which is nearly five times as common as cirrhosis in this population (Estruch et al., 1993). Furthermore, people with AUD are at greater risk for muscle damage from traumatic injury (Honkanen and Visuri, 1976). Myopathy and muscle damage both require muscle regeneration to restore normal muscle mass and structure, and myoblast differentiation is essential for myogenesis to proceed. Alcohol administration has been shown to reduce differentiation potential in myoblasts isolated from male rhesus macaques (Adler et al., 2019; Simon et al., 2017, 2014). Since sex hormones may differentially influence myogenic phases (Enns and Tiidus, 2008; Luk et al., 2019; Mangan et al., 2014; Sinha-Hikim et al., 2006, 2002), we determined whether ethanol produces differential effects in myoblast isolated from female macaques. Here, we report for the first time that alcohol decreases myogenic differentiation potential similarly in myoblasts isolated from both males and females. Using an in vivo model of chronic binge alcohol administration in male rhesus macaques, our previous studies have demonstrated a similar decrease in myoblast fusion (Simon et al., 2014). We also observed a decrease in total nuclei with ethanol, which may have been the result of either cell death, decreased proliferation, or a combination thereof, and may have contributed to the ethanol-mediated decrease in differentiation. However, ethanol decreases myogenic gene expression during differentiation both in vivo and in vitro (Adler et al., 2019; Simon et al., 2014), supporting the hypothesis that ethanol decreases myoblast differentiation potential. This impaired differentiation may synergize with decreased muscle protein synthesis (Lang, 2018) to promote a progressive decline in muscle mass and function over time in those with AUD who do not substantially decrease or eliminate alcohol use (Fernandez-Sola, 2000).
During the process of differentiation, cellular metabolic phenotype shifts to support energetic demands (McGraw and Mittal, 2010; Wanet et al., 2015). During quiescence (Barani et al., 2003) and during late differentiation (Zhu et al., 2013), energetic demands are largely met by mitochondrial metabolism. However, mitochondria still support early differentiation, as inhibiting mitochondrial translation with chloramphenicol inhibited myoblast differentiation, whereas stimulating mitochondrial activity with overexpression of p43 (mitochondrial T3 receptor) potentiated differentiation (Rochard et al., 2000). In the present study, although alcohol impaired differentiation, it did not impair mitochondrial respiration or ATP-linked oxygen consumption as measured in vitro. In fact, there was an observed increase in maximal OCR and spare capacity with 50 mM ethanol. In contrast, Kumar and colleagues (2019) reported decreased basal, ATP-linked, and maximal mitochondrial respiration and decreased spare capacity with acute alcohol treatment (100 mM ethanol, 6 hours) in differentiated C2C12 myotubes. Differences in results could be due to the stage of differentiation, dose of alcohol used, and length of administration. Alcohol is a cellular stressor, and at high doses (100 mM), was reported to induce oxidative stress in C2C12 myoblasts (Kumar et al., 2019). However, mild oxidative stress is known to promote mitochondrial adaptation by activating cellular pathways that increase transcription of antioxidant enzymes, heat shock proteins, and uncoupling proteins, among others (Ristow, 2014). Although oxidative stress was not assessed in the present study, the extent of oxidative stress was likely much lower with half the dose of alcohol. Therefore, it is possible that the lower dose of alcohol in this study applied for a longer time allowed for a hormetic or adaptive response that increased maximal OCR with alcohol. This increase in maximal OCR may have also been a compensatory response for diminished glycolytic function; however, increased mitochondrial metabolism during proliferation is not optimal for differentiation. It is noteworthy that no alcohol-mediated difference in myoblast mitochondrial content was observed in the present or previous (Kumar et al., 2019) studies, but an increase in mtDNA content over time was observed in the present study, underscoring an oxidative shift in metabolism as differentiation proceeds.
