Abstract
Purpose:
Primary brain tumor patients are vulnerable to depression and anxiety symptoms, which may affect their neurocognitive functioning. We performed a prospective longitudinal analysis to examine the association between depression and anxiety symptoms and domain-specific neurocognitive functioning in primary brain tumor patients receiving radiation therapy (RT).
Methods and Materials:
On a prospective trial, 54 primary brain tumor patients receiving RT underwent comprehensive neurocognitive evaluation at baseline (pre-RT), and 3, 6, and 12 months post-RT. Neurocognitive assessments measured attention/processing speed, verbal and visuospatial memory, and executive functioning, including Delis-Kaplan Executive Function System Trail-Making Test (DKEFS-TMT), DKEFS Verbal Fluency, and Brief Visuospatial Memory Test-Revised. Depression and anxiety symptoms were also assessed at each time point with Beck Depression and Anxiety Inventories (BDI-II and BAI), respectively. Higher scores reflect more numerous or severe depression or anxiety symptoms. Univariable and multivariable linear mixed-effects models assessed associations between BDI-II and BAI scores and domain-specific neurocognitive scores over time, controlling for pre-existing depression or anxiety disorders and other patient, tumor, and treatment characteristics.
Results:
Higher BAI scores were associated with worse attention and processing speed in univariable analyses: DKEFS-TMT visual scanning (P = .003), number sequencing (P = .011), and letter sequencing (P <.001). On multivariable analyses, these associations remained significant (all P≤.01). Higher BDI-II scores were also associated with poorer attention/processing speed (DKEFS-TMT Letter Sequencing) in univariable (P = .002) and multivariable (P = .013) models. Higher BAI scores were associated with worse visuospatial memory (Brief Visuospatial Memory Test-Revised Delayed Recall) on univariable (P = .012) but not multivariable analyses (P = .383). Similarly, higher BDI-II scores were associated with poorer executive functioning (DKEFS Verbal Fluency Category Switching) on univariable (P = .031) but not multivariable analyses (P = .198).
Conclusions:
Among primary brain tumor patients receiving RT, increased depression and anxiety were independently associated with worsened neurocognition, particularly in attention/processing speed. Depression and anxiety symptoms should be controlled for in prospective clinical trials and managed in the clinical setting to optimize neurocognitive functioning.
Introduction
Symptoms of depression and anxiety can affect up to 40% to 90% of patients with brain tumors.1,2 Brain tumor patients are at risk of developing the downstream effects of depression and anxiety symptoms, including emotional distress, decreased quality of life,3 decreased treatment adherence,4 poorer immune and hormonal responses to cancer,5 and independently worse survival outcomes.6,7
Neurocognitive functioning is a critical clinical outcome for primary brain tumor patients, and is now prioritized in clinical trials as an integrated endpoint alongside patient survival, tumor response, and quality of life.8 Within the neuropsychologic literature, depression and anxiety are often investigated as possible confounders of neurocognitive functioning.9,10 Indeed, evidence from Huntington disease,11 Parkinson disease,12 multiple sclerosis,13 and studies of older adults14,15 show that greater symptoms of depression and anxiety are associated with poorer neurocognitive and physical functioning. Among patients with cancer, there are longitudinal studies investigating patients with breast,16,17 prostate,18 and colorectal19 cancer, with findings showing mixed results that obscure clear associations. Primary brain tumor patients present a unique and complicated scenario; neurocognition can be affected by the tumor itself (location, growth, grade),20 tumor treatments, including chemotherapy,21 radiation therapy (RT),22 surgery,23 and medications (antiepileptic therapy and corticosteroid use24,25).
To our knowledge, no study has investigated the relationship between depression and anxiety symptoms and neurocognitive functioning longitudinally in primary brain tumor patients. A thorough understanding of this relationship is crucial within the brain tumor population, as clinical trials investigating novel brain tumor treatments are increasingly including neurocognition as the primary outcome in metastatic,26,27 pediatric,28 and adult primary brain tumor populations.29 We hypothesized that higher symptoms of depression and anxiety are associated with poorer neurocognitive performance within the domains of attention and processing speed, executive functioning, and visuospatial and verbal memory.
Methods
Protocol approval and consent
This study was approved by our institutional review board. All enrolled patients provided written informed consent.
Study design and participants
As part of an ongoing single institution prospective, longitudinal trial, we evaluated 54 patients with primary brain tumors treated with fractionated partial-brain RT (1.8-2.0 Gy per fraction, 50.4-60 Gy total dose). Eligibility criteria included: age >18 years, Karnofsky performance status (KPS) >70, ability to complete neurocognitive evaluation in English, and life expectancy >1 year. Patients with prior brain RT were excluded. Subjects were examined before RT, and 3, 6, and 12 months post-RT. At each time point, depression and anxiety symptoms were assessed, along with a battery of well-validated neurocognitive tests.
Depression and anxiety assessments
The Beck Depression Inventory-II30 (BDI-II) and Beck Anxiety Inventory31 (BAI) are well-validated, self-report questionnaires quantifying the number and severity of depression and anxiety symptoms experienced within the past 1 to 2 weeks. Greater number and severity of symptoms of depression and anxiety are reflected in an increased BDI-II and BAI score, respectively. These assessments are robust and have been used in other oncology studies.6,32
Neurocognitive assessments
The neurocognitive battery consisted of 12 validated tests, categorized into 3 domains. Attention and processing speed was measured by: Delis-Kaplan Executive Function System33 Trail Making Test (DKEFS-TMT) (visual scanning, number sequencing, and letter sequencing conditions) and Wechsler Adult Intelligence Scale-IV34 (digit span and coding subtests). Executive functioning included: DKEFS Verbal Fluency (letter fluency and category switching conditions) and the Wisconsin Card Sort Test35 (perseverative errors). The memory domain, assessing verbal and visuospatial memory, included: Hopkins Verbal Learning Test-Revised36 (total recall and delayed recall) and the Brief Visuospatial Memory Test37 (total recall and delayed recall). These domains were chosen because they tend to demonstrate the greatest impairment in brain tumor patients after radiation.38 These specific tests are also ideal for repeat testing (at each time point), as alternate but psychometrically equivalent forms were available. Raw neurocognitive test scores were converted to age-, sex-, and education-adjusted T-scores.38
Statistical analysis
Baseline differences in BAI and BDI-II scores based on demographic groups were tested using 2-sample t tests, one-way analysis of variance (ANOVA), and Pearson’s correlation as appropriate, whereas post hoc pairwise t tests (Holm correction) were conducted after significant associations identified via one-way ANOVA. Longitudinal BDI-II and BAI scores were fitted using linear mixed-effects models,39 with time and demographic variables as predictors and random subject intercept.
