INTRODUCTION
Celiac disease (CD) is an autoimmune enteropathy triggered by exposure to gluten proteins, leading to intestinal inflammation and villous atrophy in genetically predisposed individuals. It is associated with robust B cell and antibody responses to gluten and to the transglutaminase 2 (TG2) autoantigen 1. In contrast, non-celiac gluten sensitivity (NCGS) is a poorly understood clinical entity defined by onset of symptoms in response to ingestion of gluten-containing food without the prerequisite serologic or histologic features of CD 2. There are no established biomarkers yet for NCGS, but recent research points to a biological basis, revealing a state of systemic immune activation in conjunction with a compromised intestinal epithelium 2, 3.
We and others have demonstrated a significant increase in IgG antibody to gluten in NCGS at levels similar to CD 2, 3. Accordingly, it has been speculated that an enhanced IgG response to gluten may be a common link between CD and NCGS 2. However, whether and how B cell reactivity to gluten may differ in these conditions, especially in the context of possible relevance to intestinal pathology, have not been examined.
In this study, we extend earlier data to show that the anti-gluten IgG antibody in NCGS is significantly different from CD in subclass distribution and in its relationship to intestinal cell damage. The findings are suggestive of a sustained primary B cell response to gluten in CD despite the condition’s chronicity, and a more advanced and tolerogenic immune response to gluten in NCGS.
METHODS
Detailed methods are available in Supplementary Methods.
RESULTS
Demographic and clinical characteristics of study cohorts are included in Supplementary Table 1.
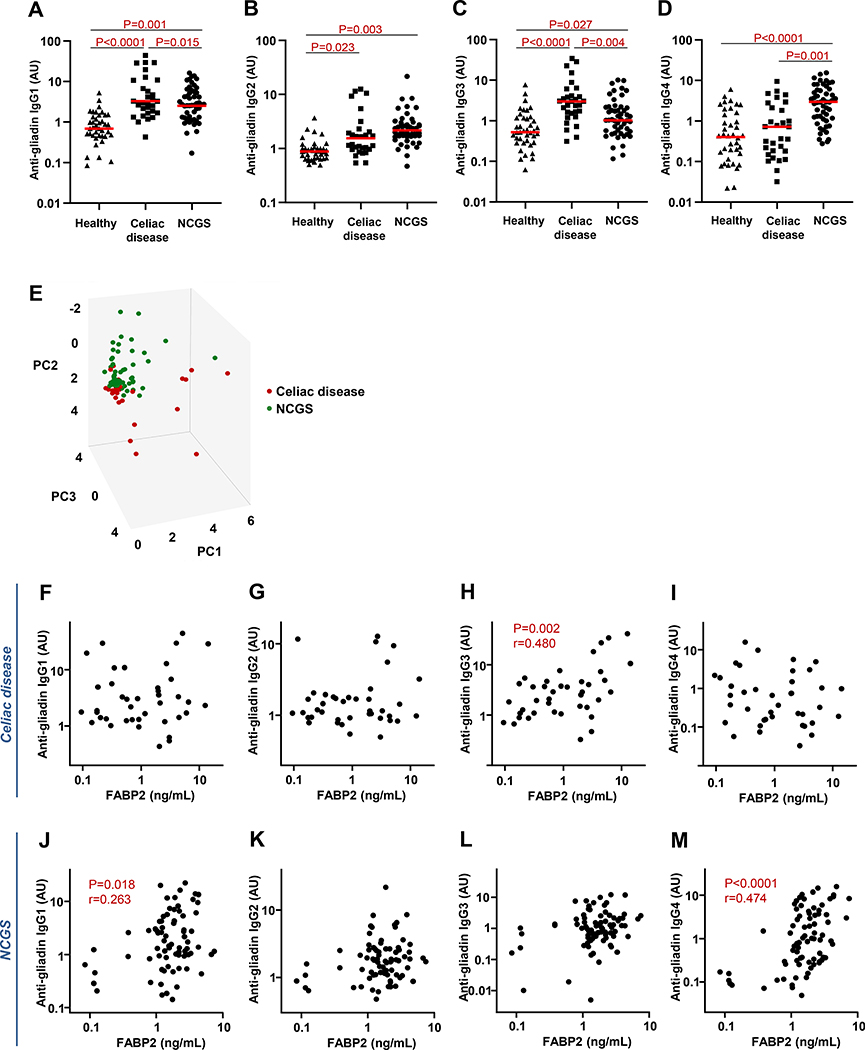
The anti-gliadin IgG response in CD patients was comprised primarily of IgG1 and IgG3, which were significantly increased in comparison with the healthy and NCGS cohorts (Fig. 1A,1C). There was a modest elevation in anti-gliadin IgG2 compared with the healthy group and no comparative increase in the IgG4 subclass (Fig. 1B,1D). Within the NCGS cohort, however, the lower contributions of anti-gliadin IgG1 and IgG3 in comparison with CD was compensated by significantly elevated IgG4 (compared with CD and healthy cohorts) and IgG2 (compared with healthy cohort) (Fig. 1A-D). No significant association was detected in this cohort between any anti-gliadin IgG subclass and the Marsh type, HLA-DQ2/DQ8 status, or eligibility for irritable bowel syndrome or functional dyspepsia diagnostic criteria.
Figure 1.