Increased maximal OCR may be due to an increased proton-motive force resulting from enhanced electron chain complex activity, increased oxygen delivery, or a combination thereof. Increased expression of PGC1A, PGC1B, and TFAM would support the synthesis of mitochondrial machinery to drive electron transport chain activity. However, the increase in maximal OCR with ethanol occurred in the absence of a concomitant ethanol-mediated change in gene expression of PGC1A, PGC1B, or TFAM. Although these genes are essential for mitochondrial biogenesis and function, their activity can be regulated post-transcriptionally and post-translationally. MicroRNAs (miRs), a class of small non-coding RNAs that act epigenomically to bind mRNAs, may decrease expression of specific mitochondria-related mRNAs. For example, miR-130b directly targets and prevents translation of PGC1A mRNA (Wang et al., 2013). Furthermore, post-translational modifications can alter the activity of the protein products of mitochondria-related genes. Acetylation is a key modification affecting activity of the PGC-1α and PGC-1β coactivators at the protein level, where SIRT1 deacetylase activates PGC-1α (Nemoto et al., 2005; Rodgers et al., 2005) and GCN5 acetyltransferase inhibits PGC1α and β (Kelly et al., 2009; Lerin et al., 2006) activity. Furthermore, TFAM DNA-binding activity is decreased by acetylation and phosphorylation (King et al., 2018). It is probable that deacetylases and phosphatases, which have yet to be identified, oppose repressive post-translational modifications for PGC-1β and TFAM. Therefore, epigenomic factors and post-translational modifications may have contributed to the increased maximal OCR and spare capacity observed with 3 days of 50 mM ethanol treatment in proliferating myoblasts. Such factors that may contribute to alcohol-mediated myoblast metabolic alterations will be the subject of future studies.
Upon myoblast activation, proliferation, and commitment to differentiation, mRNA expression and activity of glycolytic enzymes increases, with decreases as differentiation progresses and mitochondrial metabolism again prevails (Barani et al., 2003; Webster et al., 1990). In the present study, mRNA expression of glycolytic enzymes largely followed this pattern, with greater expression during the proliferative phase (day 0 of differentiation) than after 5 days of differentiation. Although the mRNA expression of these enzymes was unaffected by ethanol, ECAR, an index of glycolytic metabolism (Pala et al., 2018), was decreased with ethanol during the proliferative phase, when myoblasts rely more heavily on glycolytic metabolism. This decrease in ECAR with ethanol resulted in a greater OCR/ECAR ratio. ECAR remained lower in the ethanol-treated myoblasts after the addition of oligomycin, an ATP synthase inhibitor, suggesting that chronic alcohol decreased the ability of myoblasts to upregulate glycolytic function to compensate for the loss of aerobically supported ATP synthesis. The OCR/ECAR ratio remained higher in ethanol-treated myoblasts after oligomycin treatment. With ATP synthase inhibited, oxygen consumption during this phase was not ATP-linked; rather, it represented proton leak and non-mitochondrial oxygen consumption. Overall, our results suggest dysregulation of glycolytic metabolism with chronic alcohol during the proliferative phase, and this glycolytic dysregulation appears to occur post-transcriptionally. These results are supported by previous reports of alcohol-mediated decreases in the activity of glycolytic enzymes in skeletal muscle from individuals with alcohol-associated myopathy (Trounce et al., 1987) and from chronic alcohol-administered rodents (Trounce et al., 1990). Furthermore, a transcriptomic analysis of C2C12 myotubes treated with 100 mM ethanol for 6 or 24 hours revealed decreased expression of glycolytic pathway mRNA (Kumar et al., 2019). Combined with the results of the present study, it appears that alcohol impairs myoblast glycolytic metabolism, which may impact the ability of myoblasts to fuse during muscle regeneration in vivo.