Separate univariable linear mixed-effects models were fitted using longitudinal T-scores from each of the 12 neurocognitive assessments as the outcome, and either depression or anxiety symptoms at the same time point as the predictor, again with random subject intercepts. In total, 24 univariable models explored continuous, time-varying BDI-II or BAI symptom scores as a predictor of neurocognitive performance.
Finally, stepwise procedure was used to adjust for demographic, tumor, and treatment characteristics in the association between time-varying BDI-II or BAI symptom scores and neurocognitive performance. We assessed the following variables for inclusion in each of the models: sex, age, race or ethnicity, time point, RT type (intensity modulated RT/volumetric arc therapy vs proton therapy), concurrent treatment with chemotherapy, history of seizures, antiepileptic drug use, highest education level (categorized as up to high school (≤12 years), college (12<x≤16 years), or graduate school (>16 years), baseline KPS, tumor histology (benign tumor versus glioma), and prior history of clinical anxiety or depression. Each of the variables was first assessed as a predictor of neurocognition in univariable linear mixed-effects models including random subject intercept. Those with a P value of <.20 were then included in a multivariable stepwise backward selection procedure, using P value <.10 as the criterion for remaining in the model, while keeping either time-varying BAI or BDI-II as the main predictor in the model. This stepwise procedure was repeated for all combinations of BDI-II or BAI symptom scores and neurocognitive performance. All statistical analyses were performed in R.40
Results
Patient cohort
Of the 60 patients currently enrolled in the clinical trial, this analysis includes 54 patients with baseline neurocognitive and anxiety and depression symptom data (Fig. E1). Patient characteristics are shown in Table 1. This cohort was 60% male, predominantly non-Hispanic white race (78%), and highly educated (49% college educated, 30% graduate level education). Notably, 9 patients had been diagnosed with an anxiety disorder before their brain tumor diagnosis and 8 patients had a prior diagnosis of clinical depression.
Table 1.
Patient demographics and clinical characteristics
| Characteristic of baseline population, n = 54 | Frequency (%) or median (range) |
|---|---|
| Age, y | 47 (20-75) |
| Sex | |
| Male | 32 (60) |
| Female | 22 (40) |
| Ethnicity | |
| Non-Hispanic | |
| Asian/Pacific Islander | 3 (6) |
| Black | 1 (2) |
| Middle Eastern | 2 (4) |
| White | 42 (78) |
| Hispanic | 6 (11) |
| Highest education achieved, median (range) | 16 (10-20) |
| High school | 11 (21) |
| College | 26 (49) |
| Graduate School | 16 (30) |
| Tumor diagnosis | |
| Glioma | |
| Low-grade | 9 (17) |
| High-grade | 23 (43) |
| Meningioma | 12 (22) |
| Pituitary adenoma | 5 (9) |
| Schwannoma | 2 (4) |
| Craniopharyngioma | 2 (4) |
| Chondrosarcoma | 1 (2) |
| Tumor side | |
| Left | 27 (50) |
| Right | 23 (42) |
| Central | 4 (8) |
| Tumor region | |
| Frontal | 16 (30) |
| Temporal | 12 (22) |
| Suprasellar | 9 (17) |
| Parietal | 6 (11) |
| Base of skull | 4 (7) |
| Cerebellar | 3 (6) |
| Cavernous sinus | 3 (6) |
| Sphenoid wing | 1 (2) |
| RT type | |
| IMRT/VMAT | 40 (74) |
| Proton | 14 (26) |
| Baseline KPS | |
| 80 | 3 (6) |
| 90 | 33 (61) |
| 100 | 18 (33) |
| Surgery | |
| GTR | 11 (20) |
| STR | 33 (61) |
| Biopsy | 3 (6) |
| None | 7 (13) |
| Chemotherapy* | 28 (52) |
| Seizures† | 23 (43) |
| Antiepileptic drug use‡ | 28 (52) |
| Previously treated | |
| Anxiety | 9 (17) |
| Depression | 8 (15) |
| Baseline BDI-II, median (range)§ | 8.5 (0-24) |
| Minimal | 41 (76) |
| Mild | 9 (17) |
| Moderate | 4 (7) |
| Severe | 0 |
| Baseline BAI, median (range)§ | 5.5 (0-34) |
| Minimal | 29 (60) |
| Mild | 11 (23) |
| Moderate | 6 (13) |
| Severe | 2 (4) |
Abbreviations: GTR = gross total resection; IMRT/VMAT = intensity modulated RT/volumetric arc therapy; KPS = Karnofsky Performance Status; RT = radiation therapy; STR = subtotal resection.
Received chemotherapy during or after RT.
History of active seizures.
Taking antiepileptic drugs during study.
At baseline the median patient BDI-II score was higher than the median BAI score, at 8.5 and 5.5, respectively (Table 1). Although patients tended to report higher baseline depression symptoms than anxiety symptoms, moderate-severe BAI scores were more common (n = 8, 17%) than moderate-severe BDI-II scores (n = 4, 7%). The minimal range of symptoms was most common at baseline, comprising 75% of BDI-II responses and 58% of BAI responses.
Twelve tests among 3 cognitive domains (attention and processing speed, executive functioning, and memory) were examined, as described earlier. Changes in these neurocognitive outcomes over time have been assessed and reported previously.41,42
Depression and anxiety symptoms
BDI-II and BAI scores for the cohort over time are shown in Figure 1. There was no statistically significant change in BDI-II scores over time (Figure. 1a and b). BAI scores, however, did decrease significantly over time (linear mixed-effects, P = .003; Figure. 1c and d).