Distribution of IgG subclass antibody reactivity to wheat gluten and relationship with intestinal epithelial cell damage. A-D) Serum levels of IgG1 (A), IgG2 (B), IgG3 (C), and IgG4 (D) antibody to Prolamine Working Group (PWG) gliadin in cohorts of healthy controls and IgG anti-gliadin-positive celiac disease (CD) and non-celiac gluten sensitivity (NCGS) patients, as determined by ELISA. Horizontal red lines indicate the median for each cohort. E) Principal component analysis score plot of the entire anti-gliadin IgG subclass dataset (IgG1, IgG2, IgG3, and IgG4) for CD (red) and NCGS (green) patients. Subjects are plotted in three dimensions using the first through third principal components (PC1, PC2, and PC3). F-M) Relationship between FABP2 expression and IgG subclass antibody reactivity to gluten in CD and NCGS patients. Serum FABP2 concentrations in CD patients correlated with levels of anti-gliadin IgG3 antibody (H). In contrast, the NCGS cohort was characterized by a correlation between the levels of anti-gliadin IgG4 antibody and FABP2 concentration (M) and a weaker correlation between anti-gliadin IgG1 antibody and FABP2 (J).
The score plot for the principal component analysis of the IgG subclass data demonstrated clustering of the CD and NCGS subjects into discernible groups, further demonstrating the contrasting subclass distributions and suggesting potential biomarker value in these data (Fig. 1E).
Serum concentrations of intestinal fatty acid-binding protein (FABP2), a specific marker of intestinal epithelial cell damage 4, were similarly elevated in the CD and NCGS groups in comparison with healthy cohort (P<0.0001 for each) 3. Within the CD group, only the anti-gliadin IgG3 correlated with FABP2 (Fig. 1H). This correlation was similar in strength to that between anti-gliadin IgG3 and anti-TG2 IgA (r=0.505, P=0.001). In contrast, FABP2 levels in the NCGS group correlated with anti-gliadin IgG4 and weakly with IgG1 (Fig. 1M,1J).
DISCUSSION
The observed contrast in the IgG subclass distribution and relationship with FABP2 release in NCGS versus CD are likely reflective of differences in the evolution and disease relevance of B cell immune responses in the two conditions. Among IgG subclasses, IgG1 and IgG3 are the most potent activators of complement and efficient at binding a wide range of FcγRs 5. In contrast, IgG2 antibodies generally require higher epitope densities for complement activation and display limited binding to FcγRs 5. IgG4 antibodies contain structural properties that further distinguish them from other immunoglobulin isotypes and IgG subclasses. They bind weakly to Fc receptors and to complement, and are inefficient at crosslinking of antigens or forming immune complexes 5. IgG4 has also been shown to induce an anti-inflammatory M2-like macrophage phenotype through inhibition of IFNγ signaling 6. Considering these properties, the observed increase in the gluten-reactive IgG2 and IgG4 subclasses and the correlation between the IgG4 subclass and FABP2 in NCGS may point to a protective response aimed at dampening the inflammatory effect of other antibodies and immune cells. It is intriguing that these antibody responses are largely absent in CD, where there is instead a correlation between the IgG3 and FABP2.
The evolution in subclass switching of the IgG response to an antigen follows a 1-way direction from IgG3 to IgG1, IgG2, and IgG4 over time. Once a B cell has switched to a downstream subclass, it does not return to a preceding one 7. It has been suggested that IgG2 and IgG4 are part of the immunologic memory towards harmless and recurring antigens—an advanced immune response stimulated by a more extensive antigen exposure 8. In addition, the variable regions of IgG2 and IgG4 usually display greater levels of somatic hypermutation than IgG1 or IgG3, which can result in higher affinity for target antigens 7. As such, the prominence of the IgG3 subclass and its relationship with the autoimmune response and intestinal cell turnover in CD is suggestive of repeated activation of gluten-specific naive B cells, rather than of memory cells, in response to gluten exposure, despite the chronic nature of the disease. Pathways involved in this phenomenon may represent a source of molecular targets for therapeutic intervention. Possible shortfalls of this study include the lack of other disease controls and the fact that these observational data cannot establish a causal connection between subclass differences and the disease process.
These data warrant further examination of the evolution of gluten-reactive B cell response and subclass switching in CD and NCGS. In addition, information on other aspects of B cell and antibody variability, including affinity, glycosylation profile, and epitope specificity, is expected to contribute to a greater understanding of differences in the immune response to gluten and its relationship with disease pathophysiology in the two conditions. In conjunction with other previously identified markers, these components of the immune response to gluten are expected to provide additional biomarkers that may be informative in the context of stratifying potential disease subsets with varying mechanisms, prognoses, and responses to therapy.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Mary Ajamian for technical support and critical review of the manuscript.
FUNDING SOURCES
This study was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040 (to AA). Additional support was provided by the University of Ferrara through Fondo Incentivazione Ricerca (FIR) (to RDG) and Fondi Ateneo per la Ricerca (FAR) (to GC and RDG). The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- FABP2
intestinal fatty acid-binding protein
- FcγR
Fc-gamma receptor
- HLA
human leukocyte antigen
- IFNγ
interferon gamma
- NCGS
non-celiac gluten/wheat sensitivity
Footnotes
Competing financial interests: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med 2019;17:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volta U, De Giorgio R, Caio G, et al. Nonceliac wheat sensitivity: an immune-mediated condition with systemic manifestations. Gastroenterol Clin North Am 2019;48:165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhde M, Ajamian M, Caio G, et al. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016;65:1930–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta 2005;352:15–35. [DOI] [PubMed] [Google Scholar]
- 5.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014;5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swisher JF, Haddad DA, McGrath AG, et al. IgG4 can induce an M2-like phenotype in human monocyte-derived macrophages through FcgammaRI. MAbs 2014;6:1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela NM, Schaub S. The biology of IgG subclasses and their clinical relevance to transplantation. Transplantation 2018;102: S7–S13. [DOI] [PubMed] [Google Scholar]
- 8.de Jong BG, H IJ, Marques L, et al. Human IgG2- and IgG4-expressing memory B cells display enhanced molecular and phenotypic signs of maturity and accumulate with age. Immunol Cell Biol 2017;95:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.