Glycolysis supports myoblasts’ transition from the proliferation to differentiation phase (Barani et al., 2003; Tixier et al., 2013). Ethanol decreased glycolysis during the proliferative phase and decreased myoblast fusion; therefore, we examined the relationship between these parameters. Alcohol-mediated decrease of glycolysis during the proliferative phase was significantly correlated with decreases in fusion index and myotubes per field. We hypothesize that the glycolytic dysfunction caused by ethanol underlies subsequent decreased differentiation. Previous work demonstrated that attenuation of glycolytic enzymes during early myogenesis in skeletal muscle of drosophila decreased in vivo myoblast fusion (Tixier et al., 2013), supporting a possible causal link between ethanol-mediated impairment in glycolytic activity and decreased myoblast differentiation. Similarly, deficiencies in glycolytic enzyme activity was significantly correlated with type II muscle fiber atrophy in humans with AUD (Trounce et al., 1987). Thus, glycolytic dysfunction may play a role in promoting clinical manifestations of at-risk alcohol use. Altogether, ethanol-mediated impairment of glycolytic function appears to impair myogenesis with clinically relevant implications.
This investigation was not without limitations. One such limitation is the small sample size used in this study. However, cells were collected from higher-order primates. Unlike rodents, non-human primates have biological variability similar to humans; therefore, we believe that the consistent and statistically significant changes observed are biologically relevant. Furthermore, only one concentration of alcohol was used. This concentration of 50 mM ethanol is in the alcohol intoxication range and is physiologically relevant, but whether similar changes occur at lower or higher concentrations of alcohol remains to be determined. Further, the Seahorse XFe24 Extracellular Flux Analyzer contains probes that measure O2 and pH in real-time, allowing for simultaneous measurement of OCR and ECAR, respectively, but does not measure CO2, preventing assessment of the contribution of CO2 to ECAR. However, because mitochondrial respiration was not different between conditions at baseline and after oligomycin treatment, it is unlikely that CO2 production accounted for the observed differences in ECAR. Finally, due to technical limitations, metabolic analyses were performed only during the myoblast proliferative phase and not during differentiation. However, surface area and days in culture on Seahorse plates hinder optimal studies on primary primate myoblasts throughout differentiation. The time-dependent effects of ethanol on mitochondrial and glycolytic metabolism throughout the myogenic differentiation process are worthy of further investigation.
Altogether, our results show that ethanol decreases myoblast differentiation irrespective of sex and that this decreased differentiation is related to impaired glycolysis during the proliferative phase. These results support the notion that disturbances in glycolytic energy production during early stages of myogenesis impair skeletal muscle regenerative potential. Therefore, ethanol-mediated glycolytic impairment likely contributes to the increased prevalence of myopathy in individuals with AUD. These results can be used to inform future work dissecting the mechanisms by which ethanol alters myoblast glycolytic function and to develop therapeutic interventions targeting glycolytic energy metabolism to reverse alcoholic myopathy.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Gregory Bagby and Dr. Steve Nelson, Comprehensive Alcohol‐HIV/AIDS Research Center, LSUHSC-NO, for their administrative support and design of the original animal study; Dr. Jason Dufour, DVM, DACLAM, Tulane National Research Primate Center and Drs. Jeffrey Schumacher, DVM and Leslie Birke, DVM, LSU for veterinary expertise; and excellent veterinary and animal care from Larry Coleman, Amy Weinberg and Heather McGarrah at LSUHSC-NO and Larissa Devlin, Wayne A. Cyprian, and Nancy Dillman at TNPRC Pathology Laboratory. The authors are grateful for the technical support of Curtis Vande Stouwe, Alice Yeh, Bryant Autin, and Rhonda R. Martinez. We would like to acknowledge the Cellular Immunology and Immune Metabolism Core at the Louisiana Cancer Research Center (Grant 5P30GM114732-05 NIH/NIGMS) for access to the Seahorse XFe24 equipment, and give thanks to Dr. Dorota Wyczechowska for technical support. This work was supported by grants from the NIAAA: F32AA027982, P60AA009803, T32AA007577, and K01AA024494.
Sources of support:
This work was funded by grants from the NIH/NIAAA: F32AA027982 (DEL), P60AA009803 (PEM), T32AA007577 (PEM), and K01AA024494 (LS). The Cellular Immunology and Metabolism Core, providing access to Seahorse XFe24 equipment, is funded by a grant from the NIH/NIGMS: 5P30GM114732-05.