Fig. 1.
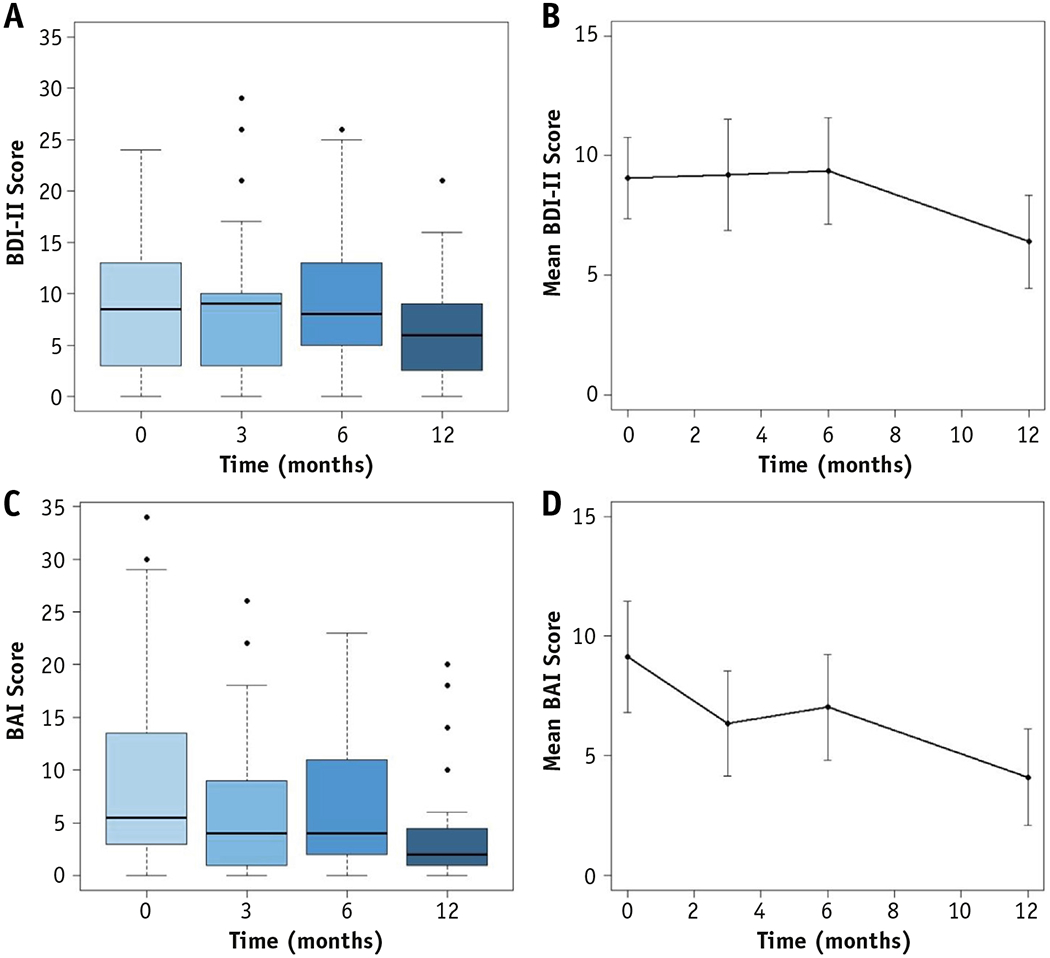
BDI and BAI scores over time. Boxplots and line plots for raw BDI and BAI scores over time. Line plots are mean scores over time with error bars representing the 95% confidence intervals. (a and b) BDI scores are not statistically different over time. (c and d) BAI scores significantly decrease over time (P = .003).
Analyses of the associations between patient characteristics and baseline BDI-II and BAI scores are shown in Table 2. History of depression was the only significant predictor of higher BDI-II at baseline (P = .011). Female sex was associated with significantly higher BAI scores at baseline (P = .048). High school education level was also significantly associated with a higher BAI score at baseline compared with groups with higher education levels (P = .028 in post hoc pairwise t test, Holm adjusted). Patients treated with protons had a lower BAI score at baseline (P = .026) compared with patients treated with intensity modulated RT and volumetric arc therapy.
Table 2.
Baseline BAI and BDI associations with patient characteristics
| Baseline BAI score |
Baseline BDI score |
|||
|---|---|---|---|---|
| Characteristic | Mean (SD) | P value | Mean (SD) | P value |
| Sex* | ||||
| Male | 6.92 (6.5) | .048 | 8.81 (5.4) | .739 |
| Female | 11.77 (9.4) | 9.45 (7.8) | ||
| Age† | ||||
| rho est: | 0.017 | .901 | −0.115 | .408 |
| Highest education‡ | ||||
| High school | 14.1 (10.9) | .028§ | 11.91 (7.9) | .234 |
| College | 6.52 (5.7) | 7.96 (5.7) | ||
| Graduate school | 11.17 (8.5) | 9.25 (6.2) | ||
| Ethnicity‡ | ||||
| Non-Hispanic | .739 | .177 | ||
| Asian | 4.06 (2.8) | 3.33 (4.1) | ||
| Black | 2.00 (NA) | 1.00 (NA) | ||
| Middle Eastern | 9.50 (0.7) | 15.5 (4.9) | ||
| White | 9.86 (8.2) | 9.48 (6.1) | ||
| Hispanic | 7.50 (11.1) | 8.33 (8.2) | ||
| History of anxiety* | ||||
| Yes | 16.50 (11.0) | .059 | 13.67 (7.2) | .055 |
| No | 7.68 (6.8) | 8.16 (5.9) | ||
| History of depression* | ||||
| Yes | 15.14 (9.1) | .093 | 14.13 (4.9) | .011 |
| No | 8.12 (7.7) | 8.20 (6.2) | ||
| Baseline KPS‡ | ||||
| 80 | 13.00 (NA) | .876 | 9.67 (8.1) | .987 |
| 90 | 9.27 (8.5) | 9.03 (6.2) | ||
| 100 | 8.71 (8.0) | 9.06 (6.9) | ||
| RT type* | ||||
| IMRT/VMAT | 10.62 (8.6) | .026 | 9.95 (6.6) | .056 |
| Protons | 5.57 (6.0) | 6.57 (5.0) | ||
| Glioma* | ||||
| Yes | 9.85 (8.4) | .526 | 9.63 (6.0) | .464 |
| No | 8.32 (8.1) | 8.27 (7.0) | ||
| Seizures* | ||||
| Yes | 10.61 (8.1) | .343 | 10.91 (4.9) | .055 |
| No | 8.27 (8.3) | 7.71 (7.1) | ||
| Antiepileptic drugs* | ||||
| Yes | 9.22 (7.7) | .702 | 9.82 (5.3) | .218 |
| No | 9.08 (8.8) | 8.27 (7.4) | ||
| Chemotherapy* | ||||
| Yes | 9.86 (7.5) | .578 | 9.54 (5.8) | .590 |
| No | 8.54 (8.8) | 8.58 (7.1) | ||
| Surgery* | ||||
| Yes | 8.53 (7.9) | .375 | 8.91 (6.3) | .726 |
| No | 11.5 (9.4) | 9.80 (7.3) | ||
Abbreviations: IMRT/VMAT = intensity modulated RT/volumetric arc therapy; KPS = Karnofsky Performance Status; RT = radiation therapy; SD = standard deviation.
