Abstract
An 80-year-old male with severe, complex mitral regurgitation after recent TAVR despite optimal medical management presented in heart failure for percutaneous mitral valve repair and possible tricuspid valve repair. TEE demonstrated mixed Carpentier Type 1 and 2 components with annular dilation, two leaflet perforations, and excessive leaflet motion (P2 flail). There were three distinct MR jets appreciated reflection a central coaptation defect and two posterior mitral valve leaflet perforations emanating from a cystic dilatation. Under TEE guidance transseptal puncture and percutaneous edge-to-edge mitral valve repair was performed with a MitraClip XTR device (Abbott, Illinois, USA). A 10mm Amplatzer Muscular VSD Occluder (Abbott, Illinois, USA) was deployed to close one of the perforations on the posterior leaflet with a significant reduction in MR severity. Attempts at crossing the remaining defect were unsuccessful and the procedure was concluded. The patient recovered uneventfully and TTE on POD 1 and again on POD 34 demonstrated normal systolic dominance on pulmonary venous Doppler interrogation, mild to moderate MR, and a mean transvalvular gradient of 5mmHg. Both devices appeared firmly attached and stable. This is the first documented use of a VSD occluder device in this clinical scenario. Management of complex MR with an approach combining edge-to-edge repair for a central coaptation defect and leaflet flail with codeployment of a VSD occluder device to address a perforated leaflet is feasible and can achieve durable results.
Index Words: MitraClip, Amplatzer, transseptal, transvenous mitral valve repair
Introduction
80-year-old male with chronic diastolic heart failure and critical aortic stenosis (AS) presented for transcatheter aortic valve replacement (TAVR) evaluation. Prior history significant for coronary artery disease treated with bypass grafting 17-years prior, chronic atrial fibrillation, and hypertension.
He had NYHA Class IV symptoms with progressively worsening dyspnea, lower extremity edema, orthopnea, weight gain, and cough with blood-tinged sputum. Transthoracic echocardiography (TTE) demonstrated severe AS with mitral annular calcification (MAC) and moderate to severe mitral regurgitation (MR). He had high surgical risk and underwent uneventful TAVR with placement of a 29mm Edwards Sapien 3 Ultra pericardial bioprosthetic valve via transaxillary approach and discharged home on post-operative day (POD) 5. Transesopheageal echocardiography (TEE) at time of TAVR demonstrate severe central and anteriorly directed MR with systolic flow reversal in the pulmonary veins, severe posterior MAC, posterior mitral valve leaflet (MVL) perforation, and moderate central tricuspid regurgitation. The interdisciplinary heart valve team evaluation deemed him at high surgical risk due to age, frailty, comorbidities, and previous cardiac surgery via sternotomy. His predicted risk of mortality using the EuroScore II was 24.21%.
He returned to clinic on POD 23 with lower extremity edema. Exam was significant for jugular venous distention, basilar crackles, a grade 3 blowing systolic murmur, and bilateral pitting edema to the knees. Chest radiograph demonstrated bilateral pleural effusions. TTE demonstrated a well-seated bioprosthetic valve with normal appearing prosthetic leaflet motion, trace paravalvular leak (PVL), and mean transvalvular pressure gradient of 6.3mmHg with severe eccentric MR. Planning TEE demonstrated central MR and two perforations in the posterior leaflet (Figures 1 and 2). Endocarditis was excluded due to lack of clinical evidence of active endocarditis, negative blood cultures, and no signs of vegetative lesions on TEE. The decision was made to pursue percutaneous mitral valve repair and possible concurrent tricuspid valve repair. Informed consent was obtained and the plan for off-label implementation of occluder devices to address the leaflet perforation was discussed in detail with the patient and family prior to proceeding. When discussing implantation of any intracardiac device we emphasize the risks of procedural failure, damage to structures within the heart, damage to blood vessels, bleeding, infection, and device embolization.