REFERENCES
- Adler K, Molina PE, Simon L, 2019. Epigenomic mechanisms of alcohol-induced impaired differentiation of skeletal muscle stem cells; role of Class IIA histone deacetylases. Physiol. Genomics 51, 471–479. 10.1152/physiolgenomics.00043.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barani AE, Sabido O, Freyssenet D, 2003. Mitotic activity of rat muscle satellite cells in response to serum stimulation: relation with cellular metabolism. Exp. Cell Res 283, 196–205. 10.1016/S0014-4827(02)00030-7 [DOI] [PubMed] [Google Scholar]
- Bhattacharya D, Scimè A, 2020. Mitochondrial function in muscle stem cell fates. Front. Cell Dev. Biol 8, 480 10.3389/fcell.2020.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplanty AA, Siggins RW, Allerton T, Simon L, Molina PE, 2018. Myoblast mitochondrial respiration is decreased in chronic binge alcohol administered simian immunodeficiency virus-infected antiretroviral-treated rhesus macaques. Physiol. Rep 6 10.14814/phy2.13625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplanty AA, Simon L, Molina PE, 2017. Chronic binge alcohol-induced dysregulation of mitochondrial-related genes in skeletal muscle of simian immunodeficiency virus-infected rhesus macaques at end-stage disease. Alcohol Alcohol. Oxf. Oxfs 52, 298–304. 10.1093/alcalc/agw107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner V, Lenaers G, Hajnóczky G, 2014. Mitochondrial fusion is frequent in skeletal muscle and supports excitation-contraction coupling. J. Cell Biol 205, 179–195. 10.1083/jcb.201312066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns DL, Tiidus PM, 2008. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J. Appl. Physiol 104, 347–353. 10.1152/japplphysiol.00128.2007 [DOI] [PubMed] [Google Scholar]
- Estruch R, Nicolás JM, Villegas E, Junqué A, Urbano-Márquez A, 1993. Relationship between ethanol-related diseases and nutritional status in chronically alcoholic men. Alcohol Alcohol. Oxf. Oxfs 28, 543–550. [PubMed] [Google Scholar]
- Fernandez-Sola J, 2000. Low-dose ethanol consumption allows strength recovery in chronic alcoholic myopathy. QJM 93, 35–40. 10.1093/qjmed/93.1.35 [DOI] [PubMed] [Google Scholar]
- Ford SM, Simon L, Vande Stouwe C, Allerton T, Mercante DE, Byerley LO, Dufour JP, Bagby GJ, Nelson S, Molina PE, 2016. Chronic binge alcohol administration impairs glucose-insulin dynamics and decreases adiponectin in asymptomatic simian immunodeficiency virus-infected macaques. Am. J. Physiol. Regul. Integr. Comp. Physiol 311, R888–R897. 10.1152/ajpregu.00142.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SM, Simon Peter L, Berner P, Cook G, Vande Stouwe C, Dufour J, Bagby G, Nelson S, Molina PE, 2018. Differential contribution of chronic binge alcohol and antiretroviral therapy to metabolic dysregulation in SIV-infected male macaques. Am. J. Physiol.-Endocrinol. Metab 315, E892–E903. 10.1152/ajpendo.00175.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Zhu M-J, Dodson MV, Du M, 2015. AMP-activated protein kinase stimulates warburg-like glycolysis and activation of satellite cells during muscle regeneration. J. Biol. Chem 290, 26445–26456. 10.1074/jbc.M115.665232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haida M, Yazaki K, Kurita D, Shinohara Y, 1998. Mitochondrial dysfunction of human muscle in chronic alcoholism detected by using 31P-magnetic resonance spectroscopy and near-infrared light absorption. Alcohol. Clin. Exp. Res 22, 108S–110S. 10.1111/acer.1998.22.s3_part1.108s [DOI] [PubMed] [Google Scholar]
- Hamai N, Nakamura M, Asano A, 1997. Inhibition of mitochondrial protein synthesis impaired C2C12 myoblast differentiation. Cell Struct. Funct 22, 421–431. 10.1247/csf.22.421 [DOI] [PubMed] [Google Scholar]