Two-sample t test.
Pearson correlation.
One-way analysis of variance.
Post hoc pairwise t test with Holm correction.
Linear mixed-effects analysis of longitudinal BDI-II and BAI scores with univariable demographic predictors are shown in Table 3. A prior history of seizures (P = .043), anxiety (P = .020), and depression (P = .041) each predicted for increased BDI-II scores over the course of the study. History of anxiety predicted for increased BAI scores over time (P = .015).
Table 3.
Univariable longitudinal Analysis of BAI and BDI associations with patient characteristics
| BAI score |
BDI score |
|||
|---|---|---|---|---|
| Characteristic | Estimate (95% CI) | LRT P value | Estimate (95% CI) | LRT P value |
| Sex | ||||
| Male | −3.06 (−6.97 to 0.85) | .128 | −0.12 (−3.56 to 3.32) | .943 |
| Age | ||||
| Continuous | 0.02 (−0.18 to 0.22) | .771 | −0.04 (−0.24 to 0.15) | .508 |
| Highest education | .063 | .420 | ||
| High school | 6.24 (1.14 to 11.3) | 2.79 (−1.72 to 7.30) | ||
| Graduate school | 1.67 (−2.64 to 5.98) | −0.10 (−4.02 to 3.82) | ||
| Ethnicity | ||||
| Non-Hispanic | .732 | .326 | ||
| Asian | −4.79 (−13.2 to 3.64) | −5.43 (−12.7 to 1.82) | ||
| Black | −5.18 (−19.3 to 8.93) | −5.78 (−17.7 to 6.18) | ||
| Middle Eastern | 2.17 (−7.83 to 12.2) | 5.34 (−3.09 to 13.8) | ||
| Hispanic | −1.40 (−7.67 to 4.87) | −0.82 (−6.11 to 4.47) | ||
| History of anxiety | ||||
| Yes | 6.35 (1.45–11.3) | .015 | 5.27 (0.96–9.58) | .020 |
| History of depression | ||||
| Yes | 4.38 (−0.91 to 9.67) | .114 | 4.90 (0.39–9.41) | .041 |
| Baseline KPS | .629 | .855 | ||
| 80 | −2.31 (−11.1 to 6.51) | 0.32 (−7.32 to 7.96) | ||
| 90 | 1.33 (−2.79 to 5.45) | 1.03 (−2.69 to 4.75) | ||
| RT type | ||||
| Protons | −3.43 (−7.74 to 0.88) | .127 | −2.41 (−6.13 to 1.31) | .217 |
| Glioma | ||||
| Yes | 1.13 (−2.79 to 5.05) | .579 | 1.93 (−1.40 to 5.26) | .271 |
| Seizures | ||||
| Yes | 1.80 (−2.12 to 5.72) | .375 | 3.47 (0.14-6.80) | .043 |
| Antiepileptic drugs | ||||
| Yes | 0.44 (−3.42 to 4.36) | .825 | 1.97 (−0.38 to 4.32) | .251 |
| Chemotherapy | ||||
| Yes | 0.83 (−3.09 to 4.75) | .680 | 1.33 (−2.00 to 4.66) | .440 |
| Surgery | ||||
| Yes | −3.78 (−8.68 to 1.12) | .137 | −0.03 (−4.34 to 4.28) | .989 |
Abbreviations: CI = confidence interval; KPS = Karnofsky performance status; LRT = likelihood ratio test; RT = radiation therapy.
Neurocognitive function as predicted by anxiety symptoms
Figure 2 shows the results of analyzing longitudinal BAI score as a predictor for each independent neurocognitive assessment, assessed at the same time as the BAI score. Univariable models (Figure. 2a) show that an increase in symptoms of anxiety significantly predicted for a decreased T-score in 3 of the 5 assessments of attention and processing speed. These assessments include DKEFS-TMT visual scanning (β = −0.388, P = .003), number sequencing (β = −0.292, P = .011), and letter sequencing (β = −0.393, P < .001). After controlling for covariates in multivariable models (Figure. 2b), BAI remained a significant independent predictor of all 3 assessments of DKEFS-TMT visual scanning (β = −0.425, P < .001), number sequencing (β = −0.261, P = .011), and letter sequencing (β = −0.327, P = .003). Inclusion of covariates in final multivariable models are shown in Table E1 and Table E2.
Fig. 2.