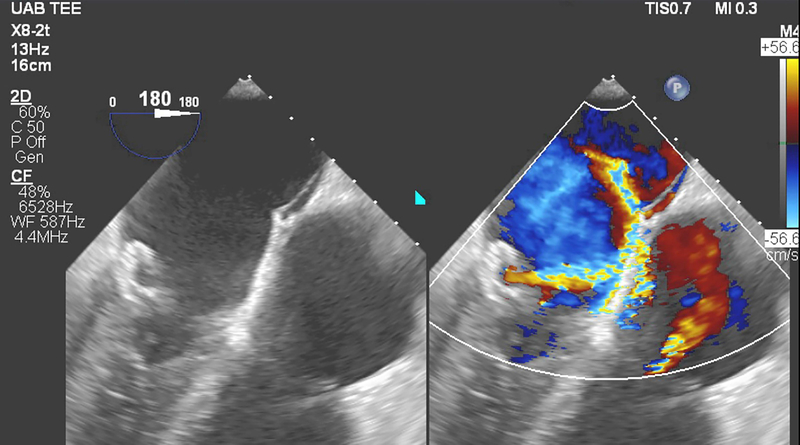
Figure 1.
Screening transesophageal echocardiography exam identified two posterior leaflet perforations. Significant color flow and coanda effect were noted through one perforation.
Figure 2.
Second, smaller posterior leaflet perforation is noted on screening echocardiography.
Case Series
He presented to the catheterization lab for percutaneous mitral valve repair. TEE under general anesthesia with positive pressure ventilation was significant for biatrial dilation, an aneurysmal interatrial septum with a patent foramen ovale, and severe MR. There was Carpentier type 1 and 2 MV dysfunction with annular dilation (41.9mm in the anterior to posterior diameter), two posterior leaflet perforations, and excessive leaflet motion. Severe posterior MAC and three distinct MR jets were present. Two distinct posterior MVL perforations arising from a large cavity or cystic dilatation of the posterior MVL was consistent with initial planning TEE. A central defect was related to poor coaptation and P2 flail. Severe eccentric jet originating from the base of the cavity noted on the posterior MVL was directed anteriorly into the left atrial surface of the anterior MVL. A third jet emanated from the antero-superior aspect of the cavity and was directed anteriorly. Figure 3 and 4 demonstrate the 3D appearance of the cystic dilation on TEE imaging. Video 1 demonstrates the 3D en face appearance of the mitral valve leaflets and Video 2 illustrates the preprocedural MR with color Doppler. Transvalvular mean gradient was 3mmHg with a mitral valve area (MVA) of 5.66cm2 on 3D analysis. Percutaneous edge-to-edge mitral valve repair was performed with a MitraClip XTR device (Abbott, Illinois, USA) with successful capture of A2 and the P2 flail segment (Video 3) and elimination of the central MR. Two eccentric jets originating from perforations in the posterior MVL remained (Figure 5 and Video 4 demonstrate the larger of the two perforations). The delivery system was removed and exchanged over wire with a 24F sheath. A steerable introducer was then advanced into the left atrium and a 6F guide catheter was directed into the larger perforation at the base using TEE guidance (Videos 5 and 6). Multiple Amplatzer (Abbott, Abbott Park, Illinois) Vascular Plug II devices were tried sequentially (8mm, 12mm, 18mm) to occlude the defect but failed to obtain satisfactory results (Video 7). Subsequently, a 10 mm Amplatzer Muscular VSD Occluder was deployed with good results and significant reduction in MR across this perforation (Video 8). Due to residual MR, attempts were made to cross the residual defect using guidewires and a multipurpose catheter without success (Video 9 demonstrates residual MR, Videos 10 and 11 illustrate attempts to advance wire across smaller perforation). At the conclusion, a stable tissue bridge between the anterior and posterior MVLs was noted with good bileaflet capture by the MitraClip XTR. There was resolution of the central MR jet with improvement in the larger posterior MVL perforation after VSD occluder deployment. The MitraClip and VSD occluder appeared stable (Figure 6, Figure 7, Videos 12–15). Post-procedure transvalvular gradient was 3mmHg. The cumulative MVA of the double orifice valve was measured at 2.23cm2 on 3D. Severe eccentric MR was noted through the remaining perforation with coanda effect around the anterior wall of the atrium (Video 16) and systolic flow reversal persisting in the left upper pulmonary vein. Despite the residual eccentric MR, the ratio of the MR jet size to the size of the LA was significantly reduced and was attributed to the elimination of the central MR jet as well as elimination of flow through the occluded larger perforation. The V-wave was reduced from 42mmHg to 29mmHg, thus the procedure was felt to have affected a significant reduction in MR and the imager and operators agreed that there was reasonable echocardiographic and hemodynamic data to support procedural closure. The decision was made to forgo attempts at percutaneous repair of the tricuspid valve and evaluate the patient for symptomatic improvement. The procedure lasted 190 minutes and the patient’s hemodynamics remained stable throughout the duration of the case with no vasoactive or inotropic infusions required at any point during the procedure.