- Honkanen R, Visuri T, 1976. Blood alcohol levels in a series of injured patients with special reference to accident and type of injury. Ann. Chir. Gynaecol 65, 287–294. [PubMed] [Google Scholar]
- Jackson AW, Lee D-C, Sui X, Morrow JR, Church TS, Maslow AL, Blair SN, 2010. Muscular strength is inversely related to prevalence and incidence of obesity in adult men. Obesity 18, 1988–1995. 10.1038/oby.2009.422 [DOI] [PubMed] [Google Scholar]
- Jackson CB, Gallati S, Schaller A, 2012. qPCR-based mitochondrial DNA quantification: Influence of template DNA fragmentation on accuracy. Biochem. Biophys. Res. Commun 423, 441–447. 10.1016/j.bbrc.2012.05.121 [DOI] [PubMed] [Google Scholar]
- Jurca R, Lamonte MJ, Church TS, Earnest CP, Fitzgerald SJ, Barlow CE, Jordan AN, Kampert JB, Blair SN, 2004. Associations of muscle strength and fitness with metabolic syndrome in men. Med. Sci. Sports Exerc 36, 1301–1307. [DOI] [PubMed] [Google Scholar]
- Kelly TJ, Lerin C, Haas W, Gygi SP, Puigserver P, 2009. GCN5-mediated transcriptional control of the metabolic coactivator PGC-1β through lysine acetylation. J. Biol. Chem 284, 19945–19952. 10.1074/jbc.M109.015164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GA, Hashemi Shabestari M, Taris K-KH, Pandey AK, Venkatesh S, Thilagavathi J, Singh K, Krishna Koppisetti R, Temiakov D, Roos WH, Suzuki CK, Wuite GJL, 2018. Acetylation and phosphorylation of human TFAM regulate TFAM–DNA interactions via contrasting mechanisms. Nucleic Acids Res. 46, 3633–3642. 10.1093/nar/gky204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Davuluri G, Welch N, Kim A, Gangadhariah M, Allawy A, Priyadarshini A, McMullen MR, Sandlers Y, Willard B, Hoppel CL, Nagy LE, Dasarathy S, 2019. Oxidative stress mediates ethanol-induced skeletal muscle mitochondrial dysfunction and dysregulated protein synthesis and autophagy. Free Radic. Biol. Med 145, 284–299. 10.1016/j.freeradbiomed.2019.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, 2018. Lack of sexual dimorphism on the inhibitory effect of alcohol on muscle protein synthesis in rats under basal conditions and after anabolic stimulation. Physiol. Rep 6, e13929 10.14814/phy2.13929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim S, Pandey A, Puigserver P, 2006. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell Metab. 3, 429–438. 10.1016/j.cmet.2006.04.013 [DOI] [PubMed] [Google Scholar]
- Levitt DE, Adler KA, Simon L, 2019. HEMA 3 staining: A simple alternative for the assessment of myoblast differentiation. Curr. Protoc. Stem Cell Biol 51 10.1002/cpsc.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt DE, Yeh AY, Prendergast MJ, Jr RGB, Adler KA, Cook G, Molina PE, Simon L, 2020. Chronic alcohol dysregulates skeletal muscle myogenic gene expression after hind limb immobilization in female rats. Biomolecules 10 10.3390/biom10030441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk H-Y, Levitt DE, Boyett JC, Rojas S, Flader SM, McFarlin BK, Vingren JL, 2019. Resistance exercise-induced hormonal response promotes satellite cell proliferation in untrained men but not in women. Am. J. Physiol. Endocrinol. Metab 10.1152/ajpendo.00473.2018 [DOI] [PubMed] [Google Scholar]
- Mangan G, Bombardier E, Mitchell AS, Quadrilatero J, Tiidus PM, 2014. Oestrogen-dependent satellite cell activation and proliferation following a running exercise occurs via the PI3K signalling pathway and not IGF-1. Acta Physiol. 212, 75–85. 10.1111/apha.12317 [DOI] [PubMed] [Google Scholar]
- McGraw TE, Mittal V, 2010. Stem cells: Metabolism regulates differentiation. Nat. Chem. Biol 6, 176–177. 10.1038/nchembio.324 [DOI] [PubMed] [Google Scholar]