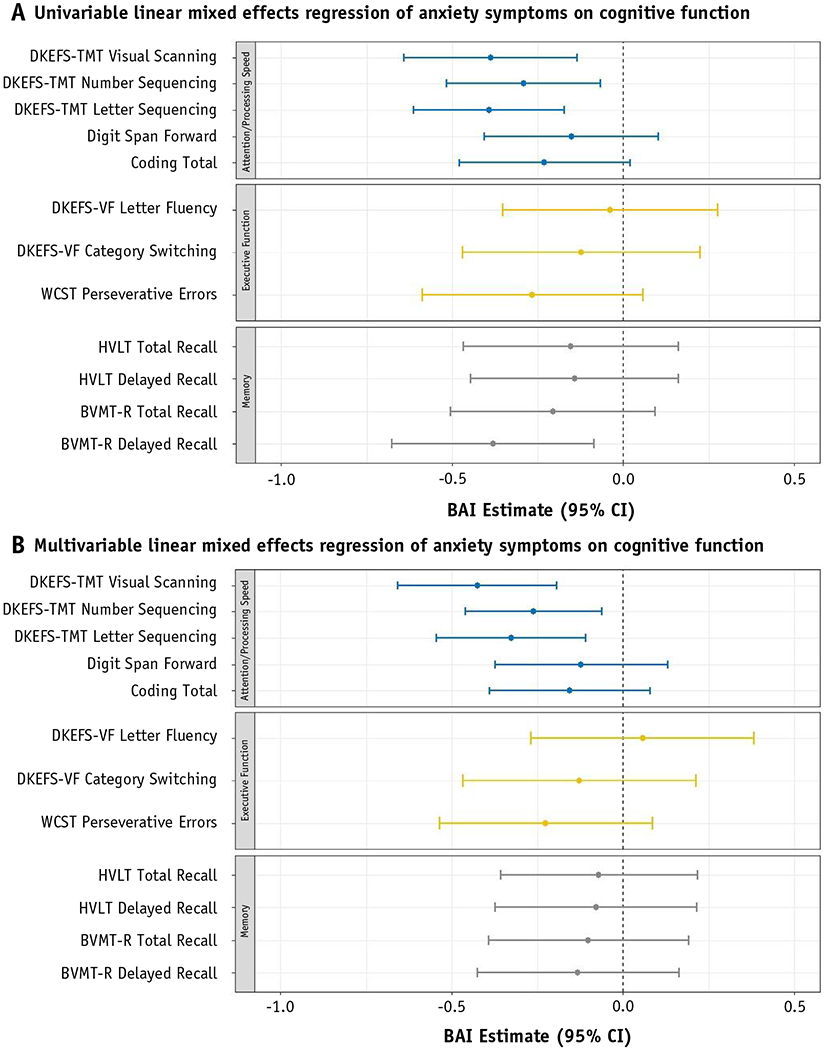
(a) Univariable linear mixed effects regression of anxiety symptoms on cognitive function. (b) Multivariable linear mixed effects regression of anxiety symptoms on cognitive function. Linear mixed effects analyses of BAI as a predictor of neurocognitive function. Assessments of attention or processing (5), executive functioning (3), and memory (4) are shown. Each assessment investigated with a unique model. β estimates are shown by dot. Whiskers reflect 95% confidence interval of the estimate. Significant associations are reflected by 95% confidence interval that do not cross the 0.0 reference line. (a) Shows univariable models. (b) Shows multivariable models, controlled for appropriate covariates. Abbreviations: BVMT-R Brief Visuospatial Memory Test-Revised; CI = confidence interval; DKEFS-TMT = Delis-Kaplan Executive Function System Trail-Making Test; DKEFS-VF = DKEFS Verbal Fluency; HVLT = Hopkins Verbal Learning Test-Revised; WCST = Wisconsin Card Sort Test.
An increase in BAI score was associated with a decrease in the Brief Visuospatial Memory Test-Revised Delayed Recall score (P = .012) in univariable analysis. In the multivariable model, which controlled for sex, time point, highest education achieved, baseline KPS, and benign versus glioma tumor histology, the relationship became nonsignificant (P = .383). In univariable analysis, BAI score approached significance for predicting the Coding Total score (β = −0.230, P = .071), but after controlling for sex, highest education level, and history or ongoing seizure activity, this association was not significant (P = .195).
Neurocognitive function as predicted by depressive symptoms
Figure 3 shows results of longitudinal BDI-II score as a predictor of each independent neurocognitive test score in univariable (Figure. 3a) and multivariable (Figure. 3b) analyses. Within the domain of attention and processing speed, BDI-II was a predictor of DKEFS-TMT letter sequencing in univariable (β = −0.381, P = .002) and multivariable analyses (β = −0.303, P = .013).
Fig. 3.