Figure 3.
Pre-intervention 3D en face view of the mitral valve oriented in the “surgeon’s view.” There is cystic dilatation of the posterior mitral valve leaflet.
Figure 4.
Pre-intervention 3D model of the mitral valve demonstrating cystic dilatation of the posterior MVL.
Figure 5.
Mid-esophageal view demonstrating one of the large eccentric jets originating from the posterior MVL perforation obtained shortly after edge-to-edge repair was achieved. The previously noted central MR jet is no longer appreciated.
Figure 6.
Post-intervention mid-esophageal mitral commissural view with the MitraClip XTR device noted at A2/P2 and the 10mm Amplatzer muscular VSD occluder appreciated posterio-medially.
Figure 7.
3D en face view of the mitral valve demonstrating the double-orifice mitral valve with the MitraClip XTR device appreciated bridging A2/P2 and the 10mm Amplatzer muscular VSD occluder positioned just medial to the MitraClip on the posterior MVL.
Follow-up
The patient recovered uneventfully and POD 1 TTE demonstrated stable devices with mild residual MR. He reported improvement in dyspnea and was discharged home on POD 5 with antibiotic therapy for presumed culture-negative endocarditis as etiology of the perforated cystic dilatation on the posterior MVL. He returned on POD 34 for follow-up and TTE demonstrated normal systolic dominance on pulmonary venous Doppler interrogation, mild to moderate MR, and mean transvalvular gradient of 5mmHg. The MitraClip and Amplatzer VSD occluder devices appeared firmly attached and stable. His weight was stable and reported improvement in shortness of breath and fatigue. The patient survived for 6 months with one hospitalization for a gastrointestinal bleed at 4 months post-intervention. At the last follow-up (2-months prior to death) there was no evidence of device failure or infection and his heart failure symptoms were stable. The cause of death was not disclosed by the family on follow-up.
Discussion
We describe a case of complex MR addressed with both percutaneous edge-to-edge repair as well as co-deployment of a VSD occluder device across a perforated region of the posterior MVL. In our preprocedural planning we anticipated that it may be possible to deploy an XTR clip to both 1) close the coaptation defect as well as 2) cross over a significant portion of the perforated section of leaflet. This could potentially address both the main perforation as well as the central MR jet. It was readily apparent that the XTR clip would not reach the perforated area. We proceeded with placement of the MitraClip believing that clip deployment would reduce leaflet motion, make perforation occlusion technically easier, and minimize the chance of occluder motion over time. The clip deployment was straightforward and effective in reducing the severity of the MR through an elimination of the central component of the jet.