- Molina PE, Amedee AM, Veazey R, Dufour J, Volaufova J, Bagby GJ, Nelson S, 2014. Chronic binge alcohol consumption does not diminish effectiveness of continuous antiretroviral suppression of viral load in simian immunodeficiency virus-infected macaques. Alcohol. Clin. Exp. Res 38, 2335–2344. 10.1111/acer.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T, 2005. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem 280, 16456–16460. 10.1074/jbc.M501485200 [DOI] [PubMed] [Google Scholar]
- Pala F, Di Girolamo D, Mella S, Yennek S, Chatre L, Ricchetti M, Tajbakhsh S, 2018. Distinct metabolic states govern skeletal muscle stem cell fates during prenatal and postnatal myogenesis. J. Cell Sci 131 10.1242/jcs.212977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy VR, Ohlendieck K, Adachi J, Koll M, Sneddon A, Hunter R, Rajendram R, Mantle D, Peters TJ, 2003. The importance of alcohol-induced muscle disease. J. Muscle Res. Cell Motil 24, 55–63. 10.1023/a:1024842817060 [DOI] [PubMed] [Google Scholar]
- Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM, 2000. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J. Gerontol. A. Biol. Sci. Med. Sci 55, M168–173. [DOI] [PubMed] [Google Scholar]
- Ristow M, 2014. Unraveling the truth about antioxidants: Mitohormesis explains ROS-induced health benefits. Nat. Med 20, 709–711. 10.1038/nm.3624 [DOI] [PubMed] [Google Scholar]
- Robichaux S, Lacour N, Bagby GJ, Amedee AM, 2016. Validation of RPS13 as a reference gene for absolute quantification of SIV RNA in tissue of rhesus macaques. J. Virol. Methods 236, 245–251. 10.1016/j.jviromet.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochard P, Rodier A, Casas F, Cassar-Malek I, Marchal-Victorion S, Daury L, Wrutniak C, Cabello G, 2000. Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression and activity of myogenic factors. J. Biol. Chem 275, 2733–2744. 10.1074/jbc.275.4.2733 [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P, 2005. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118. 10.1038/nature03354 [DOI] [PubMed] [Google Scholar]
- Romanello V, Sandri M, 2015. Mitochondrial quality control and muscle mass maintenance. Front. Physiol 6, 422 10.3389/fphys.2015.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JR, Sui X, Lobelo F, Lee D-C, Morrow JR, Jackson AW, Hébert JR, Matthews CE, Sjöström M, Blair SN, 2009. Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol 18, 1468–1476. 10.1158/1055-9965.EPI-08-1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JR, Sui X, Lobelo F, Morrow JR, Jackson AW, Sjöström M, Blair SN, 2008. Association between muscular strength and mortality in men: prospective cohort study. BMJ 337, a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyer P, Grandemange S, Busson M, Carazo A, Gamaléri F, Pessemesse L, Casas F, Cabello G, Wrutniak-Cabello C, 2006. Mitochondrial activity regulates myoblast differentiation by control of c-Myc expression. J. Cell. Physiol 207, 75–86. 10.1002/jcp.20539 [DOI] [PubMed] [Google Scholar]
- Simon L, Ford SM, Song K, Berner P, Vande Stouwe C, Nelson S, Bagby GJ, Molina PE, 2017. Decreased myoblast differentiation in chronic binge alcohol-administered simian immunodeficiency virus-infected male macaques: role of decreased miR-206. Am. J. Physiol. Regul. Integr. Comp. Physiol 313, R240–R250. 10.1152/ajpregu.00146.