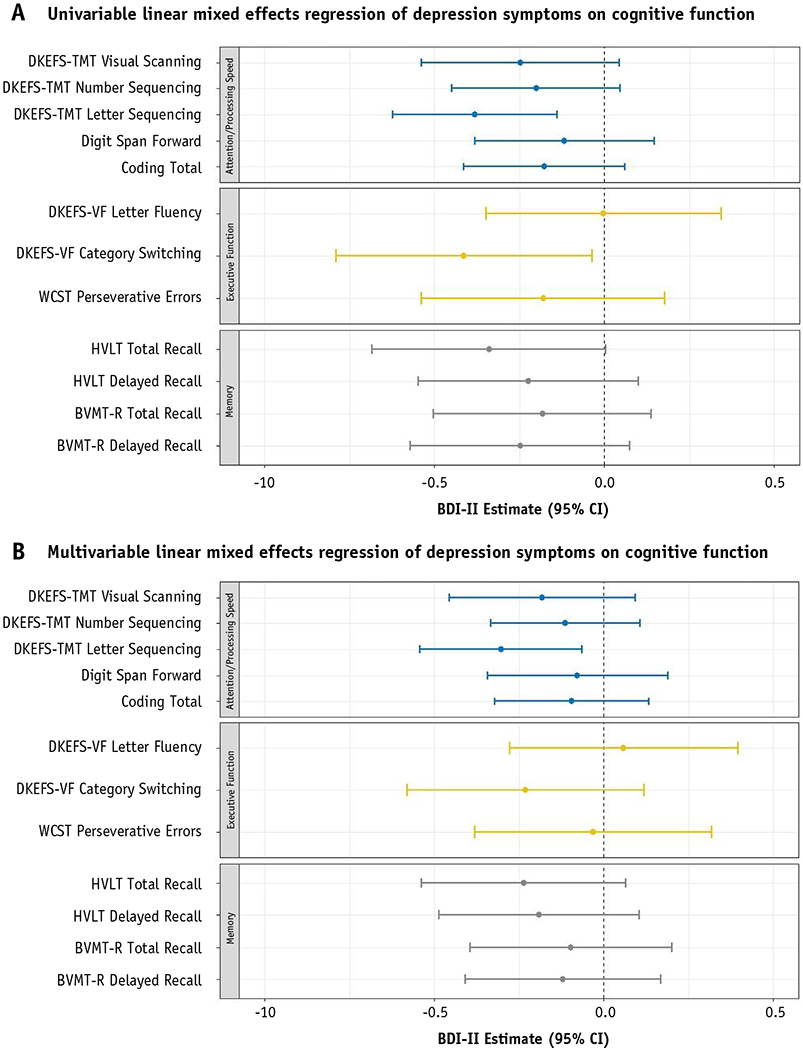
(a) Univariable linear mixed effects regression of depression symptoms on cognitive function. (b) Multivariable linear mixed effects regression of depression symptoms on cognitive function. Linear mixed effects analyses of BDI as a predictor of neurocognitive function assessments of attention and processing (5), executive functioning (3), and memory (4) are shown. Each assessment investigated with a unique model. β estimates are shown by dot. Whiskers reflect 95% confidence interval of the estimate. Significant associations are reflected by 95% confidence interval that do not cross the 0.0 reference line. (a) Shows univariable models. (b) Shows multivariable models, controlled for appropriate covariates. Abbreviations: BVMT-R Brief Visuospatial Memory Test-Revised; CI = confidence interval; DKEFS-TMT = Delis-Kaplan Executive Function System Trail-Making Test; DKEFS-VF = DKEFS Verbal Fluency; HVLT = Hopkins Verbal Learning Test-Revised; WCST = Wisconsin Card Sort Test.
Higher BDI-II score was associated with poorer DKEFS verbal fluency category switching in univariable analysis (β = −0.413, P = .031), but not multivariable analysis (P = .198), which controlled for time point, highest education level, baseline KPS, chemotherapy treatment, and history or ongoing seizures. For HVLT total recall, BDI-II trended toward significance in univariable (β = −0.339, P = .053) but not multivariable (P = .126) models, which controlled for highest education level and antiepileptic drug treatment.
Discussion
In this study, we explore how longitudinal changes in depression and anxiety symptoms can independently affect neurocognitive functioning in a cohort of primary brain tumor patients receiving RT. To our knowledge, this relationship has not been investigated among primary brain tumor patients. We found that an increase in anxiety symptoms is an independent predictor of neurocognitive functioning, specifically within the domain of attention and processing speed. Thus, to optimize neurocognition within primary brain tumor patients, standard of care should include screening for and treatment of subclinical symptoms of depression and anxiety to improve outcomes. As brain tumor clinical trials are increasingly using neurocognitive functioning as a primary endpoint26–29 commensurate with survival and quality of life, we recommend that symptoms of depression and anxiety should be quantified and controlled.
Brain tumor patients are known to be at risk of experiencing symptoms of anxiety and depression. Although our cohort had a greater median BDI-II score than BAI score at baseline, moderate-severe anxiety was more common than moderate-severe depression. We found a nonsignificant trend of depression symptoms over time, similar to previous descriptive studies of brain cancer6,43,44 and breast cancer.45 BAI scores significantly decreased over time within our cohort, also consistent with prior descriptive studies.44 Most patients in this cohort completed their baseline assessment in the postsurgical period, a time often wrought with emotional distress. Anxiety about poor prognosis and an unpredictable future contribute to pretreatment worry, which has been shown to alter brain function and contribute to neurocognitive dysfunction independent of the actual upcoming treatments.46 Ultimately these data support a high prevalence of subclinical depression and anxiety within primary brain tumor patients, and we argue for the early screening of depression and anxiety symptoms within this population.
We identified subsets of patients who may be at greater risk of experiencing symptoms of depression and anxiety. Consistent with previous studies,2,47 our results support that women and patients with lower education levels have higher baseline anxiety symptoms. Our data also shows an association between proton RT and lower baseline BAI levels; this is likely due to a higher proportion of benign and low-grade tumors treated by protons versus photons. Unsurprisingly, prior history of clinical depression and anxiety are consistently associated with elevated symptoms of these conditions.1,2 We also found that experiencing ongoing seizures is a predictor of increased BDI scores, which is not explained by antiepileptic drug use in this study or previous studies.24 Thus, seizures may present a unique neurologic risk for depressive symptoms independent of antiepileptic drug use or tumor diagnosis. We recommend universal screening of brain tumor patients for symptoms of depression and anxiety, but recognize that certain patient subsets have higher risk and may require closer follow-up.
Compared with data concerning symptoms of depression, there has been a dearth of literature regarding the relationship between anxiety symptoms and neurocognitive function. Here we investigate that relationship and find a significant correlation between the fluctuation of an individual’s anxiety symptoms score and that patient’s neurocognitive performance. The majority of the analyses within attention and processing speed show that BAI score is a significant predictor of test performance, with a greater number of symptoms associated with poorer performance. BAI score remains a significant, independent predictor after controlling for numerous characteristics, such as sex, educational attainment, tumor histology (benign versus glioma), surgical history, concurrent chemotherapy, and seizures. Anxiety manifests as a constellation of affective, cognitive, and physiologic symptoms that, beyond a certain threshold, can drain a person’s attentional reserve. This loss may in turn exert a direct influence on attention and processing speed function that we find in this study and in other neurologic diseases.48 Deficits in attention and processing speed are inextricably linked to intellectual function and a person’s perception of their intellectual function. This can effect productivity at work, relationships with friends and family, and self-image.19 We conclude that alleviating symptoms of anxiety may directly benefit patients with primary brain tumor from these cognitive and clinically important downstream effects.