There are rare reports of successful percutaneous closure of mitral valve perforations using a variety of off label closure devices. An Amplatzer duct occluder II was utilized to close a postoperative iatrogenic perforation in the anterior leaflet of the mitral valve via a retrograde approach, accomplished secondary to the ease of deliverability of this device1. Similarly, the Amplatzer vascular plug III, not available in the United States, was used to close an iatrogenic anterior leaflet perforation2. The Amplatzer vascular plug II has been utilized with limited success and in one case, success was achieved by pulling the device across the transseptal puncture to anchor the device3. Double disk atrial septal defect closure devices have offered a more attractive symmetric positioning on the mitral valve leaflet and can be stabilized by a MitraClip4–6. Secondary to hemolysis with residual flow through or alongside Amplatzer devices, the more occlusive Gore (W. L. Gore & Associates, Flagstaff, Arizona) Cardioform might offer superior short term term closure4,6. While the Gore Cardioform device was a consideration because of rapid closure with a lower risk of hemolysis we felt the VSD occluder device, which is designed for high flow and high pressure closure, would be easier to deliver and recapture if necessary with the delivery system crossing back into the left ventricle if needed.
Despite advances in the periprocedural management of structural heart patients an optimal result is not always possible at the conclusion of the case. We report a situation where in the immediate post-deployment period there was severe residual MR appreciated with coanda effect and persistent pulmonary venous flow reversal. It is possible that successive attempts to plug the defect could enlarge a perforation however the leak characteristics were not noticeably changed through the course of the procedure, the devices were deployed in a standard fashion, and there was no need for a ‘tug-test’ in each case. Interestingly, the follow up TTEs on POD 1 and POD 34 demonstrated mild residual MR and a normal pattern of systolic dominance of the pulmonary venous waveform was appreciated. Adequate windows were obtained for a comprehensive evaluation of MR severity on TTE and good Doppler alignment was noted for pulmonary vein interrogation. Further, while hospitalized the patient endorsed an improvement in his dyspnea consistent with a reduction in the extent of regurgitant flow. Hemodynamics were likely different between the immediate post-deployment period with the patient under general anesthesia (GA) with positive pressure ventilation (PPV) and the follow up TTEs performed on an awake, spontaneously ventilating patient. One would anticipate that the MR severity would be lessened under GA due to afterload reduction related to the vasodilatory effects of the volatile anesthetics. Finally, the application of PPV and PEEP (in our case only 5 cmH2O) can change left ventricular (LV) geometry, but these changes would be expected to decrease venous return (preload) potentially decreasing LV diameter and improving leaflet coaptation which would likely further decrease MR severity compared to the awake, spontaneously ventilating patient7. Taken together, the MR severity would be expected to appear worse on follow-up exam in contrast to what we observed.
An additional explanation for improvement is a shift in device, resulting in improved occlusion of the perforation. Despite the stable appearance of the devices at the conclusion of the case the VSD occluder is located immediately adjacent to the MitraClip device and normal valvular motion throughout the cardiac cycle may have resulted in friction between the two creating a small shift in position with the fortuitous effect of addressing the residual perforation flow that was not amendable to closure intraoperatively.
Conclusion
Management of complex MR with an approach combining edge-to-edge repair for a central coaptation defect and leaflet flail with deployment of a VSD occluder device to address a perforation in a cystic leaflet dilatation is feasible and can achieve durable results.
Supplementary Material
Video 1. Pre-intervention 3D en face view of the mitral valve. Cystic dilation of the posterior leaflet is appreciated.
Video 2. Pre-intervention mid-esophageal long axis view with color flow Doppler. Two of the three components of the patient’s MR can be appreciated here with the central jet, and the larger more anteriorly directed eccentric component concurrently visualized.
Video 4. Mid-esophageal view demonstrating one of the large eccentric jets originating from the posterior mitral valve leaflet perforation after edge-to-edge repair. The previously noted central jet is no longer appreciated.
Video 6. Mid-esophageal aortic valve long axis view demonstrating 6F guide across the posterior mitral valve leaflet perforation.
Video 3. Mid-esophageal long axis view and 90 degree orthogonal mid-esophageal mitral commissural view. The leaflets are grasped with a MitraClip XTR device prior to clip release.