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Hollenbach AD, Zabaleta J, Molina PE, 2015. Chronic binge alcohol administration dysregulates global regulatory gene networks associated with skeletal muscle wasting in simian immunodeficiency virus-infected macaques. BMC Genomics 16, 1097 10.1186/s12864-015-2329-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, LeCapitaine N, Berner P, Vande Stouwe C, Mussell JC, Allerton T, Primeaux SD, Dufour J, Nelson S, Bagby GJ, Cefalu W, Molina PE, 2014. Chronic binge alcohol consumption alters myogenic gene expression and reduces in vitro myogenic differentiation potential of myoblasts from rhesus macaques. Am. J. Physiol. Regul. Integr. Comp. Physiol 306, R837–844. 10.1152/ajpregu.00502.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Siggins R, Winsauer P, Brashear M, Ferguson T, Mercante D, Song K, Vande Stouwe C, Nelson S, Bagby G, Amedee A, Molina PE, 2018. Simian immunodeficiency virus infection increases blood ethanol concentration duration after both acute and chronic administration. AIDS Res. Hum. Retroviruses 34, 178–184. 10.1089/AID.2017.0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin J, Andres AM, Taylor DJR, Weston T, Hiraumi Y, Stotland A, Kim BJ, Huang C, Doran KS, Gottlieb RA, 2016. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 12, 369–380. 10.1080/15548627.2015.1115172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S, 2002. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am. J. Physiol.-Endocrinol. Metab 283, E154–E164. 10.1152/ajpendo.00502.2001 [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S, 2006. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J. Clin. Endocrinol. Metab 91, 3024–3033. 10.1210/jc.2006-0357 [DOI] [PubMed] [Google Scholar]
- Song SK, Rubin E, 1972. Ethanol produces muscle damage in human volunteers. Science 175, 327–328. 10.1126/science.175.4019.327 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), n.d. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables. [PubMed]
- Tixier V, Bataillé L, Etard C, Jagla T, Weger M, Daponte JP, Strähle U, Dickmeis T, Jagla K, 2013. Glycolysis supports embryonic muscle growth by promoting myoblast fusion. Proc. Natl. Acad. Sci. U. S. A 110, 18982–18987. 10.1073/pnas.1301262110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounce I, Byrne E, Dennett X, 1990. Biochemical and morphological studies of skeletal muscle in experimental chronic alcoholic myopathy. Acta Neurol. Scand 82, 386–391. [DOI] [PubMed] [Google Scholar]
- Trounce I, Byrne E, Dennett X, Santamaria J, Doery J, Peppard R, 1987. Chronic alcoholic proximal wasting: physiological, morphological and biochemical studies in skeletal muscle. Aust. N. Z. J. Med 17, 413–419. 10.1111/j.1445-5994.1987.tb00078.x [DOI] [PubMed] [Google Scholar]
- Urbano-Márquez A, Estruch R, Fernández-Solá J, Nicolás JM, Paré JC, Rubin E, 1995. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. JAMA 274, 149–154. [DOI] [PubMed] [Google Scholar]
- Wanet A, Arnould T, Najimi M, Renard P, 2015. Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev. 24, 1957–1971. 10.1089/scd.2015.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Wang X, Zhang D, Zhang H, Wu Q, He Y, Wang J, Zhang L, Xia H, Yan J, Li X, Ying H, 2013. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia 56, 2275–2285. 10.1007/s00125-013-2996-8 [DOI] [PubMed] [Google Scholar]
- Webster KA, Gunning P, Hardeman E, Wallace DC, Kedes L, 1990. Coordinate reciprocal trends in glycolytic and mitochondrial transcript accumulations during the in vitro differentiation of human myoblasts. J. Cell. Physiol 142, 566–573. 10.1002/jcp.1041420316 [DOI] [PubMed] [Google Scholar]
- Zhu L-N, Ren Y, Chen J-Q, Wang Y-Z, 2013. Effects of myogenin on muscle fiber types and key metabolic enzymes in gene transfer mice and C2C12 myoblasts. Gene 532, 246–252. 10.1016/j.gene.2013.09.028 [DOI] [PubMed] [Google Scholar]