We found that higher depression scores are associated with poorer performance on measures of attention and processing speed, executive functioning, and a trend within the memory domain. Although we maintain that the intrinsic nature of brain tumors causes a unique effect on neurocognition that should be investigated independently, these results are consistent with cohorts of different cancer subtypes. Among patients with breast cancer, both baseline anxiety and depression scores were significant longitudinal predictors of memory, attention, and executive functioning assessments.17 Still, other studies of patients with colorectal19 and prostate18 cancer did not find associations between symptoms of anxiety or depression and neurocognitive functioning. However, those studies explored the relationship using binary cognitive outcomes (global deficit scores, cognitive impairment), which can obscure domain-specific associations. Our analysis capitalizes on a robust neurocognitive assessment with validated measures within each cognitive domain of interest as well as validated, selfreport measures of depression and anxiety symptoms. The studies that obtained the sensitivity to detect domain-specific neurocognitive effects did in fact find significant associations between anxiety and depression symptoms and functioning within certain neurocognitive domains.17 These domain-specific effects of anxiety and depression symptoms are important to recognize, as they may have larger implications on prognosis49 and survival,6 especially within the brain tumor population.47
These results have strong implications for clinical care and ongoing clinical trial design. Due to the high prevalence of depression and anxiety symptoms within the primary brain tumor population and the emerging evidence that these symptoms affect neurocognition, quality of life, and survival, we argue for the universal screening and treatment of symptoms early in the diagnosis. Implementation may be similar to the International Prostate Symptom Score,50 in which ongoing screening becomes a major discussion point throughout treatment. Trials with neurocognitive functioning as a primary outcome should include screening and treatment protocols for depression and anxiety symptoms, as well as control for these symptoms in evaluation of cognitive assessment scores. These endpoints are not currently included in many ongoing trials.29 These steps have the potential to improve patient outcomes without RT dose modifications or treatment changes.
There are limitations to this study. The overall sample size is relatively small, though similar in size to other prospective trials of this nature.12,16,18 This cohort also underwent extensive, robust neurocognitive evaluation at each time point. As an exploratory analysis we make use of the richness of the data by exploring multiple neurocognitive domains, which provided granularity to detect important clinical correlations. Although all patients included were primary brain tumor patients receiving RT, heterogeneity existed with regard to treatment modality, tumor histology, and other characteristics. These differences were accounted for in the multivariable stepwise backward selection analysis, and thus the conclusions should be generalizable to any primary brain tumor patient presenting for treatment. Still, it is possible that there may be other unaccounted variables that could affect the relationship between mood and neurocognitive function. Although we cannot assume that the associations reported here are causative, our results suggest a clear independent association between symptoms of anxiety and depression and certain neurocognitive domains.
Conclusions
We have conducted the first prospective longitudinal analysis of the relationship between depression and anxiety symptoms and neurocognitive function among patients with primary brain tumors. Our study shows that depression and anxiety symptoms are independent predictors of neurocognitive functioning and may specifically influence attention and processing speed. These data support the evaluation and treatment of subclinical depression and anxiety symptoms throughout the disease course of primary brain tumor patients, as the relief of these symptoms may optimize cognition, quality of life, and overall survival. We urge ongoing and future clinical trials investigating neurocognitive functioning in brain tumor patients to include, treat, and control for symptoms of depression and anxiety.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (1TL1TR001444 to K.T., 1TL1TR001443 to M.D.T., UL1TR001442 of CTSA funding in support of CTRI, and 1KL2TR001444, UL1TR000100, R01 CA238783-01 to JAH-G); National Cancer Institute and UC San Diego Moores Cancer Center (P30 CA02310029 to JAH-G); and American Cancer Society (ACS-IRG 70-002 to CRM). The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies, who had no direct role in designing, conducting, or reporting the study.
Disclosures: J.A.H.-G. reports prior grant funding from Varian Medical Systems, unrelated to the present study. C.R.M. has research funding from GE Health care, unrelated to the present study. There are no financial or other relationships that might lead to a perceived conflict of interest.
Footnotes
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.07.002.
References
- 1.Litofsky SN, Resnick AG. The relationships between depression and brain tumors. J Neurooncol 2009;94:153–161. [DOI] [PubMed] [Google Scholar]
- 2.Arnold SD, Forman LM, Brigidi BD, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro Oncol 2008;10:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma: Clinical scholarship. J Nurs Scholarsh 2007;39:61–67. [DOI] [PubMed] [Google Scholar]
- 4.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment. Arch Intern Med 2000; 160:2101. [DOI] [PubMed] [Google Scholar]
- 5.Bortolato B, Hyphantis TN, Valpione S, et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat Rev 2017;52:58–70. [DOI] [PubMed] [Google Scholar]
- 6.Noll KR, Sullaway CM, Wefel JS. Depressive symptoms and executive function in relation to survival in patients with glioblastoma. J Neurooncol 2019;142:183–191. [DOI] [PubMed] [Google Scholar]
- 7.Meyers CA, Hess KR, Yung WKA, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol 2000;18:646–650. [DOI] [PubMed] [Google Scholar]
- 8.Lin NU, Lee EQ, Aoyama H, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: Neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol 2013;14:e407–e416. [DOI] [PubMed] [Google Scholar]
- 9.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord 2009;119:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Bierman EJM, Comijs HC, Rijmen F, Jonker C, Beekman ATF. Anxiety symptoms and cognitive performance in later life: Results from the longitudinal aging study Amsterdam. Aging Ment Heal 2008; 12:517–523. [DOI] [PubMed] [Google Scholar]
- 11.Smith MM, Mills JA, Epping EA, Paulsen JS, Westervelt HJ. Depressive symptom severity is related to poorer cognitive performance in prodromal Huntington disease. Neuropsychology 2012;26:664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirogovsky-Turk E, Moore RC, Filoteo JV, et al. Neuropsychiatric predictors of cognitive decline in Parkinson disease: A longitudinal study. Am J Geriatr Psychiatry 2017;25:279–289. [DOI] [PubMed] [Google Scholar]