Video 7. Mid-esophageal view with color flow Doppler demonstrated deployed Amplatzer plug device with residual MR. This device was not released.
Video 5. Mid-esophageal aortic valve long axis view demonstrating the advancement of wire to cross the perforation.
Video 8. Mid-esophageal view with color flow Doppler demonstrated larger Amplatzer plug device with residual MR appreciated. This device was not released.
Video 9. Mid-esophageal aortic valve long axis view after deployment of a 10mm Amplatzer muscular VSD occluder. This view illustrates the smaller defect located more proximally to the base of the posterior MVL.
Video 11. Mid-esophageal aortic valve long axis view demonstrating attempts to cross the smaller, more posterior defect in the posterior mitral valve leaflet with a wire.
Video 10. Mid-esophageal aortic valve long axis view demonstrating attempts to cross the smaller, more posterior defect in the posterior mitral valve leaflet with a wire. The MitraClip XTR device is appreciated bridging at A2/P2 in this clip. With respiratory motion a small portion of the Amplazter VSD device is in view at the end of the clip.
Video 13. 3D en face view of the mitral valve demonstrating the the MitraClip XTR device appreciated bridging A2/P2 and the 10mm Amplatzer muscular VSD occluder positioned just medial to the MitraClip on the posterior MVL.
Video 14. 3D en face view of the mitral valve. The light source has been optimized to highlight the 10mm Amplatzer muscular VSD occluder positioned just medial to the MitraClip on the posterior MVL.
Video 12. Post-intervention mid-esophageal mitral commissural view with the MitraClip XTR device noted at A2/P2 and the 10mm Amplatzer muscular VSD occluder appreciated posterio-medially.
Video 15. Mid-esophageal aortic valve long axis view with medial adjustment to illustrate the stable Amplatzer muscular VSD occluder on the posterior leaflet.
Video 16. Mid-esophageal aortic valve long axis view illustrating the smaller residual MVL defect.
Acknowledgments
Funding Statement: Dr. Addis is supported by the National Institutes of Health (NIH) grant number T32HL129948
Footnotes
Financial Disclosures: Dr. Addis and Dr. Law have no financial disclosures. Dr. Von Mering serves on the peripheral vascular medical advisory board for Boston Scientific Corporation and is a speaker regarding left atrial appendage closure. Dr. Ahmed serves as a consultant and proctor for Abbott, Edwards, and Medtronic.
Clinical trial number and registry URL: Not applicable
Prior Presentations: None
Contributor Information
Dylan R. Addis, University of Alabama at Birmingham School of Medicine, Department of Anesthesiology and Perioperative Medicine, Division of Cardiothoracic Anesthesiology, Division of Molecular and Translational Biomedicine, and the UAB Comprehensive Cardiovascular Center.
Mark Law, University of Alabama at Birmingham School of Medicine, Department of Pediatrics, Division of Pediatric Cardiology and Department of Medicine, Division of Cardiovascular Disease.
Gregory von Mering, University of Alabama at Birmingham School of Medicine, Department of Medicine, Division of Cardiovascular Disease, and the UAB Comprehensive Cardiovascular Center.
Mustafa Ahmed, University of Alabama at Birmingham School of Medicine, Department of Medicine, Division of Cardiovascular Disease.