- 13.Kalron A, Aloni R, Allali G. The relationship between depression, anxiety and cognition and its paradoxical impact on falls in multiple sclerosis patients. Mult Scler Relat Disord 2018;25:167–172. [DOI] [PubMed] [Google Scholar]
- 14.Williams MW, Kueider AM, Dmitrieva NO, et al. Anxiety symptoms bias memory assessment in older adults. Int J Geriatr Psychiatry 2017;32:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laukka EJ, Dykiert D, Allerhand M, Starr JM, Deary IJ. Effects of between-person differences and within-person changes in symptoms of anxiety and depression on older age cognitive performance. Psychol Med 2018;48:1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahles TA, Saykin AJ, Mcdonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol 2010;28:4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janelsins MC, Heckler CE, Peppone LJ, et al. Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol 2019;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez BD, Jim HSL, Booth-Jones M, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: A controlled comparison. J Clin Oncol 2015;33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vardy JL, Dhillon HM, Pond GR, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J Clin Oncol 2015;33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wefel JS, Noll KR, Rao G, Cahill DP. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncol 2016;18:1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep 2012;12:267–275. [DOI] [PubMed] [Google Scholar]
- 22.Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 2017;13:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irle E, Peper M, Wowra B, Kunze S. Mood changes after surgery for tumors of the cerebral cortex. Arch Neurol 1994;51:164–174. [DOI] [PubMed] [Google Scholar]
- 24.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol 2004;3:159–168. [DOI] [PubMed] [Google Scholar]
- 25.Pendergrass JC, Targum SD, Harrison JE. Cognitive impairment associated with cancer: A brief review. 2018;15:36–44. [PMC free article] [PubMed] [Google Scholar]
- 26.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases a randomized clinical trial. JAMA 2016;316:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gondi V, Deshmukh S, Brown PD, et al. Preservation of neurocognitive function (NCF) with conformal avoidance of the hippocampus during whole-brain radiotherapy (HA-WBRT) for brain metastases: Preliminary results of phase III trial NRG oncology CC001. Int J Radiat Oncol 2018;102:1607. [Google Scholar]
- 28.Warren E, Child A, Cirino P, et al. Better social, cognitive, and academic outcomes among pediatric brain tumor survivors treated with proton versus photon radiation therapy. Neuro Oncol 2018;20(suppl 2):166. [Google Scholar]
- 29.Grosshans DR, Gondi V, Shih HA, Mahajan A, Tsien CI, Tseng YD. Proton beam or intensity-modulated radiation therapy in preserving brain function in patients with IDH mutant grade II or III glioma (NRG-BN005). ClinicalTrials.gov Available at: https://clinicaltrials.gov/t2/show/study/NCT03180502 Published 2017.
- 30.Beck A, Steer R, Brown G. Beck Depression Inventory-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 31.Beck A, Epstein N, Brown G, Steer R. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 1988;56:893–897. [DOI] [PubMed] [Google Scholar]
- 32.Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: The relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol 2002; 57:41–49. [DOI] [PubMed] [Google Scholar]
- 33.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 34.Weschler D Manual for the Weschler Adult Intelligence Scale. ed 3 New York, NY: The Psychological Corporation; 1197. [Google Scholar]
- 35.Heaton R, Chelune G, Talley J, Kay G, Curtiss G. Wisconsin Card Sorting Test. Odessa, FL: Manual; 1993. [Google Scholar]
- 36.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test—revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 1998;12:43–55. [Google Scholar]
- 37.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the brief visuospatial memory test: Studies of normal performance, reliability, and validity. Psychol Assess 1996;8:145–153. [Google Scholar]
- 38.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol 2006;24:1305–1309. [DOI] [PubMed] [Google Scholar]
- 39.Bates DM, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. 2012. Available at: http://lme4.r-forge.r-project.org/. [Google Scholar]
- 40.Rs Team. RStudio: Integrated Development for R. 2015. Available at: http://www.rstudio.com/ Accessed February 15, 2020.
- 41.Tringale KR, Nguyen TT, Karunamuni R, et al. Quantitative imaging biomarkers of damage to critical memory regions are associated with post-radiation therapy memory performance in brain tumor patients. Int J Radiat Oncol Biol Phys 2019;105:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tringale KR, Nguyen T, Bahrami N, et al. Identifying early diffusion imaging biomarkers of regional white matter injury as indicators of executive function decline following brain radiotherapy: A prospective clinical trial in primary brain tumor patients. Radiother Oncol 2019; 132:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hickmann A-K, Nadji-Ohl M, Haug M, et al. Suicidal ideation, depression, and health-related quality of life in patients with benign and malignant brain tumors: A prospective observational study in 83 patients. Acta Neurochir 2016;158:1669–1682. [DOI] [PubMed] [Google Scholar]
- 44.Piil K, Jakobsen J, Christensen KB, Juhler M, Jarden M. Health-related quality of life in patients with high-grade gliomas: A quantitative longitudinal study. J Neurooncol 2015;124:185–195. [DOI] [PubMed] [Google Scholar]
- 45.Lyon DE, Cohen R, Chen H, et al. The relationship of cognitive performance to concurrent symptoms, cancer- and cancer-treatment-related variables in women with early-stage breast cancer: A 2-year longitudinal study. J Cancer Res Clin Oncol 2016;142:1461–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berman MG, Askren MK, Jung M, et al. Pretreatment worry and neurocognitive responses in women with breast cancer. Heal Psychol 2014;33:222–231. [DOI] [PubMed] [Google Scholar]
- 47.Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: A systematic review of observational studies. J Natl Cancer Inst 2011;103:61–76. [DOI] [PubMed] [Google Scholar]
- 48.Goretti B, Viterbo RG, Portaccio E, et al. Anxiety state affects information processing speed in patients with multiple sclerosis. Neurol Sci 2014;35:559–563. [DOI] [PubMed] [Google Scholar]
- 49.O’Neill BP, Meyers CA, Sawyer AM, Wefel JS, Johnson DR. Early measures of cognitive function predict survival in patients with newly diagnosed glioblastoma. Neuro Oncol 2012;14:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Fang D, Cooperberg MR, et al. Long-term follow-up of International Prostate Symptom Score (IPSS) in men following prostate brachytherapy. World J Urol 2014;32:1061–1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