References
- 1.Sengun B, Yildirim I, Yildiz O, Celiker A. Retrograde transcatheter closure of anterior mitral valve leaflet perforation. Ann Pediatr Cardiol 2019;12:312–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velasco S, Larman M, Eneriz M. Percutaneous Closure of a Native Mitral Valve Perforation. Revista Española de Cardiología (English Edition) 2010;63:597. [DOI] [PubMed] [Google Scholar]
- 3.Grant EK, Kim DW, Border WL, Lerakis S, Babaliaros V, Vincent RN. Transseptal Anchored Vascular Plug Closure of Mitral Valve Perforation. JACC Cardiovascular interventions 2017;10:e45–e6. [DOI] [PubMed] [Google Scholar]
- 4.Frisoli TM, Greenbaum A, O’Neill WW, Wang DD, Eng M. Percutaneous Repair of Mitral Valve Leaflet Perforation. JACC Cardiovascular interventions 2019;12:210–3. [DOI] [PubMed] [Google Scholar]
- 5.Klapyta A, Pregowski J, Chmielak Z, Szymanski P, Witkowski A, Demkow M. Bail-out use of the Amplatzer Septal Occluder for treatment of acute iatrogenic leaflet perforation during the MitraClip procedure in a patient with functional mitral regurgitation. Postepy Kardiol Interwencyjnej 2018;14:304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panaich SS, Qazi AH, Horwitz PA, Staffey K, Rossen JD. Transcatheter repair of anterior mitral leaflet perforation: deploy, retrieve, redeploy. JACC: Case Reports 2019;1:689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patzelt J, Zhang Y, Seizer P, et al. Effects of Mechanical Ventilation on Heart Geometry and Mitral Valve Leaflet Coaptation During Percutaneous Edge-to-Edge Mitral Valve Repair. JACC Cardiovascular interventions 2016;9:151–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Pre-intervention 3D en face view of the mitral valve. Cystic dilation of the posterior leaflet is appreciated.
Video 2. Pre-intervention mid-esophageal long axis view with color flow Doppler. Two of the three components of the patient’s MR can be appreciated here with the central jet, and the larger more anteriorly directed eccentric component concurrently visualized.
Video 4. Mid-esophageal view demonstrating one of the large eccentric jets originating from the posterior mitral valve leaflet perforation after edge-to-edge repair. The previously noted central jet is no longer appreciated.
Video 6. Mid-esophageal aortic valve long axis view demonstrating 6F guide across the posterior mitral valve leaflet perforation.
Video 3. Mid-esophageal long axis view and 90 degree orthogonal mid-esophageal mitral commissural view. The leaflets are grasped with a MitraClip XTR device prior to clip release.
Video 7. Mid-esophageal view with color flow Doppler demonstrated deployed Amplatzer plug device with residual MR. This device was not released.
Video 5. Mid-esophageal aortic valve long axis view demonstrating the advancement of wire to cross the perforation.
Video 8. Mid-esophageal view with color flow Doppler demonstrated larger Amplatzer plug device with residual MR appreciated. This device was not released.
Video 9. Mid-esophageal aortic valve long axis view after deployment of a 10mm Amplatzer muscular VSD occluder. This view illustrates the smaller defect located more proximally to the base of the posterior MVL.
Video 11. Mid-esophageal aortic valve long axis view demonstrating attempts to cross the smaller, more posterior defect in the posterior mitral valve leaflet with a wire.
Video 10. Mid-esophageal aortic valve long axis view demonstrating attempts to cross the smaller, more posterior defect in the posterior mitral valve leaflet with a wire. The MitraClip XTR device is appreciated bridging at A2/P2 in this clip. With respiratory motion a small portion of the Amplazter VSD device is in view at the end of the clip.
Video 13. 3D en face view of the mitral valve demonstrating the the MitraClip XTR device appreciated bridging A2/P2 and the 10mm Amplatzer muscular VSD occluder positioned just medial to the MitraClip on the posterior MVL.
Video 14. 3D en face view of the mitral valve. The light source has been optimized to highlight the 10mm Amplatzer muscular VSD occluder positioned just medial to the MitraClip on the posterior MVL.
Video 12. Post-intervention mid-esophageal mitral commissural view with the MitraClip XTR device noted at A2/P2 and the 10mm Amplatzer muscular VSD occluder appreciated posterio-medially.
Video 15. Mid-esophageal aortic valve long axis view with medial adjustment to illustrate the stable Amplatzer muscular VSD occluder on the posterior leaflet.
Video 16. Mid-esophageal aortic valve long axis view illustrating the smaller residual MVL defect.